Abstract
Corneal collagen cross-linking has become the preferred modality of treatment for corneal ectasia since its inception in late 1990s. Numerous studies have demonstrated the safety and efficacy of the conventional protocol. Our understanding of the cross-linking process is ever evolving, with its wide implications in the form of accelerated and pulsed protocols. Newer advancements in technology include various riboflavin formulations and the ability to deliver higher fluence protocols with customised irradiation patterns. A greater degree of customisation is likely the path forward, which will aim at achieving refractive improvements along with disease stability. The use of cross-linking for myopic correction is another avenue under exploration. Combination of half fluence cross-linking with refractive correction for high errors to prevent post LASIK regression is gaining interest. This review aims to highlight the various advancements in the cross-linking technology and its clinical applications.
Keywords: Accelerated cross-linking, corneal collagen cross-linking, customized cross-linking, photorefractive intrastromal cross-linking, pulsed cross-linking
Keratoconus is a degenerative condition associated with progressive corneal ectasia and thinning. Visual loss occurs following progressive myopia, irregular astigmatism, and corneal scarring.[1] Corneal collagen cross-linking was first described by Spoerl et al. as a modality for increasing the corneal biomechanical strength to halt disease progression.[2] Over the years, our understanding of the cross-linking process has evolved. Various advances have highlighted the opportunity to optimize the procedure improving efficacy and refractive outcomes.[3,4,5] These include new riboflavin formulations, higher ultraviolet A (UV-A) irradiance sources, and programmable UV-A patterns. The aim of this review is to detail upon the various advances and their clinical applications.
Accelerated Cross-linking
In 1998, corneal collagen cross-linking was first proposed as a treatment modality to stabilize the ectatic cornea.[2] The standard Dresden protocol entails UV-A treatment over a central 9 mm zone at an irradiance of 3.0 mW/cm2 for 30 min, delivering a fluence of 5.4 J/cm2.[6] Although various studies have demonstrated safety and efficacy of this protocol, an increased intraoperative time is a major drawback. Accelerated protocols have evolved in an attempt to overcome the limitations of conventional cross-linking, while maintaining the efficacy of results.
The Bunsen and Roscoe law of reciprocity states that the effect of a photochemical or photobiological reaction is directly proportional to the total irradiation dose, irrespective of the time span over which the dose is administered.[7] Thus, the same effect can be achieved by either applying a higher intensity for a shorter duration or a lower irradiation for a longer period. Stress–strain measurements of porcine corneal strips, comparing conventional with accelerated cross-linking (10 mW/cm2 for 9 min), demonstrated similar results in both groups.[8] Wernli et al. demonstrated a failure of the accelerated treatment for higher irradiance protocols (90 mW/cm2) in porcine eyes.[9] A significant difference in Young's modulus was noted between treatment groups up to 45 mW/cm2 and control group. However, treatment groups from 50 mw/cm2 up to 90 mW/cm2 demonstrated no significant difference. Hence, the Bunsen and Roscoe reciprocity law is valid only for illumination intensity up to 45 mW/cm2 and an irradiance time >2 min.
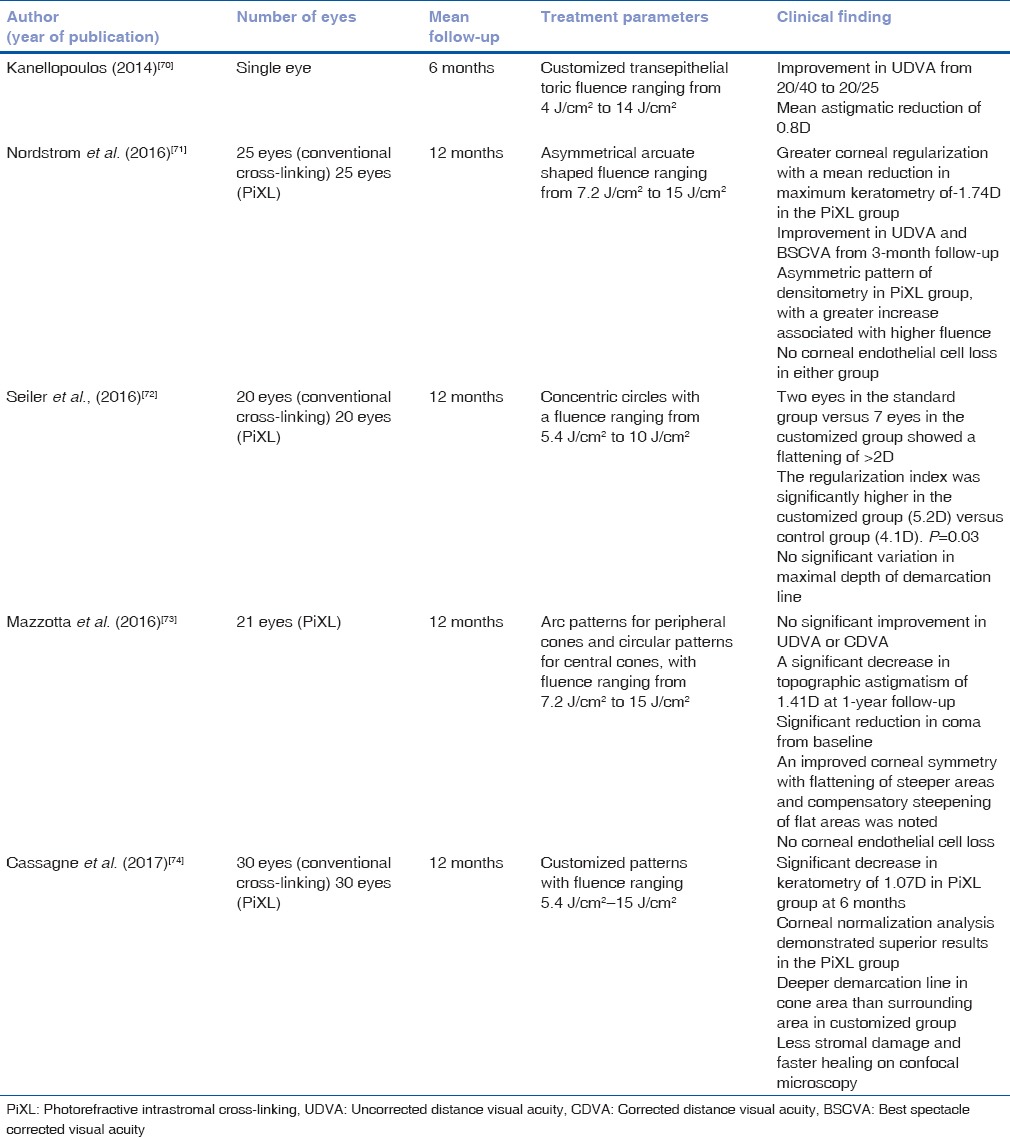
Various clinical studies demonstrated that the accelerated corneal cross-linking (CXL) provided stabilization of disease process along with significant flattening in certain protocols [Table 1].
Table 1.
Clinical results of accelerated cross-linking
Refractive and keratometric stability
The first clinical results were described by Kanellopoulos comparing conventional cross-linking to the accelerated protocol (7 mW/cm2 for 15 min) in 42 eyes with a mean follow-up of 46 months.[10] The study demonstrated a similar efficacy and refractive effect between the two groups, with no adverse effects.
Since then, numerous studies have been published using varied irradiation intensities and exposure time, with a standard total fluence of 5.4 J/cm2.
Corneal stability following a 10-min irradiation at 9 mW/cm2 was demonstrated in multiple studies with a significant improvement in visual acuity and keratometric values.[11,12,13] Similar results were noted in cases of pediatric keratoconus.[14] Shetty et al. described a posttreatment disease progression in three eyes over a 2-year follow-up.[15]
Irradiance with 18 mW/cm2 for 5 min demonstrated efficacy in halting disease progression; however, the topographic flattening was lower as compared to conventional CXL.[16,17,18]
Similar results were noted with the 3 min-30 mW/cm2 protocol.[19,20,21] Mita et al. reported loss of one line of UDVA and CDVA in two eyes each. The exact cause of visual loss was not explained.[22] Ozgurhan et al. demonstrated significant improvements in visual acuity, keratometry, and aberrations in pediatric patients.[23]
Depth of demarcation line
The demarcation line represents the transition zone between the anterior cross-linked stroma and the posterior untreated stroma. It can be appreciated at a depth of around 300 μ, as early as 2 weeks postoperatively.[24] Studies, comparing the depth of demarcation line on anterior segment optical coherence tomography (AS-OCT) in conventional and accelerated approaches while delivering the same total fluence, demonstrated greater depth in the conventional group.[25,26] As the extent of demarcation line is considered a surrogate marker for the depth of treatment, superior results of the standard protocol in comparison to the accelerated approach may be assumed. With a greater total fluence of 7.5 J/cm2 and increased riboflavin presoak, similar depths as conventional CXL were attained.[27,28,29]
While comparing the results of different accelerated protocols, a deeper and well-defined demarcation line was noted in the 3 mW/cm2 and 9 mW/cm2 group and was patchy and shallow with higher irradiances.[30] Since the anterior stromal fibers attribute to a majority of the biomechanical strength, a shallower demarcation line may still prove adequate to prevent disease progression. Long-term studies are required to further validate the results.
In conclusion, accelerated cross-linking significantly shortens the procedural time and reduces the patient discomfort. Moreover, as the UV-A irradiation time is significantly lower with the accelerated protocols, it avoids excessive stromal thinning and subsequent endothelial damage intraoperative.[31]
Pulsed Cross-linking
Shetty et al. compared the results following conventional cross-linking and accelerated protocols.[30] They demonstrated greater refractive and keratometric efficacy in the 3 mW/cm2 and 9 mW/cm2 groups as compared to the higher irradiation protocols. Moreover, a deeper and more well-defined demarcation line was observed.
Sufficient penetration of riboflavin, UV-A irradiation, and presence of oxygen are required for an effective stromal cross-linking.[2] The physiochemical basis of cross-linking lies in the photodynamic Type I and II reactions. The latter mediates cross-link formation via reactive oxygen species. It has been hypothesized that a more rapid oxygen depletion with accelerated protocols leads to a reduced efficacy.[32] Pulsed delivery of UV-A irradiation with a predetermined on and off pattern would enable better diffusion of oxygen into the stroma and a subsequent greater effect. Numerous studies compared the results of continuous versus pulsed irradiance in the accelerated protocols. A deeper demarcation line and higher apoptotic effect were noted with the pulsed approach.[33,34,35,36] Peyman et al. demonstrated a significantly deeper demarcation line following the pulsed approach (1 s on 1 s off) as compared to the 4 min of highly accelerated continuous UV-A irradiation.[33] Similar results were demonstrated by Moramarco et al. comparing the results in sixty eyes. The mean depth of demarcation line on AS-OCT in the pulsed group was 213 ± 47.38 μ as against 149.32 ± 36.03 μ in the group receiving continuous irradiation.[34] Mazzotta et al. confirmed similar findings on confocal microscopy.[35]
Although pulsed cross-linking shows promising results, the exact duration of pulsing is a question that remains unanswered. The rate of oxygen depletion in the Type II reaction is 15–20 s.[32] On the other hand, the normal tissue levels of oxygen are restored within 3–4 min of UV-A cessation. Therefore, further studies are required to determine the ideal pulsing approach.
Laser In situ Keratomileusis Xtra
Laser vision correction with concomitant cross-linking
Laser in situ keratomileusis (LASIK) is one of the more commonly performed ocular surgeries in the world. It has over the past few decades provided rapid visual recovery with improvement in quality of life and adequate patient satisfaction.[37] Despite the excellent immediate postoperative results, the long-term visual outcomes, especially in high refractive errors, have been less satisfactory due to regression of the refractive effect leading to high rate of enhancement procedures.[38] This is in part due to the corneal weakening or reduced biomechanical strength following flap creation and stromal ablation. In rare situations, corneal weakening leads to ectasia with subsequent degradation of vision.[39]
Over the past decade, corneal collagen cross-linking has become a mainstay of treatment for arresting the progression of keratoconus. CXL has shown to enhance corneal stiffening in both animal studies and clinical practice.[40,41] It does appear intuitive to combine the two procedures wherein LASIK improves quality of vision but reduces biomechanical strength of the cornea which could be partially or completely compensated by the CXL induced strengthening.
However, there are some concerns about combining the two techniques. Collagen cross-linking in addition to increasing corneal rigidity also induces a flattening effect.[42] This could limit the refractive accuracy by inducing long-term flattening and resultant overcorrection or hyperopic shift. In addition, the development of post-CXL stromal haze can further deteriorate visual quality. The advent of accelerated protocols has helped reduce treatment time by delivering higher irradiance in shorter duration. Numerous studies demonstrate the efficacy of accelerated cross-linking in stabilizing the cornea with a better safety profile as compared to conventional treatment.[10,21]
Laser in situ keratomileusis Xtra procedure
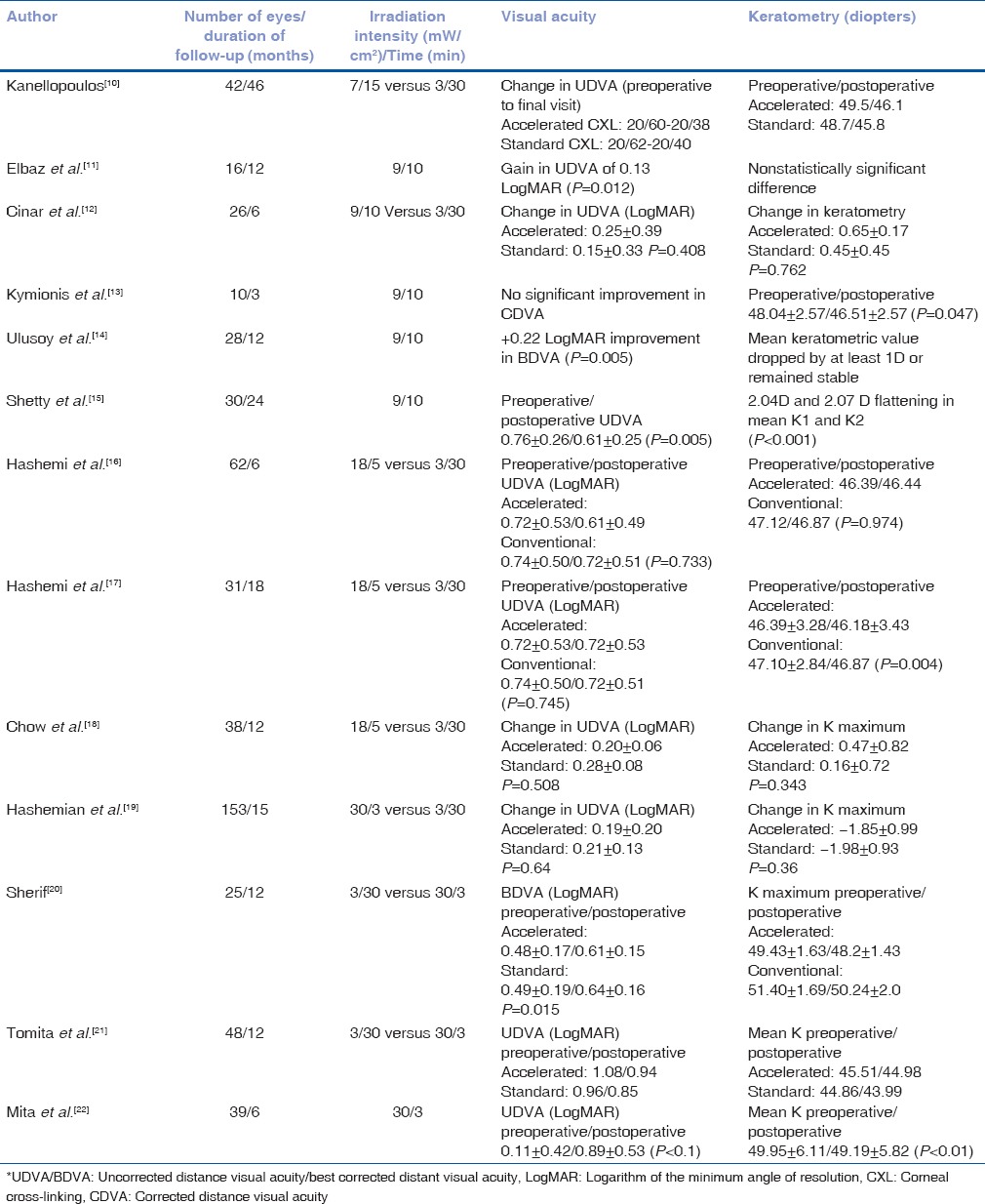
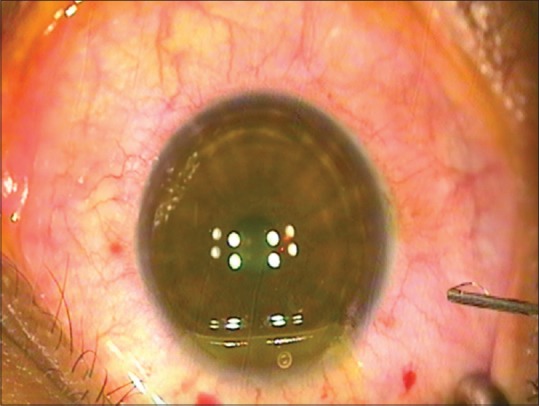
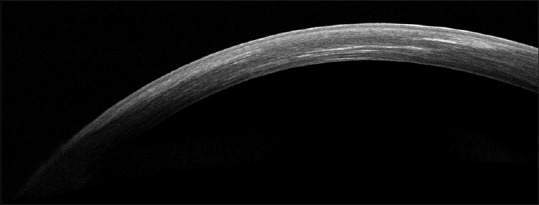
The LASIK XTRA procedure entails the administration of half fluence high irradiance cross-linking subsequent to refractive correction. A higher concentration 0.25% riboflavin is applied on the stromal bed subsequent to excimer laser ablation with a soak time of 90 s [Fig. 1]. The interface is washed thoroughly and the flap is repositioned. UV-A irradiance is delivered as a homogenous beam of 30 mW/cm2 for 90 s to deliver a total fluence of 2.7J/cm2. This is precisely half the energy delivered during conventional cross-linking in the Dresden protocol. The goal of cross-linking in this accelerated half fluence format is to restore or improve corneal strength without inducing a refractive change. The riboflavin is instilled on the stromal bed and not through the flap as it obviates the need for deepithelization to promote riboflavin diffusion. The flap itself does not contribute to the residual biomechanical strength, and therefore, cross-linking the flap would provide no advantage. In addition, cross-linking the flap may lead to subsequent shrinkage with undesirable consequences. AS-OCT is a useful tool to demonstrate thickness of flap and depth of demarcation line [Fig. 2].
Figure 1.
Instillation of 0.25% riboflavin (VibeX Xtra, Avedro, Inc.) on the stromal bed following excimer laser ablation
Figure 2.
Anterior segment optical coherence tomography demonstrating a well-apposed flap and the demarcation line in a case of femtosecond LASIK with half-fluence accelerated cross-linking
Clinical outcomes
Over the last few years, numerous studies have shown promising results with LASIK Xtra.[43]
Majority of the studies demonstrated greater stability of refraction. The incidence of post-LASIK regression is high in hyperopic eyes. Contralateral study comparing the results of LASIK with or without concomitant cross-linking in hyperopic eyes demonstrated a significantly lower regression in the LASIK Xtra group.[44] Encouraging results have been shown in the treatment of high myopia as well. Tan et al. compared the results of LASIK Xtra for high myopic correction (−8.0D–−19.0D) with a spherical equivalent matched historical cohort.[45] A greater refractive accuracy of the Xtra group was noted at 3 months with 98% of the eyes attaining a UDVA of 20/25 or better against 61% eyes in the LASIK group. A longitudinal observational study of 140 eyes with a 2-year follow-up showed lower refractive shift and greater keratometric stability in the Xtra group.[46]
The application of CXL along with refractive correction also had an influence on epithelial remodeling post-LASIK, especially while treating higher degrees of myopia. A study showed a significantly lower increase in midperipheral thickness when LASIK was combined with CXL (3.79 μ) as compared to LASIK alone (9.32 μ).[47] A greater refractive stability was noted with no progressive flattening. The procedure demonstrated a good safety profile with stable endothelial cell count and no visually significant haze development.[48] In a large study of 601 eyes, a stable uncorrected visual acuity with no significant changes in spherical equivalent or keratometry was noted at 1-year follow-up.[49]
In summary, LASIK with concomitant half fluence cross-linking is a promising treatment modality with significant improvement in refractive stability and possible reduced incidence of post-LASIK regression.
Cross-linking in Thin Corneas
Standard Dresden protocol mandates a minimal corneal thickness of 400 μ following epithelial debridement. This would limit the UV irradiance at the endothelial level to 0.18 mW/cm2, which is at least a factor of 2 smaller than the damage threshold level. Unfortunately, a number of patients have thin corneas often below the threshold of safety guidelines, making the disease not amenable to traditional cross-linking.[50]
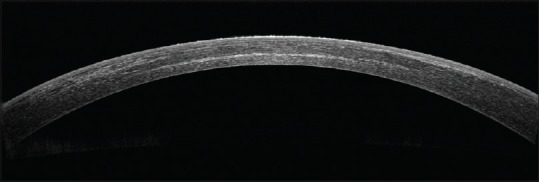
Various techniques have been described to overcome these limitations. Hypoosmolar cross-linking describes the instillation of hypotonic riboflavin to increase the corneal hydration and thickness intraoperatively.[51] Increased intraoperative time and a relatively lower concentration of collagen in the hydrated stoma are some of the limitations of this technique. Transepithelial cross-linking was introduced to prevent the adverse effects associated with epithelial debridement as well as a possible role in thinner corneas.[52] However, long-term studies demonstrated unsatisfactory results perhaps due to limited penetration of riboflavin.[53] Customized pachymetry-guided epithelial debridement entails preservation of the epithelium over the thinnest cone or area of maximal topographical steepening.[54] However, this technique demonstrated limited penetration with a demarcation line at 150 μ.[55] Jacob et al. described the use of a riboflavin-soaked bandage contact lens to augment the corneal thickness by roughly 100 μ.[56] However, the absorption properties of contact lenses differ from that of the corneal stroma. Moreover, inability to customize the contact lens thickness and intraoperative buckling were some of the associated limitations. Long-term results of this procedure are not available. Sachdev et al. described the intraoperative augmentation of stromal thickness, using refractive lenticules obtained from patients undergoing small incision lenticule extraction for myopic correction.[57] In this modified technique, the thickness of the corneal stroma is increased in the most physiological manner. Placement of the central lenticule over the apex of the cone enables one to augment the corneal thickness where required while sparing the remaining stroma to be cross-linked normally. Moreover, the relatively rough host stromal surface allows the lenticule to spread easily and buckling is avoided [Fig. 3].
Figure 3.
Anterior segment optical coherence tomography after placement of stromal lenticule and initial riboflavin soakage
In addition, the use of hydroxypropyl methylcellulose riboflavin prevents corneal dehydration induced by dextran and is more suitable for thin corneas.[58] The principle of customizing UV-A irradiance to the stromal thickness is evolving as the latest technique for thin corneas (adapted fluence). In conclusion, various advancements in procedures enable safe cross-linking in suboptimal corneal thickness.
Transepithelial Cross-linking
Conventional cross-linking entails epithelial debridement to achieve greater riboflavin penetration, which is otherwise impeded by epithelial tight junctions. Postoperative pain, increased risk of haze, and infection are associated limitations. Transepithelial application of riboflavin with numerous techniques to modify the epithelial permeability has been described including pharmacological cleavage of tight junctions and application via intrastromal pocket.[59,60,61] Although the transepithelial approach demonstrated fewer complications, efficacy was lower as compared to conventional treatment particularly in stabilizing or improving keratometry.[62]
Iontophoresis is a noninvasive technique that allows transepithelial riboflavin penetration following application of a mild electric current. Riboflavin is a negatively charged, water-soluble molecule with a relatively low molecular weight making it suitable for iontophoresis.[63,64] Numerous studies demonstrated stabilization of the disease process following iontophoresis-assisted CXL (I-CXL).[65] However, the keratometric regression was lower as compared to conventional epi-off cross-linking.[66] Similar results were noted in cases of pediatric keratoconus over a 15-month follow-up period.[67] The depth of the demarcation line noted was lower as compared to standard cross-linking. In a study by Mastropasqua et al., I-CXL demonstrated deeper saturation of riboflavin with respect to conventional epi-on but did not reach the concentrations with standard epi-off.[68] Although I-CXL-assisted riboflavin delivery is lower than the conventional approach, the effective concentration to halt disease process is not yet established.
I-CXL has the potential to become a valid alternative treatment for keratoconus while reducing treatment time, postoperative patient discomfort, and risk of infection.
Further studies are needed to establish the mechanism and efficacy of this relatively new treatment modality.
Customized Corneal Collagen Cross-linking for Keratoconus: Improved Corneal Regularization to Maximize Visual Rehabilitation
Keratoconus is a disease clinically characterized by increased corneal curvature, reduced corneal thickness, and progressive topographic irregularity. However, it has been proposed that the biomechanical modification is focal in nature, rather than a uniform generalized weakening.[69] Roy and Dupps demonstrated differential biomechanical weakening in the area of the cone using three-dimensional finite element analysis model. They additionally concluded greater efficacy of smaller diameter cone-centric treatments for the reduction of corneal curvature and higher order aberrations.[5]
Clinical application of this principle would require UV-A irradiation in customized treatment patterns localized on specific corneal zones. The Mosaic delivery system (KXL II, Avedro Inc., Waltham, MA, USA) uses an advanced pupillary tracking mechanism to offer customized cross-linking (photorefractive intrastromal cross-linking). The Mosaic device has been afforded the CE mark in Europe and Health Canada Approval.
Kanellopoulos et al. first described a case of customized cross-linking to achieve refractive results in progressive keratoconus.[70] UV-A irradiation was applied in a customized toric pattern using the transepithelial approach. A mean astigmatic reduction of 0.8D with a subsequent improvement in uncorrected visual acuity from 20/40 to 20/25 was noted at 6 months.
Various other studies have been published since demonstrating the results of the customized approach.[71,72,73,74] The use of varying treatment patterns including customized toric, asymmetric arcuates, and concentric circles has been described. An important aspect is to determine the localization of the irradiation patterns. In the previous studies, the treatment was centered on the area of greatest curvature. However, centration of irradiation around the maximum point of posterior float elevation may be more intuitive, since pachymetry as well as curvature is modulated by epithelial thickness and tear film.
Table 2 summarizes the results of customized CXL.
Table 2.
Clinical results of customized corneal collagen-cross-linking
Customized CXL may offer several advantages over the conventional approach. A greater corneal surface normalization leads to superior visual results. The propensity for haze formation was similar to conventional treatment despite the application of higher fluence. Fig. 4 demonstrates a Gaussian distribution of demarcation line on AS-OCT.
Figure 4.
Anterior segment optical coherence tomography demonstrates greater depth of demarcation line in the area of higher fluence delivery, with shallower demarcation line peripherally
Moreover, a paracentral treatment with subsequent haze will have a significantly lower impact than if the haze was central following conventional treatment. A reduced treatment zone with a smaller area of epithelial debridement would reduce postoperative discomfort and risk of infection.
In conclusion, customized CXL is a promising treatment modality. Studies are required to further customize the treatment approach and optimize outcome.
Photorefractive Intrastromal Cross-linking: High-Fluence Corneal Collagen Cross-linking for Low Myopia
Customized cross-linking for refractive correction is an emerging concept. It offers a nonincisional, nonablative treatment approach involving high-fluence irradiation to induce subsequent flattening and refractive correction. The UVA irradiation is delivered by the Mosaic device (Avedro Inc., Waltham, MA, USA) which uses advanced pupil tracking technology to deliver a customized treatment. The Mosaic device has been afforded the CE mark in Europe and the Health Canada Approval.
Kanellopoulos et al. described the preliminary results of transepithelial cross-linking for low myopic correction.[75] High-fluence UV-A irradiation of 12 J/cm2 was delivered as a customized central treatment using the KXL II system (Avedro, Waltham, MA, USA). Refractive and keratometric changes were demonstrated over a 6-month follow-up period. An average corneal flattening of 2.3 D at 1 week, with subsequent regression and stabilization to 1.4 D was noted at 1 month. No significant endothelial cell loss was noted despite the application of higher fluence.
Lim et al. demonstrated the results in a cohort of 14 eyes with a 1-year follow-up period.[76] High-fluence UV-A irradiation ranging from 10 to 15 J/cm2 was delivered over a 4.5 mm central zone. At 12 months' postprocedure, a mean reduction of 0.72 ± 0.43D was noted in the mean residual spherical error (P < 0.001). No significant regression was noted over the 1-year follow-up. Transient corneal haze subsided gradually, with no loss in best-corrected visual acuity.
PiXL for hyperopia has also been reported by Kanellopoulos and Asimellis, with a mean hyperopic correction of +0.85 D using an epithelial-on approach.[77]
Further studies are underway to determine the results of this potentially revolutionary refractive procedure. Possible advances include the development of a nomogram for astigmatic correction, addition of supplemental oxygen to enhance the efficacy of epithelium-on approach, and topographically guided treatment patterns.
Future Directions
Adapted fluence: Cross-linking in thin corneas
The standard Dresden protocol for cross-linking recommends a minimal postdebridement thickness of 400 μ, to prevent irradiation damage to the corneal endothelium.[6]
Lack of sufficient thickness for UVA absorption and attenuation is a limiting factor in thin corneas. A number of techniques with varying degrees of efficacy have been described for cross-linking the thin corneas. The three main factors than can be altered in the cross-linking process include stromal thickness, riboflavin concentration, and UV-A irradiation. However, the techniques described thus far employ the principle of increasing corneal thickness through tissue augmentation or stromal hydration.[51,56,57,78,79]
The UV-A fluence delivered in all techniques was a constant 5.4 J/cm2. Hafezi and Kling describe the principle of adapted fluence wherein customized energy is delivered by altering the irradiation time (UVA irradiation of 3 mW/cm2 for a customized irradiation time).[80] The process can be tailored based on the corneal thickness to deliver a cross-linking effect with an adequate safety zone. This eliminates the need for varying riboflavin concentrations and overcomes the limitations associated with earlier described techniques. However, the concept of adapted fluence requires validation through clinical trials before widespread use.
Scleral cross-linking for axial myopia
Scleral thinning and subsequent weakening results in axial length elongation and progressive myopia. Scleral cross-linking (SXL) using photosensitizer and blue light to mechanically reinforce the sclera may prevent progression in such cases.
Kwok et al. described the application of flexible optical waveguides around the equatorial region to induce SXL in porcine eyes.[81] They demonstrated a significant increase in Young's modulus with similar results in proximally and distally treated halves. The elastomer material with the linearly tapering design allowed a more homogenous light delivery and reduced thermal injury in comparison to earlier techniques.[82,83,84]
In vivo studies are needed to demonstrate the safety and efficacy of this method.
Photoactivated chromophore for infectious keratitis – corneal collagen crosslinking (PACK-CXL)
In addition to corneal strength augmentation, the cross-linking procedure demonstrates significant cytotoxic effect against living cells and microorganisms. UV-A irradiation has a known antimicrobial effect against bacteria, viruses, and microbes. In addition, riboflavin when photoactivated produces reactive oxygen species with subsequent microbicidal effect.[85,86] The combination of both produces cytotoxic effect which is drastically greater than the simple additive action.[87,88]
The application of CXL for keratitis was demonstrated at first for noninfectious and infectious corneal melts resistant to therapy.[89,90] The indications were subsequently extended to infectious keratitis as the first line of management in 2011.[91] Various studies demonstrated promising results for infectious keratitis excluding herpetic etiology. In addition, the results in bacterial and Acanthamoeba were superior to fungal keratitis.[92]
Further studies are required to compare the efficacy of this novel approach with traditional antimicrobial therapy in the treatment of corneal infections.
Conclusion
Cross-linking is still an evolving technology whose full potential is yet to be realized. The newer accelerated protocols and combination treatments have opened up a multitude of avenues with far reaching implications on the way we approach ectasia and refractive surgery. A greater degree of customization of treatments is likely the path forward which will enable us to achieve better refractive outcomes while maintaining a high level of safety.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Vazirani J, Basu S. Keratoconus: Current perspectives. Clin Ophthalmol. 2013;7:2019–30. doi: 10.2147/OPTH.S50119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spoerl E, Huhle M, Seiler T. Induction of cross-links in corneal tissue. Exp Eye Res. 1998;66:97–103. doi: 10.1006/exer.1997.0410. [DOI] [PubMed] [Google Scholar]
- 3.Schumacher S, Mrochen M, Wernli J, Bueeler M, Seiler T. Optimization model for UV-riboflavin corneal cross-linking. Invest Ophthalmol Vis Sci. 2012;53:762–9. doi: 10.1167/iovs.11-8059. [DOI] [PubMed] [Google Scholar]
- 4.Pertaub R, Friedman MD, Eddington WA. Computer modelling study of corneal cross-linking with riboflavin. Invest Ophthalmol Vis Sci. 2012;53:30. doi: 10.1167/iovs.11-9385. E-Abstract 6814. [DOI] [PubMed] [Google Scholar]
- 5.Roy AS, Dupps WJ., Jr Patient-specific computational modeling of keratoconus progression and differential responses to collagen cross-linking. Invest Ophthalmol Vis Sci. 2011;52:9174–87. doi: 10.1167/iovs.11-7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–7. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 7.Bunsen RW, Roscoe HE. Photochemical researches – Part V. On the measurement of the chemical action of direct and diffuse sunlight. Proc R Soc Lond. 1862;12:306–12. [Google Scholar]
- 8.Schumacher S, Oeftiger L, Mrochen M. Equivalence of biomechanical changes induced by rapid and standard corneal cross-linking, using riboflavin and ultraviolet radiation. Invest Ophthalmol Vis Sci. 2011;52:9048–52. doi: 10.1167/iovs.11-7818. [DOI] [PubMed] [Google Scholar]
- 9.Wernli J, Schumacher S, Spoerl E, Mrochen M. The efficacy of corneal cross-linking shows a sudden decrease with very high intensity UV light and short treatment time. Invest Ophthalmol Vis Sci. 2013;54:1176–80. doi: 10.1167/iovs.12-11409. [DOI] [PubMed] [Google Scholar]
- 10.Kanellopoulos AJ. Long term results of a prospective randomized bilateral eye comparison trial of higher fluence, shorter duration ultraviolet A radiation, and riboflavin collagen cross linking for progressive keratoconus. Clin Ophthalmol. 2012;6:97–101. doi: 10.2147/OPTH.S27170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elbaz U, Shen C, Lichtinger A, Zauberman NA, Goldich Y, Chan CC, et al. Accelerated (9-mW/cm2) corneal collagen crosslinking for keratoconus-A 1-year follow-up. Cornea. 2014;33:769–73. doi: 10.1097/ICO.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 12.Cınar Y, Cingü AK, Türkcü FM, Çınar T, Yüksel H, Özkurt ZG, et al. Comparison of accelerated and conventional corneal collagen cross-linking for progressive keratoconus. Cutan Ocul Toxicol. 2014;33:218–22. doi: 10.3109/15569527.2013.834497. [DOI] [PubMed] [Google Scholar]
- 13.Kymionis GD, Grentzelos MA, Kankariya VP, Liakopoulos DA, Portaliou DM, Tsoulnaras KI, et al. Safety of high-intensity corneal collagen crosslinking. J Cataract Refract Surg. 2014;40:1337–40. doi: 10.1016/j.jcrs.2013.11.041. [DOI] [PubMed] [Google Scholar]
- 14.Ulusoy DM, Göktaş E, Duru N, Özköse A, Ataş M, Yuvacı İ, et al. Accelerated corneal crosslinking for treatment of progressive keratoconus in pediatric patients. Eur J Ophthalmol. 2017;27:319–25. doi: 10.5301/ejo.5000848. [DOI] [PubMed] [Google Scholar]
- 15.Shetty R, Nagaraja H, Jayadev C, Pahuja NK, Kurian Kummelil M, Nuijts RM, et al. Accelerated corneal collagen cross-linking in pediatric patients: Two-year follow-up results. Biomed Res Int. 2014;2014:894095. doi: 10.1155/2014/894095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashemi H, Fotouhi A, Miraftab M, Bahrmandy H, Seyedian MA, Amanzadeh K, et al. Short-term comparison of accelerated and standard methods of corneal collagen crosslinking. J Cataract Refract Surg. 2015;41:533–40. doi: 10.1016/j.jcrs.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 17.Hashemi H, Miraftab M, Seyedian MA, Hafezi F, Bahrmandy H, Heidarian S, et al. Long-term results of an accelerated corneal cross-linking protocol (18 mW/cm2) for the treatment of progressive keratoconus. Am J Ophthalmol. 2015;160:1164–700. doi: 10.1016/j.ajo.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 18.Chow VW, Chan TC, Yu M, Wong VW, Jhanji V. One-year outcomes of conventional and accelerated collagen crosslinking in progressive keratoconus. Sci Rep. 2015;5:14425. doi: 10.1038/srep14425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashemian H, Jabbarvand M, Khodaparast M, Ameli K. Evaluation of corneal changes after conventional versus accelerated corneal cross-linking: A randomized controlled trial. J Refract Surg. 2014;30:837–42. doi: 10.3928/1081597X-20141117-02. [DOI] [PubMed] [Google Scholar]
- 20.Sherif AM. Accelerated versus conventional corneal collagen cross-linking in the treatment of mild keratoconus: A comparative study. Clin Ophthalmol. 2014;8:1435–40. doi: 10.2147/OPTH.S59840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomita M, Mita M, Huseynova T. Accelerated versus conventional corneal collagen crosslinking. J Cataract Refract Surg. 2014;40:1013–20. doi: 10.1016/j.jcrs.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 22.Mita M, Waring GO, 4th, Tomita M. High-irradiance accelerated collagen crosslinking for the treatment of keratoconus: Six-month results. J Cataract Refract Surg. 2014;40:1032–40. doi: 10.1016/j.jcrs.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 23.Ozgurhan EB, Kara N, Cankaya KI, Kurt T, Demirok A. Accelerated corneal cross-linking in pediatric patients with keratoconus: 24-month outcomes. J Refract Surg. 2014;30:843–9. doi: 10.3928/1081597X-20141120-01. [DOI] [PubMed] [Google Scholar]
- 24.Seiler T, Hafezi F. Corneal cross-linking-induced stromal demarcation line. Cornea. 2006;25:1057–9. doi: 10.1097/01.ico.0000225720.38748.58. [DOI] [PubMed] [Google Scholar]
- 25.Brittingham S, Tappeiner C, Frueh BE. Corneal cross-linking in keratoconus using the standard and rapid treatment protocol: Differences in demarcation line and 12-month outcomes. Invest Ophthalmol Vis Sci. 2014;55:8371–6. doi: 10.1167/iovs.14-15444. [DOI] [PubMed] [Google Scholar]
- 26.Kymionis GD, Tsoulnaras KI, Grentzelos MA, Plaka AD, Mikropoulos DG, Liakopoulos DA, et al. Corneal stroma demarcation line after standard and high-intensity collagen crosslinking determined with anterior segment optical coherence tomography. J Cataract Refract Surg. 2014;40:736–40. doi: 10.1016/j.jcrs.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 27.Kymionis GD, Tsoulnaras KI, Liakopoulos DA, Skatharoudi CA, Grentzelos MA, Tsakalis NG. Corneal stromal demarcation line depth following standard and a modified high intensity corneal collagen crosslinking protocol. J Refract Surg. 2016;32:218–22. doi: 10.3928/1081597X-20160216-01. [DOI] [PubMed] [Google Scholar]
- 28.Kymionis GD, Tsoulnaras KI, Grentzelos MA, Liakopoulos DA, Tsakalis NG, Blazaki SV, et al. Evaluation of corneal stromal demarcation line depth following standard and a modified-accelerated collagen cross-linking protocol. Am J Ophthalmol. 2014;158:671–50. doi: 10.1016/j.ajo.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Ozgurhan EB, Sezgin Akcay BI, Yildirim Y, Karatas G, Kurt T, Demirok A, et al. Evaluation of corneal stromal demarcation line after two different protocols of accelerated corneal collagen cross-linking procedures using anterior segment optical coherence tomography and confocal microscopy. J Ophthalmol. 2014;2014:981893. doi: 10.1155/2014/981893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shetty R, Pahuja NK, Nuijts RM, Ajani A, Jayadev C, Sharma C, et al. Current protocols of corneal collagen cross-linking: Visual, refractive, and tomographic outcomes. Am J Ophthalmol. 2015;160:243–9. doi: 10.1016/j.ajo.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 31.Holopainen JM, Krootila K. Transient corneal thinning in eyes undergoing corneal cross-linking. Am J Ophthalmol. 2011;152:533–6. doi: 10.1016/j.ajo.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 32.Kamaev P, Friedman MD, Sherr E, Muller D. Photochemical kinetics of corneal cross-linking with riboflavin. Invest Ophthalmol Vis Sci. 2012;53:2360–7. doi: 10.1167/iovs.11-9385. [DOI] [PubMed] [Google Scholar]
- 33.Peyman A, Nouralishahi A, Hafezi F, Kling S, Peyman M. Stromal demarcation line in pulsed versus continuous light accelerated corneal cross-linking for keratoconus. J Refract Surg. 2016;32:206–8. doi: 10.3928/1081597X-20160204-03. [DOI] [PubMed] [Google Scholar]
- 34.Moramarco A, Iovieno A, Sartori A, Fontana L. Corneal stromal demarcation line after accelerated crosslinking using continuous and pulsed light. J Cataract Refract Surg. 2015;41:2546–51. doi: 10.1016/j.jcrs.2015.04.033. [DOI] [PubMed] [Google Scholar]
- 35.Mazzotta C, Traversi C, Paradiso AL, Latronico ME, Rechichi M. Pulsed light accelerated crosslinking versus continuous light accelerated crosslinking: One-year results. J Ophthalmol. 2014;2014:604731. doi: 10.1155/2014/604731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazzotta C, Traversi C, Caragiuli S, Rechichi M. Pulsed vs.continuous light accelerated corneal collagen crosslinking:In vivo qualitative investigation by confocal microscopy and corneal OCT. Eye (Lond) 2014;28:1179–83. doi: 10.1038/eye.2014.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Solomon KD, Fernández de Castro LE, Sandoval HP, Biber JM, Groat B, Neff KD, et al. LASIK world literature review: Quality of life and patient satisfaction. Ophthalmology. 2009;116:691–701. doi: 10.1016/j.ophtha.2008.12.037. [DOI] [PubMed] [Google Scholar]
- 38.Yuen LH, Chan WK, Koh J, Mehta JS, Tan DT, et al. SingLasik Research Group. A 10-year prospective audit of LASIK outcomes for myopia in 37,932 eyes at a single institution in asia. Ophthalmology. 2010;117:1236–440. doi: 10.1016/j.ophtha.2009.10.042. [DOI] [PubMed] [Google Scholar]
- 39.Binder PS. Analysis of ectasia after laser in situ keratomileusis: Risk factors. J Cataract Refract Surg. 2007;33:1530–8. doi: 10.1016/j.jcrs.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 40.Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. J Cataract Refract Surg. 2003;29:1780–5. doi: 10.1016/s0886-3350(03)00407-3. [DOI] [PubMed] [Google Scholar]
- 41.Scarcelli G, Kling S, Quijano E, Pineda R, Marcos S, Yun SH, et al. Brillouin microscopy of collagen crosslinking: Noncontact depth-dependent analysis of corneal elastic modulus. Invest Ophthalmol Vis Sci. 2013;54:1418–25. doi: 10.1167/iovs.12-11387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sorkin N, Varssano D. Corneal collagen crosslinking: A systematic review. Ophthalmologica. 2014;232:10–27. doi: 10.1159/000357979. [DOI] [PubMed] [Google Scholar]
- 43.Rajpal RK, Wisecarver CB, Williams D, Rajpal SD, Kerzner R, Nianiaris N, et al. Lasik xtra provides corneal stability and improved outcomes. Ophthalmol Ther. 2015;4:89–102. doi: 10.1007/s40123-015-0039-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanellopoulos AJ, Kahn J. Topography-guided hyperopic LASIK with and without high irradiance collagen cross-linking: Initial comparative clinical findings in a contralateral eye study of 34 consecutive patients. J Refract Surg. 2012;28:S837–40. doi: 10.3928/1081597X-20121005-05. [DOI] [PubMed] [Google Scholar]
- 45.Tan J, Lytle GE, Marshall J. Consecutive laser in situ keratomileusis and accelerated corneal crosslinking in highly myopic patients: Preliminary results. Eur J Ophthalmol. 2014;25:101–7. doi: 10.5301/ejo.5000543. [DOI] [PubMed] [Google Scholar]
- 46.Kanellopoulos AJ, Asimellis G. Combined laser in situ keratomileusis and prophylactic high-fluence corneal collagen crosslinking for high myopia: Two-year safety and efficacy. J Cataract Refract Surg. 2015;41:1426–33. doi: 10.1016/j.jcrs.2014.10.045. [DOI] [PubMed] [Google Scholar]
- 47.Kanellopoulos AJ, Asimellis G. Epithelial remodeling after femtosecond laser-assisted high myopic LASIK: Comparison of stand-alone with LASIK combined with prophylactic high-fluence cross-linking. Cornea. 2014;33:463–9. doi: 10.1097/ICO.0000000000000087. [DOI] [PubMed] [Google Scholar]
- 48.Tomita M, Yoshida Y, Yamamoto Y, Mita M, Waring G., 4th In vivo confocal laser microscopy of morphologic changes after simultaneous LASIK and accelerated collagen crosslinking for myopia: One-year results. J Cataract Refract Surg. 2014;40:981–90. doi: 10.1016/j.jcrs.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 49.Tomita M. LASIK Xtra in clinical practice. New Orleans, USA: Presentation at: American Academy of Ophthalmology; 2013. [Google Scholar]
- 50.Pearson AR, Soneji B, Sarvananthan N, Sandford-Smith JH. Does ethnic origin influence the incidence or severity of keratoconus? Eye (Lond) 2000;14(Pt 4):625–8. doi: 10.1038/eye.2000.154. [DOI] [PubMed] [Google Scholar]
- 51.Hafezi F, Mrochen M, Iseli HP, Seiler T. Collagen crosslinking with ultraviolet-A and hypoosmolar riboflavin solution in thin corneas. J Cataract Refract Surg. 2009;35:621–4. doi: 10.1016/j.jcrs.2008.10.060. [DOI] [PubMed] [Google Scholar]
- 52.Boxer Wachler BS, Pinelli R, Ertan A, Chan CC. Safety and efficacy of transepithelial crosslinking (C3-R/CXL) J Cataract Refract Surg. 2010;36:186–8. doi: 10.1016/j.jcrs.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 53.Soeters N, Wisse RP, Imhof SM. Transepithelial vs.epithelium off collagen cross linking for progressive keratoconus. A randomised control trial. Am J Ophthalmol. 2015;151:821–9. doi: 10.1016/j.ajo.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 54.Kymionis GD, Diakonis VF, Coskunseven E, Jankov M, Yoo SH, Pallikaris IG, et al. Customized pachymetric guided epithelial debridement for corneal collagen cross linking. BMC Ophthalmol. 2009;9:10. doi: 10.1186/1471-2415-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaya V, Utine CA, Yilmaz OF. Efficacy of corneal collagen cross-linking using a custom epithelial debridement technique in thin corneas: A confocal microscopy study. J Refract Surg. 2011;27:444–50. doi: 10.3928/1081597X-20101201-01. [DOI] [PubMed] [Google Scholar]
- 56.Jacob S, Kumar DA, Agarwal A, Basu S, Sinha P, Agarwal A, et al. Contact lens-assisted collagen cross-linking (CACXL): A new technique for cross-linking thin corneas. J Refract Surg. 2014;30:366–72. doi: 10.3928/1081597X-20140523-01. [DOI] [PubMed] [Google Scholar]
- 57.Sachdev MS, Gupta D, Sachdev G, Sachdev R. Tailored stromal expansion with a refractive lenticule for crosslinking the ultrathin cornea. J Cataract Refract Surg. 2015;41:918–23. doi: 10.1016/j.jcrs.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 58.Mazzota C, Baiochhi S, Caparossi T, Craquilli S, Paradiso AL, Caparossi A. Riboflavin 0.1% (vibex) for the treatment of keratoconus. Expert Opin Orphan Drugs. 2013;1:235–40. [Google Scholar]
- 59.Leccisotti A, Islam T. Transepithelial corneal collagen cross-linking in keratoconus. J Refract Surg. 2010;26:942–8. doi: 10.3928/1081597X-20100212-09. [DOI] [PubMed] [Google Scholar]
- 60.Kissner A, Spoerl E, Jung R, Spekl K, Pillunat LE, Raiskup F, et al. Pharmacological modification of the epithelial permeability by benzalkonium chloride in UVA/Riboflavin corneal collagen cross-linking. Curr Eye Res. 2010;35:715–21. doi: 10.3109/02713683.2010.481068. [DOI] [PubMed] [Google Scholar]
- 61.Kanellopoulos AJ. Collagen cross-linking in early keratoconus with riboflavin in a femtosecond laser-created pocket: Initial clinical results. J Refract Surg. 2009;25:1034–7. doi: 10.3928/1081597X-20090901-02. [DOI] [PubMed] [Google Scholar]
- 62.Shalchi Z, Wang X, Nanavaty MA. Safety and efficacy of epithelium removal and transepithelial corneal collagen crosslinking for keratoconus. Eye (Lond) 2015;29:15–29. doi: 10.1038/eye.2014.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bikbova G, Bikbov M. Transepithelial corneal collagen cross-linking by iontophoresis of riboflavin. Acta Ophthalmol. 2014;92:e30–4. doi: 10.1111/aos.12235. [DOI] [PubMed] [Google Scholar]
- 64.Vinciguerra P, Rechichi M, Rosetta P. High fluence iontophoretic corneal collagen crosslinking:In vivo OCT imaging of riboflavin penetration. J Refract Surg. 2013;29:376–7. doi: 10.3928/1081597X-20130509-01. [DOI] [PubMed] [Google Scholar]
- 65.Vinciguerra P, Randleman JB, Romano V, Legrottaglie EF, Rosetta P, Camesasca FI, et al. Transepithelial iontophoresis corneal collagen cross-linking for progressive keratoconus: Initial clinical outcomes. J Refract Surg. 2014;30:746–53. doi: 10.3928/1081597X-20141021-06. [DOI] [PubMed] [Google Scholar]
- 66.Bikbova G, Bikbov M. Standard corneal collagen crosslinking versus transepithelial iontophoresis-assisted corneal crosslinking, 24 months follow-up: Randomized control trial. Acta Ophthalmol. 2016;94:e600–6. doi: 10.1111/aos.13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Buzzonetti L, Petrocelli G, Valente P, Iarossi G, Ardia R, Petroni S, et al. Iontophoretic transepithelial corneal cross-linking to halt keratoconus in pediatric cases: 15-month follow-up. Cornea. 2015;34:512–5. doi: 10.1097/ICO.0000000000000410. [DOI] [PubMed] [Google Scholar]
- 68.Mastropasqua L, Nubile M, Calienno R, Mattei PA, Pedrotti E, Salgari N, et al. Corneal cross-linking: Intrastromal riboflavin concentration in iontophoresis-assisted imbibition versus traditional and transepithelial techniques. Am J Ophthalmol. 2014;157:623–30. doi: 10.1016/j.ajo.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 69.Roberts CJ, Dupps WJ., Jr Biomechanics of corneal ectasia and biomechanical treatments. J Cataract Refract Surg. 2014;40:991–8. doi: 10.1016/j.jcrs.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kanellopoulos AJ, Dupps WJ, Seven I, Asimellis G. Toric topographically customized transepithelial, pulsed, very high-fluence, higher energy and higher riboflavin concentration collagen cross-linking in keratoconus. Case Rep Ophthalmol. 2014;5:172–80. doi: 10.1159/000363371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nordström M, Schiller M, Fredriksson A, Behndig A. Refractive improvements and safety with topography-guided corneal crosslinking for keratoconus: 1-year results. Br J Ophthalmol. 2017;101:920–5. doi: 10.1136/bjophthalmol-2016-309210. [DOI] [PubMed] [Google Scholar]
- 72.Seiler TG, Fischinger I, Koller T, Zapp D, Frueh BE, Seiler T, et al. Customized corneal cross-linking: One-year results. Am J Ophthalmol. 2016;166:14–21. doi: 10.1016/j.ajo.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 73.Mazzotta C, Moramarco A, Traversi C, Baiocchi S, Iovieno A, Fontana L, et al. Accelerated corneal collagen cross-linking using topography-guided UV-A energy emission: Preliminary clinical and morphological outcomes. J Ophthalmol. 2016;2016:2031031. doi: 10.1155/2016/2031031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cassagne M, Pierné K, Galiacy SD, Asfaux-Marfaing MP, Fournié P, Malecaze F, et al. Customized topography-guided corneal collagen cross-linking for keratoconus. J Refract Surg. 2017;33:290–7. doi: 10.3928/1081597X-20170201-02. [DOI] [PubMed] [Google Scholar]
- 75.Kanellopoulos AJ. Novel myopic refractive correction with transepithelial very high-fluence collagen cross-linking applied in a customized pattern: Early clinical results of a feasibility study. Clin Ophthalmol. 2014;8:697–702. doi: 10.2147/OPTH.S59934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lim WK, Soh ZD, Choi HKY, Theng JTS. Epithelium-on photorefractive intrastromal cross-linking (PiXL) for reduction of low myopia. Clin Ophthalmol. 2017;11:1205–11. doi: 10.2147/OPTH.S137712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kanellopoulos AJ, Asimellis G. Hyperopic correction: Clinical validation with epithelium-on and epithelial-off protocols, using variable fluence and topographically customized collagen cross-linking. Clin Ophthalmol. 2014;8:2425–33. doi: 10.2147/OPTH.S68222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spadea L, Mencucci R. Transepithelial corneal collagen cross-linking in ultrathin keratoconic corneas. Clin Ophthalmol. 2012;6:1785–92. doi: 10.2147/OPTH.S37335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mazzotta C, Ramovecchi V. Customized epithelial debridement for thin ectatic corneas undergoing corneal cross-linking: Epithelial island cross-linking technique. Clin Ophthalmol. 2014;8:1337–43. doi: 10.2147/OPTH.S66372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hafezi F. New Nomogram for Extremely thin KC Corneas: Clinical. CXL Experts Meet. Zurich, Switzerland; 2.3 December. 2016 [Google Scholar]
- 81.Kwok SJJ, Kim M, Lin HH, Seiler TG, Beck E, Shao P, et al. Flexible optical waveguides for uniform periscleral cross-linking. Invest Ophthalmol Vis Sci. 2017;58:2596–602. doi: 10.1167/iovs.17-21559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Iseli HP, Körber N, Koch C, Karl A, Penk A, Huster D, et al. Scleral cross-linking by riboflavin and blue light application in young rabbits: Damage threshold and eye growth inhibition. Graefes Arch Clin Exp Ophthalmol. 2016;254:109–22. doi: 10.1007/s00417-015-3213-x. [DOI] [PubMed] [Google Scholar]
- 83.Liu S, Li S, Wang B, Lin X, Wu Y, Liu H, et al. Scleral cross-linking using riboflavin UVA irradiation for the prevention of myopia progression in a guinea pig model: Blocked axial extension and altered scleral microstructure. PLoS One. 2016;11:e0165792. doi: 10.1371/journal.pone.0165792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dotan A, Kremer I, Livnat T, Zigler A, Weinberger D, Bourla D, et al. Scleral cross-linking using riboflavin and ultraviolet-a radiation for prevention of progressive myopia in a rabbit model. Exp Eye Res. 2014;127:190–5. doi: 10.1016/j.exer.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 85.Tsugita A, Okada Y, Uehara K. Photosensitized inactivation of ribonucleic acids in the presence of riboflavin. Biochim Biophys Acta. 1965;103:360–3. doi: 10.1016/0005-2787(65)90182-6. [DOI] [PubMed] [Google Scholar]
- 86.Kumar V, Lockerbie O, Keil SD, Ruane PH, Platz MS, Martin CB, et al. Riboflavin and UV-light based pathogen reduction: Extent and consequence of DNA damage at the molecular level. Photochem Photobiol. 2004;80:15–21. doi: 10.1562/2003-12-23-RA-036.1. [DOI] [PubMed] [Google Scholar]
- 87.Martins SA, Combs JC, Noguera G, Camacho W, Wittmann P, Walther R, et al. Antimicrobial efficacy of riboflavin/UVA combination (365 nm) in vitro for bacterial and fungal isolates: A potential new treatment for infectious keratitis. Invest Ophthalmol Vis Sci. 2008;49:3402–8. doi: 10.1167/iovs.07-1592. [DOI] [PubMed] [Google Scholar]
- 88.Wollensak G, Spoerl E, Reber F, Seiler T. Keratocyte cytotoxicity of riboflavin/UVA-treatment in vitro. Eye (Lond) 2004;18:718–22. doi: 10.1038/sj.eye.6700751. [DOI] [PubMed] [Google Scholar]
- 89.Schnitzler E, Spörl E, Seiler T. Irradiation of cornea with ultraviolet light and riboflavin administration as a new treatment for erosive corneal processes, preliminary results in four patients. Klin Monbl Augenheilkd. 2000;217:190–3. doi: 10.1055/s-2000-10344. [DOI] [PubMed] [Google Scholar]
- 90.Iseli HP, Thiel MA, Hafezi F, Kampmeier J, Seiler T. Ultraviolet A/riboflavin corneal cross-linking for infectious keratitis associated with corneal melts. Cornea. 2008;27:590–4. doi: 10.1097/ICO.0b013e318169d698. [DOI] [PubMed] [Google Scholar]
- 91.Makdoumi K, Mortensen J, Sorkhabi O, Malmvall BE, Crafoord S. UVA-riboflavin photochemical therapy of bacterial keratitis: A pilot study. Graefes Arch Clin Exp Ophthalmol. 2012;250:95–102. doi: 10.1007/s00417-011-1754-1. [DOI] [PubMed] [Google Scholar]
- 92.Papaioannou L, Miligkos M, Papathanassiou M. Corneal collagen cross-linking for infectious keratitis: A Systematic review and meta-analysis. Cornea. 2016;35:62–71. doi: 10.1097/ICO.0000000000000644. [DOI] [PubMed] [Google Scholar]


