Abstract
The complex pathophysiology of spinal cord injury (SCI) may explain the current lack of an effective therapeutic approach for the regeneration of damaged neuronal cells and the recovery of motor functions. A primary mechanical injury in the spinal cord triggers a cascade of secondary events, which are involved in SCI instauration and progression. The aim of the present review is to provide an overview of the therapeutic neuro-protective and neuro-regenerative approaches, which involve the use of nanofibers as local drug delivery systems. Drugs released by nanofibers aim at preventing the cascade of secondary damage (neuro-protection), whereas nanofibrous structures are intended to re-establish neuronal connectivity through axonal sprouting (neuro-regeneration) promotion, in order to achieve a rapid functional recovery of spinal cord.
Keywords: spinal cord injury, nanofibers, electrospinning, neuroprotection, neuroregeneration
1. Introduction
Spinal Cord Injury (SCI) results in devastating and debilitating conditions such as severe dysfunctions of the motor, sensory, and autonomic systems [1].
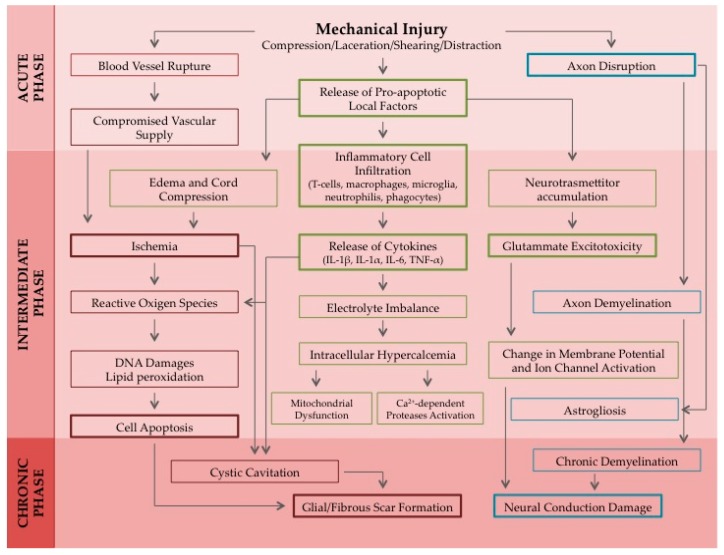
It is mainly caused by mechanical trauma of the spine, due to traffic accidents, falling from buildings, gun shots, sport injuries etc. It is considered a global issue, affecting people of every age and concerning almost 2.5 million patients worldwide. Despite the fact that, in the last decades, improvements in medical care have increased patient survival rates and reduced the impact of SCI on life quality, a therapeutic approach for the regeneration of damaged cells and for the recovery of motor functions is still lacking [2]. Therefore, effective multifaceted therapies, aiming at reducing the extent of tissue disruption and improving neurologic outgrowth after spinal cord trauma, are urgently needed. The pathophysiological response to a spinal cord injury involves a primary damage, followed by the activation of a cascade of secondary events. SCI instauration and progression are generally divided into acute (seconds to minutes after the traumatic event), intermediate (minutes to weeks after the trauma) and chronic (months to years) phases [2,3] (Figure 1).
Figure 1.
Schematic representation of the pathophysiological response to a spinal cord injury induced by a mechanical trauma. A cascade of vascular, cellular, and biochemical events brings to the progression of the spinal cord damage until the formation of a glial scar. Acronyms: IL-1α, Interleukin 1α; IL-1β, Interleukin 1β; IL-6, Interleukin 6; TNF-α, Tumor Necrosis Factor α.
Primary mechanical trauma of the spinal cord, such as compression and shear forces, produces instantaneous vascular, cellular, and axonal damages that expand from the injury site in both radial and axial directions [1,4,5].
Vertebral disc fractures produce bone fragmentation and consequent disconnection of long axonal tracts, thus damaging neurons, oligodendrocytes, and astrocytes. After capillary rupture at the injury site, an invasion of blood circulating leukocytes occurs. Such an event, in conjunction with the release of several substances from the damaged cells, determines a toxic environment that is responsible for lesion expansion (SCI secondary phase). Fibroblasts infiltrate the perilesional region while the surviving astrocytes release growth inhibitory chontroitin sulfate proteoglycans (CSPGs), such as neurocan, versican, brevican, phosphacan, as chemical barrier [6,7].
Axon distal segment, unconnected with the neuron soma, crushes and activates a process called Wallerian degeneration, which determines apoptosis of the oligodendrocytes surrounding distal segments [2,8]. The secondary damage ends with the formation of a glial scar, which can further impede axonal regeneration.
Current treatments of SCI can be classified as neuro-protective or neuro-regenerative ones. Neuro-protective therapies are intended to avoid or prevent further progression of the secondary injury; neuro-regenerative approaches are directed to recover the lost or impaired functionality of the spinal cord by repairing the broken neuronal circuitry [9,10].
The aim of the present review is to provide an overview of the therapeutic neuro-protective and neuro-regenerative approaches for SCI treatment. At first, a panorama of current pharmacological approaches is presented with particular regard to drugs whose potential in SCI treatment has been reported in the literature of the last two years. The second part of the review is devoted to emerging technological approaches based on nanofibers. They act as drug carriers, releasing the active compound/saccording to the therapeutic requirements, and provide a physical support, directing and guiding axonal regeneration. Drugs released by nanofibers aim at preventing the cascade of secondary damage (neuro-protection), whereas nanofibrous structures are intended to re-establish neuronal connectivity through axonal sprouting (neuro-regeneration) promotion, in order to obtain a rapid functional recovery of the spinal cord.
2. Overview of Current Pharmacological Approaches for the Treatment of SCI
Current pharmaceutical strategies for the treatment of SCI are intended to avoid or prevent secondary injury progression, by minimizing apoptosis, oxidative stress or inflammation [11].
Nowadays, the glucocorticoid Methylprednisolone (MP) is the only drug approved by the Food and Drug Administration (FDA) for SCI treatment. While it determines neuro-protection in the early 8 h after injury, thanks to the reduction of secondary inflammation and lipid peroxidation, it is unable to effectively prevent or stop damage progression. The use of MP is controversial due to its severe complications like pneumonia, sepsis and death [1,12,13]. In addition to MP, in recent years other drugs have obtained promising results when tested in preclinical studies. They were recently reviewed by Kabu et al. [1]. Table 1 reports a list of such drugs together with their mechanism of action and potential effect on SCI. Among them, Riluzole and Minocycline are the most promising.
Table 1.
Drugs reviewed by Kabu et al. [1] as neuro-protective agents for spinal cord injury (SCI) treatment.
| Name | Mechanism of Action | Effect on SCI |
|---|---|---|
| Atorvastatin (Lipitor) [14] | Reduction of cholesterol levels | Anti-inflammatory effect, anti-apoptosis, tissue sparing and locomotion recovery |
| Calpain inhibitors [15] | Inhibition of cytoskeletal protein degradation and apoptosis | Tissue preservation, locomotion recovery, anti-apoptosis |
| Chicago sky blue [16] | Macrophage migration inhibition | White matter increase and blood vessel integrity recovery |
| Erythropoietin (EPO) [17,18] | Activation of EPO receptor | Anti-inflammatory effect, anti-apoptosis, cytoprotection, vascular integrity recovery, lipid peroxidation inhibition |
| Estrogen [19] | Hormone replacement | Anti-apoptosis, myeloperoxidase activity reduction, microglial/macrophage accumulation |
| C3-exoenzyme, Fasudil, Y27532, Ibuprofen [1] | Rho antagonists | Locomotion recovery |
| Ferulic acid from Ferula species [20] | Antioxidant activity | Anti-inflammatory effect, locomotion recovery, axonal/myelin protection and excitotoxicity prevention |
| FTY720 [21] | Modulation of sphingosine receptor | Anti-inflammatory effect, anti-apoptosis, tissue sparing and locomotion recovery |
| Hydralazine [22,23] | Acrolein scavenger | Neuropathic pain reduction and locomotion recovery |
| Imatinib [24] | Protein-tyrosine kinase inhibitor (clinically used for leukemias and gastrointestinal stromal tumors) | Anti-inflammatory effect, anti-apoptosis, tissue sparing and locomotion recovery |
| Melatonin [25] | Antioxidant activity | Lipid peroxidation reduction, neuro-axonal and blood-spinal cord barrier (BSCB) protection, locomotion recovery |
| Minocycline [19] | Antioxidant activity | Immunomodulation of microglia, excitotoxicity, mitochondrial stabilization, anti-apoptosis |
| NSAIDs [19] | Selective cycloxygenase (COX−2) inhibitors | Anti-inflammatory effect |
| Quercitin, Deferoxamine and Ceruloplasmin [26,27,28] | Ca2+ chelation | Locomotion recovery |
| Riluzole [29] | Blockage of the sodium channels | Intracellular [Na+] and [Ca2+] modulation and excitotoxicity reduction |
| Rolipram [30] | Phosphodiesterase type 4 inhibitor | Anti-inflammatory effect, anti-apoptosis, tissue sparing and locomotion recovery |
| Vitamins C and E [31] | Antioxidant activity | Anti-inflammatory effect |
In the last two years, many research studies, focusing on the identification of drugs potentially effective in SCI treatment, have been published [32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96]; these drugs are listed in Table 2, Table 3, Table 4 and Table 5. Information about mechanism of action, potential effect on SCI and administration route, when mentioned, is reported. In particular, some bioactive vegetal extracts and compounds have been proposed as reported in Table 2. Among them, Ganoderma Lucidum (GL) seems the most promising. Histopathological evaluation and neurological examination demonstrated that GL polysaccharides have in the SCI animal model an effect comparable to that obtained in an animal control group treated with intraperitoneal injection of MP [44]. Also Caffeic acid and Mangiferin show comparable in vivo effects to MP [36,48].
Table 2.
Vegetal extract components reported in the literature over the last two years as potentially effective in the treatment of SCI.
| Name | Mechanism of Action | Effect on SCI | Administration Route in Animal Models |
|---|---|---|---|
| Allicin [32] | Increase in nuclear factor (erythroid-derived 2)-related Factor-2 (Nrf-2) nuclear translocation in neurons and astrocytes | Neuro-protection, locomotion recovery antioxidant, anti-apoptosis and anti-inflammatory effects | Intraperitoneal injection |
| Aloe vera [33] | Reduction of neuronal nitric oxide synthase (nNOS) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) protein | Anti-inflammatory, antioxidant, anti-apoptosis | Per os |
| Asiaticoside [34] | Inhibition of p38-mitogen-activated protein kinase (p38-MAPK) signaling pathway | Antioxidant and anti-inflammatory effects | Intraperitoneal injection |
| Buyang Huanwu decoction [35] | Reduction in caspase-3 and Bax expression and increase in Bcl-2 expression | Anti-apoptosis effect and hind-limb motor function recovery | Per os |
| Caffeic acid phenethyl ester (CAPE) [36] | Antioxidant activity | Neuro-protection, anti-apoptosis | Intraperitoneal injection |
| Carnosol [37] | Down-regulation of NF-κB and COX-2 levels and up-regulation of phosphorylated Akt and Nrf-2 expression | Neuro-protection, antioxidant and anti-inflammatory effects | Intraperitoneal injection |
| Crocin from Crocus sativus [38] | Down-regulation of tumor necrosis factor- α (TNF-α) and Interleukin 1β (IL-1β) and antioxidant activity | Neuro-protection and functional recovery in animal SCI | Implantation |
| Curcumin [39,40] | Reduction of inflammatory cytokine expression and antioxidant activity | Neuro-protection, anti-apoptosis, oxidative stress and lipid peroxidation reduction, locomotion recovery | Intraperitoneal injection |
| Docosahexaenoic acid (DHA) [41] | miR-21 and phosphorylated Akt up-regulation and phosphatase and tensin homologue (PTEN) down-regulation | Neuroplasticity enhancement | Tail vein injection |
| (−)-epigallocatechin-3-gallate polyphenol [42] | Down-regulation of Ras homolog gene family, member A (RhoA), fatty acid synthase (FASN) and TNF-α expression | Neuro-protection, reduction of thermal hyperalgesia and of astro- and microglia reactivity | Intraperitoneal injection |
| Glycyrrhizic acid [43] | Reduction of NF-κB and S100B expression | Neuro-protection, lipid peroxidation reduction, anti-necrotic and anti-inflammatory effects | Catheter inserted into the extradurally thoracic |
| Ganoderma lucidum polysaccharides from Basidiomycota [44] | Modulation of caspase-3 and myeloperoxidase activities, reduction of transforming growth factor- α (TGF-α), malondialdehyde and nitric oxide levels | Neuro-protection and functional recovery | Per os |
| Ginkgo biloba extract 761 [45] | Antioxidant, antiapoptosis | Neuro-protection, motor recovery | Intraperitoneal injection |
| Go-sha-jinki-Gan [46] | Anti TNF-α | Neuro-protection, analgesic and anti-necrosis effects | Implantation |
| Herba Lycopodii [47] | Increase of brain derived neurotrophic factor (BDNF) expression | Neuro-protection and motor function improvement | Intragastric injection |
| Mangiferin [48] | Reduction of malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT) activities and serum levels of glutathione peroxidase (GSH-PX), NF-κB, TNF-α, IL-1β, modulation of Bcl-2 and Bax pathway | Neuro-protection, antioxidant and anti-inflammatory effects and anti-apoptosis, locomotion recovery | Intraperitonesl injection |
| Rutin [49] | Macrophage inflammatory protein-2 (MIP-2) expression inhibition and matrix metalloproteinase-9 (MMP-9) activation, down-regulation of p-Akt expression | Neuro-protection and locomotion recovery | Intraperitoneal injection |
| Thymoquinone from Nigella sativa [50] | Antioxidant activity, modulation of cytokine, activation of antioxidant enzyme | Neuro-protection, antioxidant activity, anti-inflammatory effect, reduction of motor neuron apoptosis | Intraperitoneal injection |
Table 3.
Neuro-protective or neuro-regenerative drugs reported in the literature over the last two years as potentially effective in the treatment of SCI.
| Name | Mechanism of Action | Effect on SCI | Administration Route in Animal Models |
|---|---|---|---|
| Acetyl-L-carnitine [51] | Improvement of mitochondria respiration for adenosine tri-phosphate (ATP) production | Protection of endothelial cells of microvessels and locomotor function recovery in lumbar injury | Intrathecal (sub-arachnoid) injection in rats |
| Adalimumab [52] | Antioxidant, TNF-α, IL-1β and IL-6 serum levels | Neuro-protection and anti-inflammatory effect | Subcutaneous injection in compressive spinal cord injury |
| Alpha Lipoic Acid + N-Acetyl Cysteine [53] | TNF-α, IL-6 and malondialdehyde (MDA) inhibitor | Motor recovery and anti-inflammatory and antioxidant effects | Intraperitoneal injection |
| Aspirin [54] | Inhibition of phospholipases, nitric oxide synthetases, and cyclooxygenases | Neuro-protection and, anti-inflammatory effects, lipid peroxidation reduction and locomotion recovery | Intraperitoneal injection |
| Azithromicyn (AZM, macrolide antibiotic) [55] | Reduction of pro-inflammatory macrophage activation | Anti-inflammatory effect, tissue sparing and motor recovery | per os |
| A68930 (Dopamine D1 receptor agonist) [56] | Inhibition of NLRP3 inflammasome activation and reduction of pro-inflammatory cytokines levels and MPO activity | Neuro-protection and anti-inflammatory effect | Intraperitoneal injection |
| cAMP combined with functionalized collagen scaffold [57] | Reduction of cavitation volume, axonal and neuronal regeneration | Neuro-regeneration, remyelination, revascularization and locomotion recovery | Implantation |
| Carvedilol [58] | Increase in SOD and glutathione (GSH), reduction of MPO and malondialdehyde (MDA) | Neuro-protection, antioxidant and anti-apoptosis effects, locomotion recovery | |
| Dexamethasone [59] | Macrophages modulation | Neuro-protection and locomotor recovery | Subdural infusion |
| Dibutyryl cyclic adenosine monophosphate (db-cAMP) [60] | Activation of protein kinase A (PKA) signaling by cAMP-related pathways; reduction of apoptosis | Neuro-regeneration, axonal sprouting, functional recovery and modulation of glial scar formation | Implantation |
| 17β-estradiol (E2) [61] | Down-regulation of LC3II and beclin-1 expression and suppression of excessive autophagy | Neuro-protection and locomotion recovery | Intramuscular injection |
| Estrogen hormone [62] | Reduction of TNF-α and iNOS genes expression | Antioxidant, locomotion recovery and anti-inflammatory effect | Intraperitoneal injection |
| FK506 (Tacrolimus) + Minocycline [63] | Reduction of thiobarbituric acid–reactive species (TBARS), total glutathione (GSH) and MPO activity | Neuro-protection, functional recovery and antioxidant effect | Per os |
| Gp91ds-tat (NOX2-specific inhibitor) [64] | Inhibition of NADPH oxidase (NOX) enzyme (NOX 2 isoform) | Antioxidant and anti-inflammatory effects | Intrathecal injection |
| Histamine H4 receptor agonist [65] | Reduction of IL-1β, TNF-α, 8-hydroxy-2′-deoxyguanosine (8-OHdG) and PARP expression and restoration of MnSOD enzymatic activity |
Antioxidant, anti-inflammatory and analgesic effects | Per os |
| Histidine-Tryptophan-Ketoglutarate (HTK) solution [66] | Metabolic regulation and blood-flow maintenance agents | Locomotion recovery, neuro-protection and reduction of ischemia | Infusion into the occluded aortic segment |
| Lipoxin A4 (LXA4) [67] | Reduction of spinal expression levels of microglial markers (IBA-1) and pro-inflammatory cytokines (TNF-α) | Neuro-protection, analgesic and anti-inflammatory effects | Intrathecal injection |
| Melatonin with amniotic epithelial cells (AECs) [68] | Melatonin receptor 1 stimulation and promotion of ARC differentiation into neural cells by Wint-4 gene expression | Neuro-regeneration and locomotion recovery | Injection along the midline of spinal cord |
| Metformin [69] | Reduction of NF-κB expression and caspase 3 activation, autophagy activation via mTOR/p70S6K signaling | Neuro-protection, anti-apoptosis and anti-inflammatory effects in preconditioning treatment | Intraperitoneal injection |
| N-(4-cyanophenylmethy)-4-(2-diphenyl)-1-piperazinehexanamide (LP-211) [70] | Serotonin (5-HT7) selective agonism, hyponatremia, hyperkalemia and hypermagnesemia induction | Modulation of imbalances in serum electrolyte concentration, neuro- and renal tissue protection | Intraperitoneal injection |
| Nor-Binaltorphimine (norBNI) [71] | κ-opioid receptor (KOR) antagonism and morphine antagonism | Locomotion recovery | Intraperitoneal injection |
| PMX53 (C5aR antagonist) [72] | Inhibition of neutrophil infiltration and reduction of MPO activity | Neuro-protection from ischemia-reperfusion injury | Femoral vein injection |
| Progesterone [73] | Modulation of pro-inflammatory cytokine expression | Anti-inflammatory, remyelinating action, and analgesic effects | Subcutaneous injection |
| Propofol [74] | Reduction of superoxide dismutase 1 (SOD1) expression related to PI3K/AKT signal pathway | Reduction of spinal cord ischemia/reperfusion injury and antioxidant effect | intraperitoneal injection in rabbit with ischemia/reperfusion (I/R) spinal cord injury by aortic occlusion |
| Rapamycin [75] | Activation of Wnt/β-catenin pathway | Neuro-protection and locomotion recovery | Intraperitoneal injection |
| Retinoic acid (Vitamin A) [76] | Autophagic flux activation after trauma | Neuro-protection, functional recovery and prevention of BSCB disruption | Intraperitoneal injection |
| Rosiglitazone in combination with MP [77] | Peroxisome proliferator-activated receptor-γ (PPAR-γ) activation | Functional recovery, anti-inflammatory antioxidant and anti-apoptosis effects | Intraperitoneal injection |
| Selenium-enriched supplement (SES) [78] | Up-regulation of ciliary neurotrophic factor (CNTF) and CNTF-Rα expression | Neuro-protection | Per os |
| Simvastatin [79] | Autophagy activation by mTOR signaling pathway inhibition | Neuro-protection | |
| Stat 1 Inhibitor (S1491) [80] | Neuro-protection and anti-apoptosis effect | Intraperitoneal injection | |
| Tamoxifen [81] | Estrogen receptor modulator | Anti-apoptotic, antioxidant, anti-inflammatory, anti barrier permeability and antigliotic effects | |
| Tetramethylpyrazine (TMP) [82] | Activation of Akt/Nrf-2/HO-1 signaling pathway | Neuro-protection, locomotion recovery and reduction of BSCB permeability | Intraperitoneal injection |
Table 4.
Drugs with a neuropathic-pain target, reported in the literature over the last two years as potentially effective in the treatment of SCI.
| Name | Mechanism of Action | Effect in SCI | Administration Route |
|---|---|---|---|
| Acrolein [84] | Activation of transient receptor protein ankyrin 1 (TRPA1) in both central and peripheral systems | Reduction of both acute and chronic neuropathic pain | Injection in spinal cord |
| Botulinum Toxin type A (BTX-A) [85] | Inhibition of the release of substance P, calcitonin and glutamate | Reduction of chronic neuropathic pain | Subcutaneous injection |
| Cannabis [86] | Reduction of neuropathic pain | Vaporization | |
| GABAergic inhibitors [87] | Reduced neuronal activity in the GABAergic ZI (zona incerta) | Reduction of neuropathic pain | Cannula implantation |
| Methadone [88] | Opioid agonist | Reduction of neuropathic pain during opioid rotation for chronic pain | |
| Morphine [89] | Toll like receptor 4 (TLR4) pathway attivation and allodynia increase shortly after trauma | Prevention of amplified allodyna in a long/term administration | Subcutaneous injection |
| Neurothensin A analogue (CGX-1160) [90] | Reduction of neuropathic pain | Intrathecal injection |
Table 5.
Drugs activating the neurogenic detrusor in subject with SCI, reported in the literature over the last two years.
| Name | Mechanism of Action | Effect in SCI | Administration Route |
|---|---|---|---|
| Botulinum toxin A [91] | Upper urinary tract protection, modulation of detrusor overactivity and detrusor external sphincter dyssynergia | Injections into detrusor and external urethral sphincter in humans with suprasacral and sacral injuries | |
| Imidafenacin [92] | Anticholinergics selective for the urinary bladder, detrusor pressure reduction and cystometric volume increase | Urodynamic effects with possibly alleviation of bladder complication | Injections in patients with SCI and low cystometric volume and/or detrusor compliance |
| Inosine [93] | antioxidant by peroxynitrite disattivation, anti-inflammatory, axogenic and neurotrophic properties | Modulation of detrusor overactivity, decrease of non-voiding contraction (NVC), decrease TRPV1 in bladder tissue | Intraperitoneal injection in rat with NVC immediately after SCI |
| Mirabegron [94] | β-3 agonist | Urodynamic improvement | Administered in patients with neurogenic detrusor overactivity (NDO) after SCI |
| Naftopidil/BMY7378/Silodosin (α-adrenoceptor blockers) [95] | α-adrenoceptor blockade | Reduction of urethral resistance, voiding efficiency improvement by external urethral sphincter-electromyography(EMG) | Intravenous injection in rat with chronic SCI |
| Propiverine (antimuscarinic agent) [96] | Antagonism against muscarinic receptor, L-type Ca2+ channels and transient receptor potential vanilloid subtype 1 (TRPV1) | Amelioration of urinary tract dysfunctions and reduction of detrusor overactivity | Administered to rats with SCI and non-voiding contraction (NVC) |
Table 3 lists synthetic drugs having neuro-protective or neuro-regenerative actions, studied over the last year. Among them, Tamoxifen is of particular interest. It is an FDA approved selective estrogen receptor modulator with several neuro-protective properties. Some authors demonstrated its capability to improve functional locomotion recovery after SCI. Results suggested that the mechanism of action of Tamoxifen is to modulate antioxidant, anti-inflammatory, and anti-gliotic responses. Sex differences in response to Tamoxifen and the administration therapeutic window are still unknown [81]. Another drug that could be easily translatable in clinical trials, since it is safe for humans, is Acetyl-l-carnitine. It has a specific action mechanism, improving mitochondrial respiration in the animal model [51]. The effect of drug combination was also investigated. Recently the association of MP with Rosiglitazione showed an increase in functional recovery with respect to that observed when drugs were administrated alone [77]. Promising results were obtained for the Buspirone/Levodopa/Carbidopa combination (Spinalon™) in a double-blind randomized study of phase I/IIa involving patients with a complete spinal cord injury according to the American Spinal Injury Association Impairment Scale (AIS) [83]. Analgesic drugs with a neuropathic-pain target are shown in Table 4, whereas those effective in neurogenic detrusor activation are listed in Table 5. Such drugs are needed since SCI is frequently responsible for a disorder of the normal function of the lower urinary tract, the storage and evacuation of urine. In particular neurogenic detrusor overactivity (NDO) is characterized by a reduced bladder capacity, an elevated detrusor pressure during the storage phase and/or a reduced compliance. NDO can cause an irreversible deterioration of the upper urinary tract with subsequent renal failure [94].
3. Overview of Promising Nanotechnology Approaches for the Treatment of SCI
In the context of SCI, an effective drug dose has to be administered in order to reach the injury site and achieve the therapeutic effect. In the case of systemic administration, drugs have to cross the blood–spinal cord barrier (BSCB) to reach the injury site [97]. Oral administration undoubtedly encounters higher patient compliance than parenteral one, but limited gut absorption and/or high pre-systemic metabolism could determine a poor drug bioavailability. Drug administration via epidural and intraspinal routes could be more targeted and effective than systemic delivery, even though several limitations have to be considered. Drugs, administered via the epidural route, have to cross meninges (dura, arachnoid and pia); the use of an intrathecal catheter or repeated spinal injections can represent a high risk of infections [97]. Implanted drug delivery systems have recently emerged as a promising strategy to fill the site of injury. Recently, locally administered drug delivery carriers, such as nanofibers have been proposed in the literature. In vivo tests demonstrated that drug-loaded nanofibers show enhanced therapeutic effects as well as great potential for clinical use. As already mentioned, these systems provide a physical support, directing and guiding axonal regeneration, and, at the same time, modulating the release of the loaded drug/s in response to the therapeutic requirements [2,97].
The capability of polymer-based nanofibers to promote nerve regeneration acting as a support for cell growth and new tissue formation has been widely studied [98,99,100]. Guo et al. reviewed nanofiber scaffolds used for SCI treatment, focusing on peculiar properties such as supporting graft cells, reconstructing tissue loss, alleviating inflammation, improving axonal regeneration, and acting as drug delivery systems [101].
In a more recent work, Asghari et al. explained in detail the properties that a scaffold based on synthetic or natural biodegradable polymers must have in order to be used in tissue engineering [102].
Scaffold must be able to mimic the fascicular nerve architecture and the fibrous extracellular matrix (ECM) that characterized the native tissue in terms of both chemical composition and physical structure [103]. ECM has a complex composition: it contains proteoglycans, proteins, and signaling molecules. It is known for its role in providing structural support to cells and as a location for cells migration [104].
Aligned nanofibers are able to mimic the oriented fascicular nerve environment; in the literature, several studies demonstrated that neurites preferred aligned oriented fibers than randomly oriented ones [105]. Two explanations could clarify such cell behavior. The first one is supported by the experimental evaluation of cell growth: in particular C17.2 and PC12 neural cells were characterized by a significantly higher proliferation when cultured on aligned oriented fibers in comparison with randomly un-oriented fibers [106,107,108]. The second one is based on the reasonable hypothesis that nerve cell outgrowth does not meet any barrier to graft the aligned fibers in comparison with the randomly oriented ones [106]. Zuidema et al. demonstrated that fiber misalignment can significantly impede astrocytes migration and elongation [108].
Different categories of biomaterials were investigated for their ability to guide axonal regeneration and to deliver small molecules at the site of injury or to improve the viability of transplanted stem cells. Appropriate scaffolds for tissue engineering applications should be biocompatible, non-toxic, non-mutagenic, and non-immunogenic. Furthermore, they should be able to provide appropriate mechanical support and show favorable topographical properties to improve cell adhesion, proliferation, and differentiation [109].
The materials mainly used for nanofiber scaffolds used for SCI treatment are completely biocompatible and biodegradable to avoid a second surgical treatment to remove the implanted fibers. The nerve matrix conduits, which are Food and Drug Administration (FDA) and European Commission approved, consist of biodegradable materials, among them the Neurotube™, Neura-Gen™ and Neurolac tubes, are made of poly(glycolide) (PGA), collagen and poly(DL-lactide-ε-caprolactone), respectively.
As listed in Table 6, the common biodegradable synthetic polymers tested in therapy of SCI include: polycaprolactone (PCL), polyethylene glycol (PEG), poly(lactic acid) (PLA), poly-L-lactic acid (PLLA), poly(lactic-co-glycolic acid) (PLGA), silica (SNF), poly-d-lysine (PDL), aminopropyl-trimethoxysilane (APTS), peptide anphiphile (PA) and poly-propylene carbonate (PPC). Natural polysaccharides, such as chitosan and tragacanth gum, and proteins, such as collagen and silk fibroin (SF), are also widely used. Synthetic polymers can provide sufficient mechanical properties and improved manufacturing process, while natural polymers can improve cell attachment and orientation, mimicking the axonal environment [109].
Table 6.
Materials employed for production of nanofibers proposed for SCI treatment.
| Materials Employed | Drug Loaded | Potential Effect in SCI |
|---|---|---|
| Ac-FAQ with PCL+ PLGA [110] | - | In vivo nerve regeneration |
| Bombyx mori silk fibroin (SF) [111] | - | In vitro neurite outgrowth and astrocyte migration |
| Chitosan scaffold [112] | - | In vivo functional recovery |
| Collagen type I [113] | In vivo neurite outgrowth and astrocyte migration | |
| Collagen type I [114] | - | In vivo motor recovery |
| Graphene nanoscaffold [115] | - | In vivo biocompatibility and nerve outgrow |
| Multi-layer PCL [116] | - | In vitro axonal regeneration |
| PCL + Gum tragacanth (GT) [117] | Curcumin | In vitro biocompatibility, long-lasting release of drug and wound healing properties |
| Peptide anphiphile (PA) [118] | Dexamethasone | Achievement of long-lasting release of drug and In vivo localized anti-inflammatory effect |
| PCL [119] | Dexamethasone | Achievement of long-lasting release of drug |
| PCL + PLGA functionalized with Ac-FAQ [110] | - | In vivo nerve regeneration |
| PLA [120] | - | In vivo biocompatibility and promotion of spinal cord damage repair |
| PLGA + PCL + (RADA16, a ionic self-complementary peptide) [121] | Cytokines | In vivo axonal regeneration and neurological recovery |
| PLGA [98] | - | In vivo axonal regeneration and motor and sensory recovery |
| PLA + gum tragacanth (PLA/GT) [117] | - | In vitro neurite outgrowth and nerve cell elongation on aligned nanofibers |
| PPC [60] | Dibutyryl cyclic adenosine monophosphate (dbcAMP) | In vivo nerve regeneration, functional recovery and glial scar reduction |
| Poly(trimethylene carbonate-co-ε-caprolactone) [122] | Ibuprofen | In vivo nerve conduit and anti-inflammatory |
| Positively charged oligo[poly(ethylene glycol)fumarate] (OPF+) [123] | - | In vivo axonal regeneration and functional recovery |
| PuraMatrix nanofibrous hydrogel + honeycomb collagen sponge [107] | - | In vivo locomotion functional recovery, spinal repair and neuronal regeneration |
| Electrospun PLGA coated with polypyrrole (PPy) [124] | - | Electrical stimulation and topographical guidance In vitro on PC12 cells improved neurite outgrowth |
| PCL/collagen/nonobioglass(NBG) [125] | - | Human Endometrial Stem cells adhesion and proliferation |
| (Ser-Ile-Lys-Val-Ala-Val)-modified poly(2-hydroxethyl methacrylate) (PHEMA) [126] | - | In vivo tissue bridging and aligned axonal ingrowth |
| Poly(glycerol sebacate) (PGS) + poly(methyl methacrylate) (MMA) with and without gelatin [127] | PC12 cells proliferation | |
| Hyaluronic acid (HA) + PCL [128] | Attachment of SH-SY5Y neuroblastoma cells | |
| SNF coated with poly-d-lysine (PDL) or (3-aminopropyl) trimethoxysilane (APTS) [129] | - | Promotion of In vitro neuron growth and neurite density increase |
| Tussah silk fibroin (TSF) [130] | - | In vitro improvement of olfactory ensheathing cell (OECs) neuro-regenerative potential |
| Gelatin (GL) + polyethylene-oxide (PEO) + (3-Glycidoxypropyl) methyldiethoxysilane(GPTMS) [131] | Schwann cells proliferation | |
| PCL-Chitosan [132] | Laminin | Schwann cells grown |
Many authors proved that nanofiber scaffolds strongly improve axonal regeneration in chronic spinal cord injury [115,120,121,133,134,135,136,137].
So far, only a few studies have proposed a combined therapeutic approach, ensuring the regeneration of injured spinal cord by implanting suitable biocompatible scaffolds and by modulating secondary damage response by locally administration of neuro-protective agents. The development of drug delivery nanosystems having both neuro-protective and neuro-regenerative effect is still a challenge.
In the following paragraphs, an overview of the electrospun nanofibers proposed in recent years as drug carriers for the treatment of SCI is given. Particular attention is devoted to manufacturing strategies adopted to achieve optimal drug loading and release.
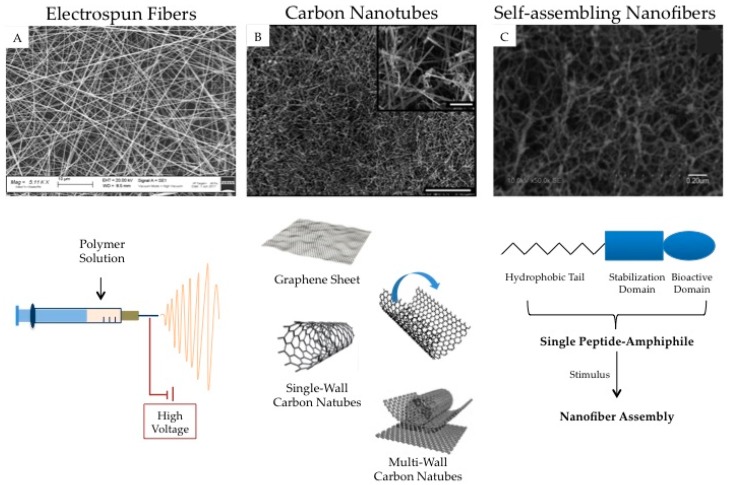
Carbon nanotubes and self-assembling nanofibers represent other interesting nanotechnology based-approach proposed for SCI treatment. A brief summary of the most meaningful experimental findings on these topics is given. The possibility of using nanostructures as cell carriers is also considered. In Figure 2, a schematic representation of electrospun nanofibers, carbon nanotubes, and self-assembling nanofibers is reported.
Figure 2.
Nanotechnological approaches for the fabrication of fibrillar structures for the treatment of SCI. (A) Scanning electron micrograph (Zeiss EVO MA10 (Carl Zeiss, Oberkochen, Germany) shows random dextran/alginate fibers; (B) Scanning electron micrograph of carbon nanotubes; scale bars: 250 and 25 μm (inset) (adapted [138]); (C) Scanning electron micrograph of self-assembling nanofibers (adapted from [139]).
3.1. Electrospun Nanofibers
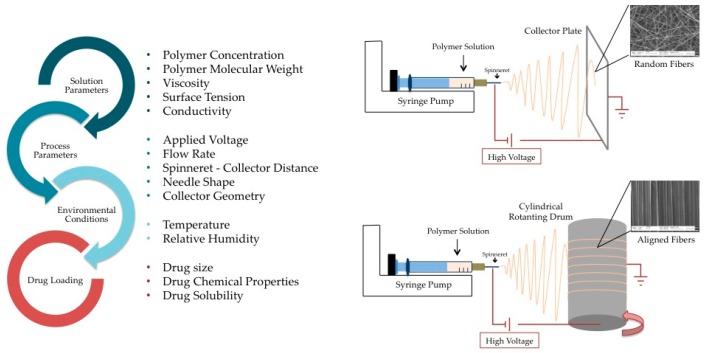
Electrospinning is of great interest in nanofiber manufacturing due to its simplicity, low cost and great versatility for drug delivery [140]. Figure 3 reports the solution, process, and environmental parameters, which can influence the morphology, size, and density of the electrospun product [140,141] Moreover, a schematic representation of the electrospinning apparatus and process is also provided.
Figure 3.
Electrospinning process. On the left, the parameters influencing fiber size, morphology, and density are listed; the physicochemical properties of the loaded drugs are also to be considered when the electrospinning technique is used for the fabrication of drug delivery systems. On the right, a schematic representation of the electrospinning apparatus with particular attention on the collector geometry, which is a crucial variable affecting fiber alignment. Scanning electron micrographs (Zeiss EVO MA10 (Carl Zeiss, Oberkochen, Germany)) show random dextran/alginate fibers and aligned polyethilenoxide/alginate ones.
The drug release profile can be modulated by using appropriate polymers and different electrospinning techniques, as hereafter described.
3.1.1. Solution Electrospinning
One-phase electrospinning technique implies that the drug is dispersed or dissolved in a polymer solution and subsequently electrospun. Experimental evidence indicated that blending a hydrophilic drug with hydrophilic polymers (i.e., PEG, PLA, PLGA, polysaccharides, collagen etc.) instead of hydrophobic ones (i.e., PCL) greatly improves drug-loading efficiency by promoting a homogeneous dispersion into the fiber matrix, avoiding the burst effect induced by an excess of drug close to the nanofiber surface [142,143,144]. Drug affinity for the selected polymeric blend should avoid drug molecule transfer towards the nanofiber surface upon storage [60].
Polymer modification and copolymerization represent optimal strategies to control the bio-erosion of the nanofiber carriers due to biological fluids [145,146,147].
Recently, Pires et al. loaded Ibuprofen (a hydrophobic anti-inflammatory drug) into electrospun fibers based on poly(trimethylenecarbonate-co-ε-caprolactone) (p(TMC-CL), by dissolving both the drug and the copolymer in a mixture of dichloromethane (DMC) and dimethyl formamide (DMF) [122]. This study demonstrated that the solvent mixture composition influences fiber morphology and diameter; in particular fiber diameter reduction was observed on increasing DMF content. Depending on the solvent used, different release mechanisms were observed. Drug release was diffusion-dependent for fibers prepared from DCM solutions, in contrast to fibers prepared from DCM/DMF mixtures where a burst release occurred. The results evidenced that the selection of an appropriate solvent or solvent mixture for drug and polymer dissolution can represent a good strategy to modulate drug release from nanofibers [122].
Recently, some interesting approaches were developed for hydrophobic drug loading into hydrophobic polymer nanofibers. In particular, Hsu et al. developed a PCL-based hybrid drug release system, consisting of nanofibers and microbeads for a month-long release of Dexamethasone (DXM) [119]. The authors evidenced that it was possible to achieve an extended release of DXM by the combination of increased crystallinity of the electrospun mats with a hybrid structure of 1.5–4 μm diameter beads and nanofibers. Such an approach may find application for neuro-regenerative drug delivery.
3.1.2. Emulsion Electrospinning
When a drug is insoluble in the polymer solution, it can be incorporated within the fiber structure using a process known as emulsion electrospinning [145]. A drug aqueous solution is mixed with a hydrophobic polymer solution, defined as the oily phase. After electrospinning, the drug-loaded aqueous phase is dispersed within the nanofiber matrix; core-shell nanofibers can be obtained when the polymer is also dispersed in the aqueous phase [148]. The main advantage of such a technique is to avoid drug stability problems possibly due to polymer and drug dissolution in respective suitable solvents. Emulsion electrospinnig provides the chemical separation of drug and polymer by employing a single vehicle instead of two different solutions as in the case of coaxial electrospinning [109].
In comparison with the coaxial electrospinning, the emulsion technique could determine degradation of unstable macromolecules, due to the interfacial tensions at the organic/aqueous interface of the emulsion. Several problems may be encountered, for example, with proteins due to their size and three-dimensional structure [146,148,149,150].
3.1.3. Coaxial Electrospinning
Coaxial electrospinning differs significantly from the emulsion method since the core-shell fibers are manufactured starting from two solutions and using two electrospinning tips [149,150]. Core-shell fibers exhibit more advantages than monolithic ones, such as modulation of drug release mechanism and kinetics via control of shell properties (i.e., thickness, porosity, biodegradation), versatility in drug selection, and preparation of multifunctional fibers (i.e., fibers characterized by a core controlling drug release and a shell improving cell adhesion). Coaxial electrospinning disadvantages are related to solvent evaporation and to the difficulty of simultaneously electrospinning two different polymer solutions with peculiar electrodynamic behavior. Since fiber morphology (core-shell structure) could prevent rapid solvent evaporation, the residual amount of organic solvents remaining in the fibers has to be detected. Due to the electrospinning process complexity, rheological and interfacial properties of the two polymer solutions as well as spinning parameters (applied voltage, spinneret-collector distance and flow rate) have to be carefully chosen [146,149,150,151].
Recently, coaxial nanofibers have been extensively investigated as biomolecule (proteins, growth factors) carriers or cell delivery scaffolds for the treatment of SCI, but only a few papers have been published on the delivery of low molecular weight drugs as nerve protective agents. Coaxial nanofibers are generally characterized by an initial burst release followed by a controlled one [146]. The initial burst effect, caused by the presence of drug molecules on fiber surface, is functional to the treatment of primary spinal cord injury, contributing to reduce even the cascade of secondary events. The subsequent prolonged drug release, guaranteed by the drug loaded in the fiber core, is useful to slow down SCI progression, particularly in the chronic phase; drug release is controlled by drug diffusion across the polymer matrix and system slow biodegradation. Different drugs can be encapsulated in both the fiber shell and core, achieving a binary release.
Nanofiber-based scaffolds, characterized by the shell and core with a peculiar morphology, have been developed also to improve the system interaction with nerve cells. In particular, Zamani et al. designed PLGA electrospun fibrous scaffolds with a nano-rough sheath and an aligned core [98]. They manufactured a three-dimensional nanofibrous scaffold by a combined electrospinning method with a water vortex and a two-nozzle system. The authors studied nerve cell morphology and proliferation in the developed scaffold. Thanks to scaffold nano-structure, nerve cells strongly attached on the fiber nanoporous shell, penetrated the inner structure and orientated along the aligned fiber direction of the core. These scaffolds were shown to support axonal regeneration of injured spinal cord in a rat model [98].
3.1.4. Drug Loading
A strategy proposed in the literature to avoid drug denaturation during fiber manufacturing or to overcome low drug solubility in polymer solution is physical drug adsorption on the fiber surface. This approach generally determines a burst release of drug that could be useful, as already mentioned, for the treatment of primary SCI. Burst release can be avoided by drug bonding to the fiber surface via covalent coupling (drug-conjugated nanofibers) [2,148,151].
Recently, Schaub et al. suggested that surface modifications may be determined through transient covalent bonds, lasting less than a few days [152]. In this study diethylenetriamine (DTA) and 2-(2-aminoethoxy)ethanol (AEO) were covalently attached to the surface of an electrospun fiber-based on PLLA. Such surface modifications improved scaffold hydrophilicity, but surprisingly no differences were observed between the modified fibrous system and the unmodified control one in terms of neurite extension. The authors evidenced that both AEO and DTA were rapidly removed from the scaffold surface [152].
Raspa et al. blended PCL and PLGA with biologically active peptide sequences (SAPs), modifying the surface properties of the electrospun scaffolds in order to improve nerve regeneration [110]. SAPs were immobilized for a longer time and nanofibers provided support for cell growth. Two systems consisting of SAPs encapsulated in PCL–PLGA coaxial electrospun scaffolds were investigated: the first was composed of a core of self-assembling peptides (SAP AC-FAQ) and by a PCL–PLGA-based shell, whereas the second one was characterized by a core containing a PCL–PLGA emulsion and a shell based on an emulsion of PCL–PLGA and a functionalized SAP AC-FAQ. The second one was characterized by the best performance in terms of cell viability and tissue response [110].
Besides maintaining drug activity, it is important to investigate if drug incorporation into nanofibers modifies fiber morphology. In fact, it has been recognized that fiber diameter could affect cellular functionality; in particular, cells adhere and expand mainly on fibers with a diameter close to the cell dimension [2]. The change in fiber diameter due to drug loading can moreover interfere with a correct interpretation of nanofiber performance: in fact, both the changes in fiber diameter and drug release can be responsible for the effects on neurite extension. Schaub and Gilbert observed a decrease of fiber diameter due to incorporation of the antimetabolite 6-Aminonicotinamide (6AN) [153]. Such a result can be explained by the impediment of polymer chain entanglement during electrospinning due to charged drug molecules. The reduction of fiber diameter is generally followed by a decrease of fiber alignment [2].
Johnson et al. proved that the inclusion of either Riluzole or neurotrophin-3(NT-3) into electrospun PLLA fibers via emulsion electrospinning had significant effects on fiber physical characteristics, in particular determined a decrease of both fiber diameter and alignment [2].
One interesting work from Pires et al. demonstrated that the polarity of the polymer solution could affect the diameter of the resulting electrospun fibers [154].
Moreover, the solid state of the drug is an important parameter that can significantly affect drug distribution and release kinetics as well as nanofiber physical stability upon storage. Seif et al. investigated the formation of Caffeine (chosen as hydrophilic model drug) crystals in electrospun fibers when two different polymers were used: the hydrophilic poly(vinyl alcohol) (PVA) and the hydrophobic PCL [155]. They proved that solvent polarity has the major effect on crystal formation, whereas a minor effect derives from the electrospinning process parameters. Therefore, uncontrolled drug crystallization can be prevented and controlled drug delivery from electrospun fibers can be achieved by adjusting the polarity of the solvent mixture and optimizing the process parameters [155].
3.1.5. Drug Release
One fundamental issue concerning material selection in the design of implantable scaffolds for the treatment of nerve regeneration is the rate of biodegradation or bioerosion. The terms biodegradable and bioerodible are frequently used as synonyms, but they represent two different concepts. Biodegradable polymers are materials able to disassemble In vivo, by forming small fragments that move away from the wound site; the term bioerodible refers instead to those polymers that are subjected to In vivo degradation, with complete elimination of the starting material [109]. Depending on the material employed, the release of the drug loaded into the implantable scaffolds can occur according to specific mechanisms and kinetics: diffusion through the polymer network or matrix pores and polymer degradation or erosion.
Slowly bioerodible scaffolds loaded with small hydrophilic molecules are reported as an example. After implantation, the initial rate of drug release will exclusively depend on drug diffusion and not on scaffold erosion, which becomes important at a later time. Conversely, if the matrix biodegradation or bioerosion rate is higher than the drug diffusion, drug release will be controlled by polymer erosion or the biodegradation process. For these reasons, slowly degrading polymers are commonly used as a support for nerve guidance in SCI animal models, considering that a period of 3–6 months is required to achieve a functional regeneration [136].
Drug release from implantable polymer-based scaffolds, such as electrospun fibers, could be moreover influenced by scaffold composition; therefore, the hydrophilicity and molecular weight of the selected polymers are crucial parameters to be considered. Cross-linking of polymer chains could be also induced to control drug diffusion, as long as nanofibers act as support of nerve outgrowth [136].
One of the first small organic molecules released from electrospun fibers intended for spinal cord repair was the antimetabolite 6-Amino-nicotinamide (6AN). This is known to inhibit astrocyte metabolism at low levels, and to exert a lower effect on neurons. In particular, Schaub and Gilbert developed 6AN loaded emulsion–electrospun PLLA nanofibers [153]. 6AN was found to inhibit astrocyte viability without interfering with neurite extension. The inhibition of astrocyte viability should reduce the negative effects due to astrocyte reactivity after SCI, but no in vivo experiments were affected [153].
A more in-depth study was carried out on Rolipram by Downing et al. [156]. Rolipram was loaded into electrospun PLLA nanofibers and implanted in a SCI rat model. The authors demonstrated that animals treated with electrospun nanofibers, able to release low drug doses of (~3 μg/cm2 over 12 days), presented significantly improved functional recovery after injury in comparison with an untreated group (controls). Interestingly, the group of animals treated with electrospun fibers releasing a low Rolipram dose showed a significantly improved motor function with respect to another group treated with electrospun fibers releasing a large amount of Rolipram (~60 μg/cm2 over 12 days). This study, even if not recent, focused on the capability of nanofibers to load and release therapeutic levels of drug for SCI treatment [156].
3.2. Carbon Nanotubes
A particular type of nanofibers is represented by carbon nanotubes (CNTs), which are composed of graphene sheets rolled up to form a cylinder made of carbon atoms. CNTs are classified according to the number of layers: single-walled (SWCNTs), double-walled (DWCNTs), and multi-walled (MWCNTs) carbon nanotubes. They are characterized by large specific surface area, electrically conductive, elastic resistance, and nanostructure mimicking the extracellular matrix (ECM). These properties make them good candidates for nerve tissue regeneration [157].
Moreover, CNTs are able to regenerate neuronal electrical activity by settling contacts with cell membranes to create electrical ‘short-cuts’ between various areas of the neuron [157,158]. Recently, Palejwala et al. prepared graphene nanoscaffolds by mild chemical reduction of graphene oxide [115]. They were implanted in a rat model immediately after hemispinal spinal cord transection, using a hydrogel matrix as vehicle. A group of animals were treated with the pure hydrogel matrix (controls). The scaffolds proved to be biocompatible and hystological evaluation pointed out the growth of connective tissue elements, blood vessels, neurofilaments, and Schwann cells around the scaffolds [115]. In another study, López-Dolado et al. proved that graphene oxide-based scaffolds promoted, after implantation, the migration of M2 macrophages into the damaged site [159].
3.3. Self-Assembling Nanofibers
A recent nanotechnology approach for spinal cord repair is represented by self-assembling (peptide) nanofibers (SAPNs). Such nanofibers are based on the synthesis of unique amphiphilic peptides, which are characterized by the periodic repetition of hydrophobic and hydrophilic aminoacids, alternatively arranged. These peptides spontaneously self-assemble into well-ordered nanofibers after contact with physiological fluids, containing electrolytes and salts. These peptides in solution can be easily injected into the nervous injury and arrange themselves in vivo into a stable nano-fiber 3D-matrix thanks to the ionic strength, pH, and temperature of the physiological environment. They fill up the site of injury without producing secondary damage, as may be the case for other more rigid scaffolds (such as nanofiber implanted system). SAPNs may be built from natural and biocompatible peptides, thus they should be promising biomaterials for regenerative medicine [135,160].
Zhang et al. discovered the sequence AcN-RADARADARADARADA-CNH2, named RADA 16-I, as artificial SAP, which can form a stable nanostructure when in contact with physiological ions [161].
Inspired by the properties of RADA 16-I on cell culture, Guo and coworkers injected the SAP RADA 16-I in a rat spinal cord injury model with very good results (six weeks after transplantation the cavity was filled up by the scaffold) [162].
Subsequently Gelain and coworkers combined microstructured electrospun nanofibers (a mixture of PLGA and PCL) with the nanostructured SAP RADA 16, thus providing in situ delivery of cytokines. In such a hybrid scaffold the SAP can self-assemble into the PLGA/PCL electrospun microguidance fibers, presoaked in PBS. Six months after transplantation in animals, conspicuous cord regeneration was still active, accomplishing functional reconstruction of chronic spinal cord injury [121].
Several research groups tested the neuro-regenerative potential of SAP alone or in combination with electrospun nanofibers and many new SAP nanofiber scaffolds were developed [135,160,161,162,163,164,165].
Gelain et al. identified a new SAP named Ac-FAQ (Ac-FAQRVPP-GGG-(LDLK)3-CONH2) which promoted significant locomotion recovery in an animal model after injection [164]. Raspa et al. as already described in Section 3.1.4 assembled the novel AC-FAQ SAP with the coaxially electrospun polymeric fibers [110].
In Table 7 examples of self-assembling peptides proposed in the literature for SCI treatment are reported
Table 7.
Self-assembling peptides reported in the literature for treatment of SCI.
| Self-Assembling Peptides | Animal Model | Potential Effect in SCI |
|---|---|---|
| Biotin B24 (GGGAFASTKT-CONH2) [166] | Murine contusion model | Low infiltration of CD68 + macrophages and iba + microglia |
| Biotin LDLK12 (LDLKLDLKLDLK-CONH2) [166] | Murine contusion model | Low infiltration of CD68 + macrophages and iba + microglia |
| Laminin epitope CQIK (Ac-(RADA)4GGCQAASIKVAV-CONH2) [167] | Motor recovery in SCI model | Higher neural differentiation of hEnSCs (human endometrial-derived stromal cells,) neurite outgrowth and myelination |
| Laminin epitope IKVAV-peptide amphiphile (PA) [163] | Murine spinal cord contusion and compression model | Promotion of functional recovery |
| SAP: K2(QL)6K2 [139] | Murine model clip compression | Improvement of locomotion function attenuation of inflammation |
It is very important to distinguish a nanofiber scaffold, formed through macromolecular self-assembly, from that prepared by coaxial, blending or emulsion electrospinning methods. The physical and chemical properties of the self-assembling structures (e.g., size, shape, internal order, stability, surface, chemistry) are strictly connected with the molecule characteristics forming the network and with the physiological conditions in which assembly occurs [142,168].
Recently, an increasing interest has been observed towards the use of drugs as possible well-defined nanostructures of various sizes and shapes (e.g., nanofibers). This strategy is based on the self-association capability of some drugs to build stable three-dimensional 3D structures [99].
Ma et al. studied how some small drugs can be employed as building blocks in the construction of 3D-nanostructures. Amphiphilic drugs, possessing hydrophobic and hydrophilic groups, could undergo reversible self-assembly, resulting in the formation of dynamic molecular micelles or even discrete supramolecular nanostructures of well-defined size and shape (such as Methotrexate used in neurodegenerative diseases) [99].
Goswami et al. demonstrated that micelles with nanofiber geometry, formed by conjugation of the hydrophobic Deferasirox (DFX) with cell-penetrating peptides (CPPs), were suitable for the delivery of Curcumin, a hydrophobic and anti-neurodegenerative drug with promising SCI applications [169].
3.4. Nanofibers as Cell Carriers
The loss of neuronal tissue at the injury site has made cell transplantation an attractive strategy for SCI treatment. Over the last decades, the use of various cell lines has been proposed due to their action in the regeneration process of nervous tissue [1].
Considering that the functional recovery of damaged nerve cells may be compromised by the hostile environment at the injury site, cell transplantation has to be performed within 7–10 days post-injury [165]. Different administration routes have been proposed: intravenous injection, that requires a great amount of cells to effectively target the injury area; intranasal, generally responsible for a lower therapeutic effect with respect to intrathecal infusion [170]; direct transplantation at the site of injury, with the risk that needle penetration produces secondary nerve damages [171]; subarachnoid route, that avoids needle damages and requires optimized procedures to impede iatrogenic problems [172].
In a recent work Kim et al. demonstrated the efficacy of mesenchymal stem cells (MSCs) transplantation in a rat model, using polymeric scaffolds as cell carrier [112]. Two different scaffolds were considered: one based on PLGA, the other one on chitosan. The control was represented by intralesional injection of MSCs without carrier. Engraftment and differentiation of the transplanted cells, expression of neurotrophic factors in the injured spinal cord and functional recovery, were evaluated for each group. It was demonstrated that the carrier-mediated approach promoted a better cell engraftment and neuroprotection than intralesional injection of cell suspension. The functional improvement was particularly evident in the animal group treated with MSC-chitosan scaffold [112].
In recent years, electrospun nanofibers encapsulating living cells have been proposed as new carriers able to improve tissue regeneration at the implantation site. Such scaffolds represent a solution to the shortage of nerve tissue useful in repair and replacement surgeries, even though the preservation of post-electrospinning viability of cells is still a challenge [172,173].
4. Conclusions
Recently, several studies have been carried out to better understand the pathological consequences of spinal cord lesion as well as the advantages and limitations of both neuro-protective and neuro-regenerative interventions. One of the most promising strategies in SCI treatment is the development of therapeutic platforms, able to ensure controlled drug delivery at the site of injury, as well as to promote neurite alignment and outgrowth. Polymer-based nanofibers represent attractive three-dimensional scaffolds for neural regeneration, mimicking the native extracellular matrix and providing topographical cues to axonal regrowth. After implantation, nanofibers should directly interact with resident cells, promoting their attachment, proliferation, and differentiation, thus establishing a pro-regenerative environment at the damaged site. Furthermore, these implanted scaffolds should hydrate upon contact with physiological fluids, forming a matrix characterized by controlled biodegradation which is able to modulate drug release, according to the therapeutic requirements. Concerning this latter aim, the design of multi-layer structures, produced by alternating layers of different polymers, should allow a controlled delivery of the loaded drug.
Currently, a combined approach ensuring both neuro-protective and neuro-regenerative outcomes, thus modulating a plethora of cellular, biochemical and vascular events, could represent a promising strategy for the treatment of spinal cord injuries. Biologists, bioengineers, chemists, pharmacists, and clinical researchers are called to share their skills and knowledge with the aim of developing innovative polymer-based delivery systems. Such systems could be loaded with neuro-protective agents, growth factors, and/or stem cells in order to promote an effective regeneration of damaged neuronal cells and the recovery of motor functions after SCI.
Author Contributions
Silvia Rossi conceived the review. Angela Faccendini and Barbara Vigani with the supervision of Silvia Rossi analyzed the references, evaluated the information relevant to the topic and wrote the paper. Giuseppina Sandri, Maria Cristina Bonferoni and Carla Marcella Caramella helped in bibliographic research. Franca Ferrari reviewed the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Kabu S., Gao Y., Kwon B.K., Labhasetwar V. Drug delivery, cell-based therapies, and tissue engineering approaches for spinal cord injury. J. Control. Release. 2015;219:141–154. doi: 10.1016/j.jconrel.2015.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson C.D., D’Amato A.R., Gilbert R.J. Electrospun fibers for drug delivery after spinal cord injury and the effects of drug incorporation on fiber properties. Cells Tissues Organs. 2016;202:116–135. doi: 10.1159/000446621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oyinbo C.A. Secondary injury mechanisms in traumatic spinal cord injury: A nugget of this multiply cascade. Acta Neurobiol. Exp. Wars. 2011;71:281–299. doi: 10.55782/ane-2011-1848. [DOI] [PubMed] [Google Scholar]
- 4.Russell C.M., Choo A.M., Tetzlaff W., Chung T.E., Oxland T.R. Maximum principal strain correlates with spinal cord tissue damage in contusion and dislocation injuries in the rat cervical spine. J. Neurotrauma. 2012;29:1574–1585. doi: 10.1089/neu.2011.2225. [DOI] [PubMed] [Google Scholar]
- 5.Choo A.M., Liu J., Dvorak M., Tetzlaff W., Oxland T.R. Secondary pathology following contusion, dislocation, and distraction spinal cord injuries. Exp. Neurol. 2008;212:490–506. doi: 10.1016/j.expneurol.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 6.Sofroniew M.V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahuja C.S., Wilson J.R., Nori S., Kotter M.R.N., Druschel C., Curt A., Fehlings M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Primers. 2017;3:17018. doi: 10.1038/nrdp.2017.18. [DOI] [PubMed] [Google Scholar]
- 8.Norenberg M.D., Smith J., Marcillo A. The pathology of human spinal cord injury: Defining the problems. J. Neurotrauma. 2004;21:429–440. doi: 10.1089/089771504323004575. [DOI] [PubMed] [Google Scholar]
- 9.Kwon B.K., Tetzlaff W., Grauer J.N., Beiner J., Vaccaro A.R. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004;4:451–464. doi: 10.1016/j.spinee.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Hamid S., Hayek R. Role of electrical stimulation for rehabilitation and regeneration after spinal cord injury: An overview. Eur. Spine J. 2008;17:1256–1269. doi: 10.1007/s00586-008-0729-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleming J.C., Norenberg M.D., Ramsay D.A., Dekaban G.A., Marcillo A.E., Saenz A.D., Pasquale-Styles M., Dietrich W.D., Weaver L.C. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129:3249–3269. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- 12.Bracken M.B., Shepard M.J., Collins W.F., Holford T.R., Young W., Baskin D.S., Eisenberg H.M., Flamm E., Leo-Summers L., Maroon J., et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury: Results of the Second National Acute Spinal Cord Injury Study. N. Engl. J. Med. 1990;322:1405–1411. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- 13.Bydon M., Lin J., Macki M., Gokaslan Z.L., Bydon A. The current role of steroids in acute spinal cord injury. World Neurosurg. 2014;82:848–854. doi: 10.1016/j.wneu.2013.02.062. [DOI] [PubMed] [Google Scholar]
- 14.Pannu R., Christie D.K., Barbosa E., Singh I., Singh A.K. Post-trauma Lipitor treatment prevents endothelial dysfunction, facilitates neuroprotection, and promotes locomotor recovery following spinal cord injury. J. Neurochem. 2007;101:182–200. doi: 10.1111/j.1471-4159.2006.04354.x. [DOI] [PubMed] [Google Scholar]
- 15.Das A., Sribnick E.A., Wingrave J.M., Del Re A.M., Woodward J.J., Appel S.H., Banik N.L., Ray S.K. Calpain activation in apoptosis of ventral spinal cord 4.1 (VSC4.1) motoneurons exposed to glutamate: Calpain inhibition provides functional neuroprotection. J. Neurosci. Res. 2005;81:551–562. doi: 10.1002/jnr.20581. [DOI] [PubMed] [Google Scholar]
- 16.Saxena T., Loomis K.H., Pai S.B., Karumbaiah L., Gaupp E., Patil K., Patkar R., Bellamkonda R.V. Nanocarrier-mediated inhibition of macrophage migration inhibitory factor attenuates secondary injury after spinal cord injury. ACS Nano. 2015;9:1492–1505. doi: 10.1021/nn505980z. [DOI] [PubMed] [Google Scholar]
- 17.Matis G.K., Birbilis T.A. Erythropoietin in spinal cord injury. Eur. Spine J. 2009;18:314–323. doi: 10.1007/s00586-008-0829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaptanoglu E., Solaroglu I., Okutan O., Surucu H.S., Akbiyik F., Beskonakli E. Erythropoietin exerts neuroprotection after acute spinal cord injury in rats: Effect on lipid peroxidation and early ultrastructural findings. Neurosurg. Rev. 2004;27:113–120. doi: 10.1007/s10143-003-0300-y. [DOI] [PubMed] [Google Scholar]
- 19.Kwon B.K., Okon E., Hillyer J., Mann C., Baptiste D., Weaver L.C., Fehlings M.G., Tetzlaff W. A systematic review of non-invasive pharmacologic neuroprotective treatments for acute spinal cord injury. J. Neurotrauma. 2011;28:1545–1588. doi: 10.1089/neu.2009.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu W., Lee S.Y., Wu X., Tyler J.Y., Wang H., Ouyang Z., Park K., Xu X.M., Cheng J.X. Neuroprotective ferulic acid (FA)-glycol chitosan (GC) nanoparticles for functional restoration of traumatically injured spinal cord. Biomaterials. 2014;35:2355–2364. doi: 10.1016/j.biomaterials.2013.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee K.D., Chow W.N., Sato-Bigbee C., Graf M.R., Graham R.S., Colello R.J., Young H.F., Mathern B.E. FTY720 reduces inflammation and promotes functional recovery after spinal cord injury. J. Neurotrauma. 2009;26:2335–2344. doi: 10.1089/neu.2008.0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamann K., Shi R. Acrolein scavenging: A potential novel mechanism of attenuating oxidative stress following spinal cord injury. J. Neurochem. 2009;111:1348–1356. doi: 10.1111/j.1471-4159.2009.06395.x. [DOI] [PubMed] [Google Scholar]
- 23.Park J., Zheng L., Marquis A., Walls M., Duerstock B., Pond A., Vega-Alvarez S., Wang H., Ouyang Z., Shi R. Neuroprotective role of hydralazine in rat spinal cord injury-attenuation of acrolein-mediated damage. J. Neurochem. 2014;129:339–349. doi: 10.1111/jnc.12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abrams M.B., Nilsson I., Lewandowski S.A., Kjell J., Codeluppi S., Olson L., Eriksson U. Imatinib enhances functional outcome after spinal cord injury. PLoS ONE. 2012;7:e38760. doi: 10.1371/journal.pone.0038760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Q., Jing Y., Yuan X., Zhang X., Li B., Liu M., Wang B., Li H., Liu S., Xiu R. Melatonin treatment protects against acute spinal cord injury-induced disruption of blood spinal cord barrier in mice. J. Mol. Neurosci. 2014;54:714–722. doi: 10.1007/s12031-014-0430-4. [DOI] [PubMed] [Google Scholar]
- 26.Paterniti I., Mazzon E., Emanuela E., Paola R.D., Galuppo M., Bramanti P., Cuzzocrea S. Modulation of inflammatory response after spinal cord trauma with deferoxamine, an iron chelator. Free Radic. Res. 2010;44:694–709. doi: 10.3109/10715761003742993. [DOI] [PubMed] [Google Scholar]
- 27.Rathore K.I., Kerr B.J., Redensek A., López-Vales R., Jeong S.Y., Ponka P., David S. Ceruloplasmin protects injured spinal cord from iron-mediated oxidative damage. J. Neurosci. 2008;28:12736–12747. doi: 10.1523/JNEUROSCI.3649-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schültke E., Griebel R.W., Juurlink B.H. Quercetin attenuates inflammatory processes after spinal cord injury in an animal model. Spinal Cord. 2010;48:857–861. doi: 10.1038/sc.2010.45. [DOI] [PubMed] [Google Scholar]
- 29.Fehlings M.G., Wilson J.R., Frankowski R.F., Toups E.G., Aarabi B., Harrop J.S., Shaffrey C.I., Harkema S.J., Guest J.D., Tator C.H., et al. Riluzole for the treatment of acute traumatic spinal cord injury: Rationale for and design of the NACTN Phase I clinical trial. J. Neurosurg. Spine. 2012;17:151–156. doi: 10.3171/2012.4.AOSPINE1259. [DOI] [PubMed] [Google Scholar]
- 30.Schaal S.M., Garg M.S., Ghosh M., Lovera L., Lopez M., Patel M., Louro J., Patel S., Tuesta L., Chan W.M., et al. The therapeutic profile of rolipram, PDE target and mechanism of action as a neuroprotectant following spinal cord injury. PLoS ONE. 2012;7:e43634. doi: 10.1371/journal.pone.0043634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cristante A.F., Barros Filho T.E., Oliveira R.P., Marcon R.M., Rocha I.D., Hanania F.R., Daci K. Antioxidative therapy in contusion spinal cord injury. Spinal Cord. 2009;47:458–463. doi: 10.1038/sc.2008.155. [DOI] [PubMed] [Google Scholar]
- 32.Lv R., Mao N., Wu J., Lu C., Ding M., Gu X., Wu Y., Shi Z. Neuroprotective effect of allicin in a rat model of acute spinal cord injury. Life Sci. 2015;143:114–123. doi: 10.1016/j.lfs.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Yuksel Y., Guven M., Kaymaz B., Sehitoglu M.H., Aras A.B., Akman T., Tosun M., Cosar M. Effects of Aloe vera on spinal cord ischemia-reperfusion injury of rats. J. Investig. Surg. 2016;29:389–398. doi: 10.1080/08941939.2016.1178358. [DOI] [PubMed] [Google Scholar]
- 34.Luo Y., Fu C., Wang Z., Zhang Z., Wang H., Liu Y. Asiaticoside attenuates the effects of spinal cord injury through antioxidant and anti-inflammatory effects, and inhibition of the p38-MAPK mechanism. Mol. Med. Rep. 2015;12:8294–8300. doi: 10.3892/mmr.2015.4425. [DOI] [PubMed] [Google Scholar]
- 35.Xian-Hui D., Xiao-Ping H., Wei-Juan G. Neuroprotective effects of the Buyang Huanwu decoction on functional recovery in rats following spinal cord injury. J. Spinal Cord Med. 2016;39:85–92. doi: 10.1179/2045772314Y.0000000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aydin H.E., Ozkara E., Ozbek Z., Vural M., Burukoglu D., Arslantas A., Atasoy M.A. Histopathological evaluation of the effects of CAPE in experimental spinal cord injury. Turk. Neurosurg. 2016;26:437–444. doi: 10.5137/1019-5149.JTN.11255-14.0. [DOI] [PubMed] [Google Scholar]
- 37.Wang Z.H., Xie Y.X., Zhang J.W., Qiu X.H., Cheng A.B., Tian L., Ma B.Y., Hou Y.B. Carnosol protects against spinal cord injury through Nrf-2 upregulation. J. Recept. Signal Transduct. Res. 2016;36:72–78. doi: 10.3109/10799893.2015.1049358. [DOI] [PubMed] [Google Scholar]
- 38.Terraf P., Kouhsari S.M., Ai J., Babaloo H. Tissue-engineered regeneration of hemisected spinal cord using human endometrial stem cells, poly ε-caprolactone scaffolds, and crocin as a neuroprotective agent. Mol. Neurobiol. 2016 doi: 10.1007/s12035-016-0089-7. [DOI] [PubMed] [Google Scholar]
- 39.Gokce E.C., Kahveci R., Gokce A., Sargon M.F., Kisa U., Aksoy N., Cemil B., Erdogan B. Curcumin attenuates inflammation, oxidative stress, and ultrastructural damage induced by spinal cord ischemia-reperfusion injury in rats. J. Stroke Cerebrovasc. Dis. 2016;25:1196–1207. doi: 10.1016/j.jstrokecerebrovasdis.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 40.Hussain Z., Thu H.E., Ng S.F., Khan S., Katas H. Nanoencapsulation, an efficient and promising approach to maximize wound healing efficacy of curcumin: A review of new trends and state-of-the-art. Colloids Surf. B Biointerfaces. 2017;150:223–241. doi: 10.1016/j.colsurfb.2016.11.036. [DOI] [PubMed] [Google Scholar]
- 41.Liu Z.H., Yip P.K., Adams L., Davies M., Lee J.W., Michael G.J., Priestley J.V., Michael-Titus A.T. A single bolus of docosahexaenoic acid promotes neuroplastic changes in the innervation of spinal cord interneurons and motor neurons and improves functional recovery after spinal cord injury. J. Neurosci. 2015;35:12733–12752. doi: 10.1523/JNEUROSCI.0605-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Álvarez-Pérez B., Homs J., Bosch-Mola M., Puig T., Reina F., Verdú E., Boadas-Vaello P. Epigallocatechin-3-gallate treatment reduces thermal hyperalgesia after spinal cord injury by down-regulating RhoA expression in mice. Eur. J. Pain. 2016;20:341–352. doi: 10.1002/ejp.722. [DOI] [PubMed] [Google Scholar]
- 43.Sehitoglu M.H., Guven M., Yüksel Y., Akman T., Bozkurt Aras A., Farooqi A.A., Cosar M. The effect of glycyrrhizic acid on traumatic spinal cord injury in rats. Cell. Mol. Biol. (Noisy-le-Grand) 2016;62:2–8. [PubMed] [Google Scholar]
- 44.Gokce E.C., Kahveci R., Atanur O.M., Gürer B., Aksoy N., Gokce A., Sargon M.F., Cemil B., Erdogan B., Kahveci O. Neuroprotective effects of Ganoderma lucidum polysaccharides against traumatic spinal cord injury in rats. Injury. 2015;46:2146–2155. doi: 10.1016/j.injury.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 45.Yan M., Liu Y.W., Shao W., Mao X.G., Yang M., Ye Z.X., Liang W., Luo Z.J. EGb761 improves histological and functional recovery in rats with acute spinal cord contusion injury. Spinal Cord. 2016;54:259–265. doi: 10.1038/sc.2015.156. [DOI] [PubMed] [Google Scholar]
- 46.Nakanishi M., Nakae A., Kishida Y., Baba K., Sakashita N., Shibata M., Yoshikawa H., Hagihara K. Go-sha-jinki-Gan (GJG) ameliorates allodynia in chronic constriction injury-model mice via suppression of TNF-α expression in the spinal cord. Mol. Pain. 2016;12 doi: 10.1177/1744806916656382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu Z.G., Yang J., Lv Z.P., Wang T.H., Li X.S., Liu J.H., Zhao N., Xiyang Y.B. Effect of Herba Lycopodii alcohol extracted granule combined methylprednisolone on expression levels of BDNF and NMDA and behavior of traumatic spinal cord injury rats. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2015;35:1004–1010. [PubMed] [Google Scholar]
- 48.Luo Y., Fu C., Wang Z., Zhang Z., Wang H., Liu Y. Mangiferin attenuates contusive spinal cord injury in rats through the regulation of oxidative stress, inflammation and the Bcl-2 and Bax pathway. Mol. Med. Rep. 2015;12:7132–7138. doi: 10.3892/mmr.2015.4274. [DOI] [PubMed] [Google Scholar]
- 49.Zhang P., Ma X. Effect of rutin on spinal cord injury through inhibition of the expression of MIP-2 and activation of MMP-9, and downregulation of Akt phosphorylation. Mol. Med. Rep. 2015;12:7554–7560. doi: 10.3892/mmr.2015.4357. [DOI] [PubMed] [Google Scholar]
- 50.Gökce E.C., Kahveci R., Gökce A., Cemil B., Aksoy N., Sargon M.F., Kısa Ü., Erdoğan B., Güvenç Y., Alagöz F., et al. Neuroprotective effects of thymoquinone against spinal cord ischemia-reperfusion injury by attenuation of inflammation, oxidative stress, and apoptosis. J. Neurosurg. Spine. 2016;24:949–959. doi: 10.3171/2015.10.SPINE15612. [DOI] [PubMed] [Google Scholar]
- 51.Ewan E.E., Hagg T. Intrathecal acetyl-l-carnitine protects tissue and improves function after a mild contusive spinal cord injury in rats. J. Neurotrauma. 2016;33:269–277. doi: 10.1089/neu.2015.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Celik H., Karatay M., Erdem Y., Yildirim A.E., Sertbas I., Karatay E., Kul H., Guvenc Y., Koksal I., Menekse G., et al. The biochemical, histopathological and clinical comparison of the neuroprotective effects of subcutaneous adalimumab and intravenous methylprednisolone in an experimental compressive spinalcord trauma model. Turk. Neurosurg. 2016;26:622–631. doi: 10.5137/1019-5149.JTN.13210-14.1. [DOI] [PubMed] [Google Scholar]
- 53.Gurcay A.G., Gurcan O., Kazanci A., Bozkurt I., Senturk S., Bodur E., Turkoglu O.F., Bavbek M. Comparative biochemical and motor function analysis of alpha lipoic acid and n-acetyl cysteine treatment on rats with experimental spinal cord injury. Turk. Neurosurg. 2016;26:119–126. doi: 10.5137/1019-5149.JTN.14594-15.0. [DOI] [PubMed] [Google Scholar]
- 54.Kermani H.R., Nakhaee N., Fatahian R., Najar A.G. Effect of aspirin on spinal cord injury: An experimental study. Iran. J. Med. Sci. 2016;41:217–222. [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang B., Bailey W.M., Kopper T.J., Orr M.B., Feola D.J., Gensel J.C. Azithromycin drives alternative macrophage activation and improves recovery and tissue sparing in contusion spinal cord injury. J. Neuroinflamm. 2015;12:218. doi: 10.1186/s12974-015-0440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang W., Li M., He F., Bian Z., Liu J., He Q., Wang X., Sun T., Zhu L. Dopamine D1 receptor agonist A-68930 inhibits NLRP3 inflammasome activation and protects rats from spinal cord injury-induced acute lung injury. Spinal Cord. 2016;54:951–956. doi: 10.1038/sc.2016.52. [DOI] [PubMed] [Google Scholar]
- 57.Li X., Han J., Zhao Y., Ding W., Wei J., Li J., Han S., Shang X., Wang B., Chen B., et al. Functionalized collagen scaffold implantation and cAMP administration collectively facilitate spinal cord regeneration. Acta Biomater. 2016;30:233–245. doi: 10.1016/j.actbio.2015.11.023. [DOI] [PubMed] [Google Scholar]
- 58.Karatas Y., Cengiz S.L., Esen H., Toker A., Savas C. Effect of carvedilol on secondary damage in experimental spinal cord injury in rats. Turk. Neurosurg. 2015;25:930–935. doi: 10.5137/1019-5149.JTN.11749-14.1. [DOI] [PubMed] [Google Scholar]
- 59.Kwiecien J.M., Jarosz B., Oakden W., Klapec M., Stanisz G.J., Delaney K.H., Kotlinska-Hasiec E., Janik R., Rola R., Dabrowski W. An in vivo model of anti-inflammatory activity of subdural dexamethasone following the spinal cord injury. Neurol. Neurochir. Polska. 2016;50:7–15. doi: 10.1016/j.pjnns.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 60.Xia T., Ni S., Li X., Yao J., Qi H., Fan X., Wang J. Sustained delivery of dbcAMP by poly(propylene carbonate) micron fibers promotes axonal regenerative sprouting and functional recovery after spinal cord hemisection. Brain Res. 2013;1538:41–50. doi: 10.1016/j.brainres.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 61.Lin C.W., Chen B., Huang K.L., Dai Y.S., Teng H.L. Inhibition of autophagy by estradiol promotes locomotor recovery after spinal cord injury in rats. Neurosci. Bull. 2016;32:137–144. doi: 10.1007/s12264-016-0017-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amini Pishva A., Akbari M., Farahabadi A., Arabkheradmand A., Beyer C., Dashti N., Moradi F., Hassanzadeh G. Effect of estrogen therapy on TNF-α and iNOS gene expression in spinal cord injury model. Acta Med. Iran. 2016;54:296–301. [PubMed] [Google Scholar]
- 63.Ahmad M., Zakaria A., Almutairi K.M. Effectiveness of minocycline and FK506 alone and in combination on enhanced behavioral and biochemical recovery from spinal cord injury in rats. Pharmacol. Biochem. Behav. 2016;145:45–54. doi: 10.1016/j.pbb.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 64.Khayrullina G., Bermudez S., Byrnes K.R. Inhibition of NOX2 reduces locomotor impairment, inflammation, and oxidative stress after spinal cord injury. J. Neuroinflamm. 2015;12:172. doi: 10.1186/s12974-015-0391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sanna M.D., Lucarini L., Durante M., Ghelardini C., Masini E., Galeotti N. Histamine H4 receptor agonist-induced relief from painful peripheral neuropathy is mediated by inhibition of spinal neuroinflammation and oxidative stress. Br. J. Pharmacol. 2017;174:28–40. doi: 10.1111/bph.13644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kang S.K., Kang M.W., Rhee Y.J., Kim C.S., Jeon B.H., Han S.J., Cho H.J., Na M.H., Yu J.H. In vivo neuroprotective effect of histidine-tryptophan-ketoglutarate solution in an ischemia/reperfusion spinal cord injury animal model. Korean J. Thorac. Cardiovasc. Surg. 2016;49:232–241. doi: 10.5090/kjtcs.2016.49.4.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martini A.C., Berta T., Forner S., Chen G., Bento A.F., Ji R.R., Rae G.A. Lipoxin A4 inhibits microglial activation and reduces neuroinflammation and neuropathic pain after spinal cord hemisection. J. Neuroinflamm. 2016;13:75. doi: 10.1186/s12974-016-0540-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gao Y., Bai C., Zheng D., Li C., Zhang W., Li M., Guan W., Ma Y. Combination of melatonin and Wnt-4 promotes neural cell differentiation in bovine amniotic epithelial cells and recovery from spinal cord injury. J. Pineal Res. 2016;60:303–312. doi: 10.1111/jpi.12311. [DOI] [PubMed] [Google Scholar]
- 69.Wang C., Liu C., Gao K., Zhao H., Zhou Z., Shen Z., Guo Y., Li Z., Yao T., Mei X. Metformin preconditioning provide neuroprotection through enhancement of autophagy and suppression of inflammation and apoptosis after spinal cord injury. Biochem. Biophys. Res. Commun. 2016;477:534–540. doi: 10.1016/j.bbrc.2016.05.148. [DOI] [PubMed] [Google Scholar]
- 70.Norouzi-Javidan A., Javanbakht J., Barati F., Fakhraei N., Mohammadi F., Dehpour A.R. Serotonin 5-HT7 receptor agonist, LP-211, exacerbates Na+, K+-ATPase/Mg2+-ATPase imbalances in spinal cord-injured male rats. Diagn. Pathol. 2015;10:157. doi: 10.1186/s13000-015-0397-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 71.Aceves M., Bancroft E.A., Aceves A.R., Hook M.A. Nor-binaltorphimine blocks the adverse effects of morphine after spinal cord injury. J. Neurotrauma. 2017;34:1164–1174. doi: 10.1089/neu.2016.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dong Q., Sun L., Peng L., Yan B., Lv J., Wang G., Gong S. PMX53 protects spinal cord from ischemia-reperfusion injury in rats in the short term. Spinal Cord. 2016;54:254–258. doi: 10.1038/sc.2015.146. [DOI] [PubMed] [Google Scholar]
- 73.Coronel M.F., Raggio M.C., Adler N.S., De Nicola A.F., Labombarda F., González S.L. Progesterone modulates pro-inflammatory cytokine expression profile after spinal cord injury: Implications for neuropathic pain. J. Neuroimmunol. 2016;292:85–92. doi: 10.1016/j.jneuroim.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 74.Yu Q.J., Yang Y. Function of SOD1, SOD2, and PI3K/AKT signalling pathways in the protection of propofol on spinal cord ischemic reperfusion injury in a rabbit model. Life Sci. 2016;148:86–92. doi: 10.1016/j.lfs.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 75.Gao K., Wang Y.S., Yuan Y.J., Wan Z.H., Yao T.C., Li H.H., Tang P.F., Mei X.F. Neuroprotective effect of rapamycin on spinal cord injury via activation of the WNT/β-catenin signalling pathway. Neural Regen. Res. 2015;10:951–957. doi: 10.4103/1673-5374.158360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou Y., Zheng B., Ye L., Zhang H., Zhu S., Zheng X., Xia Q., He Z., Wang Q., Xiao J., Xu H. Retinoic acid prevents disruption of blood-spinal cord barrier by inducing autophagic flux after spinal cord injury. Neurochem. Res. 2016;41:813–825. doi: 10.1007/s11064-015-1756-1. [DOI] [PubMed] [Google Scholar]
- 77.Li X.G., Lin X.J., Du J.H., Xu S.Z., Lou X.F., Chen Z. Combination of methylprednisolone and rosiglitazone promotes recovery of neurological function after spinal cord injury. Neural Regen. Res. 2016;11:1678–1684. doi: 10.4103/1673-5374.193250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen X.B., Yuan H., Wang F.J., Tan Z.X., Liu H., Chen N. Protective role of selenium-enriched supplement on spinal cord injury through the up-regulation of CNTF and CNTF-Ralpha. Eur. Rev. Med. Pharmacol. Sci. 2015;19:4434–4442. [PubMed] [Google Scholar]
- 79.Gao K., Wang G., Wang Y., Han D., Bi J., Yuan Y., Yao T., Wan Z., Li H., Mei X. Neuroprotective effect of simvastatin via inducing the autophagy on spinal cord injury in the rat model. Biomed. Res. Int. 2015;2015:260161. doi: 10.1155/2015/260161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu Y.X., Gao C.Z., Fan K.L., Yang L.M., Mei X.F. STAT1 inhibitor alleviates spinal cord injury by decreasing apoptosis. Genet. Mol. Res. 2016;15 doi: 10.4238/gmr.15017271. [DOI] [PubMed] [Google Scholar]
- 81.Colón J.M., Miranda J.D. Tamoxifen: An FDA approved drug with neuroprotective effects for spinal cord injury recovery. Neural Regen. Res. 2016;11:1208–1211. doi: 10.4103/1673-5374.189164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang C., Wang P., Zeng W., Li W. Tetramethylpyrazine improves the recovery of spinal cord injury via Akt/Nrf2/HO-1 pathway. Bioorg. Med. Chem. Lett. 2016;26:1287–1291. doi: 10.1016/j.bmcl.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 83.Radhakrishna M., Steuer I., Prince F., Roberts M., Mongeon D., Kia M., Dyck S., Matte G., Vaillancourt M., Guertin P.A. Double-blind, placebo-controlled, randomized phase I/IIa study (safety and efficacy) with buspirone/levodopa/carbidopa (SpinalonTM) in subjects with complete AIS A or motor-complete AIS B spinal cord injury. Curr. Pharm. Des. 2017;23:1789–1804. doi: 10.2174/1381612822666161227152200. [DOI] [PubMed] [Google Scholar]
- 84.Park J., Zheng L., Acosta G., Vega-Alvarez S., Chen Z., Muratori B., Cao P., Shi R. Acrolein contributes to TRPA1 up-regulation in peripheral and central sensory hypersensitivity following spinal cord injury. J. Neurochem. 2015;135:987–997. doi: 10.1111/jnc.13352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Han Z.A., Song D.H., Oh H.M., Chung M.E. Botulinum toxin type A for neuropathic pain in patients with spinal cord injury. Ann. Neurol. 2016;79:569–578. doi: 10.1002/ana.24605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wilsey B., Marcotte T.D., Deutsch R., Zhao H., Prasad H., Phan A. An exploratory human laboratory experiment evaluating vaporized cannabis in the treatment of neuropathic pain from spinal cord injury and disease. J. Pain. 2016;17:982–1000. doi: 10.1016/j.jpain.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moon H.C., Lee Y.J., Cho C.B., Park Y.S. Suppressed GABAergic signalling in the zona incerta causes neuropathic pain in a thoracic hemisection spinal cord injury rat model. Neurosci. Lett. 2016;632:55–61. doi: 10.1016/j.neulet.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 88.Pawasauskas J. Opioid rotation: A case example using methadone in spinal cord injury. J. Opioid Manag. 2015;11:443–448. doi: 10.5055/jom.2015.0294. [DOI] [PubMed] [Google Scholar]
- 89.Ellis A., Grace P.M., Wieseler J., Favret J., Springer K., Skarda B., Ayala M., Hutchinson M.R., Falci S., Rice K.C., et al. Morphine amplifies mechanical allodynia via TLR4 in a rat model of spinal cord injury. Brain Behav. Immun. 2016;58:348–356. doi: 10.1016/j.bbi.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sang C.N., Barnabe K.J., Kern S.E. Phase IA clinical trial evaluating the tolerability, pharmacokinetics, and analgesic efficacy of an intrathecally administered neurotensin an analogue in central neuropathic pain following spinal cord injury. Clin. Pharmacol. Drug Dev. 2016;5:250–258. doi: 10.1002/cpdd.253. [DOI] [PubMed] [Google Scholar]
- 91.Huang M., Chen H., Jiang C., Xie K., Tang P., Ou R., Zeng J., Liu Q., Li Q., Huang J., et al. Effects of botulinum toxin A injections in spinal cord injury patients with detrusor overactivity and detrusor sphincter dyssynergia. J. Rehabil. Med. 2016;48:683–687. doi: 10.2340/16501977-2132. [DOI] [PubMed] [Google Scholar]
- 92.Sugiyama H., Uemura O., Mori T., Okisio N., Unai K., Liu M. Effect of imidafenacin on the urodynamic parameters of patients with indwelling bladder catheters due to spinal cord injury. Spinal Cord. 2017;55:187–191. doi: 10.1038/sc.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chung Y.G., Seth A., Doyle C., Franck D., Kim D., Cristofaro V., Benowitz L.I., Tu D.D., Estrada C.R., Mauney J.R., et al. Inosine improves neurogenic detrusor overactivity following spinal cord injury. PLoS ONE. 2015;10:e0141492. doi: 10.1371/journal.pone.0141492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wöllner J., Pannek J. Initial experience with the treatment of neurogenic detrusor overactivity with a new β-3 agonist (mirabegron) in patients with spinal cord injury. Spinal Cord. 2016;54:78–82. doi: 10.1038/sc.2015.195. [DOI] [PubMed] [Google Scholar]
- 95.Ishida H., Yamauchi H., Ito H., Akino H., Yokoyama O. α1D-Adrenoceptor blockade increases voiding efficiency by improving external urethral sphincter activity in rats with spinal cord injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016;311:971–978. doi: 10.1152/ajpregu.00030.2016. [DOI] [PubMed] [Google Scholar]
- 96.Sakakibara F., Takahama K., Nanri M., Sasaki E. Pharmacological properties of propiverine contribute to improving lower urinary tract dysfunctions in rats with spinal cord injuries. Drug Res. (Stuttg) 2016;66:464–469. doi: 10.1055/s-0042-110855. [DOI] [PubMed] [Google Scholar]
- 97.Rossi F., Perale S., Papa S., Forloni G., Veglianese P. Current options for drug delivery to the spinal cord. Expert Opin. Drug Deliv. 2013;10:385–396. doi: 10.1517/17425247.2013.751372. [DOI] [PubMed] [Google Scholar]
- 98.Zamani F., Amani-Tehran M., Latifi M., Shokrgozar M.A., Zaminy A. Promotion of spinal cord axon regeneration by 3D nanofibrous core-sheath scaffolds. J. Biomed. Mater Res. A. 2014;102:506–513. doi: 10.1002/jbm.a.34703. [DOI] [PubMed] [Google Scholar]
- 99.Ma W., Cheethamb A.G., Cui H. Building nanostructures with drugs. Nano Today. 2016;11:13–30. doi: 10.1016/j.nantod.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kaneko A., Matsushita A., Sankai Y. A 3D nanofibrous hydrogel and collagen sponge scaffold promotes locomotor functional recovery, spinal repair, and neuronal regeneration after complete transection of the spinal cord in adult rats. Biomed. Mater. 2015;10:015008. doi: 10.1088/1748-6041/10/1/015008. [DOI] [PubMed] [Google Scholar]
- 101.Guo J.S., Quian C.H., Ling E.A., Zeng Y.S. Nanofiber scaffolds for treatment of spinal cord injury. Curr. Med. Chem. 2014;21:4282–4289. doi: 10.2174/0929867321666140815124648. [DOI] [PubMed] [Google Scholar]
- 102.Asghari F., Samiei M., Adibkia K., Akbarzadeh A., Davaran S. Biodegradable and biocompatible polymers for tissue engineering application: A review. Artif. Cells Nanomed. Biotechnol. 2017;45:185–192. doi: 10.3109/21691401.2016.1146731. [DOI] [PubMed] [Google Scholar]
- 103.Goyal R., Macri L.K., Kaplan H.M., Kohn J. Nanoparticles and nanofibers for topical drug delivery. J. Control Release. 2016;28:77–92. doi: 10.1016/j.jconrel.2015.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Owen S.C., Shoichet M.S. Design of three-dimensional biomimetic scaffolds. J. Biomed. Mater Res. A. 2010;94:1321–1331. doi: 10.1002/jbm.a.32834. [DOI] [PubMed] [Google Scholar]
- 105.Hurtado A., Cregg J.M., Wang H.B., Wendell D.F., Oudega M., Gilbert R.J., McDonald J.W. Robust CNS regeneration after complete spinal cord transection using aligned poly-l-lactic acid microfibers. Biomaterials. 2011;32:6068–6079. doi: 10.1016/j.biomaterials.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Prabhakaran M.P., Vatankhah E., Ramakrishna S. Electrospun aligned PHBV/collagen nanofibers as substrates for nerve tissue engineering. Biotechnol. Bioeng. 2013;110:2775–2784. doi: 10.1002/bit.24937. [DOI] [PubMed] [Google Scholar]
- 107.Lee J.H., Lee Y.J., Cho H.J., Shin H. Guidance of in vitro migration of human mesenchymal stem cells and in vivo guided bone regeneration using aligned electrospun fibers. Tissue Eng. Part A. 2014;20:2031–2042. doi: 10.1089/ten.tea.2013.0282. [DOI] [PubMed] [Google Scholar]
- 108.Zuidema J.M., Hyzinski-García M.C., Van Vlasselaer K., Zaccor N.W., Plopper G.E., Mongin A.A., Gilbert R.J. Enhanced GLT-1 mediated glutamate uptake and migration of primary astrocytes directed by fibronectin-coated electrospun poly-L-lactic acid fibers. Biomaterials. 2014;35:1439–1449. doi: 10.1016/j.biomaterials.2013.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lanza R.P., Langer R., Chick W.L., Peppas N.A. Principles of tissue engineering. Nature. 1997;389:453. [Google Scholar]
- 110.Raspa A., Marchini A., Pugliese R., Mauri M., Maleki M., Vasita R., Gelain F. A biocompatibility study of new nanofibrous scaffolds for nervous system regeneration. Nanoscale. 2016;8:253–265. doi: 10.1039/C5NR03698D. [DOI] [PubMed] [Google Scholar]
- 111.Qu J., Wang D., Wang H., Dong Y., Zhang F., Zuo B., Zhang H. Electrospun silk fibroin nanofibers in different diameters support neurite outgrowth and promote astrocyte migration. J. Biomed. Mater Res. A. 2013;101:2667–2678. doi: 10.1002/jbm.a.34551. [DOI] [PubMed] [Google Scholar]
- 112.Kim Y.C., Kim Y.H., Kim J.W., Ha K.Y. Transplantation of mesenchymal stem cells for acute spinal cord injury in rats: Comparative study between intralesional injection and scaffold based Transplantation. J. Korean Med. Sci. 2016;31:1373–1382. doi: 10.3346/jkms.2016.31.9.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu T., Houle J.D., Xu J., Chan B.P., Chew S.Y. Nanofibrous collagen nerve conduits for spinal cord repair. Tissue Eng. Part A. 2012;18:1057–1066. doi: 10.1089/ten.tea.2011.0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Altinova H., Möllers S., Führmann T., Deumens R., Bozkurt A., Heschel I., Damink L.H., Schügner F., Weis J., Brook G.A. Functional improvement following implantation of a microstructured, type-I collagen scaffold into experimental injuries of the adult rat spinal cord. Brain Res. 2014;1585:37–50. doi: 10.1016/j.brainres.2014.08.041. [DOI] [PubMed] [Google Scholar]
- 115.Palejwala A.H., Fridley J.S., Mata J.A., Samuel E.L., Luerssen T.G., Perlaky L., Kent T.A., Tour J.M., Jea A. Biocompatibility of reduced graphene oxide nanoscaffolds following acute spinal cord injury in rats. Surg. Neurol. Int. 2016;7:75. doi: 10.4103/2152-7806.188905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jeffries E.M., Wang Y. Biomimetic micropatterned multi-channel nerve guides by templated electrospinning. Biotechnol. Bioeng. 2012;109:1571–1582. doi: 10.1002/bit.24412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ranjbar-Mohammadi M., Prabhakaran M.P., Bahrami S.H., Ramakrishna S. Gum tragacanth/poly(l-lactic acid) nanofibrous scaffolds for application in regeneration of peripheral nerve damage. Carbohydr. Polym. 2016;140:104–112. doi: 10.1016/j.carbpol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 118.Webber M.J., Matson J.B., Tamboli V.K., Stupp S.I. Controlled release of dexamethasone from peptide nanofiber gels to modulate inflammatory response. Biomaterials. 2012;33:6823–6832. doi: 10.1016/j.biomaterials.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hsu K.H., Fang S.P., Lin C.L., Liao Y.S., Yoon Y.K., Chauhan A. Hybrid electrospun polycaprolactone mats consisting of nanofibers and microbeads for extended release of dexamethasone. Pharm. Res. 2016;33:1509–1516. doi: 10.1007/s11095-016-1894-4. [DOI] [PubMed] [Google Scholar]
- 120.Saadai P., Nout Y.S., Encinas J., Wang A., Downing T.L., Beattie M.S., Bresnahan J.C., Li S., Farmer D.L. Prenatal repair of myelomeningocele with aligned nanofibrous scaffolds-a pilot study in sheep. J. Pediatr. Surg. 2011;46:2279–2283. doi: 10.1016/j.jpedsurg.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 121.Gelain F., Panseri S., Antonini S., Cunha C., Donega M., Lowery J., Taraballi F., Cerri G., Montagna M., Baldissera F., et al. Transplantation of nanostructured composite scaffolds results in the regeneration of chronically injured spinal cords. ACS Nano. 2011;5:227–236. doi: 10.1021/nn102461w. [DOI] [PubMed] [Google Scholar]
- 122.Pires L.R., Guarino V., Oliveira M.J., Ribeiro C.C., Barbosa M.A., Ambrosio L., Pêgo A.P. Ibuprofen-loaded poly(trimethylene carbonate-co-ε-caprolactone) electrospun fibres for nerve regeneration. J. Tissue Eng. Regen. Med. 2016;10:154–166. doi: 10.1002/term.1792. [DOI] [PubMed] [Google Scholar]
- 123.Hakim J.S., Esmaeili Rad M., Grahn P.J., Chen B.K., Knight A.M., Schmeichel A.M., Isaq N.A., Dadsetan M., Yaszemski M.J., Windebank A.J. Positively charged oligo[poly(ethylene glycol) fumarate] scaffold implantation results in a permissive lesion environment after spinal cord injury in rat. Tissue Eng. Part A. 2015;21:2099–2114. doi: 10.1089/ten.tea.2015.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee Y.J., Bashur C.A., Goldstein A.S., Schmidt C.E. Polypyrrole-Coated Electrospun PLGA Nanofibers for Neural Tissue Applications. Biomaterials. 2009;30:4325–4335. doi: 10.1016/j.biomaterials.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schnell E., Klinkhammeer K., Balzer S., Brook G., Klee D., Dalton P., Mey J. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-ε-caprolactone and a collagen/poly-ε-caprolactone blend. Biomaterials. 2007;28:3012–3025. doi: 10.1016/j.biomaterials.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 126.Kubinová Š., Horák D., Hejčl A., Plichta Z., Kotek J., Proks V., Forostyak S., Syková E. SIKVAV-modified highly superporous PHEMA scaffolds with oriented pores for spinal cord injury repair. J. Tissue Eng. Regen. Med. 2015;9:1298–1309. doi: 10.1002/term.1694. [DOI] [PubMed] [Google Scholar]
- 127.Hu J., Kai D., Ye H., Tian L., Ding X., Ramakrishna S., Loh X.J. Electrospinning of poly(glycerol sebacate)-based nanofibers for nerve tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017;70:1089–1094. doi: 10.1016/j.msec.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 128.Entekhabi E., Haghbin Nazarpak M., Moztarzadeh F., Sadeghi A. Design and manufacture of neural tissue engineering scaffolds using hyaluronic acid and polycaprolactone nanofibers with controlled porosity. Mater. Sci. Eng. C Mater. Biol. Appl. 2016;69:380–387. doi: 10.1016/j.msec.2016.06.078. [DOI] [PubMed] [Google Scholar]
- 129.Feng Z.V., Chen W.S., Keratithamkul K., Stoick M., Kapala B., Johnson E., Huang A.C., Chin T.Y., Chen-Yang Y.W., Yang M.L. Degradation of the electrospun silica nanofiber in a biological medium for primary hippocampal neuron-effect of surface modification. Int. J. Nanomed. 2016;11:729–741. doi: 10.2147/IJN.S93651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fan Z., Shen Y., Zhang F., Zuo B., Lu Q., Wu P., Xie Z., Dong Q., Zhang H. Control of olfactory ensheathing cell behaviors by electrospun silk fibroin fibers. Cell Transplant. 2013;22:39–50. doi: 10.3727/096368913X672190. [DOI] [PubMed] [Google Scholar]
- 131.Gnavi S., Fornasari B.E., Tonda-Turo C., Laurano R., Zanetti M., Ciardelli G., Geuna S. The Effect of Electrospun Gelatin Fibers Alignment on Schwann Cell and Axon Behavior and Organization in the Perspective of Artificial Nerve Design. Int. J. Mol. Sci. 2015;16:12925–12942. doi: 10.3390/ijms160612925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Junka R., Valmikinathan C.M., Kalyon D.M., Yu X. Laminin Functionalized Biomimetic Nanofibers for Nerve Tissue Engineering. J. Biomater. Tissue Eng. 2013;3:494–502. doi: 10.1166/jbt.2013.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dwivedi C., Pandey H., Pandey A.C., Ramteke P.W. Repair and regenerations. Curr. Pharm. Des. 2016;22:1460–1471. doi: 10.2174/1381612822666151215103553. [DOI] [PubMed] [Google Scholar]
- 134.Ranjbar-Mohammadi M., Bahrami S.H. Electrospun curcumin loaded poly(ε-caprolactone)/gum tragacanth nanofibers for biomedical application. Int. J. Biol. Macromol. 2016;84:448–456. doi: 10.1016/j.ijbiomac.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 135.Kubinová Š., Syková E. Nanotechnology for trearment of stroke spinal cord injury. Nanomedicine. 2010;5:99–108. doi: 10.2217/nnm.09.93. [DOI] [PubMed] [Google Scholar]
- 136.Schaub N.J., Johnson C.D., Cooper B., Gilbert R.J. Electrospun fibers for spinal cord injury research and regeneration. J. Neurotrauma. 2016;33:1405–1415. doi: 10.1089/neu.2015.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rochkind S., Shahar A., Fliss D., El-Ani D., Astachov L., Hayon T., Alon M., Zamostiano R., Ayalon O., Biton I.E., et al. Development of a tissue-engineered composite implant for treating traumatic paraplegia in rats. Eur. Spine J. 2006;15:234–245. doi: 10.1007/s00586-005-0981-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Usmani S., Aurand E.R., Medelin M., Fabbro A., Scaini D., Laishram J., Rosselli F.B., Ansuini A., Zoccolan D., Scarselli M., et al. 3D meshes of carbon nanotubes guide functional reconnection of segregated spinal explants. Sci. Adv. 2016;2:e1600087. doi: 10.1126/sciadv.1600087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu Y., Ye H., Satkunendrarajah K., Yao G.S., Bayon Y., Fehlings M.G. A self-assembling peptide reduces glial scarring, attenuates post-traumatic inflammation and promotes neurological recovery following spinal cord injury. Acta Biomater. 2013;9:8075–8088. doi: 10.1016/j.actbio.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 140.Rogina A. Electrospinning process: Versatile preparation method for biodegradable and natural polymers and biocomposite systems applied in tissue engineering and drug. Appl. Surf. Sci. 2014;296:221–230. doi: 10.1016/j.apsusc.2014.01.098. [DOI] [Google Scholar]
- 141.Pelipenko J., Kristl J., Janković B., Baumgartner S., Kocbek P. The impact of relative humidity during electrospinning on the morphology and mechanical properties of nanofibers. Int. J. Pharm. 2013;456:125–134. doi: 10.1016/j.ijpharm.2013.07.078. [DOI] [PubMed] [Google Scholar]
- 142.Kim K., Luu Y.K., Chang C., Fang D., Hsiao B.S., Chu B., Hadjiargyrou M. Incorporation and controlled release of a hydrophilic antibiotic using poly(lactide-co-glycolide)-based electrospun nanofibrous scaffolds. J. Control Release. 2004;98:47–56. doi: 10.1016/j.jconrel.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 143.Jannesari M., Varshosaz J., Morshed M., Zamani M. Composite poly (vinyl alcohol)/poly (vinyl acetate) electrospun nanofibrous mats as a novel wound dressing matrix for controlled release of drugs. Int. J. Nanomed. 2011;6:993–1003. doi: 10.2147/IJN.S17595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Meng Z., Xu X.X., Zheng W., Zhou H.M., Li L., Zheng Y.F., Lou X. Preparation and characterization of electrospun PLGA/gelatin nanofibers as a potential drug delivery system. Colloids Surf. B Biointerfaces. 2011;84:97–102. doi: 10.1016/j.colsurfb.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 145.Bhattarai N., Cha D.I., Bhattarai S.R., Khil M.S., Kim H.Y. Biodegradable electrospun mat: Novel block copolymer of poly (p-dioxanone-co-l-lactide)-block-poly(ethylene glycol) J. Polym. Sci. B Polym. Phys. 2003;41:1955–1964. doi: 10.1002/polb.10547. [DOI] [Google Scholar]
- 146.Lu Y., Huang J., Yu G., Cardenas R., Wei S., Wujcik E.K., Guo Z. Coaxial electrospun fibers: Applications in drug delivery and tissue engineering. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016;8:654–677. doi: 10.1002/wnan.1391. [DOI] [PubMed] [Google Scholar]
- 147.Siafaka P.I., Barmbalexis P., Bikiaris D.N. Novel electrospun nanofibrous matrices prepared from poly(lactic acid)/poly(butylene adipate) blends for controlled release formulations of an anti-rheumatoid agent. Eur. J. Pharm. Sci. 2016;88:12–25. doi: 10.1016/j.ejps.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 148.Mohammadian F., Eatemadi A. Drug loading and delivery using nanofibers scaffolds. Artif. Cells Nanomed. Biotechnol. 2016;17:1–8. doi: 10.1080/21691401.2016.1185726. [DOI] [PubMed] [Google Scholar]
- 149.Yarin A.L. Coaxial electrospinning and emulsion electrospinning of core-shell fibers. Polym. Adv. Technol. 2011;22:310–317. doi: 10.1002/pat.1781. [DOI] [Google Scholar]
- 150.McClellan P., Landis W.J. Recent applications of coaxial and emulsion electrospinning methods in the field of tissue engineering. BioRes. Open Access. 2016;5:212–227. doi: 10.1089/biores.2016.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sperling L.E., Reis K.P., Pranke P., Wendorff J.H. Advantages and challenges offered by biofunctional core-shell fiber systems for tissue engineering and drug delivery. Drug Discov. Today. 2016;21:1243–1256. doi: 10.1016/j.drudis.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 152.Schaub N.J., Le Beux C., Miao J., Linhardt R.J., Alauzun J.G., Laurencin D., Gilbert R.J. The effect of surface modification of aligned poly-l-lactic acid electrospun fibers on fiber degradation and neurite extension. PLoS ONE. 2015;10:e0136780. doi: 10.1371/journal.pone.0136780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schaub N.J., Gilbert R.J. Controlled release of 6-aminonicotinamide from aligned, electrospun fibers alters astrocyte metabolism and dorsal root ganglia neurite outgrowth. J. Neural Eng. 2011;8:046026. doi: 10.1088/1741-2560/8/4/046026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pires L.R., Rocha D.N., Ambrosio L., Pêgo A.P. The role of the surface on microglia function: Implications for central nervous system tissue engineering. J. R. Soc. Interface. 2015;12 doi: 10.1098/rsif.2014.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Seif S., Franzen L., Windbergs M. Overcoming drug crystallization in electrospun fibers—Elucidating key parameters and developing strategies for drug delivery. Int. J. Pharm. 2015;478:390–397. doi: 10.1016/j.ijpharm.2014.11.045. [DOI] [PubMed] [Google Scholar]
- 156.Downing T.L., Wang A., Yan Z.Q., Nout Y., Lee A.L., Beattie M.S., Bresnahan J.C., Farmer D.L., Li S. Drug-eluting microfibrous patches for the local delivery of rolipram in spinal cord repair. J. Control Release. 2012;161:910–917. doi: 10.1016/j.jconrel.2012.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Stout D.A. Recent advancements in carbon nanofiber and carbon nanotube applications in drug delivery and tissue engineering. Curr. Pharm. Des. 2015;21:2037–2044. doi: 10.2174/1381612821666150302153406. [DOI] [PubMed] [Google Scholar]
- 158.Su W.T., Shih Y.A. Nanofiber containing carbon nanotubes enhanced PC12 cell proliferation and neuritogenesis by electrical stimulation. Bio. Med. Mater. Eng. 2015;26:189–195. doi: 10.3233/BME-151305. [DOI] [PubMed] [Google Scholar]
- 159.López-Dolado E., González-Mayorga A., Gutiérrez M.C., Serrano M.C. Immunomodulatory and angiogenic responses induced by graphene oxide scaffolds in chronic spinal hemisected rats. Biomaterials. 2016;99:72–81. doi: 10.1016/j.biomaterials.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 160.Raspa A., Pugliese R., Maleki M., Gelain F. Recent Therapeutic Approaches for Spinal Cord Injury. Biotechnol. Bioeng. 2016;113:253–259. doi: 10.1002/bit.25689. [DOI] [PubMed] [Google Scholar]
- 161.Zhang S., Gelain F., Zhao X. Designer self assembling peptide nanofiber scaffold for 3D tissue cell cultures. Semin Cancer Biol. 2005;15:410–420. doi: 10.1016/j.semcancer.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 162.Guo J., Su H., Zeng Y., Liang Y.X., Wong W.M., Ellis-Behnke R.G., So K.F., Wu W. Reknitting the injured spinal cord by self-assembling peptide nanofiber scaffold. Nanomedicine. 2007;3:311–321. doi: 10.1016/j.nano.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 163.Tysseling-Mattiace V.M., Sahni V., Niece K.L., Birch D., Czeisler C., Fehlings M.G., Stupp S.I., Kessler J.A. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. J. Neurosci. 2008;28:3814–3823. doi: 10.1523/JNEUROSCI.0143-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gelain F., Cigognioni D., Caprini A., Silva D., Colleoni B., Donegà M., Antonini S., Choen B.E., Vescovi A. New bioactive motif and their use in functionalized self-assembling peptides for NSC differentiation and neural tissue engineering. Nanoscale. 2012;4:2946–2957. doi: 10.1039/c2nr30220a. [DOI] [PubMed] [Google Scholar]
- 165.Tetzlaff W., Okon E.B., Karimi-Abdolrezaee S., Hill C.E., Sparling J.S., Plemel J.R., Plunet W.T., Tsai E.C., Baptiste D., Smithson L.J., et al. A systematic review of cellular transplantation therapies for spinal cord injury. J. Neurotrauma. 2011;28:1611–1682. doi: 10.1089/neu.2009.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cigognini D., Silva D., Paloppi S., Gelain F. Evaluation of mechanical properties and therapeutic effect of injectable self-assembling hydrogels for spinal cord injury. J. Biomed. Nanotechnol. 2014;10:309–323. doi: 10.1166/jbn.2014.1759. [DOI] [PubMed] [Google Scholar]
- 167.Tavakol S., Saber R., Hoveizi E., Tavakol B., Aligholi H., Ai J., Rezayat S.M. Motif of Laminin Induces Neural Differentiation, Tubulin Polymerization, and Neurogenesis: In Vitro, Ex Vivo, and In Vivo Studies. Mol. Neurobiol. 2016;53:5288–5299. doi: 10.1007/s12035-015-9448-z. [DOI] [PubMed] [Google Scholar]
- 168.Gerth D.J., Tashiro J., Thaller S.R. Clinical outcomes for conduits and scaffolds in peripheral nerve repair. World J. Clin. Cases. 2015;3:141–147. doi: 10.12998/wjcc.v3.i2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Goswami D., Vitorino H.A., Machini M.T., Espòsito B.P. Self-Assembled Penetratin-Deferasirox Micelles as Potential Carriers for Hydrophobic Drug Delivery. Pept. Sci. 2015;104:712–719. doi: 10.1002/bip.22672. [DOI] [PubMed] [Google Scholar]
- 170.Ninomiya K., Iwatsuki K., Ohnishi Y., Ohkawa T., Yoshimine T. Intranasal delivery of bone marrow stromal cells to spinal cord lesions. J. Neurosurg. Spine. 2015;23:111–119. doi: 10.3171/2014.10.SPINE14690. [DOI] [PubMed] [Google Scholar]
- 171.Lee H.Y., Lee H.L., Yun Y., Kim J.S., Ha Y., Yoon D.H., Lee S.H., Shin D.A. Human adipose stem cells improve mechanical allodynia and enhance functional recovery in a rat model of neuropathic pain. Tissue Eng. A. 2015;21:2044–2052. doi: 10.1089/ten.tea.2014.0713. [DOI] [PubMed] [Google Scholar]
- 172.Mehta T., Feroz A., Thakkar U., Vanikar A., Shah V., Trivedi H. Subarachnoid placement of stem cells in neurological disorders. Transplant Proc. 2008;40:1145–1147. doi: 10.1016/j.transproceed.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 173.Mei L., Wang Y., Tong A., Guo G. Facile electrospinning of an efficient drug delivery system. Expert Opin. Drug Deliv. 2016;13:741–753. doi: 10.1517/17425247.2016.1142525. [DOI] [PubMed] [Google Scholar]