Abstract
Purpose:
To compare the outcomes of intraoperative wavefront aberrometry versus optical biometry alone for intraocular lens (IOL) power calculation in eyes undergoing cataract surgery with monofocal IOL implantation.
Methods:
Preoperative data were obtained with the IOLMaster. Intraoperative aphakic measurements and IOL power calculations were obtained in some patients with the optiwave refractive analysis (ORA) system. Analysis was performed to determine the accuracy of monofocal IOL power prediction and postoperative manifest refraction at 1 month of the ORA versus IOLMaster.
Results:
Two hundred and ninety-five eyes reviewed, 61 had only preoperative IOLMaster measurements and 234 had both IOLMaster and ORA measurements. Of these 234 eyes, 6 were excluded, 107 had the same recommended IOL power by ORA and IOLMaster. Sixty-four percent of these eyes were within ±0.5D. 95 eyes had IOL power implantation based on ORA instead of IOLMaster. Seventy percent of these eyes were within ±0.5D of target refraction. 26 eyes had IOL power chosen based on IOLMaster predictions instead of ORA. Sixty-five percent were within ±0.5D. In the group with IOLMaster without ORA measurements, 80% of eyes were within ±0.5D of target refraction. The absolute error was statistically smaller in those eyes where the ORA and IOLMaster recommended the same IOL power based on preoperative target refraction compared to instances in which IOL selection was based on ORA or IOLMaster alone. Neither prediction errors were statistically different between the ORA and IOLMaster alone.
Conclusion:
Intraoperative wavefront aberrometry with the ORA system provides postoperative refractive results comparable to conventional biometry with the IOLMaster for monofocal IOL selection.
Keywords: Intraocular lens master, intraoperative wavefront aberrometry, optiwave refractive analysis
Intraoperative wavefront aberrometry has become increasingly used in cataract surgery in recent years, with the goal of obtaining more predictable postoperative results. One such method is the optiwave refractive analysis (ORA) (Alcon, Fort Worth, TX, USA) system, which measures phakic, aphakic, and/or pseudophakic refraction at the time of cataract surgery.[1,2] The ORA system takes into account both the anterior and posterior corneal astigmatism, which may improve astigmatic outcomes by accounting for refractive contribution from the posterior cornea. Intraocular lens (IOL) power calculations using newer generation IOL formulae rely on preoperative biometry including axial length, keratometry, and often additional measurements such as white-to-white diameter and anterior chamber depth. The previous studies with older intraoperative wavefront aberrometry systems, ORange Gen 1 and 2 (WaveTec Vision Inc, Aliso Viejo, CA, USA) demonstrate similar accuracy compared to conventional biometry.[2,3] In addition, recent studies suggest that intraoperative wavefront aberrometry is useful for IOL power selection after refractive surgery[1,4,5] and may in some cases even be superior to conventional methods for determining IOL selection.[5]
The purpose of this study was to evaluate the accuracy of intraoperative wavefront aberrometry for IOL power selection in virgin eyes compared to conventional methods with partial coherence interferometry (IOLMaster, Carl Zeiss Meditec, Jena, Germany) using a surgeon's best choice method to select IOL power (i.e., surgeon's choice based on Holladay 1, SRK/T, and Hoffer Q formulas depending on the preoperative axial length and keratometry). Specifically, the goal of the study was to determine whether the use of ORA in addition to preoperative data from IOLMaster for IOL selection provides a more accurate postoperative result compared to using IOLMaster alone for monofocal IOL implantation.
Methods
This was a nonrandomized, consecutive retrospective study from a single center; one surgeon (SG) performed all the cases. Approval for this study was obtained from the Institutional Review Board of the University of California, Irvine, and the study was in accordance with the Helsinki Declaration of 1975, as revised in 2000.
Inclusion criteria were patients with monofocal IOL implants and those who also had postoperative manifest refraction (MRx) at approximately 1 month. Exclusion criteria included the presence of significant ocular comorbidities that could limit best corrected visual acuity, eyes with poor visual potential, corneal ectasias or opacities, inability to cooperate with postoperative refraction or insufficient follow-up, inability to fixate intraoperatively, previous refractive surgery, or intraoperative complications.
All patients underwent preoperative biometry with IOLMaster in two locations affiliated with the University of California, Irvine. Various technicians performed IOLmaster measurements using either the IOLMaster 300 or IOLMaster 500, depending on the location. All measurements were captured before any contact with the patient's cornea (e.g., intraocular pressure check) to avoid affecting the numerical outcomes. All patients' eye surgeries were performed under topical anesthesia, and phacoemulsification was performed through temporal, clear corneal incision with IOL implantation in the capsular bag. During measurements with ORA, the speculum was carefully adjusted to avoid extra pressure on the eyelid. ORA measurements were obtained after the cataract was removed, and the anterior chamber was inflated to normotensive level with a cohesive viscoelastic (Healon– Abbott Medical Optics, Santa Ana, CA, USA), as previous studies have indicated successful measurements in aphakia with the presence of viscoelastic.[6,7] This study was completed with the ORA before the more recent upgrade to VerifEye+™ Technology.
We examined a cohort of 61 patients who had cataract surgery and preoperative IOLMaster measurements between January 2011 and January 2013 with the IOLMaster only (pre-ORA group). The IOLMaster was used to select the appropriate power of IOL implantation by surgeon's best choice, and the target postoperative spherical equivalent (SE) was obtained. This group was included to serve as a reference before ORA technology use. Another cohort of 234 patients from July 2012 to February 2014 who had preoperative IOLMaster as well as ORA measurements intraoperatively was included in this study. Again, the target postoperative SE refraction was obtained using IOLMaster. When the ORA provided several different IOL powers during aphakic measurements, the IOL with the ORA-predicted power closest to the preoperative IOLMaster selected target was chosen, and the predicted SE refraction given by the ORA was also recorded. Between one to four capture attempts were made for each patient with the ORA, depending on the quality of the measurements, averaging about 2.2 captures per patient. In many cases, the surgeon chose a different IOL power than was originally planned by the IOL Master, based on the ORA reading, either to a higher or lower lens power.
Postoperative uncorrected visual acuity, best-corrected visual acuity, and subjective MRx were performed, and the SE was recorded. The prediction error for IOLMaster was calculated as the difference between the postoperative MRx in SE and preoperative target refraction in SE. The prediction error for ORA was calculated based on the difference between the postoperative MRx SE and the ORA predicted postoperative refraction SE. Finally, in cases where the ORA and IOLMaster recommended different IOL powers for the same target postoperative refraction, the IOLMaster “error” was calculated as the difference between final postoperative MRx SE, and the IOLMaster target postoperative SE based on the final IOL power that was implanted.
Statistical analysis
Some subjects in the study had both eyes operated on and the outcomes from the same subject might be correlated. Thus, the group difference in real prediction errors on postsurgery MRx were analyzed using the linear mixed model, and the group difference in absolute prediction errors was analyzed using the generalized linear-mixed model with gamma distribution. Operated eye (right eye, left eye) and number of days' postsurgery were included as covariates in both models. The post hoc pairwise comparisons were performed with Bonferroni's multiple comparison adjustment method. All analyses were performed using SAS 9.4 (Cary, NC, USA), and the significance level was set at 0.05.
Results
Two hundred and ninety-five eyes of patients (50.2% male) with median age 71 (range 22–95) years old were identified. Among them, 61 eyes (20.7%) had cataract surgery with IOLMaster measurements, but without ORA (pre-ORA), and 234 eyes had both IOLMaster and ORA measurements. Of these, 107 (36.3%) eyes had the same IOL power recommendation from IOLMaster and ORA (BOTH group). For 95 (32.2%) eyes, the final IOL power implanted was chosen from ORA recommendations rather than IOLMaster (ORA group). Thus, for the ORA group, the final implanted IOL power was changed from what surgeon's best choice from IOLMaster measurements once ORA measurements were obtained. The decision to change was made by the surgeon to meet individual patient preference for near, intermediate, or distance refractive goals. For 26 (8.8%) patients, the final IOL power implanted was based on surgeon's best choice from IOLmaster measurements rather than ORA (IOLMaster group). Six patients were excluded from the data analyses; three had the same IOL power chosen by ORA and surgeon's best choice from IOLMaster, but the surgeon ended up placing a different IOL type than previously planned, two had IOL power implantation different from that chosen by ORA, and surgeon's best choice and one had postsurgical myopic surprise likely related to a corneal condition that was diagnosed after surgery.
Of the 95 patients where final IOL power implantation was made based on the ORA measurements rather than preoperative IOLMaster measurements, the chosen IOL power was higher (HIGHER) than IOLMaster for 39 (41.1%) subjects and lower (LOWER) for 56 (58.9%) subjects. Postoperative MRx was obtained at approximately 1 month after surgery. The median days (min, max) postsurgery for postoperative MRx were 27 (8, 139), 25 (6, 97), 23.5 (11, 110), and 22 (5, 57) for ORA, BOTH, IOLMaster, and pre- ORA group, respectively, and the difference was not significant. Other sample characteristics including axial length, keratometry, anterior chamber depth, implanted IOL power and predicted refraction in SE for each study group were summarized in Table 1 and as shown the values were similar between groups.
Table 1.
Sample characteristics
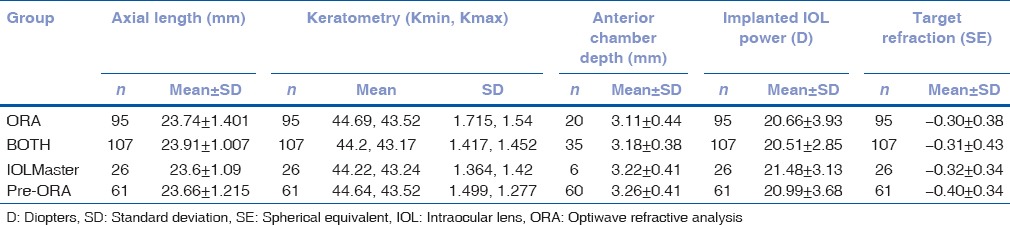
Prediction error of intraocular lens master target manifest refraction
Table 2 shows the prediction errors of the postoperative MRx SE compared to the IOL master's target refraction SE and are expressed either in absolute error (only positive values) or real error (includes both positive and negative values, referring to either a more hyperopic or myopic result than targeted). The results showed that a statistically significant difference between the 4 groups in absolute error (P = 0.0049) but not in real error (P = 0.57). The post hoc comparisons identified that the absolute error in the BOTH group was significantly smaller than the ORA (P = 0.002) and pre-ORA (P = 0.0037) groups, but not than the IOLMaster group (P = 0.35). There was no significant difference between ORA, IOLMaster and pre-ORA groups (all P > 0.25). In addition, there were 3 (2.8%), 0 (0%), 5 (8.2%), and 9 (9.5%) eyes with large absolute errors (>1D) in the BOTH, IOLMaster, pre-ORA, and ORA groups, respectively.
Table 2.
Prediction error of intraocular lens Master target manifest refraction
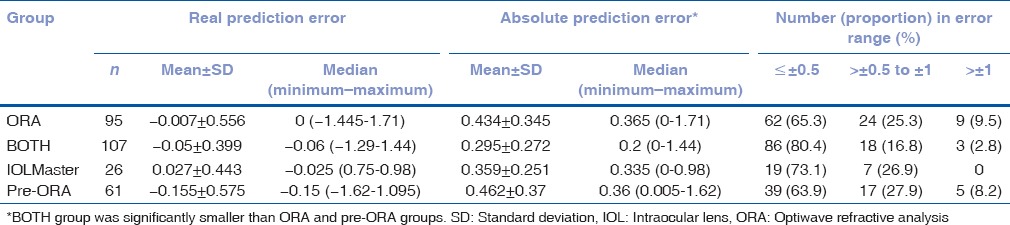
Prediction error of optiwave refractive analysis predicted manifest refraction
When the ORA was used intraoperatively, before IOL implantation, it gave a predicted final refraction in SE for different IOL powers of the specified lens type that may be chosen. Table 3 shows the distribution of the ORA's prediction error for postoperative refraction, in both real and absolute error. The error is the difference between the actual postoperative MRx and the ORA's-predicted postoperative refraction. There was a significant group difference in absolute prediction error (P = 0.0053) but not in real prediction error (P = 0.91). The absolute prediction error in the BOTH group was significantly less than the ORA group (P = 0.004) but not the IOLMaster group (P = 0.12), and there is no difference between ORA and IOLMaster groups (P = 0.75). Thus, the absolute prediction error was significantly smaller in cases where the surgeon's best choice (based on IOLMaster), and ORA recommended the same IOL power based on the preoperative target refraction, compared to cases where the final IOL power was changed based on ORA measurements. In addition, there were 3 (2.8%), 1 (3.8%), and 7 (7.4%) eyes with large absolute errors (>1D) in the BOTH, IOLMaster, and ORA groups, respectively.
Table 3.
Prediction error of optiwave refractive analysis predicted manifest refraction
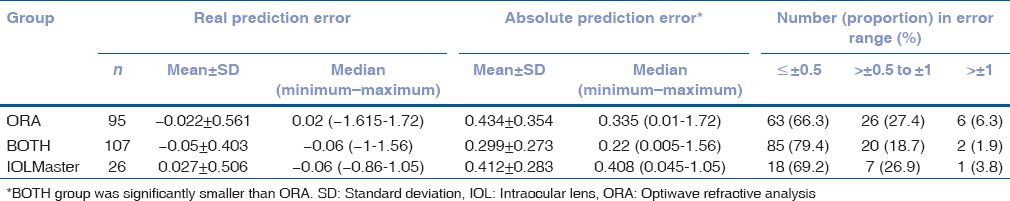
Change in intraocular lens power based on optiwave refractive analysis measurements
In 95 eyes, the ORA and IOLMaster measurement did not agree on the same IOL power for the same target refraction, and the surgeon chose the final IOL power based on the ORA's recommendation rather than preoperative IOL measurements. Therefore, the IOL power was changed based on the intraoperative ORA measurements, in 39 eyes for a higher-powered lens and in 56 eyes for a lower-powered lens than previously planned, as shown in Table 4. The difference between higher and lower power in prediction error of IOLMaster target MRx was not significant in real prediction error (P = 0.44) or absolute prediction error (P = 0.4).
Table 4.
Prediction error of intraocular lens Master target manifest refraction for optiwave refractive analysis group
Discussion
With improvements in cataract surgery techniques and technology arises the demand to provide more accurate postoperative results similar to the precision offered by refractive surgery. Intraoperative wavefront aberrometry is one tool that may help with improving surgeons' accuracy, and while, it has shown to be helpful in postrefractive cases.[1] In our study, the use of intraoperative wavefront aberrometry combined with IOLMaster was comparable, but not superior, to the use of conventional biometry with IOLMaster alone. Our postoperative outcomes show that for 107 patients in the group that had both IOLMaster and ORA recommend the same IOL power for the same target refraction (BOTH), the mean absolute error of postoperative SE compared to IOLMaster target SE is statistically smaller than 61 patients who had IOLMaster measurements without the use of ORA (pre-ORA). In addition, in this group where the IOLMaster and ORA agreed (BOTH), the mean absolute error was also statistically smaller than the group of 95 patients where the IOL power was changed based on ORA measurements (ORA). When the mean absolute prediction error was used to compare postoperative SE to ORA-predicted SE, the group of 107 patients where IOLMaster and ORA recommend the same IOL power (BOTH) had a statistically smaller mean absolute error than the group of 95 patients where the IOL power was changed based on ORA measurements (ORA). However, there was no statistical difference in mean absolute error between the BOTH group and the group of 26 patients where the IOL power was chosen based on the original IOLMaster target, despite ORA recommendations (IOLMaster). It is worth noting that there is a theoretical systematic difference between the measurements obtained using the IOLMaster 300 versus the IOLMaster 500, although we suspect this difference would be minimal. Changing the previously planned IOL power (based on preoperative IOLMaster) to another IOL power, whether higher or lower, based on ORA measurements did not lead to a better postoperative result in our study. This suggests that in cases, in which the IOLMaster and ORA agree, the postoperative outcome will be closer to target. However, this may be due to a variety of factors, including patient cooperation, technique of intraoperative aberrometry capture, stability of intraoperative IOP, lack of ocular surface disease, or other associated ocular comorbidities.
The differences in our study were small because the ORA and IOLMaster often provided very similar IOL power recommendations based on their predicted refractions. Out of 121 cases in which the ORA and IOLMaster did not agree on the same IOL power, and final IOL selection was made based on either the ORA or surgeon's best choice based on IOLMaster, in 87% (106 eyes) the variation was only ±0.5D. In only three cases, the difference in power between surgeon's best choice and ORA was ±1.5D, and in 12 cases, it was ±1.0D. The study could identify significantly lower absolute error in BOTH group compared to ORA group and pre-ORA group, but not compared to IOLMaster group, probably due to the small sample size in the IOLMaster group. While this was limited to a retrospective study at a single center with a single experienced surgeon, this likely also served to decrease any differences caused by surgeon technique and decision-making in IOL selection.
A limitation of the study is that there was no standardized preoperative IOL calculation method, instead relying on surgeon's best choice. Most cases relied on the Holladay 1 formula, with some preference to SRK/T for eyes with longer axial length, and Hoffer Q for eyes with shorter axial length. This study did not compare the predictive errors of each individual prediction formula (i.e., Haigis vs. Holladay 1 vs. Hoffer Q, etc.), and this may prove beneficial in the future research by providing additional data to aid in comparison between groups. In general, the surgeon looks for agreement in various formulas when choosing an IOL. Regarding the few instances where ORA gave several different IOL powers during aphakic measurements, the IOL was selected to closely match that of the initial prediction by the IOL master. This may influence the data by introducing confounding and bias. However, these cases comprised a small portion of our study, and the IOL powers provided by ORA were also chosen with the patients' postoperative goals in mind. In addition, in our study, several different types of IOLs were used although the majority consisted of ZCB00 or ZA9003 (Abbott Medical Optics, Santa Ana, CA), or SN60WF (Alcon, Fort Worth, TX, USA). The use of different IOL types and A-constant modification may have influenced the preoperative or intraoperative predictions. Other factors, such as patient fixation, intraocular pressure, external pressure from the eyelid speculum, and viscoelastic versus balanced saline solution in the anterior chamber may also affect the accuracy of the ORA measurements.[6] In our study, Healon was used in the anterior chamber for all ORA measurements, which very well may impact the predictions by ORA. In the future, additional studies to optimize these variables may be needed to determine the best conditions for intraoperative biometry.
Another limitation of this study centers arounds the cases when ORA recommended several different IOL powers during aphakic measurements. ORA depends on several variables (e.g., intraocular pressure, hydration, and external pressure), and these data were not recorded in this retrospective study. It may be useful in the future studies to focus on this issue as a possible limitation of ORA. Another limitation is only 121 patients out of 289 patients had recorded anterior chamber lengths in chart review.
Other uses for intraoperative wavefront aberrometry include the measurement of cylindrical power and axes to determine the placement of limbal relaxing incisions (LRI) for astigmatism and as well as orientation and power of toric IOL implants. Our study examined the accuracy of ORA in standard monofocal nontoric IOLs only. While some surgeons already use this technology for LRIs,[8] further studies to elucidate the value of intraoperative aberrometry specifically for toric IOL implantation, rotation, and residual postoperative cylinder would be useful.
A recent retrospective case series demonstrated the utility of intraoperative wavefront aberrometry for toric IOL placement in eyes with a history of prior refractive surgery.[9] Although limited by a small number of patients in the study, the study by Yesilirmak et al.[9] did demonstrate successful use of the ORA in eyes with significant astigmatism after both myopic and hyperopic LASIK that underwent toric IOL implantation, compared to IOLMaster and the online ASCRS calculator. Another recent retrospective study examined the use of the ORA with the Trulign accommodative toric IOL (Bausch + Lomb) and demonstrated good uncorrected distance visual acuity and low refractive cylinder on postoperative refraction.[10] Although this study was also limited by small sample size and did not have a control group, the ability of the ORA to consider anterior as well as posterior astigmatism likely makes it useful for toric IOL implantation and orientation. Additional comparative studies on whether intraoperative wavefront aberrometry provides more accurate results for toric IOL implantation would be important.
Conclusion
Intraoperative wavefront aberrometry with the ORA system provides comparable postoperative refractive results relative to conventional biometry with IOLMaster in patients undergoing routine cataract surgery with monofocal IOL implantation.
Financial support and sponsorship
This project was partially supported by grant UL1 TR000153 and UL1 TR001414 from the National Center for Advancing Translational Sciences through the Biostatistics, Epidemiology and Research Design Unit of UC Irvine Institute for Clinical Translational Science and an unrestricted grant from Research to Prevent Blindness (RPB).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Fram NR, Masket S, Wang L. Comparison of intraoperative aberrometry, OCT-based IOL formula, haigis-L, and masket formulae for IOL power calculation after laser vision correction. Ophthalmology. 2015;122:1096–101. doi: 10.1016/j.ophtha.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 2.Chen M. Correlation between ORange (Gen 1, pseudophakic) intraoperative refraction and 1-week postcataract surgery autorefraction. Clin Ophthalmol. 2011;5:197–9. doi: 10.2147/OPTH.S17489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen M. An evaluation of the accuracy of the ORange (Gen II) by comparing it to the IOLMaster in the prediction of postoperative refraction. Clin Ophthalmol. 2012;6:397–401. doi: 10.2147/OPTH.S30153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canto AP, Chhadva P, Cabot F, Galor A, Yoo SH, Vaddavalli PK, et al. Comparison of IOL power calculation methods and intraoperative wavefront aberrometer in eyes after refractive surgery. J Refract Surg. 2013;29:484–9. doi: 10.3928/1081597X-20130617-07. [DOI] [PubMed] [Google Scholar]
- 5.Ianchulev T, Hoffer KJ, Yoo SH, Chang DF, Breen M, Padrick T, et al. Intraoperative refractive biometry for predicting intraocular lens power calculation after prior myopic refractive surgery. Ophthalmology. 2014;121:56–60. doi: 10.1016/j.ophtha.2013.08.041. [DOI] [PubMed] [Google Scholar]
- 6.Huelle JO, Katz T, Druchkiv V, Pahlitzsch M, Steinberg J, Richard G, et al. First clinicial results on the feasibility, quality and reproducibility of aberrometry-based intraoperative refraction during cataract surgery. Br J Ophthalmol. 2014;98:1484–91. doi: 10.1136/bjophthalmol-2013-304786. [DOI] [PubMed] [Google Scholar]
- 7.Masket S, Fram NR. Influence of OVD Use on Intraoperative Aberrometry. Presented at the 2014 American Society of Cataract and Refractive Surgery Symposium and Congress; 25-2 April, 2014. Boston, MA. 2014 Abstract No. 3634. [Google Scholar]
- 8.Packer M. Effect of intraoperative aberrometry on the rate of postoperative enhancement: Retrospective study. J Cataract Refract Surg. 2010;36:747–55. doi: 10.1016/j.jcrs.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 9.Yesilirmak N, Palioura S, Culbertson W, Yoo SH, Donaldson K. Intraoperative wavefront aberrometry for toric intraocular lens placement in eyes with a history of refractive surgery. J Refract Surg. 2016;32:69–70. doi: 10.3928/1081597X-20151210-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epitropoulos AT. Visual and refractive outcomes of a toric presbyopia-correcting intraocular lens. J Ophthalmol. 2016;2016:7458210. doi: 10.1155/2016/7458210. [DOI] [PMC free article] [PubMed] [Google Scholar]


