Abstract
Estrogens are commonly used in gynecologic area, such as oral contraception, hormone replacement therapy, and in vitro fertilization-embryo transfer. Although estrogen is a common cause of acute drug-induced pancreatitis, there has been paucity of report in Korea. Clinical course of estrogen-induced acute pancreatitis is usually mild to moderate, but fetal case can occur. In addition, there can be a latency from the first administration to the symptom. Therefore, physicians should consider the possibility of the disease when a woman taking estrogen or previous history of taking estrogen presents with acute abdominal pain. Here, we report a case of estrogen-induced acute pancreatitis that occurred during the preparation for embryo transfer.
Keywords: Acute pancreatitis, Drug-induced acute pancreatitis, Estrogen
Introduction
There are more than 100 drugs that have been implicated in causing acute pancreatitis [1]. Estrogen is a rare cause of drug-induced acute pancreatitis and only about 40 cases of acute pancreatitis have been reported due to use of estrogen worldwide [1].
Estrogen-containing oral contraceptive is considered a safe method of birth control. Estrogen is also commonly used for postmenopausal hormone replacement therapy (HRT) and endometrial development in preparation for embryo transfer (ET). Although estrogen is used safely in most of the cases, it is one of the causative agents of drug-induced acute pancreatitis. Although more than 40 cases of pancreatitis due to HRT or oral contraceptive use have been reported worldwide, there has been no case report of estrogen-induced acute pancreatitis in Korea. In this report, we describe a case of estrogen-induced acute pancreatitis that occurred when estrogen was used for the preparation of ET.
Case report
A 40-year-old woman presented to the emergency department with severe epigastric pain and nausea. She had been trying to get pregnant with assisted reproductive technology for 2 years due to unexplained infertility. Previously, she received 2 cycles of intrauterine insemination and 6 cycles of in vitro fertilization-embryo transfer (IVF-ET), which all failed. She underwent cryo-thawed ET a week ago, and she had been on estradiol valerate (Progynova®; Bayer-Shering Pharma, Berlin, Germany) 2 mg per os every 8 hours for 24 days, vaginal progesterone 90 mg (Crinone® gel 8%; Merck Serono SA, Geneva, Switzerland) every 12 hours for 10 days, and enoxaparin sodium (Clexane®; Sanofi-Aventis, Paris, France) 40 mg subcutaneous injection once a day for 7 days before presenting. She had taken estrogens in previous IVF-ET cycles, but denied any severe abdominal pain like this time. She had 3-year history of diabetes mellitus, which was under control with insulin injection. Hemoglobin A1c concentration was 6.8% and the serum cholesterol levels were within normal range on blood test which was performed 1 month before the symptom. She denied smoking and drinking and she did not have familial hypercholesterolemia.
On physical examination, her blood pressure was 145/87 mmHg, pulse rate was 65/min and body temperature was 37.5℃. Abdominal examination revealed that the abdomen was distended with whole abdomen tenderness and bowel sound was hypoactive. Her weight and height were 77.25 kg/156.2 cm, and body mass index was 29.91 kg/m2.
Blood tests revealed elevated levels of amylase (416 U/L), lipase (790 U/L), white blood cell count (12.24×103/uL), lactate dehydrogenase (442 IU/L), total cholesterol (723 mg/dL), triglycerides (4,051 mg/dL) and C-reactive protein (5.54 ng/mL). Serum blood urea nitrogen, creatinine, alkaline phosphatase, alanine aminotransferase, and bilirubin level were within reference range.
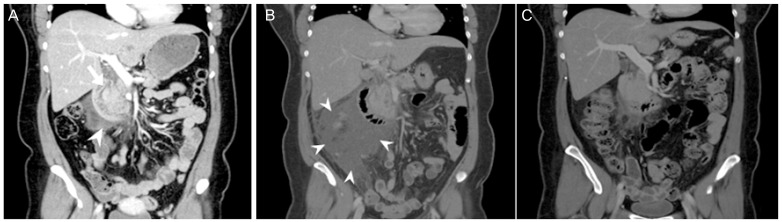
An abdominal computed tomographic (CT) scan was performed showing mild swelling of pancreas head with peripancreatic infiltration, the presence of peripancreatic fluid and fluid in the upper mesentery which was categorized into grade D of Balthazar CT grade (Fig. 1A).
Fig. 1. (A) Mild swelling of pancreas head (arrow) with peripancreatic infiltration, the presence of peripancreatic fluid (arrow head) and fluid in the upper mesentery (admission day). (B) Increased of peripancreatic fluid collection (arrow head) (4th hospital day). (C) Decreased peripancreatic fluid collection (2 months after discharge).
The patient was managed with supportive therapy including intravenous hydration, antibiotics, and analgesics. As her abdominal pain still remained and fever sustained at fourth hospital day, follow-up abdominal CT scan was performed and the result revealed increased peripancreatic fluid collection (Fig. 1B). We changed antibiotics from third generation cephalosporin to ertapenem and clinical condition improved. She was discharged on the 10th hospital day. Follow-up CT scan performed 2 months after discharge showed much decreased peripancreatic fluid collection (Fig. 1C). Fortunately, she became pregnant naturally after the treatment of pancreatitis and has been maintaining her pregnancy for about 36 weeks without complication.
Discussion
Acute pancreatitis has been described in subjects using oral contraceptives, IVF-patients without using estrogen, and there is a report of increase risk of acute pancreatitis in postmenopausal women using hormone therapy [2,3,4]. Here we report a case of estrogen-induced acute pancreatitis in which estrogen was administered for the cryo-thaw ET.
The unique feature of this case is that the symptom did not develop until the last cycle of estrogen administration. There is a report describing the latency between initiating the drug and the onset of acute pancreatitis [1]. In this article which analyzed case reports, estrogens were classified as a category in which a consistent latency has been reported in more than 75% of cases. The latency of estrogen ranged from 2 months to 2 years (Table 1). In addition, there was a recent case report of hypertriglyceridemic pancreatitis caused by oral contraceptive agent. In this case, the patient had been taking the oral pill for 9 years without any adverse effect [2]. Based on the above evidence, it is possible that estrogen-induced pancreatitis can develop in women who had taken estrogen without any problem.
Table 1. Summary of estrogen-induced acute pancreatitis cases with IVF, and with latency.
| Case report | Age (yr) | Sex | The purpose of using estrogen | Latency | Initial serum triglyceride level (mg/dL) | Follow-up serum triglyceride level (mg/dL) |
|---|---|---|---|---|---|---|
| Lee and Goldberg [3] | 33 | Female | IVF ovulation induction | - | >2,400 | <80 (2 mon) |
| 22 | Female | IVF ovulation induction | >7,000 | 1,083 (7 mon) | ||
| 28 | Female | IVF ovulation induction | 1,500 | 420 (4 mon) | ||
| Perego et al. [5] | 37 | Male | Preparatory to sex change surgery | 2 mon | 5,174 | 181 (22 day) |
| Abraham et al. [2] | 24 | Female | - | 9 yr | 2,230 | 355 (7 day) |
| Bank and Marks [13] | 29 | Female | - | 2 mon | 7,450 | - |
| Davidoff et al. [14] | 33 | Female | - | 23 mon | 3,560 | - |
| Ruman et al. [15] | 30 | Female | Preparation for ET | - | 8,062 | 353 (7 mon) |
IVF, in vitro fertilization; ET, embryo transfer.
Although the exact mechanism of estrogen-induced acute pancreatitis is still unclear, estrogen-induced acute pancreatitis is thought to be caused by hypertriglyceridemia. Most of the reported cases show elevated serum triglyceride level of up to 1,000 mg (Table 1). Hypertriglyceridemic pancreatitis is reported to be responsible for 1.3–3.8% of patients with acute pancreatitis [5]. Hyperlipidemia is divided into primary hyperlipidemia and secondary hyperlipidemia. Estrogen-induced hyperlipidemia is secondary hyperlipidemia. Other causes of secondary hyperlipidemia include diabetes, alcohol, and pregnancy. The triglycerides transported by chylomicron and very low density lipoprotein are hydrolyzed by the high concentration of lipase in the capillaries of the pancreas and form a large amount of toxic free fatty acids, which causes lipotoxicity during acute pancreatitis. [6,7] The risk factors for estrogen-induced acute pancreatitis include genetic abnormality of lipid metabolism, insulin resistance, obesity, alcohol use, and pregnancy. Infants, elderly, and women patients with advanced acquired immune deficiency syndrome, and patients with inflammatory bowel disease are also considered as high risk groups that are susceptible to drug-induced pancreatitis [7,8]. Although the patient did not have a history of hypercholesterolemia, she was 40 years old which can be identified as a risk factor for the drug-induced acute pancreatitis.
As most of the estrogen-induced pancreatitis are case-reports, it is hard to assess the magnitude of the risk. The fact that pancreatitis can be caused in woman who had no problem taking the oral contraceptives for long time before the development of disease [2] also adds to the difficulty in assessing the risk. Although the amount of estrogen used in case-reports in the 1970's was relatively high (0.1 mg of ethinyl estradiol), lower dose of estrogen was associated with acute pancreatitis recently [2]. There is a prospective cohort study addressing the risk of acute pancreatitis in postmenopausal women with HRT. In the report, adjusted risk ratio was 1.92 (1.38–2.66), and the risk increases as the duration of hormone use increases [4]. However, locally administered therapy did not increase the risk of acute pancreatitis, which can be expected from the fact that transdermal hormone therapy does not change plasma lipoproteins very much [4,9]. Overall, systemic estrogen administration seems to increase the risk of acute pancreatitis. However, we do not know the magnitude of the risk increase when one or risk factors are present.
In this case, the patient was taking estrogen for ET, and the blood test confirmed a high concentration of triglyceride as in other previously reported estrogen-induced acute pancreatitis cases. Although the patient had diabetes which could be the cause of secondary hyperlipidemia, her diabetes was under control with insulin. Gallstone or structural abnormality which are major causes of pancreatitis were not visualized in CT scan and no history of alcohol abuse was found. In addition, her symptom improved after stopping taking estrogens. All the evidence above supports the diagnosis of estrogen-induced acute pancreatitis.
The clinical presentation of estrogen-induced acute pancreatitis is similar to that from other causes. The majority of estrogen-induced acute pancreatitis cases are mild to moderate in severity, but severe and fatal cases can occur [5]. There are several indicators for the severity of acute pancreatitis and we used Balthazar CT grade which classified as grade A to E on CT findings according to severity. Usually estrogen-induced acute pancreatitis has a short episode of abdominal pain and there is a moderate to severe increase of pancreatic enzymes and triglycerides which normalize soon after estrogen withdrawal [5].
Physicians should consider the possibility of estrogen-induced acute pancreatitis in estrogen-taking women who present with acute abdominal pain. Furthermore, if a physician chooses to prescribe estrogen to a patient who is old, obese and/or with a family history of hyperlipidemia, the possibility of estrogen-induced acute pancreatitis should be borne in mind [8]. Especially when the patient is obese, and has a family history of hypercholesterolemia, the total cholesterol and triglyceride level should be checked and corrected before the initiation of treatment. In addition, they should also be monitored during treatment. These measures could be helpful in preventing estrogen-induced acute pancreatitis.
There can be several options of route of estrogen administration for the IVF-ET patients who previously suffered from estrogen-induced acute pancreatitis. Endometrial preparation with transdermal estrogen were reported to be comparable to oral estrogen in pregnancy rate [10] in a randomized clinical trial, and live birth rate [11] in a large retrospective cohort study. Gonadotropin-releasing hormone agonists [12] were also used successfully. However, as there are reports of hyperlipidemia and acute pancreatitis in the IVF-ET cycle without using oral estrogen, it would be prudent not to use estrogen if possible, or to use for a short period of time if it should be used. Although one researcher reported the use of surrogate carrier [3], it may not be feasible. The most important points are to monitor serum lipid level, to detect abnormality, and to take appropriate action.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Badalov N, Baradarian R, Iswara K, Li J, Steinberg W, Tenner S. Drug-induced acute pancreatitis: an evidence-based review. Clin Gastroenterol Hepatol. 2007;5:648–661. doi: 10.1016/j.cgh.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 2.Abraham M, Mitchell J, Simsovits D, Gasperino J. Hypertriglyceridemic pancreatitis caused by the oral contraceptive agent estrostep. J Intensive Care Med. 2015;30:303–307. doi: 10.1177/0885066614528083. [DOI] [PubMed] [Google Scholar]
- 3.Lee J, Goldberg IJ. Hypertriglyceridemia-induced pancreatitis created by oral estrogen and in vitro fertilization ovulation induction. J Clin Lipidol. 2008;2:63–66. doi: 10.1016/j.jacl.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oskarsson V, Orsini N, Sadr-Azodi O, Wolk A. Postmenopausal hormone replacement therapy and risk of acute pancreatitis: a prospective cohort study. CMAJ. 2014;186:338–344. doi: 10.1503/cmaj.131064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perego E, Scaini A, Romano F, Franciosi C, Uggeri F. Estrogen-induced severe acute pancreatitis in a male. JOP. 2004;5:353–356. [PubMed] [Google Scholar]
- 6.Gan SI, Edwards AL, Symonds CJ, Beck PL. Hypertriglyceridemia-induced pancreatitis: a case-based review. World J Gastroenterol. 2006;12:7197–7202. doi: 10.3748/wjg.v12.i44.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yadav D, Pitchumoni CS. Issues in hyperlipidemic pancreatitis. J Clin Gastroenterol. 2003;36:54–62. doi: 10.1097/00004836-200301000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Nitsche CJ, Jamieson N, Lerch MM, Mayerle JV. Drug induced pancreatitis. Best Pract Res Clin Gastroenterol. 2010;24:143–155. doi: 10.1016/j.bpg.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Vrablik M, Fait T, Kovar J, Poledne R, Ceska R. Oral but not transdermal estrogen replacement therapy changes the composition of plasma lipoproteins. Metabolism. 2008;57:1088–1092. doi: 10.1016/j.metabol.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 10.Davar R, Janati S, Mohseni F, Khabazkhoob M, Asgari S. A comparison of the effects of transdermal estradiol and estradiol valerate on endometrial receptivity in frozen-thawed embryo transfer cycles: a randomized clinical trial. J Reprod Infertil. 2016;17:97–103. [PMC free article] [PubMed] [Google Scholar]
- 11.Madero S, Rodriguez A, Vassena R, Vernaeve V. Endometrial preparation: effect of estrogen dose and administration route on reproductive outcomes in oocyte donation cycles with fresh embryo transfer. Hum Reprod. 2016;31:1755–1764. doi: 10.1093/humrep/dew099. [DOI] [PubMed] [Google Scholar]
- 12.Dal Prato L, Borini A, Cattoli M, Bonu MA, Sciajno R, Flamigni C. Endometrial preparation for frozen-thawed embryo transfer with or without pretreatment with gonadotropin-releasing hormone agonist. Fertil Steril. 2002;77:956–960. doi: 10.1016/s0015-0282(02)02960-6. [DOI] [PubMed] [Google Scholar]
- 13.Bank S, Marks IN. Case reports. Hyperlipaemic pancreatitis and the pill. Postgrad Med J. 1970;46:576–578. doi: 10.1136/pgmj.46.539.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davidoff F, Tishler S, Rosoff C. Marked hyperlipidemia and pancreatitis associated with oral contraceptive therapy. N Engl J Med. 1973;289:552–555. doi: 10.1056/NEJM197309132891103. [DOI] [PubMed] [Google Scholar]
- 15.Ruman J, Brenner S, Sauer MV. Severe hypertriglyceridemia and pancreatitis following hormone replacement prior to cryothaw transfer. J Assist Reprod Genet. 2002;19:94–97. doi: 10.1023/A:1014404016609. [DOI] [PMC free article] [PubMed] [Google Scholar]