Abstract
Rationale: Respiratory-related hospitalizations of patients with idiopathic pulmonary fibrosis (IPF) are more frequent than those for acute IPF exacerbations and are associated with poor outcomes.
Objectives: To compare the risk of nonelective hospitalization by type (all-cause, respiratory related, and non–respiratory related) and death after hospitalization with use of pirfenidone versus placebo over 52 weeks using data derived from three phase III IPF clinical trials.
Methods: Individual patient data was pooled from three phase III randomized, placebo-controlled studies of pirfenidone for IPF (the two CAPACITY [Clinical Studies Assessing Pirfenidone in IPF: Research of Efficacy and Safety Outcomes] trials and the ASCEND [Assessment of Pirfenidone to Confirm Efficacy and Safety in Idiopathic Pulmonary Fibrosis] trial), including all patients randomized to pirfenidone 2,403 mg/d (n = 623) or placebo (n = 624). The risk of hospitalization over 52 weeks was compared using standard time-to-event methods. Among those hospitalized, the risk of death after hospitalization was compared with adjustment for treatment group propensity.
Measurements and Main Results: A total of 1,247 patients (692 from the CAPACITY trials and 555 from the ASCEND trial) were included in the pooled analysis. Pirfenidone was associated with lower risk of respiratory-related hospitalization than placebo (7% vs. 12%; hazard ratio [HR], 0.52; 95% confidence interval [CI], 0.36–0.77; P = 0.001), but all-cause (HR, 0.91; 95% CI, 0.70–1.19; P = 0.528) or non–respiratory-related hospitalization (HR, 1.32; 95% CI, 0.92–1.88; P = 0.145) was not. Among those hospitalized for any reason, treatment with pirfenidone was associated with lower risk of death after hospitalization up to 52 weeks after randomization, but this association was no longer significant with longer follow-up.
Conclusions: In a pooled analysis of three phase III IPF clinical trials, patients receiving pirfenidone had a lower risk of nonelective respiratory-related hospitalization over the course of 1 year. The effect of pirfenidone on death after hospitalization is uncertain.
Keywords: idiopathic pulmonary fibrosis, pirfenidone, hospitalization, mortality
At a Glance Summary
Scientific Knowledge on the Subject
Nonelective hospitalizations, especially those for respiratory reasons, are associated with increased mortality and health care costs in idiopathic pulmonary fibrosis (IPF). Pirfenidone is proven to reduce the decline in FVC in IPF, but the impact of pirfenidone on hospitalizations is unknown.
What This Study Adds to the Field
Analysis of pooled individual patient data from three phase III IPF trials demonstrated that pirfenidone treatment is associated with a reduced risk of respiratory-related hospitalization and possibly death after hospitalization of any type among patients with IPF.
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive lung disease occurring primarily in older adults, with a median survival of 3–5 years from diagnosis (1). During their disease course, most patients with IPF will be hospitalized several times, for both respiratory and non–respiratory-related reasons (2–5). Respiratory-related hospitalizations are particularly important clinical events in IPF because of the associated morbidity, cost, and short-term mortality risk (2, 3, 5–12). Respiratory-related hospitalizations are more easily identified than acute IPF exacerbations and are directly relevant to how patients feel, function, and survive (13–16). Therefore, treatments that reduce the risk and/or morbidity of respiratory-related hospitalizations could have a major impact in the field.
Pirfenidone is a U.S. Food and Drug Administration–approved treatment for IPF that is proven to slow disease progression as defined by 1-year decline in FVC (17–19). Pirfenidone has also been shown to reduce decline in exercise capacity as defined by 6-minute-walk distance (6MWD) and progression-free survival over the course of 1 year as defined by decline in FVC, decline in 6MWD, or death (18). Pooled analysis of three phase III clinical trials in IPF has also suggested a significant reduction in 1-year mortality (18, 20, 21). However, the impact of pirfenidone on the risk of respiratory hospitalization and subsequent mortality remains unknown.
The objective of this study was to evaluate the 1-year risk of nonelective hospitalization (including all-cause, respiratory related, and non–respiratory related) and death after hospital admission among patients randomized to pirfenidone versus placebo, using data derived from three large phase III clinical trials of pirfenidone in IPF.
Methods
Study Population
The study population included all patients randomized to pirfenidone 2,403 mg daily or placebo in three completed phase III trials: the two CAPACITY (Clinical Studies Assessing Pirfenidone in IPF: Research of Efficacy and Safety Outcomes) trials (004 and 006) (17) and the ASCEND (Assessment of Pirfenidone to Confirm Efficacy and Safety in Idiopathic Pulmonary Fibrosis) trial (18). The enrollment criteria for CAPACITY and ASCEND are described in more detail elsewhere (17, 18). All patients were required to have a diagnosis of IPF (confident diagnosis by the local investigator for CAPACITY and centrally confirmed diagnosis according to international criteria [1] for ASCEND), an FVC percent predicted of at least 50%, and a 6MWD of at least 150 m. Key differences were in the diffusing capacity of the lung for carbon monoxide (DlCO) percent predicted cutoff (≥35% for CAPACITY vs. ≥30% for ASCEND), post-bronchodilator FEV1/FVC ratio (≥70% for CAPACITY vs. ≥80% for ASCEND), and upper limits allowed for FVC and DlCO percent predicted (either required to be ≤90% for CAPACITY vs. both required to be ≤90% for ASCEND).
Study Design
Patients were followed for 72–120 weeks and 52 weeks from the time of randomization in the CAPACITY and ASCEND trials, respectively. Clinical assessments, including pulmonary function testing, were performed at randomization and every 12 weeks in the CAPACITY trials and every 13 weeks in the ASCEND trial for the duration of the trials. The primary endpoint for both CAPACITY and ASCEND was change in FVC percent predicted from randomization to the end of the trial (72 weeks in CAPACITY vs. 52 weeks in ASCEND). CAPACITY 004 and ASCEND were positive studies, whereas CAPACITY trial 006 failed to meet its primary endpoint (16). In CAPACITY, hospitalization was a prespecified secondary endpoint, and the local site investigator selected the primary reason for hospitalization from among acute respiratory decompensation; IPF exacerbation; pneumonia; respiratory related, other; or non–respiratory related. For this analysis, all but non–respiratory related were considered respiratory-related hospitalizations. In ASCEND, hospitalizations were recorded as serious adverse events. For the purposes of this study, all hospitalizations in ASCEND were retrospectively reviewed independently by two experienced pulmonologists blinded to treatment group and categorized as either respiratory related or non–respiratory related. Discordance (n = 1) was independently adjudicated by a third experienced pulmonologist blinded to treatment group.
Statistical Analysis
Individual patient data from all randomized patients were pooled and analyzed according to treatment assignment at randomization (i.e., based on intention to treat). Follow-up was censored at the time of loss to follow-up, death, or administratively at 52 weeks. Deaths after admission to the first hospitalization were recorded. Elective hospitalizations for lung transplant were excluded.
Cox proportional hazards models stratified by trial, Kaplan-Meier curves, and the log-rank test were used to compare time to first nonelective hospitalization (including all-cause, non–respiratory related, and respiratory related) in pirfenidone-treated versus placebo-treated patients. Sensitivity analyses were performed, including (1) a repeated failure analysis for hospitalizations (i.e., not restricted to time to first hospitalization), (2) including trial as a covariate in Cox models with testing for trial-by-treatment interactions, and (3) extending follow-up time to 72 weeks for the CAPACITY trials.
To evaluate the risk of death after admission for the first nonelective hospitalization, we compared the time to death after hospital admission by treatment group among those patients hospitalized. Because this subgroup of patients was selected by a postrandomization factor (hospitalization), we performed a propensity score–adjusted analysis to account for potential confounding between treatment assignment and postadmission death. The propensity score model was constructed using a multivariable logistic regression model for treatment assignment (pirfenidone vs. placebo) among those hospitalized with inclusion of all demographic and baseline characteristics that had a P value less than 0.25 in univariate analysis for each hospitalization type. A Cox proportional hazards model was then used for time to death after hospital admission, including treatment assignment (pirfenidone vs. placebo) and adjustment for the propensity score. The primary analysis was again restricted to 52 weeks after randomization to align trials, and a sensitivity analysis was performed using up to 72 weeks of follow-up in the CAPACITY trials.
Results
Cohort Characteristics and Hospitalizations
A total of 1,247 patients were enrolled (CAPACITY, n = 692; ASCEND, n = 555). Of these, 623 were randomized to treatment with pirfenidone 2,403 mg daily, and 624 were randomized to placebo. Baseline characteristics of the pooled cohort are shown in Table 1 and are well matched, as expected for randomized trials. There were a total of 139 nonelective hospitalizations (54 respiratory related and 85 non–respiratory related) in pirfenidone-treated patients and 151 nonelective hospitalizations (87 respiratory related and 64 non–respiratory related) in placebo-treated patients (Table 2). Hospitalization rates were similar for centers in the United States (18.4% [165 of 897] of patients had one or more all-cause hospitalizations and 8.7% [78 of 897] had one or more respiratory-related hospitalizations) and non-U.S. centers (16.0% [56 of 350] of patients had one or more all-cause hospitalizations and 10.6% [37 of 350] had one or more respiratory-related hospitalizations).
Table 1.
Characteristics of Patients Pooled from Three Phase III Trials of Pirfenidone versus Placebo in Idiopathic Pulmonary Fibrosis
| Characteristic | Pirfenidone (n = 623) | Placebo (n = 624) |
|---|---|---|
| Age, yr, mean (SD) | 67.2 (7.6) | 67.1 (7.5) |
| Male sex, n (%) | 463 (74.3) | 465 (74.5) |
| White race, n (%) | 592 (95.0) | 590 (94.6) |
| FVC, % predicted, mean (SD) | 71.6 (13.2) | 72.0 (13.6) |
| DlCO, % predicted, mean (SD) | 45.6 (10.2) | 45.6 (11.1) |
| FEV1/FVC ratio, mean (SD) | 0.84 (0.04) | 0.83 (0.05) |
| 6MWD, m, mean (SD) | 403.8 (93.7) | 411.8 (94.2) |
| UCSD SOBQ, mean (SD) | 34.2 (21.4) | 34.9 (21.6) |
| Supplemental oxygen, n (%) | 155 (24.9) | 150 (24.0) |
| HRCT-based definite UIP, n (%) | 574 (92.3) | 584 (93.6) |
| Time since diagnosis, yr, median (range) | 1.1 (>0–5) | 1.1 (>0–4) |
Definition of abbreviations: 6MWD = 6-minute-walk distance; DlCO = diffusing capacity of the lung for carbon monoxide; HRCT = high-resolution computed tomography; UCSD SOBQ = University of California, San Diego Shortness of Breath Questionnaire; UIP = usual interstitial pneumonia.
Table 2.
Nonelective Hospitalization Events by Type (All-Cause, Respiratory Related, and Non–Respiratory Related) and by Treatment Group
| All-Cause Hospitalizations |
Respiratory Hospitalizations |
Nonrespiratory Hospitalizations |
||||
|---|---|---|---|---|---|---|
| Pirfenidone (n = 623) | Placebo (n = 624) | Pirfenidone (n = 623) | Placebo (n = 624) | Pirfenidone (n = 623) | Placebo (n = 624) | |
| Total events, n | 139 | 151 | 54 | 87 | 85 | 64 |
| Patients with at least one event, n (%) | 106 (17) | 115 (18) | 41 (7) | 74 (12) | 70 (11) | 54 (9) |
| Events per 100 person-years | 23.1 | 25.3 | 9.0 | 14.6 | 14.1 | 10.7 |
Risk of Hospitalization
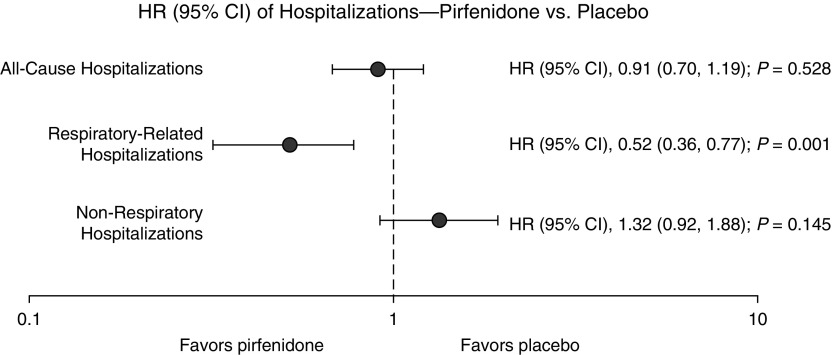
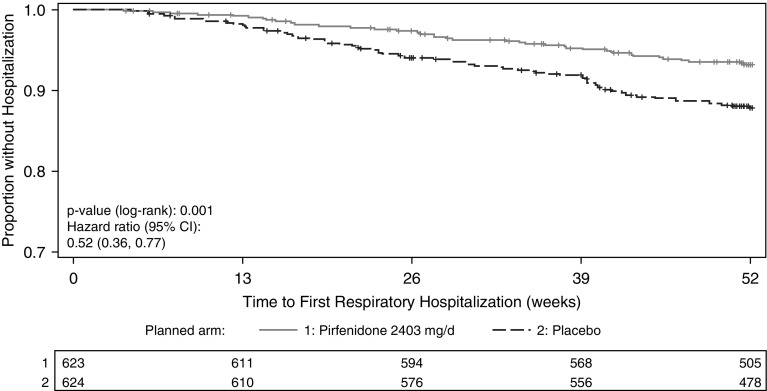
In pirfenidone-treated patients, 17% (106 of 623) experienced at least one nonelective hospitalization, 7% (41 of 623) experienced at least one respiratory-related hospitalization, and 11% (70 of 623) experienced at least one non–respiratory-related hospitalization (Table 2). In placebo-treated patients, 18% (115 of 624) experienced at least one nonelective hospitalization, 12% (74 of 624) experienced at least one respiratory-related hospitalization, and 9% (54 of 624) experienced at least one non–respiratory-related hospitalization. Pirfenidone-treated patients were less likely than placebo-treated patients to experience respiratory-related hospitalization (hazard ratio [HR], 0.52; 95% confidence interval [CI], 0.36–077; P = 0.001 by log-rank test) (Figures 1 and 2). There were no significant differences in risk of all-cause hospitalization (HR, 0.91; 95% CI, 0.70–1.19; P = 0.528 by log-rank test) or non–respiratory-related hospitalizations (HR, 1.32; 95% CI, 0.92–1.88; P = 0.145 by log-rank test) (Figure 1). The results were similar when we used a repeated-failures analysis for all hospitalizations (see Table E1 in the online supplement) and after extending follow-up time in CAPACITY to 72 weeks (Table E2). We did not find evidence for significant treatment-by-trial interactions on risk of hospitalization of any type (Table E3).
Figure 1.
Forest plot demonstrating risk of first nonelective hospitalization by type (all-cause, respiratory related, and non–respiratory related) in pirfenidone-treated patients compared with placebo-treated patients. CI = confidence interval; HR = hazard ratio.
Figure 2.
Kaplan-Meier plot for time to first nonelective respiratory-related hospitalization in pirfenidone-treated patients compared with placebo-treated patients. CI = confidence interval.
Death after Hospitalization
Among those with at least one nonelective hospitalization, 18 of 106 (17%) died in the pirfenidone group and 37 of 115 (32%) died in the placebo group (Table 3), which was consistent with an association between allocation to the pirfenidone arm and lower risk of death in unadjusted (HR, 0.49; 95% CI, 0.28–0.86; P = 0.013) and propensity score–adjusted (HR, 0.56; 95% CI, 0.32–0.99; P = 0.047) analyses (Table 4). This difference appeared to be driven largely by reduced risk of death among those hospitalized for respiratory-related reasons (Tables 3 and 4). However, this association was no longer significant when we considered follow-up to 72 weeks in the CAPACITY trials (Table E4).
Table 3.
Deaths Occurring after Nonelective Admission to the Hospital, by Hospitalization Type (All-Cause, Respiratory Related, and Non–Respiratory Related) and by Treatment Group
| All-Cause Hospitalizations |
Respiratory Hospitalizations |
Nonrespiratory Hospitalizations |
||||
|---|---|---|---|---|---|---|
| Pirfenidone (n = 106) | Placebo (n = 115) | Pirfenidone (n = 41) | Placebo (n = 74) | Pirfenidone (n = 70) | Placebo (n = 54) | |
| Deaths, n (%) | 18 (17) | 37 (32) | 11 (27) | 34 (46) | 8 (11) | 9 (17) |
The n values given in column heads are the number of patients with at least one hospitalization.
Table 4.
Risk of Death after Nonelective Hospitalization, by Type (All-Cause, Respiratory Related, and Non–Respiratory Related) in Pirfenidone-treated Patients versus Placebo-treated Patients, Unadjusted and Adjusted for Treatment Propensity
| Hazard Ratio | 95% Confidence Interval | P Value | |
|---|---|---|---|
| All-cause hospitalization (n = 221*) | |||
| Unadjusted | 0.49 | 0.28–0.86 | 0.013 |
| Adjusted for propensity score† | 0.56 | 0.32–0.99 | 0.047 |
| Respiratory-related hospitalization (n = 115*) | |||
| Unadjusted | 0.55 | 0.28–1.08 | 0.082 |
| Adjusted for propensity score† | 0.50 | 0.25–1.03 | 0.061 |
| Non–respiratory-related hospitalization (n = 124*) | |||
| Unadjusted | 0.67 | 0.26–1.74 | 0.412 |
| Adjusted for propensity score† | 0.73 | 0.27–1.97 | 0.537 |
Definition of abbreviations: DlCO% = diffusing capacity of the lung for carbon monoxide percent predicted; GAP = Gender-Age-Physiology Index.
Number of patients experiencing at least one event.
Propensity score models were constructed using multivariable logistic regression for treatment assignment (pirfenidone vs. placebo) among those hospitalized with inclusion of demographic and baseline characteristics that had a P value less than 0.25 in univariate analysis for each hospitalization type. For all-cause hospitalization, the propensity score included GAP stage, baseline oxygen use, years since idiopathic pulmonary fibrosis diagnosis, and baseline DlCO%. For respiratory-related hospitalization, the propensity score included study, GAP stage, racial group, baseline DlCO%, baseline 6-minute-walk distance, and baseline University of California, San Diego Shortness of Breath Questionnaire score. For nonrespiratory hospitalization, the propensity score included GAP stage, age, baseline body mass index, years since idiopathic pulmonary fibrosis diagnosis, baseline DlCO%, and baseline 6-minute-walk distance.
Discussion
On the basis of pooled individual patient data from three phase III clinical trials of pirfenidone in IPF, this study demonstrates that treatment of IPF with pirfenidone is associated with a significant reduction in the risk of hospitalization for respiratory reasons over the course of 1 year of therapy. It also suggests a reduced risk of death after any hospital admission (respiratory related or not) over the course of 1 year for those taking pirfenidone compared with placebo. To our knowledge, this is the first study reporting a beneficial effect of any treatment on respiratory hospitalizations in IPF. Respiratory hospitalizations are clinically relevant events for patients with IPF with a high risk of short-term mortality as well as excessive morbidity and additional health care costs; therefore, prevention of respiratory hospitalizations should be regarded as an important goal of therapy.
How pirfenidone results in reduced risk of respiratory hospitalization is unclear, considering the multiple reasons that a patient with IPF may be hospitalized with worsening respiratory symptoms (2). One plausible explanation is that pirfenidone, by preserving lung function (physiological reserve) (17, 18), reduces the relative risk of acute IPF exacerbation. Unfortunately, we were unable to examine this hypothesis directly, because acute exacerbations were not systematically collected and adjudicated in the source trials. Another possibility is that pirfenidone reduces disease activity, thereby limiting the collateral damage resulting from respiratory stressors such as infection that may drive admissions. Regardless of the reason, the effect of pirfenidone on reducing nonelective hospitalizations seems to be specific to those resulting from worsening respiratory symptoms and not other nonpulmonary acute events that result in hospitalization in the older IPF population. Although not statistically significant, a numerical increase in nonrespiratory hospitalizations in pirfenidone compared with placebo-treated patients was observed that is of unclear clinical significance. It is reassuring that despite this, the risk of death after nonrespiratory hospitalization was not increased in pirfenidone-treated patients, and in contrast to respiratory hospitalizations, nonrespiratory reasons for hospitalization in patients with IPF do not appear to be associated with increased risk of subsequent mortality (6). We also did not observe an increase in any specific reason for nonrespiratory hospitalization in pirfenidone-treated versus placebo-treated patients, but our study lacked statistical power for definitive conclusions to be drawn.
Although decline in FVC has served as an important surrogate endpoint in IPF trials, there is a strong desire to identify clinically meaningful endpoints more directly (14–16, 22). This study lends further support for respiratory hospitalization as a modifiable and clinically meaningful endpoint in IPF trials (6, 12). Respiratory hospitalizations can be enriched by including patients with greater physiological impairment, an important aspect of efficient clinical trial design lacking for FVC decline (23). Concerns regarding varying rationales for hospitalization across countries are not supported by our data, which show consistent rates of respiratory hospitalization, regardless of region.
There are important limitations to this study. First, it is a post hoc analysis of pooled data. However, this is a large dataset of well-characterized patients with IPF and high-quality prospective data collection, and in our study we analyzed individual patient–level data, allowing for more flexible accounting for confounding factors (e.g., propensity scores) than is possible in conventional meta-analyses. Also, the observed reduction in respiratory hospitalizations with pirfenidone versus placebo is consistent with the positive findings for other disease progression events, such as decline in FVC, observed in these trials. Second, the methods of collection and adjudication of hospitalization events differed between the source studies, which may have affected the accuracy of hospitalization classification. We believe this to be unlikely, considering the near-complete agreement between independent adjudicators for the ASCEND study. Although misclassification of hospitalization type could reduce the observed effect size, investigative sites (CAPACITY) and adjudicators (ASCEND) were blinded to treatment assignment, and thus their classification of hospital type should not have biased our results. Importantly, we found no significant effect of trial or trial-by-treatment interaction in the analyses. Third, in the analysis of death after hospitalization, we selected patients on a postrandomization factor (hospitalization), thus introducing the possibility of unmeasured confounders. Our use of a propensity score helps limit, but does not eliminate, the possibility of confounding in this analysis. We acknowledge that it is also difficult to explain how pirfenidone might reduce the risk of death after hospitalization of any type, especially in light of the fact that we observed a nonsignificant increase in rates of nonrespiratory hospitalization in the pirfenidone group. Although not statistically significant in isolation, this association appears to be driven primarily by lower risk of death after respiratory hospitalizations. These results should be interpreted with caution because the association is no longer significant with longer-term follow-up. Finally, the small number of respiratory hospitalization events and the retrospective data review limit our ability to decipher which types of respiratory events were and were not impacted by pirfenidone therapy.
In conclusion, pooled data from three phase III clinical trials in IPF indicate that pirfenidone reduces the risk of hospitalization for respiratory reasons. Future studies are needed to examine the long-term effects of pirfenidone on hospitalization rates, outcomes of hospitalization, and health care costs in IPF. We believe that reducing the risk of respiratory hospitalization is an important goal of therapy, and it should be included as a key endpoint in future IPF trials.
Acknowledgments
Acknowledgment
The authors thank Elizabeth Morgenthien for providing valuable statistical support in this study.
Footnotes
This study, including data collection and statistical analysis, was funded by Genentech, Inc. Support was also received through National Institutes of Health grants KL2TR001870 (B.L.) and K24 HL127131 (H.R.C.).
Author Contributions: B.D. and K.R.: collected data; B.L., J.L.S., and H.R.C.: adjudicated events; B.D.: performed the data analysis; and B.L. and H.R.C.: wrote the original manuscript. All authors designed the study; all authors contributed to data interpretation; and all authors contributed to revisions of the manuscript, provided final approval of the version to be published, and agree to be accountable for all aspects of the work.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201701-0091OC on May 4, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu YF, Wu N, Chuang CC, Wang R, Pan X, Benjamin NN, Devercelli G, Coultas DB. Patterns and economic burden of hospitalizations and exacerbations among patients diagnosed with idiopathic pulmonary fibrosis. J Manag Care Spec Pharm. 2016;22:414–423. doi: 10.18553/jmcp.2016.22.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raimundo K, Chang E, Broder MS, Alexander K, Zazzali J, Swigris JJ. Clinical and economic burden of idiopathic pulmonary fibrosis: a retrospective cohort study. BMC Pulm Med. 2016;16:2. doi: 10.1186/s12890-015-0165-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collard HR, Ward AJ, Lanes S, Cortney Hayflinger D, Rosenberg DM, Hunsche E. Burden of illness in idiopathic pulmonary fibrosis. J Med Econ. 2012;15:829–835. doi: 10.3111/13696998.2012.680553. [DOI] [PubMed] [Google Scholar]
- 5.Collard HR, Chen SY, Yeh WS, Li Q, Lee YC, Wang A, Raghu G. Health care utilization and costs of idiopathic pulmonary fibrosis in U.S. Medicare beneficiaries aged 65 years and older. Ann Am Thorac Soc. 2015;12:981–987. doi: 10.1513/AnnalsATS.201412-553OC. [DOI] [PubMed] [Google Scholar]
- 6.Durheim MT, Collard HR, Roberts RS, Brown KK, Flaherty KR, King TE, Jr, Palmer SM, Raghu G, Snyder LD, Anstrom KJ, et al. IPFnet investigators. Association of hospital admission and forced vital capacity endpoints with survival in patients with idiopathic pulmonary fibrosis: analysis of a pooled cohort from three clinical trials. Lancet Respir Med. 2015;3:388–396. doi: 10.1016/S2213-2600(15)00093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song JW, Hong SB, Lim CM, Koh Y, Kim DS. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J. 2011;37:356–363. doi: 10.1183/09031936.00159709. [DOI] [PubMed] [Google Scholar]
- 8.du Bois RM, Weycker D, Albera C, Bradford WZ, Costabel U, Kartashov A, Lancaster L, Noble PW, Raghu G, Sahn SA, et al. Ascertainment of individual risk of mortality for patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184:459–466. doi: 10.1164/rccm.201011-1790OC. [DOI] [PubMed] [Google Scholar]
- 9.Brown AW, Fischer CP, Shlobin OA, Buhr RG, Ahmad S, Weir NA, Nathan SD. Outcomes after hospitalization in idiopathic pulmonary fibrosis: a cohort study. Chest. 2015;147:173–179. doi: 10.1378/chest.13-2424. [DOI] [PubMed] [Google Scholar]
- 10.Navaratnam V, Fogarty AW, Glendening R, McKeever T, Hubbard RB. The increasing secondary care burden of idiopathic pulmonary fibrosis: hospital admission trends in England from 1998 to 2010. Chest. 2013;143:1078–1084. doi: 10.1378/chest.12-0803. [DOI] [PubMed] [Google Scholar]
- 11.Yu YF, Macaulay DS, Reichmann WM, Wu EQ, Nathan SD. Association of early suspected acute exacerbations of idiopathic pulmonary fibrosis with subsequent clinical outcomes and healthcare resource utilization. Respir Med. 2015;109:1582–1588. doi: 10.1016/j.rmed.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Paterniti MO, Bi Y, Rekić D, Wang Y, Karimi-Shah BA, Chowdhury BA. Acute exacerbation and decline in forced vital capacity are associated with increased mortality in idiopathic pulmonary fibrosis. Ann Am Thorac Soc. doi: 10.1513/AnnalsATS.201606-458OC. [online ahead of print] 7 Apr 2017; DOI: 10.1513/AnnalsATS.201606-458OC. [DOI] [PubMed] [Google Scholar]
- 13.Collard HR, Ryerson CJ, Corte TJ, Jenkins G, Kondoh Y, Lederer DJ, Lee JS, Maher TM, Wells AU, Antoniou KM, et al. Acute Exacerbation of idiopathic pulmonary fibrosis: an international working group report. Am J Respir Crit Care Med. 2016;194:265–275. doi: 10.1164/rccm.201604-0801CI. [DOI] [PubMed] [Google Scholar]
- 14.Raghu G, Collard HR, Anstrom KJ, Flaherty KR, Fleming TR, King TE, Jr, Martinez FJ, Brown KK. Idiopathic pulmonary fibrosis: clinically meaningful primary endpoints in phase 3 clinical trials. Am J Respir Crit Care Med. 2012;185:1044–1048. doi: 10.1164/rccm.201201-0006PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collard HR, Brown KK, Martinez FJ, Raghu G, Roberts RS, Anstrom KJ IPFnet Investigators. Study design implications of death and hospitalization as end points in idiopathic pulmonary fibrosis. Chest. 2014;146:1256–1262. doi: 10.1378/chest.14-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nathan SD, Meyer KC. IPF clinical trial design and endpoints. Curr Opin Pulm Med. 2014;20:463–471. doi: 10.1097/MCP.0000000000000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, King TE, Jr, Lancaster L, Sahn SA, Szwarcberg J, et al. CAPACITY Study Group. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377:1760–1769. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 18.King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L, et al. ASCEND Study Group. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 19.Karimi-Shah BA, Chowdhury BA. Forced vital capacity in idiopathic pulmonary fibrosis—FDA review of pirfenidone and nintedanib. N Engl J Med. 2015;372:1189–1191. doi: 10.1056/NEJMp1500526. [DOI] [PubMed] [Google Scholar]
- 20.Noble PW, Albera C, Bradford WZ, Costabel U, du Bois RM, Fagan EA, Fishman RS, Glaspole I, Glassberg MK, Lancaster L, et al. Pirfenidone for idiopathic pulmonary fibrosis: analysis of pooled data from three multinational phase 3 trials. Eur Respir J. 2016;47:243–253. doi: 10.1183/13993003.00026-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nathan SD, Albera C, Bradford WZ, Costabel U, Glaspole I, Glassberg MK, Kardatzke DR, Daigl M, Kirchgaessler KU, Lancaster LH, et al. Effect of pirfenidone on mortality: pooled analyses and meta-analyses of clinical trials in idiopathic pulmonary fibrosis. Lancet Respir Med. 2017;5:33–41. doi: 10.1016/S2213-2600(16)30326-5. [DOI] [PubMed] [Google Scholar]
- 22.Collard HR, Bradford WZ, Cottin V, Flaherty KR, King TE, Jr, Koch GG, Kolb M, Martinez FJ, Montgomery B, Raghu G, et al. A new era in idiopathic pulmonary fibrosis: considerations for future clinical trials. Eur Respir J. 2015;46:243–249. doi: 10.1183/09031936.00200614. [DOI] [PubMed] [Google Scholar]
- 23.Ley B, Bradford WZ, Vittinghoff E, Weycker D, du Bois RM, Collard HR. Predictors of mortality poorly predict common measures of disease progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2016;194:711–718. doi: 10.1164/rccm.201508-1546OC. [DOI] [PMC free article] [PubMed] [Google Scholar]