Abstract
MTX is a standard component of acute GVHD prophylaxis. However, its use can be limited by toxicity. On the basis of disease risk, we prospectively assigned 132 consecutive patients from January 2005 to February 2011 undergoing first allogeneic hematopoietic cell transplant after conditioning with fludarabine and melphalan to acute GVHD prophylaxis with tacrolimus/MTX (TAC/MTX, N = 22), TAC/micro-dose MTX/mycophenolate mofetil (TAC/μMTX/MMF, N = 78) or TAC/MMF (TAC/MMF, N = 32), to optimize acute GVHD prevention and decrease mortality. The median (range) follow-up was 24 (0.8–60) months. The median patient ages (range) were 37 (23–63), 56 (20–68) and 54 (22–68) years (P < 0.0001) for TAC/MTX, TAC/μMTX/MMF and TAC/MMF, respectively. The 100-day cumulative incidences of grade III–IV acute GVHD were 19, 23 and 49% (P < 0.015), respectively. The cumulative incidences of severe chronic GVHD at 1 year were 38, 29 and 79% (P < 0.001), respectively. Regimen-related toxicities were not significantly different among the three prophylaxis regimens. PFS and OS were equivalent between the TAC/MTX and TAC/μMTX/MMF arms despite significantly older patients in the latter arm, and both had superior PFS and OS than the TAC/MMF arm. Acute GVHD prophylaxis with TAC/μMTX/MMF is as effective as TAC/MTX and superior to TAC/MMF.
Keywords: acute GVHD, reduced-intensity conditioning, MTX
INTRODUCTION
Acute GVHD is the major impediment to successful allogeneic hematopoietic cell transplant (alloHCT) and occurs among 35–47% of recipients of related and unrelated allogeneic HLA-matched grafts despite prophylaxis.1,2 Starting from single-agent regimens, the combination of tacrolimus (TAC) and MTX has become a standard acute GVHD prophylaxis regimen.3–6 However, because of MTX-related toxicities such as liver/kidney abnormalities, delayed neutrophil/platelet engraftment and stomatitis, MTX-sparing regimens were developed in an attempt to improve acute GVHD prophylaxis and long-term outcomes. MTX doses were initially reduced without decreased efficacy compared with historical controls.7 Later, MTX was completely eliminated in regimens using TAC/sirolimus (SIR) as well as TAC/mycophenolate mofetil (MMF).8–13 On the basis of disease risk factors, we prospectively assigned alloHCT patients, who received reduced-intensity conditioning with fludarabine (FLU) and melphalan (MEL), to acute GVHD prophylaxis with TAC/MTX, TAC/μMTX/MMF or TAC/MMF with the intent to develop a MTX-sparing prophylaxis regimen to reduce toxicity while maintaining efficacy.
PATIENTS AND METHODS
A total of 132 adult (>18 years) alloHCT patients received conditioning with FLU (25 mg/m2 i.v. on days −6, −5, −4, −3 and −2) and MEL (70 mg/m2 i.v. on days −3 and −2) and MEL for the treatment of hematologic malignancies from 2005 to 2011 at Roswell Park Cancer Institute (RPCI).14 Patients received FLU/MEL conditioning if they were not eligible for a myeloablative alloHCT due to disease, HLA mismatch, performance status or prior receipt of an autologous or alloHCT. The clinical protocol for FLU/MEL conditioning was approved by the RPCI institutional review board, and all participants provided written informed consent.
A minimum of 2 × 106 CD34 + cells/kg (peripheral blood) or 2 × 108 total nucleated cells/kg (BM) were infused. Before 6 January 2008, G-CSF was administered to all patients from day 6 after cell infusion until neutrophil engraftment. Patients with disease-related monosomy 7 cytogenetic abnormalities received GM-CSF instead. Scheduled G-CSF administration was discontinued because of theoretical concerns that it might increase the risk of acute GVHD.15,16
On the basis of disease status at transplant, patients were prospectively assigned in a non-randomized manner to a standard (TAC/MTX) or alternative (TAC/μMTX/MMF or TAC/MMF) acute GVHD prophylaxis regimen (Table 1). Patients at a low risk for disease relapse were assigned to TAC/MTX. Patients at a high and very high risk for disease relapse were assigned to TAC/μMTX/MMF or TAC/MMF. Low risk was defined as ALL or non-Hodgkin lymphoma (NHL)/Hodgkin lymphoma (HL) in CR, AML in CR within 1 month of transplant or myelodysplastic syndrome (MDS) with ≤10% blasts. High-risk disease was defined as AML in early untreated relapse or with a hypoplastic BM, MDS with 10–20% blasts or secondary etiology or HL/NHL in PR or stable disease after relapse therapy. Very high-risk disease was defined as AML with measurable histopathological disease after reinduction therapy, NHL/HL that progressed/never responded despite therapy (primary induction failure or refractory relapse), ALL not in CR at transplant and AML, ALL, MDS or NHL/HL that relapsed after prior alloHCT. The disease status categories used for assigning acute GVHD prophylaxis were internally derived and partially overlapped with the Center for International Blood and Marrow Transplant Research (CIBMTR) risk categories.17 Patients were also retrospectively classified according to CIBMTR risk categories as follows: low: AML/ALL in CR1, CML in first chronic phase or CR1, CLL in CR⩾1, HL/NHL in CR1; MDS RA, RARS, RCMD, RCMD-RS and unclassified or isolated 5q; MM in CR1, VGPR1 and PR1; intermediate: AML/ALL in CR⩾2, CML in second chronic phase, CR2 or first accelerated phase; CLL in PR, untreated relapse or never treated; HL/NHL in CR⩾2, chemo-sensitive relapse or PR1; high: AML/ALL not in remission, CML in third or greater chronic phase, CR⩾3, second accelerated phase or blast crisis; CLL progression or no response to treatment; HL/NHL not in remission, never treated or less than PR to initial therapy; MDS RAEB-1, RAEB-2 and CMML; MM in CR⩾2, VGPR⩾2, PR⩾2, stable disease, progression and never treated; non-malignant: severe aplastic anemia, Fanconi anemia and Diamond–Blackfan anemia.
Table 1.
Acute GVHD prophylaxis regimens
| TACa/MTX | MTX 10 mg/m2 on day 1, 3, 6 and 11 |
| TACa/MMF | MMF 1000 mg q12 or q8h from day −1 to day 60 |
| TACa/μMTX/MMF | μMTX 2.5 mg/m2 on day 1, 3 and 6 |
| MMF 1000 mg q12 or q8h from day −1 to day 60 |
Abbreviations: TAC = tacrolimus; MMF = mycophenolate mofetil; μMTX = micro-dose MTX; MTX = standard-dose MTX.
TAC 0.005 mg/kg i.v. bid with later conversion to oral doses maintained at a serum concentration of 5–10 ng/mL from day −1 to day 100. In the absence of acute GVHD, TAC was tapered by 10% weekly beginning on day 60 (if high-risk disease) with the goal to taper off by day 100 or day 100 with the goal to taper off by day 180 and MMF was discontinued on day 60 without taper.
All regimens started with a dose of 0.005 mg/kg of TAC i.v. twice daily, with conversion to oral dosing once tolerated. TAC serum concentrations were maintained at 5–10 ng/mL from day −1 to day 100. TAC/MTX used 10 mg/m2 MTX i.v. on days 1, 3, 6 and 11 (total 40 mg/m2) for unrelated donors and 1, 3 and 6 (total 30 mg/m2) for related donors. TAC/μMTX/MMF used 2.5 mg/m2 MTX i.v. on days 1, 3 and 6 (total 7.5 mg/m2) for unrelated and related donors combined with MMF dosed at 1000 mg orally or i.v. every 8 h for 60 days. TAC/MMF used MMF dosed at 1000 mg orally or i.v. every 8 h for 60 days. Assignment to TAC/MTX occurred from January 2005 to February 2011. Assignment to TAC/MMF occurred from September 2005 to December 2008. Early analysis of acute GVHD incidence concluded that TAC/MMF acute GVHD prophylaxis was inferior. Therefore, this regimen was discontinued. Consequently, TAC/μMTX/MMF superseded TAC/MMF and was used from December 2007 to January 2011. The same criteria were used for assignment to the TAC/MMF and TAC/μMTX/MMF regimens. Acute GVHD prophylaxis regimens were implemented as a standard-of-care therapy.
Definitions
Neutrophil engraftment was defined as the first of three consecutive days with an ANC ⩾0.5 × 106 cells/mL. Platelet engraftment was defined as the first day with a platelet count of ⩾20 × 106 platelets/mL without platelet transfusions in the preceding 7 days. Acute GVHD stage and grade were defined according to the Glucksberg criteria, toxicity was graded according to the Bearman criteria and chronic GVHD severity was defined according to the CIBMTR criteria.18–20 Acute GVHD organ stage/grade, toxicity data and chronic GVHD (cGvHD) severity grading were prospectively collected. The analysis presented here is retrospective and IRB approved.
Statistical analysis
The Pearson’s χ2-test or Fisher’s exact text was used for univariate comparisons of categorical variables. Observation time for acute GVHD was calculated from the date of BMT (day 0) until the date of acute GVHD onset or day +100 post BMT and was analyzed as a competing risk with disease progression/relapse or death without GVHD. Observation time for cGVHD was defined as the time from day 0 to cGVHD onset and was analyzed as a competing risk with disease progression/relapse or death without cGVHD. Patients who died before day + 100 post BMT were excluded from the analysis of cGVHD. Relapse incidence was calculated from day 0 to the date of either relapse (if in CR pre-alloHCT) or progression (if not in CR pre-alloHCT) and was analyzed as a competing risk with GVHD. OS time was calculated from day 0 to the date of death owing to any cause; patients were censored at the last follow-up. PFS time was calculated from day 0 to the first date of disease progression/relapse or death owing to any cause; patients were censored at the last follow-up in the absence of disease progression/relapse. Kaplan–Meier survival curves were constructed and the difference among the three prophylaxis groups was tested by the log-rank statistic. Pairwise comparisons of all outcomes were also performed to test the statistical significance between any two prophylaxis groups. An explanatory Cox proportional hazard model was built for OS, PFS and grade III–IV acute GVHD incorporating the following factors CIBMTR risk category, HLA-match, age at alloHCT and acute GVHD prophylaxis regimen. All statistical analyses were performed using SPSS version 16 (Chicago, IL, USA) with a two-sided type I error rate of 0.05.
RESULTS
Patient characteristics
Patient characteristics are presented in Table 2. A total of 132 patients received TAC/MTX (n = 22), TAC/μMTX/MMF (n = 78) or TAC/MMF (n = 32). Patients who received TAC/MTX were significantly younger than those receiving TAC/μMTX/MMF or TAC/MMF owing to assignment criteria. Twenty percent of patients received a graft matched at <8/8 HLA loci. All CIBMTR risk categories were represented in each acute GVHD prophylaxis regimen group and did not significantly differ by regimen. The proportion of high/intermediate/low-CIBMTR-risk patients in the TAC/μMTX/MMF group was 27 (35%)/18 (23%)/30 (38%), respectively. The proportion of high/intermediate/low-CIBMTR-risk patients in the TAC/MMF group was 16 (50%)/4 (13%)/12 (38%), respectively. The CIBMTR high-/low-risk ratios were not significantly different between the TAC/μMTX/MMF or TAC/MMF groups. The median (range) follow-up was 24 (0.8–60) months.
Table 2.
Patient characteristics
| TAC/MTX, N = 22, N (%) | TAC/μMTX/MMF, N = 78, N (%) | TAC/MMF, N = 32, N (%) | P-value |
|---|---|---|---|
| Age in years | <0.0001 | ||
| Median (range) | 37 (23–63) | 54 (22–68) | 56 (20–68) |
| 19–39 | 12 (55) | 11 (14) | 2 (6) |
| ⩾40 | 10 (46) | 67 (86) | 30 (94) |
| Gender | NS | ||
| Female | 9 (41) | 30 (39) | 17 (53) |
| Male | 13 (59) | 48 (62) | 15 (47) |
| Donor/recipient gender | NS | ||
| Female/female | 3 (14) | 16 (21) | 10 (31) |
| Female/male | 4 (18) | 17 (22) | 6 (19) |
| Male/female | 6 (27) | 14 (18) | 7 (22) |
| Male/male | 9 (41) | 31 (40) | 9 (28) |
| Diagnosis | NS | ||
| ALL | 1 (3) | 5 (6) | 1 (3) |
| AML | 9 (41) | 33 (42) | 18 (56) |
| MDS | 3 (14) | 9 (12) | 4 (13) |
| NHL | 4 (18) | 14 (18) | 8 (25) |
| Other | 5 (23) | 17 (22) | 1 (3) |
| KPS | NS | ||
| 50–80 | 18 (82) | 66 (85) | 26 (81) |
| 90–100 | 4 (18) | 12 (15) | 6 (19) |
| CIBMTR risk category | NS | ||
| High | 5 (23) | 27 (35) | 16 (50) |
| Intermediate | 7 (32) | 18 (23) | 4 (13) |
| Low | 8 (36) | 30 (39) | 12 (38) |
| Non-malignancy | 2 (9) | 3 (4) | 0 (0) |
| Stem cell type | NS | ||
| BM | 1 (5) | 12 (15) | 3 (9) |
| Peripheral blood | 21 (96) | 66 (85) | 29 (91) |
| Donor source | NS | ||
| Related | 9 (41) | 19 (24) | 12 (38) |
| Unrelated | 13 (59) | 59 (76) | 20 (63) |
| HLA matcha | NS | ||
| Match (8/8 or 6/6) | 17 (77) | 62 (80) | 26 (81) |
| Mismatch (<8/8) | 5 (23) | 16 (21) | 6 (19) |
| ABO match | NS | ||
| Match | 10 (46) | 29 (37) | 11 (34) |
| Major mismatch | 4 (18) | 18 (23) | 9 (28) |
| Minor mismatch | 6 (27) | 22 (28) | 8 (25) |
| Bidirectional mismatch | 2 (9) | 9 (12) | 4 (13) |
| CMV recipient/donor status-pre BMT | NS | ||
| Negative/negative | 6 (27) | 27 (35) | 14 (44) |
| Negative/positive | 3 (14) | 11 (14) | 2 (6) |
| Positive/negative | 5 (23) | 25 (32) | 10 (31) |
| Positive/positive | 8 (36) | 15 (19) | 6 (19) |
Abbreviations: CIBMTR = Center for International Blood and Marrow Transplant Research; KPS = Karnofsky Performance Status; MDS = myelodysplastic syndrome; MMF = Mycophenolate mofetil; μMTX = micro-dose MTX (7.5 mg/m2); MTX = standard-dose MTX (30–40 mg/m2); NHL = non-Hodgkin lymphoma; TAC = tacrolimus. P>0.1.
8/8 HLA (A, B, C and DRB1) matching only in unrelated donors; 6/6 HLA (A, B and DRB1) matching only in related donors.
Engraftment
Before 6 January 2008, during a period of routine use of G- or GM-CSF, the median (range) times for neutrophil engraftment for TAC/MTX, TAC/μMTX/MMF and TAC/MMF were 14 (11–66), 12 (5–19) and 11 (3–86) days, respectively. The median (range) times for platelet engraftment were 21 (11–66), 17 (3–92) and 18 (5–36) days, respectively. Neutrophil and platelet engraftment were not significantly different between the three acute GVHD prophylaxis regimens. After 6 January 2008, when routine G- or GM-CSF administration was discontinued, the median (range) times for neutrophil and platelet engraftment for TAC/μMTX/MMF were 17 (10–30) and 19 (10–390) days, respectively. Engraftment times for the TAC/MTX and TAC/MMF prophylaxis regimens could not be calculated owing to small sample sizes (n = 1 and 1).
Acute GVHD
The incidence of grade III–IV acute GVHD between the TAC/MTX (19%, 95% CI 2–36%) and TAC/μMTX/MMF (23%, 95% CI 13–33%) groups was not significantly different. In contrast, TAC/MMF (49%, 95% CI 31–67%) resulted in a significantly higher incidence of grade III–IV acute GVHD (P = 0.015) than either MTX-containing regimen (Figure 1a). Organ-specific involvement by acute GVHD was examined, and a trend toward a higher incidence of stage 2–4 gastrointestinal tract acute GVHD (P = 0.078, Figure 1b) for TAC/MMF (42%, 95% CI 24–60%) than for TAC/MTX (14%, 95% CI 0–29%) or TAC/μMTX/MMF (23%, 95% CI 13–33%) was observed. Little difference between the three prophylaxis groups was observed in the incidence of stage 2–4 liver or that of stage 3–4 skin acute GVHD. (Figures 1c and d) The incidences of grade IV acute GVHD for TAC/MTX, TAC/μMTX/MMF and TAC/MMF were 9%, 6% and 19%, respectively. Death due to acute GVHD occurred in 5, 3 and 19% by day 100, respectively.
Figure 1.
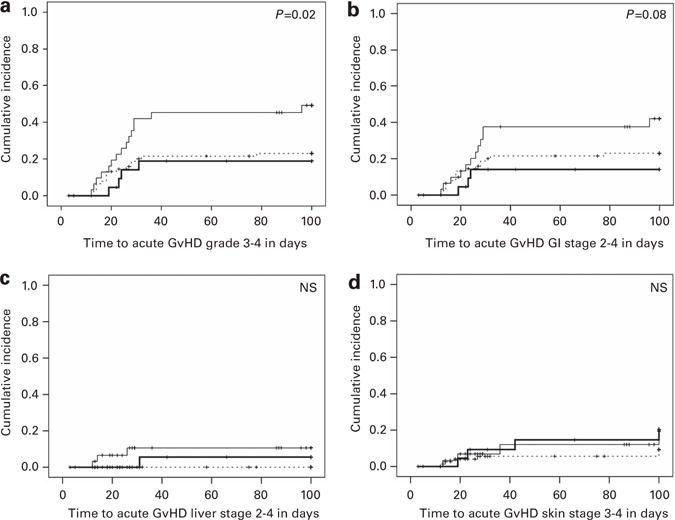
Cumulative incidences of grade III–IV acute GVHD for TAC/MTX, TAC/μMTX/MMF and TAC/MMF. TAC/MMF results in significantly greater incidence of grade III–IV acute GVHD than TAC/MTX or TAC/μMTX/MMF (a) Acute GVHD organ involvement is greater for TAC/MMF than TAC/MTX or TAC/μMTX/MMF in the gastrointestinal tract (b) but not liver (c) or skin (d) thick line—TAC/MTX, dashed line—TAC/μMTX/MMF, thin line—TAC/MMF. Abbreviations: μMTX = micro-dose MTX (7.5 mg/m2); MMF = mycophenolate mofetil; MTX = standard-dose MTX (30–40 mg/m2); TAC = tacrolimus.
A multivariate analysis to determine the contribution of acute GVHD prophylaxis regimen (TAC/μMTX/MMF vs TAC/MMF), CIBMTR risk category, HLA matching and age to grade III–IV acute GVHD risk was performed (Table 3). TAC/MTX was not included owing to significant differences in disease status and age, which could confound the analysis. Patients who received TAC/MMF were at a significantly higher risk (HR = 2.36, P = 0.02) for developing grade III–IV acute GVHD than those who received TAC/μMTX/MMF, after controlling for age at alloHCT, HLA match and CIBMTR risk category. As expected, there was a trend toward increased risk of grade III–IV acute GVHD in patients with 7/8 HLA-mismatched unrelated donor grafts compared with a 6/6 HLA-matched related or 8/8 HLA-matched unrelated donor grafts.21 CIBMTR risk and age did not significantly affect grade III–IV acute GVHD risk.
Table 3.
Explanatory multivariate model of grade III–IV acute GVHD, PFS AND OS
| Variable | N |
Grade III–IV acute GVHD
|
PFS
|
OS
|
|||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| Acute GVHD prophylaxis regimen | |||||||
| TAC/μMTX/MMF | 78 | 1.0 | 1.0 | 1.0 | |||
| TAC/MMF | 32 | 2.36 (1.14–4.88) | 0.02 | 1.47 (0.85–2.53) | 0.2 | 1.62 (0.93–2.84) | 0.09 |
| CIBMTR risk | |||||||
| Low | 45 | 1.0 | 1.0 | 1.0 | |||
| Intermediate | 22 | 1.49 (0.54–4.13) | NS | 2.4 (1.11–5.17) | 0.03 | 2.09 (0.93–4.69) | 0.08 |
| High | 43 | 1.5 (0.68–3.32) | NS | 3.6 (1.96–6.6) | <0.0001 | 3.48 (1.88–6.45) | <0.0001 |
| HLA match | |||||||
| Match | 88 | 1.0 | 1.0 | 1.0 | |||
| Mismatch | 22 | 1.8 (0.81–4) | 0.15 | 1.81 (1–3.29) | 0.05 | 1.93 (1.05–3.52) | 0.03 |
| Age, years | |||||||
| 19–39 | 13 | 1.0 | 1.0 | 1.0 | |||
| ⩾40 | 97 | 1.1 (0.32–3.72) | NS | 1.3 (0.58–2.93) | NS | 3.31 (1.01–10.83) | 0.05 |
Abbreviations: CIBMTR = Center for International Blood and Marrow Transplant Research; HR = hazard ratio; MMF = mycophenolate mofetil; μMTX = micro-dose MTX (7.5 mg/m2); TAC = tacrolimus. P>0.2.
The theoretical association between G- or GM-CSF use after alloHCT and acute GVHD was evaluated by examining the incidence of acute GVHD in alloHCTs performed before and after 6 January 2008, the date on which routine, planned growth factor use was discontinued. No significant differences were observed in the incidence of grade III–IV acute GVHD (data not shown).
Sampling bias was evaluated by comparing the proportion of corroborating biopsy samples obtained from the TAC/MTX, TAC/μMTX/MMF and TAC/MMF groups. Gastrointestinal tract biopsy samples were obtained from 50, 53 and 56% of patients, respectively. Skin biopsy samples were obtained from 45, 42 and 37% of patients, respectively. Only 8% of patients had a liver biopsy. No significant differences in the biopsy rate were observed between the three prophylaxis groups.
Transplant-related toxicities
All three regimens were well tolerated. Transplant-related toxicities were not significantly different between the three regimens. The rate of grade II–IV stomatitis was lowest in the TAC/MMF group (16%) compared with the TAC/μMTX/MMF (22%) and TAC/MTX (32%) groups; however, these differences were not statistically significant. The incidences of transplant-related mortality for TAC/MTX, TAC/μMTX/MMF and TAC/MMF were 18%, 10% and 38% at day 100, and 18%, 26% and 59% at 1 year, respectively.
Chronic GVHD
The cumulative incidences of severe cGVHD were significantly higher (P<0.0001, Figure 2) for TAC/MMF (79%, 95% CI 60–97%) compared with TAC/MTX (38%, 95% CI 13–62%) and TAC/μMTX/MMF (29%, 95% CI 17–41%). The cumulative incidences of moderate/severe cGVHD at 1 year were 46% (95% CI 20–71%), 45% (95% CI 32–57%) and 95% (95% CI 85–100%), for TAC/MTX, TAC/μMTX/MMF and TAC/MMF, respectively. Death due to cGVHD occurred in 5, 12 and 38% at 1 year in the TAC/MTX, TAC/μMTX/MMF and TAC/MMF groups, respectively.
Figure 2.
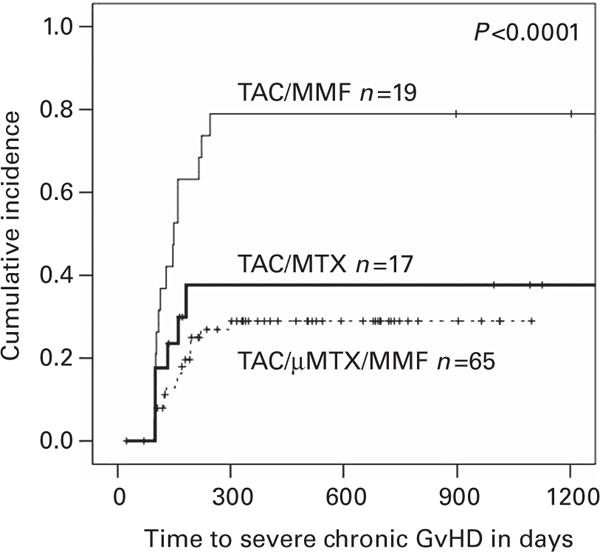
Cumulative incidences of severe cGVHD. TAC/MMF results in significantly increased incidence of severe cGVHD than TAC/MTX or TAC/μMTX/MMF. Abbreviations: cGVHD = chronic GVHD; μMTX micro-dose MTX (7.5 mg/m2); MMF = mycophenolate mofetil; MTX = standard-dose MTX (30–40 mg/m2); TAC = tacrolimus.
Relapse
The incidences of relapse/progression for TAC/MTX, TAC/μMTX/MMF and TAC/MMF were 21, 11 and <1% at day 100, respectively, and 27, 19 and 9% at 1 year, respectively.
PFS and OS
When TAC/μMTX/MMF was compared with TAC/MMF, OS was significantly better (P = 0.05) and PFS showed a trend (P = 0.2) toward improvement for TAC/μMTX/MMF (Figures 3a and b). There was no significant difference between TAC/MTX and TAC/μMTX/MMF in 1-year OS (64% vs 66%, respectively) or 1-year PFS (59% vs 59%, respectively).
Figure 3.
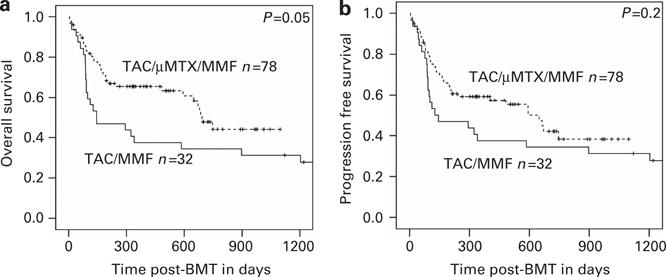
OS and PFS. (a) TAC/μMTX/MMF results in significantly better OS than TAC/MMF. (b) There is a trend toward improved PFS for TAC/μMTX/MMF when compared to TAC/MMF. Abbreviations: μMTX = micro-dose MTX (7.5 mg/m2); MMF = mycophenolate mofetil; MTX = standard-dose MTX (30–40 mg/m2); TAC = tacrolimus.
A multivariate analysis of PFS found only CIBMTR intermediate/high-risk, and HLA mismatch was significantly associated with a lower PFS (Table 3). GVHD prophylaxis regimen and age were not significantly associated with PFS. The multivariate analysis of OS demonstrated an increased risk of death for patients aged ⩾40 years (HR 3.31, P = 0.05), HLA-mismatched donors (HR 1.93, P = 0.03) and CIBMTR high-risk disease (HR 3.48, P<0.0001), with a minor effect of CIBMTR intermediate-risk disease (HR 2.09, P = 0.08) and FK/MMF (HR 1.62, P = 0.09) on the mortality risk (Table 3).
DISCUSSION
TAC/μMTX/MMF is as effective as TAC/MTX for acute GVHD prophylaxis in patients conditioned with FLU/MEL receiving an alloHCT. Both regimens are superior to TAC/MMF; thus, we were unable to confirm the adequacy of TAC/MMF for GVHD prophylaxis.11–13
We observed a higher incidence of grade III–IV acute GVHD for all three prophylaxis regimens than previously reported by other groups.11–13,22,23 These differences were likely due to older patient age and higher percentage of HLA-mismatched transplants and CIBMTR high-risk patients in our cohort. Despite this, our PFS and OS estimates are comparable to previous reports.24–26
One trend in acute GVHD prophylaxis has been to reduce or eliminate the dose of MTX. TAC/SIR has been tested as a MTX-sparing acute GVHD prophylaxis regimen in reduced intensity alloHCT using matched related donors.27,28 TAC/SIR has only been studied in HLA-matched siblings with a low-risk disease who received different conditioning regimens and graft sources than our cohort.
We suggest that TAC/μMTX/MMF may be an alternative to SIR-based acute GVHD prophylaxis regimens because it does not require SIR-level monitoring, has fewer interactions with azole drugs and has less pneumonitis.29 BMT CTN 0402, a phase-3 randomized trial comparing acute GVHD prophylaxis with TAC/MTX versus TAC/SIR, in a preliminary report showed no significant differences between the two regimens.30
The superiority of TAC/MTX and TAC/μMTX/MMF over TAC/MMF acute GVHD prophylaxis may be explained by the differential pharmacology of MTX and MMF. T and B cells do not use the salvage pathway for purine synthesis but rely upon de novo synthesis, and MMF is an inhibitor of IMP dehydrogenase, an enzyme crucial to de novo purine nucleotide synthesis.31,32 Therefore, MMF’s effects are specific to T and B lymphocytes.33 MTX nonspecifically inhibits cellular proliferation by antagonizing folate metabolism.34 During acute GVHD development, MTX may inhibit the spectrum of immunologically active cells. This broader inhibition of the immune response may have resulted in our lower observed incidence of acute GVHD and longer OS in the MTX-containing prophylaxis arms.
The strength of this study is that it compares three prospectively assigned acute GVHD prophylaxis regimens using different doses of MTX after a standard reduced-intensity conditioning regimen. Although the TAC/μMTX/MMF group included older patients at higher risk for acute GVHD and poor transplant outcomes compared with the TAC/MTX group, the incidences of acute and cGVHD, OS and PFS were equivalent between these two regimens. Thus, TAC/μMTX/MMF may be a superior acute GVHD prophylaxis regimen. A limitation of this study is a possible cohort effect due to the enrollment of patients treated with TAC/μMTX/MMF and TAC/MMF in sequential cohorts. However, the characteristics of patients in the TAC/MMF and TAC/μMTX/MMF groups, including relative proportions of high- and low-CIBMTR-risk patients and an older patient age, did not significantly differ, and we did not find evidence of a cohort effect in sensitivity and subgroup analyses (data not shown), grade III–IV acute GVHD incidence, OS and PFS. These data indicate an advantage for patients receiving acute GVHD prophylaxis with TAC/μMTX/MMF compared with TAC/MMF.
In summary, we observe that TAC/μMTX/MMF is as effective as TAC/MTX and superior to TAC/MMF for acute GVHD prophylaxis. Although improved toxicity or engraftment was not seen in the TAC/μMTX/MMF arm compared with the TAC/MTX arm, the observation of an equivalent incidence of acute and cGVHD, PFS and OS in a higher-risk population suggests that TAC/μMTX/MMF may be superior to TAC/MTX and acceptable acute GVHD prophylaxis. These data challenge the paradigm of completely eliminating MTX from acute GVHD prophylaxis regimens to improve tolerability while maintaining efficacy. Dose reduction of MTX in combination with MMF is another strategy that may result in improved outcomes.
Acknowledgments
We thank the patients who participated in this study, the BMT clinical team for excellent patient care and the BMT data managers. This work was supported by RPCI and in part by NCI grant #P30-CA016056.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
GLC, YZ, TH and PLM designed the study and analyzed the data. All authors contributed to this paper.
References
- 1.Valcarcel D, Sierra J, Wang T, Kan F, Gupta V, Hale GA, et al. One-antigen mismatched related versus HLA-matched unrelated donor hematopoietic stem cell transplantation in adults with acute leukemia: Center for International Blood and Marrow Transplant Research results in the era of molecular HLA typing. Biol Blood Marrow Transplant. 2011;17:640–648. doi: 10.1016/j.bbmt.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hahn T, McCarthy PL, Jr, Zhang MJ, Wang D, Arora M, Frangoul H, et al. Risk factors for acute graft-versus-host disease after human leukocyte antigen-identical sibling transplants for adults with leukemia. J Clin Oncol. 2008;26:5728–5734. doi: 10.1200/JCO.2008.17.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ratanatharathorn V, Nash RA, Przepiorka D, Devine SM, Klein JL, Weisdorf D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92:2303–2314. [PubMed] [Google Scholar]
- 4.Nash RA, Antin JH, Karanes C, Fay JW, Avalos BR, Yeager AM, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–2068. [PubMed] [Google Scholar]
- 5.Storb R, Deeg HJ, Pepe M, Appelbaum F, Anasetti C, Beatty P, et al. Methotrexate and cyclosporine versus cyclosporine alone for prophylaxis of graft-versus-host disease in patients given HLA-identical marrow grafts for leukemia: long-term follow-up of a controlled trial. Blood. 1989;73:1729–1734. [PubMed] [Google Scholar]
- 6.Nash RA, Etzioni R, Storb R, Furlong T, Gooley T, Anasetti C, et al. Tacrolimus (FK506) alone or in combination with methotrexate or methylprednisolone for the prevention of acute graft-versus-host disease after marrow transplantation from HLA-matched siblings: a single-center study. Blood. 1995;85:3746–3753. [PubMed] [Google Scholar]
- 7.Przepiorka D, Ippoliti C, Khouri I, Woo M, Mehra R, Le Bherz D, et al. Tacrolimus and minidose methotrexate for prevention of acute graft-versus-host disease after matched unrelated donor marrow transplantation. Blood. 1996;88:4383–4389. [PubMed] [Google Scholar]
- 8.Cutler C, Li S, Ho VT, Koreth J, Alyea E, Soiffer RJ, et al. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood. 2007;109:3108–3114. doi: 10.1182/blood-2006-09-046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zohren F, Schroeder T, Czibere A, Fenk R, Bruns I, Kondakci M, et al. Tacrolimus and mycofenolate mofetil as GvHD prophylaxis following nonmyeloablative conditioning and unrelated hematopoietic SCT for adult patients with advanced hematologic diseases. Bone Marrow Transplant. 2011;46:747–755. doi: 10.1038/bmt.2010.167. [DOI] [PubMed] [Google Scholar]
- 10.Uchida N, Wake A, Nakano N, Ishiwata K, Takagi S, Tsuji M, et al. Mycophenolate and tacrolimus for graft-versus-host disease prophylaxis for elderly after cord blood transplantation: a matched pair comparison with tacrolimus alone. Transplantation. 2011;92:366–371. doi: 10.1097/TP.0b013e318223d7ac. [DOI] [PubMed] [Google Scholar]
- 11.Perkins J, Field T, Kim J, Kharfan-Dabaja MA, Fernandez H, Ayala E, et al. A randomized phase II trial comparing tacrolimus and mycophenolate mofetil to tacrolimus and methotrexate for acute graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant. 2010;16:937–947. doi: 10.1016/j.bbmt.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Sabry W, Le Blanc R, Labbe AC, Sauvageau G, Couban S, Kiss T, et al. Graft-versus-host disease prophylaxis with tacrolimus and mycophenolate mofetil in HLA-matched nonmyeloablative transplant recipients is associated with very low incidence of GVHD and nonrelapse mortality. Biol Blood Marrow Transplant. 2009;15:919–929. doi: 10.1016/j.bbmt.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Nieto Y, Patton N, Hawkins T, Spearing R, Bearman SI, Jones RB, et al. Tacrolimus and mycophenolate mofetil after nonmyeloablative matched-sibling donor allogeneic stem-cell transplantations conditioned with fludarabine and low-dose total body irradiation. Biol Blood Marrow Transplant. 2006;12:217–225. doi: 10.1016/j.bbmt.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Giralt S, Thall PF, Khouri I, Wang X, Braunschweig I, Ippolitti C, et al. Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood. 2001;97:631–637. doi: 10.1182/blood.v97.3.631. [DOI] [PubMed] [Google Scholar]
- 15.Ringden O, Labopin M, Gorin NC, Le Blanc K, Rocha V, Gluckman E, et al. Treatment with granulocyte colony-stimulating factor after allogeneic bone marrow transplantation for acute leukemia increases the risk of graft-versus-host disease and death: a study from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2004;22:416–423. doi: 10.1200/JCO.2004.06.102. [DOI] [PubMed] [Google Scholar]
- 16.Battiwalla M, McCarthy PL. Filgrastim support in allogeneic HSCT for myeloid malignancies: a review of the role of G-CSF and the implications for current practice. Bone Marrow Transplant. 2009;43:351–356. doi: 10.1038/bmt.2008.443. [DOI] [PubMed] [Google Scholar]
- 17.http://www.asbmt.org/displaycommon.cfm?an=1&subarticlenbr=35 2013
- 18.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Lee SJ, Klein JP, Barrett AJ, Ringden O, Antin JH, Cahn JY, et al. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood. 2002;100:406–414. doi: 10.1182/blood.v100.2.406. [DOI] [PubMed] [Google Scholar]
- 20.Bearman SI, Appelbaum FR, Buckner CD, Petersen FB, Fisher LD, Clift RA, et al. Regimen-related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol. 1988;6:1562–1568. doi: 10.1200/JCO.1988.6.10.1562. [DOI] [PubMed] [Google Scholar]
- 21.Jagasia M, Arora M, Flowers ME, Chao NJ, McCarthy PL, Cutler CS, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119:296–307. doi: 10.1182/blood-2011-06-364265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasper C, Sayer HG, Mugge LO, Schilling K, Scholl S, Issa MC, et al. Combined standard graft-versus-host disease (GvHD) prophylaxis with mycophenolate mofetil (MMF) in allogeneic peripheral blood stem cell transplantation from unrelated donors. Bone Marrow Transplant. 2004;33:65–69. doi: 10.1038/sj.bmt.1704299. [DOI] [PubMed] [Google Scholar]
- 23.Lai Y, Ma J, Schwarzenberger P, Li W, Cai Z, Zhou J, et al. Combination of CsA, MTX and low-dose, short-course mycophenolate mofetil for GVHD prophylaxis. Bone Marrow Transplant. 2009;43:61–67. doi: 10.1038/bmt.2008.265. [DOI] [PubMed] [Google Scholar]
- 24.Pasquini MC, Wang Z. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR Summary Slides. 2012 Available at: http://www.cibmtr.org.
- 25.Marks DI, Wang T, Perez WS, Antin JH, Copelan E, Gale RP, et al. The outcome of full-intensity and reduced-intensity conditioning matched sibling or unrelated donor transplantation in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia in first and second complete remission. Blood. 2010;116:366–374. doi: 10.1182/blood-2010-01-264077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horan JT, Logan BR, Agovi-Johnson MA, Lazarus HM, Bacigalupo AA, Ballen KK, et al. Reducing the risk for transplantation-related mortality after allogeneic hematopoietic cell transplantation: how much progress has been made? J Clin Oncol. 2011;29:805–813. doi: 10.1200/JCO.2010.32.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho VT, Aldridge J, Kim HT, Cutler C, Koreth J, Armand P, et al. Comparison of Tacrolimus and Sirolimus (Tac/Sir) versus Tacrolimus, Sirolimus, and mini-methotrexate (Tac/Sir/MTX) as acute graft-versus-host disease prophylaxis after reduced-intensity conditioning allogeneic peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:844–850. doi: 10.1016/j.bbmt.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez R, Nakamura R, Palmer JM, Parker P, Shayani S, Nademanee A, et al. A phase II pilot study of tacrolimus/sirolimus GVHD prophylaxis for sibling donor hematopoietic stem cell transplantation using 3 conditioning regimens. Blood. 2010;115:1098–1105. doi: 10.1182/blood-2009-03-207563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Euvrard S, Morelon E, Rostaing L, Goffin E, Brocard A, Tromme I, et al. Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med. 2012;367:329–339. doi: 10.1056/NEJMoa1204166. [DOI] [PubMed] [Google Scholar]
- 30.Cutler C, Logan BR, Nakamure R, Johnston L, Choi SW, Porter DL, et al. Tacrolimus/Sirolimus vs. Tacrolimus/Methotrexate for Graft-Vs-Host Disease Prophylaxis After HLA-Matched, Related Donor Hematopoietic Stem Cell Transplantation: Results of Blood and Marrow Transplant Clinical Trials Network Trial 0402. Blood (ASH Annu Meet Abstr) 2012;120:739. [Google Scholar]
- 31.Allison AC, Hovi T, Watts RW, Webster AD. Immunological observations on patients with Lesch-Nyhan syndrome, and on the role of de-novo purine synthesis in lymphocyte transformation. Lancet. 1975;2:1179–1183. doi: 10.1016/s0140-6736(75)92661-6. [DOI] [PubMed] [Google Scholar]
- 32.Carr SF, Papp E, Wu JC, Natsumeda Y. Characterization of human type I and type II IMP dehydrogenases. J Biol Chem. 1993;268:27286–27290. [PubMed] [Google Scholar]
- 33.Eugui EM, Almquist SJ, Muller CD, Allison AC. Lymphocyte-selective cytostatic and immunosuppressive effects of mycophenolic acid in vitro: role of deoxyguanosine nucleotide depletion. Scand J Immunol. 1991;33:161–173. doi: 10.1111/j.1365-3083.1991.tb03746.x. [DOI] [PubMed] [Google Scholar]
- 34.Jolivet J, Cowan KH, Curt GA, Clendeninn NJ, Chabner BA. The pharmacology and clinical use of methotrexate. N Engl J Med. 1983;309:1094–1104. doi: 10.1056/NEJM198311033091805. [DOI] [PubMed] [Google Scholar]


