Abstract
Most studies on termite food selection have focused on a single nutrient per choice, however, termites, like all animals, must balance multiple nutrients in their diet. While most studies that use multi-nutrient approaches focus on macromolecules, the ability to balance the intake of inorganic nutrients is also vital to organisms. In this study, we used the geometric framework to test the effects of multiple inorganic nutrients on termite feeding. We presented the subsets of Reticulitermes flavipes colonies with food enriched with varying in levels of KCl, MgSO4, and FePO4. Each trial varied two of the three nutrients while the third nutrient was kept constant. The amount of food consumed was measured over two weeks. The termites’ feeding patterns during the study suggested that they fed until they reached a limit for MgSO4. This result suggests that the termites were using the rule of compromise such that the termites would over consume KCl or FePO4 in order to avoid overeating MgSO4. Thus, the termite colonies are able to regulate the intake of inorganic nutrients, and by doing so, adjust their intake from multiple resources in order to maintain an intake target.
Keywords: termites, Reticulitermes flavipes, geometric framework, inorganic nutrients, feeding regulation
1. Introduction
Social insect colonies must regulate the intake of nutrients in order to optimize their forging force. Rather than single individuals making food selections, colonies draw from multiple food sources in order for the colony as a whole to meet its nutritional needs. Bee and ant colonies will distribute their foraging force to their maximize energy intake [1,2] and also regulate the flow of different nutrients [3,4,5,6] into their colonies based on season and colony composition. Ant colonies have been specifically shown to be capable of regulating their feeding to maintain a balance of multiple nutrients [7]. Most of foraging regulation studies on social insect colonies have focused on social Hymenoptera. However, most feeding groups of termites also access nutrients from multiple sources [8,9,10] and individuals can recruit others to a food source [11]. Termites, like social Hymenoptera, are able to distribute nutrients to other colony members [12,13,14,15]. Thus, it would seem likely that termite colonies should also maintain the balance of multiple nutrients and regulate their feeding accordingly.
A number of studies have examined the effects of individual nutrients and other substances on the food selection of termites. Sugars, amino acids, ions, and other small compounds have been found to influence the feeding of different species of termites [16,17,18,19,20,21,22]. However, it has been found that different species have different responses to the same molecules [18,23,24], and in some cases the same species will have seasonal or even geographical differences in its response [25]. The effect of the interaction of different nutrients on feeding responses in termites has had very little attention in termite foraging studies, even though it is unlikely that termites are responding to a single nutrient when regulating their feeding. One model that was developed to examine the regulation of multiple nutrients is the geometric framework [26]. This framework examines the ratio of nutrients that organisms consume to reach an intake target, the ideal ratio, and amount of all the nutrients. When presented with multiple foods that do not have the same ratio as the intake target, organisms will use multiple food sources to reach the target or use different strategies in order to approximate the target as close as possible [27]. This model has been successfully used to demonstrate that a number of species from various trophic levels, are regulating their diets based on the availability of several nutrients in some cases from multiple food sources [28,29,30,31,32,33]. Up to this point, most of the geometric framework studies have focused on macromolecules (carbohydrates, lipids, and protein), however the model could be applied to other nutrients.
Termites are excellent models for examining inorganic nutrient balance because they feed on cellulose based sources [8] and can access monosaccharides found in cellulose and hemicelluloses with the aid of microorganisms or through the production of their own enzymes [34,35,36]. Thus, the energy compounds are not a limiting factor in the termite diet. However, cellulose-based foods are generally a poor source of other nutrients [12]. How termites are able to acquire the correct levels of other nutrients is of interest. In many cases, the termites access nitrogen with the aid of nitrogen fixing bacteria [37,38,39,40,41] or consuming fungi [12,15]. Thus, other inorganic nutrients would be ideal candidates to examine the nutrient regulation. Subterranean termites have two potential sources for inorganic nutrients, the soil and food [42]. It is from these sources that termites can balance the intake of the other nutrients required in their diet.
Here, we used the geometric framework [26] to compare the effects of several inorganic nutrients on the feeding regulation of the eastern subterranean termite Reticulitermes flavipes. Lab colonies were placed in nutrient poor soil so they were forced to draw inorganic nutrients from artificial food sources enriched with different levels of KCl, MgSO4, and FePO4. KCl was chosen because both potassium (K) and chloride (Cl) are used for homeostasis in insects [43,44]. Furthermore, termites have been shown to be attracted to K in the soil [45]. Magnesium (Mg) is an important cofactor in glycolysis and sulfur (S), and is necessary for the production of proteins. Several symbionts in termite guts process sulfates [46,47,48]. FePO4 is a source of phosphates which are important for the production of nucleic acids. Shortages in phosphates can potentially limit protein production [49]. R. flavipes has been shown to select foods with phosphates in the fall [17]. Iron (Fe) is important for protein function and essential for the nitrogen fixation process used by termite symbionts [50]. It is likely that these nutrients are not needed in similar amounts and termites’ ability to process these nutrients differ. Both factors could affect how termite colonies reach their intake targets. It was not the goal of this study to isolate the effects of individual nutrients, but instead to examine the interplay of these nutrients when the termites are faced with food varying levels of multiple nutrients.
2. Materials and Methods
2.1. Collections
Ten colonies were sampled from the Juden Creak Natural Area, Gape Girardeau, MO using termite traps as described in [25]. Colonies were removed from the traps in the laboratory and placed in a sealable container half filled with soil. All colonies were used within 48 h of collection.
2.2. Feeding Trials
All food was prepared by dissolving 1.5 g of agar (Sigma-Aldrich, Arvada, CO, USA) in 100 mL of hot deionized (ddi) H2O. Each food type had different levels of KCl, MgSO4, and FePO4 (Sigma-Aldrich, Arvada, CO, USA, Table 1) dissolved into the solution followed by the addition of 7.5 g of α-cellulose (Sigma-Aldrich, Arvada, CO, USA). The mixtures were then poured into petri dishes and because α-cellulose is non-soluble, the petri dishes were placed on a shaker until the mixture solidified in order to ensure the cellulose remained suspended and evenly distributed throughout the food.
Table 1.
Total amounts of KCl, MgSO4, and FePO4 added to each food type. Amounts are listed as mg and resulting mM for each diet. For each food label, the element in lower case refers to the diet that was the lowest level in the enrichment and the element in upper case refers to the diet that was highest in the enrichment.
| Food Type | KCl mg (mM) | MgSO4 mg (mM) | FePO4 mg (mM) |
|---|---|---|---|
| kFE | 0.025 (3.35 × 10−3) | 0.050 (4.15 × 10−3) | 0.100 (6.63 × 10−3) |
| kMG | 0.025 (3.35 × 10−3) | 0.100 (8.31 × 10−3) | 0.050 (3.32 × 10−3) |
| mgFE | 0.050 (6.71 × 10−3) | 0.025 (2.08 × 10−3) | 0.100 (6.63 × 10−3) |
| Mgfe | 0.050 (6.71 × 10−3) | 0.100 (8.31 × 10−3) | 0.025 (1.66 × 10−3) |
| Kmg | 0.100 (13.4 × 10−2) | 0.025 (2.08 × 10−3) | 0.050 (3.32 × 10−3) |
| Kfe | 0.100 (13.4 × 10−2) | 0.050 (4.15 × 10−3) | 0.025 (1.66 × 10−3) |
Three separate bins were created for each colony and designated kFE/Kfe, kMg/Kmg, and mgFE/MGfe based types of food used in each trial. Two of the three nutrients were varied, while the third was kept constant. Each bin was a 17.8 × 17.8 × 5 cm sealable plastic container ¼ filled with play-sand. Sand was used to minimize any uptake of inorganic nutrients from the soil [42]. After the sand was added, 100 worker termites, hereafter called a “subcolony”, were added from the main colony (Figure 1). 3.0 g of each of two food types were placed on a 3.5 × 3.5 cm note card and placed in the opposite corners of the bin. R. flavipes creates a small hole through the card to get to the food. For each of the three trials, three control bins were created containing everything except termites to control for water loss in the food [51]. Food was weighed every two days to determine loss of mass using a balance (Denver Instrument XP-300, Bohemia, NY, USA). All termites and sand were removed from the food prior to weighing. The feeding trials were terminated after two weeks. All three trials were run simultaneously. The termites used in the trials were not returned to the main colony.
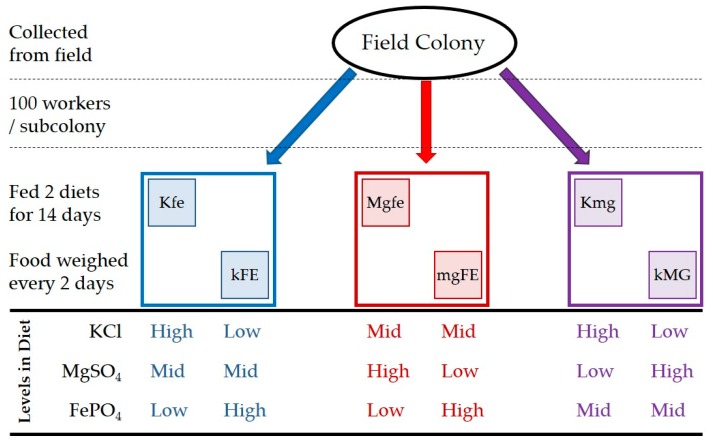
Figure 1.
Setup of the experiment. The top portion outlines the experimental procedure for each of the ten colonies used in the experiment. Large boxes represent a subcolony and small boxes represent diets fed to the subcolonies. Each subcolony received two diets simultaneously. The lower portion of the figure indicates the relative levels of KCl, MgSO4, and FePO4 in each diet.
Calculations for water loss were performed in the same manner as Judd et al. 2009 [51]. The mean weight change of the controls was subtracted from the change in weight for each food type. The result was the weight loss due to termite feeding. In rare instances (a total of 5 times throughout the experiment, the difference was less than 0.06 g) the calculated consumption was negative and they were changed to a zeros to indicate that none of the weight loss was due to feeding. Although the possibility existed that the termites could have transported pellets of agar to the surface rather than eating them, this was not noted during the study. Previous studies have shown that the termite workers do not readily create pellets of agar less than 2% [52]. The level of agar in the solution used in this study was less than 2% making it unlikely that the transportation of agar contributed to the weight loss in the food.
Once the weight loss due to termite feeding was calculated, the total amount of each diet (KCl, MgSO4, and FePO4) that was consumed by each subcolony was determined from a proportion of the mass of each diet represented in the food. The result was converted to mmol. Nutrient rails, which represent the ratio of the diets in individual food types, were also determined by converting the amounts of each nutrient in the diets to mmols.
2.3. Data Analysis
The results of each individual trials were compared using the Wilcoxon Signed Rank Test (WSR) [53] to determine if there was a preference for either diet within a trial. In order to estimate the intake target, only colonies that were represented in all of the three experiments were used (N = 7). The total amounts of KCl, MgSO4, and FePO4 consumed were calculated based on the percent amount of each nutrient in each of the food consumed. The intake target was estimated by taking the centroid of the triangle formed by the average intake of nutrients in all three trials in three-dimensional spaces.
3. Results
3.1. Results of Individual Tests
3.1.1. Kfe vs. kFE Trial
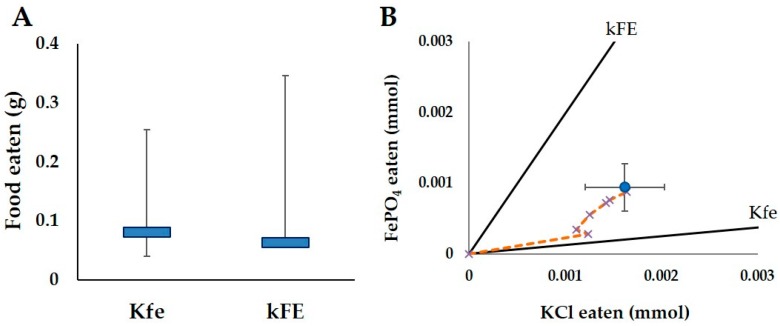
There was no significant difference between the amount of Kfe or kFE foods consumed (T = 25, p > 0.05, WSR, Figure 2A). Based on the daily trajectory, the termites primarily took in KCl for the first two days, and then abruptly switch to consuming both KCl and FePO4 equally (Figure 2B). The final trajectory was a balance between KCl and FePO4.
Figure 2.
Results of the feeding trial in termites exposed to the food varying in KCl and FePO4 (Kfe and kFE). (A) Medians (blue bar) and quartiles (error bars) of the total amount of food eaten for all colonies (N = 10) after 14 days; (B) The nutritional intake of the termites for KCl and FePO4 over the 14 d period. The large blue circle with error bars indicates the average total intake of KCl and FePO4 for all colonies. The small x’s indicate the average cumulative intake per day (including day 0) for all colonies and are connected by the orange dashed line to represent the average intake trajectory. The black lines represent the nutrient rails, the ratio of FePO4 and KCl in each diet (Kfe and kFE). These rails represent the intake trajectory if the termites were to exclusively feed on that diet. The closer the feeding trajectory is to a rail, the more similar the nutrient intake was to the diet represented by that rail.
3.1.2. MGfe vs. mgFE Trial
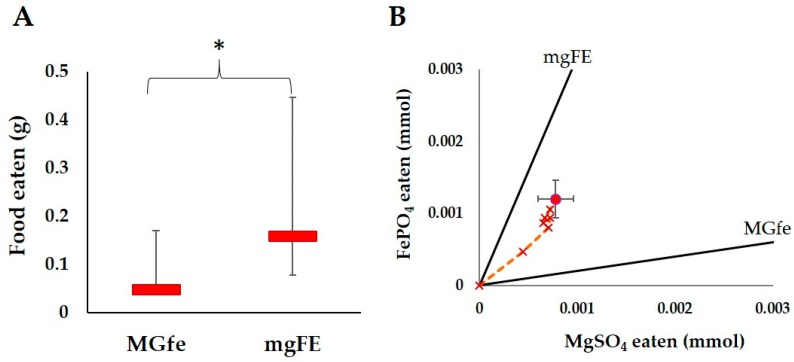
Termites in this study fed significantly more on the mgFE food source than the MGfe source (T = 0, p < 0.01, WSR, Figure 3A). The termites initially consumed equal amounts of MgSO4 and FePO4. After day four, the termites increased the intake of FePO4 (Figure 3B). Thus, the termites appeared to be limiting the intake of MgSO4 in favor of FePO4.
Figure 3.
Results of the feeding trial in termites exposed to the food varying in MgSO4 and FePO4 (MGfe and mgFE diets). (A) Medians (red bar) and quartiles (error bars) of the total amount of food eaten for all colonies (N = 10) after 14 days. The “*” indicates a significant difference between the amount of each diet consumed (p < 0.01); (B) The nutritional intake of the termites for MgSO4 and FePO4 over a 14 days period. The lines and symbols are the same as in Figure 2, except the large circle and x’s are in red and the nutrient rails represent the ratio of MgSO4 and FePO4 in both food types.
3.1.3. Kmg/kMG Trial
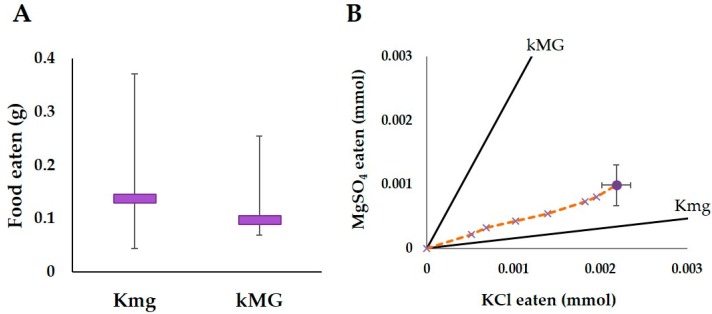
There was no significant difference in the overall consumption of the Kmg or kMG foods (T = 13, p > 0.05, WSR, Figure 4A). However, when the intake of both KCl and MgSO4 are compared, the termite colonies seemed to consume more of the KCl then MgSO4 (Figure 4B). Thus, the termite colonies were on track to limit the intake of MgSO4 relative to KCl.
Figure 4.
Results of the feeding trial in termites exposed to the food varying in MgSO4 and KCl (Kmg and kMG diets). (A) Medians (purple bar) and quartiles (error bars) of the total amount of food eaten for all colonies (N = 10) after 14 days; (B) The nutritional intake of the termites for MgSO4 and KCl over a 14 days period. The lines and symbols are the same as in Figure 2, except for the large circle and x’s are in purple and the nutrient rails represent the ratio of MgSO4 and KCl in both food types.
3.2. Combined Analysis and Intake Target
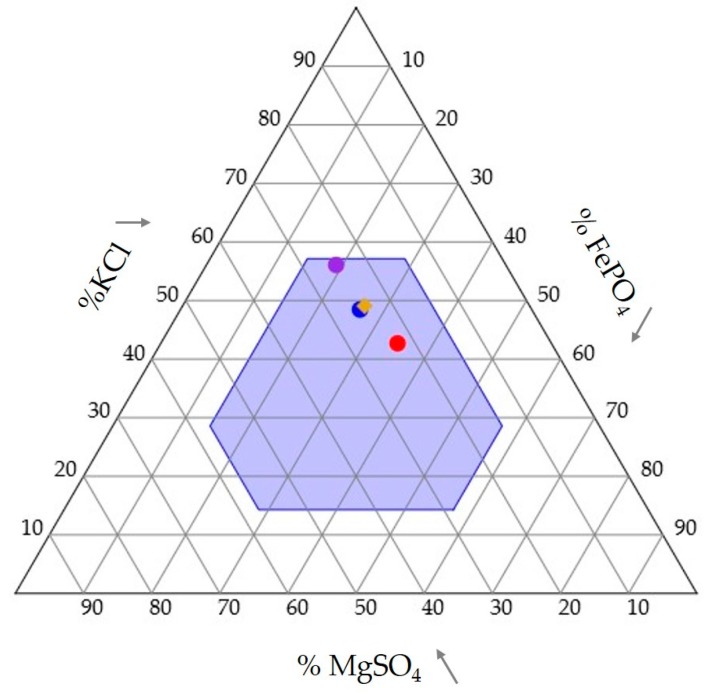
Due to a labeling error for three of the colonies in one of the trials, seven colonies were represented in the results of all three trials to ensure the same colonies were represented in all trials. The means and estimated intake target are plotted in Figure 5. The colonies from the Kfe/kFE trials came closest to the intake target as compared to the other two trials.
Figure 5.
Equilateral mixture triangle summarizing the intake of KCl, MgSO4, and FePO4 for all three trials. The blue, red, and purple dots represent the average intake of the same seven colonies from the Kfe/kFE, MGfe/mgFE, and Kmg/kMG trials, respectively. The light blue area is the area of possible accessibility from the combined trials. The gold diamond is the estimated intake target based on the results for all 7 colonies.
4. Discussion
The results of this study support the hypothesis that termites are regulating their feeding based on the nutrient content in available foods. If the termites were not regulating their feeding we should have seen termites eating all foods equally [54]. Instead, the termites in this study did not feed on all of the food equally. The termites in this study preferentially fed on the FEmg food to the feMG food source. However, with the use of additional nutrients and the geometric framework, we were able to see that there may be more to the story than a simple preference of one substance over another.
The termites fed until they reached an upper threshold for the intake of MgSO4. Based on the MGfe/mgFE and Kmg/kMG trials, the ceiling is around 0.001 mmol. In the Kfe/kFE trial, both foods had an equal amount of MgSO4. The final result was an approximate of the estimated intake target (Figure 5). It is unknown if it is Mg or SO4 (or both) that are responsible for the ceiling, a question for further study. In the MGfe/mgFE and Kmg/kMG trials, the termites increased the intake of FePO4 and KCl, respectively, before hitting the MgSO4 ceiling (Figure 5). One possible explanation for these results is the rule of compromise [27]. In studies using macronutrients, many carnivores will consume extra protein in order to gain enough carbohydrates [29,30,32]. Domestic cats, for example, will consume extra protein and fats, but will limit the level of carbohydrates in their diet [29]. It appears that a similar response is occurring in termites with the inorganic nutrients used here. The overconsumption of KCl and FePO4 is similar to what occurred with protein and fats in the domestic cats [29]. In both the MGfe/mgFE and Kmg/kMG trials, the intake trajectories were moving away from the food enriched with high levels of MgSO4 (MGfe and kMG, Figure 3B and Figure 4B). Thus, it is possible the termites were operating under the rule of compromise as well.
The threshold for MgSO4 can potentially be caused by either Mg or SO4. Mg is an important cofactor in glycolysis [43]. However, an excess of Mg may be unhealthy for termites. Mg is regulated by the Malpighian tubules [55,56], and in Hayiphora cercropia it is also been shown to be stored in the midgut [55], thus it is possible that the same is true for termites. If the levels of Mg in the hemolymph and midgut epithelia reach capacity, it may cause termites to reduce their intake. On the other hand, sulfates are mainly processed by symbiotic bacteria Desulfovibrio in termite guts [46,48]. Increases in the sulfate content of termite diets will cause an increase in populations of these bacteria in termite guts [48]. Thus, it is possible that these bacteria are essential for processing sulfates and the size of the population of Desulfovibrio in termites may affect the levels of sulfates that termites can consume. If the bacteria are involved in setting the intake target for sulfates, then changes in the population of these bacteria could change the intake target. Whether or not bacteria can influence the intake of nutrients by its host remains to be seen.
Potassium is found in higher concentrations in termites than most other elements, including Mg and Fe [14,57]. Although Cl levels have not been measured in termites, Cl and phosphates are generally the most common anions in insects [44]. Both K and Cl are important for homeostasis in insects and can be processed by multiple tissues, thus, consuming excess amounts of both nutrients may be more tolerable [58,59]. Phosphorus levels have been shown to be higher than Mg levels in Nasutitermes [57]. Phosphates are needed for energetic functions, protein synthesis, and even used to store Mg [44,55]. Iron could be potentially toxic at higher levels, but insects generally have transferrins and ferritins to transport and store Fe, respectively [50]. These molecules also are involved in the immune defense in insects [60]. Thus, it is possible that termites may be able to handle a larger intake of Fe. Iron is also important for metabolic enzymes for several symbiotic bacteria [61].
Termite colonies involve multiple individuals, and the intake target represents the combination of the nutritional needs of all of the individuals. Termites will pass inorganic nutrients to other individuals and different castes will retain different levels of these nutrients [14]. Thus, the intake target is reached when all individuals have reached their individual target. The presence of soldiers and reproductives that are not present in this study could affect the intake target. The maintenance of an intake target requires internal sensing mechanisms that would regulate foraging behavior. Insects have been shown to forage for inorganic nutrients to meet nutritional demands. A number of insects will collect sodium from water sources [62,63,64,65] until their needs are met. Honeybees will forage for several inorganic nutrients from water sources when they cannot get those nutrients from pollen or nectar sources [66]. In this study, the termite workers were forced to gain their nutrients from the food sources because they were housed in nutrient poor soil (sand). They too changed their foraging patterns during the course of the study, presumably as members of the subcolony met their intake targets. The change in food consumption suggests that termites also have internal systems to detect the levels of several inorganic nutrients, and these levels may influence foraging behavior and recruitment. It is possible that the termites in this study simply prefer foods with higher levels of FePO4 or possibly KCl, regardless of their nutritional state. However, the fact that in the MGfe/mgFE and Kmg/kMG trials the threshold of the consumption of MgSO4 was extremely close (Figure 5), this suggests that some form of regulation may be taking place.
The development of baits for monitoring and control is an important aspect of applied termite nutritional ecology because some species are pests. The single-attractant approach may not be the most effective method to produce a reliable bait. Subterranean termites are drawing nutrients from multiple sources, and thus other dietary components may limit the amount of bait termites they are able to consume even if there is a viable phagostimulant or important nutrient in the bait. Analyzing the forging behavior of termites using a geometric approach may lead to a better understanding on how to detect termites using baits.
Acknowledgments
We thank three anonymous reviewers and the academic editor for their helpful comments. This work was supported by Southeast Missouri State University.
Author Contributions
Timothy M. Judd conceived and designed the experiments, analyzed the data and wrote the paper. James R. Landes, Hanruna Ohara and Alex W. Riley performed the experiments and are listed in alphabetical order, not by level of contribution.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Seeley T.D., Camazine S., Sneyd J. Collective decision-making in honey bees: How colonies choose among nectar sources. Behav. Ecol. Sociobiol. 1991;28:277–290. doi: 10.1007/BF00175101. [DOI] [Google Scholar]
- 2.Sudd J.H., Sudd M.E. Seasonal changes in the response of wood-ants (Formica lugubris) to sucrose baits. Ecol. Entmol. 1985;10:89–97. doi: 10.1111/j.1365-2311.1985.tb00538.x. [DOI] [Google Scholar]
- 3.Cook S.C., Eubanks M.D., Gold R.E., Behmer S.T. Colony-level macronutrient regulation in ants: mechanisms, hoarding and associated costs. Anim. Behav. 2010;79:429–437. doi: 10.1016/j.anbehav.2009.11.022. [DOI] [Google Scholar]
- 4.Cook S.C., Eubanks M.D., Gold R.E., Behmer S.T. Seasonality directs contrasting food collection behavior and nutrient regulation strategies in ants. PLoS ONE. 2011;6:e25407. doi: 10.1371/journal.pone.0025407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Judd T.M. Relationship between food stores and foraging behavior of Pheidole ceres (Hymenoptera: Formicidae) Ann. Entomol. Soc. Am. 2006;99:398–406. doi: 10.1603/0013-8746(2006)099[0398:RBFSAF]2.0.CO;2. [DOI] [Google Scholar]
- 6.Sorensen A.A., Busch T.M., Vinson S.B. Behavioral flexibility of temporal subcastes in the fire ant, Solenopsis invicta in response to food. Psyche. 1985;91:316–331. doi: 10.1155/1984/39236. [DOI] [Google Scholar]
- 7.Dussutour A., Simpson S.J. Carbohydrate regulation in relation to colony growth in ants. J. Exp. Biol. 2008;211:2224–2232. doi: 10.1242/jeb.017509. [DOI] [PubMed] [Google Scholar]
- 8.Donovan S.E., Eggleton P., Bignell D.E. Gut content analysis and a new feeding group classification of termites. Ecol. Entmol. 2001;26:356–366. doi: 10.1046/j.1365-2311.2001.00342.x. [DOI] [Google Scholar]
- 9.Inward D.J.G., Vogler A.P., Eggleton P.A. Comprehensive phylogenetic analysis of termites (Isoptera) illuminates key aspects of their evolutionary biology. Mol. Phylogenet. Evol. 2007;44:953–967. doi: 10.1016/j.ympev.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Eggleton P., Tayasu I. Feeding groups, lifetypes and the global ecology of termites. Ecol. Res. 2001;16:941–960. doi: 10.1046/j.1440-1703.2001.00444.x. [DOI] [Google Scholar]
- 11.Reinhard J., Kaib M. Trail communication during foraging and recruitment in the subterranean termite Reticulitermes santonensis De Feytaud (Isoptera, Rhinotermitidae) J. Insect Behav. 2001;14:157–171. doi: 10.1023/A:1007881510237. [DOI] [Google Scholar]
- 12.La Fage J.P., Nutting W.L. Nutrient dynamics of termites. In: Brian M.V., editor. Production Ecology of Ants and Termites. Cambridge University Press; Cambridge, UK: 1978. pp. 165–232. [Google Scholar]
- 13.Suárez M.E., Thorne B.L. Rate, amount, and distribution pattern of alimentary fluid transfer via trophallaxis in three species of termites (Isoptera: Rhinotermitidae, Termopsidae) Ann. Entomol. Soc. Am. 2000;93:145–155. doi: 10.1603/0013-8746(2000)093[0145:RAADPO]2.0.CO;2. [DOI] [Google Scholar]
- 14.Judd T.M., Fasnacht M.P. Distribution of micronutrients in social insects: A test in the termite Reticulitermes flavipes (Isoptera: Rhinotermitidae) and the ant Myrmica punctiventris (Hymenoptera: Formicidae) Ann. Entomol. Soc. Am. 2007;100:893–899. doi: 10.1603/0013-8746(2007)100[893:DOMISI]2.0.CO;2. [DOI] [Google Scholar]
- 15.Waller D.A., La Fage J.P. Nutritional ecology of termites. In: Slansky F. Jr., Rodriguez J.G., editors. Nutritional Ecology of Insects, Mites, Spiders and Related Invertebrates. John Wiley & Sons; New York, NY, USA: 1987. pp. 487–532. [Google Scholar]
- 16.Abushama F.T., Kamal M.A. The role of sugars in the food-selection of termite Microtermes traegardhi (Sjostedt) J. Appl. Entomol. 1977;84:250–255. [Google Scholar]
- 17.Botch P.S., Brennan C.L., Judd T.M. Seasonal effects of calcium and phosphate on the feeding preference of the termite Reticulitermes flavipes (Isoptera: Rhinotermitidae) Sociobiology. 2010;55:42–56. [Google Scholar]
- 18.Haifig I., Costa-Leonardo A.M., Marchetti F.F. Effects of nutrients on feeding activities of the pest termite Heterotermes tenuis (Isoptera: Rhinotermitidae) J. Appl. Entomol. 2008;132:497–501. doi: 10.1111/j.1439-0418.2008.01288.x. [DOI] [Google Scholar]
- 19.Haifig I., Marchetti F.F., Costa-Leonardo A.M. Nutrients affecting food choice by the pest subterranean termite Coptotermes gestroi (Isoptera: Rhinotermitidae) Int. J. Pest Manag. 2010;56:371–375. doi: 10.1080/09670874.2010.505304. [DOI] [Google Scholar]
- 20.Saran R.K., Rust M.K. Feeding, uptake, and utilization of carbohydrates by western subterranean termite (Isoptera: Rhinotermitidae) J. Econ. Entomol. 2005;98:1284–1293. doi: 10.1603/0022-0493-98.4.1284. [DOI] [PubMed] [Google Scholar]
- 21.Swoboda L.E., Miller D.M., Fell R.J., Mullins D.E. The effect of nutrient compounds (sugars and amino-acids) on bait consumption by Reticulitermes spp. (Isoptera: Rhinotermitidae) Sociobiology. 2004;44:547–563. [Google Scholar]
- 22.Waller D.A., Morlino S.E., Matkins N. Factors affecting termite recruitment to baits in laboratory and field studies. In: Robinson W.M., Rettich F., Rambo G.W., editors. Proceedings of the 3rd International Conference on Urban Pests, Czech University of Agriculture; Prague, Czech Republic. 19–22 July 1999; Prague, Czech Republic: Grafické Závody Hronov; 1999. pp. 597–600. [Google Scholar]
- 23.Castillo V.P., Sajap A.S., Sahri M.H. Feeding response of subterranean termites Coptotermes curvignathus and Coptotermes gestroi (Blattodea: Rhinotermitidae) to baits supplemented with sugars, amino acids, and cassava. J. Econ. Entomol. 2013;106:1794–1801. doi: 10.1603/EC12301. [DOI] [PubMed] [Google Scholar]
- 24.Haifig I., Jost C., Fourcassie V., Zana Y., Costa-Leonardo A. Dynamics of foraging trails in the Neotropical termite Velocitermes heteropterus (Isoptera: Termitidae) Behav. Proc. 2015;118:123–129. doi: 10.1016/j.beproc.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Wallace B.A., Judd T.M. A test of seasonal responses to sugars in four populations of the termite Reticulitermes flavipes. J. Econ. Entomol. 2010;103:2126–2131. doi: 10.1603/EC09326. [DOI] [PubMed] [Google Scholar]
- 26.Simpson S.J., Raubenheimer D. A multi-level analysis of feeding behaviour: The geometry of nutritional decisions. Philos. Trans. R. Soc. B. 1993;342:381–402. doi: 10.1098/rstb.1993.0166. [DOI] [Google Scholar]
- 27.Simpson S.J., Raubenheimer D. The geometric analysis of feeding and nutrition: A user’s guide. J. Insect Physiol. 1995;41:545–553. doi: 10.1016/0022-1910(95)00006-G. [DOI] [Google Scholar]
- 28.Behmer S.T., Raubenheimer D., Simpson S.J. Frequency-dependent food selection in locusts: a geometric analysis of the role of nutrient balancing. Anim. Behav. 2001;61:995. doi: 10.1006/anbe.2000.1695. [DOI] [Google Scholar]
- 29.Hewson-Hughes A.K., Hewson-Hughes V.L., Miller A.T., Hall S.R., Simpson S.J., Raubenheimer D. Geometric analysis of macronutrient selection in the adult domestic cat, Felis catus. J. Exp. Biol. 2011;214:1039–1051. doi: 10.1242/jeb.049429. [DOI] [PubMed] [Google Scholar]
- 30.Hewson-Hughes A.K., Hewson-Hughes V.L., Coyler A., Miller A.T., McGrane S.J., Hall S.R., Butterwick R.F., Simpson S.J., Raubenheimer D. Geometric analysis of macronutrient selection in breeds of the domestic dog, Canis lupus familiaris. Behav. Ecol. 2013;24:293–304. doi: 10.1093/beheco/ars168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang X., Hancock D.P., Gosby A.K., McMahon A.C., Solom S.M.C., Le Couteur D.G., Conigrave A.D., Raubenheimer D., Simpson S.J. Effects of dietary protein to carbohydrate balance on energy intake, fat storage, and heat production in mice. Obesity. 2013;21:85–92. doi: 10.1002/oby.20007. [DOI] [PubMed] [Google Scholar]
- 32.Jensen K., Mayntz D., Toft S., Clissold F.J., Hunt J., Raubenheimer D., Simpson S.J. Optimal foraging for specific nutrients in predatory beetles. Proc. Biol. Sci. 2012;279:2212–2218. doi: 10.1098/rspb.2011.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee K.P., Behmer S.T., Simpson S.J., Raubenheimer D. A geometric analysis of nutrient regulation in the generalist caterpillar Spodoptera littoralis (Boisduval) J. Insect Physiol. 2002;48:655–665. doi: 10.1016/S0022-1910(02)00088-4. [DOI] [PubMed] [Google Scholar]
- 34.Brune A. Symbiotic digestion of lignocellulose in termite guts. Nat. Rev. Microbiol. 2014;12:168–180. doi: 10.1038/nrmicro3182. [DOI] [PubMed] [Google Scholar]
- 35.König H., Li L., Fröhlich J. The cellulolytic system of the termite gut. Appl. Microbiol. Biotechnol. 2013;97:7943–7962. doi: 10.1007/s00253-013-5119-z. [DOI] [PubMed] [Google Scholar]
- 36.Ni J., Tokuda G. Lignocellulose-degrading enzymes from termites and their symbiotic microbiota. Biotechnol. Adv. 2013;31:838–850. doi: 10.1016/j.biotechadv.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Bentley B.L. Nitrogen fixation in termites: Fate of newly fixed nitrogen. J. Insect Physiol. 1984;30:653–655. doi: 10.1016/0022-1910(84)90050-7. [DOI] [Google Scholar]
- 38.Breznak J.A., Brill W.J., Mertins J.W., Coppel H.C. Nitrogen fixation in termites. Nature. 1973;244:577–580. doi: 10.1038/244577a0. [DOI] [PubMed] [Google Scholar]
- 39.Curtis A.D., Waller D.A. Seasonal patterns of nitrogen fixation in termites. Funct. Ecol. 1998;12:803–807. doi: 10.1046/j.1365-2435.1998.00248.x. [DOI] [Google Scholar]
- 40.Ohkuma M., Noda S., Kudo T. Phylogenetic diversity of nitrogen fixation genes in the symbiotic microbial community in the gut of diverse termites. Appl. Environ. Microbiol. 1999;65:4926–4934. doi: 10.1128/aem.65.11.4926-4934.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prestwich G.D., Bentley B.L., Carpenter E.J. Nitrogen sources for neotropical nasute termites: Fixation and selective foraging. Oecologia. 1980;46:397–401. doi: 10.1007/BF00346270. [DOI] [PubMed] [Google Scholar]
- 42.Janzow M.P., Judd T.M. The termite Reticulitermes flavipes (Rhinotermitidae: Isoptera) can acquire micronutrients from soil. Environ. Entomol. 2015;44:814–820. doi: 10.1093/ee/nvv041. [DOI] [PubMed] [Google Scholar]
- 43.Cohen A.C. Insect Diets Science and Technology. CRC Press; New York, NY, USA: 2004. [Google Scholar]
- 44.Nation J.L. Insect Physiology and Biochemistry. CRC Press; Boca Raton, FL, USA: 2002. [Google Scholar]
- 45.Botch P.S., Judd T.M. The effects of soil cations on the foraging behavior of the termite Reticulitermes flavipes. J. Econ. Entomol. 2011;104:425–435. doi: 10.1603/EC10118. [DOI] [PubMed] [Google Scholar]
- 46.Brauman A., Koenig J.F., Dutreix J., Garcia J.L. Characterization of two sulfate-reducing bacteria from the gut of the soil-feeding termite, Cubitermes speciosus. Antonie Van Leeuwenhoek. 1990;58:271–275. doi: 10.1007/BF00399339. [DOI] [PubMed] [Google Scholar]
- 47.Droge S., Limper U., Emtiazi F., Schonig I., Pavlus N., Dryzga O., Fischer U., Konig H. In vitro and in vivo sulfate reduction in the gut contents of the termite Mastotermes darwiniensis and the rose-chafer Pachnoda marginata. J. Gen. Appl. Microbiol. 2005;51:57–64. doi: 10.2323/jgam.51.57. [DOI] [PubMed] [Google Scholar]
- 48.Kuhnigk T., Branke J., Krekeler D., Cypionka H., König H. A feasible role of sulfate-reducing bacteria in the termite gut. Syst. Appl. Microbiol. 1996;19:139–149. doi: 10.1016/S0723-2020(96)80039-7. [DOI] [Google Scholar]
- 49.Elser J.J., Sterner R.W., Gorokhova E., Fagan W.F., Markow T.A., Cotner J.B., Harrison J.F., Hobbie S.E., Odell G.M., Weider L.W. Biological stoichiometry from genes to ecosystems. Ecol. Lett. 2000;3:540–550. doi: 10.1046/j.1461-0248.2000.00185.x. [DOI] [Google Scholar]
- 50.Nichol H., Law J.H., Winzerling J.J. Iron metabolism in insects. Annu. Rev. Entomol. 2002;47:535–559. doi: 10.1146/annurev.ento.47.091201.145237. [DOI] [PubMed] [Google Scholar]
- 51.Judd T.M., Corbin C.C. Effect of cellulose concentration on the feeding preferences of the termite Reticulitermes flavipes (Isoptera: Rhinotermitidae) Sociobiology. 2009;53:775–784. [Google Scholar]
- 52.Zachariah N., Das A., Murthy T.G., Borges R.M. Building mud castles: A perspective from brick-laying termites. Sci. Rep. 2017;7:4692. doi: 10.1038/s41598-017-04295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daniel W.W. Applied Nonparametric Statistics. 2nd ed. PWS-Kent; Boston, MA, USA: 1990. [Google Scholar]
- 54.Emmans G.C. Diet selection by animals: Theory and experimental design. Proc. Nutr. Soc. 1991;50:59–64. doi: 10.1079/PNS19910010. [DOI] [PubMed] [Google Scholar]
- 55.Jungreis A.M. Distribution of magnesium in tissues of silkmoth Hyalophora cecropia. Am. J. Physiol. 1973;224:27–30. doi: 10.1152/ajplegacy.1973.224.1.21. [DOI] [PubMed] [Google Scholar]
- 56.Kiceniuk J., Phillips J.E. Magnesium regulation in mosquito larvae (Aedes compestris) living in waters of high MgSO4 content. J. Exp. Biol. 1974;61:749–760. doi: 10.1242/jeb.61.3.749. [DOI] [PubMed] [Google Scholar]
- 57.Oyarzun S.E., Crawshaw G.J., Valdes E.V. Nutrition of the tamandua: I. Nutrient composition of termites (Nasutitermes spp.) and stomach contents from wild tamanduas (Tamandua tetradactyla) Zoo Biol. 1996;15:509–524. doi: 10.1002/(SICI)1098-2361(1996)15:5<509::AID-ZOO7>3.0.CO;2-F. [DOI] [Google Scholar]
- 58.Blumenthal E.M. Regulation of chloride permeability by endogenously produced tyramine in the Drosophila Malpighian tubule. Am. J. Physiol. 2003;284:C718–C728. doi: 10.1152/ajpcell.00359.2002. [DOI] [PubMed] [Google Scholar]
- 59.Harvey W.R., Cioffi M., Dow J.A., Wolfersberger M.G. Potassium ion transport ATPase in insect epithelia. J. Exp. Biol. 1983;106:91–117. doi: 10.1242/jeb.106.1.91. [DOI] [PubMed] [Google Scholar]
- 60.Popham H.J., Sun R., Shelby K.S., Robertson J.D. Changes in trace metals in hemolymph of baculovirus-infected noctuid larvae. Biol. Trace Elem. Res. 2012;146:325–334. doi: 10.1007/s12011-011-9257-9. [DOI] [PubMed] [Google Scholar]
- 61.Inoue J.I., Saita K., Kudo T., Ui S., Ohkuma M. Hydrogen production by termite gut protists: Characterization of iron hydrogenases of parabasalian symbionts of the termite Coptotermes formosanus. Eukaryot. Cell. 2007;6:1925–1932. doi: 10.1128/EC.00251-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arms K., Feeny P., Lederhouse R.C. Sodium: Stimulus for puddling behavior by tiger swallowtail butterflies, Papilio glaucus. Science. 1974;185:372–374. doi: 10.1126/science.185.4148.372. [DOI] [PubMed] [Google Scholar]
- 63.Barrows E.M. Aggregation behavior and response to sodium chloride in females of a solitary bee, Augochlora pura (Hymenoptera: Halictidae) Fla. Entomol. 1974;57:189–193. doi: 10.2307/3493482. [DOI] [Google Scholar]
- 64.Pivnick K.A., McNeil J.M. Puddling in butterflies: Sodium affects reproductive success in Thymelicus lineola. Physiol. Entomol. 1987;12:461–472. doi: 10.1111/j.1365-3032.1987.tb00773.x. [DOI] [Google Scholar]
- 65.Spring J.H., Hyatt A.D., Marshall A.T. Uptake and release of sodium and potassium by the fat body of the American cockroach in vitro. J. Insect Physiol. 1986;32:439–444. doi: 10.1016/0022-1910(86)90004-1. [DOI] [Google Scholar]
- 66.Bonoan R.E., Tai T.M., Tagle Rodriguez M., Feller L., Daddario S.R., Czaja R.A., O’Connor L.D., Burruess G., Starks P.T. Seasonality of salt foraging in honey bees (Apis mellifera) Ecol. Entomol. 2017;42:195–201. doi: 10.1111/een.12375. [DOI] [Google Scholar]