Abstract
Five varieties of Ocimum basilicum L. namely lettuce, cinnamon, minimum, latifolia, and violetto were separately cultivated in field and greenhouse in the island Kefalonia (Greece). The effect of successive harvesting to the essential oil content was evaluated. In total 23 samples of essential oils (EOs) were analyzed by GC-FID and GC-MS. Ninety-six constituents, which accounted for almost 99% of the oils, were identified. Cluster analysis was performed for all of the varieties in greenhouse and field conditions, in order to investigate the possible differentiation on the chemical composition of the essential oils, obtained between harvests during growing period. Each basil variety showed a unique chemical profile, but also the essential oil composition within each variety seems to be differentiated, affected by the harvests and the cultivation site.
Keywords: basil varieties, essential oil, GC-MS, harvest, cluster analysis
1. Introduction
Basil (Ocimum basilicum L.) is an annual herb growing in several regions around the world. The genus Ocimum consists of more than 150 species whereof basil is cultivated in many countries as a major essential oil crop [1]. The species most commonly used as spices and medicinal herbs are O. basilicum L., O. americanum L. (syn. O. canum Sims.), O. gratissimum L., O. kilimandscharicum Guerke, O. tenuiflorum L. (syn. O. sanctum L.), and O. africanum Lour. (syn. O. × citriodorum Vis.), that is a hybrid of O. basilicum and O. americanum [2]. The taxonomy of the genus Ocimum is very complicated due to the occurrence of interspecific hybridization, polyploidy, aneuploidy, the existence of numerous botanical varieties, and chemotypes, as well as the many synonymous names [3,4,5]. Plants of the species O. basilicum have square stems, fragrant opposite leaves, and whorled flowers on spiked inflorescences [6]. O. basilicum L. is widely used in the culinary arts and in the food processing industry [7]. Traditionally, it has been used as medicinal plant for the treatment of headaches, coughs, diarrhea, constipation, warts, worms, and kidney malfunction [8]. A plethora of biological activities have been attributed to basil essential oils like antimicrobial, insecticidal, and recently was found to exhibit in vivo anti-malarial activity [9]. In addition, extracts from leaves and flowers can be used as aroma additives in food, pharmaceutical, and cosmetic industry [10]. Economical data clearly indicate the commercial importance of basil essential oil, since the world production is estimated to 1,200,000 € [8]. Basil essential oils contain a broad array of chemical compounds depending on variations in chemotypes, flower, and leaf colours, aroma and especially the origin of the plant [7]. The essential oil constituents vary among sweet basil cultivars, with linalool, methyl chavicol, eugenol, 1,8-cineole, geranial, neral, methyl cinnamate recognized as main components [11,12,13]. Several scientists have classified basil to different chemotypes according to the main components of the essential oil. Thereby, Marotti et al. (1996) divided essential oil from Italian basil varieties to three chemotypes “linalool”, “linalool-methyl chavicol”, and “linalool-eugenol” [14]. According to the plant origin, basils are grouped to European chemotype that has linalool and methyl chavicol as main components and Tropical chemotype having methyl chavicol as a main compound [15]. Concerning the cultivation of basil, the plants can be harvested one to three times during cropping season depending on the climate [16]. Generally, basil is harvested for the leaves that are sold fresh or dried. In the cases that basil is cultivated for dried leaves and extraction of essential oil, the plants are cut just prior the appearance of the flowers [17]. Scientific data concerning how harvest and number of harvests during the cultivation of aromatic plants affect essential oil quality and composition are lacking either in field or greenhouse conditions. To the best of our knowledge, so far Carlo et al. (2013) studied the effect of cut number in quality traits of sweet basil [18]. Also Zheljazkof et al. (2008) studied the effect of harvests in O. basilicum L. (cvs. German and Mesten) and O. sanctum L. (syn. O. tenuiflorum L.) (cv. Local) cultivated in Mississippi [9]. Taking into account that geographic position and the environmental characteristics of the habitat should be taken as factors affecting the chemical composition of the essential oils [19], the current study is the first including total chemical analysis of the essential oils from five basil varieties cultivated and harvested in the field and greenhouse in the island of Kefalonia, (Greece). The aim of our study is the chemical analysis of essential oils from the five varieties of basil considering also the cultivation site as well as how the ability of the plant to rejuvenate after successive cuttings during growing cycle, affects the obtained essential oil yield and composition. In order to simulate the natural growing conditions, successive cuttings were performed in every variety just before flowering stage, as done practically from aromatic plants’ growers. Moreover the cluster analysis of all basil varieties for greenhouse and field samples is given.
2. Results and Discussion
2.1. Chemical Analysis
In the greenhouse conditions the essential oil (EO) content of varieties violetto, latifolia, and minimum increased in the second and/or third harvest, whereas in var. lettuce the second harvest produced less essential oil content when comparing to the first and third harvest where the essential oil content was in the same levels. Noteworthy is the fact, that in the field conditions, the essential oil content in the varieties of latifolia, minimum and lettuce decreased gradually after the successive harvests while in var. cinnamon sequential harvests didn’t affect overall essential oil yield (Table 1).
Table 1.
Samples from O. basilicum varieties and essential oil yield.
| O. basilicum L. Varieties | Harvest | Cultivation Site | EO (% Fresh Weight) |
|---|---|---|---|
| O. basilicum L. var. violetto | OCK1 | greenhouse | 0.017 |
| OCK2 | greenhouse | 0.04 | |
| OCK3 | greenhouse | 0.08 | |
| O. basilicum L. var. latifolia | OCP4 | greenhouse | 0.026 |
| OCP5 | greenhouse | 0.14 | |
| O. basilicum L. var. minimum | OCS6 | greenhouse | 0.033 |
| OCS7 | greenhouse | 0.08 | |
| O. basilicum L. var. lettuce | OCM8 | greenhouse | 0.10 |
| OCM9 | greenhouse | 0.04 | |
| OCM10 | greenhouse | 0.15 | |
| O. basilicum L. var. latifolia | OCP11 | field | 0.18 |
| OCP12 | field | 0.20 | |
| OCP13 | field | 0.12 | |
| OCP14 | field | 0.08 | |
| O. basilicum L. var. minimum | OCS15 | field | 0.11 |
| OCS16 | field | 0.04 | |
| OCS17 | field | 0.036 | |
| O. basilicum L. var lettuce | OCM18 | field | 0.12 |
| OCM19 | field | 0.07 | |
| OCM20 | field | 0.08 | |
| O. basilicum L. var. cinnamon | OCC21 | field | 0.21 |
| OCC22 | field | 0.21 | |
| OCC23 | field | 0.20 |
Results indicate that different cultivation conditions affect plant’s response to successive harvests and consequently the essential oil content, even of the same variety. As such controlled greenhouse conditions favor or have no impact to the total production of essential oil in the varieties latifolia, minimum, and lettuce when comparing to the field conditions.
All obtained essential oils were analyzed with GC/FID and GC/MS. In total, 96 individual constituents were identified representing 98.0–99.9% of the total essential oil. The detailed chemical analysis of the essential oils of the greenhouse samples showed that the major constituents, in var. violetto, were linalool (22.5–26.5%) and trans-bergamontene (15.2–20.0%), followed by eugenol (4.5–11.9%). Variety latifolia has as major compounds linalool (17.1–35.6%), eugenol (10.1–23.3%) and trans-bergamontene (8.8–16.1%). In var. minimum linalool (27.4–28.3%) and eugenol (14.5–23.4%) were the main constituents while in var. lettuce linalool (21.0–23.6%), methyl chavicol (12.1–17.5%), trans-bergamontene (11.4–12.9%) and epi-α-cadinol (6.0–9.2%) (Table 2).
Table 2.
Chemical composition of the essential oils from O. basilicum L. varieties, violetto, latifolia, minimum and lettuce, cultivated in the greenhouse conditions.
| Compound | RI a | OCK1 | OCK2 | OCK3 | OCP4 | OCP5 | OCS6 | OCS7 | OCM8 | OCM9 | OCM10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | α-thujene | 923 | - | - | tr | - | - | tr | tr | tr | tr | tr |
| 2 | α-pinene | 932 | 0.9 | 0.1 | 0.4 | 0.8 | 0.2 | 0.8 | 0.3 | 0.5 | 0.3 | 0.4 |
| 3 | camphene | 946 | - | - | tr | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | tr | tr |
| 4 | sabinene | 968 | 0.3 | 0.1 | 0.3 | 0.4 | 0.2 | 0.1 | 0.1 | 0.2 | 0.1 | 0.2 |
| 5 | β-pinene | 973 | 0.7 | - | 0.6 | 0.7 | 0.4 | 0.3 | 0.2 | 0.5 | 0.2 | 0.5 |
| 6 | myrcene | 984 | 1.0 | 0.1 | 0.7 | 0.8 | 0.5 | 0.4 | 0.3 | 0.3 | 0.2 | 0.4 |
| 7 | α-phellandrene | 1001 | - | - | 0.2 | - | - | - | 0.1 | - | 0.1 | 0.1 |
| 8 | δ-3-carene | 1004 | 0.3 | - | - | 0.4 | 0.1 | 0.4 | tr | 0.2 | 0.1 | - |
| 9 | α-terpinene | 1011 | - | - | 0.1 | 0.1 | - | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| 10 | limonene | 1019 | - | 0.2 | - | - | - | - | - | - | - | - |
| 11 | p-cymene | 1017 | - | - | - | - | - | - | 0.1 | - | - | - |
| 12 | 1,8-cineole | 1027 | 6.6 | 2.3 | 6.3 | 4.9 | 7.1 | 2.7 | 2.7 | 5.2 | 3.5 | 4.8 |
| 13 | trans-β-ocimene | 1043 | - | - | tr | 1.7 | 1.7 | 1.1 | 1.3 | 0.9 | 0.8 | 1.1 |
| 14 | γ-terpinene | 1051 | 0.1 | - | 0.2 | 0.1 | 0.1 | 0.2 | 0.4 | 0.3 | 0.4 | 0.4 |
| 15 | α-terpinolene | 1078 | 0.1 | 0.6 | 0.1 | 0.4 | 0.2 | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 |
| 16 | linalool | 1100 | 26.5 | 22.5 | 23.5 | 17.1 | 35.6 | 27.4 | 28.3 | 21.0 | 23.1 | 23.6 |
| 17 | allo-ocimene | 1133 | - | - | - | - | - | - | - | tr | - | - |
| 18 | trans-epoxy-ocimene | 1134 | - | - | - | 0.2 | 0.4 | 0.1 | - | - | - | - |
| 19 | camphor | 1142 | - | - | tr | 0.2 | 0.3 | 0.3 | 0.3 | 0.4 | 0.6 | 0.5 |
| 20 | borneol | 1166 | - | - | 0.2 | 0.5 | 0.8 | 0.3 | 0.3 | - | - | - |
| 21 | terpinen-4-ol | 1172 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.5 | 1.2 | - | 2.0 | 1.9 |
| 22 | α-terpineol | 1184 | 0.7 | - | 1.4 | 0.7 | 1.3 | 0.4 | - | 1.5 | - | - |
| 23 | methyl chavicol | 1193 | - | 0.5 | - | - | - | - | 1.9 | 17.5 | 12.1 | 16.0 |
| 24 | myrtenal | 1205 | - | - | - | - | - | - | - | - | tr | - |
| 25 | octyl acetate | 1207 | 0.2 | - | 0.2 | 0.4 | 0.1 | 0.2 | 0.2 | - | - | - |
| 26 | endo-fenchyl acetate | 1213 | 0.7 | - | 0.4 | - | - | - | - | - | - | |
| 27 | neral | 1231 | - | - | - | - | - | 0.1 | - | - | - | - |
| 28 | chavicol | 1245 | - | - | - | - | - | - | - | 1.0 | 0.3 | 0.8 |
| 29 | geraniol | 1247 | - | - | - | - | - | - | 0.9 | - | - | - |
| 30 | trans-anethole | 1280 | - | - | 0.7 | - | - | - | 0.2 | - | 0.3 | - |
| 31 | bornyl-acetate | 1282 | - | - | - | 3.2 | 1.4 | 1.5 | 1.1 | 0.7 | 0.5 | 0.6 |
| 32 | carvacrol | 1296 | 10.0 | 0.8 | 2.9 | 4.4 | 0.7 | 4.3 | 2.7 | 2.3 | 1.1 | 1.5 |
| 33 | α-cubebene | 1334 | - | - | 0.1 | 0.1 | - | 0.1 | tr | 0.1 | - | tr |
| 34 | eugenol | 1356 | 8.6 | 4.5 | 11.9 | 10.1 | 23.3 | 14.5 | 23.4 | 7.2 | 2.1 | 9.1 |
| 35 | α-copaene | 1369 | 0.2 | 0.2 | - | tr | 0.1 | - | - | 0.3 | 0.2 | - |
| 36 | β-patchoulene | 1372 | - | - | - | - | - | - | - | 0.1 | 0.1 | 0.1 |
| 37 | β-cubebene | 1375 | - | - | - | - | - | tr | 0.1 | - | - | - |
| 38 | β-elemene | 1387 | 1.4 | 1.9 | 1.9 | 1.3 | 0.9 | 3.6 | 2.3 | 2.6 | 3.8 | 2.4 |
| 39 | methyl eugenol | 1399 | 0.1 | - | 0.4 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| 40 | cis-α-bergamotene | 1398 | - | - | - | - | 0.1 | - | 0.1 | - | - | - |
| 41 | trans-β-caryophyllene | 1409 | 1.3 | 1.6 | 1.9 | 1.1 | 0.2 | 0.8 | 0.4 | 0.6 | 0.6 | 0.4 |
| 42 | β-gurjunene | 1417 | - | 0.7 | - | - | - | 0.5 | 0.3 | - | - | - |
| 43 | trans-α-bergamotene | 1432 | 15.6 | 20.0 | 15.2 | 16.1 | 8.8 | 3.8 | 5.1 | 11.4 | 12.9 | 11.7 |
| 44 | aromadendrene | 1437 | 0.2 | 0.3 | 0.3 | 0.4 | 0.2 | 0.6 | 0.1 | 0.4 | 0.5 | 0.4 |
| 45 | cis-muurola-3,5-diene | 1439 | - | - | - | - | 0.3 | 0.6 | 0.5 | - | - | - |
| 46 | trans-β-farnesene | 1446 | 0.3 | 0.4 | - | 1.3 | - | - | - | - | - | 0.4 |
| 47 | α-humulene | 1448 | 1.1 | 2.6 | 1.4 | 0.4 | 0.9 | 1.5 | 0.9 | 0.4 | 1.4 | 0.6 |
| 48 | epi-bicyclosesquiphellandrene | 1453 | 0.4 | 0.6 | 0.5 | 0.9 | 0.4 | 1.0 | 0.8 | 0.7 | 0.9 | 0.7 |
| 49 | allo-aromadendrene | 1459 | - | - | 0.2 | 0.2 | - | 0.2 | 0.1 | tr | - | - |
| 50 | α-amorphene | 1460 | - | - | 0.3 | - | - | - | - | tr | - | - |
| 51 | germacrene-D | 1476 | 3.7 | 9.4 | 5.4 | 6.7 | 3.6 | 7.0 | 4.5 | 5.4 | 8.2 | 5.4 |
| 52 | β-selinene | 1475 | 2.9 | 3.5 | - | - | - | - | - | 0.2 | - | - |
| 53 | bicyclogermacrene | 1486 | - | - | 3.0 | 2.2 | 1.1 | 2.5 | 2.0 | 1.3 | 1.2 | 1.3 |
| 54 | δ-guaiene | 1496 | 2.7 | 7.1 | 3.8 | 3.1 | 1.2 | 3.8 | 2.1 | 2.3 | 3.3 | 1.7 |
| 55 | germacrene-A | 1499 | 2.6 | 5.4 | 1.5 | 1.4 | 1.1 | 1.1 | 1.0 | 0.8 | 1.0 | 0.7 |
| 56 | γ-cadinene | 1506 | 1.9 | 2.8 | 1.7 | 2.7 | 1.6 | 3.2 | 3.4 | 2.7 | 3.5 | 2.8 |
| 57 | β-sesquiphellandrene | 1514 | 0.7 | 1.0 | 0.9 | 0.9 | 0.4 | 0.2 | 0.2 | 0.6 | 0.7 | 0.6 |
| 58 | δ-cadinene | 1516 | 0.2 | 0.3 | 0.1 | 0.2 | 0.1 | 0.2 | 0.1 | 0.1 | 0.2 | 0.1 |
| 59 | α-cadinene | 1520 | - | - | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | tr | 0.1 |
| 60 | trans-γ-bisabolene | 1530 | 0.2 | - | - | 0.1 | - | - | - | 0.1 | 0.1 | 0.1 |
| 61 | γ-cuprenene | 1535 | 0.1 | 0.2 | 0.1 | 0.1 | - | - | - | tr | 0.1 | tr |
| 62 | trans-cadina-1,4-diene | 1534 | - | - | 0.1 | 0.1 | - | 0.1 | - | 0.1 | 0.2 | tr |
| 63 | germacrene B | 1548 | - | - | - | 0.1 | - | - | - | - | - | - |
| 64 | trans-nerodilol | 1543 | - | - | 0.4 | - | - | 0.2 | 0.1 | 0.1 | 0.1 | 0.2 |
| 65 | maaliol | 1550 | - | 1.5 | 0.5 | - | - | - | - | 0.1 | 0.9 | 0.2 |
| 66 | caryophyllene oxide | 1557 | - | - | - | - | - | - | - | tr | - | - |
| 67 | spathulenol | 1562 | - | - | 0.1 | 0.1 | - | - | tr | tr | 0.1 | tr |
| 68 | viridiflorol | 1595 | 0.5 | 0.7 | 0.9 | 1.1 | - | - | - | 0.9 | 1.1 | 0.8 |
| 69 | 1,10-di-epi-cubenol | 1596 | - | - | - | - | 0.4 | 1.0 | 0.8 | - | - | - |
| 70 | epi-α-cadinol | 1638 | 3.7 | 5.9 | 5.0 | 6.7 | 3.2 | 7.2 | 6.3 | 6.2 | 9.2 | 6.0 |
| 71 | vulgarone B | 1641 | - | - | 0.2 | - | - | - | - | - | - | - |
| 72 | α-eudesmol | 1651 | - | - | 0.1 | - | - | - | - | - | - | - |
| 73 | α-cadinol | 1652 | 0.2 | 0.5 | 0.7 | 0.8 | 0.2 | 0.7 | 0.5 | 0.7 | 0.9 | 0.6 |
| 74 | 7-epi-α-eudesmol | 1659 | - | - | - | - | - | - | - | tr | - | - |
| 75 | α-bisabolol | 1685 | 0.6 | - | 0.2 | 0.2 | - | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| 76 | germacrone | 1691 | - | - | - | 0.3 | - | - | - | - | - | - |
| 77 | cis-farnesol | 1692 | - | - | 0.3 | - | - | - | - | 0.1 | 0.2 | 0.1 |
| 78 | β-sinensal | 1693 | - | - | 0.4 | - | - | 0.2 | - | 0.2 | 0.2 | 0.1 |
| 79 | mint sulfide | 1733 | - | - | tr | 0.1 | - | 0.1 | - | tr | - | - |
| 80 | neo phytadiene | 1838 | - | - | - | 0.8 | - | - | - | - | - | - |
| 81 | nonadecane | 1900 | - | - | - | tr | - | - | - | - | - | - |
| 82 | eicosane | 2000 | - | - | - | 0.4 | - | - | - | - | - | - |
| 83 | heneicosane | 2100 | - | - | - | 0.2 | - | - | - | - | - | - |
| 84 | docosane | 2200 | - | - | - | 0.2 | - | - | - | - | - | - |
| 85 | tricosane | 2300 | - | - | - | 0.1 | - | - | - | - | - | - |
| 86 | tetracosane | 2400 | - | - | - | - | - | tr | - | - | - | - |
| 87 | pentacosane | 2500 | - | - | - | - | - | 0.2 | - | tr | - | - |
| 88 | hexacosane | 2600 | - | - | - | - | - | tr | - | - | - | - |
| 89 | heptacosane | 2700 | 0.3 | 0.5 | 0.1 | - | - | 0.2 | - | 0.1 | - | - |
| 90 | nonacosane | 2900 | 0.3 | 0.5 | 0.1 | - | - | - | - | 0.1 | - | - |
| 91 | triacontane | 3000 | 0.2 | 0.3 | 0.1 | - | - | - | - | 0.1 | - | - |
| Total | 98.3 | 99.8 | 98.3 | 98.0 | 99.8 | 97.1 | 98.5 | 99.2 | 99.9 | 99.9 | ||
a Retention indices were calculated against C9-C23 n-alkanes on the HP 5MS capillary column.
In the field conditions, the main constituents of var. latifolia were linalool (32.2–49.5%), eugenol (19.8–34.9%), trans-bergamontene (5.9–9.5%), and 1,8-cineole (4.2–6.7%). In var. minimun linalool (30.2–52.0%) and eugenol (28.8–36.0%), were the dominating compounds. Var. lettuce has as major components linalool (20.4–26.0%) and methyl-chavicol (45.4–54.1%). The latter constituent was dominating also in the essential oil of var. cinnamon in a range 61.2–75.1% among harvests (Table 3). Between harvests and cultivation sites, the main compounds of the essential oils displayed varying concentrations. The highest concentration for linalool (52.0%) was measured in var. minimum in the field cultivation, in the second harvest in contrast to what has been stated from Zheljazkof et al. (2008) that measures a higher content of linalool in the third cutting of O. basilicum varieties [9]. Interesting is the fact that eugenol showed the highest concentration in var. minimum in the first harvest in the field conditions. Noteworthy, from var. minimum methyl-chavicol is lacking or is present in small amounts. Given that methyl-chavicol has a structural resemblance to potential carcinogenic phenylpropanoids such as safrole, chemotypes rich in linalool are preferred for cultivation when used in food and perfume industries [15,20]. Methyl-chavicol showed the highest concentration (75.1%) in var. cinnamon in the field conditions from the first harvest followed by var. lettuce (54.1%) from the first harvest in the same conditions. In addition, it has been observed that the intensity of purple leaf colour is positively correlated in varieties rich in methyl chavicol [21]. However, in compliance with Liber et al. (2011), our results show that the biosynthesis of phenylopropanoids e.g., methyl chavicol, is not characteristic exclusively for the purple morfotypes. Also, green morfotypes (var. lettuce and cinnamon) are rich sources of methyl chavicol as well. [22].
Table 3.
Chemical composition of the essential oils from O. basilicum L. varieties latifolia, minimum, lettuce and cinnamon, cultivated in the field conditions.
| Compound | RI a | OCP11 | OCP12 | OCP13 | OCP14 | OCS15 | OCS16 | OCS17 | OCM18 | OCM19 | OCM20 | OCC21 | OCC22 | OCC23 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | α-thujene | 922 | - | - | - | - | - | - | tr | tr | tr | tr | tr | tr | tr |
| 2 | α-pinene | 931 | 0.2 | 0.2 | 0.2 | 0.5 | tr | 0.3 | 0.3 | 0.3 | 0.3 | 0.2 | 0.2 | 0.5 | 0.5 |
| 3 | Camphene | 946 | 0.1 | tr | tr | 0.1 | tr | 0.1 | 0.1 | tr | tr | tr | tr | 0.2 | 0.2 |
| 4 | sabinene | 967 | 0.2 | 0.2 | 0.2 | 0.2 | tr | 0.2 | 0.1 | 0.1 | 0.2 | 0.1 | 0.2 | 0.4 | 0.4 |
| 5 | β-pinene | 974 | 0.3 | 0.3 | 0.3 | 0.5 | 0.1 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.7 | 0.8 |
| 6 | myrcene | 984 | 0.3 | 0.3 | 0.5 | 0.7 | 0.1 | - | 0.5 | 0.2 | 0.3 | 0.3 | 0.4 | 0.7 | 0.8 |
| 7 | 3-octanol | 988 | - | - | - | - | - | - | - | - | - | tr | - | - | - |
| 8 | n-decane | 1000 | - | - | - | - | - | - | - | - | - | 0,1 | - | - | - |
| 9 | α-phellan-drene | 1002 | - | - | - | - | tr | - | tr | - | - | tr | tr | 0.1 | 0.1 |
| 10 | δ-3-carene | 1004 | tr | tr | 0.1 | 0.1 | tr | - | tr | tr | 0.1 | - | - | - | - |
| 11 | α-terpinene | 1013 | - | - | tr | 0.1 | 0.1 | - | tr | 0.1 | 0.1 | 0.1 | tr | 0.1 | 0.1 |
| 12 | p-cymene | 1019 | - | - | - | - | 0.1 | - | tr | - | - | 0.1 | - | - | - |
| 13 | limonene | 1022 | - | - | - | - | 0.1 | - | 0.2 | - | - | - | - | - | - |
| 14 | 1,8-cineole | 1026 | 4.2 | 4.2 | 5.1 | 6.7 | 1.6 | 3.8 | 2.7 | 5.7 | 4.8 | 4.6 | 4.8 | 6.8 | 7.6 |
| 15 | cis-β-ocimene | 1035 | - | - | - | - | - | - | - | - | tr | - | - | - | - |
| 16 | trans-β-ocimene | 1043 | 1.4 | 1.1 | 0.9 | 0.6 | 0.8 | 1.4 | 0.9 | 0.7 | 0.5 | 0.4 | 1.9 | 3.0 | 2.9 |
| 17 | γ-terpinene | 1053 | 0.1 | 0.1 | 0.1 | tr | 0.2 | 0.2 | 0.2 | 0.3 | 0.3 | 0.4 | 0.1 | 0.1 | 0.1 |
| 18 | cis-sabinene hydrate | 1064 | - | - | - | - | - | - | - | - | - | tr | - | tr | tr |
| 19 | α-terpinolene | 1081 | 0.2 | 0.2 | 0.1 | 0.2 | 0.2 | 0.1 | 0.3 | 0.1 | 0.1 | 0.1 | 0.1 | 0.3 | 0.3 |
| 20 | fenchone | 1081 | - | - | - | - | - | - | - | - | - | tr | - | - | - |
| 21 | linalool | 1100 | 32.2 | 34.0 | 35.5 | 49.5 | 36.8 | 52.0 | 30.2 | 26.0 | 21.8 | 20.4 | 5.7 | 0.6 | 1.3 |
| 22 | allo-ocimene | 1132 | - | - | - | - | - | - | - | tr | tr | tr | - | - | - |
| 23 | trans-epoxy-ocimene | 1134 | 0.3 | 0.3 | 0.1 | tr | 0.1 | - | tr | - | - | - | tr | 0.1 | 0.1 |
| 24 | camphor | 1141 | 0.3 | 0.3 | 0.1 | 0.1 | 0.3 | 0.2 | 0.3 | 0.3 | 0.2 | 0.3 | 0.6 | 2.1 | 1.9 |
| 25 | borneol | 1165 | 0.6 | 0.6 | 0.5 | 0.4 | 0.4 | 0.2 | 0.4 | - | - | 0.2 | - | - | tr |
| 26 | terpinen-4-ol | 1173 | 0.1 | 0.1 | 0.2 | 0.1 | 1.2 | 1.1 | 0.8 | - | tr | 2.1 | - | - | - |
| 27 | α-terpineol | 1184 | 0.7 | 1.0 | 1.0 | 0.8 | 0.8 | 0.5 | 0.9 | - | - | - | - | - | - |
| 28 | methyl chavicol | 1186 | - | - | - | - | - | 0.3 | - | 45.4 | 54.1 | 46.7 | 75.1 | 60.2 | 64.9 |
| 29 | octyl acetate | 1208 | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | 0.1 | 0.3 | - | - | - | - | - | - |
| 30 | nerol | 1224 | - | - | - | - | - | - | 0.1 | tr | tr | tr | - | - | - |
| 31 | citronellol | 1225 | - | - | - | - | - | - | 0.1 | - | - | tr | - | - | - |
| 32 | neral | 1234 | - | - | - | - | - | - | 0.1 | - | - | - | - | - | - |
| 33 | carvone | 1239 | - | - | - | - | - | - | 0.1 | - | tr | - | tr | - | - |
| 34 | chavicol | 1243 | - | - | - | - | - | - | - | 0.2 | 0.2 | 0.5 | tr | 0.1 | 0.1 |
| 35 | geraniol | 1245 | - | - | - | - | 0.6 | 0.7 | 1.5 | - | - | - | - | - | - |
| 36 | citronellyl formate | 1277 | - | - | - | - | - | - | - | - | - | - | - | - | tr |
| 37 | trans-anethole | 1280 | - | - | - | - | tr | tr | - | - | tr | tr | |||
| 38 | bornyl acetate | 1289 | 0.8 | 0.8 | 0.6 | 1.2 | 0.6 | 0.3 | 1.3 | 0.1 | 0.1 | 0.2 | 0.2 | 0.6 | 0.5 |
| 39 | carvacrol | 1298 | 1.0 | 0.4 | 0.3 | tr | 0.7 | 0.3 | 0.3 | 0.7 | 1.1 | 0.2 | 0.2 | 0.4 | 0.2 |
| 40 | δ-elemene | 1336 | - | - | - | - | - | - | - | - | - | - | tr | tr | tr |
| 41 | carvyl acetate | 1333 | - | - | - | - | - | - | - | - | - | - | - | tr | tr |
| 42 | α-cubebene | 1341 | - | - | - | - | - | - | 0.1 | - | - | 0.1 | - | 0.1 | 0.1 |
| 43 | eugenol | 1356 | 26.9 | 32.2 | 34.9 | 19.8 | 36.0 | 28.8 | 28.9 | 10.0 | 2.5 | 5.6 | 0.1 | 0.1 | 0.2 |
| 44 | neryl acetate | 1359 | - | - | - | - | 0.2 | - | - | - | - | - | - | - | - |
| 45 | α-copaene | 1369 | 0.1 | - | - | - | - | tr | - | - | tr | 0.1 | tr | 0.2 | 0.2 |
| 46 | geranyl acetate | 1378 | - | - | - | - | - | - | 0.8 | - | - | - | - | - | - |
| 47 | β-bourbonene | 1386 | - | - | - | - | - | - | - | tr | tr | 0.1 | 0.1 | 0.2 | 0.1 |
| 48 | β-cubebene | 1387 | - | - | - | - | - | - | - | - | - | tr | - | - | - |
| 49 | β-elemene | 1391 | 1.0 | - | tr | 0.7 | 1.9 | 1.1 | 1.1 | 0.5 | 0.6 | 0.8 | 0.9 | 1.9 | 1.3 |
| 50 | methyl-eugenol | 1402 | 0.1 | 0.2 | 0.2 | 0.1 | 0.1 | - | 0.2 | tr | tr | 0.1 | 0.1 | 0.5 | 0.5 |
| 51 | α-gurjunene | 1398 | - | - | - | - | - | - | - | - | - | - | - | tr | tr |
| 52 | cis-α-bergamotene | 1401 | 0.1 | - | - | - | - | - | tr | tr | 0.1 | - | tr | tr | |
| 53 | trans-β-caryophyllene | 1415 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.4 | 0.3 |
| 54 | β-copaene | 1414 | - | - | - | - | - | - | - | - | - | 0.1 | - | 0.1 | 0.1 |
| 55 | β-gurjunene | 1420 | 0.4 | - | - | - | - | - | 0.1 | - | - | - | tr | 0.2 | - |
| 56 | trans-α-bergamotene | 1431 | 9.5 | 7.6 | 6.5 | 5.9 | 2.2 | 2.1 | 3.1 | 4.0 | 5.1 | 4.4 | 0.4 | 1.0 | 1.5 |
| 57 | α-guaiene | 1427 | - | - | - | - | - | - | - | - | - | - | 0.1 | 0.4 | - |
| 58 | aromadendrene | 1435 | 0.2 | - | - | 0.1 | - | - | 0.1 | tr | 0.1 | 0.1 | tr | tr | - |
| 59 | cis-muurola-3.5-diene | 1439 | 0.3 | 0.2 | 0.2 | - | 0.2 | - | 0.3 | - | - | - | - | - | - |
| 60 | trans-β-farnesene | 1445 | - | - | 0.2 | 0.2 | - | - | 0.2 | - | 0.1 | - | - | - | - |
| 61 | α-humulene | 1448 | 0.8 | 0.8 | 0.2 | 0.2 | 0.5 | 0.2 | 0.3 | 0.2 | 0.1 | 0.4 | 0.5 | 1.4 | 1.0 |
| 62 | epi-bicyclosesquiphellandrene | 1454 | 0.5 | 0.4 | 0.3 | 0.2 | 0.4 | - | 0.6 | 0.1 | 0.1 | 0.3 | 0.1 | 0.5 | 0.4 |
| 63 | allo-aromadendrene | 1456 | - | - | 2.6 | tr | - | 0.1 | tr | - | - | - | - | - | - |
| 64 | α-amorphene | 1460 | - | - | - | - | - | - | - | - | - | 0.1 | - | 0.1 | 0.1 |
| 65 | germacrene-D | 1475 | 4.2 | 3.5 | 0.1 | 1.9 | 3.0 | 1.5 | 2.8 | 1.2 | 1.6 | 2.0 | 3.2 | 5.0 | 3.2 |
| 66 | β-selinene | 1479 | - | 0.1 | 0.8 | 0.1 | tr | - | 0.1 | 0.2 | tr | 0.1 | - | - | - |
| 67 | bicyclogermacrene | 1486 | 1.0 | 0.9 | 0.9 | 0.4 | 0.9 | - | 1.2 | 0.2 | 0.3 | 0.5 | 0.3 | 0.3 | 0.2 |
| 68 | δ-guaiene | 1493 | 1.4 | 1.3 | 0.4 | 0.5 | 1.4 | - | 1.3 | 0.1 | 0.3 | 0.6 | 0.5 | 1.4 | 1.0 |
| 69 | germacrene-A | 1498 | 1.2 | 0.7 | 1.7 | 0.2 | 0.5 | - | 0.4 | - | 0.1 | 0.3 | 0.2 | 0.5 | 0.3 |
| 70 | γ-cadinene | 1505 | 1.9 | 1.6 | 0.3 | 1.5 | 2.0 | 0.9 | 3.2 | 0.7 | 0.9 | 1.2 | 0.9 | 1.9 | 1.6 |
| 71 | trans-calamenene | 1506 | - | - | - | - | tr | - | 0.2 | - | tr | - | tr | 0.1 | - |
| 72 | β-sesquiphellandrene | 1508 | 0.5 | 0.4 | tr | 0.2 | 0.1 | - | 0.1 | 0.1 | 0.1 | 0.2 | - | - | - |
| 73 | δ-cadinene | 1514 | 0.2 | 0.1 | tr | tr | tr | - | 0.1 | - | tr | 0.1 | tr | 0.1 | 0.1 |
| 74 | α-cadinene | 1520 | - | tr | - | tr | tr | - | - | - | tr | tr | tr | 0.2 | 0.1 |
| 75 | γ-cuprenene | 1528 | - | 0.1 | - | - | - | - | tr | - | - | - | - | tr | tr |
| 76 | trans-γ-bisabolene | 1530 | 0.1 | - | - | - | - | - | - | - | tr | - | tr | tr | tr |
| 77 | germacrene B | 1551 | 0.7 | 0.5 | - | - | - | - | - | - | - | - | - | - | - |
| 78 | trans-nerolidol | 1557 | - | - | - | tr | 0.1 | - | 0.1 | tr | tr | 0.1 | tr | tr | tr |
| 79 | maaliol | 1565 | - | - | - | - | - | - | - | tr | tr | 0.1 | tr | 0.2 | 0.1 |
| 80 | spathulenol | 1571 | - | tr | - | 0.1 | - | - | tr | - | tr | tr | - | 0.1 | tr |
| 81 | caryophyllene-oxide | 1585 | - | - | - | - | - | - | - | - | - | tr | - | tr | tr |
| 82 | viridiflorol | 1592 | - | - | - | - | - | - | 0.2 | 0.1 | 0.2 | 0.4 | 0.2 | 0.6 | 0.5 |
| 83 | guaiol | 1600 | - | - | - | - | - | - | - | - | - | - | - | tr | - |
| 84 | 1,10-di-epi-cubenol | 1616 | 0.5 | 0.5 | 0.5 | 0.5 | 0.6 | - | 1.0 | - | - | - | - | - | - |
| 85 | epi-α-cadinol | 1637 | 4.5 | 3.8 | 3.7 | 4.4 | 4.1 | 1.8 | 6.6 | 1.8 | 2.9 | 3.2 | 2.3 | 4.1 | 3.1 |
| 86 | α-cadinol | 1653 | 0.3 | 0.3 | 0.3 | 0.2 | 0.2 | - | 0.5 | 0.1 | 0.1 | 0.3 | 0.1 | 0.4 | 0.3 |
| 87 | allo himachalol | 1654 | - | - | - | - | - | - | - | - | - | - | - | tr | tr |
| 88 | β-bisabolol | 1681 | - | - | - | - | - | - | 0.2 | - | - | - | - | - | - |
| 89 | α-bisabolol | 1683 | - | 0.1 | tr | 0.1 | 0.1 | - | 0.1 | tr | tr | 0.1 | tr | 0.1 | 0.1 |
| 90 | cis-farnesol | 1680 | - | - | - | - | - | - | - | - | tr | - | - | - | - |
| 91 | β-sinensal | 1686 | - | 0.1 | - | - | - | - | - | - | - | - | tr | tr | |
| 92 | pentadecanal | 1711 | - | - | - | - | - | - | 0.1 | - | - | - | - | - | - |
| 93 | mintsulfide | 1731 | - | - | - | - | - | - | - | - | - | - | - | tr | tr |
| 94 | 6,10,14-trimethyl pendadecanone | 1836 | - | - | - | - | - | - | tr | - | - | - | - | - | - |
| 95 | E.E-farnesyl acetone | 1904 | - | - | - | - | - | - | tr | - | - | - | - | - | - |
| 96 | palmitic acid | 1961 | - | - | - | - | - | - | tr | - | - | - | - | - | - |
| Total | 99.7 | 99.8 | 99.9 | 99.5 | 99.7 | 99.2 | 95.7 | 99.9 | 99.7 | 98.9 | 99.9 | 99.1 | 99.2 | ||
a Retention indices were calculated against C9–C23 n-alkanes on the HP 5MS capillary column.
Despite the observation that in the field conditions the total essential oil yield was decreasing after successive harvests the individual components, prevalent in basil, like linalool and eugenol seem to follow an opposite pattern. Linalool in var. latifolia was beyond 32.2% in all harvests and in the last harvest reached 49.5% while in var. minimum the percentage of linalool was in all harvests above 30.0% and in the second harvest reached 52.0% in the total essential oil concentration. Furtermore, although the concentrations of linalool in var. lettuce for both cultivation conditions were similar, compound methyl-chavicol showed a remarkable difference reaching 54.1% of the EO in the second harvest in the field conditions, whereas in the greenhouse conditions the higher concentration measured, was 17.5% in the first harvest (Table 2 and Table 3). These data suggest that changes in the environmental conditions can alter the biosynthesis of individual essential oils’ components. Ložienė et al. (2004) have reached similar conclusion while studying the influence of environmental and genetic factors in Thymus pulegioides’ essential oil [23]. Similar conclusions were reached also by Awadh Ali et al. (2017) while studying the EO content of O. forskolei and Teucrium yemense collected from different regions of Yemen [24]. In parallel, var. cinnamon grown in the field conditions has methyl-chavicol as major constituent reaching 75.1% of the EO content, in the first harvest (Table 3). It seems that the unstable field conditions, in combination with successive harvests affect and promote the metabolic pathway of L-phenylalanine and cinnamic acid [25], in var. latifolia, minimum and lettuce that leads to the production of methyl chavicol and eugenol. Moreover, the combination of the harvest stress with the unstable field conditions seems to promote the biosynthesis of individual components. In addition to this, the observed decrease in the EO yield in the field conditions during harvests can be attributed to the partial evaporation of the essential oil from the plant surface.
2.2. Statistical Analysis
The statistical analysis of our results confirmed the laborious interaction between the essential oil composition, different varieties, and cultivation conditions. After thorough study of the cluster analyses, it is obvious that during the growing period, varieties can alter the composition of individual components of the essential oil by shifting their chemotype [23].
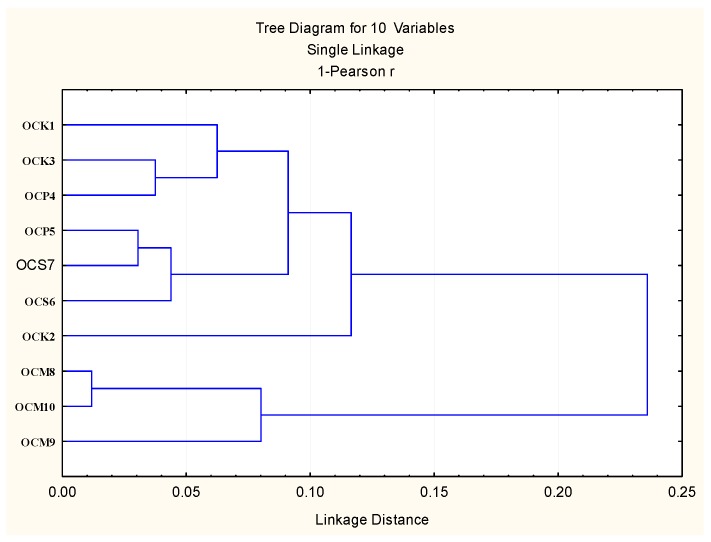
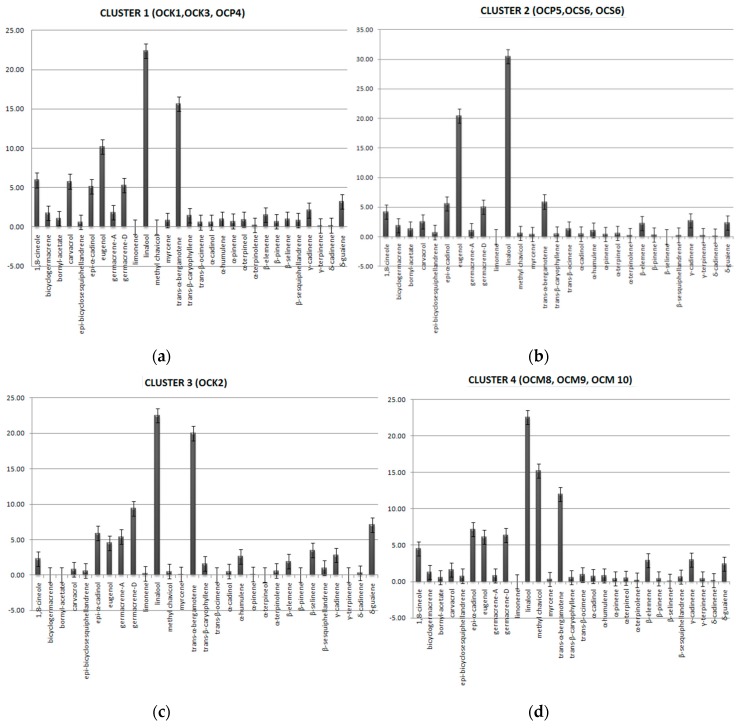
Taking into account the EO content similarities between harvests for the varieties cultivated in the greenhouse, four clusters were formed (Figure 1). Cluster 1 consists of samples OCK1, OCK3, and OCP4, with linalool, trans-α-bergamontene, and eugenol as the dominant components. Cluster 2 consists of samples OCP5, OCS6, and OCS7, with linalool and eugenol as the dominant components. Cluster 3 is “simplicifolious” and consists of OCK2 with major constituents linalool and trans-α-bergamontene. Finally, cluster 4 consists of OCM8, OCM9, and OCM10 with major components linalool, methyl chavichol, and trans-α-bergamontene (Figure 2).
Figure 1.
Bidimensional dendrogram representing the similarity in the main components of the essential oils, between 10 harvests of 4 basil varieties growing in the greenhouse conditions.
Figure 2.
Clusters formatted according to the dominant compounds identified, in varieties violetto, latifolia, minimum and lettuce, cultivated in the greenhouse conditions. (a) Dominant compounds in var. violetto (1st and 3rd harvest) and var. latifolia (1st harvest); (b) Dominant compounds in var. latifolia (2nd harvest) and var. minimum (1st and 2nd harvest); (c) Dominant compounds in var. violetto (2nd harvest); (d) Dominant compounds in var. lettuce (1st, 2nd and 3rd harvest).
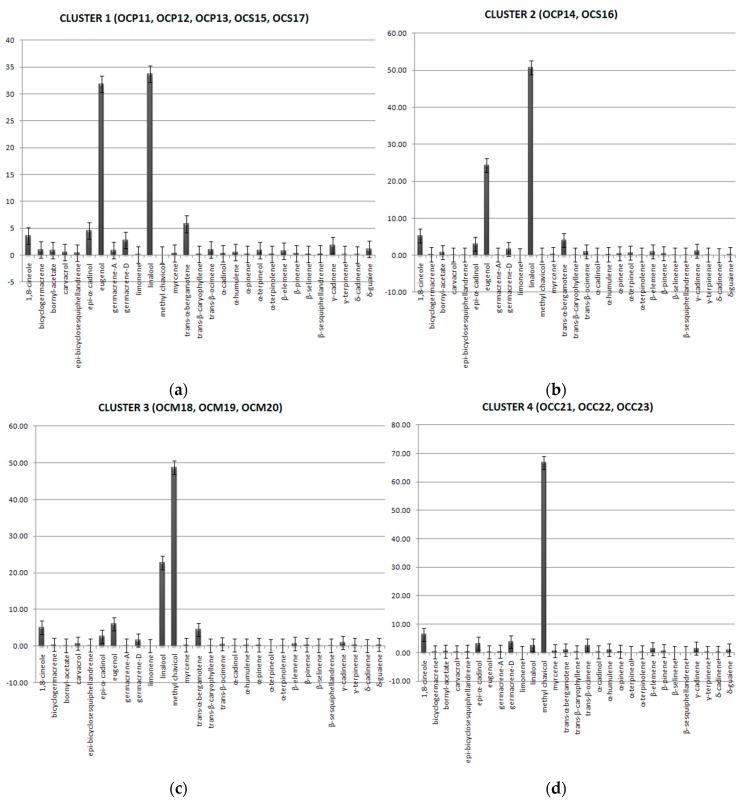
Cultivation conditions in the field defined the dominant constituents for the formation of four clusters in the dendrogram (Figure 3). The similarities observed between harvests and varieties characterized the clusters as: Cluster 1 consisting of samples OCP11, OCP12, OCP13, OCS15, and OCS17 with linalool, eugenol, and trans-α-bergamontene as major components. Cluster 2 consists of OCP14 and OCS16 with linalool and eugenol as major components. Cluster 3 consists of OCM18, OCM19, and OCM20, with methyl chavicol and linalool as major components. Finally, cluster 4 is “simplicifolious” and consists of samples OCC21, OCC22, and OCC23, with methyl-chavicol as the dominant component (Figure 4).
Figure 3.
Bidimensional dendrogram representing the similarity in the main components of the essential oils, between 13 harvests of 4 basil varieties growing in the field conditions.
Figure 4.
Clusters formatted according to the dominant compounds identified in varieties latifolia, minimum, lettuce and cinnamon, cultivated in the field conditions. (a) Dominant compounds in var. latifolia (1st, 2nd and 3rd harvest) and var. minimum (1st and 3rd harvest); (b) Dominant compounds in var. latifolia (4th harvest) and var. minimum (2nd harvest); (c) Dominant compounds in var. lettuce (1st , 2nd, and 3rd harvest); (d) Dominant compounds in var. cinnamon (1st, 2nd and 3rd harvest).
Considering that in both field and greenhouse the conditions were same for all of the varieties, the observed variations can be attributed to the ability of each variety to react to the stressing factor of harvesting. The grouping obtained for the examined basil varieties in greenhouse and field conditions, provides significant data about the effect of harvesting and cultivation site in the chemical composition of basil essential oils. A comparison of Cluster 1 between field and greenhouse conditions reveals that in var. latifolia, between harvests, shifts are observed in the chemotype by alterations in the biosynthesis of essential oils’ components. These results are in compliance with the study of Božović et al. (2017), in EOs obtained from Calamintha nepeta (L.) Savi subsp. glandulosa, indicating that the main compounds were stable, but the ratio between them varied greatly according to the growth stage [26]. Especially in var. lettuce it is obvious that apart from the harvest factor, the cultivation site also affects the chemical profile of the obtained EO. Observation of cluster 3 from field cultivation and cluster 4 from greenhouse cultivation reveals a different chemical profile in the EOs obtained for the same variety. As such, these results imply that the cultivation site should also be considered as factor affecting the composition of EOs, apart from the number of harvests. The variations of the chemical profile of the five basil varieties are in accordance with the results of Verma et al. (2013) where different Ocimum populations belonging to the “basilicum” group are distributed to different clusters [27]. The alteration in the chemical profile of the essential oils obtained from Foeniculum vulgare Miller between harvests was also indicated by Garzoli et al. (2017). Researchers showed that while o-cymene and a-phellandrene were dominating compounds in the first two harvests, EO from the last harvest was characterized by a high content of methyl-chavichol (estragole) [28]. Cluster analysis has been proved as a useful tool in the attempt of an evaluation of chemotypes among essential oil bearing plants. However, in O. basilicum’s case, the numerous synonymous names, varieties, and inerspecific hybridization along with the several factors that affecting essential oils’ composition make this process extremely difficult [29].
The obtained data can serve as a guide for cultivation conditions and the harvesting of basil varieties in this geographical region in order to obtain maximum essential oil yield along with certain metabolites or mixtures of them in the composition of the essential oil. Comparing to the literature data about basil chemical composition, current results are in accordance about the yield of essential oil produced. Additionally, they are very promising concerning the composition of them to important constituents like linalool, eugenol, and methyl chavicol. Environmental conditions, like temperature, humidity, and soil conditions, in the island of Kefalonia affect the chemical profile of the cultivated basil varieties and favor the production of bioactive compounds like linalool [30]. Practically, this implies that essential oils from aromatic plants cultivated in the climatic conditions of Kefalonia have the quality characteristics needed for commercial exploitation.
3. Materials and Methods
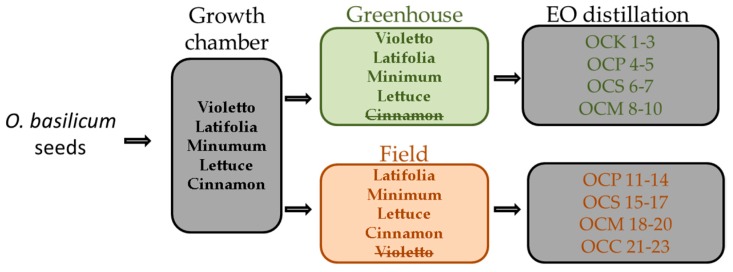
3.1. Cultivation Conditions and Plant Material
Certified Ocimum basilicum L. (basil) seeds, belonging to varieties lettuce, cinnamon, minimum, latifolia, and violetto, were purchased from the local market. Basil seeds of the five varieties were sown into sowing boxes filled with universal soil for sowing seeds in an environmentally controlled growth chamber. Environmental conditions were: photoperiod 12/12, air temperature 18/24 °C, and air relative humidity 50/70% (day/night). When the plants formed true leaves were transplanted into small plastic pots. When they reached 12–15 cm height, they were transplanted to their final position either into plastic 20 L pots in the greenhouse or directly to the land in the experimental field. For each variety 20 plants were planted in each cultivation site. For the field conditions, basil transplants were planted into rows with 0.50 m in-row and 1.00 m between row spacing, covering a total area of 15 m2. Plants were irrigated every second day with an irrigation tape providing 5–7 L of water, in both field and greenhouse conditions. During the growing period, no pests or diseases were observed apart from the inability of var. cinnamon to grow in the greenhouse conditions and that var. violetto failed to grow in the field conditions, thus only the results from one cultivation site are given. Harvesting of the plants was performed almost two months after transplantation, prior to flowering stage [17]. The field cultivation conducted in the experimental field of the Department of Food Technology (Technological Educational Institute of Ionian Islands) in the island of Kefalonia (Figure 5). Plant material was hand harvested by removing the apical portion of the herbage 20 cm above ground and leaving 1–2 internodes in order to promote rejuvenation from the sleeping buds. For each variety and cultivation site, 2–3 harvests were performed depending on the variety and its ability to regenerate stems and flowers. Practically, this includes a period of 30–40 days between each cutting. In every collection, plant material was collected the same time of the day in order to eliminate day variations to the yield of essential oil, transferred to the laboratory and fresh weight was measured, and the essential oil yield to fresh weight was calculated. In total, 23 samples were collected extracted and analysed, namely 10 essential oils obtained from 4 four different varieties cultivated in the greenhouse, and 13 essential oils obtained from four varieties cultivated in the field (Table 1). The plant materials have been deposited in the herbarium of the Department of Food Technology (Technological Educational Institute of Ionian Islands) under the number 737.
Figure 5.
Diagrammatic presentation of the cultivation of basil seeds belonging to five varieties in field and greenhouse, along with the relevant obtained essential oils (EOs).
3.2. Essential Oil Distillation
The collected plant material from the five varieties was separately subjected to hydro distillation in a modified Clevenger-type apparatus according to the Hellenic Pharmacopoeia [31]. Each plant material sample (about 2.0 kg) was extracted for 3 h. The essential oil content (% v/w) was estimated on a fresh weight basis. The oil samples were dehydrated over anhydrous Na2SO4. The obtained essential oils were of yellowish colour and a pleasant odor, and were deposited in vials at −20 °C prior to further chemical analyses.
3.3. GC Analysis
Quantification was performed using gas-chromatography coupled with flame ionization detection (GC-FID). Analyses were carried out on a Perkin Elmer Clarus 500 gas chromatograph with FID, fitted with a fused silica Rtx-5 MS capillary column (30 m × 0.25 mm (i.d.), film thickness: 0.25 μm). The column temperature was programmed from 60 °C to 250 °C at a rate of 3 °C/min. The injector and detector temperatures were programmed at 230 °C and 280 °C, respectively. 0.5 µL of each sample were diluted in 500 µL GC grade n-pentane and 2 μL of the obtained solution was further injected in the GC apparatus. Identification of constituents was achieved by calculating the arithmetic indices relative to linear alkanes from C9–C23 and comparing with data from GC-MS identifications.
3.4. GC-MS Analysis
GC-MS analyses were performed on a Hewlett-Packard 5973-6890 system operating in EI mode (70 eV) equipped with a split/splitless injector (220 °C), a split ratio 1/10, using a fused silica HP-5 MS capillary column (30 m × 0.25 mm (i.d.), film thickness: 0.25 μm). The temperature program for HP-5 MS column was from 60 °C (5 min) to 280 °C at a rate of 4 °C/min. Helium was used as a carrier gas at a flow rate of 1.0 mL/min. Injection volume for all samples, diluted as previously described, was 2 μL.
3.5. Identification of Components
Retention indices for all compounds were determined according to the Van der Dool approach [32] with reference to a homologous series of normal n-alkanes from C9 to C23. The identification of the components was based on a comparison of their mass spectra with those of Wiley and NBS Libraries and those described by Adams (2007), as well as by comparison of their retention indices with literature data [33]. Component relative percentages were calculated based on GC-FID peak areas without using correction factors.
3.6. Statistical Analysis
Data obtained from the chemical analysis of the essential oils from all varieties were analyzed by Multivariate analysis method using Statistica software version 7.0 Statsoft Company. Cluster analyses were performed based on the similarity between harvests from all varieties and their constituent distribution. These analyses were performed on complete data sets. The unweighted pair-group average linkage clustering method based on Pearson distances was used to measure the similarities between each measured unit.
Author Contributions
Y.S. and H.S. conceived and design the experiments of the selection of the varieties, cultivation and evaluation experimental results, respectively. In addition, H.S. supervised the chemical analyses. E.S. performed the cultivation of the basil varieties in the greenhouse and field, the harvesting and the extractions of all essentials oils. T.M. and A.D performed the chemical analyses of the obtained EOs. P.R. performed the statistical analysis of the results. G.T analyzed the data obtained from the chemical analysis, collection of results andwriting of the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Sajjadi S.E. Analysis of the essential oils of two cultivated basil (Ocimum basilicum L.) from Iran. Daru. 2006;14:128–130. [Google Scholar]
- 2.Paton W., Harley R.M., Harley M.M. Ocimum—An overview of relationshipsand classification. In: Holm Y., Hiltuen R., editors. Basil: The Genus Ocimum. Hawood Academic; Amsterdam, The Netherlands: 1999. pp. 1–33. [Google Scholar]
- 3.Paton A., Putievsky E. Taxonomic problems and cytotaxonomic relationships between and within varieties of Ocimum basilicum and related species (Labiatae) Kew Bull. 1996;51:509–524. doi: 10.2307/4117026. [DOI] [Google Scholar]
- 4.Putievsky E., Paton A., Lewinsohn E., Ravid U., Haimovich D., Katzir I., Saadi D., Dudai N. Crossability and relationship between morphological and chemical varieties of Ocimum basilicum L. J. Herbs Spices Med. Plants. 1999;6:11–24. doi: 10.1300/J044v06n03_02. [DOI] [Google Scholar]
- 5.Labra M., Miele M., Ledda B., Grassi F., Mazzei M., Sala F. Morphological characterization: Essential oil composition and DNA genotyping of Ocimum basilicum L. cultivars. Plant Sci. 2004;167:725–731. doi: 10.1016/j.plantsci.2004.04.026. [DOI] [Google Scholar]
- 6.Darrah H.H. The Cultivated Basils. Buckeye Printing Company; Winter Haven, FL, USA: 1980. p. 82. [Google Scholar]
- 7.Beatovic D., Krstic-Miloševic D., Trifunovic S., Šiljegovic J., Glamoclija J., Ristic M., Jelacic S. Chemical composition, antioxidant and antimicrobial activities of the essential oils of twelve Ocimum basilicum L. cultivars grown in Serbia. Rec. Nat. Prod. 2015;9:62–75. [Google Scholar]
- 8.Khalid K.A. Influence of water stress on growth, essential oil, and chemical composition of herbs (Ocimum sp.) Int. Agrophys. 2006;20:289–296. [Google Scholar]
- 9.Zheljazkov V.D., Cantrell C.L., Tekwani B., Khan S.I. Content, composition, and bioactivity of the essential oils of three basil genotypes as a function of harvesting. J. Agric. Food Chem. 2008;56:380–385. doi: 10.1021/jf0725629. [DOI] [PubMed] [Google Scholar]
- 10.Simon J.E., Quinn J., Murray R.G. Basil: A source of essential oils. In: Janick J., Simon J.E., editors. Advanced in New Crops. Timber Press; Portland, OR, USA: 1999. pp. 484–489. [Google Scholar]
- 11.Nurzyńska-Wierdak R. Morphological and chemical variability of Ocimum basilicum L. (Lamiaceae) Mod. Phytomorphol. 2013;3:115–118. [Google Scholar]
- 12.Koba K., Poutouli P.W., Raynaud C., Chaumont J.P., Sanda K. Chemical composition and antimicrobial properties of different basil essential oils chemotypes from Togo. Bangladesh J. Pharmacol. 2009;4:1–8. doi: 10.3329/bjp.v4i1.998. [DOI] [Google Scholar]
- 13.Singh S., Singh M., Singh A.K., Kalra A., Yadav A., Patra D.D. Enhancing productivity of Indian basil (Ocimum basilicum L.) through harvest management under rainfed conditions of subtropical north India plains. Ind. Crops Prod. 2010;32:601–606. doi: 10.1016/j.indcrop.2010.07.007. [DOI] [Google Scholar]
- 14.Marotti M., Piccaglia R., Giovanelli E. Differences in essential oil composition of basil (Ocimum basilicum L.) Italian cultivars related to morphological characteristics. J. Agric. Food Chem. 1996;44:3926–3929. doi: 10.1021/jf9601067. [DOI] [Google Scholar]
- 15.Telci I., Bayram E., Yılmaz G., Avcı B. Variability in essential oil composition of Turkish basils (Ocimum basilicum L.) Biochem. Syst. Ecol. 2006;34:489–497. doi: 10.1016/j.bse.2006.01.009. [DOI] [Google Scholar]
- 16.Topalov V.D. Basil. In: Topalov V.D., editor. Essential Oil and Medicinal Plant. Hr. G. Danov Press; Plovdiv, Bulgaria: 1962. pp. 200–209. [Google Scholar]
- 17.Makri O., Kintzios S. Ocimum sp. (Basil): Botany, Cultivation, Pharmaceutical Properties, and Biotechnology. J. Herbs. Spices Med. Plants. 2007;13:123–150. [Google Scholar]
- 18.Carlo N., Silvia S., Stefano B., Paolo S. Influence of cut number on qualitative traits in different cultivars of sweet basil. Ind. Crops Prod. 2013;44:465–472. doi: 10.1016/j.indcrop.2012.10.009. [DOI] [Google Scholar]
- 19.Lakušić D., Ristić M., Slavkovska V., Lakušić B. Seasonal Variations in the Composition of the Essential Oils of Rosemary (Rosmarinus officinalis, Lamiaceae) Nat. Prod. Commun. 2013;8:131–134. [PubMed] [Google Scholar]
- 20.Public Statement on the Use of Herbal Medicinal Products Containing Estragole. [(accessed on 17 September 2017)]; Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/04/WC500089960.pdf.
- 21.Saran P.L., Tripathy V., Meena R.P., Kumar J., Vasara R.P. Chemotypic characterization and development of morphological markers in Ocimum basilicum L. germplasm. Sci. Hortic. 2017;215:164–171. doi: 10.1016/j.scienta.2016.12.007. [DOI] [Google Scholar]
- 22.Liber Z., Carović-Stanko K., Politeo O., Strikić F., Kolak I., Milos M., Satovic Z. Chemical characterization and genetic relationships among Ocimum basilicum L. cultivars. Chem. Biodivers. 2011;8:1978–1989. doi: 10.1002/cbdv.201100039. [DOI] [PubMed] [Google Scholar]
- 23.Ložienė K., Venskutonis P.R. Influence of environmental and genetic factors on the stability of essential oil composition of Thymus pulegioides. Biochem. Syst. Ecol. 2005;33:517–525. doi: 10.1016/j.bse.2004.10.004. [DOI] [Google Scholar]
- 24.Awadh Ali N.A., Chhetri B.K., Dosoky N.S., Shari K., Al-Fahad A.J.A., Wessjohann L., Setzer W.N. Antimicrobial, Antioxidant, and Cytotoxic Activities of Ocimum forskolei and Teucrium yemense (Lamiaceae) Essential Oils. Medicines. 2017;4:17. doi: 10.3390/medicines4020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nykanen I. The Effect of Cultivation Conditions on the Composition of Basil Oil. Flavour Fragr. J. 1989;4:125–128. doi: 10.1002/ffj.2730040309. [DOI] [Google Scholar]
- 26.Božović M., Garzoli S., Sabatino M., Pepi F., Baldisserotto A., Andreotti E., Ramagnoli C., Mai A., Manfredini S., Ragno R. Essential oil extraction, chemical analysis and anti-Candida activity of Calamintha nepeta (L.) Savi subsp. glandulosa (Req.) Ball—New approaches. Molecules. 2017;22:203. doi: 10.3390/molecules22020203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verma R.S., Padalia R.C., Chauhan A., Thul S.T. Exploring compositional diversity in the essential oils of 34 Ocimum taxa from Indian flora. Ind. Crops Prod. 2013;45:7–19. doi: 10.1016/j.indcrop.2012.12.005. [DOI] [Google Scholar]
- 28.Garzoli S., Božović M., Baldisserotto A., Sabatino M., Cesa S., Pepi F., Vincentini C.B., Manfredini S., Ragno R. Essential oil extraction, chemical analysis and anti-Candida activity of Foeniculum vulgare Miller—New approaches. Nat. Prod. Res. 2017 doi: 10.1080/14786419.2017.1340291. [DOI] [PubMed] [Google Scholar]
- 29.Sharopova F.S., Satyal P., Awadh A.N.A., Pokharel S., Zhang H., Wink M., Kukanieve M.A., Setzer W.N. The Essential Oil Compositions of Ocimum basilicum from three different Regions: Nepal, Tajikistan, and Yemen. Chem. Biodivers. 2016;13:241–248. doi: 10.1002/cbdv.201500108. [DOI] [PubMed] [Google Scholar]
- 30.Aprotosoaie A.C., Hăncianu M., Costache I.I., Miron A. Linalool: A review on a key odorant molecule with valuable biological properties. Flavour Fragr. J. 2014;29:193–219. doi: 10.1002/ffj.3197. [DOI] [Google Scholar]
- 31.Scientific Committee of the Hellenic Pharmacopoeia . Hellenic Pharmacopoeia. 5th ed. National Organization for Medicines of Greece; Athens, Greece: 2002. Chapter 28.12. [Google Scholar]
- 32.Van den Dool H., Kratz P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A. 1963;11:463–471. doi: 10.1016/S0021-9673(01)80947-X. [DOI] [PubMed] [Google Scholar]
- 33.Adams R.P. Identification of Essential Oils Components by Gas Chromatography/Quadrupole Mass Spectroscopy. Allured Publishing Corp; Carol Stream, IL, USA: 2007. [Google Scholar]