Abstract
Rationale: The rate of decline of lung function is greater than age-related change in a substantial proportion of patients with chronic obstructive pulmonary disease, even after smoking cessation. Regions of the lung adjacent to emphysematous areas are subject to abnormal stretch during respiration, and this biomechanical stress likely influences emphysema initiation and progression.
Objectives: To assess whether quantifying this penumbra of lung at risk would predict FEV1 decline.
Methods: We analyzed paired inspiratory-expiratory computed tomography images at baseline of 680 subjects participating in a large multicenter study (COPDGene) over approximately 5 years. By matching inspiratory and expiratory images voxel by voxel using image registration, we calculated the Jacobian determinant, a measure of local lung expansion and contraction with respiration. We measured the distance between each normal voxel to the nearest emphysematous voxel, and quantified the percentage of normal voxels within each millimeter distance from emphysematous voxels as mechanically affected lung (MAL). Multivariable regression analyses were performed to assess the relationship between the Jacobian determinant, MAL, and FEV1 decline.
Measurements and Main Results: The mean (SD) rate of decline in FEV1 was 39.0 (58.6) ml/yr. There was a progressive decrease in the mean Jacobian determinant of both emphysematous and normal voxels with increasing disease stage (P < 0.001). On multivariable analyses, the mean Jacobian determinant of normal voxels within 2 mm of emphysematous voxels (MAL2) was significantly associated with FEV1 decline. In mild-moderate disease, for participants at or above the median MAL2 (threshold, 36.9%), the mean decline in FEV1 was 56.4 (68.0) ml/yr versus 43.2 (59.9) ml/yr for those below the median (P = 0.044).
Conclusions: Areas of normal-appearing lung are mechanically influenced by emphysematous areas and this lung at risk is associated with lung function decline.
Clinical trial registered with www.clinicaltrials.gov (NCT00608764).
Keywords: FEV1, emphysema, image registration, biomechanical, lung at risk
At a Glance Commentary
Scientific Knowledge on the Subject
The rate of decline of lung function is greater than age-related change in a substantial proportion of patients with chronic obstructive pulmonary disease, even after smoking cessation. Regions of the lung adjacent to emphysematous areas are subject to abnormal stretch during respiration, and this biomechanical stress likely influences emphysema initiation and progression. Whether the regions of the lung surrounding emphysematous areas constitute lung at risk for disease progression is not known.
What This Study Adds to the Field
In participants with emphysema, normal-appearing lung regions on computed tomography have abnormal regional expansion, and there is a spatial relationship between areas of emphysema and abnormal lung mechanics resulting in a penumbra of mechanically affected lung. Quantifying the amount of mechanically affected lung can independently predict lung function change over time.
Chronic obstructive pulmonary disease (COPD) is characterized by airflow obstruction, and the rate of decline of lung function is greater than age-related changes in a substantial proportion of patients, even after smoking cessation (1). COPD is the third leading cause of death in the United States and is associated with significant morbidity and health care costs (1). Progression of disease in COPD is variable, and although some patients have a relatively slow rate of lung function deterioration, others suffer an inexorable decline resulting in significant symptoms, respiratory failure, and mortality (2). Despite significant advances in phenotyping COPD and the development of markers to predict exacerbations and mortality (3), there is unfortunately a distinct lack of biomarkers that can help identify early disease and predict disease progression (4).
FEV1 is affected by resistance to airflow in the small airways and the elastic recoil of the lung parenchyma (5). The dominant pathogenetic mechanisms for COPD have involved proteinase-antiproteinase imbalance, oxidative stress, and inflammation (6, 7); however, biomarkers related to these pathways have not been shown to be useful in predicting disease progression. A less explored mechanism for emphysema initiation and progression is mechanical forces and stress fatigue (8). It is plausible that emphysema begets more emphysema, as regions of the lung adjacent to emphysematous regions are subject to abnormal forces of stretch during tidal respiration (9–13). At end expiration, the alveolar walls are under significant mechanical stress, and cyclical breathing imposes additional mechanical forces on already weakened elastin and collagen fibers, which over a long time can result in rupture of the alveolar wall in surrounding susceptible regions, effectively creating a penumbra (9, 12). Heterogeneity in regional lung expansion in COPD might be a reflection of these mechanical forces exerted by emphysematous areas.
Using image registration on paired inspiratory-expiratory images, voxels on inspiratory images can be matched with corresponding voxels on expiratory images, and a biomechanical metric called the Jacobian determinant, a measure of regional parenchymal volume change with respiration, can be calculated (14, 15). We hypothesized that regions of the lung in the penumbra around the areas of emphysematous lung would have abnormal mechanics, and that heterogeneity of regional lung expansion is associated with lung function decline. Using this metric for estimating lung mechanics, we assessed the areas surrounding emphysematous regions with normal appearance but with abnormal mechanics, which we called the mechanically affected lung (MAL). We also hypothesized that by enabling estimation of the regions subject to abnormal stretch, the percentage of MAL would predict lung function decline.
Methods
Study Population
We included 714 participants with Global Initiative for Chronic Obstructive Lung Disease (GOLD) 1–4 disease enrolled in the COPDGene (Genetic Epidemiology of COPD) study and who completed a second COPDGene visit approximately 5 years after the first visit by November 2014 (see Figure E1 in the online supplement). COPDGene is a large multicenter cohort of current and former smokers aged 45–80 years and with 10 or more pack-year smoking history; details of this study have been previously published (16). Participants with known lung disease other than COPD and asthma, or prior lobar excision, active malignancy, and chest radiation were excluded. All participants provided written informed consent and the study was approved by the institutional review boards of all 21 centers.
At enrollment and at follow-up, spirometry was performed before and after administration of 180 μg of albuterol (Easy-One spirometer; ndd, Andover, MA). Post-bronchodilator ratio of FEV1 to FVC less than 0.70 at baseline was used to diagnose COPD, and severity of the disease was graded per the GOLD recommendations (1). Volumetric computed tomography (CT) scans were obtained at full inspiration (total lung capacity [TLC]) and end-tidal expiration (FRC). Subjects with CT images acquired at residual volume at one center were excluded (see online supplement). CT images were analyzed using Apollo software (VIDA Diagnostics, Inc., Coralville, IA) (16). Percentage emphysema was quantified by the low attenuation units less than −950 Hounsfield units (HU) at TLC, and gas trapping using the percentage of low attenuation units less than −856 HU at FRC using density mask analyses. Airway disease was quantified by the wall area percentage of segmental airways (WA%) (16).
CT Image Registration
Using image registration, we quantified regional lung expansion over 1 mm3 volumes. Image registration is a technique that finds the spatial relationship between two images (see online supplement) (14, 17). Briefly, after extraction of the airways, the FRC image is held fixed and the TLC image is transformed into the same coordinate system as the fixed image, thereby matching the images voxel for voxel. A lung mass-preserving registration method is used to capture local volume changes between the two phases. A deformation matrix is created to measure the deformation of lung tissue between inspiration and expiration (14, 17). We calculated a derived variable termed the Jacobian determinant (see Figure E2), a measure of the regional volume change (across 1 mm3) caused by deformation of the lung from TLC to FRC; values range from 0 to infinity. Jacobian determinant greater than one indicates local expansion and less than one implies local contraction. We created deformation maps for the entire lung using the Jacobian determinant that included regions of emphysema and normal lung without emphysema. To test our hypothesis that areas of emphysematous lung influence the mechanics of normal regions of the lungs, we calculated the Jacobian determinant separately for emphysematous and normal regions of the lung, and the coefficient of variation of the Jacobian determinant (CV Jacobian) to estimate the heterogeneity of regional expansion of the lung.
Spatial Relationship: Emphysema and Normal Regions
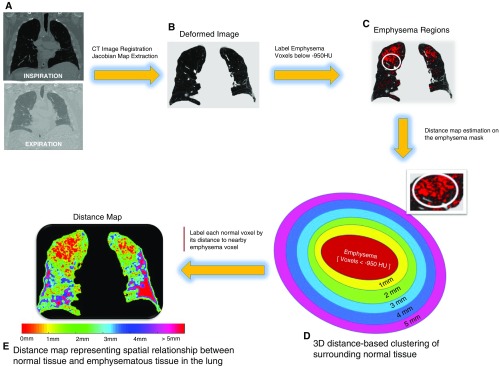
To improve anatomic localization, we created a distance map by plotting the Euclidean distance between each normal voxel to the nearest voxel with emphysema (Figure 1). The percentage of normal voxels within each millimeter distance from emphysematous voxel was quantified (MAL).
Figure 1.
Graphic representation of the spatial mapping methodology showing spatial mapping for a representative subject. (A) Deformed inspiratory image with (B) emphysema regions (low-attenuation areas less than −950 Hounsfield units [HU]) highlighted in red on inspiratory computed tomography scan. (C) Example of emphysematous region in the lung. (D) Three-dimensional Euclidean distance-based clustering: distance was calculated for each normal voxel to the nearest emphysema voxel in three dimensions, and the surrounding normal tissue was clustered into different groups based on their spatial distance to the nearest emphysema voxels. Clustering of the surrounding tissue was performed in 1-mm increments up to 5 mm based on distance to nearest emphysematous voxels. (E) Color map representing the distances of normal voxels to the nearby emphysematous regions, from 1 to 5 mm. Red represents emphysema regions, labeled as 0 mm in the color scale. CT = computed tomography.
Statistical Analyses
Univariate comparisons were made using two-tailed independent Student’s t tests and analyses of variance with Tukey post hoc comparisons for continuous variables, and chi-square test for categorical variables. The primary outcome of change in FEV1 was calculated by subtracting FEV1 at enrollment from FEV1 at follow-up, and annualized. Univariate and multivariable associations were tested between the CV of Jacobian determinant (separately for whole lung and normal areas) and change in FEV1, after adjustment for age, race, sex, body mass index (BMI), current smoking status, pack-years of smoking, and CT scanner protocol, as well as baseline FEV1 and CT emphysema, gas trapping, and WA%. To test for association between spatial distributions of normal voxels in relation to emphysematous voxels, multivariable regression analysis was performed to find independent associations between change in FEV1 and mean Jacobian determinant of MAL in 1-mm incremental distance from emphysematous voxels. No adjustments were made for multiple comparisons. All analyses were performed using Statistical Package for the Social Sciences (SPSS 22.0; SPSS Inc., Chicago, IL) and R statistical software version 3.2.
Results
Subject Characteristics
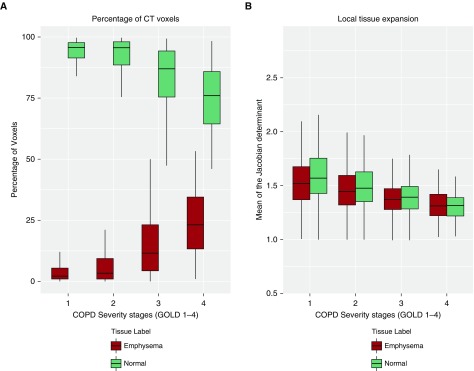
Baseline data for 680 participants with complete CT data are reported in Table 1 (see Figure E1). The mean (SD) age was 63.0 (8.4), 305 (44.9%) were female, and 167 (24.6%) were of African American race (see Table E1). A total of 256 (37.6%) were current smokers, and the average smoking pack-years was 49.8 (23.9). Participants encompassed the spectrum of disease severity with 120 (17.6%), 308 (45.3%), 190 (27.9%), and 62 (9.1%) having GOLD stages 1–4, respectively. Participants were followed for a median duration of 63 months (interquartile range, 60–66). The mean (SD) rate of decline in FEV1 was 39.0 (58.6) ml/yr. Those with GOLD grade 1 had the most rapid rate of decline, 54.3 (61.2) ml/yr. The rate of FEV1 decline was slower with increasing GOLD grades: 45.6 (63.9), 28.8 (48.5), and 8.0 (33.3) for GOLD grades 2–4, respectively (trend test for grades 1–4; P < 0.001). Figure 2A shows that with increasing disease severity, there was progressive increase in CT emphysema and a decrease in the percentage of normal voxels.
Table 1.
Univariate and Multivariable Associations with FEV1
| Parameter | Univariate Regression |
Multivariable Regression |
||
|---|---|---|---|---|
| Regression Coefficient β (95% CI) | P Value | Regression Coefficient β (95% CI) | P Value | |
| Age, yr | −0.017 (−0.024 to −0.011) | <0.001 | −0.017 (−0.022 to −0.012) | <0.001 |
| African American race | −0.100 (−0.230 to 0.030) | 0.132 | −0.212 (−0.302 to −0.123) | <0.001 |
| Female sex | −0.597 (−0.701 to −0.494) | <0.001 | −0.570 (−0.644 to −0.496) | <0.001 |
| BMI, kg/m2 | −0.001 (−0.011 to 0.009) | 0.888 | −0.016 (−0.024 to −0.009) | <0.001 |
| Smoking pack-years | −0.002 (−0.004 to 0.001) | 0.201 | −0.001 (−0.125 to 0.001) | 0.701 |
| Current smoker | 0.276 (0.162 to 0.390) | <0.001 | −0.033 (−0.125 to 0.058) | 0.473 |
| CT scanner type | −0.144 (−0.306 to 0.016) | 0.078 | 0.117 (0.011 to 0.222) | 0.029 |
| CT emphysema, % | −0.027 (−0.031 to −0.022) | <0.001 | −0.015 (−0.020 to −0.009) | <0.001 |
| CT gas trapping, % | −0.019 (−0.022 to −0.017) | <0.001 | −0.012 (−0.016 to −0.009) | <0.001 |
| Segmental airway wall area, % | −0.081 (−0.099 to −0.064) | <0.001 | −0.066 (−0.079 to −0.052) | <0.001 |
| CV of Jacobian determinant, whole lung | 3.412 (2.658 to 3.626) | <0.001 | 0.821 (0.364 to 1.278) | <0.001 |
Definition of abbreviations: BMI = body mass index; CI = confidence interval; CT = computed tomography; CV = coefficient of variation.
Adjusted R2 = 0.61.
Figure 2.
Comparison of lung density and mechanics by Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage for emphysematous and normal voxels. (A) With increasing severity of chronic obstructive pulmonary disease by GOLD grade, the percentage of voxels of the lung parenchyma with density less than −950 Hounsfield units (computed tomography emphysema) increases, whereas the number of normal voxels progressively decreases. (B) With progressive disease severity the mean Jacobian determinant, a measure of regional lung expansion, of the emphysematous voxels progressively decreases. Of note, the mean Jacobian determinant of the normal voxels also decreases with progressive GOLD grade. COPD = chronic obstructive pulmonary disease; CT = computed tomography.
Mechanics of Lung Parenchyma and Lung Function
Figure 2B shows that there was a progressive decrease in the mean Jacobian determinant of voxels with emphysema with increasing GOLD grade (P < 0.001 for overall trend and between group differences). Notably, there was also a similar decrease in the mean Jacobian determinant of normal voxels with increasing disease severity (P < 0.001). The mean Jacobian determinant of the entire lung was associated with FEV1 on univariate analysis (regression coefficient β = 1.063; 95% confidence interval [CI], 0.834–1.291; P < 0.001) but not after adjustment for age, race, sex, BMI, current smoking status, pack-years of smoking, CT scanner protocol, CT emphysema, CT gas trapping, and WA% (adjusted β = 0.021; 95% CI, −0.171 to 0.213; P = 0.830).
To test our hypothesis of emphysematous voxels affecting the mechanics of normal voxels with the assumption that a greater number of emphysematous areas would result in more heterogeneous regional mechanics, we tested the association between the CV of Jacobian determinant and FEV1. CV of Jacobian determinant for the entire lung, including both emphysematous and normal voxels, was associated with FEV1 on univariate and multivariable analyses, after adjustment for age, race, sex, BMI, current smoking status, pack-years of smoking, CT scanner protocol, CT emphysema, CT gas trapping, and WA% (adjusted β = 0.821; 95% CI, 0.364 to 1.278; P < 0.001), consistent with observations with progressive GOLD stage (Table 1). The CV of Jacobian determinant for normal voxels was also associated with FEV1 on multivariable analyses (adjusted β = 0.010; 95% CI, 0.005–0.014; P < 0.001). This association was mainly seen in participants with GOLD stages 1 and 2, and not in GOLD stages 3 and 4 (see Table E2).
Spatial Relationship of Normal Voxels and FEV1 Change
In predicting FEV1 change, the CV of Jacobian determinant for the entire lung did not perform as well (adjusted β = −38.083; 95% CI, −91.384 to 15.218; P = 0.161) after adjustment for the previously mentioned variables and baseline FEV1 (see Table E3). The CV of Jacobian determinant of normal voxels also did not predict lung function change (adjusted β = −0.316; 95% CI, −0.846 to 0.214; P = 0.242).
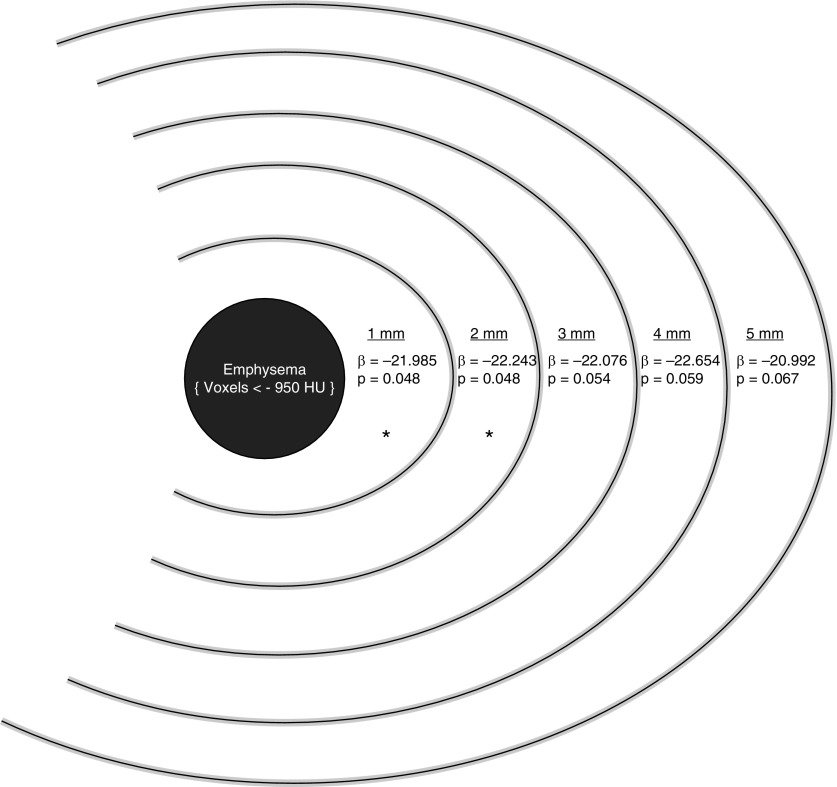
To improve the anatomic localization and clinical applicability of these mechanistic relationships between heterogeneity of lung mechanics and FEV1 change, we examined the spatial distribution of normal voxels in relationship to emphysematous voxels. The mean percentage of voxels within 1 mm (MAL1), 2 mm (MAL2), and 3 mm (MAL3) of emphysematous voxels was 17.1% (8.2%), 35.4% (13.9%), and 52.8% (15.9%), respectively. After adjustment for age, race, sex, BMI, current smoking status, pack-years of smoking, baseline FEV1, CT scanner protocol, CT emphysema, CT gas trapping, and WA%, the mean Jacobian determinant of voxels within MAL1 was significantly associated with FEV1 change (adjusted β = −21.985; 95% CI, −43.791 to −0.179; P = 0.048). Similar relationships were seen for the mean Jacobian determinant of normal voxels within MAL2 (adjusted β = −22.243; 95% CI, −44.317 to −0.169; P = 0.048). However, this relationship was no longer significant for voxels within MAL3 and beyond (Figure 3). The spatial relationship held true for severe emphysema (less than −950 HU) but not for milder emphysema (less than −910 HU) (see Table E4). To test whether MAL areas were influenced by lung densities borderline for emphysema, we converted HU to lung mass (g/ml) and found that there was no difference in lung density of each MAL area going from MAL 1 through 3 (see Table E5) (18).
Figure 3.
Spatial mapping of emphysema and surrounding normal areas depicting relative contributions of mechanically affected lung to FEV1 change. Graphic of a hypothetical central emphysematous area and surrounding normal voxels. β represents adjusted regression coefficient for mean Jacobian determinant for each 1-mm incremental distance of normal voxels from emphysematous voxels. Computed tomography (CT) emphysema was defined as percentage of voxels less than −950 Hounsfield units (HU). All models for predicting FEV1 change were adjusted for age, race, sex, body mass index, smoking pack-years, current smoking status, FEV1 at baseline, CT emphysema, CT gas trapping, segmental wall area %, and CT scanner protocol. *Significant at P < 0.05.
To understand the significance of MAL2 and its implications for lung function change in earlier disease stages, we also examined the rate of change of FEV1 for varying levels of MAL2 in GOLD grades 1 and 2 for whom the mean change of FEV1 was −54.3 (61.2) ml. For participants at or above the median MAL2 (36.9%), the mean decline in FEV1 was 56.4 (68.0) ml/yr versus 43.2 (59.9) ml/yr for those below the median (P = 0.044). For participants with greater than or equal to 75th percentile of MAL2 (47.2%), the mean decline in FEV1 was 65.3 (75.7) ml/yr compared with those below the 75th percentile (44.9 [60.2]; P = 0.039).
Discussion
We demonstrated that in participants with emphysema, normal-appearing lung regions on CT have abnormal regional expansion, and that there is a spatial relationship between areas of emphysema and abnormal lung mechanics resulting in a penumbra of MAL. In addition, we showed that quantifying the amount of MAL can independently predict lung function change over time.
Our results expand on previous preclinical studies that examined the relationship between mechanical stress and remodeling of lung parenchyma (9, 11, 13, 19). Cyclical stretching of the alveolar walls during respiration in the milieu of higher levels of degradative enzymes, as seen in emphysema, is associated with alveolar wall destruction seen on electron microscopy (19, 20). The extracellular matrix of the lung is constantly subject to transpulmonary pressure (21), and to intermittent increases during tidal breathing. The degree of pressure exposure is higher during bouts of coughing and exacerbations. In emphysematous areas, where the extracellular matrix is already damaged, these pressure changes can cause sequential destruction of surrounding alveolar walls resulting in creation of fewer but larger clusters of emphysematous areas with disease progression (22). These findings are consistent with the zipper network model proposed for the failure of the extracellular matrix over time, wherein with decreasing number of fibrils, the remaining fibrils become exposed to progressively more pressure load and stretch and hence are more likely to rupture (12, 22). Here, we demonstrate the impact of emphysematous voxels on the regional expansion of surrounding voxels noninvasively in human subjects, and extend the findings of previous pathobiologic studies. This “penumbra” of MAL likely represents areas of “lung at risk” for emphysema progression. Although we did not match images on follow-up scans voxel by voxel, we showed that these areas of at-risk lung, when quantified, independently predict lung function decline over time.
It follows that the effects of emphysema on surrounding lung are likely to be greater in mild to moderate emphysema than in more severe emphysema where the alveolar breaks tend to coalesce and progressively lesser number of surrounding normal alveoli are exposed to the effects of emphysematous regions (23). Indeed, we found that the percentage of voxels in MAL2 decreased with progressively increasing GOLD grade. It is also possible that this effect is caused by lesser amount of remaining normal lung. A greater amount of emphysema can result in a decline in transpulmonary pressure as the lung matrix weakens, which in turn can result in reduced regional mechanical forces. In either case, these findings support the faster rate of lung function decline seen in the earlier phases of the disease reported in several large studies (15, 24–26).
Previous CT studies have shown that the presence and severity of emphysema on baseline scans predict lung function decline over time (4, 27). Indirect estimates of small airways disease are also associated with lung function change (15). These, however, represent established disease and do not inform about structural disease progression. Whether the at-risk lung can be modulated remains to be tested in future studies. Our results suggest that MAL2 offers new information beyond that offered by previous CT metrics, and may serve as a CT-based biomarker of lung function decline in COPD. Although we calculated Jacobian metrics to mechanistically assess the influence of emphysematous areas on surrounding voxels, MAL2 is based on spatial distribution of normal voxels and hence is an easily calculated measure on inspiratory scans.
The main strength of our study is that we included participants across a range of severity of disease in a well-characterized cohort with rigorous CT and spirometry quality control. Our study also had some limitations. Lung function measurements were made at two time points separated by 5 years; however, the rate of change of lung function is consistent with other large studies and the within-person variability is likely reduced by the large sample size (4, 15, 28). We analyzed data from the first 847 GOLD 1–4 subjects who returned for follow up, and it is possible that this introduces a healthy survivor selection bias. We did not adjust FEV1 decline for medication usage but no existing pharmacologic therapy has been unequivocally shown to alter lung function decline. CT image acquisition was not spirometrically gated but all participants were carefully coached to full inspiration and end expiration. We acknowledge that the P values were just below the 0.05 threshold, and no corrections were made for multiple comparisons.
Finally, we used the less than −950 HU density threshold to define severe emphysema. Although it is possible that the penumbra effect could be caused by milder surrounding emphysema defined using lower thresholds, our models did not hold true using a −910 HU for mild emphysema, suggesting that MAL is a function of more severe emphysema. We also found that the lung density of MAL1 through MAL3 was not different, suggesting our results were not driven by changes in lung density in the areas surrounding emphysematous regions. In addition, although the use of other thresholds to define less severe emphysema might influence results, we were interested in the effects of severe emphysema for which −950 HU is the most accepted threshold and is validated by microscopy (29).
Conclusions
Our findings suggest that areas of normal-appearing lung are mechanically influenced by emphysematous areas, and these regions of lung at risk are associated with lung function decline, thus providing a novel biomarker for disease progression in COPD that is measurable on clinical CT scans. More studies are needed to confirm these findings and to determine whether the lung at risk represents a target for interventions.
Footnotes
Supported by the COPDGene study (National Institutes of Health grants R01 HL089897 and R01 HL089856) and National Institutes of Health grants R01 HL112986 (E.A.H.), R01 HL079406 (J.M.R.), and K23HL133438 (S.P.B.). The COPDGene project is also supported by the COPD Foundation through contributions made to an Industry Advisory Board comprised of AstraZeneca, Boehringer Ingelheim, Novartis, Pfizer, Siemens, Sunovion, and GlaxoSmithKline.
Author Contributions: S.P.B. had full access to all of the data in the study, takes responsibility for the integrity of the data and the accuracy of the data analysis, and had authority over manuscript preparation and the decision to submit the manuscript for publication. Study concept and design, S.P.B., S.B., and J.M.R. Acquisition, analysis, or interpretation of data, S.P.B., S.B., E.A.H., J.D.N., J.C.S., M.T.D., and J.M.R. Drafting of the manuscript, S.P.B. and S.B. Critical revision of the manuscript for important intellectual content, all authors. Statistical analysis, S.P.B. and S.B. Study supervision, all authors.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201701-0050OC on May 8, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura M, Makita H, Nagai K, Konno S, Nasuhara Y, Hasegawa M, Shimizu K, Betsuyaku T, Ito YM, Fuke S, et al. Hokkaido COPD Cohort Study Investigators. Annual change in pulmonary function and clinical phenotype in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185:44–52. doi: 10.1164/rccm.201106-0992OC. [DOI] [PubMed] [Google Scholar]
- 3.Duvoix A, Dickens J, Haq I, Mannino D, Miller B, Tal-Singer R, Lomas DA. Blood fibrinogen as a biomarker of chronic obstructive pulmonary disease. Thorax. 2013;68:670–676. doi: 10.1136/thoraxjnl-2012-201871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, Calverley PM, Celli B, Coxson HO, Crim C, et al. ECLIPSE Investigators. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365:1184–1192. doi: 10.1056/NEJMoa1105482. [DOI] [PubMed] [Google Scholar]
- 5.Mead J, Turner JM, Macklem PT, Little JB. Significance of the relationship between lung recoil and maximum expiratory flow. J Appl Physiol. 1967;22:95–108. doi: 10.1152/jappl.1967.22.1.95. [DOI] [PubMed] [Google Scholar]
- 6.Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343:269–280. doi: 10.1056/NEJM200007273430407. [DOI] [PubMed] [Google Scholar]
- 7.Alford SK, van Beek EJ, McLennan G, Hoffman EA. Heterogeneity of pulmonary perfusion as a mechanistic image-based phenotype in emphysema susceptible smokers. Proc Natl Acad Sci USA. 2010;107:7485–7490. doi: 10.1073/pnas.0913880107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suki B, Ito S, Stamenovic D, Lutchen KR, Ingenito EP. Biomechanics of the lung parenchyma: critical roles of collagen and mechanical forces. J Appl Physiol. 1985;2005:1892–1899. doi: 10.1152/japplphysiol.01087.2004. [DOI] [PubMed] [Google Scholar]
- 9.Kononov S, Brewer K, Sakai H, Cavalcante FS, Sabayanagam CR, Ingenito EP, Suki B. Roles of mechanical forces and collagen failure in the development of elastase-induced emphysema. Am J Respir Crit Care Med. 2001;164:1920–1926. doi: 10.1164/ajrccm.164.10.2101083. [DOI] [PubMed] [Google Scholar]
- 10.Maksym GN, Bates JH. A distributed nonlinear model of lung tissue elasticity. J Appl Physiol. 1985;1997:32–41. doi: 10.1152/jappl.1997.82.1.32. [DOI] [PubMed] [Google Scholar]
- 11.Ito S, Ingenito EP, Brewer KK, Black LD, Parameswaran H, Lutchen KR, Suki B. Mechanics, nonlinearity, and failure strength of lung tissue in a mouse model of emphysema: possible role of collagen remodeling. J Appl Physiol. 1985;2005:503–511. doi: 10.1152/japplphysiol.00590.2004. [DOI] [PubMed] [Google Scholar]
- 12.Ritter MC, Jesudason R, Majumdar A, Stamenovic D, Buczek-Thomas JA, Stone PJ, Nugent MA, Suki B. A zipper network model of the failure mechanics of extracellular matrices. Proc Natl Acad Sci USA. 2009;106:1081–1086. doi: 10.1073/pnas.0808414106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suki B, Jesudason R, Sato S, Parameswaran H, Araujo AD, Majumdar A, Allen PG, Bartolák-Suki E. Mechanical failure, stress redistribution, elastase activity and binding site availability on elastin during the progression of emphysema. Pulm Pharmacol Ther. 2012;25:268–275. doi: 10.1016/j.pupt.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 14.Bodduluri S, Newell JD, Jr, Hoffman EA, Reinhardt JM. Registration-based lung mechanical analysis of chronic obstructive pulmonary disease (COPD) using a supervised machine learning framework. Acad Radiol. 2013;20:527–536. doi: 10.1016/j.acra.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatt SP, Soler X, Wang X, Murray S, Anzueto AR, Beaty TH, Boriek AM, Casaburi R, Criner GJ, Diaz AA, et al. COPDGene Investigators. Association between functional small airway disease and FEV1 decline in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;194:178–184. doi: 10.1164/rccm.201511-2219OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, Curran-Everett D, Silverman EK, Crapo JD. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7:32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin Y, Hoffman EA, Lin CL. Mass preserving nonrigid registration of CT lung images using cubic B-spline. Med Phys. 2009;36:4213–4222. doi: 10.1118/1.3193526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Washko GR, Kinney GL, Ross JC, San José Estépar R, Han MK, Dransfield MT, Kim V, Hatabu H, Come CE, Bowler RP, et al. COPDGene Investigators. Lung mass in smokers. Acad Radiol. 2017;24:386–392. doi: 10.1016/j.acra.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yi E, Sato S, Takahashi A, Parameswaran H, Blute TA, Bartolák-Suki E, Suki B. Mechanical forces accelerate collagen digestion by bacterial collagenase in lung tissue strips. Front Physiol. 2016;7:287. doi: 10.3389/fphys.2016.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jesudason R, Sato S, Parameswaran H, Araujo AD, Majumdar A, Allen PG, Bartolák-Suki E, Suki B. Mechanical forces regulate elastase activity and binding site availability in lung elastin. Biophys J. 2010;99:3076–3083. doi: 10.1016/j.bpj.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carson J. On the elasticity of the lungs. Philos Trans R Soc Lond. 1820;110:29–44. [Google Scholar]
- 22.Mishima M, Hirai T, Itoh H, Nakano Y, Sakai H, Muro S, Nishimura K, Oku Y, Chin K, Ohi M, et al. Complexity of terminal airspace geometry assessed by lung computed tomography in normal subjects and patients with chronic obstructive pulmonary disease. Proc Natl Acad Sci USA. 1999;96:8829–8834. doi: 10.1073/pnas.96.16.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanabe N, Muro S, Sato S, Tanaka S, Oguma T, Kiyokawa H, Takahashi T, Kinose D, Hoshino Y, Kubo T, et al. Longitudinal study of spatially heterogeneous emphysema progression in current smokers with chronic obstructive pulmonary disease. PLoS One. 2012;7:e44993. doi: 10.1371/journal.pone.0044993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, Decramer M, Investigators US UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543–1554. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 25.Celli BR, Thomas NE, Anderson JA, Ferguson GT, Jenkins CR, Jones PW, Vestbo J, Knobil K, Yates JC, Calverley PM. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med. 2008;178:332–338. doi: 10.1164/rccm.200712-1869OC. [DOI] [PubMed] [Google Scholar]
- 26.Mohamed Hoesein FA, Zanen P, Boezen HM, Groen HJ, van Ginneken B, de Jong PA, Postma DS, Lammers JW. Lung function decline in male heavy smokers relates to baseline airflow obstruction severity. Chest. 2012;142:1530–1538. doi: 10.1378/chest.11-2837. [DOI] [PubMed] [Google Scholar]
- 27.Mohamed Hoesein FA, de Hoop B, Zanen P, Gietema H, Kruitwagen CL, van Ginneken B, Isgum I, Mol C, van Klaveren RJ, Dijkstra AE, et al. CT-quantified emphysema in male heavy smokers: association with lung function decline. Thorax. 2011;66:782–787. doi: 10.1136/thx.2010.145995. [DOI] [PubMed] [Google Scholar]
- 28.Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med. 2002;166:675–679. doi: 10.1164/rccm.2112096. [DOI] [PubMed] [Google Scholar]
- 29.Gevenois PA, De Vuyst P, de Maertelaer V, Zanen J, Jacobovitz D, Cosio MG, Yernault JC. Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1996;154:187–192. doi: 10.1164/ajrccm.154.1.8680679. [DOI] [PubMed] [Google Scholar]