Abstract
Intraspecific variation in crop responses to global climate change conditions would provide opportunities to adapt crops to future climates. These experiments explored intraspecific variation in response to elevated CO2 and to high temperature during anthesis in Chenopodium quinoa Wild. Three cultivars of quinoa were grown to maturity at 400 (“ambient”) and 600 (“elevated”) μmol·mol−1 CO2 concentrations at 20/14 °C day/night (“control”) temperatures, with or without exposure to day/night temperatures of 35/29 °C (“high” temperatures) for seven days during anthesis. At control temperatures, the elevated CO2 concentration increased the total aboveground dry mass at maturity similarly in all cultivars, but by only about 10%. A large down-regulation of photosynthesis at elevated CO2 occurred during grain filling. In contrast to shoot mass, the increase in seed dry mass at elevated CO2 ranged from 12% to 44% among cultivars at the control temperature. At ambient CO2, the week-long high temperature treatment greatly decreased (0.30 × control) or increased (1.70 × control) seed yield, depending on the cultivar. At elevated CO2, the high temperature treatment increased seed yield moderately in all cultivars. These quinoa cultivars had a wide range of responses to both elevated CO2 and to high temperatures during anthesis, and much more variation in harvest index responses to elevated CO2 than other crops that have been examined.
Keywords: quinoa, elevated CO2, high temperature stress, photosynthesis, harvest index, seed yield
1. Introduction
The yearly mean concentration of carbon dioxide in the atmosphere has increased from about 320 μmol·mol−1 in 1965 to about 400 μmol·mol−1 currently, and continues to increase rapidly. Research has shown that concentrations above the current ambient concentration generally increase the growth and yield of C3 crop species, and could stimulate future crop yields, unless other changes in climate, such as rising temperatures or altered precipitation, interfere. Intraspecific variation in the stimulation in yield at elevated carbon dioxide concentrations has been detected in many crops species, including barley [1], common bean [2], cowpea [3], soybean [4,5], wheat [6,7], oats [8], rapeseed [1,9], and rice [10]. Identification of traits associated with larger yield increases at elevated carbon dioxide would be desirable for breeding cultivars better able to exploit the rising carbon dioxide concentration. In most cases, reasons for cultivar differences in yield response to elevated carbon dioxide, beyond differential increases in seed number, have not been identified [11]. Harvest index, defined as the ratio of the economic product relative to the total aboveground biomass at maturity, generally decreases slightly or is unchanged at elevated CO2 in crops where the economic product results from sexual reproduction [12,13,14].
Rising mean temperature and increased frequency of extreme high temperature events may reduce crop yields [15,16,17,18,19]. Many annual seed-producing crops are grown in regions where temperatures during reproductive development are near or even above the optimum for seed yield [19], and increases in temperature tend to decrease yields because of high temperature stress, in spite of increasing the growing season length. Despite predictions that elevated CO2 might mitigate reductions in yield caused by high temperatures based on increasing the optimum temperature for photosynthesis [19,20], several studies in various crop species have found that elevated CO2 exacerbated yield reductions caused by high temperatures [21,22,23,24,25,26,27,28,29]. With the exception that elevated CO2 may increase tissue temperatures by reducing stomatal conductance and transpiration [26], reasons why elevated CO2 may exacerbate yield reductions caused by high temperatures remain largely unknown. There is little information about differences among cultivars in response to high temperature and elevated CO2 treatments [28].
Quinoa (Chenopodium quinoa Willd) is an annual grain crop native to the Andes of South America, with Peru and Bolivia currently being the largest producers. It is increasing in popularity because of its high nutritional quality. It has been studied for variation in adaptation to salinity, drought, and elevation [30,31,32,33,34], but not with regard to climate change factors. In this study, three cultivars were studied for responses to elevated CO2 and to high temperature stress during anthesis, which is typically the phase of development in which seed yield is most sensitive to high temperature stress [18].
2. Results
The beginning of anthesis of main stem flowers occurred at 40 to 44 days after planting of seeds, depending upon the cultivar, and CO2 concentration had no effect on this timing (not shown). Most main stem leaves had senesced and seeds were mature at about 90 days after planting, except that the high temperature treatment at the lower CO2 concentration resulted in a slower progression of anthesis up the main stem in Salcedo, which prolonged seed filling and delayed maturity by about 10 days. The failure of seed development resulting from the high temperature treatment was evident in Cherry Vanilla grown at the lower CO2 concentration, but not in other cases. In Cherry Vanilla with the high temperature treatment, there was no flower abortion, but flowers often produced no seeds.
Leaf photosynthetic rates were very similar for the three cultivars under all conditions (Table 1). Increasing CO2 from 400 to 600 μmol·mol−1 increased leaf photosynthesis of plants grown at the lower CO2 concentration by about 14% at 20 °C, at the growth photosynthetic photon flux density (PPFD) (Table 1). At 35 °C, the increase was about 25%. Prior to anthesis, and during the high temperature treatment, there was little difference in photosynthesis between plants grown at the two CO2 concentrations when measured at the higher CO2 (Table 1), indicating no significant down-regulation of photosynthesis due to growth at elevated CO2 at these stages of development. However, after the stress period, and during grain filling, there was no difference in photosynthesis between plants grown at 400 and 600 μmol·mol−1 CO2 measured at their growth CO2 and PPFD conditions, while plants grown at the lower CO2 concentration had higher rates when measured at 600 μmol·mol−1 (Table 1). This is indicative of the down-regulation of photosynthesis during the grain filling period, resulting from growth at the elevated CO2 concentration. There was no after-effect of the heat stress on leaf photosynthetic rates in any cultivar (Table 1).
Table 1.
Photosynthesis (in μmol CO2 m−2·s−1) of upper leaves of three cultivars of quinoa grown at ambient (400 μmol·mol−1) or elevated (600 μmol·mol−1) CO2 concentrations, with or without a high temperature stress treatment during anthesis. Photosynthesis was measured at the growth PPFD of 1000 μmol·m−2·s−1, and at the current daytime growth temperature (20 °C before and after the stress, and 35 °C during the stress). Photosynthesis was measured at the growth CO2, ambient or elevated, and ambient plants were also measured at elevated CO2 (ambient at elevated). Numbers followed by the same letters within a growth stage were not significantly different at p = 0.05.
| Growth Stage | Cultivar | Ambient | Elevated | Ambient at Elevated |
|---|---|---|---|---|
| Before stress | Cherry Vanilla | 38.8a | 42.6b | 44.2b |
| Red Head | 39.5a | 42.5b | 45.0b | |
| Salcedo | 37.8a | 43.6b | 42.0b | |
| During stress | Cherry Vanilla | 32.7a | 39.8b | 40.8b |
| Stressed plants | Red Head | 34.1a | 41.6b | 41.7b |
| Salcedo | 32.2a | 37.8b | 38.9b | |
| After stress | Cherry Vanilla | 35.2a | 34.2a | 40.3b |
| Stressed plants | Red Head | 36.3a | 37.1a | 41.1b |
| Salcedo | 34.1a | 35.0a | 39.6b | |
| After stress | Cherry Vanilla | 34.7a | 35.4a | 40.8b |
| Control plants | Red Head | 35.8a | 36.2a | 41.0b |
| Salcedo | 35.1a | 35.8a | 39.3b |
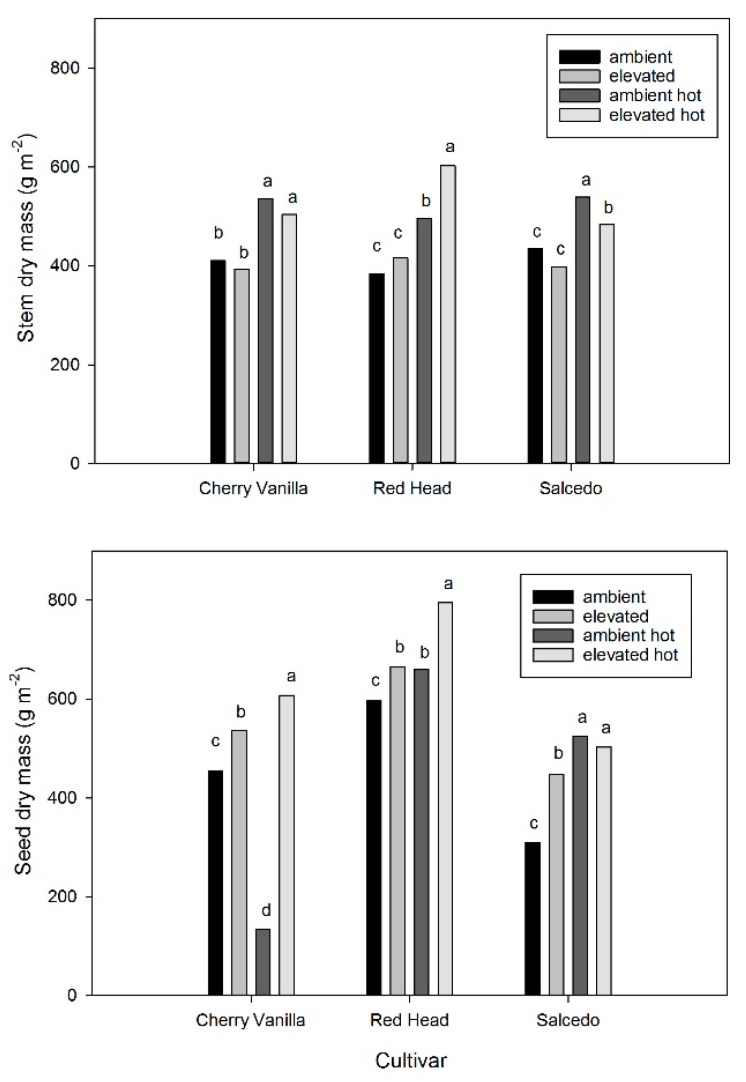
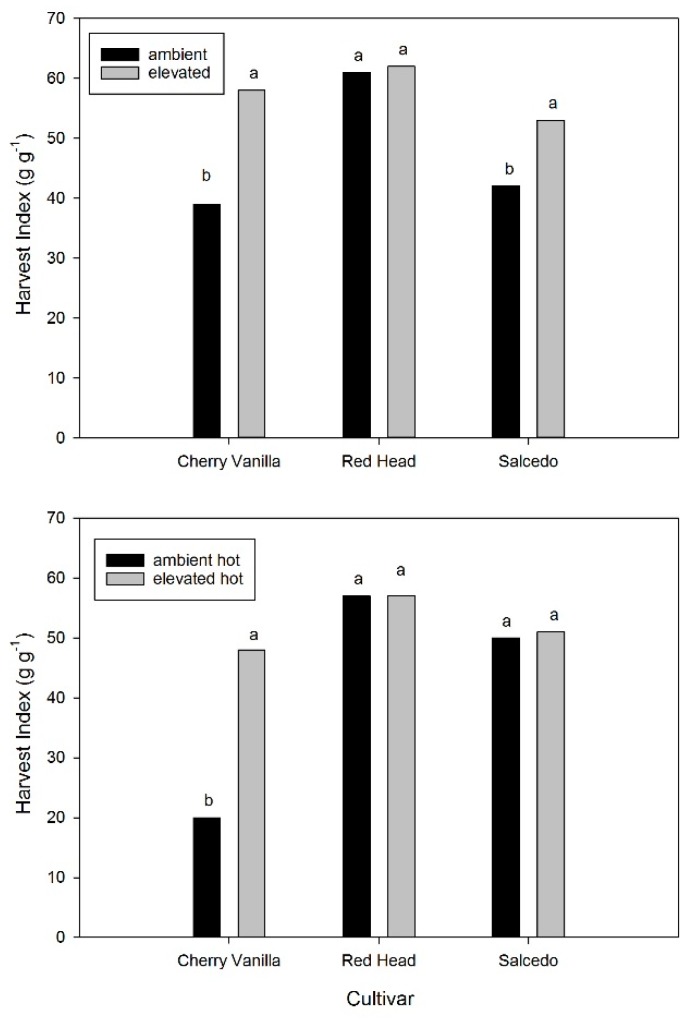
Seed yield was affected by the CO2 and high temperature treatments in a complex manner, as indicated by a significant CO2 × T × cultivar interaction (p = 0.023). At the control temperature, stem dry mass at maturity was not increased significantly by elevated CO2 in any cultivar (Figure 1). In contrast to the lack of increase in stem dry mass, seed dry mass was increased by elevated CO2 in all three cultivars, ranging from 12% in Red Head to 44% in Salcedo (Figure 1). Total shoot dry mass was increased by the high CO2 treatment in all cultivars, but only by 8% to 13%. Harvest index was significantly increased by the elevated CO2 treatment in both Salcedo and Cherry Vanilla for the control temperature treatment, but unchanged in Red Head (Figure 2).
Figure 1.
Stem and seed dry mass responses of three cultivars of quinoa to ambient (400 μmol·mol−1) or elevated (600 μmol·mol−1) CO2 concentrations, with or without a high temperature treatment for 7 days beginning at anthesis. The control growth day/night temperatures were 20/14 °C, and the high temperature treatment was 35/29 °C. Within cultivars, different letters indicate significant differences in mean values, based on analysis of variance (ANOVA).
Figure 2.
Harvest index (seed dry mass/total aboveground dry mass) at final harvest for three cultivars of quinoa grown at ambient (400 μmol·mol−1) or elevated (600 μmol·mol−1) CO2 concentrations, with or without a high temperature treatment for 7 days beginning at anthesis. The control growth day/night temperatures were 20/14 °C, and the high temperature treatment was 35/29 °C. Within cultivars and temperature treatments, different letters indicate significant differences in mean values, based on ANOVA.
The high temperature at anthesis treatment produced a wider range of responses to elevated CO2 than had occurred at the control temperature. With the high temperature treatment, elevated CO2 greatly increased seed mass in Cherry Vanilla, moderately increased seed mass in Red Head, and did not change seed mass in Salcedo (Figure 1). Stem mass was increased by the high temperature treatment in all cultivars and for both CO2 levels (Figure 1). For the high temperature stress regime, stem mass was increased by elevated CO2 only in the case of Red Head (Figure 1). At ambient CO2, the high temperature treatment either decreased (0.30 × control, Cherry Vanilla) or increased (1.70 × control, Salcedo) seed yield, or had little effect (Red Head), depending on the cultivar (Figure 1). In contrast, at elevated CO2, the high temperature treatment increased seed yield by 12% to 19% in all cultivars (Figure 1). With the high temperature treatment, the harvest index was greatly increased by elevated CO2 in Cherry Vanilla, but unchanged in Red Head and Salcedo (Figure 2). The mean mass per seed was unaffected by either the elevated CO2 treatment or the heat stress treatment in any of the cultivars.
3. Discussion
The decrease in photosynthesis during the high temperature treatment was less at elevated than at ambient CO2 concentrations (Table 1), consistent with expectations based on Rubisco thermal kinetics [35]. The similar, small increase in total aboveground biomass at harvest of all cultivars at the control temperature was consistent with the large down-regulation of photosynthesis at elevated CO2, which occurred during grain filling in all the cultivars. However, relationships between photosynthetic and growth responses for the high temperature treatments were unclear, based on the very similar photosynthetic responses to the treatments among the three cultivars and their divergent total biomass responses to the treatments.
The significant differences in yield response among these three cultivars of quinoa to the elevated CO2 and high temperature treatments offer promise for adapting this crop to changes in climate. At the control temperature, two of the cultivars, Cherry Vanilla and Salcedo, had substantially increased yield at elevated CO2 because of increased harvest index. For other crops where agronomic yield results from sexual reproduction, increases in harvest index with elevated CO2 have not generally been found, with slight decreases in harvest index being very common [12,13,14,24]. This is despite the generalization that elevated CO2 stimulates flower production and/or reduces flower and seed abortion by increasing whole plant photosynthate supply [36]. Gomez et al. [37] found that the harvest index in quinoa can be quite flexible in response to the manipulation of gibberellin synthesis. For the two quinoa cultivars which had an increased harvest index at elevated CO2 in this experiment, it was not investigated whether the increased harvest index resulted from increased numbers of flowers or reduced losses of reproductive structures. However, consistent with other grain crops [36], seed size was unaffected by the elevated CO2 treatment in all of the cultivars.
The lack of decrease in total seed mass at elevated CO2 when plants were exposed to the high temperature treatments observed here in quinoa (significant increases in two cultivars, Figure 1) contrasts with the response found in several studies with other species. For example, in rice and wheat, even small increases in temperature in combination with elevated CO2 decreased yields in free air carbon dioxide enrichment (FACE) experiments [22]. The same decrease in yield due to high temperatures for plants at elevated CO2 also occurred in soybean [26] and maize [27] in FACE experiments. These results are consistent with several earlier studies using other exposure systems [24]. Wang et al. [28] showed that brief high temperatures at anthesis caused more yield reductions than did longer-term, milder high temperature treatments in rice, and that yield reductions caused by high temperatures in both cases were greater at elevated than at ambient CO2. Prassad et al. [25] showed in glasshouse experiments with sorghum that elevated CO2 resulted in higher tissue temperatures during heat stress than occurred at ambient CO2, which could potentially explain why elevated CO2 made crops more sensitive to heat stress. However, in contrast to these responses, anecdotal observations on wheat indicated that elevated CO2 could reduce the negative impact of heat waves on yield [38]. Additionally, Ferris et al. [39] found that brief high temperature treatments which decreased soybean seed yield at ambient CO2 increased yield at elevated CO2 in glasshouse experiments, evidenced by a significant CO2 × T interaction. These observations as well as the results presented here for quinoa provide hope that cultivars may be found in several crops in which rising atmospheric CO2 concentrations may mitigate the negative effects of high temperature stress on yield.
4. Materials and Methods
Three open pollinated cultivars of quinoa, Chenopodium quinoa Willd. Red Head, Cherry Vanilla, and Salcedo, were grown from seed in indoor controlled environment chambers. The Red Head and Cherry Vanilla cultivars were developed in the USA. from Peruvian germplasm, and Salcedo is a commercial cultivar from Peru. The cultivars from the USA. have reduced yields in hot climates, by informal reputation. Plants were grown with 12 h of light per day at 1000 μmol·m−2·s−1 PPFD from a mixture of high pressure sodium and metal halide lamps in Environmental Growth Chambers (M-18 Chambers). Temperature control was ±0.3 °C. Initial day/night temperatures were 20/14 °C, with a dew point temperature of 10 °C. Plants were grown with one plant per pot in 25 cm diameter plastic pots filled with vermiculite, and flushed to the drip point once or twice per day with a complete nutrient solution containing 14.5 mM nitrogen. The chemical composition of the nutrient solution is given in Shimono and Bunce [40], except that for quinoa all nutrient concentrations were increased by a factor of 4. The stand density was maintained at eight plants per m2 throughout. There were two CO2 treatments, 400 and 600 μmol·mol−1, each ±20 μmol·mol−1 maintained 24 h per day by the injection of pure CO2 or CO2-free air under the control of WMA-4 or WMA-5 infrared CO2 analyzers, which sampled chamber air continuously. When the first mainstem flowers had reached anthesis for a given cultivar, half of the plants of that cultivar were placed in other chambers with day/night temperatures of 35/29 °C, with a dew point temperature of 25 °C for 7 days. This high temperature treatment was chosen based on our observation of poor field yields of Cherry Vanilla in Beltsville, where those temperatures are typical of summer hot spells. The initial CO2 treatments were maintained during the high temperature treatments. After the high temperature treatments, the plants were returned to the original growth conditions, and all plants were grown to seed maturity.
Leaf photosynthesis and stomatal conductance were measured before, during, and after the high temperature treatments, using a CIRAS-3 portable photosynthesis system with CO2, light, temperature, and humidity control (PP Systems, Amesbury, MA, USA). On each growth stage, for each replicate chamber run, three leaves from different plants per cultivar per treatment were measured. Measurements were made on upper canopy leaves which were fully expanded and fully exposed to light. Measurements were made near midday at the current daytime growth temperature (either 20 or 35 °C), at 400 or 600 μmol·mol−1 CO2 concentrations, at the daytime growth PPFD of 1000 μmol·m−2 s−1. For plants grown at the lower CO2, photosynthesis measurements were also made at the higher CO2, to assess the short-term response. The water vapor pressure inside the leaf cuvette was set to match that corresponding to the current growth dew point temperature (either 10 or 25 °C).
A total of eight different controlled environment chambers were utilized, with treatments randomly assigned to chambers over a span of 24 months. There were three replicate chambers per treatment per cultivar, with five or six plants per treatment replicate per cultivar. At seed maturity, the aboveground biomass of each plant was separately dried at 70 °C in a forced air oven, and seed and stem dry mass were separated and weighed. Any remaining leaf material was discarded. Harvest index was calculated as the seed dry mass divided by the total seed and stem dry mass. Analysis of variance was conducted using the three chamber replicates of each treatment and cultivar. Because ANOVA indicated a significant Cultivar × CO2 × Temperature interaction (p = 0.023) for seed mass, the CO2 and temperature treatments were analyzed separately for each cultivar.
Acknowledgments
This study was funded by the United States Department of Agriculture-Agricultural Research Service.
Conflicts of Interest
The author declares no conflict of interest.
References
- 1.Clausen S.K., Frenck G., Linden L.G., Mikkelsen T.N., Lunde C., Jorgensen R.B. Effects of single and multifactor treatments with elevated temperature, CO2 and ozone on oilseed rape and barley. J. Agron. Crop Sci. 2011;197:442–453. doi: 10.1111/j.1439-037X.2011.00478.x. [DOI] [Google Scholar]
- 2.Bunce J.A. Contrasting responses of seed yield to elevated carbon dioxide under field conditions within Ph. Vulg. Agric. Ecosyst. Environ. 2008;128:219–234. doi: 10.1016/j.agee.2008.06.003. [DOI] [Google Scholar]
- 3.Ahmed F.E., Hall A.E., Madore M.A. Interactive effects of high temperature and elevated carbon dioxide concentration on cowpea [(Vigna unguiculata (L.) Walp.] Plant Cell Environ. 1993;16:835–842. doi: 10.1111/j.1365-3040.1993.tb00505.x. [DOI] [Google Scholar]
- 4.Bishop K.A., Betzelberger A.M., Long S.P., Ainsworth E.A. Is there potential to adapt soybean (Glycine max Merr.) to future CO2? An analysis of the yield response of 18 genotypes in free-air CO2 enrichment. Plant Cell Environ. 2015;38:1765–1774. doi: 10.1111/pce.12443. [DOI] [PubMed] [Google Scholar]
- 5.Bunce J.A. Variable responses to CO2 of the duration of vegetative growth and yield within a maturity group in soybeans. Am. J. Plant Sci. 2016;7:1759–1764. doi: 10.4236/ajps.2016.713164. [DOI] [Google Scholar]
- 6.Batts G.R., Ellis R.H., Morison J.I.L., Nkemka P.N., Gregory P.J., Hadley P. Yield and partitioning in crops of contrasting cultivars of winter wheat in response to CO2 and temperature in field studies using temperature gradient tunnels. J. Agric. Sci. 1998;130:17–27. doi: 10.1017/S0021859697005017. [DOI] [Google Scholar]
- 7.Bunce J.A. Using FACE systems to screen wheat cultivars for yield increases at elevated CO2. Agronomy. 2017;7:20. doi: 10.3390/agronomy7010020. [DOI] [Google Scholar]
- 8.Johannessen M.M., Mikkelsen T.N., Nersting L.G., Gullord M., von Bothmer R., Jorgenses R.B. Effect of increased atmospheric CO2 on varieties of oat. Plant Breed. 2005;124:253–256. doi: 10.1111/j.1439-0523.2005.01096.x. [DOI] [Google Scholar]
- 9.Johannessen M.M., Mikkelsen T.N., Jorgensen R.B. CO2 exploitation and genetic diversity in winter varieties of oilseed rape (Brassica napus); varieties of tomorrow. Euphytica. 2002;128:75–86. doi: 10.1023/A:1020672116652. [DOI] [Google Scholar]
- 10.Hasegawa T., Tokida T., Nakamura H., Zhu C., Usui Y., Yoshimoto M., Fukuoka M., Fukuoka M., Wakatsuki H., Katayanagi N., et al. Rice cultivar responses to elevated CO2 at two free–air CO2 enrichment (FACE) site in Japan. Funct. Plant Biol. 2013;40:148–159. doi: 10.1071/FP12357. [DOI] [PubMed] [Google Scholar]
- 11.Ziska L.H., Bunce J.A., Shimono H., Gealy D.R., Baker J.T., Newton P.C.D., Reynolds M.P., Jagadish K.S.V., Zhu C., Howden M., et al. Food Security and climate change: On the potential to adapt global crop production by active selection to rising atmospheric carbon dioxide. Proc. Biol. Sci. 2012;279:4097–4105. doi: 10.1098/rspb.2012.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ainsworth L.A., Davey P.A., Bernacchi C.J., Dermody O.C., Heaton E.A., Moore D.J., Morgan P.B., Naidu S.L., Yoora H.S., Zhu X.G., et al. A meta-analysis of elevated [CO2] effect on soybean (Glycine max) physiology, growth and yield. Glob. Chang. Biol. 2002;8:695–709. doi: 10.1046/j.1365-2486.2002.00498.x. [DOI] [Google Scholar]
- 13.Wang J., Wang C., Xiong Z., Wolfe D., Zou J. Response of rice production to elevated [CO2] and its interaction with rising temperature or nitrogen supply: A meta-analysis. Clim. Chang. 2015;130:529–543. doi: 10.1007/s10584-015-1374-6. [DOI] [Google Scholar]
- 14.Wang L., Feng Z.Z., Schjoerring K.J. Effects of elevated atmospheric CO2 on physiology and yield of wheat (Triticum aestivum L.): A meta-analytic test of current hypotheses. Agric. Ecosyst. Environ. 2013;178:57–63. doi: 10.1016/j.agee.2013.06.013. [DOI] [Google Scholar]
- 15.Wheeler T., von Braun J. Climate change impacts on global food security. Science. 2013;341:508–513. doi: 10.1126/science.1239402. [DOI] [PubMed] [Google Scholar]
- 16.Schlenker W., Roberts M.J. Nonlinear temperature effects indicate severe damages to U.S. crop yield under climate change. Proc. Natl. Acad. Sci. USA. 2009;106:15594–15598. doi: 10.1073/pnas.0906865106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urban D., Robers M., Schlenker W., Lobell D. Projected temperature changes indicate significant increase in interannual variability of U.S. maize yields. Clim. Chang. 2012;112:525–595. doi: 10.1007/s10584-012-0428-2. [DOI] [Google Scholar]
- 18.Hatfield J.L., Boote K.J., Kimball B.A., Ziska L.H., Izaurralde R.C., Ort D., Thomson A.M., Wolfe D. Climate impacts on agriculture: Implication for crop production. Agron. J. 2011;103:351–370. doi: 10.2134/agronj2010.0303. [DOI] [Google Scholar]
- 19.Lobell D.B., Field C.B. Global scale climate-crop yield relationships and the impacts of recent warming. Environ. Res. Lett. 2007;2:014002. doi: 10.1088/1748-9326/2/1/014002. [DOI] [Google Scholar]
- 20.Bishop K.A., Leakey A.D.B., Ainsworth E.A. How seasonal temperature or water inputs affect the relative response of C3 crops to elevated [CO2]: A global analysis of open top chamber and free air CO2 enrichment studies. Food Energy Secur. 2014;3:33–45. doi: 10.1002/fes3.44. [DOI] [Google Scholar]
- 21.Broughton K.J., Smith R.A., Duursma R.A., Tan D.K.Y., Payton P., Bange M.P., Tissue D.T. Warming alters the positive impact of elevated CO2 concentration on cotton growth and physiology during soil water deficit. Funct. Plant Biol. 2017;44:267–278. doi: 10.1071/FP16189. [DOI] [PubMed] [Google Scholar]
- 22.Cai C., Yin X., He S., Jiang W., Si C., Struik P.C., Luo W., Li G., Xie Y., Xiong Y., et al. Responses of wheat and rice to factorial combinations of ambient and elevated CO2 and temperature in FACE experiments. Glob. Chang. Biol. 2015;22:856–874. doi: 10.1111/gcb.13065. [DOI] [PubMed] [Google Scholar]
- 23.Dias de Oliveira E., Bramley J., Siddique K.H.M., Berger H.S. Can elevated CO2 combined with high temperature ameliorate the effect of terminal drought in wheat? Funct. Plant Biol. 2013;40:160–171. doi: 10.1071/FP12206. [DOI] [PubMed] [Google Scholar]
- 24.Kimball B.A. Crop responses to elevated CO2 and interactions with H2O, N, and temperature. Curr. Opin. Plant Biol. 2016;31:36–43. doi: 10.1016/j.pbi.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Prasad P.V.V., Pisipati S.R., Mutava R.N., Tuinstra M.R. Sensitivity of grain sorghum to high temperature stress during reproductive development. Crop Sci. 2008;48:1911–1917. doi: 10.2135/cropsci2008.01.0036. [DOI] [Google Scholar]
- 26.Ruiz-Vera U.M., Siebers M., Gray S.B., Drag D.W., Rosenthal D.M., Kimball B.A., Ort D.R., Bernacchi C.J. Global warming can negate the expected CO2 stimulation in photosynthesis and productivity for soybean grown in the Midwestern United States. Plant Physiol. 2013;162:410–423. doi: 10.1104/pp.112.211938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruiz-Vera U.M., Siebers M.H., Drag D.W., Ort D.R., Bernacchi C.J. Canopy warming caused photosynthetic acclimation and reduced seed yield in maize grown at ambient and elevated [CO2] Glob. Chang. Biol. 2015;21:4237–4249. doi: 10.1111/gcb.13013. [DOI] [PubMed] [Google Scholar]
- 28.Wang D.R., Bunce J.A., Tomecek M.B., Gealy D., McClung A., McCouch S., Ziska L.H. Evidence for divergence of response in Indica, Japonica, and wild rice to high CO2 × temperature interaction. Glob. Chang. Biol. 2016;22:2620–2632. doi: 10.1111/gcb.13279. [DOI] [PubMed] [Google Scholar]
- 29.Wang D., Heckathorn S.A., Wang X., Philpott S.M. A meta-analysis of plant physiological and growth responses to temperature and elevated CO2. Oecologia. 2012;169:1–13. doi: 10.1007/s00442-011-2172-0. [DOI] [PubMed] [Google Scholar]
- 30.Pulvento C., Riccardi M., Lavini A., Iafelice G., Marconi E., d’Andria R. Yield and quality characteristics of quinoa grown in open field under different saline and non-saline irrigation regimes. J. Agron. Crop Sci. 2012;198:254–263. doi: 10.1111/j.1439-037X.2012.00509.x. [DOI] [Google Scholar]
- 31.Curti R.N., de la Vega A.J., Andrade A.J., Bramardi S.J., Bertero H.D. Multi-environment evaluation for grain yield and its physiological determinants of quinoa genotypes across Northwest Argentina. Field Crops Res. 2014;166:46–57. doi: 10.1016/j.fcr.2014.06.011. [DOI] [Google Scholar]
- 32.Curti R.N., de la Vega A.J., Andrade A.J., Bramardi S.J., Bertero H.D. Adaptive responses of quinoa to diverse agro-ecological environments along an altitudinal gradient in North West Argentina. Field Crop. Res. 2016;189:10–18. doi: 10.1016/j.fcr.2016.01.014. [DOI] [Google Scholar]
- 33.Gonzalez J.A., Bruno M., Valoy M., Prado F.E. Genotypic variation of gas exchange parameters and leaf stable carbon and nitrogen isotopes in ten quinoa cultivars grown under drought. J. Agron. Crop Sci. 2011;197:81–93. doi: 10.1111/j.1439-037X.2010.00446.x. [DOI] [Google Scholar]
- 34.Lavini A., Pulvento C., d’Andria R., Riccardi M., Choukr-Allad R., Belhabib O., Yazar A., Incekaya C., Metin Sezen S., Qudir M., Jacobsen S.E. Quinoa’s potential in the Mediterranean region. J. Agron. Crop Sci. 2014;200:344–360. doi: 10.1111/jac.12069. [DOI] [Google Scholar]
- 35.Lloyd J., Farquhar G.D. Effects of rising temperature and CO2 on the physiology of tropical forest trees. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008;363:1811–1817. doi: 10.1098/rstb.2007.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patrick J.W., Colyvas K. Crop yield components–photoassimilate supply- or utilization limted-organ development? Funct. Plant Biol. 2014;41:893–913. doi: 10.1071/FP14048. [DOI] [PubMed] [Google Scholar]
- 37.Gomez M.B., Aguirre Castro P., Mignone C., Bertero H.D. Can yield potential be increased by manipulation of reproductive partitioning in quinoa (Chenopodium quinoa)? Evidence from gibberellic acid synthesis inhibition using paclobrutrazol. Funct. Plant Biol. 2011;38:420–430. doi: 10.1071/FP10168. [DOI] [PubMed] [Google Scholar]
- 38.Fitzgerald G.J., Tausz M., O’Leary G., Mollah M.R., Tausz-Pausch S., Seneweera S., Mock I., Low M., Partington D.L., McNeil D., et al. Elevated atmospheric [CO2] can dramatically increase wheat yields in semi-arid environments and buffer against heat waves. Glob. Chang. Biol. 2016;22:2269–2284. doi: 10.1111/gcb.13263. [DOI] [PubMed] [Google Scholar]
- 39.Ferris R., Wheeler T.R., Ellis R.H., Hadley P. Seed yield after environmental stress in soybean grown under elevated CO2. Crop Sci. 1999;39:710–718. doi: 10.2135/cropsci1999.0011183X003900030018x. [DOI] [Google Scholar]
- 40.Shimono H., Bunce J.A. Acclimation of nitrogen uptake capacity of rice to elevated atmospheric CO2 concentration. Ann. Bot. 2009;103:87–94. doi: 10.1093/aob/mcn209. [DOI] [PMC free article] [PubMed] [Google Scholar]