Abstract
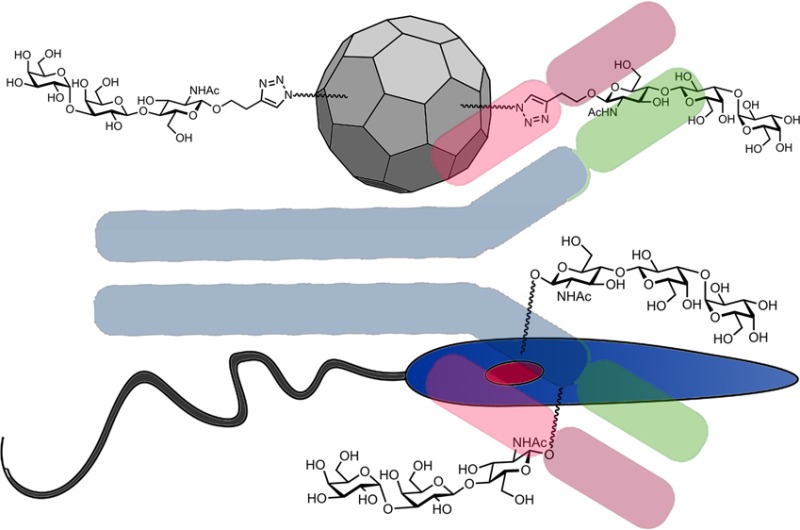
Secreted and surface-displayed carbohydrates are essential for virulence and viability of many parasites, including for immune system evasion. We have identified the α-Gal trisaccharide epitope on the surface of the protozoan parasites Leishmania infantum and Leishmania amazonensis, the etiological agents of visceral and cutaneous leishmaniasis, respectively, with the latter bearing larger amounts of α-Gal than the former. A polyvalent α-Gal conjugate on the immunogenic Qβ virus-like particle was tested as a vaccine against Leishmania infection in a C57BL/6 α-galactosyltransferase knockout mouse model, which mimics human hosts in producing high titers of anti-α-Gal antibodies. As expected, α-Gal-T knockout mice infected with promastigotes of both Leishmania species showed significantly lower parasite load in the liver and slightly decreased levels in the spleen, compared with wild-type mice. Vaccination with Qβ–α-Gal nanoparticles protected the knockout mice against Leishmania challenge, eliminating the infection and proliferation of parasites in the liver and spleen as probed by qPCR. The α-Gal epitope may therefore be considered as a vaccine candidate to block human cutaneous and visceral leishmaniasis.
Short abstract
Leishmania protozoa display the immunogenic α-Gal carbohydrate epitope. Immunization against this structure confers protective immunity against these parasites in a mouse model that mimics human α-Gal biochemistry.
Leishmaniasis, a major human health problem endemic in more than 88 countries, is spread by minute insects called sand flies, which regurgitate the parasite into the skin of their vertebrate hosts.1Leishmania species cause a spectrum of clinical symptoms2,3 depending on the nature of the infecting strain and its interaction with the host immune system and other genetic factors.4 In Mediterranean and South American countries, cutaneous leishmaniasis (CL) in humans is caused primarily by L. mexicana, L. amazonensis, and L. braziliensis, all of which induce ulcers localized to the skin. L. amazonensis is also responsible for diffuse cutaneous leishmaniasis, whereas L. braziliensis, in some circumstances, may cause lesions in mucosal surfaces. Leishmania infantum (in Europe, North Africa, and Latin America) and Leishmania donovani (in East Africa and the Indian subcontinent) are the causative agents of visceral leishmaniasis (VL),5 in which the parasite spreads to internal organs and can be lethal if not treated.6,7
The carbohydrate-rich molecules found on the surface of Leishmania parasites include lipophosphoglycans, glycosylphosphatidylinositol-anchored proteins, glycosylphosphatidylinositol lipids, and proteophosphoglycans.8 These glycoproteins are part of the glycocalyx of the promastigote infective form, playing an important role in infectivity9,10 and sand fly interaction.11 After transmission by blood-feeding sand flies of the genus Lutzomyia (in the New World), metacyclic Leishmania parasites interact primarily with phagocytic cells and then may be distributed to their resident tissues and organs, depending of the Leishmania species. While carbohydrates present on the parasite surface are likely to play an important role in mediating cell host adhesion,12 the molecular mechanism of virulence of this parasitic disease is poorly understood.
Among the most interesting pathogen-related carbohydrates is the α-Gal epitope, a name given to both the Gal-α1,3-Gal disaccharide13 and the Gal-α1,3-Gal-β1,4-GlcNAc trisaccharide;14 we use the latter designation here. Recently, high levels of anti-α-Gal antibodies were detected in cured patients with cutaneous leishmaniasis,15 raising the possibility that this highly immunogenic16,17 structure could be present on the parasite surface, stimulating anti-α-Gal antibody production beyond the levels normally present in healthy individuals.14,18 We describe here the identification of the α-Gal epitope on the promastigote form of Leishmania amazonensis and Leishmania infantum, the use of the α-galactosyltransferase knockout mouse (αGalT-KO) of Thall and colleagues19 as a murine model for leishmaniasis, and a polyvalent α-Gal conjugate as an effective vaccine candidate against parasite infection in this model. Since only humans and higher apes among all mammals lack the α-galactosyltransferase gene,20 the αGalT-KO mouse mimics the human immune system’s ability to generate anti-α-Gal antibodies to a much greater degree than wild-type rodents. These mice have been used primarily in studies of xenotransplant rejection and fertilization,21,22 but are also of great potential interest for studies of any pathogen that displays α-Gal.
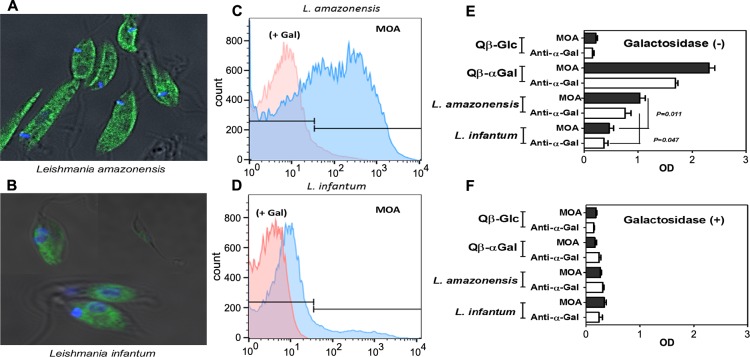
To see if Leishmania falls into this category, we investigated the presence of the α-Gal epitope on the promastigote forms of L. infantum (MCAN/BR/2002/BH401) and L. amazonensis (IFLA/BR/1967/PH8). The Marasmius oreades agglutinin (MOA) lectin, which binds strongly to the α-Gal trisaccharide, the related type B branched trisaccharide, and the Gal-α1,3-Gal disaccharide (but not the isomeric α1,2-α1,4- and α1,6-disaccharides),23,24 was used as the key reagent for these studies. Promastigote cells (108 per mL) of L. infantum and L. amazonensis were labeled with 3–5 μg/mL of fluorescein-labeled MOA and examined by fluorescence microscopy25 and flow cytometry.26 Strong staining of the L. amazonensis cell surface, compared to moderate staining of L. infantum, was observed (Figure 1A,B), which was confirmed by flow cytometry (Figure 1C,D). The addition of a large excess (0.2 M) of galactose largely abrogated binding, as expected. Lastly, L. amazonensis and L. infantum lysates were plated and incubated either with biotinylated MOA or purified anti-α-Gal antibody in an ELISA-type assay (Figure 1E).27 As before, the presence of α-Gal was confirmed for both species, but to a greater degree for L. amazonensis. Preincubation with an α-galactosidase enzyme reduced binding by more than 90% (Figure 1F), showing that the terminal galactose residue is critical. Taken together, these results provide strong evidence that both L. infantum and L. amazonensis express the α-Gal epitope on their surfaces, the latter in greater amount or accessibility.
Figure 1.
α-Gal epitope identification. (A, B) Fluorescence microscopy of L. amazonensis and L. infantum promastigote cells labeled with 3–5 μg/mL of FITC-labeled MOA (green = labeled MOA, blue = DAPI-stained nuclei, scale bar = 10 μm). (C–D) Flow cytometry (FACScan) of the cells treated as in panels A and B; x-axis = fluorescein fluorescence (488 nm excitation). “+ Gal” represents the same samples pretreated with 0.2 M galactose before addition of MOA-FITC. (E) ELISA performed with the indicated Leishmania extracts (10 μg/mL) as antigen, and biotinylated MOA (2 μg/mL) and anti-α-Gal antibody (3 μg/mL) as probe reagents. Qβ virus-like particles (2 μg/mL) bearing high density of either α-Gal or glucose were used as positive and negative controls, respectively. (F) ELISA as in panel E, with pretreatment of the antigens with green coffee bean α-galactosidase (overnight, 10 U/mL). All experiments were performed in triplicate.
In visceral leishmaniasis, caused by L. infantum, parasites are able to escape from the host immune system and reach internal organs such as liver or spleen, whereas in cutaneous leishmaniasis L. amazonensis usually stay at the site of the infection. C57BL/6 mice have been used to study pathogenesis of experimental cutaneous and visceral leishmaniasis.28 However, in spite of intense effort with this model, greater consistency is needed, particularly over multiple Leishmania strains and numbers of parasites used for infection. The presence of α-Gal on the parasite makes these experiments even less reliable as an indicator of the human response since α-Gal is a self sugar in these wild-type C57BL/6 mice.
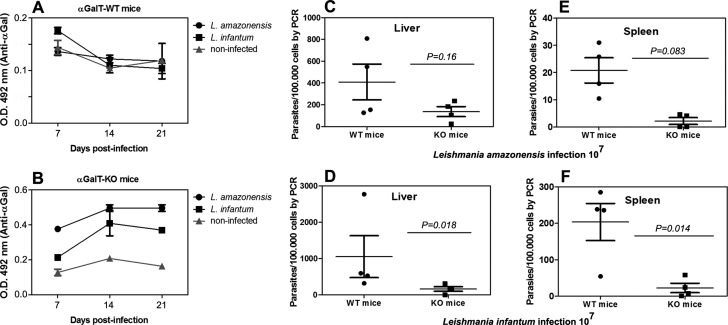
We therefore compared the response of α-GalT-KO vs α-GalT-WT mice to intraperitoneal infection with 107 promastigotes of L. infantum and subcutaneously with the same number of L. amazonensis. Blood samples for ELISA were collected at days 7, 14, and 21; liver and spleen samples were collected for quantitative PCR (qPCR) 10 weeks postinfection. The Leishmania-infected α-GalT-WT produced levels of anti-α-Gal antibodies identical to uninfected controls, representing a negligible response against the α-Gal epitope (Figure 2A). In contrast, α-GalT-KO infected mice produced anti-α-Gal antibodies to a moderate extent, significantly greater than noninfected controls (Figure 2B). Infection with L. amazonensis induced a more intense anti-α-Gal response, consistent with its higher level of α-Gal display. Parasite loads in liver and spleen were assessed by qPCR, with significantly greater variability for α-GalT-WT mice than α-GalT-KO mice. Differences in liver infection at 10 weeks were not significant for either Leishmania strain between the two mouse models (p > 0.05, Figure 2C,E), but both parasites were found in the spleens of α-GalT-WT mice to a significantly greater extent than α-GalT-KO mice (Figure 2D,F).
Figure 2.
Infection with Leishmania parasites. (A) Anti-α-Gal serum IgG antibody levels in infected α-galactosyltransferase wild type mice (αGalT-WT, serum dilution 1/100). (B) Anti-α-Gal serum IgG antibody levels in infected α-galactosyltransferase knockout mice (αGalT-KO, serum dilution 1/100); same key as panel A. (C, E) Parasite load levels (qPCR) in liver and spleen samples of αGalT-WT and αGalT-KO mice infected with 107Leishmania amazonensis cells. (D, F) Parasite load levels (qPCR) in liver and spleen samples of αGalT-WT and αGalT-KO infected mice with 107leishmania infantum cells. Three or more independent experiments were performed in each group containing 3–7 mice.
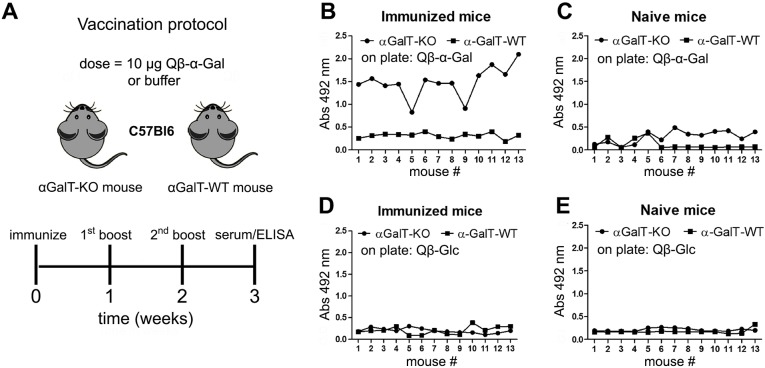
These findings suggested that α-GalT-KO mice were more resistant than α-GalT-WT toward Leishmania infection, perhaps because of the anti-α-Gal antibodies produced by the knockout animals. The highly variable nature of the response of WT mice (panels C, D, E, F) is to be expected given the many factors that contribute to the extent of infection in the absence of a controlling factor, including parasite dissemination from the site of injection and the draining lymph nodes to other organs.29−32 To further test the proposed role of an induced anti-α-Gal immune response, we immunized both mouse strains with the previously reported33,34 Qβ virus-like particle bearing approximately 540 copies of the α-Gal trisaccharide, designated Qβ–α-Gal. We hoped that high anti-α-Gal antibody levels would be generated against the glycoconjugate, and that this response would protect these mice more effectively from Leishmania infection. The immunization protocol is shown in Figure 3A, in which groups of mice were immunized with 10 μg Qβ–α-Gal particles (containing approximately 1 μg of attached α-Gal) and boosted twice (at one-week intervals) with the same dose.
Figure 3.
Anti-α-Gal IgG antibody production by mice vaccinated with Qβ–α-Gal antigen. (A) Protocol employing 13 mice per group. (B–E) ELISA for detection of serum antibodies (serum dilution 1/100); x-axis = each mouse in the group, arbitrarily numbered. (B) Serum from immunized mice, against Qβ–α-Gal immobilized on the ELISA plate; (C) serum from naïve mice, against plated Qβ–α-Gal; (D) serum from immunized mice, against plated Qβ-Glc; (E) serum from naïve mice, against plated Qβ-Glc.
The immune response of these animals compared to nonimmunized control mice was assessed by ELISA against two reagents: the Qβ–α-Gal immunogen and an analogous particle (Qβ-Glc) bearing glucose moieties (approximately 500 per particle) in place of α-Gal. As shown in Figure 3B, the immunized α-GalT-KO mice produced a strong IgG anti-α-Gal response, whereas α-GalT-WT gave no such response above background. The immune response against the carrier Qβ protein was not independently assessed, but is unlikely to have contributed significantly to the results, as evidenced by the modest intensities of ELISA signals against Qβ-Glc (Figure 3D). As expected, nonimmunized mice showed no IgG antibodies against Qβ–α-Gal or Qβ-Glc particles (Figure 3C,E).
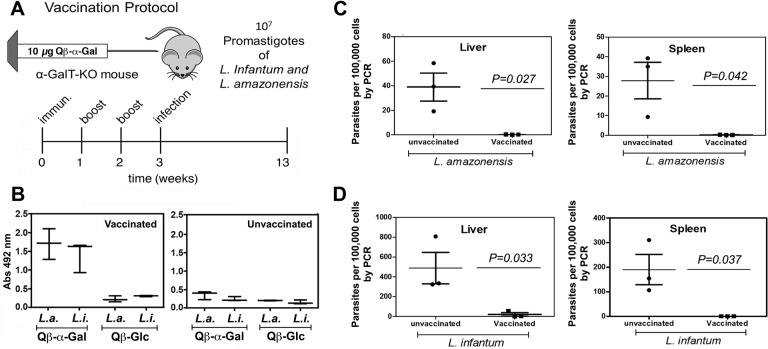
Following vaccination as above with Qβ–α-Gal (immunization + two boosts), groups of 3–5 mice were assessed for anti-α-Gal antibody production (Figure 4B) and then were infected with 107L. amazonensis or L. infantum by the appropriate route. Ten weeks later, the animals were sacrificed and qPCR was performed on samples collected from liver and spleen from all groups. Parasite loads in both organs were found to be undetectable, or nearly so, for all vaccinated and infected α-GalT-KO mice, whereas the unvaccinated mice all harbored the parasite, in widely varying amounts (Figure 4C–D). Preliminary tests showed no difference between unvaccinated mice and mice immunized with Qβ-Glc in Leishmania challenge (data not shown).
Figure 4.
Protection from Leishmania infection in α-GalT-KO mice. (A) Vaccination and infection protocol. (B) Detection of anti-α-Gal IgG antibody response by ELISA at week 2.5, for animals immunized with Qβ–α-Gal vs Qβ-Glc. “L.a.” = mice later infected with L. amazonensis; “L.i.” = mice later infected with L. infantum. (C) qPCR determination of parasite load from liver and spleen of vaccinated and unvaccinated αGalT-KO mice infected subcutaneously with 107 promastigotes of Leishmania amazonensis. (D) qPCR determination of parasite load from liver and spleen of vaccinated and unvaccinated αGalT-KO mice infected intraperitoneally with 107 promastigotes of Leishmania infantum. Three or more independent experiments were performed in groups containing from 3 to 7 mice.
Conclusions
Carbohydrates present on the surface of the Leishmania parasite have been proposed to accomplish several functions, many of which contribute to immune system evasion and pathogenesis.9,10 Since the α-Gal epitope has been found on many pathogens including organisms related to Leishmania, and since an α-Gal immune response had recently been found in Leishmania patients, we looked for and found evidence of α-Gal on two representative Leishmania species.
Antibodies against α-Gal have been reported to protect against experimental malaria infection,35 assist in the healing of burn wounds,36 improve immunogenicity in HIV vaccination,37 show lytic action against Trypanosoma cruzi parasites,38 and enhance immunogenicity in anticancer vaccine development.16 These diverse functions show that the anti-α-Gal immune response, while strong in all healthy humans, is also more complex than may be commonly appreciated. To study this for Leishmania, the α-galactosyltransferase knockout mouse can be a useful tool. Indeed, we showed here that these animals are susceptible to immunization against causative organisms of cutaneous and visceral leishmaniasis with a conjugate vaccine that presents only the α-Gal carbohydrate in common with the parasites.
Methods
Details of the handling of Leishmania samples, extract preparation, ELISA, Qβ virus-like particle preparation, qPCR, and immunization are provided in Supporting Information.
Virus-like Particles
Qβ virus-like particles (of “wild type” sequence) were prepared, purified,39 chemically derivatized,34 and characterized as previously described.
Mice
All animals and experiments were handled in strict accordance with the guidelines of the Research Ethics Committee of the UFMG, approved under the protocol number 137/2011. Female C57BL/6 mice (6–8 weeks old) having disrupted alleles of the α1,3-GalT gene19 (α1,3GalT-KO) were used. These mice have the H-2b genetic background and are bred and maintained at the animal facility of Federal University of Minas Gerais, Belo Horizonte, Brazil.
Fluorescence Microscopy and Flow Cytometry
Cells were fixed for 40 min in 2% paraformaldehyde in 0.1 mM phosphate buffer (pH 7.4), and washed three times in phosphate buffer at 4 °C for 15 min. Cells were then labeled with 3–5 μg/mL of MOA lectin-fluorescein isothiocyanate (FITC) for 60 min, washed, mounted on slides with Mowiol reagent (DABCO, Sigma a 2,3%), and observed using a fluorescent Nikon Eclipse Ti, (USA), with fluorescence illumination and a FITC filter-barrier system. Pictures were taken with Kodak Tri-X pan 400 ASA film. Lectins conjugated with FITC were obtained from EY laboratories, San Mateo, CA.
Fluorescent signals were quantified using a Becton Dickinson FACScan (Becton Dickinson), excitation 488 nm, emission 530 nm (±15 nm), adjusted to a fixed channel using standard Brite Beads (Coulter) prior to determining fluorescence. Samples were briefly vortexed before introduction to sheath fluid. Data acquisition and manipulation were performed with CellQuest and FlowJo version X.0.7 (Tree Star Inc.). Lectin binding was blocked by the addition of 0.2 M galactose to verify specificity.
Mice Immunization for Antibody Detection
We initially verified the competence of α1,3-GalT-KO mice, previously immunized, to produce antibodies (primarily IgG) against α-Gal epitopes. Immunization was performed in groups of 13 mice by subcutaneous injection of 10 μg doses of Qβ–α-Gal or unmodified Qβ particle at the base of the tail (not injection into the tail vein), followed by subcutaneous boost injections of the same dose at 1 week intervals in the flank. A control group of 13 mice was similarly treated with unmodified Qβ virus-like particle.
Only α-GalT-KO mice were used for vaccination and parasite challenge given the tolerance of α-GalT-WT mice to α-Gal epitopes.40 Groups of 3–5 female α-GalT-KO mice were immunized (initial and two boost injections) as described above; control groups received PBS only. One week after the third injection, serum antibody levels were checked by ELISA. Each each animal was then challenged with 107Leishmania parasites prepared as described in Supporting Information: L. amazonensis, MCAN/BR/2002/BH401, causing cutaneous leishmaniasis, so injected into the footpad in 10 μL volume and L. infantum, MCAN/BR/2002/BH401, causing visceral leishmaniasis, so injected i.p. in 200 μL volume.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscentsci.7b00311.
Experimental details of parasite isolation, characterization, immunization, and challenge (PDF)
This work was supported by a research partnership between Children’s Healthcare of Atlanta and the Georgia Institute of Technology, the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ) Brazil, and the National Institutes of Health (R01 GM101421).
The authors declare no competing financial interest.
Supplementary Material
References
- Bates P. A. Transmission of Leishmania metacyclic promastigotes by phlebotomine sand flies. Int. J. Parasitol. 2007, 37, 1097–1106. 10.1016/j.ijpara.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjeux P. Leishmaniasis: current situation and new perspectives. Comp. Immunol. Microbiol. Infect. Dis. 2004, 27, 305–318. 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Goihman-Yahr M. American mucocutaneous leishmaniasis. Dermatol. Clin. 1994, 12, 703–712. [PubMed] [Google Scholar]
- Handman E. Leishmaniasis: current status of vaccine development. Clin. Microbiol. Rev. 2001, 14, 229–243. 10.1128/CMR.14.2.229-243.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukes J.; Mauricio I. L.; Schönian G.; Dujardin J. C.; Soteriadou K.; Dedet J. P.; Kuhls K.; Tintaya K. W.; Jirků M.; Chocholova E.; Haralambous C.; Pratlong F.; Obornik M.; Horák A.; Ayala F. J.; Miles M. A. Evolutionary and geographical history of the Leishmania donovani complex with a revision of current taxonomy. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 9375–9380. 10.1073/pnas.0703678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W.; Berman J. D.; Davies C. R.; Saravia N. G. Advances in leishmaniasis. Lancet 2005, 366, 1561–1577. 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- Silveira F. T.; Lainson R.; Corbett C. E. Clinical and immunopathological spectrum of American cutaneous leishmaniasis with special reference to the disease in Amazonian Brazil: a review. Mem. Inst. Oswaldo Cruz 2004, 99, 239–251. 10.1590/S0074-02762004000300001. [DOI] [PubMed] [Google Scholar]
- Ilg T. Proteophosphoglycans of Leishmania. Parasitol. Today 2000, 16, 489–497. 10.1016/S0169-4758(00)01791-9. [DOI] [PubMed] [Google Scholar]
- Naderer T.; Vince J. E.; McConville M. J. Surface determinants of Leishmania parasites and their role in infectivity in the mammalian host. Curr. Mol. Med. 2004, 4, 649–665. 10.2174/1566524043360069. [DOI] [PubMed] [Google Scholar]
- Descoteaux A.; Turco S. J. Glycoconjugates in Leishmania infectivity. Biochim. Biophys. Acta, Mol. Basis Dis. 1999, 1455, 341–352. 10.1016/S0925-4439(99)00065-4. [DOI] [PubMed] [Google Scholar]
- Sacks D. L. Leishmania-sand fly interactions controlling species-specific vector competence. Cell. Microbiol. 2001, 3, 189–196. 10.1046/j.1462-5822.2001.00115.x. [DOI] [PubMed] [Google Scholar]
- Desjardins M.; Descoteaux A. Inhibition of phagolysosomal biogenesis by the Leishmania lipophosphoglycan. J. Exp. Med. 1997, 185, 2061–2068. 10.1084/jem.185.12.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinke J. W.; Platts-Mills T. A. E.; Commins S. P. The alpha-gal story: Lessons learned from connecting the dots. J. Allergy Clin. Immunol. 2015, 135, 589–596. 10.1016/j.jaci.2014.12.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili U. Anti-Gal: an abundant human natural antibody of multiple pathogeneses and clinical benefits. Immunology 2013, 140, 1–11. 10.1111/imm.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Salem W. S.; Ferreira D. M.; Dyer N. A.; Alyamani E. J.; Balghonaim S. M.; Al-Mehna A. Y.; Al-Zubiany S.; Ibrahim E. K.; Al Shahrani A. M.; Alkhuailed H.; Aldahan M. A.; Al Jarallh A. M.; Abdelhady S. S.; Al-Zahrani M. H.; Almeida I. C.; Acosta-Serrano A. Detection of high levels of anti-alpha-galactosyl antibodies in sera of patients with Old World cutaneous leishmaniasis: a possible tool for diagnosis and biomarker for cure in an elimination setting. Parasitology 2014, 141, 1898–1903. 10.1017/S0031182014001607. [DOI] [PubMed] [Google Scholar]
- LaTemple D. C.; Abrams J. T.; Zhang S. Y.; Galili U. Increased immunogenicity of tumor vaccines complexed with anti-Gal: studies in knockout mice for alpha1,3galactosyltransferase. Cancer Res. 1999, 59, 3417–3423. [PubMed] [Google Scholar]
- Rother R. P.; Galili U. alpha-Gal epitopes on viral glycoproteins. Subcell. Biochem. 1999, 32, 143–172. 10.1007/978-1-4615-4771-6_7. [DOI] [PubMed] [Google Scholar]
- Rother R. P.; Squinto S. P. The alpha-galactosyl epitope: a sugar coating that makes viruses and cells unpalatable. Cell 1996, 86, 185–188. 10.1016/S0092-8674(00)80090-2. [DOI] [PubMed] [Google Scholar]
- Thall A. D.; Maly P.; Lowe J. B. Oocyte Gal alpha 1,3Gal epitopes implicated in sperm adhesion to the zona pellucida glycoprotein ZP3 are not required for fertilization in the mouse. J. Biol. Chem. 1995, 270, 21437–21440. 10.1074/jbc.270.37.21437. [DOI] [PubMed] [Google Scholar]
- Macher B. A.; Galili U. The Galalpha1,3Galbeta1,4GlcNAc-R (alpha-Gal) epitope: a carbohydrate of unique evolution and clinical relevance. Biochim. Biophys. Acta, Gen. Subj. 2008, 1780, 75–88. 10.1016/j.bbagen.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. M.; O’Donnell C. L.; Reed D. J.; Rother R. P. Evaluation of the Galalpha1–3Gal epitope as a host modification factor eliciting natural humoral immunity to enveloped viruses. J. Virol. 1998, 72, 4650–4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez I. A.; Welsh R. M. Possible Role of a Cell Surface Carbohydrate in Evolution of Resistance to Viral Infections in Old World Primates. J. Virol. 2013, 87, 8317–8326. 10.1128/JVI.01118-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel B. P.; Winter H. C.; Goldstein I. J.; Hindsgaul O. Characterization of the recognition of blood group B trisaccharide derivatives by the lectin from Marasmius oreades using frontal affinity chromatography-mass spectrometry. Glycoconjugate J. 2002, 19, 175–180. 10.1023/A:1024297623445. [DOI] [PubMed] [Google Scholar]
- Winter H. C.; Mostafapour K.; Goldstein I. J. The mushroom Marasmius oreades lectin is a blood group type B agglutinin that recognizes the Galalpha 1,3Gal and Galalpha 1,3Galbeta 1,4GlcNAc porcine xenotransplantation epitopes with high affinity. J. Biol. Chem. 2002, 277, 14996–15001. 10.1074/jbc.M200161200. [DOI] [PubMed] [Google Scholar]
- Ramoino P. Lectin-binding glycoconjugates in Paramecium primaurelia: changes with cellular age and starvation. Histochem. Cell Biol. 1997, 107, 321–329. 10.1007/s004180050117. [DOI] [PubMed] [Google Scholar]
- Wanchoo A.; Lewis M. W.; Keyhani N. O. Lectin mapping reveals stage-specific display of surface carbohydrates in in vitro and haemolymph-derived cells of the entomopathogenic fungus Beauveria bassiana. Microbiology 2009, 155, 3121–3133. 10.1099/mic.0.029157-0. [DOI] [PubMed] [Google Scholar]
- McCoy J. P. Jr.; Varani J.; Goldstein I. J. Enzyme-linked lectin assay (ELLA). II. Detection of carbohydrate groups on the surface of unfixed cells. Exp. Cell Res. 1984, 151, 96–103. 10.1016/0014-4827(84)90359-8. [DOI] [PubMed] [Google Scholar]
- Sacks D. L.; Melby P. C., Animal models for the analysis of immune responses to leishmaniasis. Curr. Prot. Immun. 2001, Ch. 19, Unit 19 2. 10.1002/0471142735.im1902s28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley A. C.; Engwerda C. R. Balancing immunity and pathology in visceral leishmaniasis. Immunol. Cell Biol. 2007, 85, 138–147. 10.1038/sj.icb7100011. [DOI] [PubMed] [Google Scholar]
- Blackwell J. M.; Fakiola M.; Ibrahim M. E.; Jamieson S. E.; Jeronimo S. B.; Miller E. N.; Mishra A.; Mohamed H. S.; Peacock C. S.; Raju M.; Sundar S.; Wilson M. E. Genetics and visceral leishmaniasis: of mice and man. Parasite Immunol. 2009, 31, 254–266. 10.1111/j.1365-3024.2009.01102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeuillet C.; Banuls A. L.; Hide M. Study of Leishmania pathogenesis in mice: experimental considerations. Parasites Vectors 2016, 9, 144. 10.1186/s13071-016-1413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues V.; Cordeiro da Silva A.; Laforge M.; Silvestre R.; Estaquier J. Regulation of immunity during visceral Leishmania infection. Parasites Vectors 2016, 9, 118. 10.1186/s13071-016-1412-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo R. N.; Franco P. F.; Rodrigues H.; Santos L. C. B.; McKay C. S.; Sanhueza C. A.; Brito C. R. N.; Azevedo M. A.; Venuto A. P.; Cowan P. J.; Almeida I. C.; Finn M. G.; Marques A. F. Amblyomma sculptum tick saliva: α-Gal identification, antibody response and possible association with red meat allergy in Brazil. Int. J. Parasitol. 2016, 46, 213–220. 10.1016/j.ijpara.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito C. R. N.; McKay C. S.; Azevedo M. A.; Santos L. C. B.; Nunes D. F.; D’Avila D. A.; Rodrigues de Cunha G. M.; Almeida I. C.; Gazzinelli R. T.; Galvao L. M. C.; Chiari E.; Sanhueza C. A.; Finn M. G.; Marques A. F.; Venuto A. P. Virus-like particle display of the α-Gal epitope for diagnostic assessment of Chagas disease. ACS Infect. Dis. 2016, 2, 917–922. 10.1021/acsinfecdis.6b00114. [DOI] [PubMed] [Google Scholar]
- Yilmaz B.; Portugal S.; Tran T. M.; Gozzelino R.; Ramos S.; Gomes J.; Regalado A.; Cowan P. J.; d’Apice A. J.; Chong A. S.; Doumbo O. K.; Traore B.; Crompton P. D.; Silveira H.; Soares M. P. Gut microbiota elicits a protective immune response against malaria transmission. Cell 2014, 159, 1277–1289. 10.1016/j.cell.2014.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili U.; Wigglesworth K.; Abdel-Motal U. M. Accelerated healing of skin burns by anti-Gal/alpha-gal liposomes interaction. Burns: journal of the International Society for Burn Injuries 2010, 36, 239–251. 10.1016/j.burns.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Abdel-Motal U. M.; Wang S.; Awad A.; Lu S.; Wigglesworth K.; Galili U. Increased immunogenicity of HIV-1 p24 and gp120 following immunization with gp120/p24 fusion protein vaccine expressing alpha-gal epitopes. Vaccine 2010, 28, 1758–1765. 10.1016/j.vaccine.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida I. C.; Milani S. R.; Gorin P. A.; Travassos L. R. Complement-mediated lysis of Trypanosoma cruzi trypomastigotes by human anti-alpha-galactosyl antibodies. J. Immunol. 1991, 146, 2394–2400. [PubMed] [Google Scholar]
- Fiedler J. D.; Higginson C.; Hovlid M. L.; Kislukhin A. A.; Castillejos A.; Manzenrieder F.; Campbell M. G.; Voss N. R.; Potter C. S.; Carragher B.; Finn M. G. Engineered Mutations Change the Stability and Structure of a Virus-Like Particle. Biomacromolecules 2012, 13, 2339–2348. 10.1021/bm300590x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili U. Immune response, accommodation, and tolerance to transplantation carbohydrate antigens. Transplantation 2004, 78, 1093–1098. 10.1097/01.TP.0000142673.32394.95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.