Abstract
Glutamate neurotransmission in the nucleus accumbens core (NAc) mediates ethanol consumption. Previous studies using non-contingent and voluntary alcohol administration in inbred rodents have reported increased basal extracellular glutamate levels in the NAc. Here, we assessed basal glutamate levels in the NAc following intermittent alcohol consumption in male Sprague-Dawley rats that had access to ethanol for 7 weeks on alternating days. We found increased basal NAc glutamate at 24 h withdrawal from ethanol and thus sought to identify the source of this glutamate. To do so, we employed a combination of microdialysis, slice electrophysiology and western blotting. Reverse dialysis of the voltage-gated sodium channel blocker tetrodotoxin did not affect glutamate levels in either group. Electrophysiological recordings in slices made after 24 h withdrawal revealed a decrease in spontaneous excitatory postsynaptic current (sEPSC) frequency relative to controls, with no change in sEPSC amplitude. No change in metabotropic glutamate receptor 2/3 (mGlu2/3) function was detected as bath application of the mGlu2/3 agonist LY379268 decreased spontaneous and miniature EPSC frequency in slices from both control and ethanol-consuming rats. The increase in basal glutamate was not associated with changes in the surface expression of GLT-1, however, a decrease in slope of the no-net-flux dialysis function was observed following ethanol consumption, indicating a potential decrease in glutamate reuptake. Taken together, these findings indicate that the increase in basal extracellular glutamate occurring after chronic ethanol consumption is not mediated by an increase in action potential-dependent glutamate release or a failure of mGlu2/3 autoreceptors to regulate such release.
Keywords: GLT-1, GluA1, mGluR2/3, microdialysis
Introduction
A critical component of the circuitry involved in alcohol addiction is the nucleus accumbens (NA). NA glutamate transmission mediates both alcohol consumption (Kapasova & Szumlinski, 2008; Griffin et al., 2014) and relapse to operant alcohol-seeking after a period of withdrawal (Gass et al., 2011). Basal extracellular glutamate levels are increased in the NA core (NAc; Melendez et al., 2005) following 24 h withdrawal from experimenter-administered alcohol. NAc basal extracellular glutamate is also elevated after a combination of non-contingent (vapor chamber) alcohol exposure and voluntary alcohol consumption for up to 7 days after the last vapor chamber exposure (Griffin et al., 2014). In male alcohol-preferring (P) rats, basal glutamate is elevated in the NAc when assessed within 20 h of the last drinking session (Das et al., 2015).
Glutamate levels in the NA are regulated by multiple mechanisms. Glutamate reuptake is mediated by the excitatory amino acid transporters (EAATs). EAAT2 (rodent GLT-1) is responsible for 90% of total CNS glutamate uptake (Tanaka et al., 1997) and is abundantly expressed in the NA (Lehre et al., 1995). Basal glutamate levels in the NA are primarily determined by system xC-(Baker et al., 2002), which exchanges extracellular cystine for intracellular glutamate. System xC- is composed of a generic 4FH2 protein and the unique subunit xCT. The contribution of system xC-and GLT-1 to the increased basal extracellular glutamate observed after alcohol is presently unclear. Melendez et al. (2005) found a deficit in glutamate uptake and GLT-1 expression accompanying elevated NAc glutamate. However, Griffin et al. (2015) demonstrated elevated basal extracellular glutamate with no accompanying alterations in EAAT or system xc-function. Ding et al. (2013) found no changes in GLT-1 or xCT expression in a model in which basal extracellular glutamate was elevated in the NA shell. Thus, there remains some controversy regarding the contribution of glutamate transporters to the increase in basal extracellular glutamate levels following alcohol consumption.
In addition to altered glutamate transport into or out of the extrasynaptic space, the presynaptic neuron is another potential source of glutamate. The Gi-coupled group II metabotropic glutamate receptors (mGlu2/3) serve as autoreceptors for synaptically released glutamate (Cartmell & Schoepp, 2000; Schoepp, 2001). These receptors also regulate glutamate released via system xC- in the NAc as an mGlu2/3 agonist prevents this release (Baker et al., 2002). A deficit in mGlu2/3 function and an increase in glutamate levels via action potential-dependent release have been proposed as mechanisms for increased NA glutamate after alcohol (Griffin et al., 2014). The present set of experiments is the first to directly investigate these presynaptic mechanisms as a source of increased NAc glutamate after alcohol.
We hypothesized that basal extracellular glutamate would be increased in the NAc of outbred rats which had intermittent access to alcohol (IAA) for 7 weeks and underwent 24 h withdrawal. We predicted that the increase in glutamate would result in synaptic potentiation and assessed this possibility with ex vivo slice electro-physiology to compare excitatory postsynaptic current (EPSC) frequency and amplitude, as well as mGlu2/3 function.
Materials and methods
Subjects
Adult male Sprague–Dawley rats (n = 64; Charles Rivers Laboratories, Raleigh, NC, USA) were housed in a temperature- and humidity-controlled virarium. Rats were single-housed and placed on a reverse light cycle with lights off at 7 am and on at 7 pm. Animals were food-restricted to 20 g/day. The experiments which yielded the data graphed in Figs 1 and 2 were conducted partially at the Medical University of South Carolina and partially at the University of Florida, while those yielding the remaining data were conducted at the University of Florida. All procedures were pre-approved by the Medical University of South Carolina and University of Florida Institutional Animal Care and Use Committees and were in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals. Efforts were made to minimize animal suffering and reduce the number of animals used. No alternatives to the in vivo techniques used here are available. A total of 14 control (Ctrl) and 12 ethanol-consuming (EtOH) rats were used for the microdialysis experiments depicted in Fig. 2. Thirteen additional rats were excluded from these experiments due to misplaced cannulae or probe failure during microdialysis procedures; their data are not graphed in either Fig. 1 or 2 or used in data analysis. A total of 5 Ctrl and 5 EtOH rats were used for the electrophysiological experiments comprising Fig. 3 and 7 Ctrl and 9 EtOH rats were used for the western blotting study (Fig. 4 results).
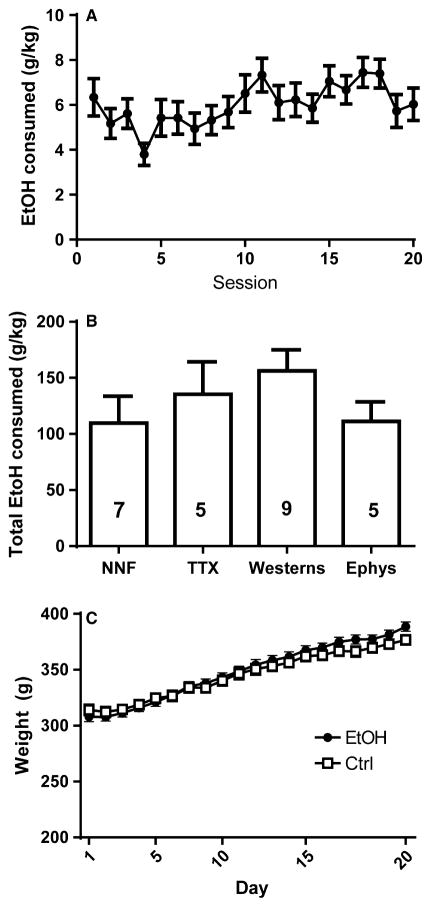
Fig. 1.
Alcohol consumption and body weight for both control (Ctrl) and ethanol-consuming (EtOH) rats over the course of the experiment. (A) Mean alcohol consumption for all rats (n = 25). (B) Total alcohol consumption did not differ between the four cohorts of rats used for different experimental endpoints: NNF = no-net-flux dialysis to determine basal extracellular glutamate; TTX = the infusion of tetrodotoxin during microdialysis; Westerns = western blot analysis; Ephys = electrophysiological recordings. The numbers inside the bars indicate the number of EtOH rats/experiment. (C) No differences were observed in body weight between EtOH (n = 25) and Ctrl (n = 26) rats.
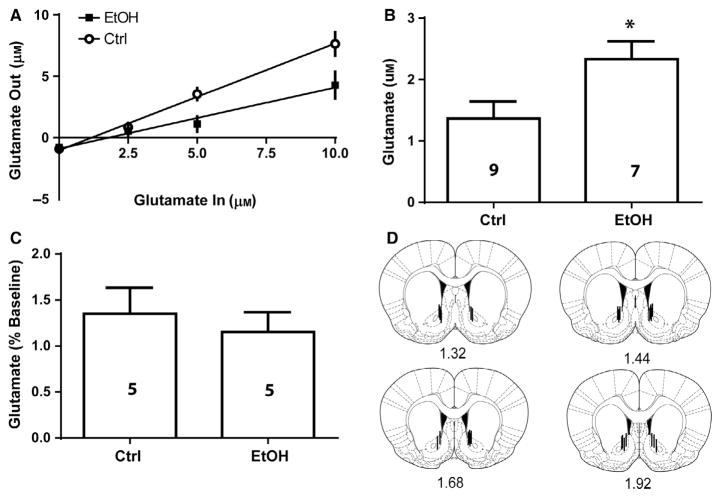
Fig. 2.
No-net-flux microdialysis revealed that basal extracellular glutamate is elevated in the NAc after 24 h withdrawal from 7 weeks of intermittent access to EtOH. (A) The amount of glutamate perfused was plotted against the amount of glutamate extracted to calculate the basal extracellular level of glutamate as the point of no net-flux of glutamate across the microdialysis membrane (the x-intercept). (B) Computing the point of no net-flux across the dialysis membrane in (A) and comparing between groups revealed that EtOH rats displayed significantly elevated NAc basal extracellular glutamate relative to controls (Ctrl). (C) In a separate group of rats, reverse dialysis of the voltage-gated sodium channel blocker tetrodotoxin (TTX) did not alter NAc glutamate levels in control or EtOH rats. (D) Diagrams showing dialysis probe tracks through the NA core; representative examples of dialysis probes are depicted along the rostro-caudal axis of the NAc. * indicates significant difference between groups (P < 0.05).
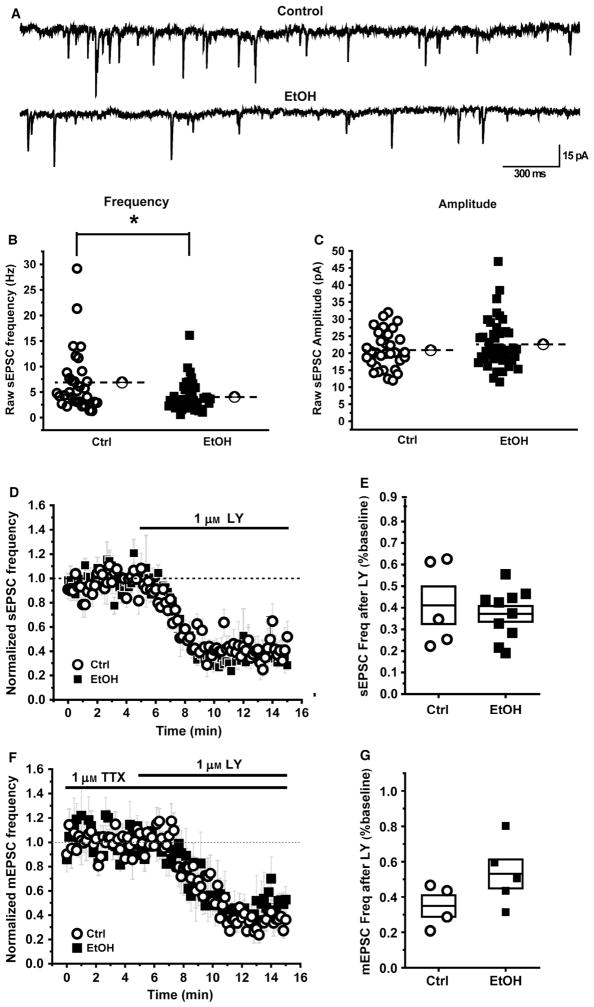
Fig. 3.
Decrease in spontaneous glutamatergic transmission in the NAc of rats following 24 h withdrawal from 7 weeks of alcohol consumption. (A) Example sEPSC traces from control (Ctrl) and alcohol-consuming (EtOH) rats. (B, C) Summary data for all the cells recorded from in both the groups. Open circles represent cells from control rats (n = 32 cells) and filled squares represent cells from alcohol-consuming rats (n = 44 cells). There is a modest but statistically significant decrease in sEPSC frequency in alcohol-consuming rats compared to the controls (B) without any change in sEPSC amplitude (C). Dotted lines represent the population mean. (D, E) The mGlur2/3 agonist LY379268 equally decreases the sEPSC frequency in both groups. (D) sEPSC frequency decreases following bath application of 1 μM LY at 5 min. The raw data was normalized to baseline period (2–5 min). (E) Box plots summarizing the effects of LY on sEPSC frequency in cells recorded from control and alcohol-consuming rats. Note that no additional inhibition of sEPSCs was observed at 10 μM LY (n = 5 cells, data not illustrated). (F, G) LY equally decreases mEPSC frequency in both groups. Recordings were made in the presence of 1 μM TTX to isolate mEPSC and 1 μM LY was bath applied at 5 min. (F) mEPSC frequency was decreased in both the groups by LY. The raw data were normalized to baseline period (2–5 min). (G) Box plots summarizing the effects of LY on mEPSC frequency in cells recorded from alcohol-consuming and control rats. In panels E and G, the horizontal line within the box indicates the mean and the top and bottom edges represent the mean ± the SEM. * indicates significant difference between groups (P < 0.05).
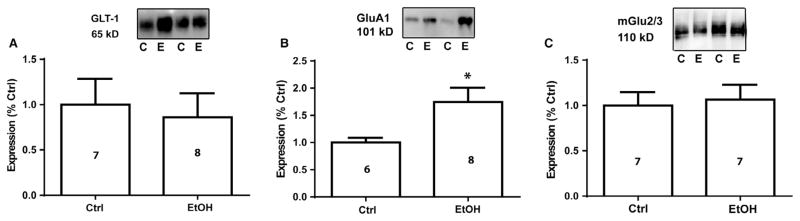
Fig. 4.
The surface expression of GluA1 was significantly increased in the NAc following 24 h withdrawal from alcohol, whereas mGlu2/3 and GLT-1 were unchanged. (A) GLT-1 surface expression was not altered by alcohol consumption. (B) Surface expression of GluA1 was significantly increased after 24 h withdrawal from alcohol consumption. There were no differences in mGlu2/3 surface expression (C). * indicates significant difference between groups (P < 0.05).
Intermittent access to alcohol paradigm
The IAA paradigm has been shown to be effective at inducing alcohol drinking in rats without sucrose-fading (Simms et al., 2008). This method permits 24-h access to unsweetened ethanol on alternating days (3 day/week) for 20 sessions without water-deprivation (Simms et al., 2008). Animals were weighed and then presented with ethanol (20% v/v) in graduated bottles with sipper tubes within the first hour of the dark cycle. The daily allotment of food (20 g) was also given at this time. Food restriction began the night prior to the 3rst alcohol presentation. Water was available ad libitum at all times. Bottles were checked for leaks after 2 h of drinking (none were detected). The amount of alcohol consumed was recorded at 24 h post presentation. For all experiments, animals experienced 20 drinking sessions over the course of 7 weeks as in Simms et al. (2008). Ctrl animals were age-matched to the EtOH rats and were treated identically to the EtOH rats, with the exception of being provided ethanol. Thus, they were identically food-restricted, weighed daily and underwent microdialysis and euthanasia (for western blotting or electrophysiological recording) at identical ages and time of day as the EtOH rats.
Microdialysis and HPLC for quantification of glutamate
Stereotaxic surgery to implant intracranial guide cannulas occurred prior to the initiation of drinking procedures. Animals were placed in a stereotaxic frame (Stoelting, Wood Dale, IL, USA) and a stainless guide cannula (22 gauge; Synaptech, Marquette, MI, USA) was unilaterally aimed 2 mm above the NAc according to the following coordinates (AP + 1.2 mm, ML ± 1.6 mm, DV −5.5 mm; Paxinos & Watson, 2007). Cannulas were secured to the skull with stainless steel skull screws and dental acrylic (Co-Oral-Ite Dental MFG. Co., Diamond Springs, CA, USA). Postoperatively, animals were administered the analgesic ketorolac (2 mg/kg, i.p.) for 3 days. Following 7 weeks of drinking via the IAA, subjects were implanted with a microdialysis probe the night prior to microdialysis procedures. Probes were constructed of a 2 mm cuprophane membrane (20 kDa cut-off weight, outer diameter 0.36 mm; Synaptech). Subjects were placed into a plexiglass blox (45 cm × 45 cm) and perfused overnight with artificial cerebral spinal fluid (aCSF) with food and water available. All microdialysis sample collections began at 24 h withdrawal from the last drinking session. For no-net flux dialysis, increasing concentrations of glutamate (0, 2.5, 5 and 10 μM) were perfused and the efflux collected at 20 min intervals for 80 min. Liquid switches were used to minimize the pressure fiuctuations while changing glutamate/aCSF buffers. The first sample collected after switching to a new glutamate concentration was discarded, as it likely contained a mixture of the old and new concentration. Sixteen animals completed NNF microdialysis: nine control and seven alcohol-consuming rats.
A separate group of animals (5 Ctrl and 5 EtOH) received reverse dialysis of the voltage-gated sodium channel blocker tetrodotoxin, (TTX; 1 μM) following baseline sample collections. This concentration was chosen based on previous microdialysis studies that found this concentration to be ineffective at reducing basal extracellular glutamate in the NAc of drug-naïve animals (Baker et al., 2002) while preventing synaptic glutamate release in this brain region (McFarland et al., 2003). Furthermore, this concentration suppresses action potentials in the striatum in vitro (Jiang & North, 1991) The hemisphere of probe insertion was counterbalanced, a flow rate of 2 μL/min was used and samples collected in 20 min intervals as described above.
Glutamate levels were analyzed using a high-pressure liquid chromatography (HPLC) system with electrochemical detection (Thermo Scientific, Dionex). Samples were derivatized with o-pthalaldehyde (Sigma-Aldrich) by an autosampler (Thermo Scientific, Inc.) immediately before injection onto a CAPLCELL PAK C18 column (5 μm, 2.0 mm I.D. × 50 mm; Shiseido Inc., Tokyo, Japan). The mobile phase consisted of 100 mM Na2HPO4, 16% (vol/vol) methanol, and 2.5% (vol/vol) acetonitrile (pH = 6.5). Glutamate content in each sample was analyzed by peak area and compared with an external standard curve for quantification.
Measuring surface protein expression via biotinylation and western blotting
Twenty-four hours after conclusion of the last drinking session (EtOH n = 9) or control condition (Ctrl n = 7), rats were rapidly decapitated without anesthesia. The NAc was dissected on ice, sliced with a McIllewan tissue chopper and incubated in Sulfo-NHS-SS-Biotin (Pierce) and the reaction quenched by glycine. A portion of the sample lysate was incubated with streptavidin agarose beads. The remainder of the sample was stored as the total protein fraction. Biotinylated proteins attached to streptavidin-coated beads were separated by centrifugation and eluted with Laemli buffer. The amount of target proteins in the total and biotinylated fractions was be analyzed by Western blotting. Briefly, proteins were separated using 10% SDS-PAGE and transferred to PVDF membrane. Membranes were blocked in 5% milk and probed with primary antibody against xCT (Novus 1 : 5000), GLT-1 (EMD Millipore 1 : 1000), GluA1 (EMD Millipore 1 : 2000) and mGlu2/3 (EMD Millipore 1 : 5000). For total protein expression, blots were re-probed with either calnexin (Millipore 1 : 20 000) or beta-tubulin (Abcam, 1 : 50 000) to serve as loading controls. Membranes were incubated with HRP-conjugated secondary antiserum (Jackson ImmunoResearch Lab) at room temperature. Secondary antibody concentrations and species were as follows, xct: anti-rabbit 1 : 50 000; GLT-1: anti-guinea pig 1 : 50 000; GluA1: anti-mouse 1 : 50 000; mGlu2/3: anti-rabbit 1 : 50 000, calnexin: anti-rabbit 1 : 50 000; beta-tubulin: anti-rabbit 1 : 50 000. Bands were visualized using ECL Plus (GE Healthcare). Band density was quantified with NIH IMAGE J software.
Electrophysiology
Slice preparation
Rats were anesthetized with ketamine (75 mg/kg IP) and xylazine (5 mg/kg IP). Once a surgical plane of anesthesia was achieved, animals were transcardially perfused with ice-cold oxygenated artificial cerebrospinal fluid (aCSF) which contained in mM: 206 sucrose, 2 KCl, 25 NaHCO3, 1.25 NaH2PO4, 1 CaCl2, 1 MgSO4, 0.01 Glycine, 10 D-glucose, saturated with 95% O2 and 5% CO2. Immediately after perfusion, animals were decapitated, the brain was rapidly removed, and 300 μM thick coronal slices containing the NAc were made in the same ice-cold aCSF using a Leica VT 1000s vibratome. Slices were then transferred to an incubator containing aCSF that contained in mM: 124 NaCl, 2.5 KCl, 25 NaHCO3, 1.23 NaH2PO4, 1 CaCl2, 3 MgSO4, 10 D-glucose. This aCSF was preheated to 30–35°C, and was also saturated with 95% O2 and 5% CO2. Slices were allowed to equilibrate to room temperature for a minimum of 30 min before experimental use. A total of 10 rats were used for electrophysiology experiments: 5 EtOH, and 5 Ctrl rats.
Whole-cell recording
After incubation, slices were transferred to a recording chamber where they were perfused at 2 mL/min with aCSF that contained in mM: 124 NaCl, 5 KCl, 25 NaHCO3, 1.25 NaH2PO4, 2.5 CaCl2, 1.2 MgSO4, 25 D-glucose. This aCSF was also saturated with 95% O2 and 5% CO2. Temperature in the recording chamber was maintained at 30 ± 2 °C. Whole-cell patch clamp recordings were made from medium spiny neurons in NAc using patch electrodes filled with an internal solution which contained in mM: 140 CsMeSO3, 3 NaCl, 1 MgCl2, 0.2 Cs-EGTA, 4 Na2-ATP, 0.3 Na-GTP, 10 HEPES, 5 QX-314, pH adjusted to 7.3 with CsOH, and volume adjusted to 285–300 mOSm. Slices were visualized with infrared differential interference contrast microscopy (IR DIC) using an Olympus BX51WI microscope. Whole-cell voltage clamp recordings were performed using micropipettes pulled from borosilicate glass using a Flaming/Brown electrode puller (Sutter P-97; Sutter Instruments, Novato, CA, USA). Electrode tip resistance was 3–5 MΩ. Access resistance, membrane resistance, and whole-cell capacitance were measured in voltage clamp mode in response to a −10 mV hyperpolarizing step delivered every 10 s. Cells were discarded if access resistance increased by −30% or more during the course of an experiment, or if sudden changes in access resistance were temporally related to changes in parameters of experimental interest (e.g. spontaneous synaptic currents or holding current). Cells were voltage clamped at −70 mV and spontaneous excitatory postsynaptic currents (sEPSCs) were isolated by bath application of 100 μM picrotoxin (PTX). Voltage clamp recordings were performed using an Axon Multiclamp 700B amplifier (Molecular Devices, Sunny-vale, CA, USA). Data were sampled at 20 kHz, filtered at 2 kHz, and digitally recorded by a Digidata 1440AtoD converter using CLAMPEX v. 10 (Molecular Devices). In some experiments, LY354740 was bath applied at a concentration of 1 μM (Kłodzinska et al., 2002; Zhai et al., 2003). Chemicals were obtained from Tocris Cookson, Sigma-Aldrich, or Fisher Scientific.
Data analysis
GRAPHPAD PRISM (version 5.00; GraphPad Software, La Jolla, CA, USA) was used for statistical analyses of data depicted in Figs 1, 2 and 4. For all statistical analyses, the alpha level was set at P < 0.05. Alcohol consumption data were analyzed with mixed-factorial two-way analyses of variance with time as the repeated measure (two-way RM ANOVA). Group differences in alcohol consumption between cohorts of animals with different experimental endpoints were investigated using one-way ANOVA.
For the no-net-flux microdialysis experiment, we first subtracted the known amount of glutamate (GluR) added to the perfusate from the amount measured in the dialysate by HPLC analysis ([Glu]in–[Glu]out). These values were plotted against the [Glu]in and a line of regression was drawn. A 2-way RM ANOVA was used to determine group and time differences. The X intercepts reflect basal extracellular glutamate concentration and we examined group differences in this measure between EtOH and control groups using an independent samples t-test. The slope of the line of regression reflects the extraction fraction (Ed) and thus the slopes were compared between groups with an independent samples t-test. A separate group of animals received TTX during microdialysis procedures. Microdialysis samples were converted to a percent baseline by averaging the dialysate glutamate in the samples collected for 30 immediately prior to the perfusion of TTX and setting that value as baseline. The glutamate values for each individual rat for the 40 min post-TTX were normalized to its own baseline value and that data was compared between groups with an independent samples t-test.
Electrophysiological data was analyzed using custom software written in OriginC (OriginLab, Northampton, MA, USA) by CJF. For experiments that involved spontaneous synaptic currents, events were detected using a parameter based event detection algorithm, adjusted to allow for reliable detection of fast, small events typical in these neurons (typical peak threshold: ~10 pA, typical area threshold: ~20 pA). In all cases an algorithm for detecting complex peaks (peaks that occur during the decay period of a previous event) was employed. This algorithm calculates event amplitude for the second (and subsequent) peaks within the decay period based on an extrapolated monoexponential decay from the peak of the initial event. For the kinetic analysis, individual sEPSCs were extracted from raw data over a 3 min baseline period in each cell, and aligned by the midpoint of the rising phase. As a result, one average sEPSC was created for each cell. Data reported for rise time, decay time and area are the mean of data from all individual events in each cell, rather than data from the average event. For these analyses, the rise time of each event was calculated from the time during the rising phase at which amplitude reached 50% of the peak amplitude. Similarly, the decay time for each event was calculated from the time during the decay phase at which amplitude reached 25% of the peak amplitude. Area under the curve was calculated between these two points. A standard unpaired Student’s two-sample t-test for means7 was used to compare corresponding event parameters (e.g. frequency or amplitude, rise time, decay time or area) in like conditions across groups (Ctrl vs. EtOH). Equal variance was assumed unless otherwise indicated by a two sample test for variance on the same data. A one sample t-test for data normalized to the baseline mean was used to evaluate the effect of LY354740 within a group (null hypothesis: mean = 1.0). These tests used data obtained during a 3-min period before application of LY354740, and compared to data obtained 12–15 min after initiation of the bath application. These times were chosen to allow complete wash-in of the compound.
For western blotting data, the total protein integrated optical density was divided by the density of calnexin or beta-tubulin immunoreactivity within the same sample. For both surface and total protein expression data, the integrated density of individual protein bands were first normalized to the control group by computing the average Ctrl density and dividing all samples by this average. This yielded a ‘percent control value’ and these values were compared between groups with independent samples t-tests.
Results
Alcohol consumption
Animals were provided access to alcohol for 24 h/day on alternating days for 20 sessions (Fig. 1A). To confirm that cohorts of rats used in different experiments did not differ in the amount of alcohol consumed, we used a two-way RM ANOVA to compare alcohol consumption over the 20 sessions in each group (data not shown). We found no effect of Group (F4,28 = 1.800, P = 0.530) on g/kg alcohol consumed, no Time × Group interaction (F76,532 = 1.180, P = 0.1551), but a significant effect of Time was detected (F19,532 = 2.100, P = 0.004). A one-way ANOVA revealed no group differences in total amount of alcohol consumed (g/kg) between animals later used for microdialysis, TTX microdialysis, electrophysiology and western blotting studies (F3,22 = 1.113, P = 0.2844; Fig. 1B). Blood was collected from a subset of animals (n = 4) for determination of blood alcohol level following 2 h of drinking: blood alcohol levels ranged from 33.6 to 76.7 mg%. Both controls and ethanol-consuming rats were allotted 20 g/day standard chow, resulting in no significant Group differences in weight (F1,67 = 0.377, P = 0.541; Fig. 1C) over the course of the experiment. Both groups increased body weight over time, reflected by a significant effect of Time (F19,1273 = 233.4, P = 0.000).
Microdialysis
Basal glutamate in the NAc was elevated following 24 h withdrawal from intermittent alcohol consumption. A two-way RM ANOVA conducted on the no-net-flux dialysis data (Fig. 2A) revealed a significant Group × Concentration interaction (F3,42 = 4.614, P = 0.000), a significant effect of Group (F1,14 = 7.068, P = 0.000), and a significant effect of Concentration (F3,42 = 77.70, P = 0.000). Using the point of y = 0 to determine basal glutamate, increased basal extracellular glutamate was observed in EtOH relative to Ctrl animals [Fig. 2B; t(1,14) = 2.704, P = 0.0171]. The slope of the regression line was significantly reduced in the EtOH group relative to the Ctrl group (F1,60 = 12.007, P = 0.000).
To determine whether TTX perfusion altered glutamate levels, a paired samples t-test was used to compare baseline glutamate values (average of three samples prior to TTX) to the average values after TTX (Fig. 2C). We found no effect of TTX perfusion on glutamate levels in either EtOH or Ctrl animals [Fig. 2C; t(1,8) = 0.560, P = 0.5908]. Accurate placement was achieved when 2/3 of the active dialysis membrane was within the NA core. Several rats were excluded from final data analysis of both microdialysis studies due to probe failure during dialysis or misplaced cannulae (n = 13); these rats are not included in the Fig. 1 analysis. Figure 2D indicates the placement of microdialysis probes for the 26 animals whose data comprises Fig. 2A–C.
Electrophysiology
Whole-cell patch clamp recordings were made from medium spiny neurons in the NAc using slices extracted from 5 Ctrl and 5 EtOH animals. We recorded from a total of 44 cells from EtOH rats and 32 cells from Ctrl rats. Representative traces are presented in Fig. 3A. A two-sample test for variance revealed unequal variance for basal sEPSC frequency (t(21,41) = 0.345, P = 0.003) and so an unpaired two sample t-test with Welch’s correction was conducted and revealed that the sEPSC frequency recorded from medium spiny neurons was reduced in slices from EtOH animals relative to controls [Ctrl: 6.9 ± 1.1 Hz, EtOH: 4.0 ± 0.4 Hz, Fig. 3B; t(1,28.79) = 2.388, P = 0.0237]. sEPSC amplitude was unaltered [Fig. 3C; t(1,74) = 1.184, P = 0.240]. Bath application of 1 μM TTX did not have a statistically significant effect on sEPSC frequency in slices from either Ctrl or EtOH animals [Ctrl: 5.7 ± 1.2 Hz during baseline period, 5.7 ± 1.2 Hz after TTX, t(4) = 0.12, P = 0.909; EtOH: 4.6 ± 0.7 Hz during baseline period, 4.1 ± 0.9 Hz after TTX, t(5) = 1.45, P = 0.207], suggesting the majority of sEPSC recorded from medium spiny neurons in this slice preparation are action potential independent. One sample t-tests revealed that bath application of the mGlu2/3 receptor agonist LY354740 strongly reduced sEPSC frequency [Fig. 3D and E; Ctrl: t(4) = 6.77, P = 0.002; EtOH: t(9) = 17.04, P = 0.000] and mEPSC frequency [Fig. 3F and G; Ctrl: t(3) = 10.64, P = 0.002; EtOH: t(4) = 5.73, P = 0.005] relative to baseline in slices from Ctrl animals. Student’s t-tests were used to compare the effects of LY on sEPSC and mEPSC frequency and these effects were unaltered in slices from control and EtOH rats [Fig. 3E and G; sEPSC: t(1,13) = 0.506, P = 0.621); mEPSC: t(1,7) = 1.690, P = 0.135]. In contrast, LY354740 had no significant effect on sEPSC [Ctrl: t(4) = 2.12, P = 0.10; EtOH: t(9) = 1.15, P = 0.28, data not shown] or mEPSC amplitude [Ctrl: t(3) = 0.36, P = 0.74; EtOH: t(4) = 1.05, P = 0.92, data not shown). We also performed a detailed kinetic analysis of spontaneous sEPSCs observed in control vs. alcohol-consuming animals, but noted no significant difference in rise time [t(1,74) = 0.9736, P = 0.3515], decay time [t(1,74) = 0.08036, P = 0.4242] or area [t(1,74) = 0.9867, P = 0.327].
Western blotting
The NAc membrane surface expression of GluA1, mGlu2/3 and GLT-1 were quantified following 24 h withdrawal from alcohol. The surface expression of GLT-1 was not altered following 24 h withdrawal from intermittent access to alcohol [Fig. 4A; t(1,13) = 0.682, P = 0.507]. Surface expression of GluA1 was significantly increased in the NAc after 24 h withdrawal from EtoH [Fig. 4B; t (1,12) = 2.371, P = 0.0353]. There were no differences in mGlu2/3 surface expression [Fig. 4C, t(1,12) = 0.301, P = 0.7687]. There were no significant group differences in total protein expression of these proteins [GLT-1: t(1,14) = 0.89, P = 0.5357; mGlu2/3: t (1,12) = 1.171, P = 0.2642; GluA1: t(1,12) = 0.799, P = 0.4387] nor of xCT: t(1,14) = 0.925, P = 0.3703, data not shown].
Discussion
The IAA paradigm provides rodents access to high concentrations of unsweetened EtOH intermittently, with periods of withdrawal interspersed with 2-bottle choice (EtOH and water). Previously, in Long-Evans, Sprague–Dawley and Wistar rats, this method has been demonstrated to engender EtOH consumption in the range of 4.5 g/kg/24 h only after 9–12 sessions (for review see Carnicella et al., 2014). Initial EtOH intake here was much higher than that observed in previous studies (Fig. 1A). This effect may have been due to food restriction as the rats in this study were restricted to 20 g chow/day while those in previous studies utilizing the IAA paradigm were permitted ad libitum access to food (e.g. Carnicella et al., 2008; Simms et al., 2008). Food restriction has previously been shown to increase cocaine self-administration in rodents (e.g. Carroll, 1985) and psychostimulants in monkeys (de la Garza & Johanson, 1987). Thus, it is possible that food restriction contributed to the high level consumption of EtOH here in the first IAA sessions. However, the level of food restriction utilized here permitted normal growth patterns, with average weights falling within the standard deviation for Charles River Sprague–Dawley rats (Fig. 1C). It is also possible that the high initial intake observed here is due to factors related to our laboratory procedures or supplier, as these inter-laboratory factors have been shown to affect EtOH intake in rats (for review see Carnicella et al., 2014).
Here, we demonstrate for the first time that voluntary alcohol consumption in outbred rats increases basal extracellular glutamate in the NA core (Fig. 2A and B). Similar results have been found after 24 h withdrawal from systemic alcohol injections in the NA core (Melendez et al., 2005) and shell (Kapasova & Szumlinski, 2008). Basal glutamate has also been reported to be increased in the NA shell following 3–4 h withdrawal from voluntary alcohol consumption in inbred female P rats (Ding et al., 2013). The same effect was observed in male P rats within 20 h of the last drinking session (Das et al., 2015). Furthermore, in mice exposed to non-contingent alcohol via vapor chambers in combination with voluntary consumption, basal extracellular glutamate in the NA is elevated relative to animals that self-administered alcohol without vapor chamber exposure (Griffin et al., 2014). Thus, regardless of strain or alcohol exposure regimen, brief withdrawal (24 h or less) from alcohol administration consistently increases basal extracellular glutamate in the NA as detected by no-net-flux microdialysis. Confirming that this increase occurs after both contingent and non-contingent administration is important, as the contingency of administration of other drugs such as cocaine has been demonstrated to determine the direction of glutamate change during self-administration (Suto et al., 2010). The increase in basal extracellular glutamate levels at the time at which animals would typically be presented with alcohol and initiate drinking indicates that the increase in glutamate may drive the consumption of alcohol. In support of this idea, pharmacological inhibition of glutamate uptake in the NA significantly increases in the amount of alcohol consumed in mice which had a history of alcohol consumption (Kapasova & Szumlinski, 2008; Griffin et al., 2014). While these researchers targeted the NA shell in these studies, the use of a pharmacological strategy does not allow the discrimination between shell and core of the NA in mice and thus it is unclear if both NA subcompartments mediate this effect. Other reports have found that the increase in basal extracellular glutamate does not persist into late withdrawal (Ding et al., 2013), or even 48 h after the last alcohol exposure (Szumlinski et al., 2008), implying that elevated basal extracellular NA glutamate is not necessary for drinking. However, based on the extensive literature implicating long-term glutamate changes following alcohol in the motivation to seek alcohol (for review, see Gass & Olive, 2008), it is possible that increased basal extracellular glutamate early in withdrawal sets the stage for subsequent glutamate adaptations that do promote alcohol intake.
Upon confirming the increase in NAc basal extracellular glutamate levels observed in early withdrawal from EtOH exposure in other rodent strains following multiple EtOH administration regimens, we next sought to determine the source of this glutamate. One such proposed source has been increased synaptic release of glutamate. Reverse dialysis of TTX into the NAc did not alter glutamate levels (Fig. 2C), indicating that increased action potential-dependent release of glutamate does not account for increased basal extracellular glutamate following intermittent alcohol consumption.
While we did not observe decreased surface GLT-1 expression following 24 h withdrawal from EtOH (Fig. 4A), we did find a significant reduction in slope of the line of regression for the no-net-flux (NNF) dialysis plots at this time (Fig. 2A), which reflects a change in the extraction fraction (Ed). For the neurotransmitters dopamine and acetylcholine, Ed has been empirically demonstrated to be a measure of clearance (Smith & Justice, 1994; Vinson & Justice, 1997) but this may not be the case for glutamate. Glutamate concentrations in the NA are regulated by multiple mechanisms, including reuptake via the sodium-dependent transporters (e.g. GLT-1) and release via glia (e.g. system xC-). Chen (2006) reported that the Ed is determined by the ratio of neurotransmitter release to reuptake. Thus, in the case where a reduction in glutamate reuptake is not accompanied by changes in other glutamate release mechanisms, a relationship between Ed and slope may be an accurate index of clearance and thus transporter function. But in the case where multiple mediators of glutamate release and reuptake are altered, this is not an accurate index. A reduction in slope of the no-net-flux function was observed following repeated injections of alcohol in B2 mice without an effect on basal extracellular glutamate levels (Kapasova & Szumlinski, 2008). Thus, while the reduction in slope demonstrated here (Fig. 2A) indicates that GLT-1 function may be reduced after alcohol, a clear relationship between Ed and glutamate uptake has yet to be established.
There are contradicting reports regarding the contribution of GLT-1 adaptations to the increased basal extracellular glutamate observed after short withdrawal from EtOH exposure. We recently demonstrated that mice with elevated NA basal extracellular glutamate following chronic intermittent alcohol vapor exposure did not demonstrate alterations in glutamate transport as assessed by a glutamate uptake assay that quantifies GLT-1 and GLAST activity (Griffin et al., 2015). Melendez et al. (2005) reported decreased sodium-dependent glutamate transport and GLT-1 protein expression accompanying increased NAc basal extracellular glutamate levels after 24 h withdrawal from 7 days of alcohol injections. GLT-1 expression in the NA is significantly reduced in continuously drinking male P rats (Sari & Sreemantula, 2012; Sari et al., 2013; Alhaddad et al., 2014a,b) but not in female P rats (Ding et al., 2013). Here, we did not find a change in GLT-1 surface expression following intermittent EtOH consumption. It is possible that the intermittent nature of EtOH access utilized here and our previous work (Griffin et al., 2015) underlies the lack of changes in GLT-1 expression, as the reports of GLT-1 downregulation have occurred following continuous non-contingent administration (Melendez et al., 2005) or home-cage consumption (Sari & Sreemantula, 2012; Sari et al., 2013; Alhaddad et al., 2014a,b). Furthermore, the GLT-1 assessments made in male P rats did not involve withdrawal; rats were killed following continuous access (Sari & Sreemantula, 2012; Sari et al., 2013; Alhaddad et al., 2014a,b). It should be noted that Melendez et al. (2005) is the only report in which the functional consequences of decreased GLT-1 expression was confirmed with a glutamate uptake assay. Thus, GLT-1 expression following alcohol is likely influenced by withdrawal periods.
Here, we did not find changes in total xCT expression following 24 h withdrawal from EtOH (data not shown). We have previously shown that the increased NA basal extracellular glutamate induced by chronic intermittent EtOH exposure via vapor chambers does not arise from increased system xC-activity (Griffin et al., 2015). Ding et al. (2013) also found no changes in NA xCT expression in female P rats at a time when basal extracellular glutamate levels were increased, indicating that system xC-upregulation is likely not the source of increased basal extracellular glutamate. Interestingly, Alhaddad et al. (2014a) found decreases in xCT expression in the NA at a time when the same group observes increases in NA glutamate in continuously drinking male P rats (Das et al., 2015). Thus, the present results are consistent with the literature in finding that xCT/system xC- upregulation likely does not account for the increase in basal extracellular glutamate levels observed during brief withdrawal from ethanol consumption or chronic intermittent EtOH exposure.
Surprisingly, we did not find a potentiation of synaptic transmission in the NAc following 24 h withdrawal from 7 weeks of alcohol consumption as has been demonstrated after withdrawal from 2 weeks of cocaine (Trantham-Davidson et al., 2012), nicotine (Gipson et al., 2013) and heroin (Shen et al., 2011) self-administration. Instead we observed a small but significant decrease in sEPSC frequency and no change in sEPSC amplitude (Fig. 3). Considering both the lack of effect of TTX on glutamate levels (Fig. 2C) and the failure to observe an increase in sEPSC frequency in EtOH rats, the increase in NAc basal extracellular glutamate observed here in EtOH rats does not arise from an increase in action potential-dependent presynaptic glutamate release. In agreement with our data, following 12 h withdrawal from forced alcohol consumption (via liquid diet), a reduction in mEPSC frequency in the NA shell has been observed (Spiga et al., 2014). As bath application of the mGlu2/3 agonist LY379268 decreased EPSC frequency in both control and alcohol conditions (Fig. 3D–G), a loss of function of these release-regulating receptors is also not contributing to increased basal extracellular glutamate in our model. In line with this finding, there was no change in surface expression of mGlu2/3 receptors (Fig. 4C). Thus, our results are the first to negate the possibility that increased action potential-dependent release of glutamate underlies increases in basal extracellular glutamate in early withdrawal from EtOH.
We found an increase in surface expression of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor subunit GluA1 (Fig. 4B) in EtOH animals which indicates possible changes in AMPA receptor function after alcohol. Spiga et al. (2014) reported an increase in AMPA-mediated currents in the NA shell at 12 h withdrawal from alcohol. In light of these findings, we performed post hoc analysis of the sEPSC kinetics and found no differences between controls and alcohol-consuming rats in rise time, decay time or area of sEPSCs (data not shown). These results are not necessarily in conflict as AMPA receptor trafficking is a complicated process and increased GluA1 expression may partially or wholly reflect expression at perisynaptic or extrasynaptic sites that would be expected to have minimal impact on kinetics of spontaneous EPSCs. For example, in hippocampal slices, increased surface GluA1 did not result in changes in AMPA currents (He et al., 2011). Furthermore, work in cultured hippocampal neurons indicates that GluA1 is initially inserted into extrasynpatic sites and subsequently recruited to the synapse (Passafaro et al., 2001). Future experiments could test the possibility that changes in GluA1 expression would impact overall AMPA receptor rectification index, or impact induction of synaptic plasticity. In agreement with our finding of elevated GluA1 expression, Ary et al. (2012) observed a similar effect in the NA of B6 mice after 1 month of alcohol consumption.
In conclusion, we have shown that the increase in NA core basal extracellular glutamate previously demonstrated only in alcohol-preferring strains or following non-contingent alcohol administration is also present following 24 h withdrawal from intermittent access to alcohol in the outbred Sprague–Dawley rat. The increased basal extracellular glutamate did not stem from changes in glutamate transporter expression, however, the no-net-flux microdialysis results indicate a possible reduction in glutamate uptake in EtOH rats. Decreased GLT-1 expression has been reported in studies utilizing continuous daily administration of EtOH without withdrawal (Sari & Sreemantula, 2012; Sari et al., 2013; Alhaddad et al., 2014a,b) but not in those utilizing intermittent EtOH exposure and a period of withdrawal (Fig. 4A and Griffin et al., 2015). Thus, periods of withdrawal from EtOH likely prevent GLT-1 adaptations. While we were not able to conclusively determine the source of NAc glutamate, the present results rule out two sources that have been previously proposed to account for this increase in glutamate: a loss of function of mGlu2/3 autoreceptors and sodium-dependent presynaptic release. Other possibilities for mediators of elevated basal extra-cellular glutamate following EtOH include calcium-dependent release from the presynaptic terminal or glia, as the N-type calcium channel has also been found to regulate basal extracellular glutamate in drug-naïve rats (Baker et al., 2002). Furthermore, the mGlu5 antagonist MPEP prevents the EtOH-induced rise in NA glutamate observed in vehicle-treated animals (Lominac et al., 2006). As astrocytic mGlu5 regulate calcium-dependent glutamate release in the NA (D’Ascenzo et al., 2007), future work examining the role of mGlu5 receptors in basal extracellular glutamate increases in the NA is warranted. Identifying the source of increased basal extracellular glutamate would be an important preclinical finding, as medications to reduce postalcohol glutamate levels would like reduce alcohol-seeking.
Acknowledgments
The authors thank Lizhen Wu and Kristine Hill for their technical assistance. This work was funded in part by a pilot award from the Charleston Alcohol Research Center (P50 AA010761).
Abbreviations
- Ctrl
control
- Ed
extraction fraction
- EtOH
ethanol
- IAA
intermittent access to alcohol
- mEPSC
miniature excitatory postsynaptic current
- mGlu2/3
metabotropic glutamate receptor 2/3
- mGlu5
metabotropic glutamate receptor 5
- NA
nucleus accumbens
- RM ANOVA
repeated measures two-way analyses of variance
- sEPSC
spontaneous excitatory postsynaptic current
- TTX
tetrodotoxin
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
References
- Alhaddad H, Das SC, Sari Y. Effects of ceftriaxone on ethanol intake: a possible role for xCT and GLT-1 isoforms modulation of glutamate levels in P rats. Psychopharmacology. 2014a;231:4049–4057. doi: 10.1007/s00213-014-3545-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaddad H, Kim NT, Aal-Aaboda M, Althobaiti YS, Leighton J, Boddu SH, Wei Y, Sari Y. Effects of MS-153 on chronic ethanol consumption and GLT1 modulation of glutamate levels in male alcohol-preferring rats. Front Behav Neurosci. 2014b;8:366. doi: 10.3389/fnbeh.2014.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ary AW, Cozzoli DK, Finn DA, Crabbe JC, Dehoff MH, Worley PF, Szumlinski KK. Ethanol up-regulates nucleus accumbens neuronal activity dependent pentraxin (Narp): implications for alcohol-induced behavioral plasticity. Alcohol. 2012;46:377–387. doi: 10.1016/j.alcohol.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Kharazia V, Jeanblanc J, Janak PH, Ron D. GDNF is a fast-acting potent inhibitor of alcohol consumption and relapse. Proc Natl Acad Sci USA. 2008;23:8114–8119. doi: 10.1073/pnas.0711755105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Ron D, Barak S. Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol. 2014;48:243–252. doi: 10.1016/j.alcohol.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME. The role of food deprivation in the maintenance and reinstatement of cocaine-seeking behavior in rats. Drug Alcohol Depen. 1985;16:95–109. doi: 10.1016/0376-8716(85)90109-7. [DOI] [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Chen KC. Effects of tissue trauma on the characteristics of micro-dialysis zero-net-flux method sampling neurotransmitters. J Theor Biol. 2006;238:863–881. doi: 10.1016/j.jtbi.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Das SC, Yamamoto BK, Hristov AM, Sari Y. Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology. 2015;97:67–74. doi: 10.1016/j.neuropharm.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ascenzo M, Fellin T, Terunuma M, Revilla-Sanchez R, Meaney DF, Auberson YP, Moss SJ, Haydon PJ. mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc Natl Acad Sci USA. 2007;104:1995–2000. doi: 10.1073/pnas.0609408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Rodd ZA, Engleman EA, Bailey JA, Lahiri DK, McBride WJ. Alcohol drinking and deprivation alter basal extra-cellular glutamate concentrations and clearance in the mesolimbic system of alcohol-preferring (P) rats. Addict Biol. 2013;18:297–306. doi: 10.1111/adb.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Garza R, Johanson CE. The effects of food deprivation on the self-administration of psychoactive drugs. Drug Alcohol Depen. 1987;19:17–27. doi: 10.1016/0376-8716(87)90083-4. [DOI] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Sinclair CM, Cleva RM, Widholm JJ, Olive MF. Alcohol-seeking behavior is associated with increased glutamate transmission in basolateral amygdala and nucleus accumbens as measured by glutamate-oxidase-coated biosensors. Addict Biol. 2011;16:215–228. doi: 10.1111/j.1369-1600.2010.00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Reissner KJ, Kupchik YM, Smith AC, Stankeviciute N, Hensley-Simon ME, Kalivas PW. Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proc Natl Acad Sci USA. 2013;110:9124–9129. doi: 10.1073/pnas.1220591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, Haun HL, Hazelbaker CL, Ramachandra VS, Becker HC. Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice. Neuropsy-chopharmacol. 2014;39:707–717. doi: 10.1038/npp.2013.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, Ramachandra VS, Knackstedt LA, Becker HC. Repeated cycles of chronic intermittent ethanol exposure increases basal extracellular glutamate in the nucleus accumbens of mice without affecting glutamate transport. Front Pharmacol. 2015;6:27. doi: 10.3389/fphar.2015.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K, Goel A, Ciarkowski CE, Song L, Lee HK. Brain area specific regulation of synaptic AMPA receptors by phosphorylation. Commun Integr Biol. 2011;4:569–572. doi: 10.4161/cib.4.5.15890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZG, North RA. Membrane properties and synaptic responses of rat striatal neurones in vitro. J Physiol. 1991;443:533–553. doi: 10.1113/jphysiol.1991.sp018850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapasova Z, Szumlinski KK. Strain differences in alcohol-induced neurochemical plasticity, a role for accumbens glutamate in alcohol intake. Alcohol Clin Exp Res. 2008;32:617–631. doi: 10.1111/j.1530-0277.2008.00620.x. [DOI] [PubMed] [Google Scholar]
- Kłodzinska A, Bijak M, Tokarski K, Pilc A. Group II mGlu receptor agonists inhibit behavioural and electrophysiological effects of DOI in mice. Pharmacol Biochem Be. 2002;73:327–332. doi: 10.1016/s0091-3057(02)00845-6. [DOI] [PubMed] [Google Scholar]
- Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC. Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. J Neurosci. 1995;15:1835–1853. doi: 10.1523/JNEUROSCI.15-03-01835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lominac KD, Kapasova Z, Hannun RA, Patterson C, Middaugh LD, Szumlinski KK. Behavioral and neurochemical interactions between Group 1 mGluR antagonists and ethanol: potential insight into their anti-addictive properties. Drug Alcohol Depen. 2006;85:142–156. doi: 10.1016/j.drugalcdep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, Hicks MP, Cagle SS, Kalivas PW. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcohol Clin Exp Res. 2005;29:326–333. doi: 10.1097/01.alc.0000156086.65665.4d. [DOI] [PubMed] [Google Scholar]
- Passafaro M, Piëch V, Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci. 2001;4:917–926. doi: 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6. Academic/Elsevier; Amsterdam: 2007. [Google Scholar]
- Sari Y, Sreemantula SN. Neuroimmunophilin GPI-1046 reduces ethanol consumption in part through activation of GLT1 in alcohol-preferring rats. Neuroscience. 2012;227:327–335. doi: 10.1016/j.neuroscience.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sreemantula SN, Lee MR, Choi DS. Ceftriaxone treatment affects the levels of GLT1 and ENT1 as well as ethanol intake in alcohol-preferring rats. J Mol Neurosci. 2013;51:779–787. doi: 10.1007/s12031-013-0064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther. 2001;299:12–20. [PubMed] [Google Scholar]
- Shen H, Moussawi K, Zhou W, Toda S, Kalivas PW. Heroin relapse requires long-term potentiation-like plasticity mediated by NMDA2b-containing receptors. Proc Natl Acad Sci USA. 2011;108:19407–19412. doi: 10.1073/pnas.1112052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AD, Justice JB. The effect of inhibition of synthesis, release, metabolism and uptake on the microdialysis extraction fraction of dopamine. J Neurosci Meth. 1994;54:75–82. doi: 10.1016/0165-0270(94)90161-9. [DOI] [PubMed] [Google Scholar]
- Spiga S, Talani G, Mulas G, Licheri V, Fois GR, Muggironi G, Masala N, Cannizzaro C, et al. Hampered long-term depression and thin spine loss in the nucleus accumbens of ethanol-dependent rats. Proc Natl Acad Sci USA. 2014;111:E3745–E3754. doi: 10.1073/pnas.1406768111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto N, Ecke LE, You ZB, Wise RA. Extracellular fluctuations of dopamine and glutamate in the nucleus accumbens core and shell associated with lever-pressing during cocaine self-administration, extinction, and yoked cocaine administration. Psychopharmacology. 2010;211:267–275. doi: 10.1007/s00213-010-1890-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Ary AW, Lominac KD, Klugmann M, Kippin TE. Accumbens Homer2 overexpression facilitates alcohol-induced neuroplasticity in C57BL/6J mice. Neuropsychopharmacol. 2008;33:1365–1378. doi: 10.1038/sj.npp.1301473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, LaLumiere RT, Reissner KJ, Kalivas PW, Knackstedt LA. Ceftriaxone normalizes nucleus accumbens synaptic transmission, glutamate transport, and export following cocaine self-administration and extinction training. J Neurosci. 2012;32:12406–12410. doi: 10.1523/JNEUROSCI.1976-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson PN, Justice JB., Jr Effect of neostigmine on concentration and extraction fraction of acetylcholine using quantitative microdialysis. J Neurosci Meth. 1997;73:61–67. doi: 10.1016/s0165-0270(96)02213-3. [DOI] [PubMed] [Google Scholar]
- Zhai Y, George CA, Zhai J, Nisenbaum ES, Johnson MP, Nisenbaum LK. Group II metabotropic glutamate receptor modulation of DOI-induced c-fos mRNA and excitatory responses in the cerebral cortex. Neuropsychopharmacol. 2003;28:45–52. doi: 10.1038/sj.npp.1300013. [DOI] [PubMed] [Google Scholar]