Abstract
Rationale: Extracorporeal membrane oxygenation (ECMO) is used for respiratory and cardiac failure in children but is complicated by bleeding and thrombosis.
Objectives: (1) To measure the incidence of bleeding (blood loss requiring transfusion or intracranial hemorrhage) and thrombosis during ECMO support; (2) to identify factors associated with these complications; and (3) to determine the impact of these complications on patient outcome.
Methods: This was a prospective, observational cohort study in pediatric, cardiac, and neonatal intensive care units in eight hospitals, carried out from December 2012 to September 2014.
Measurements and Main Results: ECMO was used on 514 consecutive patients under age 19 years. Demographics, anticoagulation practices, severity of illness, circuitry components, bleeding, thrombotic events, and outcome were recorded. Survival was 54.9%. Bleeding occurred in 70.2%, including intracranial hemorrhage in 16%, and was independently associated with higher daily risk of mortality. Circuit component changes were required in 31.1%, and patient-related clots occurred in 12.8%. Laboratory sampling contributed to transfusion requirement in 56.6%, and was the sole reason for at least one transfusion in 42.2% of patients. Pump type was not associated with bleeding, thrombosis, hemolysis, or mortality. Hemolysis was predictive of subsequent thrombotic events. Neither hemolysis nor thrombotic events increased the risk of mortality.
Conclusions: The incidences of bleeding and thrombosis are high during ECMO support. Laboratory sampling is a major contributor to transfusion during ECMO. Strategies to reduce the daily risk of bleeding and thrombosis, and different thresholds for transfusion, may be appropriate subjects of future trials to improve outcomes of children requiring this supportive therapy.
Keywords: extracorporeal life support, cardiorespiratory failure, hemolysis, transfusion, outcome
At a Glance Commentary
Scientific Knowledge on the Subject
Although bleeding and thrombosis are known events during extracorporeal membrane oxygenation, a prospective, multicenter study of factors that are associated with these events is lacking. Establishing factors related to need for transfusion (due to blood loss) or thrombosis would be of benefit to the field and pave the way for more research to reduce these events.
What This Study Adds to the Field
This study provides a prospective, consecutive patient evaluation from large children’s hospitals within the Eunice Kennedy Shriver National Institute for Child Health and Development Collaborative Pediatric Critical Care Research Network of factors associated with bleeding and thrombosis. The variability between centers in the results highlights the need for standardization to perform further specific studies. The finding that many transfusions were attributed to blood loss from laboratory sampling is novel and an area for more research. No optimal anticoagulation regimen was found, nor was a difference between centrifugal and roller devices.
Extracorporeal membrane oxygenation (ECMO) is used to support critically ill infants and children with severe respiratory or cardiac failure. Despite improvements in ECMO technology, bleeding and thrombosis remain significant complications (1, 2), as the interaction between the patient’s native blood and the foreign surface of the ECMO circuit activates the coagulation cascade (3). Bleeding and thrombotic complications may be associated with morbidity and mortality related to ECMO (1), but the precise incidence of these complications is not known.
Improving the outcome of patients requiring ECMO support requires understanding the causes of complications, such as bleeding and thrombosis, but this is complicated by variation of practice between ECMO centers. Anticoagulation regimens (4), laboratory monitoring, patient selection, and types of circuit components (such as oxygenator and pump) are different between centers. Technological changes have resulted in a transition from roller head to centrifugal pumps, and the impact of this remains unclear (5–7). Optimum management of children requiring ECMO, especially related to anticoagulation management and the significance of hemolysis (7–9), remains controversial and elusive. Finally, the relatively small number of patients treated at single centers mandates multicenter observational and interventional ECMO studies, but the signal-to-noise ratio of such studies will be poor unless ECMO practice can be better standardized between centers.
In an attempt to overcome these issues, the Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network (CPCCRN) first conducted a retrospective analysis of bleeding and thrombosis complications using data submitted from its sites to the Extracorporeal Life Support Organization (ELSO) International Registry (1), and then implemented this prospective, observational cohort study to measure the incidence of bleeding and thrombosis to assess factors associated with these complications, and to determine the impact of bleeding and thrombosis on survival and functional outcome after ECMO support. We hypothesized that bleeding and thrombotic events would adversely affect survival and functional outcome after adjustment for duration of ECMO support, similar to our retrospective observations from the ELSO Registry. This article reports the results of this prospective study. Excerpts of data have been presented in oral form, but no prior publication of data has occurred.
Methods
Consecutive patients under 19 years of age treated with ECMO initiated in a pediatric, cardiac, or neonatal intensive care unit of eight CPCCRN institutions between December 2012 and September 2014 were included in the study. The study was limited to the initial ECMO course for patients who required multiple episodes of ECMO. The project was approved with waiver of informed consent by the responsible Institutional Review Board for every clinical site and the Data Coordinating Center (DCC) at the University of Utah (Salt Lake City, UT).
Bleeding complications were defined as blood loss requiring transfusion or intracranial hemorrhage. The bedside ECMO specialist recorded the reasons for each transfusion as bleeding from the surgical or cannula sites, sanguineous chest tube output, pulmonary hemorrhage, gastrointestinal or genitourinary bleeding, intracranial bleeding, or blood loss from laboratory sampling. Training for data collection was conducted by research personnel from the DCC, as well as research coordinators at each site. Questions on the identification of bleeding and thrombotic events were directed first to on-site research coordinators and then centrally to the project manager (S. Bisping) based at the DCC and the principal investigator (H.J.D.). The principal investigator made the final adjudication as needed after conferring with the DCC and center personnel. Data on efforts to control bleeding were not specifically obtained, although data on use of agents, such as aminocaproic acid, antithrombin III, and factor VII, were collected daily. Bleeding events from the same source that occurred over multiple days were not recorded as “continuing” events. The source(s) of bleeding that contributed to need for transfusion was recorded daily. No strict guidelines for transfusing to maintain a specific hemoglobin level were mandated by the study.
Patient-related thrombotic complications were classified as intracranial infarction, limb ischemia, pulmonary embolus, intracardiac thrombus, aortopulmonary shunt thrombus, or other sites of thrombosis. Circuit-related thrombotic events requiring change-out of one or more circuit components were recorded by location of the clots and the replaced circuit components.
Hemolysis was defined as a plasma-free hemoglobin level greater than 50 mg/dl, which was regularly monitored in four of eight participating sites.
Data Collection
Baseline data reflected the patient status before and closest to ECMO initiation. Data included patient demographics, diagnoses, primary indication for ECMO, number of failed organs, PRISM (Pediatric Risk of Mortality Score) III score in the 12 hours before ECMO initiation (10, 11), vasoactive inotrope score (12, 13), ventilator settings, and relevant laboratory data. Indications for ECMO were categorized as respiratory, cardiac, or extracorporeal cardiopulmonary resuscitation. Type of membrane lung, pump, and other components of the ECMO circuit were recorded.
Clinical data collected on a daily basis included all administered blood product transfusions, laboratory results closest to 7:00 a.m. (arterial blood gas values, lactate levels, coagulation studies, and hemoglobin and plasma-free hemoglobin concentrations), ventilator settings, bleeding and thrombotic complications, circuit clots, and circuit component changes.
Patient outcomes were recorded at hospital discharge and included vital status, Functional Status Scale (FSS) (14–16), the Pediatric Overall Performance Category (POPC), and the Pediatric Cerebral Performance Category (PCPC) (17).
Statistical Analysis
The incidence of bleeding (and thrombotic) events was calculated as the proportion of total subjects and separately as the proportion of total study days on which a bleeding event occurred. Incidence was evaluated overall and by age and indication for ECMO. Age was categorized as neonatal (≤31 d) versus pediatric (32 d–19 yr).
Univariable subject-level associations with bleeding and thrombotic events were assessed with the Wilcoxon rank-sum test for continuous or ordinal variables and with Fisher’s exact test (Monte Carlo approximation) for categorical variables. Reported P values are based on a two-sided alternative.
Multivariable models were developed for mortality, hemolysis, bleeding, and thrombotic events. Variables were considered potential predictors if they were associated with the outcome in univariable analysis (P < 0.10) and available for at least 90% of the study days. The final model was selected using bidirectional step-wise selection on the potential predictors with a significance criterion of P less than 0.05 to enter and stay in the model. No variables were forced into the model.
Poisson regression models with robust error estimates based on generalized estimating equations were used to evaluate predictors of daily hemolysis, bleeding, and thrombotic events. An autoregressive covariance structure of order 1 was specified to account for correlation between different study days on the same subject. In particular, this accounts for a higher correlation between study days that are close together, but relatively lower correlation between study days that are far apart. The model for hemolysis was restricted to the four clinical sites that monitored plasma-free hemoglobin. An additional model restricted to these sites was created for daily thrombotic events in which recent hemolysis was included as a potential predictor.
A Cox proportional hazards model was used to evaluate factors associated with mortality. In addition to the predictors assessed in the Poisson models, this model also incorporated daily bleeding and thrombotic events as potential time-varying covariates. Subjects alive at the end of the last day on ECMO were considered right censored; deaths occurring after the last day of ECMO were not captured in this model.
Anticoagulation monitoring practices were summarized by comparing the proportion of total study days on which each test was measured. Practices were summarized overall and by age and indication for ECMO within each site.
Summaries and analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
Patient Population
The study enrolled 514 patients. Table E1 in the online supplement summarizes the demographics, baseline physiologic status, length of stay, and outcome of patients categorized by age and primary indication for ECMO.
ECMO Support
The ECMO pump type and mode of support are shown in Table E2, categorized by age and primary indication for ECMO. Overall, 65.0% of patients were supported with centrifugal pumps, but pump selection was different between sites. Two sites only used centrifugal pumps, one site only used roller head pumps, and five sites used both types of pump. Venoarterial support was provided to the majority of patients. A silicone membrane lung was used in only 20 patients at one center, and the remainder of patients were oxygenated with hollow fiber membranes (data not shown).
Bleeding and Thrombotic Events
The rates of bleeding and thrombotic events varied significantly between sites (Table 1, P < 0.001). Bleeding and thrombotic events are summarized at a patient level in Table 2. Bleeding events occurred in 70.2% of the cohort, including intracranial hemorrhage in 16.0%. Blood sampling for laboratory analyses contributed to transfusion requirements in 56.6% of patients. Notably, in 42.2% of patients, at least one transfusion was attributed solely to blood loss from laboratory sampling. Thrombotic events occurred in 37.5% of patients; 12.8% of subjects suffered a patient-related clot and 31.1% had thrombotic complications involving the circuit. The specific circuit components involved are shown in Table 2. Hemolysis was diagnosed in 32.9% of all patients, but plasma-free hemoglobin was not monitored at four sites. Within the sites that regularly monitored free hemoglobin, hemolysis was diagnosed in 57.5% of the patients.
Table 1.
Percent of Patients Suffering Bleeding, Thrombotic Complications, or Death
| Hospital |
P Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| A |
B |
C |
D |
E |
F |
G |
H |
||
| (n = 67) | (n = 37) | (n = 104) | (n = 63) | (n = 88) | (n = 67) | (n = 28) | (n = 60) | ||
| Bleeding event, n (%) | 60 (89.6) | 19 (51.4) | 60 (57.7) | 46 (73.0) | 70 (79.5) | 42 (62.7) | 24 (85.7) | 40 (66.7) | <0.001* |
| Thrombotic event, n (%) | 43 (64.2) | 12 (32.4) | 33 (31.7) | 29 (46.0) | 34 (38.6) | 11 (16.4) | 16 (57.1) | 15 (25.0) | <0.001* |
| Mortality, n (%) | 28 (41.8) | 15 (40.5) | 44 (42.3) | 30 (47.6) | 47 (53.4) | 33 (49.3) | 15 (53.6) | 20 (33.3) | 0.314* |
Fisher’s exact test (Monte Carlo approximation).
Table 2.
Percent of Patients with Bleeding or Thrombotic Events by Age and Primary Indication for Extracorporeal Membrane Oxygenation
| Respiratory |
Cardiac |
ECPR |
|||||
|---|---|---|---|---|---|---|---|
| Neonatal (n = 151) | Pediatric (n = 86) | Neonatal (n = 92) | Pediatric (n = 115) | Neonatal (n = 24) | Pediatric (n = 46) | Overall (n = 514) | |
| Bleeding events, n (%) | 91 (60.3) | 63 (73.3) | 71 (77.2) | 91 (79.1) | 17 (70.8) | 28 (60.9) | 361 (70.2) |
| Surgical site bleeding, n (%) | 37 (24.5) | 24 (27.9) | 46 (50.0) | 51 (44.3) | 12 (50.0) | 15 (32.6) | 185 (36.0) |
| Chest tube bleeding, n (%) | 21 (13.9) | 24 (27.9) | 51 (55.4) | 60 (52.2) | 9 (37.5) | 16 (34.8) | 181 (35.2) |
| ml/kg, median (IQR) | 0.0 (0.0–5.7) | 0.0 (0.0–4.4) | 26.9 (9.3–53.5) | 17.0 (0.0–46.5) | 18.7 (7.2–55.2) | 1.6 (0.0–28.2) | 3.9 (0.0–25.9) |
| Cannula site bleeding, n (%) | 39 (25.8) | 39 (45.3) | 31 (33.7) | 40 (34.8) | 9 (37.5) | 18 (39.1) | 176 (34.2) |
| Pulmonary hemorrhage, n (%) | 17 (11.3) | 17 (19.8) | 5 (5.4) | 13 (11.3) | 2 (8.3) | 3 (6.5) | 57 (11.1) |
| Gastrointestinal bleeding, n (%) | 9 (6.0) | 14 (16.3) | 1 (1.1) | 2 (1.7) | 1 (4.2) | 5 (10.9) | 32 (6.2) |
| Genitourinary bleeding, n (%) | 9 (6.0) | 14 (16.3) | 2 (2.2) | 4 (3.5) | 0 (0.0) | 2 (4.3) | 31 (6.0) |
| Intracranial bleeding, n (%) | 34 (22.5) | 14 (16.3) | 17 (18.5) | 8 (7.0) | 4 (16.7) | 5 (10.9) | 82 (16.0) |
| Laboratory sample bleeding, n (%) | 113 (74.8) | 47 (54.7) | 48 (52.2) | 53 (46.1) | 10 (41.7) | 20 (43.5) | 291 (56.6) |
| ml/kg, median (IQR) | 7.6 (5.2–10.3) | 1.1 (0.4–2.7) | 5.4 (3.3–7.8) | 2.0 (0.8–4.4) | 5.9 (4.2–8.2) | 2.4 (0.6–5.3) | 4.4 (1.5–7.7) |
| Only laboratory sample bleeding, n (%) | 100 (66.2) | 34 (39.5) | 33 (35.9) | 31 (27.0) | 5 (20.8) | 14 (30.4) | 217 (42.2) |
| Thrombotic events, n (%) | 66 (43.7) | 33 (38.4) | 29 (31.5) | 43 (37.4) | 6 (25.0) | 16 (34.8) | 193 (37.5) |
| Patient-related thromboses, n (%) | 11 (7.3) | 11 (12.8) | 12 (13.0) | 23 (20.0) | 1 (4.2) | 8 (17.4) | 66 (12.8) |
| Intracranial infarction, n (%) | 5 (3.3) | 4 (4.7) | 3 (3.3) | 6 (5.2) | 0 (0.0) | 4 (8.7) | 22 (4.3) |
| Limb ischemia, n (%) | 1 (0.7) | 4 (4.7) | 5 (5.4) | 4 (3.5) | 0 (0.0) | 3 (6.5) | 17 (3.3) |
| Intracardiac clot, n (%) | 0 (0.0) | 1 (1.2) | 0 (0.0) | 9 (7.8) | 0 (0.0) | 0 (0.0) | 10 (1.9) |
| Aortopulmonary shunt clot, n (%) | 1 (0.7) | 0 (0.0) | 1 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.4) |
| Pulmonary embolus, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.9) | 0 (0.0) | 0 (0.0) | 1 (0.2) |
| Other, n (%) | 4 (2.6) | 6 (7.0) | 3 (3.3) | 6 (5.2) | 1 (4.2) | 1 (2.2) | 21 (4.1) |
| Circuit-related thromboses (requiring change-out), n (%) | 61 (40.4) | 29 (33.7) | 23 (25.0) | 30 (26.1) | 6 (25.0) | 11 (23.9) | 160 (31.1) |
| Entire circuit, n (%) | 42 (27.8) | 13 (15.1) | 7 (7.6) | 10 (8.7) | 1 (4.2) | 6 (13.0) | 79 (15.4) |
| Oxygenator, n (%) | 8 (5.3) | 5 (5.8) | 9 (9.8) | 12 (10.4) | 3 (12.5) | 3 (6.5) | 40 (7.8) |
| Bladder, n (%) | 13 (8.6) | 4 (4.7) | 3 (3.3) | 4 (3.5) | 2 (8.3) | 0 (0.0) | 26 (5.1) |
| CVVH, n (%) | 1 (0.7) | 1 (1.2 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.2) | 3 (0.6) |
| Tubing, n (%) | 6 (4.0) | 6 (7.0) | 2 (2.2) | 1 (0.9) | 1 (4.2) | 0 (0.0) | 16 (3.1) |
| Arterial cannula, n (%) | 1 (0.7) | 3 (3.5) | 1 (1.1) | 1 (0.9) | 0 (0.0) | 0 (0.0) | 6 (1.2) |
| Bridge, n (%) | 1 (0.7) | 0 (0.0) | 0 (0.0) | 1 (0.9) | 1 (4.2) | 0 (0.0) | 3 (0.6) |
| Hemofilter, n (%) | 12 (7.9) | 3 (3.5) | 3 (3.3) | 0 (0.0) | 1 (4.2) | 2 (4.3) | 21 (4.1) |
| Pump head, n (%) | 0 (0.0) | 2 (2.3) | 0 (0.0) | 2 (1.7) | 0 (0.0) | 1 (2.2) | 5 (1.0) |
| Venous cannula, n (%) | 1 (0.7) | 3 (3.5) | 1 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (1.0) |
| Other, n (%) | 20 (13.2) | 11 (12.8) | 8 (8.7) | 13 (11.3) | 2 (8.3) | 4 (8.7) | 58 (11.3) |
| Hemolysis, n (%) | 60 (39.7) | 23 (26.7) | 38 (41.3) | 26 (22.6) | 7 (29.2) | 15 (32.6) | 169 (32.9) |
Definition of abbreviations: CVVH = continuous venovenous hemofiltration; ECPR = extracorporeal cardiopulmonary resuscitation; IQR = interquartile range.
Volume of sanguineous chest tube drainage has three (1%) missing values. Volume of laboratory sample bleeding has 140 (27%) missing values.
Table 3 summarizes bleeding and thrombotic events at the level of days of exposure (e.g., bleeding events occurred at a rate of 27.5 per 100 days of ECMO). The rate of overall thrombotic events was 11.0 per 100 days. Patient-related and circuit-related thromboses occurred at rates of 4.5 and 7.0 per 100 days, respectively.
Table 3.
New Bleeding and Thrombotic Events per 100 Days by Age and Primary Indication for Extracorporeal Membrane Oxygenation
| Respiratory |
Cardiac |
ECPR |
|||||
|---|---|---|---|---|---|---|---|
| Neonatal (1,724 d) | Pediatric (1,123 d) | Neonatal (583 d) | Pediatric (771 d) | Neonatal (153 d) | Pediatric (306 d) | Overall (4,660 d) | |
| Bleeding events, n (%) | 282 (16.4) | 335 (29.8) | 218 (37.4) | 297 (38.5) | 51 (33.3) | 100 (32.7) | 1283 (27.5) |
| Surgical site bleeding, n (%) | 84 (4.9) | 90 (8.0) | 105 (18.0) | 116 (15.0) | 28 (18.3) | 31 (10.1) | 454 (9.7) |
| Chest tube bleeding, n (%) | 75 (4.4) | 127 (11.3) | 139 (23.8) | 185 (24.0) | 34 (22.2) | 62 (20.3) | 622 (13.3) |
| ml/kg, median (IQR) | 0.0 (0.0–1.8) | 0.0 (0.0–1.3) | 17.3 (0.0–48.8) | 7.4 (0.0–33.5) | 16.7 (3.2–42.4) | 0.9 (0.0–15.4) | 0.0 (0.0–13.2) |
| Cannula site bleeding, n (%) | 85 (4.9) | 124 (11.0) | 46 (7.9) | 94 (12.2) | 13 (8.5) | 35 (11.4) | 397 (8.5) |
| Pulmonary hemorrhage, n (%) | 33 (1.9) | 66 (5.9) | 6 (1.0) | 26 (3.4) | 5 (3.3) | 8 (2.6) | 144 (3.1) |
| Gastrointestinal bleeding, n (%) | 15 (0.9) | 49 (4.4) | 1 (0.2) | 4 (0.5) | 1 (0.7) | 11 (3.6) | 81 (1.7) |
| Genitourinary bleeding, n (%) | 13 (0.8) | 26 (2.3) | 2 (0.3) | 6 (0.8) | 0 (0.0) | 3 (1.0) | 50 (1.1) |
| Intracranial bleeding, n (%) | 57 (3.3) | 16 (1.4) | 25 (4.3) | 13 (1.7) | 6 (3.9) | 5 (1.6) | 122 (2.6) |
| Laboratory sample bleeding, n (%) | 483 (28.0) | 203 (18.1) | 145 (24.9) | 180 (23.3) | 41 (26.8) | 73 (23.9) | 1125 (24.1) |
| ml/kg, median (IQR) | 9.0 (6.3–11.6) | 1.4 (0.6–3.2) | 7.1 (4.2–10.3) | 2.7 (1.0–5.5) | 7.6 (3.7–9.6) | 2.4 (1.4–4.5) | 6.0 (2.1–9.7) |
| Only laboratory sample bleeding, n (%) | 349 (20.2) | 137 (12.2) | 63 (10.8) | 79 (10.2) | 19 (12.4) | 32 (10.5) | 679 (14.6) |
| Thrombotic events, n (%) | 154 (8.9) | 104 (9.3) | 66 (11.3) | 122 (15.8) | 17 (11.1) | 48 (15.7) | 511 (11.0) |
| Patient-related thromboses, n (%) | 27 (1.6) | 39 (3.5) | 33 (5.7) | 75 (9.7) | 7 (4.6) | 29 (9.5) | 210 (4.5) |
| Intracranial infarction, n (%) | 17 (1.0) | 17 (1.5) | 5 (0.9) | 15 (1.9) | 0 (0.0) | 17 (5.6) | 71 (1.5) |
| Limb ischemia, n (%) | 2 (0.1) | 15 (1.3) | 21 (3.6) | 26 (3.4) | 0 (0.0) | 10 (3.3) | 74 (1.6) |
| Intracardiac clot, n (%) | 0 (0.0) | 1 (0.1) | 0 (0.0) | 25 (3.2) | 0 (0.0) | 0 (0.0) | 26 (0.6) |
| Aortopulmonary shunt clot, n (%) | 1 (0.1) | 0 (0.0) | 2 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (0.1) |
| Pulmonary embolus, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (0.8) | 0 (0.0) | 0 (0.0) | 6 (0.1) |
| Other, n (%) | 7 (0.4) | 18 (1.6) | 5 (0.9) | 10 (1.3) | 7 (4.6) | 2 (0.7) | 49 (1.1) |
| Circuit-related thromboses (requiring change-out), n (%) | 129 (7.5) | 70 (6.2) | 36 (6.2) | 55 (7.1) | 12 (7.8) | 22 (7.2) | 324 (7.0) |
| Entire circuit, n (%) | 51 (3.0) | 20 (1.8) | 8 (1.4) | 12 (1.6) | 3 (2.0) | 7 (2.3) | 101 (2.2) |
| Oxygenator, n (%) | 8 (0.5) | 10 (0.9) | 9 (1.5) | 17 (2.2) | 4 (2.6) | 4 (1.3) | 52 (1.1) |
| Bladder, n (%) | 16 (0.9) | 5 (0.4) | 3 (0.5) | 6 (0.8) | 2 (1.3) | 0 (0.0) | 32 (0.7) |
| CVVH, n (%) | 1 (0.1) | 1 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.3) | 3 (0.1) |
| Tubing, n (%) | 7 (0.4) | 14 (1.2) | 2 (0.3) | 1 (0.1) | 1 (0.7) | 0 (0.0) | 25 (0.5) |
| Arterial cannula, n (%) | 1 (0.1) | 3 (0.3) | 1 (0.2) | 1 (0.1) | 0 (0.0) | 0 (0.0) | 6 (0.1) |
| Bridge, n (%) | 1 (0.1) | 0 (0.0) | 0 (0.0) | 1 (0.1) | 1 (0.7) | 0 (0.0) | 3 (0.1) |
| Hemofilter, n (%) | 19 (1.1) | 4 (0.4) | 4 (0.7) | 0 (0.0) | 2 (1.3) | 2 (0.7) | 31 (0.7) |
| Pump head, n (%) | 0 (0.0) | 2 (0.2) | 0 (0.0) | 2 (0.3) | 0 (0.0) | 1 (0.3) | 5 (0.1) |
| Venous cannula, n (%) | 1 (0.1) | 3 (0.3) | 1 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (0.1) |
| Other, n (%) | 32 (1.9) | 20 (1.8) | 11 (1.9) | 22 (2.9) | 2 (1.3) | 9 (2.9) | 96 (2.1) |
| Hemolysis, n (%) | 275 (16.0) | 145 (12.9) | 108 (18.5) | 78 (10.1) | 24 (15.7) | 35 (11.4) | 665 (14.3) |
For definition of abbreviations, see Table 2.
Volume of sanguineous chest tube drainage has 12 (<1%) missing values. Volume of laboratory sample bleeding has 1,722 (37%) missing values.
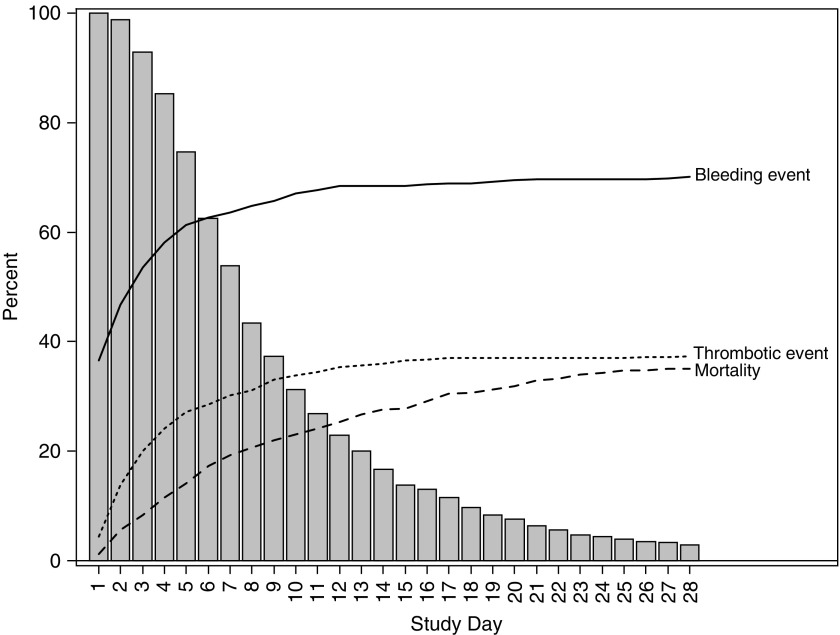
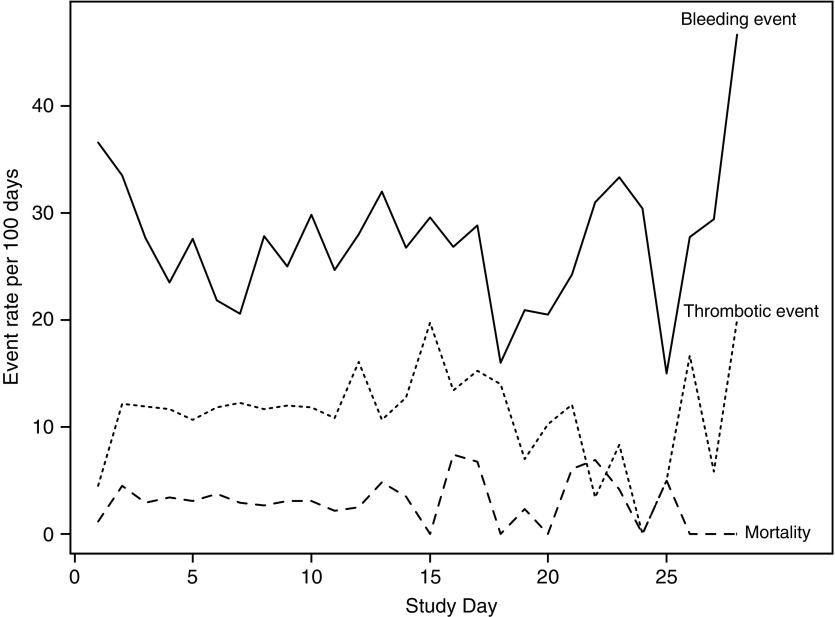
The distribution of bleeding and thrombotic events by age group and primary indication for ECMO is summarized in Figure E1. Combinations of bleeding and thrombotic events were frequent in all categories. Most subjects that experienced a bleeding event or a thrombotic event, or mortality, did so early on (Figure 1). This result is largely due to the fact that 64% of subjects remained on ECMO for less than 1 week. However, the daily event rates are relatively stable as duration increases (Figure 2). For example, the mortality risk was 1–3% per day of ECMO support. By Day 28, 35% of patients had died.
Figure 1.
The cumulative percent of patients who have suffered mortality, bleeding events, or thrombotic events by day of extracorporeal membrane oxygenation support. The histogram shows the percent of patients who remain on extracorporeal membrane oxygenation support as the duration of support increases.
Figure 2.
The event rates per 100 days of extracorporeal membrane oxygenation support by day of extracorporeal membrane oxygenation support. Rates of bleeding and thrombotic events, and mortality, are relatively constant across time.
Laboratory Monitoring
Laboratory testing for anticoagulation monitoring and detection of hemolysis is summarized by age group and primary indication for ECMO in Table E3. Fibrinogen and activated clotting time were monitored most frequently (>90% of ECMO days). Monitoring approaches were highly variable between sites (Table E4); for example, fibrinogen was monitored only 42% of the time at institution B, but in over 90% of the time in the remaining sites. Similarly, evaluation and administration of antithrombin III (ATIII) varied between sites. ATIII was monitored on 48% of study days overall, and ranged from 2 to 92% of study days within centers. Administration of ATIII was noted in 11% of study days, and ranged from 0 to 39% in centers. Centers that monitored ATIII had higher rates of administration. Only four of eight sites regularly monitored plasma-free hemoglobin, and three sites never measured this parameter.
Factors Associated with Bleeding and Thrombotic Events
Multivariable logistic regression models (data not shown) indicated a strong association of hospital and ECMO duration with the occurrence of bleeding and thrombotic complications during the entire ECMO episode. Factors associated with bleeding, thrombotic events, or hemolysis, on a daily basis, as assessed by Poisson regression models, are reported in Tables 4–7. The clinical site was a significant factor in all of the models. Certain baseline characteristics (chronic neurological condition, requirement for tracheostomy or home ventilation, primary diagnosis of respiratory failure) and venovenous ECMO support were all associated with less risk of thrombotic events when all institutions were included (Table 4). Table 5 displays the model for the prediction of hemolysis, and only includes hospitals that monitor free hemoglobin. The presence of a bladder/venous reservoir in the ECMO circuit was strongly associated with subsequent hemolysis. Table 6 models the prediction of thrombosis in hospitals that monitored free hemoglobin, and the occurrence of hemolysis within the previous 3 days was strongly associated with a thrombotic complication. Finally, predictors of a bleeding event included cardiac or extracorporeal cardiopulmonary resuscitation indications and direct transition to ECMO from cardiopulmonary bypass (Table 7). Due to the diverse mechanisms of intracranial hemorrhage, the robustness of the predictors for bleeding was assessed by reconstructing the multivariable model excluding intracranial hemorrhage. With the exception of organ failure index, which was no longer significantly associated with bleeding, the same predictors were identified, and the estimates of relative risk were not substantially altered.
Table 4.
Multivariable Model for Daily Thrombotic Event
| Adjusted Relative Risk (95% CI) | P Value | |
|---|---|---|
| Baseline ventilation (home ventilator or tracheostomy) | 0.025 | |
| No | Reference | |
| Yes | 0.11 (0.02–0.79) | |
| Chronic diagnosis: neurologic, other chronic condition | 0.023 | |
| No | Reference | |
| Yes | 0.51 (0.23–1.13) | |
| Mode of ECMO | 0.024 | |
| VA | Reference | |
| VV | 0.62 (0.40–0.95) | |
| Primary diagnosis | 0.018 | |
| CV/shock | Reference | |
| Other | 1.22 (0.52–2.89) | |
| Respiratory | 0.67 (0.50–0.88) | |
| Clinical site | <0.001 | |
| A | 2.32 (1.60–3.36) | |
| B | 1.54 (0.74–3.19) | |
| C | 1.01 (0.64–1.60) | |
| D | 1.15 (0.73–1.80) | |
| E | Reference | |
| F | 1.03 (0.48–2.22) | |
| G | 2.71 (1.61–4.57) | |
| H | 0.83 (0.45, 1.54) |
Definition of abbreviations: CI = confidence interval; CV = cardiovascular; ECMO = extracorporeal membrane oxygenation; VA = venoarterial; VV = venovenous.
Table 7.
Multivariate Model for Daily Bleeding Event
| Adjusted Relative Risk (95% CI) | P Value | |
|---|---|---|
| Age, yr | 1.04 (1.02–1.05) | <0.001 |
| Patient placed on ECMO directly from CPB | <0.001 | |
| No | Reference | |
| Yes | 1.76 (1.45–2.13) | |
| Primary ECMO indication | 0.002 | |
| Cardiac | 1.34 (1.11–1.63) | |
| ECPR | 1.52 (1.16–1.98) | |
| Respiratory | Reference | |
| Patient has MAS | 0.002 | |
| No | Reference | |
| Yes | 0.54 (0.34–0.84) | |
| Organ Failure Index on day of ECMO initiation | 0.013 | |
| 1 | 1.00 (0.65–1.53) | |
| 2 | Reference | |
| 3 | 1.28 (1.08–1.51) | |
| 4–5 | 1.47 (1.09–1.97) | |
| Clinical site | <0.001 | |
| A | 1.53 (1.24–1.90) | |
| B | 0.58 (0.35–0.95) | |
| C | 0.44 (0.33–0.58) | |
| D | 0.77 (0.59–1.01) | |
| E | Reference | |
| F | 0.86 (0.64–1.16) | |
| G | 1.21 (0.84–1.73) | |
| H | 0.90 (0.66–1.21) |
Definition of abbreviations: CI = confidence interval; CPB = cardiopulmonary bypass; ECMO = extracorporeal membrane oxygenation; ECPR = extracorporeal cardiopulmonary resuscitation; MAS = meconium aspiration syndrome.
Table 5.
Multivariable Model for Daily Hemolysis (Only Sites That Measure Hemolysis)
| Adjusted Relative Risk (95% CI) | P Value | |
|---|---|---|
| Age, yr | 0.94 (0.90–0.98) | <0.001 |
| Setup includes bladder/venous reservoir | 0.001 | |
| No | Reference | |
| Yes | 1.71 (1.25–2.34) | |
| Clinical site | <0.001 | |
| A | 2.10 (1.51–2.90) | |
| D | 1.16 (0.80–1.69) | |
| E | Reference | |
| F | 1.71 (1.04–2.81) |
Definition of abbreviation: CI = confidence interval.
Table 6.
Multivariable Model for Daily Thrombosis with Recent Hemolysis as a Predictor (Only Sites That Measure Hemolysis)
| Adjusted Relative Risk (95% CI) | P Value | |
|---|---|---|
| Mode of ECMO | 0.046 | |
| VA | Reference | |
| VV | 0.63 (0.40–0.99) | |
| Primary diagnosis | <0.001 | |
| CV/shock | Reference | |
| Other | 1.02 (0.33–3.11) | |
| Respiratory | 0.51 (0.38–0.69) | |
| Hemolysis in the last 3 d | <0.001 | |
| No | Reference | |
| Yes | 1.69 (1.30–2.19) | |
| Clinical site | 0.003 | |
| A | 2.07 (1.44–2.98) | |
| D | 1.08 (0.71–1.66) | |
| E | Reference | |
| F | 0.92 (0.44–1.92) |
For definition of abbreviations, see Table 4.
Outcomes
Bleeding and thrombotic events had adverse effects on the discharge status measured by the POPC and PCPC scores, primarily related to higher rates of brain death in patients with these complications. Bleeding and thrombotic events were not associated with a worse FSS of patients who survived to hospital discharge (Tables 8 and 9). Hemolysis had no effect on the discharge status (data not shown).
Table 8.
Discharge Functional Status Scale, Pediatric Overall Performance Category, and Pediatric Cerebral Performance Category by Bleeding
| Bleeding Event |
|||
|---|---|---|---|
| No (n = 153) | Yes (n = 361) | P Value | |
| Total FSS at hospital discharge | 0.323* | ||
| n | 101 | 181 | |
| Mean (SD) | 8.7 (2.95) | 9.1 (3.11) | |
| Minimum, maximum | 6.0, 24.0 | 6.0, 21.0 | |
| Median (IQR) | 8.0 (6.0–10.0) | 8.0 (7.0–10.0) | |
| POPC score at hospital discharge, n (%) | <0.001* | ||
| 1 (good) | 21 (13.7) | 31 (8.6) | |
| 2 (mild disability) | 46 (30.1) | 82 (22.7) | |
| 3 (moderate disability) | 29 (19.0) | 49 (13.6) | |
| 4 (severe disability) | 5 (3.3) | 19 (5.3) | |
| 5 (Coma/vegetative) | 0 (0) | 0 (0) | |
| 6 (brain death) | 52 (34.0) | 180 (49.9) | |
| PCPC score at hospital discharge, n (%) | <0.001* | ||
| 1 (normal) | 56 (36.6) | 89 (24.7) | |
| 2 (mild disability) | 33 (21.6) | 64 (17.7) | |
| 3 (moderate disability) | 10 (6.5) | 17 (4.7%) | |
| 4 (severe disability) | 2 (1.3) | 11 (3.0) | |
| 5 (Coma/vegetative) | 0 (0) | 0 (0) | |
| 6 (brain death) | 52 (34.0) | 180 (49.9) | |
Definition of abbreviations: FSS = Functional Status Scale; IQR = interquartile range; PCPC = Pediatric Cerebral Performance Category; POPC = Pediatric Overall Performance Category.
FSS is only measured on survivors, whereas POPC and PCPC include death as the worst possible score.
P values are based on the Wilcoxon rank-sum test.
Table 9.
Discharge Functional Status Scale, Pediatric Overall Performance Category, and Pediatric Cerebral Performance Category by Thrombosis
| Thrombotic Event |
|||
|---|---|---|---|
| No (n = 321) | Yes (n = 193) | P Value | |
| Total FSS at hospital discharge | 0.101* | ||
| n | 190 | 92 | |
| Mean (SD) | 8.8 (3.17) | 9.2 (2.81) | |
| Minimum, maximum | 6.0, 24.0 | 6.0, 20.0 | |
| Median (IQR) | 8.0 (6.0–10.0) | 8.0 (7.5–10.0) | |
| POPC score at hospital discharge, n (%) | 0.005* | ||
| 1 (good) | 39 (12.1) | 13 (6.7) | |
| 2 (mild disability) | 84 (26.2) | 44 (22.8) | |
| 3 (moderate disability) | 55 (17.1) | 23 (11.9) | |
| 4 (severe disability) | 12 (3.7) | 12 (6.2) | |
| 5 (Coma/vegetative) | 0 (0) | 0 (0) | |
| 6 (brain death) | 131 (40.8) | 101 (52.3) | |
| PCPC score at hospital discharge, n (%) | 0.001* | ||
| 1 (normal) | 108 (33.6) | 37 (19.2) | |
| 2 (mild disability) | 58 (18.1) | 39 (20.2) | |
| 3 (moderate disability) | 17 (5.3) | 10 (5.2) | |
| 4 (severe disability) | 7 (2.2) | 6 (3.1) | |
| 5 (Coma/vegetative) | 0 (0) | 0 (0) | |
| 6 (brain death) | 131 (40.8) | 101 (52.3) | |
For definition of abbreviations, see Table 8.
FSS is only measured on survivors, whereas POPC and PCPC include death as the worst possible score.
P values are based on the Wilcoxon rank-sum test.
Overall survival for the cohort was 54.9% and significantly differed by indication, but not by age or site (Table E1 and Table 1). In the multivariable Cox model, bleeding events (among other factors) were associated with increased risk of death (Table 10; hazard ratio, 1.75).
Table 10.
Multivariable Cox Model of Time to Death with Time-Varying Covariates
| Adjusted Hazard Ratio (95% CI) | P Value | |
|---|---|---|
| Primary diagnosis | <0.001 | |
| Other | 1.15 (0.51–2.57) | |
| Respiratory | 0.36 (0.23–0.56) | |
| CV/shock | Reference | |
| Baseline hepatic failure | 2.26 (1.50–3.41) | <0.001 |
| Clinical site | 0.006 | |
| A | 1.13 (0.56–2.28) | |
| B | 1.18 (0.49–2.85) | |
| C | 1.00 (0.54–1.86) | |
| D | 0.61 (0.29–1.27) | |
| E | Reference | |
| F | 2.66 (1.39–5.09) | |
| G | 1.83 (0.83–4.03) | |
| H | 1.10 (0.52–2.32) | |
| Premature | 2.30 (1.40–3.79) | 0.001 |
| Baseline feeding tube | 2.01 (1.17–3.45) | 0.012 |
| Bleeding event | 1.75 (1.20–2.55) | 0.004 |
| MAS | 0.23 (0.06–0.97) | 0.045 |
| Baseline temperature (°C) | 0.86 (0.76–0.97) | 0.015 |
Definition of abbreviations: CI = confidence interval; CV = cardiovascular; MAS = meconium aspiration syndrome.
Discussion
In this prospective cohort of 514 consecutively enrolled patients on ECMO, bleeding and thrombotic complications occurred in 70.2 and 37.5% of subjects, respectively, and differed significantly between sites. Intracranial hemorrhage occurred in 16% of subjects. Bleeding and thrombotic complications occurred at rates of 27.5 and 11 per 100 days of ECMO exposure, respectively. These risk rates were relatively constant across the duration of ECMO support, although the majority of events occurred early in the course, as the majority of patients were on ECMO at that time. Laboratory monitoring to manage anticoagulation was highly variable between sites. Laboratory sampling contributed to significant blood loss, being the sole cause of at least one transfusion in 42.2% of subjects. Hemolysis occurred in 57.5% of subjects in centers that regularly monitored plasma-free hemoglobin, and was predictive of a subsequent thrombotic event. The centrifugal pump was not associated with hemolysis, bleeding or thrombotic events, mortality, or morbidity. Overall survival was 54.9% and significantly differed by primary indication for ECMO, but not by age or site. Finally, bleeding and thrombotic events were associated with worse outcomes assessed by POPC and PCPC, but not FSS, in univariable analyses. Bleeding events were associated with increased mortality, but neither hemolysis nor thrombotic complications were associated with increased mortality.
The rates of bleeding and thrombotic events and the mortality of this prospective study population are similar to those reported in previous publications based on ELSO Registry data (1, 18). However, the ELSO Registry is limited by collecting only data on whether a complication occurred at any time during ECMO support, and does not record the number or the timing of events. Because complications were recorded on a daily basis in this prospective study, the risk of each complication per 100 days could be discerned. The estimated incidence of clots by circuit location was also able to be provided (Table 3). The daily incidences (Figure 2) were relatively constant across the first 28 days of ECMO, supporting the argument that the cumulative prevalence of these complications in patients is related to the risk exposure (duration of support).
An unexpected finding of this detailed prospective study was that laboratory blood sampling was the sole contributor to a transfusion requirement at least once in 42.2% of the patients (Table 2), and the incidence of transfusion solely because of laboratory sampling was 14.6 per 100 days of ECMO support (Table 3). Although this was a qualitative assessment by the ECMO specialist at the bedside, data collection included all daily contributors to transfusion in a systematic manner. Because transfusion exposure has demonstrated morbidity and may contribute to multiple organ failure in numerous critical care contexts (19–32), it is plausible that reducing transfusions in patients on ECMO may improve outcome (33).
The variability of anticoagulation management and monitoring has been documented previously (4), and is confirmed in this study. Our study included the frequency of monitoring every day, enabling a better assessment of this variability than is possible with survey data, single-center reports, or registry data (Table E3). Table E4 illustrates the variability of daily monitoring between centers. The variability is striking, and it is clear that prospective, randomized trials in ECMO will require standardization of monitoring practices between participating trial sites. Although anticoagulation monitoring variance between sites may affect bleeding and thrombotic events, other factors, such as indication for ECMO, pump type, flow rates, and age, may also be factors. Center-related volume and patient population have been associated with events and outcome in other reports (33). Further analysis of center variances and observed outcomes is underway.
Earlier publications, including our own retrospective analysis of ELSO data from CPCCRN sites, have reported associations between bleeding and thrombotic complications with adverse outcome (1). Brain death occurred more frequently in patients with these complications (Tables 8 and 9). However, this prospective evaluation found that these associations were not independent of the duration of support and the clinical site. Duration of support (and hence the exposure risk for incurring a complication) is predominantly determined by the underlying disease process, and is not an easily or electively modifiable risk factor for survival. However, the incidence rates per 100 days of bleeding and thrombotic complications, although relatively constant across the first 28 days of ECMO support in this study, may be alterable with standardized and rational management of anticoagulation, ECMO circuitry, and patient care practices. Bleeding events, unlike thrombotic complications or hemolysis, were associated with higher prospective mortality.
Limitations
Several important limitations should be considered when evaluating the results of this study. First, due to the observational design, practice varied between sites in terms of anticoagulation regimen, transfusion triggers, and equipment. To avoid detecting spurious relationships, multivariable models adjusted for clinical site when appropriate, but this may have prevented us from detecting important factors of patient care. For example, other, predominantly single-center, reports have noted conflicting results of adverse effects of hemolysis on outcome as well as linkage to type of pump, membrane lung, inlet pressures, and other factors (5–9). Because ECMO component, pump selection (Table E2), and monitoring for hemolysis (Table E4) differed by site, our understanding of the potential relationships between circuit- and pump-related factors and hemolysis, and their impact on outcome, is severely hampered. In our study, two sites that exclusively used centrifugal pumps monitored plasma-free hemoglobin regularly, and one site that exclusively used the roller pump never monitored free hemoglobin, confounding the assessment of potential relationships between pump type and hemolysis. Our multivariable models do not support any relationship between the centrifugal pump and complications or outcomes, but a definitive answer to these questions will require prospective randomized trials with standardized ECMO circuitry and monitoring of hemolysis.
Second, the variation of monitoring significantly confounds an understanding of the importance of hemolysis. Because our definition of hemolysis was based on plasma-free hemoglobin levels, hemolysis can only be analyzed in sites that measured plasma-free hemoglobin. Hemolysis occurred in 57.5% of patients in these hospitals, and hemolysis was predictive of impending thrombotic events. This suggests that standardized monitoring for hemolysis might allow prediction of ECMO component failure or impending thrombosis, warning of the need for circuit component replacement, and potentially making component replacement less emergent. The extent that elevation in plasma hemoglobin represents unseen patient thrombosis is also unknown. Postmortem evaluations have noted clinically unsuspected major thrombosis, such as pulmonary embolus (34). We did not mandate any specific patient-related investigation if elevated plasma hemoglobin was noted. Future work that examines associations between patient thrombosis, as determined by ultrasound or similar, and plasma hemoglobin has potential benefits (35, 36). Given that half of the centers in our cohort did not monitor plasma hemoglobin, the true incidence of thrombosis is likely underestimated. It is clear that prospective interventional trials in ECMO will need to standardize monitoring for and acting upon evidence of hemolysis.
Third, with the exception of intracranial bleeding, the identification of bleeding events was based on the need for transfusion. Site personnel were trained on the collection of these events, but the subjective nature of our definition may increase the variability in reported rates between sites. Site was considered in multivariable models when appropriate to adjust for this and other potential differences in outcome rates.
Conclusions
Patients receiving ECMO support are subject to risks from their underlying disease, as well as from complications that are related to the duration of ECMO support. The majority of complications occur early in the ECMO course, as many children are on support for less than 1 week. The absolute rate of complications over time, however, remains stable as duration increases. Strategies to improve the outcome of patients receiving ECMO must concentrate on reducing the daily risk of complications. Further investigation into arbitrary blood product transfusion trigger points, as well as the effects of hemolysis and the components used during ECMO, is warranted.
Standardized anticoagulation regimens and monitoring for hemolysis may help identify optimal care practices. Limiting laboratory blood sampling to identified “best practice” may reduce exogenous blood product exposure. Before designing and implementing randomized trials to evaluate specific variables, such as hemolysis or target hemoglobin concentrations for triggering transfusion, standardization of anticoagulation monitoring, circuitry, and other care practices between participating clinical sites will be critically important.
Acknowledgments
Acknowledgment
The authors acknowledge the important contributions of the following research coordinators and Data Coordinating Center staff: Stephanie Bisping, B.S.N., R.N., C.C.R.P., Alecia Peterson, B.S., and Jeri Burr, M.S., R.N.-B.C., C.C.R.C., from University of Utah; Mary Ann DiLiberto, B.S., R.N., C.C.R.C., and Carol Ann Twelves, B.S., R.N., from the Children’s Hospital of Philadelphia; Jean Reardon, M.A., B.S.N., R.N., and Elyse Tomanio, B.S.N., R.N., from Children’s National Medical Center; Aimee Labell, M.S., R.N., from Phoenix Children’s Hospital; Margaret Villa, R.N., and Jeni Kwok, J.D., from Children’s Hospital of Los Angeles; Mary Ann Nyc, B.S., from University of California Los Angeles Mattel Children’s Hospital; Ann Pawluszka, B.S.N., R.N., and Melanie Lulic, B.S., from Children’s Hospital of Michigan; Monica S. Weber, R.N., B.S.N., C.C.R.P., and Lauren Conlin, B.S.N., R.N., C.C.R.P., from University of Michigan; and Alan C. Abraham, B.A., C.C.R.C., from University of Pittsburgh Medical Center.
Footnotes
Supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services cooperative agreements U10HD050096, U10HD049981, U10HD049983, U10HD050012, U10HD063108, U10HD063106, U10HD063114, and U01HD049934. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions: All authors made substantial contributions to the conception and design of the study, reviewed and revised the manuscript critically for important intellectual content, approved the final version submitted for publication, and are accountable for all aspects of the work; H.J.D., R.R., P.G.-F., R. Holubkov, and J.M.D. conducted the experimental and statistical analyses; J.M.D. and R. Holubkov managed data collection throughout the study; H.J.D., R.A.B., A.Z., F.W.M., T.S., M.M.P., C. Newth, J.B., D.W., J.C., M.B., S.H., K.L.M., and R. Harrison enrolled patients and supervised data collection at their clinical sites; R.A.B., T.S., M.M.P., C. Newth, D.W., J.C., K.L.M., J.M.D., and C. Nicholson obtained funding for the study.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201609-1945OC on March 22, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Dalton HJ, Garcia-Filion P, Holubkov R, Moler FW, Shanley T, Heidemann S, Meert K, Berg RA, Berger J, Carcillo J, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network. Association of bleeding and thrombosis with outcome in extracorporeal life support. Pediatr Crit Care Med. 2015;16:167–174. doi: 10.1097/PCC.0000000000000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Werho DK, Pasquali SK, Yu S, Donohue J, Annich GM, Thiagarajan RR, Hirsch-Romano JC, Gaies MG Extracorporeal Life Support Organization Member Centers. Hemorrhagic complications in pediatric cardiac patients on extracorporeal membrane oxygenation: an analysis of the Extracorporeal Life Support Organization Registry. Pediatr Crit Care Med. 2015;16:276–288. doi: 10.1097/PCC.0000000000000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Annich GM. Extracorporeal life support: the precarious balance of hemostasis. J Thromb Haemost. 2015;13(Suppl 1):S336–S342. doi: 10.1111/jth.12963. [DOI] [PubMed] [Google Scholar]
- 4.Bembea MM, Annich G, Rycus P, Oldenburg G, Berkowitz I, Pronovost P. Variability in anticoagulation management of patients on extracorporeal membrane oxygenation: an international survey. Pediatr Crit Care Med. 2013;14:e77–e84. doi: 10.1097/PCC.0b013e31827127e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrett CS, Jaggers JJ, Cook EF, Graham DA, Yarlagadda VV, Teele SA, Almond CS, Bratton SL, Seeger JD, Dalton HJ, et al. Pediatric ECMO outcomes: comparison of centrifugal versus roller blood pumps using propensity score matching. ASAIO J. 2013;59:145–151. doi: 10.1097/MAT.0b013e31828387cd. [DOI] [PubMed] [Google Scholar]
- 6.Halaweish I, Cole A, Cooley E, Lynch WR, Haft JW. Roller and centrifugal pumps: a retrospective comparison of bleeding complications in extracorporeal membrane oxygenation. ASAIO J. 2015;61:496–501. doi: 10.1097/MAT.0000000000000243. [DOI] [PubMed] [Google Scholar]
- 7.Lou S, MacLaren G, Best D, Delzoppo C, Butt W. Hemolysis in pediatric patients receiving centrifugal-pump extracorporeal membrane oxygenation: prevalence, risk factors, and outcomes. Crit Care Med. 2014;42:1213–1220. doi: 10.1097/CCM.0000000000000128. [DOI] [PubMed] [Google Scholar]
- 8.Byrnes J, McKamie W, Swearingen C, Prodhan P, Bhutta A, Jaquiss R, Imamura M, Fiser R. Hemolysis during cardiac extracorporeal membrane oxygenation: a case–control comparison of roller pumps and centrifugal pumps in a pediatric population. ASAIO J. 2011;57:456–461. doi: 10.1097/MAT.0b013e31822e2475. [DOI] [PubMed] [Google Scholar]
- 9.Toomasian JM, Bartlett RH. Hemolysis and ECMO pumps in the 21st century. Perfusion. 2011;26:5–6. doi: 10.1177/0267659110396015. [DOI] [PubMed] [Google Scholar]
- 10.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated pediatric risk of mortality score. Crit Care Med. 1996;24:743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Pollack MM, Patel KM, Ruttimann UE. The Pediatric Risk of Mortality III–Acute Physiology Score (PRISM III–APS): a method of assessing physiologic instability for pediatric intensive care unit patients. J Pediatr. 1997;131:575–581. doi: 10.1016/s0022-3476(97)70065-9. [DOI] [PubMed] [Google Scholar]
- 12.Gaies MG, Gurney JG, Yen AH, Napoli ML, Gajarski RJ, Ohye RG, Charpie JR, Hirsch JC. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med. 2010;11:234–238. doi: 10.1097/PCC.0b013e3181b806fc. [DOI] [PubMed] [Google Scholar]
- 13.Gaies MG, Jeffries HE, Niebler RA, Pasquali SK, Donohue JE, Yu S, Gall C, Rice TB, Thiagarajan RR. Vasoactive-inotropic score is associated with outcome after infant cardiac surgery: an analysis from the Pediatric Cardiac Critical Care Consortium and Virtual PICU System registries. Pediatr Crit Care Med. 2014;15:529–537. doi: 10.1097/PCC.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollack MM, Holubkov R, Funai T, Clark A, Berger JT, Meert K, Newth CJ, Shanley T, Moler F, Carcillo J, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network. Pediatric intensive care outcomes: development of new morbidities during pediatric critical care. Pediatr Crit Care Med. 2014;15:821–827. doi: 10.1097/PCC.0000000000000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pollack MM, Holubkov R, Funai T, Clark A, Moler F, Shanley T, Meert K, Newth CJ, Carcillo J, Berger JT, et al. Relationship between the functional status scale and the pediatric overall performance category and pediatric cerebral performance category scales. JAMA Pediatr. 2014;168:671–676. doi: 10.1001/jamapediatrics.2013.5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pollack MM, Holubkov R, Glass P, Dean JM, Meert KL, Zimmerman J, Anand KJ, Carcillo J, Newth CJ, Harrison R, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network. Functional Status Scale: new pediatric outcome measure. Pediatrics. 2009;124:e18–e28. doi: 10.1542/peds.2008-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121:68–74. doi: 10.1016/s0022-3476(05)82544-2. [DOI] [PubMed] [Google Scholar]
- 18.Joffe AR, Lequier L, Robertson CM. Pediatric outcomes after extracorporeal membrane oxygenation for cardiac disease and for cardiac arrest: a review. ASAIO J. 2012;58:297–310. doi: 10.1097/MAT.0b013e31825a21ff. [DOI] [PubMed] [Google Scholar]
- 19.Bateman ST, Lacroix J, Boven K, Forbes P, Barton R, Thomas NJ, Jacobs B, Markovitz B, Goldstein B, Hanson JH, et al. Pediatric Acute Lung Injury and Sepsis Investigators Network. Anemia, blood loss, and blood transfusions in North American children in the intensive care unit. Am J Respir Crit Care Med. 2008;178:26–33. doi: 10.1164/rccm.200711-1637OC. [DOI] [PubMed] [Google Scholar]
- 20.Boutin A, Chassé M, Shemilt M, Lauzier F, Moore L, Zarychanski R, Griesdale D, Desjardins P, Lacroix J, Fergusson D, et al. Red blood cell transfusion in patients with traumatic brain injury: a systematic review and meta-analysis. Transfus Med Rev. 2016;30:15–24. doi: 10.1016/j.tmrv.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Corwin HL, Gettinger A, Pearl RG, Fink MP, Levy MM, Abraham E, MacIntyre NR, Shabot MM, Duh MS, Shapiro MJ. The CRIT Study: anemia and blood transfusion in the critically ill—current clinical practice in the United States. Crit Care Med. 2004;32:39–52. doi: 10.1097/01.CCM.0000104112.34142.79. [DOI] [PubMed] [Google Scholar]
- 22.Ferraris VA, Davenport DL, Saha SP, Bernard A, Austin PC, Zwischenberger JB. Intraoperative transfusion of small amounts of blood heralds worse postoperative outcome in patients having noncardiac thoracic operations. Ann Thorac Surg. 2011;91:1674–1680. doi: 10.1016/j.athoracsur.2011.01.025. [Discussion, p. 80.] [DOI] [PubMed] [Google Scholar]
- 23.Gauvin F, Lacroix J. Should we avoid transfusion in the pediatric intensive care unit? Pediatr Crit Care Med. 2009;10:400–401. doi: 10.1097/PCC.0b013e31819adef8. [DOI] [PubMed] [Google Scholar]
- 24.Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care: Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 25.Hébert PC, Wells G, Martin C, Tweeddale M, Marshall J, Blajchman M, Pagliarello G, Sandham D, Schweitzer I, Boisvert D, et al. Variation in red cell transfusion practice in the intensive care unit: a multicentre cohort study. Crit Care. 1999;3:57–63. doi: 10.1186/cc310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karam O, Tucci M, Ducruet T, Hume HA, Lacroix J, Gauvin F Canadian Critical Care Trials Group; PALISI Network. Red blood cell transfusion thresholds in pediatric patients with sepsis. Pediatr Crit Care Med. 2011;12:512–518. doi: 10.1097/PCC.0b013e3181fe344b. [DOI] [PubMed] [Google Scholar]
- 27.Kleiber N, Lefebvre É, Gauvin F, Tucci M, Robitaille N, Trottier H, Jouvet P, Ducruet T, Poitras N, Lacroix J, et al. Respiratory dysfunction associated with RBC transfusion in critically ill children: a prospective cohort study. Pediatr Crit Care Med. 2015;16:325–334. doi: 10.1097/PCC.0000000000000365. [DOI] [PubMed] [Google Scholar]
- 28.Lacroix J, Demaret P, Tucci M. Red blood cell transfusion: decision making in pediatric intensive care units. Semin Perinatol. 2012;36:225–231. doi: 10.1053/j.semperi.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Lacroix J, Hébert PC, Hutchison JS, Hume HA, Tucci M, Ducruet T, Gauvin F, Collet JP, Toledano BJ, Robillard P, et al. TRIPICU Investigators; Canadian Critical Care Trials Group; Pediatric Acute Lung Injury and Sepsis Investigators Network. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609–1619. doi: 10.1056/NEJMoa066240. [DOI] [PubMed] [Google Scholar]
- 30.Napolitano LM, Corwin HL. Efficacy of red blood cell transfusion in the critically ill. Crit Care Clin. 2004;20:255–268. doi: 10.1016/j.ccc.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Toledano B, Tucci M, Lacroix J. Red cell transfusion to cardiac patients: facts and fallacies. Pediatr Crit Care Med. 2011;12:107–108. doi: 10.1097/PCC.0b013e3181e289eb. [DOI] [PubMed] [Google Scholar]
- 32.Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A, Meier-Hellmann A, Nollet G, Peres-Bota D ABC (Anemia and Blood Transfusion in Critical Care) Investigators. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288:1499–1507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- 33.Agerstrand CL, Burkart KM, Abrams DC, Bacchetta MD, Brodie D. Blood conservation in extracorporeal membrane oxygenation for acute respiratory distress syndrome. Ann Thorac Surg. 2015;99:590–595. doi: 10.1016/j.athoracsur.2014.08.039. [DOI] [PubMed] [Google Scholar]
- 34.Barbaro RP, Odetola FO, Kidwell KM, Paden ML, Bartlett RH, Davis MM, Annich GM. Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality: analysis of the extracorporeal life support organization registry. Am J Respir Crit Care Med. 2015;191:894–901. doi: 10.1164/rccm.201409-1634OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rastan AJ, Lachmann N, Walther T, Doll N, Gradistanac T, Gommert JF, Lehmann S, Wittekind C, Mohr FW. Autopsy findings in patients on postcardiotomy extracorporeal membrane oxygenation (ECMO) Int J Artif Organs. 2006;29:1121–1131. doi: 10.1177/039139880602901205. [DOI] [PubMed] [Google Scholar]
- 36.Sud S, Mittmann N, Cook DJ, Geerts W, Chan B, Dodek P, Gould MK, Guyatt G, Arabi Y, Fowler RA Canadian Critical Care Trials Group; E-PROTECT Investigators. Screening and prevention of venous thromboembolism in critically ill patients: a decision analysis and economic evaluation. Am J Respir Crit Care Med. 2011;184:1289–1298. doi: 10.1164/rccm.201106-1059OC. [DOI] [PubMed] [Google Scholar]