Abstract
Chronic posterior glenohumeral joint instability can be a challenging clinical entity for patients and surgeons alike. In the setting of a posterior dislocation, a large anterior humeral impaction injury (reverse Hill-Sachs [HS]) may occur, leading to engagement of the humerus with the posterior glenoid bone, especially during internal rotation of the joint. A reverse HS is especially debilitating because of the significant portion of affected humeral head cartilage, and is made worse in the setting of ligamentous disruption such as a posterior humeral avulsion of the glenohumeral ligament (HAGL) lesions. Although several nonanatomic procedures to address these defects have been previously described, recent interest in anatomic reconstructions capable of restoring the cartilage surface of the humeral head has led to the use of bone grafts (autografts and allografts) to restore the articular contour of the humeral head in conjunction with anatomic repair of associated soft tissue injuries. We present our preferred technique for an anatomic repair of a posterior HAGL lesion in combination with reconstruction of an engaging reverse HS lesion using an unmatched hemitalar allograft.
Posterior shoulder instability has become increasingly recognized as a cause of pain and dysfunction, especially in young contact sport athletes.1 Cases of recurrent posterior instability can lead to complications including laxity of the surrounding soft tissues, glenoid bone loss, and osteochondral lesions of the humeral head (reverse Hill-Sachs [HS] lesions).2 In patients with a first-time traumatic posterior shoulder dislocation, 86% were found to have a reverse HS lesion identified via magnetic resonance imaging.3 Recurrent posterior instability with a reverse HS lesion will lead to repetitive engagement with the posterior glenoid rim, which will lead to patient morbidity and an increased risk of early-onset osteoarthritis. Unlike the posterior cartilage damage seen in traditional HS lesions, reverse HS lesions are associated with significantly greater cartilage damage at the anterior aspect of the humeral head.4 Because of the location and size of these lesions, it is essential that reverse HS repair include a method for chondral restoration. Moreover, cartilage viability is a key determinant of successful clinical outcomes following osteochondral allograft transplantation.
When a significant bony defect is present on the humeral head, restoration of the humeral contour or placement of an anatomic block to prevent engagement with the posterior glenoid may be necessary to reduce pain, restore motion, and prevent recurrent instability. However, this anatomic block must not only restore the native contour of the humeral head but also address the engaging chondral defect. The articular portion of the talus bone acts as a potential allograft source for repair of a reverse HS lesion with several advantages, including its thick and dense bony quality, cartilage surface, and radius of curvature that closely approximates that of the humeral head. Additionally, the talus does not require size matching and represents an almost limitless bone source to harvest an appropriate-sized graft.
In cases of chronic posterior instability, the soft tissues must also be addressed. In particular, posterior humeral avulsion of the glenohumeral ligament (HAGL) lesions can be seen in posterior instability, leading to persistent instability and pain. However, these lesions are often not appreciated on imaging, with only 50% of the lesions identified preoperatively by radiologists. In addition, posterior HAGL lesions are often seen in association with other pathology, which can distract from this diagnosis.5
In this Technical Note, we describe soft tissue management of posterior shoulder instability completed via arthroscopic HAGL and labrum repair, followed by anatomic resurfacing of the humeral head with an unmatched talus allograft for reconstruction of a large reverse HS lesion.
Objective Diagnosis
Physical Examination Findings
A thorough physical examination is key for an effective evaluation and accurate diagnosis of a reverse HS lesion associated with posterior shoulder instability. The physical examination should be bilateral to compare to the contralateral shoulder. For an accurate diagnosis of instability, evaluation for possible joint laxity must first be completed. Despite excessive range of motion and joint translation, laxity will not be associated with pain or apprehension. As a result of posterior instability, the patient will feel discomfort and report a sensation of the shoulder “popping out” during extreme translation. Examination of the shoulder will reveal an inability to externally rotate because of a mechanical block with limited flexion and abduction. Given that the humeral head is fixed on the posterior glenoid rim, the dislocated shoulder is locked in internal rotation. However, abduction and forward elevation may be preserved up to 80° or more. Aside from the routine shoulder examination, several provocative tests can be completed for diagnosis of posterior shoulder instability, including the posterior drawer, jerk, and Kim tests.
Radiographic Findings
If posterior instability is suspected, a standard 3-view radiographic series should first be used confirm the presence of a reverse HS lesion, including a Y view, an axillary lateral view, and an apical oblique view with the arm in external rotation. Of all views, the apical oblique view coupled with external rotation will be most useful, allowing visualization of the anterosuperomedial aspect of the humeral head. However, several other imaging modalities can be used to assess for a possible reverse HS lesion. Computed tomography, specifically 3-dimensional computed tomography, is the best imaging tool used for evaluation of bone loss. With 3-dimensional computed tomography, the presence, orientation, and size of a reverse HS lesion can be visualized. Furthermore, the humeral head can be digitally subtracted to assess for possible bipolar bone loss. When evaluating for presence of an HS lesion, the radiographic findings must be used in combination with the physical examination, as we consider the glenoid track concept more clinically relevant than the percentage of the humeral head defect. The clinician must also determine whether or not glenoid bone loss is present, as it will heighten the effect of even a mild HS lesion. Without the use of advanced imaging, it is not possible to accurately quantify the size, orientation, and location of a bony lesion affecting the glenohumeral joint.
With the use of magnetic resonance imaging, posterior HAGL lesions can be identified. In addition, associated soft tissue injuries can be identified. A high level of suspicion is necessary to diagnose a posterior HAGL lesion. Therefore, if a patient presents with posterior instability, a posterior HAGL lesion should always be considered.
Indications
The indications for combination procedures in the setting of both soft tissue pathology and bony loss continue to be more clearly defined. At present, the procedure is considered when given the following indications: failed nonoperative management, symptoms of recurrent posterior instability, catching and pain, greater than 20% to 40% of humeral head articular surface involvement, and off-track lesion leading to engagement of the HS lesion with the glenoid rim, thereby producing an instability event.
Surgical Technique
Patient Positioning to Lateral Decubitus Position
The patient is positioned supine on the operating table and an examination under anesthesia is performed to confirm the diagnosis and document the affected shoulder range of motion (Video 1). The patient is then placed in the lateral decubitus position for the initial, arthroscopic portion of the case (Fig 1). All bony prominences are well padded and the arm is placed in a STaR Sleeve (Arthrex, Naples, FL) under 10 pounds of balanced suspension. The patient is later repositioned and redraped in a modified beach chair position for the second, open portion of the case.
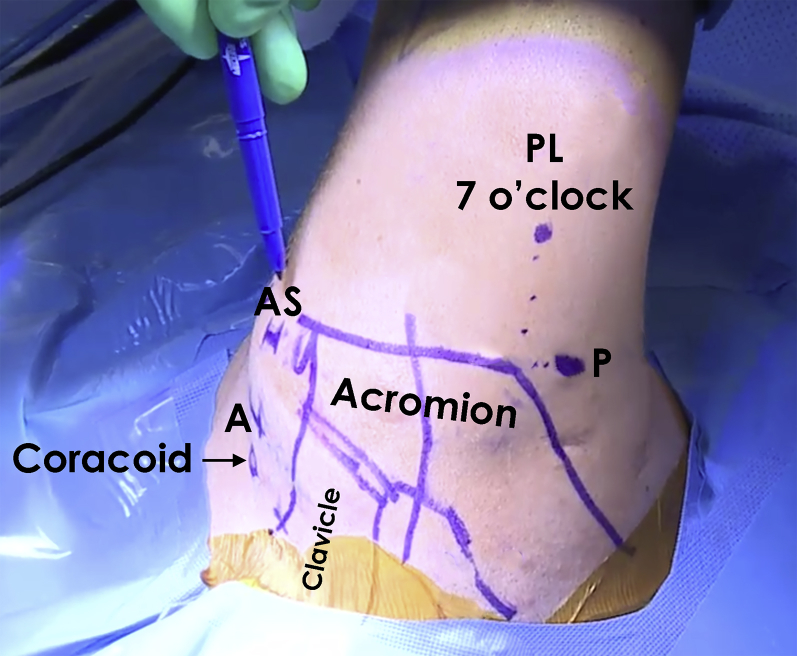
Fig 1.
Image of a right shoulder following preparation and draping with the patient in lateral decubitus position. The osseous landmarks of the clavicle and acromion as well as the areas of portal placement are marked prior to commencing surgery. (A, anterior portal; AS, anterosuperior portal; P, posterior portal; PL, posterolateral portal.)
Arthroscopic Portal Placement
The arthroscopic portion of the case starts by establishing a standard posterior portal. The arthroscope is inserted into the joint and then a midanterior glenoid portal is established under direct visualization. Diagnostic arthroscopy is performed at this point. The arthroscope is then moved from the posterior to the midanterior glenoid portal. The diagnostic arthroscopy is completed at this point. A switching stick is then inserted into the previously established posterior portal, and a 5-mm cannula is inserted over this. A posterior lateral portal is then established under direct visualization with a spinal needle. A switching stick is then inserted, and a second 5-mm cannula is inserted over this into the joint.
Labral Repair
The labrum is addressed first. The extent of the tear is delineated with an arthroscopic probe. An elevator is then inserted through the posterior portal, and the labrum and capsular layer is elevated off the face and neck of the glenoid. Once the labrum has been fully mobilized (Fig 2), an arthroscopic shaver is inserted to debride all loose frayed tissue. The bony surface is then freshened with the shaver on burr mode to provide an excellent healing surface for repair. Attention is then turned to delineating the posterior glenohumeral ligament and capsule from the overlying muscle fibers (Fig 3). The defect in these structures should be clearly visualized. Once the ligaments and capsule have been freed, the labral repair and capsulorraphy should be performed.
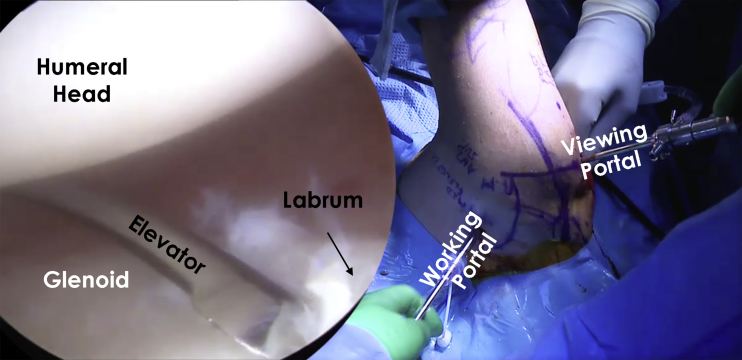
Fig 2.
Intraoperative image of the right shoulder with the patient in the lateral decubitus position viewing from the posterior portal. The labrum and the glenoid can be visualized. To access the posterior labrum and glenoid with an elevator, the anterior portal is used as a working portal. It is important to use the elevator to release the labrum, which is often scarred and retracted in chronic injuries. The labrum should be mobilized and fixed in the optimal position to provide stability. A fresh glenoid bony surface is necessary for healing.
Fig 3.
A view of the posterior labrum and capsule of the right shoulder from the anterior portal with the patient in the lateral decubitus position is shown. The 7-o'clock posterolateral portal is a good working portal, and is used here for easy access to the posterior and posteroinferior labrum for anchor placement, suture passage, and knot tying.
A ReelPass (Arthrex) suture lasso is used to capture both the mobilized capsule and labrum. Labral tape is then shuttled through both structures with the ReelPass. A guide is then inserted and a hole is pre-drilled for a 2.9-mm short PushLock suture anchor (Arthrex). The labral tape is then loaded into the anchor. Following this, the anchor is inserted with appropriate tensioning of the labral tape. The appropriate number of anchors is determined based on the extent of the labral tear.
Repair of HAGL Lesion
Once the labral repair has been completed, the HAGL lesion can then be addressed (Fig 4). Often times, the defect is fairly extensive, and approximation of the ligaments and capsule can be first made with a side-to-side repair. The ReelPass suture lasso is again used to shuttle no. 2 FiberWire suture (Arthrex) in a mattress fashion. An arthroscopic knot is then tied to secure both leaflets. The posteroinferior aspect of the humerus is then cleared of all soft tissues. The surface is freshened with the arthroscopic shaver on burr mode, which provides an excellent repair surface for anchor placement. Two free FiberTapes (Arthrex) are then shuttled through the approximated ligaments and capsule. Each end of the FiberTape is retrieved and passed through a 5.5-mm SwiveLock (Arthrex) suture anchor. A tamp is used to pre-tap the posterior inferior neck of the humerus. It is important to place the anchor as inferior as possible to allow for easier placement of the second anchor. The SwiveLock anchor is then inserted. Afterwards, the FiberTapes are appropriately tensioned. An arthroscopic tape cutter is used to transect the remaining tape. A second anchor is then placed in similar fashion more superior in relation to the first anchor. At the completion of the HAGL repair, a nonabsorbable PDS suture is passed through the capsular leaflets of the posterior portal. Both ends of the suture are retrieved, and an arthroscopic knot is tied. Examination consisting of a posterior load and shift is performed under direct visualization to ensure restoration of posterior stability.
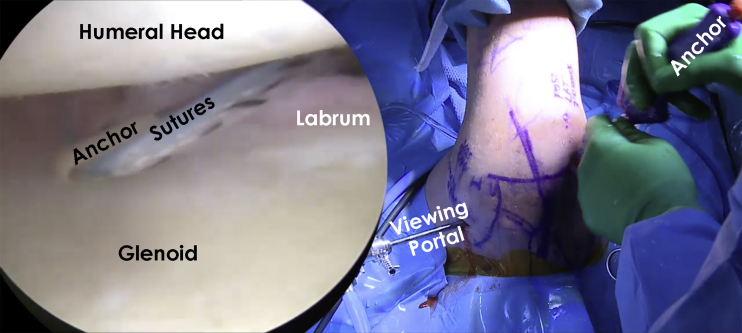
Fig 4.
From the anterior portal, the posterior labrum and capsule of this right shoulder is visualized with the patient in the lateral decubitus position. The working portal is the 7-o'clock posterolateral portal, which makes it easier to address the posterior glenohumeral defect and tie the necessary sutures. A suture lasso device is used to pass the sutures to repair the glenohumeral defect. The sutures are tied arthroscopically outside the joint.
Patient Repositioning to Beach Chair Position
At the conclusion of the arthroscopic procedure, all wounds are closed in layered fashion with absorbable sutures except the wound of the anterior portal, which is sterilely covered during repositioning to the beach chair position (Fig 5). With the patient in the supine position, the head of the bed is elevated to approximately 40°. The arm is then reprepped and redraped in standard sterile fashion.
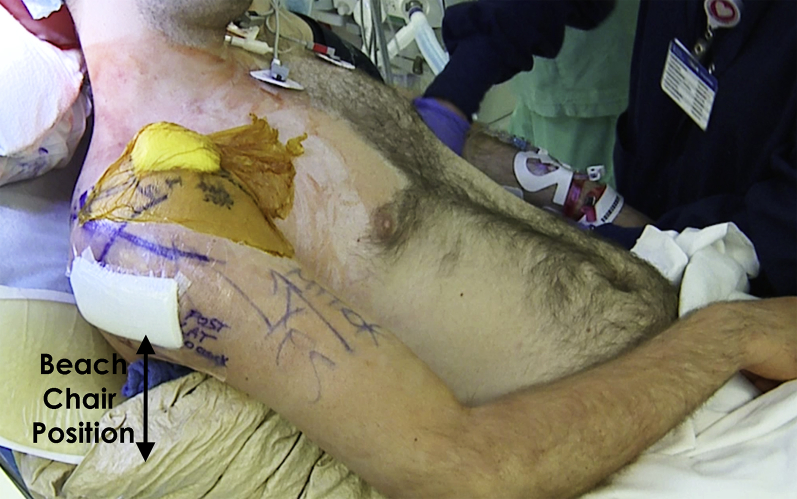
Fig 5.
After addressing the intra-articular pathology (labrum and HAGL), the portals are closed and draped in a sterile fashion. The patient is placed in a beach chair position to transition to the necessary open procedure undertaken to address the bone defect of the right shoulder's humeral head. (HAGL, humeral avulsion of the glenohumeral ligament.)
Surgical Approach and Exposure
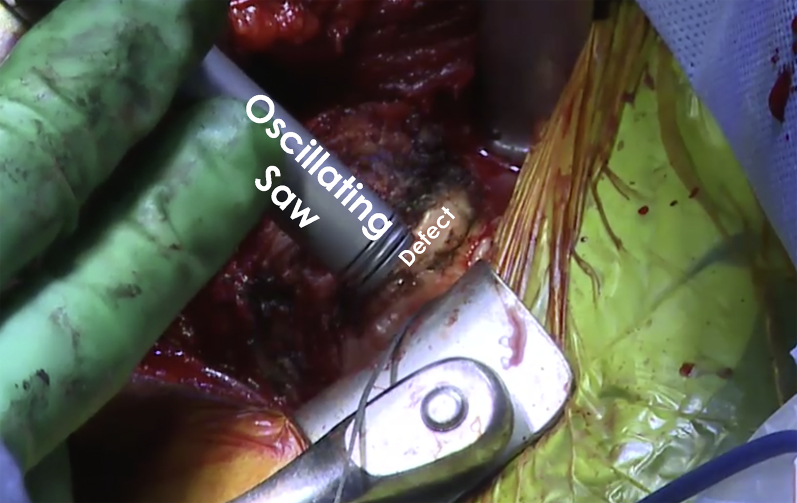
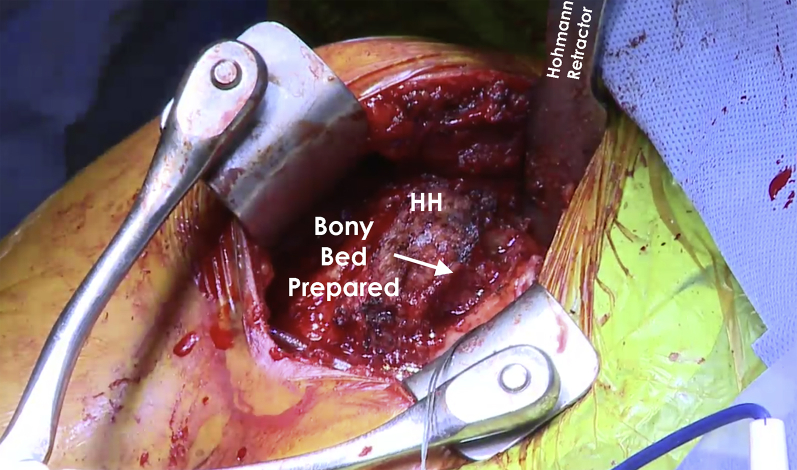
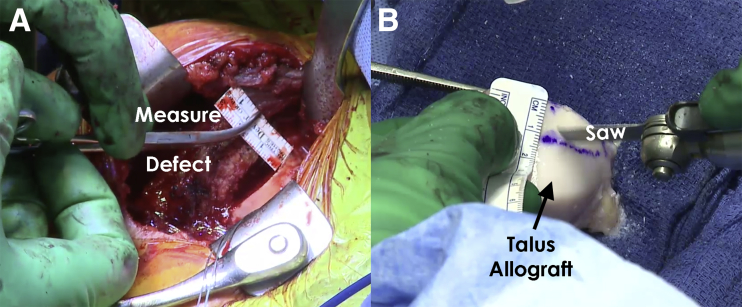
An incision is made over the deltopectoral interval and dissection is carried down to identify the cephalic vein. The vein is protected and mobilized medially. The clavipectoral fascia is then incised and the interval between the deltoid and conjoint tendon is bluntly developed. The undersurface of the deltoid is then freed of all attachments overlying the rotator cuff. This allows placement of a cobra retractor in the superior aspect of the wound (Fig 6). The arm is then internally and externally rotated to confirm identification of the long head of the biceps tendon and the bicipital groove. The bicipital sheath is opened and the biceps tendon is released and then tagged for later tenodesis. A pair of curved mayo scissors is then used to open the rotator interval. The subscapularis attachment on the lesser tuberosity should then be clearly delineated. The bicipital groove is cleared of all soft tissues and the lateralmost aspect of the subscapularis footprint is identified. At this time, a peel technique is employed to release the upper two thirds of the subscapularis and capsule as one unit. As the release is performed superiorly to inferiorly, a blunt retractor should be placed inferiorly to protect the axillary nerve. Once both have been released, the arm can be adducted and externally rotated to expose the reverse HS lesion of the humeral head. The lesion should be thoroughly debrided of all soft tissue to allow full exposure of the lesion (Fig 7). The lesion is then outlined. Following this, quarter-and-half-inch curved osteotomes are used to excise the defect. The bed of the resected bone is then prepared (Fig 8) with use of a power rasp. Once the defect has been sufficiently prepared, measurements of the defect are completed using a ruler for preparation of the bone graft (Fig 9).
Fig 6.
Intraoperative image of the right shoulder showing a deltopectoral approach, which is used for exposure of the humeral head to address the Hill-Sachs lesion. A Hohmann retractor is used to hold the soft tissue away for better visualization. Following this, the subscapularis is then detached from its insertion to access the joint. The subscapularis is later reinserted using suture anchors and FiberTape with a SpeedBridge construct (Arthrex).
Fig 7.
Following thorough exposure, the humeral head of the right shoulder is viewed. The bony defect of the humeral head is visualized. Prior to bone grafting, the bone defect is prepared with an oscillating saw and rongeur to have fresh flat surfaces for better fixation of the graft and optimal healing.
Fig 8.
Intraoperative image of the right shoulder showing the humeral head following preparation of the defect. An “orange-slice” shape of the defect is preferred to get a press fit of the bone graft into the defect to provide optimal stability. (HH, humeral head.)
Fig 9.
Intraoperative image showing the humeral head of a right shoulder following exposure via a deltopectoral approach. After preparing the defect bed on the humeral head, the defect is measured (A) prior to preparing the talus bone graft (B). When preparing the bone graft, the area with the appropriate curvature is marked to the correct dimensions. Then, an oscillating saw is used to complete the cuts and harvest the bone graft. The harvested bone graft is irrigated with normal saline to remove debris. Following this, the graft is soaked in platelet-rich plasma to stimulate integration into the humeral head and maximize healing.
Talar Allograft Preparation
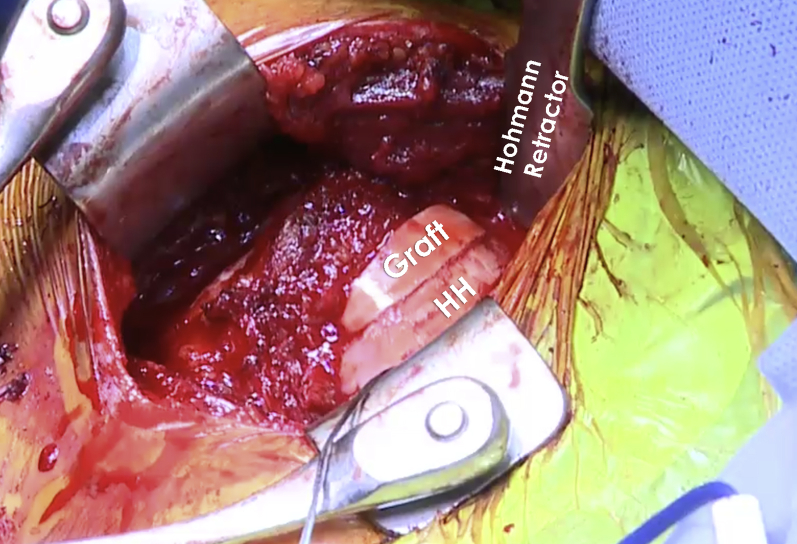
The base of a fresh talar allograft is then removed with use of an oscillating saw. Based on the dimensions of the prepared humeral head defect, an outline is then drawn on the graft for its width, length, and depth. Marks are made 1 to 2 mm larger than the resected lesion. The graft is then firmly secured onto an OATS allograft vice. Using a handheld oscillating saw, the graft is cut in an “orange slice” shape. Because of slight oversizing of the graft, a press fit of the graft into the prepared bed is possible. The graft and humeral head are then thoroughly pulse lavaged for 10 minutes. Following pulse lavage, the graft is soaked in a combination of autologous conditioned plasma (ACP) and platelet-rich plasma (PRP) using a Double Syringe System (Arthrex). To prepare the autologous conditioned and platelet-rich plasma, 60 mL of blood is drawn from the patient by the anesthesiologist and spun down using an Arthrex autologous conditioned plasma spinning machine for approximately 10 minutes. After application of the autologous conditioned and platelet-rich plasma, the graft is then press-fit into the defect. Two 2.4-mm Kirschner wires are used to secure the graft. These are overdrilled, and then two 2.5-mm Acutrak headless compression screws (Acumed, Hillsboro, OR) are inserted to complete the graft fixation (Fig 10).
Fig 10.
Intraoperative image of the humeral head of the right shoulder after fixation of the talus bone graft. The bone graft is fixed to the humeral head with two 2.5-mm headless compression screws. It is important to ensure that the screws do not protrude to avoid potential damage to the glenoid. After fixing the talus bone graft to the humeral head (HH), the shoulder is taken through a range of motion to ensure that shoulder instability has been resolved. (HH, humeral head.)
Subscapularis Tendon Repair and Closure
The subscapularis tendon and capsule is then repaired back to the lesser tuberosity footprint (Fig 11). Using 3 medial Suretak (Arthrex) suture anchors in a SpeedBridge configuration (Arthrex) with 2 additional 5.5-mm PEEK SwiveLock (Arthrex) suture anchors. The anchor construct is then oversewed with no. 2 FiberWire (Arthrex) suture. This ultimately results in a tape configuration double-row subscapularis repair. Following this, the wound is then closed in layered fashion with absorbable suture. The patient is then immobilized in a padded abduction sling. Table 1 summarizes the steps of our surgical technique, whereas Table 2 describes the pearls and pitfalls associated with the technique. Lastly, Table 3 describes the benefits and limitations of the presented technique.
Fig 11.
(A) Intraoperative image of the right shoulder following exposure via deltopectoral approach showing the repair of the subscapularis. The subscapularis (SS) is sutured back onto the lesser tuberosity (LT) using FiberTape (Arthrex, Naples, FL) and suture anchors. (B) A SpeedBridge construct (Arthrex) is used to repair the subscapularis tendon. After the subscapularis repair, the shoulder is taken through a limited range of motion to ensure that the repair is stable. The subcutaneous tissue and skin are then closed in layers. (LT, lesser tuberosity; SS, subscapularis.)
Table 1.
Abbreviated Surgical Outline
| Basic Surgical Plan |
|---|
| Lateral decubitus position for the initial, arthroscopic portion |
| Standard posterior portal is established with a 5-mm cannula followed by an anterior midglenoid portal. |
| Anterior viewing portal is established to assess the posterior portion of the glenohumeral ligaments. |
| An elevator is used to detach the capsule from the scarred muscle fibers. |
| Side-to-side repair of the glenohumeral ligaments is performed with a suture lasso, no. 2 Ethibond sutures, and labral tape. |
| The main portion of the HAGL repair is performed. |
| Repositioning to the beach chair position. |
| A deltopectoral approach is done and dissection until the conjoint tendon is reached is performed. |
| Dissection is then continued until the subscapularis tendon can be identified through the deltopectoral interval. |
| The subacromial space is identified and debrided. |
| The subscapularis tendon and the anterior joint capsule are partially resected. |
| The reverse Hill-Sachs lesion is identified. |
| The bone defect is excised with an osteotome and smoothed with a power rasp. |
| A fresh talus allograft is molded and prepared for insertion into the Hill-Sachs defect. |
| The “orange slice”–shaped graft is press-fitted into the humeral head defect. |
| The subscapularis tendon is then repaired. |
| The wound is closed in layered fashion with absorbable suture. |
| The patient is then immobilized in a padded abduction sling. |
HAGL, humeral avulsion of the glenohumeral ligament.
Table 2.
Pearls and Pitfalls
| Pearls | Pitfalls |
|---|---|
| Perform a complete preoperative examination with the patient under anesthesia to evaluate the extent of posterior instability. | Failure to identify a HAGL lesion can result in lower outcomes. The presence of instability symptoms with a normal labral appearance should raise suspicion. |
| Verification of adequate patient positioning is needed to ensure optimal preoperative setup. Landmark identification and portal placement demarcation should be performed before starting the procedure. | Incorrect patient positioning may worsen visualization and reduce the ease of work. |
| Use a spinal needle with an outside-in technique previous to portal creation to verify proper working and viewing portals. | The axillary nerve might be at risk when working with shavers or hooks in the anteroinferior quadrant. Moreover, injury to the cephalic vein can occur with low percutaneous subscapularis anchor insertion. |
| Meticulous bony preparation with the aid of shavers and curettes is advocated to establish an optimal biologic environment of the repair. | Inadequate restoration of HAGL tension might lead to higher risk of recurrence. |
| The use of cannulas can facilitate the interchanging of the viewing and working portals with ease. | Because of the number and position of the anchors, suture tangling is a concern. Mindful suture managing should be carried out. |
| Verify a good bony apposition of the bony avulsion to its anatomic attachment in the humerus. | Allograft shaping errors can be detrimental to postoperative outcomes. Moreover, it would prolong surgical time and exposure. |
| Use of fresh allograft (less than 28 days after deceased) has been reported as important in consideration of chondrocyte vitality. | |
| Careful measurement and shaping of the defect should be performed before molding the allograft to ensure a good press fit. |
HAGL, humeral avulsion of the glenohumeral ligament.
Table 3.
Benefits and Risks/Limitations
| Benefits | Risks/Limitations |
|---|---|
| Anatomic HAGL and labrum repair | Requirement of advanced arthroscopic skills and thorough knowledge of the anatomy |
| One procedure to address all pathology at once | Fresh osteochondral allografts is not widely available in all regions |
| Large Hill-Sachs defects can be addressed with this fresh talar allograft technique | Failure of proper bone graft shaping may lead to a negative postoperative outcome |
| No additional fixation is required after the graft is press fitted | A trained team is necessary to keep the anterior portal sterile while achieving a good position for the second part of the surgery |
| Changing of patient positioning allows for optimal viewing of each pathology |
HAGL, humeral avulsion of the glenohumeral ligament.
Rehabilitation
The patient is allowed to perform passive range of motion exercises on the scapular plane in the immediate postoperative period. However, the patient is restricted in internal rotation, shoulder flexion, and any posterior loading for 5 to 6 weeks. Following these initial weeks, formal functional rehabilitation with a physical therapist will begin.
Discussion
Posterior glenohumeral joint instability accounts for only a small subset of all known cases of instability. Therefore, it poses a diagnostic and treatment dilemma for orthopaedic surgeons.6, 7 Similar to anterior instability, patients with chronic or recurrent posterior instability may present with complaints of multiple subjective subluxation events, apprehension, or frank dislocation. These findings can lead to misdiagnosis and, in some cases, inappropriate treatment.8 In cases of multiple posterior dislocations, the likelihood of erosion of the posterior glenoid or anterior humerus (reverse HS lesions) increases.9 This places the shoulder at risk for pathologic bony instability secondary to loss of the primary static glenohumeral restraint.
Although surgical options for posterior glenoid deficiency have largely focused on open surgical approaches using autograft or allograft bone blocks,10, 11, 12, 13, 14 techniques to address the humeral-sided deficiency in the setting of a reverse HS lesion remain more variable and controversial. Although unengaging lesions measuring less than 25% of the articular surface of the humeral head may be amenable to nonsurgical treatment, some authors suggest that anterior impression fractures involving more than 50% of the articular surface of the humeral head should be treated either by hemiarthroplasty or total shoulder replacement. However, this may not be desirable in younger and more active patients.15, 16, 17, 18 This also reveals limited treatment options for patients with anterior humeral impaction lesions measuring greater than 25% and less than 50% of the humeral head, which likely require an alternative option to restore normal function. For these larger lesions, a wide variety of operations have been reported.
Surgical options to correct an engaging reverse HS lesion can be largely categorized into nonanatomic and anatomic reconstruction. These options can be performed either in isolation, or in combination with posterior soft tissue stabilization procedures. McLaughlin originally proposed a nonanatomic soft tissue procedure to prevent engagement with the anterior glenoid by transferring and securing the subscapularis tendon directly into the defect.19 Since this original description, several similar nonanatomic techniques have been proposed to address these defects in both open and arthroscopic fashion including the remplissage procedure by placing soft tissue structures into the impaction, which act to fill the defect and limit engagement against the glenoid.20, 21, 22
Recently, many authors have recommended an anatomic procedure that restores the normal contour of the humeral articular cartilage in lieu of remplissage-type procedures.16, 23, 24 These procedures originally attempted to elevate the anterior impaction similar to the reduction of a fracture. However, given the loss of integrity in the subchondral bone that occurs with these techniques as well as the persistent damage to the articular cartilage, many have begun to favor anatomic reconstruction with osteochondral allograft. The potential difficulty in using allograft tissue lies in the requirement for a matched donor to resemble the recipient's articular contour as well as prolonged wait times for recipients. Because of this, techniques using unmatched grafts have gained recent popularity. Prior evidence has suggested that the use of unmatched allograft tissue from remote locations in the body can closely match the anatomy of the glenoid and humeral surface. In this regard, the use of an unmatched hemitalar allograft may be used to reconstruct the humeral articular surface as described here.
Given this recent trend in clinical practice, we recommend our approach for anatomic reconstruction of the humeral head in the setting of a large engaging reverse HS lesion. In addition, patients with posterior instability almost always have soft tissue pathology, such as labrum, capsule, posterior HAGL, and rotator cuff lesions, which heighten symptoms of instability and pain. These soft tissue lesions should be addressed concurrently with any bony pathology. Doing so likely improves patient outcomes given that failure to address soft tissue pathology can lead to persistent symptoms of instability.5 Posterior HAGL lesions are often missed on preoperative magnetic resonance imaging and should be actively sought during diagnostic arthroscopy. Viewing from the anterosuperior portal can aid visualization of the posterior joint pathology. In the majority of cases, the presented technique would be completed in a revision setting following previous posterior stabilization procedures. However, this technique could also be considered in acute, traumatic cases with large reverse HS lesions. Future long-term studies with large cohorts are needed to assess the efficacy of this technique based on patient-reported outcome measures.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: G.M. has received research grants from Health South East, Norway, and from Arthrex; the financial support is not related to this work. M.T.P. is a consultant for Arthrex and JRF Ortho; has patents issued (9226743, 20150164498, 20150150594, 20110040339); and receives publishing royalties from Arthrex and SLACK.
Supplementary Data
The patient is positioned supine on the operating table while the right shoulder is placed in the lateral decubitus position for the initial, arthroscopic portion of the case. The patient is later repositioned and redraped in a modified beach chair position for the second, open portion of the case. A standard posterior portal is established with a 5-mm cannula followed by an anterior midglenoid portal. With the arthroscope in the posterior portal, pathology of the posterior labrum can be addressed from the 6- to 9-o'clock position with a series of elevators and shavers. The arthroscope is then removed and repositioned in the anterior portal to assess the glenohumeral ligaments. An elevator can be used to separate the capsule from the scarred muscle fibers. Then, side-to-side repair of the glenohumeral ligaments is performed with a suture lasso, no. 2 Ethibond sutures, and labral tape. The sutures are tied while the knots are left between the capsule and rotator cuff. The main portion of the HAGL repair is performed through placement of a 5.5-mm SwiveLock suture anchor in the humerus and using 2 FiberTapes to reapproximate the glenohumeral ligaments to the humerus. A thorough debridement of the humeral head should be performed to facilitate suture anchor placement. Once the HAGL lesion is repaired with capsular closure, any present labral pathology can be addressed. Following completion of the arthroscopic portion of the procedure, all wounds are closed in layered fashion with absorbable suture except the wound of the anterior portal, which is sterilely covered during repositioning to the beach chair position. With the patient in the supine position, the head of the bed is elevated to approximately 40°. The arm is then reprepped and redraped in standard sterile fashion. A deltopectoral approach is done and dissection is carried out down to the level of the conjoint tendon. Dissection is continued until the subscapularis tendon can be identified through the deltopectoral interval. Following this, the subacromial space is identified and debrided. At this time, the pectoralis minor tendon is also debrided using electrocautery and a key elevator. The subscapularis tendon (approximately two-thirds of the superior portion) and the anterior joint capsule are partially resected using electrocautery. At this point, the reverse Hill-Sachs lesion is identified, which may be filled in with scar tissue. The bony defect is then excised with an osteotome and smoothed with a power rasp to maximize healing potential in preparation of bone graft fixation. A fresh talus allograft is then molded and prepared for insertion into the humeral head Hill-Sachs defect. Then, the “orange slice” shaped graft is placed into the humeral defect. Once the graft is properly fixed through use of 2 headless compression screws, the subscapularis tendon is repaired using 3 medial FiberTape suture anchors in a bridge configuration with two 5.5-mm PEEK SwiveLock suture anchors. The anchor construct is then oversewed with no. 2 FiberWire suture. This ultimately results in a tape configuration double-row subscapularis repair. Following this, the wound is then closed in layered fashion with absorbable suture. The patient is then immobilized in a padded abduction sling. (HAGL, humeral avulsion of the glenohumeral ligament; PEEK, polyether ether ketone.)
References
- 1.Owens B.D., Campbell S.E., Cameron K.L. Risk factors for posterior shoulder instability in young athletes. Am J Sports Med. 2013;41:2645–2649. doi: 10.1177/0363546513501508. [DOI] [PubMed] [Google Scholar]
- 2.Owens B.D., Duffey M.L., Nelson B.J., DeBerardino T.M., Taylor D.C., Mountcastle S.B. The incidence and characteristics of shoulder instability at the United States Military Academy. Am J Sports Med. 2007;35:1168–1173. doi: 10.1177/0363546506295179. [DOI] [PubMed] [Google Scholar]
- 3.Saupe N., White L.M., Bleakney R. Acute traumatic posterior shoulder dislocation: MR findings. Radiology. 2008;248:185–193. doi: 10.1148/radiol.2481071003. [DOI] [PubMed] [Google Scholar]
- 4.Moroder P., Tauber M., Scheibel M. Defect characteristics of reverse Hill-Sachs lesions. Am J Sports Med. 2016;44:708–714. doi: 10.1177/0363546515621286. [DOI] [PubMed] [Google Scholar]
- 5.Bui-Mansfield L.T., Banks K.P., Taylor D.C. Humeral avulsion of the glenohumeral ligaments: The HAGL lesion. Am J Sports Med. 2007;35:1960–1966. doi: 10.1177/0363546507301081. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz E., Warren R.F., O'Brien S.J., Fronek J. Posterior shoulder instability. Orthop Clin North Am. 1987;18:409–419. [PubMed] [Google Scholar]
- 7.Provencher M.T., King S., Solomon D.J., Josh Bell S., Mologne Recurrent posterior shoulder instability: Diagnosis and management operative techniques in sports medicine. Oper Tech Sports Med. 2005;13:196–205. [Google Scholar]
- 8.Paul J., Buchmann S., Beitzel K., Solovyova O., Imhoff A.B. Posterior shoulder dislocation: Systematic review and treatment algorithm. Arthroscopy. 2011;27:1562–1572. doi: 10.1016/j.arthro.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Van Tongel A., Karelse A., Berghs B., Verdonk R., De Wilde L. Posterior shoulder instability: Current concepts review. Knee Surg Sports Traumatol Arthrosc. 2011;19:1547–1553. doi: 10.1007/s00167-010-1293-z. [DOI] [PubMed] [Google Scholar]
- 10.Gupta A.K., Chalmers P.N., Klosterman E., Harris J.D., Provencher M.T., Romeo A.A. Arthroscopic distal tibial allograft augmentation for posterior shoulder instability with glenoid bone loss. Arthrosc Tech. 2013;2:e405–e411. doi: 10.1016/j.eats.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank R.M., Provencher M.T., Romeo A.A. Arthroscopic reconstruction of posterior glenoid bone loss with distal tibia allograft. Orthop Today. 2015;35:14. (serial online) [Google Scholar]
- 12.Mulcahey M.K., Campbell K.J., Golijanan P., Gross D., Provencher M.T. Posterior bone grafting for glenoid defects of the shoulder. Oper Tech Sports Med. 2015;23:32–42. [Google Scholar]
- 13.Cvetanovich G.L., Bhatia S., Provencher M.T., Cole B.J. Treatment of bone defects in posterior instability. Oper Tech Sports Med. 2014;22:10–17. [Google Scholar]
- 14.Cofield R.H. Bone grafting for glenoid bone deficiencies in shoulder arthritis: A review. J Shoulder Elbow Surg. 2007;16(5 suppl):S273–S281. doi: 10.1016/j.jse.2007.03.005. (5 suppl) [DOI] [PubMed] [Google Scholar]
- 15.Checchia S.L., Santos P.D., Miyazaki A.N. Surgical treatment of acute and chronic posterior fracture-dislocation of the shoulder. J Shoulder Elbow Surg. 1998;7:53–65. doi: 10.1016/s1058-2746(98)90183-5. [DOI] [PubMed] [Google Scholar]
- 16.Gavriilidis I., Magosch P., Lichtenberg S., Habermeyer P., Kircher J. Chronic locked posterior shoulder dislocation with severe head involvement. Int Orthop. 2010;34:79–84. doi: 10.1007/s00264-009-0762-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawkins R.J., Neer C.S., 2nd, Pianta R.M., Mendoza F.X. Locked posterior dislocation of the shoulder. J Bone Joint Surg Am. 1987;69:9–18. [PubMed] [Google Scholar]
- 18.Robinson C.M., Aderinto J. Posterior shoulder dislocations and fracture-dislocations. J Bone Joint Surg Am. 2005;87:639–650. doi: 10.2106/JBJS.D.02371. [DOI] [PubMed] [Google Scholar]
- 19.McLaughlin H.L. Posterior dislocation of the shoulder. J Bone Joint Surg Am. 1952;24:584–590. [PubMed] [Google Scholar]
- 20.Martetschläger F., Padalecki J.R., Millett P.J. Modified arthroscopic McLaughlin procedure for treatment of posterior instability of the shoulder with an associated reverse Hill-Sachs lesion. Knee Surg Sports Traumatol Arthrosc. 2013;21:1642–1646. doi: 10.1007/s00167-012-2237-6. [DOI] [PubMed] [Google Scholar]
- 21.Krackhardt T., Schewe B., Albrecht D., Weise K. Arthroscopic fixation of the subscapularis tendon in the reverse Hill-Sachs lesion for traumatic unidirectional posterior dislocation of the shoulder. Arthroscopy. 2006;22:227.e1–227.e6. doi: 10.1016/j.arthro.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Lavender C.D., Hanzlik S.R., Pearson S.E., Caldwell P.E. Arthroscopic reverse remplissage for posterior instability. Arthrosc Tech. 2016;5:e43–e47. doi: 10.1016/j.eats.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuchs B., Jost B., Gerber C. Posterior-inferior capsular shift for the treatment of recurrent, voluntary posterior subluxation of the shoulder. J Bone Joint Surg Am. 2000;82:16–25. doi: 10.2106/00004623-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Gerber C., Lambert S.M. Allograft reconstruction of segmental defects of the humeral head for the treatment of chronic locked posterior dislocation of the shoulder. J Bone Joint Surg Am. 1996;78:376–382. doi: 10.2106/00004623-199603000-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The patient is positioned supine on the operating table while the right shoulder is placed in the lateral decubitus position for the initial, arthroscopic portion of the case. The patient is later repositioned and redraped in a modified beach chair position for the second, open portion of the case. A standard posterior portal is established with a 5-mm cannula followed by an anterior midglenoid portal. With the arthroscope in the posterior portal, pathology of the posterior labrum can be addressed from the 6- to 9-o'clock position with a series of elevators and shavers. The arthroscope is then removed and repositioned in the anterior portal to assess the glenohumeral ligaments. An elevator can be used to separate the capsule from the scarred muscle fibers. Then, side-to-side repair of the glenohumeral ligaments is performed with a suture lasso, no. 2 Ethibond sutures, and labral tape. The sutures are tied while the knots are left between the capsule and rotator cuff. The main portion of the HAGL repair is performed through placement of a 5.5-mm SwiveLock suture anchor in the humerus and using 2 FiberTapes to reapproximate the glenohumeral ligaments to the humerus. A thorough debridement of the humeral head should be performed to facilitate suture anchor placement. Once the HAGL lesion is repaired with capsular closure, any present labral pathology can be addressed. Following completion of the arthroscopic portion of the procedure, all wounds are closed in layered fashion with absorbable suture except the wound of the anterior portal, which is sterilely covered during repositioning to the beach chair position. With the patient in the supine position, the head of the bed is elevated to approximately 40°. The arm is then reprepped and redraped in standard sterile fashion. A deltopectoral approach is done and dissection is carried out down to the level of the conjoint tendon. Dissection is continued until the subscapularis tendon can be identified through the deltopectoral interval. Following this, the subacromial space is identified and debrided. At this time, the pectoralis minor tendon is also debrided using electrocautery and a key elevator. The subscapularis tendon (approximately two-thirds of the superior portion) and the anterior joint capsule are partially resected using electrocautery. At this point, the reverse Hill-Sachs lesion is identified, which may be filled in with scar tissue. The bony defect is then excised with an osteotome and smoothed with a power rasp to maximize healing potential in preparation of bone graft fixation. A fresh talus allograft is then molded and prepared for insertion into the humeral head Hill-Sachs defect. Then, the “orange slice” shaped graft is placed into the humeral defect. Once the graft is properly fixed through use of 2 headless compression screws, the subscapularis tendon is repaired using 3 medial FiberTape suture anchors in a bridge configuration with two 5.5-mm PEEK SwiveLock suture anchors. The anchor construct is then oversewed with no. 2 FiberWire suture. This ultimately results in a tape configuration double-row subscapularis repair. Following this, the wound is then closed in layered fashion with absorbable suture. The patient is then immobilized in a padded abduction sling. (HAGL, humeral avulsion of the glenohumeral ligament; PEEK, polyether ether ketone.)