Abstract
The high rate of failure during drug development is well-known, however recent advances in tissue engineering and microfabrication have contributed to the development of microphysiological systems (MPS), or ‘organs-on-chips’ that recapitulate the function of human organs. These ‘tissue chips’ could be utilized for drug screening and safety testing to potentially transform the early stages of the drug development process. They can also be used to model disease states, providing new tools for the understanding of disease mechanisms and pathologies, and assessing effectiveness of new therapies. In the future, they could be used to test new treatments and therapeutics in populations - via clinical trials-on-chips - and individuals, paving the way for precision medicine. Here we will discuss the wide-ranging and promising future of tissue chips, as well as challenges facing their development.
1. Introduction
1.1 What are tissue chips?
Tissue chips, or microphysiological systems (MPS), are devices designed to position cells in a three-dimensional structure that mimic the function of organs of the body, and react in a physiological manner to exposure to drugs, hormones, cell signaling molecules and biomechanical stressors. Platforms vary in design, with some systems allowing cells to self-organize into organoid-type structures, and others providing scaffolding for cells to proliferate and grow in a structurally-defined way. Some have highly prescriptive designs, where specific cell types are placed in well-defined positions or compartments to recapitulate functional units of organs, such as the kidney proximal tubule or liver sinusoid. The wide range of designs means a wide range of platform sizes too, ranging from cell compartments of a few hundred micrometers resting on microscope slide-sized platforms, to multi-organ systems with a footprint of a few centimeters. What all systems have in common is the recapitulation of miniaturized functional units of human organ systems, many thousands or millions of times smaller than the actual organ, and the employment of microfluidic technology to allow for fluid flow through the system to deliver nutrients and remove cellular waste, either by pump or gravity. Also common to all MPS platforms is the three-dimensional (3D) cellular arrangement of multiple cell types, which allows functional tissue-tissue interfaces and complex cellular communication to be recapitulated in vitro. These last two design features – fluid flow and 3D spatial cellular arrangements – subject tissues to shear and stretch forces that mimic in vivo conditions and are difficult to model in two dimensions. Additionally, chips are often designed from clear plastics or materials that enable cells to be visualised through the device via microscopy, allowing for real-time imaging and monitoring of cell function and health over a longer period of time. This kind of longitudinal monitoring of cell function allows the time course of response and recovery to be modeled, and the effects of cyclical hormone patterns on drug response over time investigated. Additionally, the flow of fluid through the systems allows for collection of platform effluent for further enzymatic or biochemical assays. The wide diversity of platform designs (see Figure 1) allows a broad range of biological questions to be addressed in novel and innovative ways.
Figure 1.

A broad array of tissue chip platforms have been developed. These include (clockwise from top right) a blood-brain barrier (Wikswo lab at Vanderbilt University), cardiac muscle (Parker lab at Harvard), kidney proximal tubule (www.nortis.com), female reproductive tract (DRAPER laboratories), vascularized tumor (George lab at Washington University), skin epidermis (Christiano lab at Columbia), vasculature (George lab at Washington University), liver (Taylor lab at University of Pittsburgh), and lung (www.emulatebio.com). Center image from www.ncats.nih.gov/tissuechip. All images reproduced with permission from the developers.
Broadly speaking, the ability to recreate functional human (and animal) organs in vitro could transform the drug discovery process, and open new avenues for the study of physiology and disease pathology in both humans and animals. This review will discuss the current state of the technology, drug development and disease modeling applications, and challenges faced by the field moving into the future.
1.2 Organs-on-chips mimic biologically relevant environments
Over the past decade, the MPS field has expanded from early systems of “microscale cell culture analogs” (“μCCA”) with microfluidic channels, used for drug toxicity testing1–4, to the advent of multi-organ complex 3D cell interaction systems that are becoming commercially available5. Coining the term “μCCA”, Sin and colleagues1 described a system of interconnected cell cultures from lung, liver and “other” in an early microfluidic platform, which was supplied with recirculating tissue culture medium to act as blood. Incorporating an oxygen sensor, this model was used along with a physiologically based pharmacokinetic (PBPK) model to predict the absorption, distribution metabolism and excretion (ADME) profile of chemicals. This early system was useful for predicting effective concentrations and dosages of drugs in animal and human studies, and paved the way for the nascent microphysiological systems field. However, it required all the pathways involved in metabolism of a particular drug to be incorporated into the model, which proved complex and limited its use.
Developing the use of microfluidics platforms further, Huh and colleagues6 created the more structurally complex “lung-on-a-chip”, which mimics the inhalation and exhalation of the lung alveolus and allows gas exchange between microvascular endothelial and alveolar epithelial cells. The two-chamber design, containing the two cell types separated by a thin porous membrane, proved a seminal contribution to the microfluidics field and was highly publicized. The device models the stretch forces seen in vivo in the lung airway (alveoli) by the placement of vacuum chambers which run alongside the cell chambers. As vacuum forces are applied or removed, this causes the membrane, and lung tissues, to stretch and relax the cells mimicking breathing. Importantly, the model showed physiologically relevant reactions to challenges such as bacteria and nanoparticle introduction, and produced an edemic response to inflammation from the anticancer drug interleukin-2 (IL-2) in timeframes similar to those seen in humans7. This was one of the first systems showing that MPS devices could accurately and faithfully model biological responses. Since then, the two-chamber design has been adapted to model other organs such as the gut8–10, liver and skin, and forms the basis of a system employed by a spin-off company, Emulate, which is starting to market the system for a variety of industrial uses.
MPS design requires consideration of a number of attributes during development. For example, the inclusion of innervation, vasculature, and immune components must be carefully considered in order to establish a physiologically relevant system; gradients of growth hormones, cytokines and other cell signaling pathways are likely to be highly relevant to modeling any organ system; and the readout methods for monitoring cell health and extracting information from the systems must be considered (i.e. whether biosensors are included in the platforms, or multi-electrode arrays (MEA) built into the systems, where appropriate). Additionally, the length of use of platforms can vary widely – some systems are designed to get fast readouts from a perturbation, whereas other are designed to maintain viable cells for many months, allowing for longitudinal study. While still a long way from replicating years of organ function in an adult human, maintenance of tissue culture of a few weeks or more extends the timeframes for useful physiologically-relevant information to be gleaned from these in vitro models further than that seen in two-dimensional cell culture models.
Microfabrication techniques (such as soft lithography and replica molding) are often used to manufacture tissue chips, which allows for precise designs that dictate careful spatiotemporal control over cell growth and interaction, fluid flow rates, and drug and compound exposures. Microcontact printing methods allow for inclusion of extracellular matrix (ECM) proteins, often composed of proteoglycans and fibrous proteins, to act as scaffolding for cell growth11, 12. The innovative inclusion of leading technologies such as biosensors, recent stem cell advances and gene editing techniques such as CRISPR, plus advances in microengineering of robust and reliable pumping mechanisms, places tissue chip research at the forefront of cutting-edge translational research. Together with the flexibility with complexity and design, there is a growing interest in MPS development, and support from governmental funding agencies13, 14 and private industry has led to a wide, and ever-expanding, range of organ tissues being modeled. These include liver15, 16, skin17, heart18–20, vasculature21, 22, brain23, 24, blood-brain barrier25, 26, bone27, the female reproductive system28–30, gut31, muscle32, kidney33, 34, pancreas35, 36, and cornea37, amongst others (see Bhatia and Ingber38).
2. Tissue Chips as tools for drug development
2.1 Addressing a critical need in drug development
The drug development pipeline has well-documented high failure rates, and is expensive and time-consuming39–41. A recent PhRMA (Pharmaceutical Research and Manufacturers of America) report stated that the average R&D cost for each new medicine is estimated at $2.6 billion, and can take at least ten years from the early discovery to successful approval stage. Even when a drug reaches the clinical trials stage, the probability of clinical success is around only 12%. Once a biological target for a potential medicine is found, and a lead compound is identified, the pharmacokinetics (how the body processes the drug) and pharmacodynamics (what the drug does to body functions) must be profiled before the compound can be taken further to preclinical studies. At these stages, much of the testing is done in two-dimensional cell cultures and animal models. Two-dimensional cell culture tests can be extremely useful for fast, cheap and high-throughput testing of potentially toxic compounds, and animal testing gives important readouts of the organism-level effects in vivo. However, neither method adequately models human physiological responses. Human tissues are 3D in nature, and this structure and heterogeneous environment, from the presence of multiple cell types and cell-cell interactions, cannot be replicated in a 96 (or higher)-well plate (see Breslin and O’Driscoll42). Animal models can miss important off-target effects or toxicities that would be manifested in humans, because of species differences in physiology and drug response. For example, genetic differences between species can lead to differences in drug target binding, metabolizing enzymes, and metabolites43. Indeed, these species-specific differences may prove catastrophic if not realized early on – a small difference in CD28 receptor expression patterns between humans and monkeys caused multiple organ failure in phase 1 clinical trials of six human volunteers for the drug TGN1412, being developed for immune system disorders44.
At early preclinical drug development stages, safety is the biggest reason for a lead target being pulled, whereas efficacy (the ability of the drug to show effectiveness for the indicated disorder) is a much larger source of failure at the phase II and beyond stages of clinical trials, where human testing continues in small patient groups39–41. By this time, multiple years and millions of dollars would have been spent on compound development, and this is before the compound even enters large clinical trials and gains regulatory approval, which can take many more years. Clearly there is a need for improved predictive tools for risk assessment in the drug development pipelines, and organs-on-chips are uniquely placed to help potential therapies cross the “valley of death” with more assurance of success45.
2.2 Organs-on-chips provide tools for drug screening and toxicity testing
The screening of compounds during the drug development pipeline is a key point in reducing the number of molecules of interest to a smaller pool of “hit” and subsequently “lead” compounds, and involves high-throughput methods where toxicity is gauged in simple 2D cellular models. Two-dimensional cell culture methods are widely used to initially screen out compounds that are obviously toxic. Three-dimensional organ systems may have a useful role to contribute at this stage in the development pipeline by providing more information on ‘borderline’ drugs – those that may show a certain toxicity-associated profile, but it is minor or limited, or effects could be mitigated by further development. Tissue systems with greater levels of structural and cellular complexity, giving readouts of interactions of multiple tissue types, could provide more physiologically-relevant outcomes on compounds of interest.
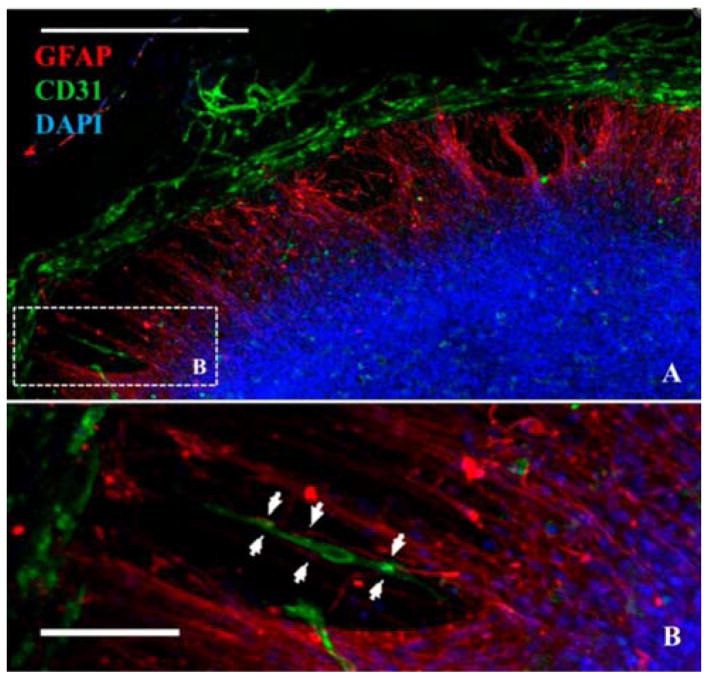
The development of neural microsystems has illustrated one example of MPS’ potential use for toxicity screening. Neural constructs, containing multiple cell types seen in the brain (neurons, glia, neuronal-specific endothelial cells) were developed, and the genetic readout to chemicals provided data for machine learning algorithms, in turn producing a predictive neurodevelopmental toxicity screening model23. The human brain is very sensitive to toxic insults during development, but the assessment of toxicity mechanisms is difficult because cellular interactions change rapidly as tissues mature, and mechanisms of diverse cell populations are not well understood. In this 3D model, human pluripotent neural progenitor cells, endothelial cells, mesenchymal stem cells and microglial precursors were cultured on hydrogels and allowed to self-assemble over the course of three weeks into “neurospheres”, which displayed vascular networks with functional glial integration (Figure 2). The constructs were then exposed to 70 compounds of known toxicity, and global gene expression pattern changes were measured by RNA-Seq readouts. The extensive data sets generated were used, along with machine learning algorithms, to build a predictive model for neurotoxicity that correctly predicted the likely toxicity of 9 of 10 blinded compounds (the remaining compound was later identified as a false positive). This combinatorial use of machine learning and medium-throughput platforms could have a transformative impact on drug screening methods, as well as inform regulators and chemical manufacturers on the developmental neurotoxicity of existing and future products.
Figure 2.
Neural constructs generated from human embryonic stem cell-derived precursor cells form vascular networks. A) Immunofluorescence for endothelial cells (CD31, green), glial cells (GFAP, red) and nuclei (DAPI, blue) shows multiple cell types growing together. B) Zoom in of the boxed region in A shows association and alignment of a capillary tubule and radially oriented glial cells (arrowheads). Scale bars A-250μm, B-100μm. Figure adapted from Schwartz et al23 with permission.
Also of interest to pharmaceuticals companies are 3D models of the liver and kidney, as the risk of drug-induced liver injury (DILI) and kidney toxicity accounts for a large number of compound failures46, 47. Microfluidic devices have advantages over cell culture models as the prolonged physiological impacts of drugs can be studied more informatively, because cells remain viable for several weeks and are subject to continuous fluid flow. For example, kidney proximal tubule models have been developed that replicate the polarity of the proximal tubule and show secretory and reabsorptive processes that replicate in vivo functions34, 48. Liver sinusoid platforms such as that described by Vernetti et al16, combine multiple liver cell types in 3D layers, which produce albumin and urea and show stable lactate dehydrogenase (LDH) leakage levels over the course of a month. Importantly, they produce expected metabolites from drugs of known action such as diclofenac. Additionally, virally transfected ‘sentinel’ cells, containing fluorescent biosensors for cell events such as mitochondrial reactive oxygen species (ROS) production and apoptosis, were incorporated into this platform. This enables fluorescence imaging to give real-time readouts on cell health and status. The inclusion of ‘biosensors’ can be done in many platforms49. As an example, using cell sources that have been genetically modified with fluorescent calcium sensors such as GCaMP6 into cardiac myocytes highlights another way that tissues can provide functional information on cell activity19.
2.3 Integrated organ systems can model in vivo-like ADME-TOX profiles
It is important to know the effects of potential therapeutics and metabolites on multiple organ systems, and this is an area where adverse outcome pathway (AOP) modeling could be made easier by MPS. For example, 98% of small molecules cannot cross the blood-brain barrier (BBB)50, but the 2% that can must be predicted and understood in order to prevent brain damage, either from drugs themselves or from their metabolites. To address this, as well as to assess the ability of candidate therapeutics for treatment of brain disorders, a number of microfluidic devices have been developed to model the BBB51. One of these systems from the VIBRE Center at Vanderbilt consists of chambers of neurons cultured separately from chambers of endothelial cells, to mimic the neurovascular environment and enable study of BBB function and dysfunction25, 26.
While a model of the BBB alone can be helpful in assessing BBB permeability for molecular studies, the power of microfluidics platforms is illustrated by the coupling of multiple organ systems to model sequential drug metabolism. For example, Vernetti and colleagues52 functionally coupled the gut, liver, kidney and BBB by exposing organs sequentially to compounds with known actions, collecting the conditioned media from platforms after metabolism by the tissues and feeding it into the next system. In this manner they showed that organ-specific processing of terfenadine, trimethylamine (TMA) and vitamin D3 was in line with clinical data, but also that the TMA metabolite, trimethylamine-N-oxide (TMAO), crosses the BBB. While the results are limited by their lack of real-time kinetic responses and use of primary cells from multiple individuals, this new finding illustrates how sequential coupling of MPS could predict drug responses and uncover previously unforeseen or unexpected effects of metabolites5, 53–55.
Another integrated system modeling the female reproductive tract was recently published30, and could be a powerful tool in drug development pipelines that historically focus heavily on toxicity and safety screening in male-derived cells and organisms. The system, dubbed “EVATAR”, consists of organ modules for the ovary, fallopian tube, uterus, cervix and liver in a single MPS platform, and could simulate a 28-day menstrual cycle hormone profile (Figure 3). The importance of fluid flow for tissue culture was also highlighted – ciliary beating of fallopian tube cells is difficult to maintain in static culture, but was maintained for 21 days in the EVATAR system, where each organ system is microfluidically connected to the next. The complex platform design, with microengineered pumping mechanisms enabling integration of multiple organs on one chip, illustrates how biomechanical engineering experts working in parallel with field leaders in female reproductive biology can create cutting-edge technology at the leading edge of MPS development. A number of other integrated systems are currently under development, and start-up and spin-off companies from academic environments are beginning to bring commercialized multi-systems to the market for industrial usages (Figure 4)5, 56, 57. Some of the challenges associated with platform integration and commercialization will be discussed in more detail later in this review.
Figure 3.
The “EVATAR” system, modeling the human reproductive tract. Liver, ovary, ectocervix, uterus and fallopian tube modules are linked by microfluidic channels (left). Ovarian hormone secretion over 28 days in culture with oestradiol and progesterone mimics the human menstrual cycle30. Image adapted under a Creative Commons 4.0 license from Xiao et al30.
Figure 4.
A four-organ chip device from TissUse (Germany). The device consists of two polycarbonate coverplates with a PDMS chip that incorporates intestine (1), liver (2), skin (3) and kidney (4) tissue. Pink represents a surrogate blood flow; yellow represents an excretory flow circuit. Each microphysiological fluid flow circuit is operated by a separate peristaltic micropump. Image adapted under a Creative Commons 3.0 license from Maschmeyer et al57.
2.4 Tissue chips for efficacy testing could help reduce drug development failures
Once toxicity screens are complete, drugs move to the stages of testing in animals and humans, and here a lack of efficacy may be another roadblock as drugs do not show expected clinical effects 39. Human volunteers may suffer unexpected and potentially catastrophic side effects if efficacy data is not adequately presented before drugs move to first-in-human trials58. Cook et al41 investigated the reasons for failure of 142 small molecule drug development programs between 2005 and 2010 at AstraZeneca, and found that lack of efficacy caused failure rates of an astonishing 57–88% at phase II clinical trials.
MPS platforms can help reduce this failure rate in a several ways. For example, they can help screen out damaging compounds to human tissues earlier, so fewer but more promising compounds reach human trials. Additionally, the range of tissue platforms means a variety of human organ systems can be screened for drug effectiveness BEFORE reaching human trials, and mechanisms of action confirmed and modeled appropriately. Another advantage for the use of tissue chip models in early drug development pipelines is an ethical one, as use of these platforms could significantly reduce the use of animals in preclinical stages. There will still be a critical need for animal models as whole-organism models, but as individual and integrated organ microsystems become more readily available, cheaper, and capable of medium- to high-throughput screening, the use of rodents and other mammals for drug development may be streamlined and significantly reduced.
3. Tissue Chips for disease modelling
The modeling of disease states on MPS opens new avenues for understanding of pathologies and potential treatments. Diseases could be modeled from patient donors, either from primary or induced stem cell sources, or using genetic tools to induce a disease phenotype that can be studied in vitro against an isogenic background.
3.1 Understanding disease mechanisms on tissue chips
A considerable number of disease states have already been modeled on tissue chips. For example, cancer tumor cells show different phenotypes when cultured in 2D as compared to 3D, and the microenvironment in which they are cultured critically dictates their behaviors including growth and spread59, 60. Metastatic spread accounts for a large proportion of cancer-related mortality, and as the liver is a major site of metastasis for a number of carcinomas, as well as a key site of drug metabolism, it is an important target for MPS cancer modeling61. Clark et al62 describe a liver bioreactor of human liver tissues, cultured for a number of weeks, which can be seeded with carcinoma cells that proliferate and grow into small tumors. The system has been used to investigate breast cancer metastatic growth, and importantly, showed that a proportion of cancerous cells entered a dormant state within the systems. This is a significant problem in human populations as microtumors can remain dormant for many years before beginning to grow again63. Additionally, the presence of nonparenchymal cells (stellate, Kupffer and endothelial) in the system altered cancer cell behaviors, illustrating the complexity of the heterogeneous cell microenvironment.
Tumors both small and large rely on vascular support for proliferation, and MPS modeling of vascularized microtumors (“VMTs”) has demonstrated vigorous growth of both colorectal and breast cancer cells64. Exposure of the VMTs to standard chemotherapeutics also displayed the different known modes of action of the drugs. For example, vincristine caused disruption in the microvasculature and resulting decreases in microtumor size, whereas oxaliplatin acted on cancer cells but left the vasculature intact. This platform has now been adapted to 96-well arrays for higher-throughput screening and toxicity testing of angiogenic and chemotherapeutic agents65.
Tissue chips can also be used to better understand bacterial and viral infections. For example, a “gut-on-a-chip” platform, which houses human Caco2 intestinal cells in a two-chamber device of similar design to the lung-on-a-chip described previously8, was recently used to demonstrate the response of these intestinal epithelial cells to a viral infection by the enterovirus coxsackievirus B166. The authors showed viral entry and replication in the epithelium, as well as inflammatory cytokine release, helping understand enterovirus mechanisms of action. Both of these last examples highlight how data from MPS were validated against known clinical results, and is an important step for the field, helping to prove the utility of platforms for understanding of disease pathology and therapeutic development.
3.2 Rare disease patients may benefit from tissue chip technology
Treating rare disease patients can be a huge challenge for a number of reasons, and fewer than 5% of around 7000 currently identified rare diseases have effective drug therapies67. However, MPS technology may not only enable better understanding of many under-studied disorders, but also provide new drug screening tools for existing therapeutics, kick-start the continued development of orphan drugs, and provide platforms for testing of repurposed existing drugs68.
One high profile use of MPS platforms to date describes the use of a cardiac MPS to model a rare disease, Barth syndrome, which causes cardiac and skeletal myopathy and immune deficiencies due to a mutation in the TAZ gene. Wang and colleagues69 used ‘muscular thin films’ (MTFs) of cardiac tissue on elastomeric thin films that ‘twitch’ in a measurable way as tissue contracts18, 70. The team generated induced pluripotent stem cells (iPSCs) from two Barth syndrome patients, and differentiated cells into cardiomyocytes, which they seeded and cultured on the MTFs. The resulting MTFs showed lower twitch and peak systolic stress, similar to the pathophysiology seen in the disease. Importantly, the team then ‘rescued’ a normal phenotype in the tissue by introduction of TAZ RNA, showing the power of gene editing technologies on MPS. To the authors’ knowledge, a variety of rare diseases are now being modeled in MPS platforms, including Hutchinson-Gilford progeria syndrome, hereditary hemorrhagic telangiectasia (HHT), Rett syndrome, and Alpers-Huttenlocher syndrome (personal communications).
3.3 Tissue chips for precision medicine and clinical trials on chips
The applications for MPS use in disease research offers promise to further our understanding of a broad range of disorders, and could lead to potentially ground-breaking discoveries of disease mechanisms and treatments. Indeed, the National Institutes of Health (NIH) in the US in investing around $75M over the next five years in a program specifically for use of the chips for disease modeling. In the future, the ability to populate MPS with primary or iPS cells from patients opens avenues for treatments that are tailored to an individual – in other words, MPS could be useful tools for precision medicine efforts. Chips could be used to model tissues from individual patients for therapeutic assessment. Chips could also be created containing tissues with known genetic polymorphisms, in order to model pharmacogenetic variations within populations. Taking this concept further, tissue chips offer the possibility to model population-wide variation, enabling genetic factors such as gender, as well as environmental factors such as exposure to infectious agents or toxins, to be modeled. Together, these possibilities open the way for clinical trials-on-chips in the coming years, where both new and existing drugs can be tested on MPS from different populations. This could streamline the drug development process by accurately representing population variation at preclinical and early clinical trial stages, reducing the attrition rate of promising drugs.
4. Questions and issues around continued MPS development
The potential uses for MPS are promising, but as with all new technologies, there are issues that remain to be resolved. Continued investment of expertise and research funding from a variety of stakeholders will be crucial to address these challenges in forthcoming years.
4.1 What cells can be used in tissue chips?
One issue facing the field is the challenge of cell sourcing. Currently, researchers can populate platforms with commercially available immortalized cell lines, primary tissues from donors, or iPSC sources (either from donors or from commercial sources). Each of these has advantages and disadvantages. For example, commercially available cell lines facilitate ease of access for researchers, but these immortalized lines may have been cultured for many generations, resulting in substantial genetic/epigenetic drift71, 72. Primary tissues from donors are ideal for MPS seeding, particularly for rare diseases. However, they are difficult to obtain from small population sizes and may only be available in small amounts, or from diseased populations. Healthy tissues are difficult or unethical to source, or may be from deceased donors and therefore compromised, depending on the length of time post mortem before it is available. In addition, some tissues are not readily available from donors (i.e. nervous system tissue). Furthermore, when using primary cells from multiple individuals, or cell lines with unknown donor demographics, increased genetic variability is seen in resulting tissues, increasing the variability of results and confounding some data interpretations.
The most promising cell sources for MPS platforms are from stem cells, particularly induced pluripotent types. Technological advances now allow renewable cell sources for a variety of tissues, and the reprogramming of tissue types from skin fibroblasts or blood cells (to create iPSCs) provides remarkable opportunities and has many advantages over primary cell use. Firstly, multiple tissue types can be created from individuals where each tissue has the same genetic background i.e. isogenic. Another advantage is that with the advent of gene editing techniques such as CRISPR, both the creation AND genetic therapy of tissues is feasible for monogenic or Mendelian diseases in ways never before possible69. Finally, iPSC advances hold promise for validation of other population-wide studies such as genome-wide association studies (GWAS). For example, iPSC-derived cardiac and fat tissue was recently produced from peripheral blood cells of 34 donor participants in the Framingham heart study cohort. Genetic variants with known phenotypic signatures were investigated and results were in line with those from previous analyses using different approaches – this being one of the first studies to functionally validate variants found from a GWAS73. A number of successful 3D models utilizing human iPS-derived cells have been developed, and include gut and stomach organoids that have allowed investigation of enteric infections such as pathogen-induced diarrhea and stomach ulcers74, 75; iPSC-derived human brain organoids and MPS which illustrate features of human cortical development76 and can be used to model demyelinating disorders such as multiple sclerosis77; and human embryonic-derived iPS neural constructs described above for developmental neurotoxicity screening23.
It is now widely known that the biomechanical environment in which stem cells are cultured can have critical effects on their phenotype78, and that 3D culture promotes higher yields of differentiated stem cells, sometimes into more mature cell phenotypes, than culture in two-dimensions79–81. One potential application for MPS to help advance the field of iPSC was detailed recently82, with the precisely controlled microfluidic environment of tissue chips being highlighted as a benefit for accurately controlling and exploring the fluid environment surrounding differentiating cells. This kind of technological crosstalk has the potential to significantly advance the stem cell field, and benefit MPS researchers’ needs for renewable cell sources.
Challenges remain in this area, however. Differentiation protocols for iPS-derived cells differ widely between labs, and the resulting cells resemble fetal phenotypes. A move towards standardization for specific cell types would be helpful in many instances, but the field itself is relatively young so it may take many years before robust protocols are available for some tissues. Epigenetic memory, whereby iPS cells retain certain characteristics of their original phenotype, is also a factor to consider as it may affect phenotypic responses to potential therapeutics83, 84. Additionally, the fast culture of iPSCs is not optimal for modeling late-onset or chronic disorders associated with aging, although aspects of some late onset neurodegenerative diseases such Alzheimer’s and Parkinson’s diseases have been successfully modeled in iPSC-derived tissues85. The pharmaceutical industry has moved towards using neural iPSCs for lead validation and optimization, which when used in conjunction with cell culture and animal models, can yield useful information86. However, the complexity of many diseases such as diabetes and chronic obstructive pulmonary disease – which manifest in multiple tissues and mature over many years – makes modeling of these phenotypes currently very challenging.
Additional questions that remain to be answered in the future include addressing how well reprogrammed and redifferentiated cell lines replicate normal and disease phenotypes in vivo. For example, does the process of reprogramming, with exposure to different growth factors, alter the genetic and epigenetic code of the cells? How closely do in vitro phenotypes compare to in vivo ones? And for disease modeling, for example for neurological disease, how do we validate iPSC neuron phenotypes when the drugs for many neurological diseases (such as Huntington’s disease) may not even exist? Currently, there is still much progress being made with iPSC use, and over the coming years, the use of iPSC-derived sources is likely to become more widespread in microphysiological systems.
4.2 What materials are MPS fabricated from?
The materials used to create chips allow much flexibility in terms of design, but can have large effects on functionality. The flexible clear plastic polydimethylsiloxane (PDMS) is ideal for MPS fabrication, as it allows real-time optical imaging of cells due to its transparency, and is cheap and easy to work with in the laboratory (for example through the use of soft lithography). However, it also absorbs hydrophobic drugs and is gas permeable87. This potentially decreases drug concentrations reaching tissues, or cross-contaminates neighboring microfluidic channels or cell-containing chambers. Additionally, its hydrophobicity has been cited as a factor causing poor cell adhesion in MPS devices, leading to cell aggregation which can interfere with fluidic flow88. Researchers have addressed this issue in a number of ways. These include oxidizing the PDMS, which creates a barrier layer of silicon dioxide on the surface89, and plasma treating90 or coating it with proteins91, 92, to prevent cell adhesion and drug loss. A variety of mathematical modeling approaches have been developed to account for absorption93, 94, and some groups are moving away from PDMS altogether, or are using alternative techniques such as inclusion of carrier proteins to enable transport of hydrophobic molecules such as vitamin D52.
4.3 Key challenges facing platform integration
As described above, the coupling of MPS platforms holds much promise as a tool for toxicity screening in drug development. However, some key biological and technical challenges remain. One biological challenge is that of organ scaling between systems, i.e. organ systems need to produce outputs that are at physiologically-relevant levels in vivo to other organs. For example, linking a liver and kidney system would not give appropriate readouts of a metabolite if the liver module were five times “larger” than the kidney module within the system. Allometric scaling can be used to quantify the relationship between organs of different sizes. However, this type of ‘simple’ scaling does not necessarily hold true when considering the complexities and size differentials between human in vivo tissues and MPS95. Functional scaling may be a more appropriate strategy for determining meaningful ratios between organ masses, as the function of the tissue is taken into account when designing the system (e.g. gas exchange for lung, molecular filtration for kidney). This more straightforward approach allows preservation of organ-specific functions at appropriate relative magnitudes, although relating platform results to in vivo physiology (or “in vitro-in vivo translation”) is still a critical consideration when physically linking platform systems96.
That MPS could be used to model population variance in vitro means, as mentioned above, MPS could be powerful tools for precision medicine efforts. However, variance in physiological responses can depend on environment, age, genetic, demographic, immune capabilities, and hundreds of other variables, making designing and manufacturing reliable, robust and reproducible platforms even more challenging. Additionally, the linkage of multiple organ systems requires some sort of blood mimetic or universal medium, which can be pumped through multiple organ systems and adequately supply disparate cell types with the appropriate nutrients. Some coupled systems currently mix effluent from one system with the optimal media for the next to lessen the impact of metabolic waste products52, or provide barriers between organ systems that mimic endothelial barriers between the stromal cells and circulating perfusate57, 97.
Technical challenges associated with platform integration include: 1) How to connect microfluidic systems without bubble introduction, which can impede fluid flow much more than in larger systems, 2) How to maintain sterility when connecting systems that are seeded and matured independently, and 3) How to control different oxygenation levels between organ systems, or even within organ systems. For example, liver zonation produces different physiological functionality therefore multi-organ systems may require sophisticated engineering, such as the use of local recirculation reservoirs that can be selectively oxygenated or deoxygenated to appropriately supply target tissues. Additionally, media flow rates are highly variable between organ systems, and indeed tissues such as the kidney proximal tubule function in part due to the high pressure of blood entering the nephron, so physical scaling of fluid supply needs to be accounted for appropriately.
4.4 The move towards validation and commercialization of MPS
For tissue chip technology to be accessible and available for researchers and the pharmaceutical industry, the platforms need to be validated and commercialized for community-wide access. This requires validation at multiple levels. To begin, platforms must show they produce robust, reliable and replicable results in the hands of investigators other than the developers. Technology transfer can be challenging, as detailed protocols are required to be made public and skilled technicians and scientists versed in cell culture, microfluidics, and the target organ system may be required to recreate functional platforms. Once platforms are seeded and tissues can survive, basic outcomes such as tissue composition, and physiologic and pharmacologic responses to well-known agents, should be examined to ensure that the results produced from the tissues are physiologically correct (e.g. that the antibiotic polymyxin B shows the same nephrotoxicity in a kidney proximal tubule platform as it does in vivo). To begin this early translational and face validity testing, the National Center for Advancing Translational Sciences at the National Institutes of Health in the US is currently funding a Tissue Chip Testing Centers initiative, for tissue chip developers funded under a larger Tissue Chip for Drug Screening program. The creation of centralized testing centers, in partnership with the US Food and Drug Administration (FDA) and the IQ Consortium (a group of pharmaceutical industry representatives), will allow broader testing of chip capability98. Throughput capabilities of platforms can be independently assessed, producing realistic readouts of intra- and inter-laboratory reproducibility, and collating and integrating operating procedures and processes for wider use. In the future, these centers would be well-positioned to offer services testing the development of new platforms, or as contract research organizations (CROs) for a variety of platform end-users. This removes the need for organizations to set up their own core tissue chip facilities.
In the shorter term, the data from these centers can be made readily available to a variety of stakeholders, including the pharmaceutical and biotechnology industries, and provide information for continued evolution of chip design that is fit-for-purpose. For example, chips for toxicity screens for environmental toxins may result in very different platform designs than those for therapeutic testing of an orphan drug in multi-tissue platforms. The former will require easily fabricated fast, cheap and reliable designs for high-throughput testing of simpler tissue systems, and the latter may necessitate lower-throughput but significantly more complex platform designs. The balance between simplicity and complexity, and lower versus higher throughput processes, will be a continuing conversation between chip developers and the end-users over the coming years. End-user perspectives will also become increasingly important because currently the broad array of device formats, operational procedures and endpoints gathered requires often wide-ranging and/or expensive expertise, which limits the easy translatability of platforms to the wider research community. Few systems combine user-friendliness, plus integration of more than one or two standard assays (such as immunohistochemistry and off-chip assays such as ELISA), and are market-ready, suggesting that ongoing dialogue will be crucial for platform developers and engineers to create systems that are broadly accessible to all users99.
For applications such as drug screening - especially if chips are to complement current cell culture, animal studies, and/or early human clinical trials - assay validation by regulatory agencies will be required. For this, MPS may need to produce results conforming to guidelines from international bodies such as the Organisation for Economic Cooperation and Development (OECD) and European Medicines Agency (EMA) and, in the US, the National Toxicology Program’s Interagency Center for the Evaluation of Alternative Toxicological Methods (NICEATM). Then, results from MPS can be carefully assessed by agencies such as the FDA. These processes require input and expertise from all levels, and will take time and cooperation to successfully launch any MPS platform for wider commercial use.
Conclusions
Tissue chips hold much promise for use as tools in the drug therapeutic development and screening arena, as well as to model human disease pathologies. Depending on design and use, they can be utilised to ask and answer questions that cannot be answered by conventional means. These include how the sequential metabolism of certain drugs may differ between animals and humans, and how that species difference should inform drug developers. For drug development processes such as toxicity screening and efficacy testing, animal models and two-dimensional cell culture techniques are currently the widely-used “gold standards”. Further uptake and validation by the research community will be needed for widespread adoption by the pharmaceutical industry. Currently, interest is high and many pharmaceutical companies are actively pursuing the research, or looking into how to adopt it for their own means.
Precision medicine efforts remain in relatively early stages. However, the power of using tissue chips to model organ systems in specific individuals will likely mean increased visibility in future years, as patient groups are modeled ex vivo, or therapeutics are tested in populations with specific genetic polymorphisms and genotypes. Over the next few years, we predict that there will be a divergence in platform development, with drug companies and the biomedical industry moving towards validation and commercialization of higher throughput, relatively simple systems for cheap, fast, reliable toxicity screening. There will also be continued development of physiologically complex “body-on-a-chip” systems for multi-organ diseases such as metabolic and cardiovascular disorders. We see much potential for this exciting and rapidly expanding field, to contribute insight for biomedical researchers and the pharmaceutical industry into treatments for some of the most prevalent and intractable and disorders of our time.
Acknowledgments
We gratefully thank Dobrila Rudnicki for critical reading of the manuscript.
Footnotes
Conflict of Interest
There are no conflicts of interest to declare.
References
- 1.Sin A, Chin KC, Jamil MF, Kostov Y, Rao G, Shuler ML. Biotechnology Progress. 2004;20:338–345. doi: 10.1021/bp034077d. [DOI] [PubMed] [Google Scholar]
- 2.Wang Z, Kim M-C, Marquez M, Thorsen T. Lab on a Chip. 2007;7:740–745. doi: 10.1039/b618734j. [DOI] [PubMed] [Google Scholar]
- 3.Park JW, Vahidi B, Taylor AM, Rhee SW, Jeon NL. Nat Protocols. 2006;1:2128–2136. doi: 10.1038/nprot.2006.316. [DOI] [PubMed] [Google Scholar]
- 4.Huh D, Fujioka H, Tung Y-C, Futai N, Paine R, Grotberg JB, Takayama S. Proceedings of the National Academy of Sciences. 2007;104:18886–18891. doi: 10.1073/pnas.0610868104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oleaga C, Bernabini C, Smith AST, Srinivasan B, Jackson M, McLamb W, Platt V, Bridges R, Cai Y, Santhanam N, Berry B, Najjar S, Akanda N, Guo X, Martin C, Ekman G, Esch MB, Langer J, Ouedraogo G, Cotovio J, Breton L, Shuler ML, Hickman JJ. Scientific Reports. 2016;6:20030. doi: 10.1038/srep20030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Science. 2010;328:1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huh D, Leslie DC, Matthews BD, Fraser JP, Jurek S, Hamilton GA, Thorneloe KS, McAlexander MA, Ingber DE. Science Translational Medicine. 2012;4:159ra147–159ra147. doi: 10.1126/scitranslmed.3004249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HJ, Huh D, Hamilton G, Ingber DE. Lab on a Chip. 2012;12:2165–2174. doi: 10.1039/c2lc40074j. [DOI] [PubMed] [Google Scholar]
- 9.Kim HJ, Ingber DE. Integrative Biology. 2013;5:1130–1140. doi: 10.1039/c3ib40126j. [DOI] [PubMed] [Google Scholar]
- 10.Kim HJ, Li H, Collins JJ, Ingber DE. Proceedings of the National Academy of Sciences. 113:E7–E15. doi: 10.1073/pnas.1522193112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huh D, Hamilton GA, Ingber DE. Trends in Cell Biology. 2011;21:745–754. doi: 10.1016/j.tcb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huh D, Kim HJ, Fraser JP, Shea DE, Khan M, Bahinski A, Hamilton GA, Ingber DE. Nat Protocols. 2013;8:2135–2157. doi: 10.1038/nprot.2013.137. [DOI] [PubMed] [Google Scholar]
- 13.Sutherland ML, Fabre KM, Tagle DA. Stem Cell Research & Therapy. 2013;4:1–5. doi: 10.1186/scrt361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fabre KM, Livingston C, Tagle DA. Experimental Biology and Medicine. 2014;239:1073–1077. doi: 10.1177/1535370214538916. [DOI] [PubMed] [Google Scholar]
- 15.Domansky K, Inman W, Serdy J, Dash A, Lim MHM, Griffith LG. Lab on a Chip. 2010;10:51–58. doi: 10.1039/b913221j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vernetti LA, Senutovitch N, Boltz R, DeBiasio R, Ying Shun T, Gough A, Taylor DL. Experimental Biology and Medicine. 2016;241:101–114. doi: 10.1177/1535370215592121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abaci HE, Guo Z, Coffman A, Gillette B, Lee W-h, Sia SK, Christiano AM. Advanced Healthcare Materials. 2016;5:1800–1807. doi: 10.1002/adhm.201500936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agarwal A, Goss JA, Cho A, McCain ML, Parker KK. Lab on a chip. 2013;13:3599–3608. doi: 10.1039/c3lc50350j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathur A, Loskill P, Shao K, Huebsch N, Hong S, Marcus SG, Marks N, Mandegar M, Conklin BR, Lee LP, Healy KE. Scientific Reports. 2015;5:8883. doi: 10.1038/srep08883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capulli AK, MacQueen LA, O’Connor BB, Dauth S, Parker KK. Cardiovascular Pathology. 2016;25:316–324. doi: 10.1016/j.carpath.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belair DG, Whisler JA, Valdez J, Velazquez J, Molenda JA, Vickerman V, Lewis R, Daigh C, Hansen TD, Mann DA, Thomson JA, Griffith LG, Kamm RD, Schwartz MP, Murphy WL. Stem cell reviews. 2015;11:511–525. doi: 10.1007/s12015-014-9549-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandez CE, Yen RW, Perez SM, Bedell HW, Povsic TJ, Reichert WM, Truskey GA. Scientific Reports. 2016;6:21579. doi: 10.1038/srep21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz MP, Hou Z, Propson NE, Zhang J, Engstrom CJ, Costa VS, Jiang P, Nguyen BK, Bolin JM, Daly W, Wang Y, Stewart R, Page CD, Murphy WL, Thomson JA. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:12516–12521. doi: 10.1073/pnas.1516645112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown JA, Sherrod SD, Goodwin CR, Brewer B, Yang L, Garbett KA, Li D, McLean JA, Wikswo JP, Mirnics K. Journal of Neuroinflammation. 2014;11:183. doi: 10.1186/s12974-014-0183-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown JA, Pensabene V, Markov DA, Allwardt V, Neely MD, Shi M, Britt CM, Hoilett OS, Yang Q, Brewer BM, Samson PC, McCawley LJ, May JM, Webb DJ, Li D, Bowman AB, Reiserer RS, Wikswo JP. Biomicrofluidics. 2015;9:054124. doi: 10.1063/1.4934713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown JA, Codreanu SG, Shi M, Sherrod SD, Markov DA, Neely MD, Britt CM, Hoilett OS, Reiserer RS, Samson PC, McCawley LJ, Webb DJ, Bowman AB, McLean JA, Wikswo JP. Journal of Neuroinflammation. 2016;13:306. doi: 10.1186/s12974-016-0760-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Peppo GM, Marcos-Campos I, Kahler DJ, Alsalman D, Shang L, Vunjak-Novakovic G, Marolt D. Proceedings of the National Academy of Sciences. 2013;110:8680–8685. doi: 10.1073/pnas.1301190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eddie SL, Kim JJ, Woodruff TK, Burdette JE. Experimental biology and medicine (Maywood, NJ) 2014;239:1192–1202. doi: 10.1177/1535370214529387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu J, Xu Y, Rashedi AS, Pavone ME, Kim JJ, Woodruff TK, Burdette JE. Molecular Human Reproduction. 2016 doi: 10.1093/molehr/gaw041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao S, Coppeta JR, Rogers HB, Isenberg BC, Zhu J, Olalekan SA, McKinnon KE, Dokic D, Rashedi AS, Haisenleder DJ, Malpani SS, Arnold-Murray CA, Chen K, Jiang M, Bai L, Nguyen CT, Zhang J, Laronda MM, Hope TJ, Maniar KP, Pavone ME, Avram MJ, Sefton EC, Getsios S, Burdette JE, Kim JJ, Borenstein JT, Woodruff TK. Nature Communications. 2017;8:14584. doi: 10.1038/ncomms14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foulke-Abel J, In J, Yin J, Zachos NC, Kovbasnjuk O, Estes MK, de Jonge H, Donowitz M. Gastroenterology. 2016;150:638–649.e638. doi: 10.1053/j.gastro.2015.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madden L, Juhas M, Kraus WE, Truskey GA, Bursac N. eLife. 2015;4:e04885. doi: 10.7554/eLife.04885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jang K-J, Mehr AP, Hamilton GA, McPartlin LA, Chung S, Suh K-Y, Ingber DE. Integrative Biology. 2013;5:1119–1129. doi: 10.1039/c3ib40049b. [DOI] [PubMed] [Google Scholar]
- 34.Weber EJ, Chapron A, Chapron BD, Voellinger JL, Lidberg KA, Yeung CK, Wang Z, Yamaura Y, Hailey DW, Neumann T, Shen DD, Thummel KE, Muczynski KA, Himmelfarb J, Kelly EJ. Kidney International. 2016;90:627–637. doi: 10.1016/j.kint.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silva PN, Green BJ, Altamentova SM, Rocheleau JV. Lab on a Chip. 2013;13:4374–4384. doi: 10.1039/c3lc50680k. [DOI] [PubMed] [Google Scholar]
- 36.Jun Y, Kim MJ, Hwang YH, Jeon EA, Kang AR, Lee S-H, Lee DY. Biomaterials. 2013;34:8122–8130. doi: 10.1016/j.biomaterials.2013.07.079. [DOI] [PubMed] [Google Scholar]
- 37.Puleo CM, McIntosh Ambrose W, Takezawa T, Elisseeff J, Wang T-H. Lab on a Chip. 2009;9:3221–3227. doi: 10.1039/b908332d. [DOI] [PubMed] [Google Scholar]
- 38.Bhatia SN, Ingber DE. Nat Biotech. 2014;32:760–772. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 39.Arrowsmith J. Nat Rev Drug Discov. 2011;10:87–87. doi: 10.1038/nrd3375. [DOI] [PubMed] [Google Scholar]
- 40.Arrowsmith J. Nat Rev Drug Discov. 2011;10:328–329. doi: 10.1038/nrd3439. [DOI] [PubMed] [Google Scholar]
- 41.Cook D, Brown D, Alexander R, March R, Morgan P, Satterthwaite G, Pangalos MN. Nat Rev Drug Discov. 2014;13:419–431. doi: 10.1038/nrd4309. [DOI] [PubMed] [Google Scholar]
- 42.Breslin S, O’Driscoll L. Drug Discovery Today. 2013;18:240–249. doi: 10.1016/j.drudis.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, López CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG. t. Inflammation and L. S. C. R. P. Host Response to Injury. Proceedings of the National Academy of Sciences. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eastwood D, Findlay L, Poole S, Bird C, Wadhwa M, Moore M, Burns C, Thorpe R, Stebbings R. British Journal of Pharmacology. 2010;161:512–526. doi: 10.1111/j.1476-5381.2010.00922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coller BS, Califf RM. Science Translational Medicine. 2009;1:10cm19–10cm19. doi: 10.1126/scitranslmed.3000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fuchs TC, Hewitt P. The AAPS Journal. 2011;13:615–631. doi: 10.1208/s12248-011-9301-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Materne E-M, Tonevitsky AG, Marx U. Lab on a Chip. 2013;13:3481–3495. doi: 10.1039/c3lc50240f. [DOI] [PubMed] [Google Scholar]
- 48.Kim S, Takayama S. Kidney Research and Clinical Practice. 2015;34:165–169. doi: 10.1016/j.krcp.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Senutovitch N, Vernetti L, Boltz R, DeBiasio R, Gough A, Taylor DL. Experimental Biology and Medicine. 2015;240:795–808. doi: 10.1177/1535370215584934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pardridge WM. NeuroRX. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van der Helm MW, van der Meer AD, Eijkel JCT, van den Berg A, Segerink LI. Tissue Barriers. 2016;4:e1142493. doi: 10.1080/21688370.2016.1142493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vernetti L, Gough A, Baetz N, Blutt S, Broughman JR, Brown JA, Foulke-Abel J, Hasan N, In J, Kelly E, Kovbasnjuk O, Repper J, Senutovitch N, Stabb J, Yeung C, Zachos NC, Donowitz M, Estes M, Himmelfarb J, Truskey G, Wikswo JP, Taylor DL. Scientific Reports. 2017;7:42296. doi: 10.1038/srep42296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wagner I, Materne E-M, Brincker, Su S, Fradrich C, Busek M, Sonntag F, Sakharov DA, Trushkin EV, Tonevitsky AG, Lauster R, Marx U. Lab on a Chip. 2013;13:3538–3547. doi: 10.1039/c3lc50234a. [DOI] [PubMed] [Google Scholar]
- 54.Iori E, Vinci B, Murphy E, Marescotti MC, Avogaro A, Ahluwalia A. PLOS ONE. 2012;7:e34704. doi: 10.1371/journal.pone.0034704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang C, Zhao Z, Abdul Rahim NA, van Noort D, Yu H. Lab on a Chip. 2009;9:3185–3192. doi: 10.1039/b915147h. [DOI] [PubMed] [Google Scholar]
- 56.Loskill P, Marcus SG, Mathur A, Reese WM, Healy KE. PLOS ONE. 2015;10:e0139587. doi: 10.1371/journal.pone.0139587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maschmeyer I, Lorenz AK, Schimek K, Hasenberg T, Ramme AP, Hubner J, Lindner M, Drewell C, Bauer S, Thomas A, Sambo NS, Sonntag F, Lauster R, Marx U. Lab on a Chip. 2015;15:2688–2699. doi: 10.1039/c5lc00392j. [DOI] [PubMed] [Google Scholar]
- 58.Kimmelman J, Federico C. Nature. 2017;542:25–27. doi: 10.1038/542025a. [DOI] [PubMed] [Google Scholar]
- 59.Nelson CM, Bissell MJ. Annual Review of Cell and Developmental Biology. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Villasante A, Vunjak-Novakovic G. Expert Opinion on Drug Discovery. 2015;10:257–268. doi: 10.1517/17460441.2015.1009442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clark AM, Ma B, Taylor DL, Griffith L, Wells A. Experimental Biology and Medicine. 2016 doi: 10.1177/1535370216658144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clark AM, Wheeler SE, Taylor DP, Pillai VC, Young CL, Prantil-Baun R, Nguyen T, Stolz DB, Borenstein JT, Lauffenburger DA, Venkataramanan R, Griffith LG, Wells A. Experimental biology and medicine (Maywood, NJ) 2014;239:1170–1179. doi: 10.1177/1535370214532596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wheeler SE, Clark AM, Taylor DP, Young CL, Pillai VC, Stolz DB, Venkataramanan R, Lauffenburger D, Griffith L, Wells A. Br J Cancer. 2014;111:2342–2350. doi: 10.1038/bjc.2014.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sobrino A, Phan DTT, Datta R, Wang X, Hachey SJ, Romero-López M, Gratton E, Lee AP, George SC, Hughes CCW. Scientific Reports. 2016;6:31589. doi: 10.1038/srep31589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Phan DTT, Wang X, Craver BM, Sobrino A, Zhao D, Chen JC, Lee LYN, George SC, Lee AP, Hughes CCW. Lab on a Chip. 2017;17:511–520. doi: 10.1039/c6lc01422d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Villenave R, Wales SQ, Hamkins-Indik T, Papafragkou E, Weaver JC, Ferrante TC, Bahinski A, Elkins CA, Kulka M, Ingber DE. PLOS ONE. 2017;12:e0169412. doi: 10.1371/journal.pone.0169412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fajgenbaum DC, Ruth JR, Kelleher D, Rubenstein AH. The Lancet Haematology. 2016;3:e150–e152. doi: 10.1016/S2352-3026(16)00007-7. [DOI] [PubMed] [Google Scholar]
- 68.Low LA, Tagle DA. Expert Opin Orphan D. 2016;4:1113–1121. doi: 10.1080/21678707.2016.1244479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang G, McCain ML, Yang L, He A, Pasqualini FS, Agarwal A, Yuan H, Jiang D, Zhang D, Zangi L, Geva J, Roberts AE, Ma Q, Ding J, Chen J, Wang D-Z, Li K, Wang J, Wanders RJA, Kulik W, Vaz FM, Laflamme MA, Murry CE, Chien KR, Kelley RI, Church GM, Parker KK, Pu WT. Nat Med. 2014;20:616–623. doi: 10.1038/nm.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grosberg A, Alford PW, McCain ML, Parker KK. Lab on a Chip. 2011;11:4165–4173. doi: 10.1039/c1lc20557a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nestor CE, Ottaviano R, Reinhardt D, Cruickshanks HA, Mjoseng HK, McPherson RC, Lentini A, Thomson JP, Dunican DS, Pennings S, Anderton SM, Benson M, Meehan RR. Genome Biology. 2015;16:11. doi: 10.1186/s13059-014-0576-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Horvath P, Aulner N, Bickle M, Davies AM, Nery ED, Ebner D, Montoya MC, Ostling P, Pietiainen V, Price LS, Shorte SL, Turcatti G, von Schantz C, Carragher NO. Nat Rev Drug Discov. 2016;15:751–769. doi: 10.1038/nrd.2016.175. [DOI] [PubMed] [Google Scholar]
- 73.Warren CR, O’Sullivan JF, Friesen M, Becker CE, Zhang X, Liu P, Wakabayashi Y, Morningstar JE, Shi X, Choi J, Xia F, Peters DT, Florido MHC, Tsankov AM, Duberow E, Comisar L, Shay J, Jiang X, Meissner A, Musunuru K, Kathiresan S, Daheron L, Zhu J, Gerszten RE, Deo RC, Vasan RS, O’Donnell CJ, Cowan CA. Cell Stem Cell. 2017;20:547–557.e547. doi: 10.1016/j.stem.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 74.McCracken KW, Howell JC, Wells JM, Spence JR. Nat Protocols. 2011;6:1920–1928. doi: 10.1038/nprot.2011.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McCracken KW, Cata EM, Crawford CM, Sinagoga KL, Schumacher M, Rockich BE, Tsai Y-H, Mayhew CN, Spence JR, Zavros Y, Wells JM. Nature. 2014;516:400–404. doi: 10.1038/nature13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lancaster MA, Renner M, Martin C-A, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pamies D, Barreras P, Block K, Makri G, Kumar A, Wiersma D, Smirnova L, Zhang C, Bressler J, Christian KM, Harris G, Ming GL, Berlinicke CJ, Kyro K, Song H, Pardo CA, Hartung T, Hogberg HT. ALTEX. 2016 doi: 10.14573/altex.1609122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Downing TL, Soto J, Morez C, Houssin T, Fritz A, Yuan F, Chu J, Patel S, Schaffer DV, Li S. Nat Mater. 2013;12:1154–1162. doi: 10.1038/nmat3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lei Y, Schaffer DV. Proceedings of the National Academy of Sciences. 2013;110:E5039–E5048. doi: 10.1073/pnas.1309408110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Caiazzo M, Okawa Y, Ranga A, Piersigilli A, Tabata Y, Lutolf MP. Nat Mater. 2016;15:344–352. doi: 10.1038/nmat4536. [DOI] [PubMed] [Google Scholar]
- 81.Abilez OJ, Wu JC. Nat Mater. 2016;15:259–261. doi: 10.1038/nmat4583. [DOI] [PubMed] [Google Scholar]
- 82.Gagliano O, Elvassore N, Luni C. Biochemical and Biophysical Research Communications. 2016;473:683–687. doi: 10.1016/j.bbrc.2015.12.058. [DOI] [PubMed] [Google Scholar]
- 83.Kim K, Zhao R, Doi A, Ng K, Unternaehrer J, Cahan P, Hongguang H, Loh Y-H, Aryee MJ, Lensch MW, Li H, Collins JJ, Feinberg AP, Daley GQ. Nat Biotech. 2011;29:1117–1119. doi: 10.1038/nbt.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bar-Nur O, Russ Holger A, Efrat S, Benvenisty N. Cell Stem Cell. 2011;9:17–23. doi: 10.1016/j.stem.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 85.Hung SSC, Khan S, Lo CY, Hewitt AW, Wong RCB. Pharmacology & Therapeutics. 2017 doi: 10.1016/j.pharmthera.2017.02.026. DOI: https://doi.org/10.1016/j.pharmthera.2017.02.026. [DOI] [PubMed]
- 86.Mullard A. Nat Rev Drug Discov. 2015;14:589–591. doi: 10.1038/nrd4708. [DOI] [PubMed] [Google Scholar]
- 87.Toepke MW, Beebe DJ. Lab on a Chip. 2006;6:1484–1486. doi: 10.1039/b612140c. [DOI] [PubMed] [Google Scholar]
- 88.Chuah YJ, Kuddannaya S, Lee MHA, Zhang Y, Kang Y. Biomaterials Science. 2015;3:383–390. doi: 10.1039/c4bm00268g. [DOI] [PubMed] [Google Scholar]
- 89.Markov DA, Lillie EM, Garbett SP, McCawley LJ. Biomedical microdevices. 2014;16:91–96. doi: 10.1007/s10544-013-9808-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tan SH, Nguyen N-T, Chua YC, Kang TG. Biomicrofluidics. 2010;4:032204. doi: 10.1063/1.3466882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kuddannaya S, Chuah YJ, Lee MHA, Menon NV, Kang Y, Zhang Y. ACS Applied Materials & Interfaces. 2013;5:9777–9784. doi: 10.1021/am402903e. [DOI] [PubMed] [Google Scholar]
- 92.Chuah YJ, Koh YT, Lim K, Menon NV, Wu Y, Kang Y. Scientific Reports. 2015;5:18162. doi: 10.1038/srep18162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang JD, Douville NJ, Takayama S, ElSayed M. Annals of Biomedical Engineering. 2012;40:1862–1873. doi: 10.1007/s10439-012-0562-z. [DOI] [PubMed] [Google Scholar]
- 94.Shirure VS, George SC. Lab on a Chip. 2017 doi: 10.1039/C6LC01401A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wikswo JP, Curtis EL, Eagleton ZE, Evans BC, Kole A, Hofmeister LH, Matloff WJ. Lab on a Chip. 2013;13:3496–3511. doi: 10.1039/c3lc50243k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stokes CL, Cirit M, Lauffenburger DA. CPT: Pharmacometrics & Systems Pharmacology. 2015;4:559–562. doi: 10.1002/psp4.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Smith AS, Long CJ, Berry BJ, McAleer C, Stancescu M, Molnar P, Miller PG, Esch MB, Prot J-M, Hickman JJ, Shuler ML. Stem Cell Research & Therapy. 2013;4:1–5. doi: 10.1186/scrt370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ewart L, Fabre K, Chakilam A, Dragan Y, Duignan DB, Eswaraka J, Gan J, Guzzie-Peck P, Otieno M, Jeong CG, Keller DA, Morais SMd, Phillips JA, Proctor W, Sura R, Vleet TV, Watson D, Will Y, Tagle D, Berridge B. Experimental Biology and Medicine. 2017;0:1535370217715441. doi: 10.1177/1535370217715441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Junaid A, Mashaghi A, Hankemeier T, Vulto P. Current Opinion in Biomedical Engineering. 2017;1:15–22. [Google Scholar]