Abstract
Purpose:
The purpose of this study is to compare the refractive error outcomes in the eyes of premature babies with retinopathy of prematurity (ROP) who underwent laser plus lens-sparing vitrectomy (LSV) in one eye and laser alone in the fellow eye.
Methods:
This is a retrospective study. Fourteen babies with Stage 4A of ROP or worse who underwent laser plus LSV in one eye (Group 1) and laser alone in the fellow eye (Group 2) were followed at 2 months, 6 months, 1 year, one and a half year, and 2 years. The main outcome variable studied was cycloplegic refraction at the baseline and follow-up visits. The change in spherical and cylindrical power at each visit was compared in Groups 1 and 2. The changes in spherical equivalent in subgroups were analyzed.
Results:
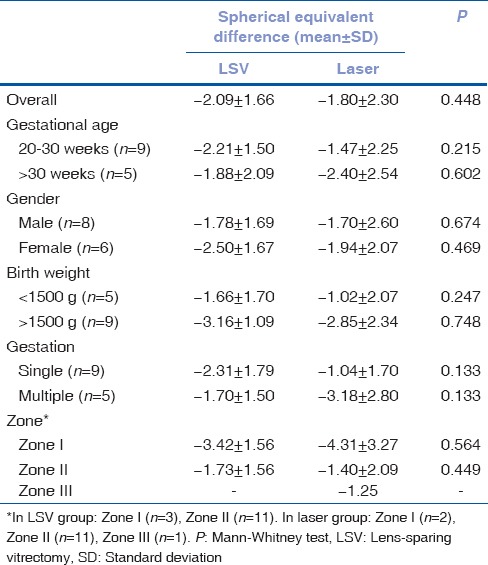
Mean gestational age at birth was 29.43 ± 2.10 weeks (range 26–32 weeks). Mean chronological age at the time of surgery was 4.11 ± 3.00 months (range 2–10 months). Mean postmenstrual age was 45.86 ± 12.13 weeks (range 39–75 weeks). Mean birth weight was 1340.71 ± 361.59 g (range 860–1980 g). All the babies in both groups had progressive myopia till 2 years follow-up; laser group had less myopia than LSV group till 1 year, thereafter, there was no difference in median till 2-year follow-up. The mean ± standard deviation of spherical equivalent in LSV versus laser group was: −4.36 ± 5.52 versus −3.21 ± 4.59 at 2 months; −5.09 ± 5.82 versus −4.04 ± 4.68 at 6 months; −7.14 ± 5.36 versus −5.36 ± 5.09 at 1 year; and −7.47 ± 1.38 versus −6.41 ± 1.91 at 2 years. Spherical equivalent difference across the visits did not differ significantly between Groups 1 and Group 2 in children whose birth weight was <1500 g (P = 0.247) and those who had more than 1500 g (P = 0.748), in those with gestational age between 20 and 30 weeks (P = 0.215) compared to those >30 weeks (P = 0.602).
Conclusion:
No difference in the progression of myopia was noted in eyes that underwent additional LSV following laser photocoagulation in one eye and laser alone in the fellow eye.
Keywords: Laser, lens-sparing vitrectomy, myopia, retinopathy of prematurity
Premature birth is associated with physiological myopia or myopia without retinopathy of prematurity (ROP) and myopia associated with severe ROP.[1] Evaluating the cryotherapy for ROP study (CRYO-ROP study) showed that the incidence of myopia in eyes with severe ROP and sequels is 80%.[2] There is evidence to show that the degree of myopia is significantly less following laser treatment as compared to CRYO.[3] Petrol Carvounis et al. studied the refractive outcome of 3-port lens-sparing vitrectomy (LSV) in nine infants with Stage 4A retinal detachment and found that these eyes develop less myopia than fellow eyes which were treated by ablative laser alone.[4] However, these studies did not include eyes with severe ROP (>Stage 4A). Moreover, the refractive outcomes were not studied separately for spherical and cylindrical powers. The change in myopia over a period in these eyes undergoing LSV has also been rarely reported.[4]
The purpose of this study was to compare the refractive outcomes both spherical as well as cylindrical in severe ROP (Stage 4A or worse) who underwent laser along with LSV in one eye and laser alone in the fellow eye over a 2-year follow-up.
Methods
This retrospective study was done at a tertiary eye care center in South India, where the data were obtained from the electronic medical records of the babies who underwent ablative laser treatment in one eye and LSV in the other eye for advanced ROP between January 2003 and December 2008. A total of 14 patients were included in the study, all babies with initial ablative laser treatment in both eyes and subsequent LSV in one eye. The decision of LSV was taken when the disease progressed despite laser, causing tractional retinal detachment. Details of birth history, ROP grades (early treatment for ROP (ETROP) classification), and zones involved were noted. Six out of 14 eyes had Stage 4A and eight out of 14 eyes had Stage 4B. In LSV group, three eyes had involvement up to zone I, and 11 eyes had involvement up to zone II. In the laser group, two eyes had involvement up to zone I, 11 eyes up to zone II, and one eye up to zone III. The extent and stage of disease were comparable between eyes.
The surgical techniques used here have been described in detail elsewhere.[5] Three-port pars plicata vitrectomy using 25-gauge instrumentation (Alcon, Constellation, a Novartis Division, Texas, United States) was performed by the surgeon. Not all surgeries were performed by a single surgeon, rather three surgeons performed the surgeries in our series, but the instrument used and techniques followed were similar. Sclerotomies were made 0.5–1.0 mm posterior to the limbus through the pars plicata. The 25-gauge cannula for the infusion line was placed inferotemporally unless the configuration of the tractional retinal detachment precluded placement in that quadrant. If so, the infusion port was placed away from the anteriorly displaced retina. The 25-gauge vitreous cutter was used in all cases. Lens-sparing procedures were used in all cases. The Binocular Indirect Ophthalmic Microscope was used for wide-angle viewing. The goal of the surgery was to release vitreous adhesions between the ridge and pars plicata, ridge and lens, and ridge and optic nerve.
Data such as birth history, clinical diagnosis, visual acuity measured with Snellen chart, Lea symbols, and refractive error were collected. The main outcome variable studied was cycloplegic refraction at subsequent follow-up visits. Cycloplegic refraction was done manually using a streak retinoscope. Cycloplegia was achieved by two instillations of 1% cyclopentolate or homatropine 2%, with one of 1% tropicamide in between. All instillations were spaced 5 min apart. Refraction was done 45 min after instillation of the last drop. The same protocol of cycloplegia was followed in all cases across all follow-ups. Institutional review board approval was obtained to analyze the hospital-based data, and the tenets of Helsinki were followed.
Statistical analysis
Data entry was done by a single investigator; statistical analysis was performed using statistical software (SPSS for Windows, ver. 17.0 SPSS Science, Chicago, IL, USA). P < 0.05 was set as statistical significant. Tests for normality were performed; as the data were not normally distributed nonparametric tests were used.
Results
A total of 28 eyes of 14 children were included in the study: 16 eyes from eight boys and 12 eyes from six girls. Mean gestational age at birth was 29.43 ± 2.10 weeks (range 26–32 weeks). Mean postmenstrual age was 45.86 ± 12.13 weeks (range 39–75 weeks). Mean birth weight was 1340.71 ± 361.59 g (range 860–1980 g). The demographic and general patient data of the study group has been presented in Table 1. Of the 14, 12 (85.71%) children had a history of birth apnea and respiratory distress syndrome, jaundice, or anemia. Three (21.42%) children were one of a pair of twins and two (14.28%) were one of triplets. The mean chronological age at the time of surgery was 4.11 ± 3.00 months (range 2–10 months).
Table 1.
Baseline characteristics of study patients
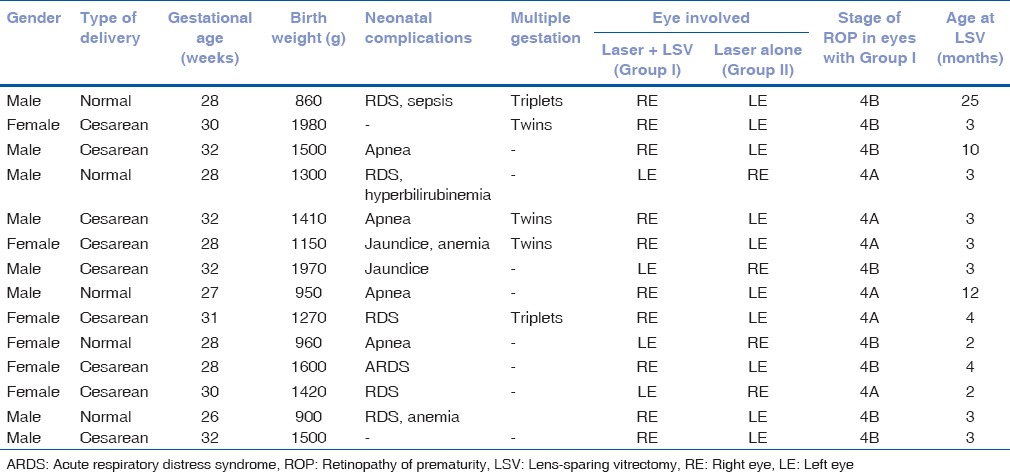
Fig. 1 shows the median spherical equivalent in LSV and laser group at 2 months, 6 months, 18 months, and 2-year follow-up. Myopia (spherical equivalent) increases with increasing age (2 months to 2 years) in both LSV and laser group. Patients in the laser group had less myopia than those in the LSV group till year 1; however, after that both groups had similar myopia (median). The mean ± standard deviation of spherical equivalent in LSV versus laser group were −4.36 ± 5.52 versus −3.21 ± 4.59 at 2 months; −5.09 ± 5.82 versus −4.04 ± 4.68 at 6 months; −7.14 ± 5.36 versus −5.36 ± 5.09 at 1 year; −9.13 ± 5.82 versus −7.48 ± 4.17 at one and a half years; and −7.47 ± 1.38 versus −6.41 ± 1.91 at 2 years.
Figure 1.
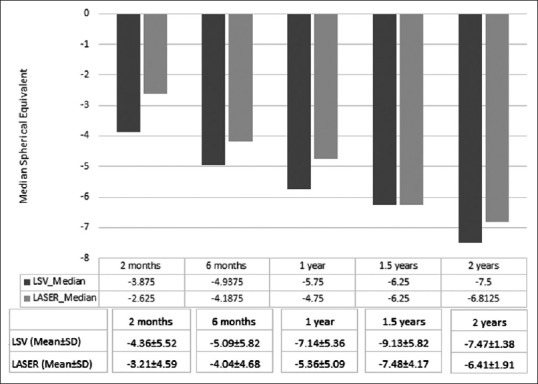
Shows comparison of spherical equivalent in lens-sparing vitrectomy and laser group
Refractive error assessment was done in 14 children at the baseline and 14 children at 6 months, 9 at 1 year and four at follow-up visit after 2 years. There was no statistically significant difference in refractive error either spherical or cylindrical between the eyes that underwent LSV following laser photocoagulation (Group 1), and the eyes that underwent laser alone (Group 2) at all the visits P > 0.05 [Table 2].
Table 2.
Change in refraction at 2 months versus 6 months, 1 year, 1.5 years and 2 years in both groups
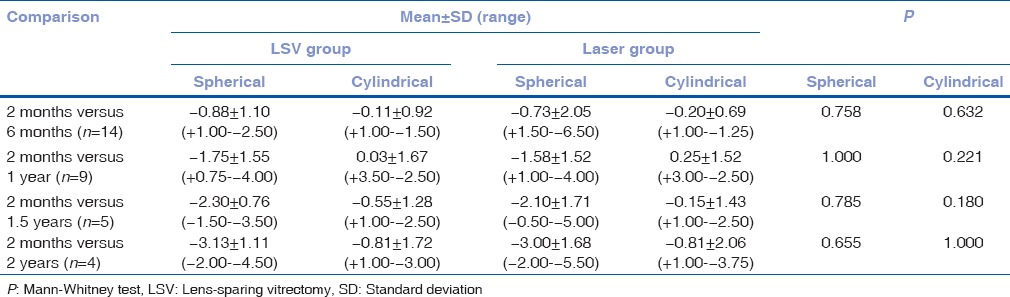
Table 3 shows the changes in refractive error in the two groups in different subgroups. Spherical equivalent difference across the visits did not differ significantly in group 1 and group 2 in children whose birth weight was <1500 g as compared to those who had more than 1500 g, in those with gestational age between 20 and 30 weeks compared to those >30 weeks, gender (male vs. female) and single gestation versus multiple gestation between the groups.
Table 3.
Difference in spherical equivalence between baseline and final follow-up between lens-sparing vitrectomy and laser groups
Discussion
In this study, we reported the refractive error outcomes (spherical and cylindrical) in severe ROP (Stage 4A and 4B) who had underwent laser along with LSV in one eye and laser alone in the fellow eye over 2 years follow-up. We found that these babies have myopia which increases with increasing age. However, there is no difference in change in spherical and cylindrical refraction with increasing follow-up between the LSV and laser group. The difference was also not seen with different ROP influencers, such as birth weight, gestational age, gender, and gestation status (single vs. multiple).
Table 4 shows the comparison with other studies regarding refraction status in ROP and with different treatment modalities. Enrique Garcia-Valenzuela and Kaufman studied the amount of myopia between ROP and full-term infants and found the prevalence to be higher in ROP infants.[6] In our study, myopic refractive error was found in all the infants with ROP, which is in accordance with that reported in the literature. Although the reasons for myopic refraction in ROP infants is not clearly understood the possible reasons quoted in the literature are steeper corneal curvature,[7,8] decrease in anterior chamber depth[9,10] and increase in the power of the crystalline lens.[11]
Table 4.
Refractive error outcomes reported in the literature
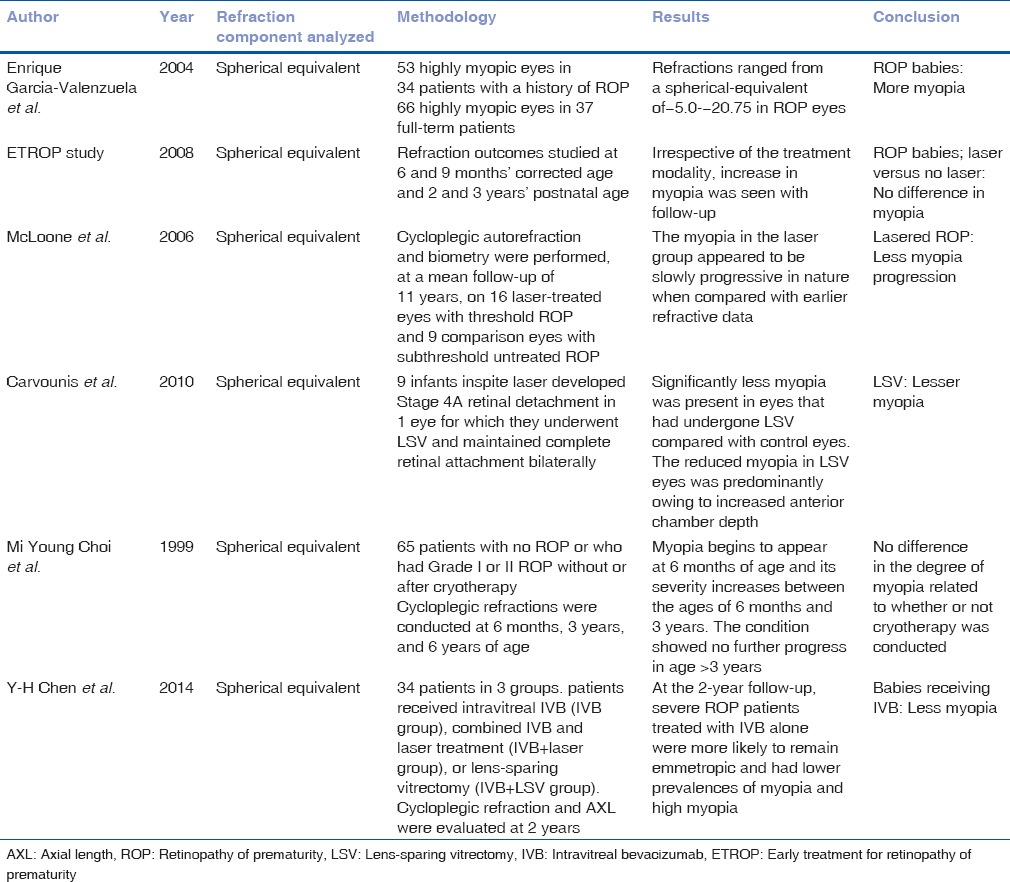
Progression of myopia in ROP in infants has been assessed with different treatment modalities such as CRYO, photocoagulation, LSV, and also Intravitreal injections.[12,13,14] The ETROP[15] study, which is the first randomized study to study the progression of myopia with treatment, found no difference in myopia progression in infants who underwent laser therapy, whereas McLoone et al. and associates found higher myopia in eyes that underwent diode laser therapy.[12] Choi et al. studied the refractive outcome in the eyes of preterm infants with and without ROP and suggested that the occurrence of myopia is related to the degree of cicatricial retinopathy.[13]
Chen et al. studied the refractive error outcomes after the use of bevacizumab injection in the treatment of ROP infants, and after a follow-up period of 2 years, patients treated with intravitreal bevacizumab injection alone were more likely to remain emmetropic and had lower prevalences of myopia and high myopia.[14] However, in the present study, none of the patients received intravitreal injections.
We did not find any difference in the amount of myopia on comparing between LSV and Laser group; Carvounis et al., who studied the refractive outcomes of the eyes that underwent LSV with fellow eyes that underwent laser alone and found decrease in myopia in the infants who underwent LSV.[4] However, the possible reasons for the difference between groups could be due to the fact that the measurement of refractive error was done at a single point of time. Moreover, Carvounis et al. included infants who had Stage 4A ROP while infants in our series had more severe disease (4A and 4B) which may explain this difference in refractive outcomes.
The present study has few limitations such as variable sample size at each visit and lack of biometric data. Due to the inherent retrospective design, the difference in number of subjects in each follow-up and lack of any statistical corrections for the difference in number of subjects were major limitation. The power of the study is 60% during initial follow-up and drastically reduced to 10% during final follow-up as the number of patients reduced, and standard deviation increased at final follow-up. Hence, it will be worthwhile to study the data of myopia in cases with laser regressed in both eyes; myopia after laser plus surgery regressed in both eyes and compare these cases with large sample size. However, as the comparison was made between both eyes of the same subject, the differences due to patient characteristics were avoided.
Another major limitation of our study was the absence of visual acuity, due to which the correlation of structural outcome and functional outcome following LASER and LSV could not be obtained.
Conclusion
In summary, we found that eyes with advanced ROP show myopia progression as age advances; however, there was no difference in the progression of myopia in those who underwent laser versus laser followed by LSV. Although this series is the largest dataset comparing myopia progression between the two treatment modalities, prospective studies with biometric data would give us better insights into the progression of myopia in this vulnerable population.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Fielder AR, Quinn GE. Myopia of prematurity: Nature, nurture, or disease? Br J Ophthalmol. 1997;81:2–3. doi: 10.1136/bjo.81.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quinn GE, Dobson V, Kivlin J, Kaufman LM, Repka MX, Reynolds JD, et al. Prevalence of myopia between 3 months and 5 1/2 years in preterm infants with and without retinopathy of prematurity. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology. 1998;105:1292–300. doi: 10.1016/s0161-6420(98)97036-1. [DOI] [PubMed] [Google Scholar]
- 3.Laws F, Laws D, Clark D. Cryotherapy and laser treatment for acute retinopathy of prematurity: Refractive outcomes, a longitudinal study. Br J Ophthalmol. 1997;81:12–5. doi: 10.1136/bjo.81.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carvounis PE, Poll J, Weikert MP, Wilhelmus K, Lakhanpal RR, Holz ER. Refractive outcomes of lens-sparing vitrectomy for retinopathy of prematurity. Arch Ophthalmol. 2010;128:843–6. doi: 10.1001/archophthalmol.2010.118. [DOI] [PubMed] [Google Scholar]
- 5.Bhende P, Gopal L, Sharma T, Verma A, Biswas RK. Functional and anatomical outcomes after primary lens-sparing pars plana vitrectomy for Stage 4 retinopathy of prematurity. Indian J Ophthalmol. 2009;57:267–71. doi: 10.4103/0301-4738.53050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Valenzuela E, Kaufman LM. High myopia associated with retinopathy of prematurity is primarily lenticular. J AAPOS. 2005;9:121–8. doi: 10.1016/j.jaapos.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto M, Tatsugami H, Bun J. A follow-up study of refractive errors in premature infants. Jpn J Ophthalmol. 1979;23:435–43. [Google Scholar]
- 8.Gallo JE, Fagerholm P. Low-grade myopia in children with regressed retinopathy of prematurity. Acta Ophthalmol (Copenh) 1993;71:519–23. doi: 10.1111/j.1755-3768.1993.tb04629.x. [DOI] [PubMed] [Google Scholar]
- 9.Majima A. Studies on retinopathy of prematurity II. Fundus appearance and ocular functions in cicatricial phase of very low birth weight infants. Jpn J Ophthalmol. 1977;21:421–35. [Google Scholar]
- 10.Koraszewska-Matuszewska B, Samochowiec-Donocik E, Pieczara E, Papiez M. Myopia as a complication of retinopathy of prematurity. Klin Oczna. 1993;95:339–42. [PubMed] [Google Scholar]
- 11.Gordon RA, Donzis PB. Myopia associated with retinopathy of prematurity. Ophthalmology. 1986;93:1593–8. doi: 10.1016/s0161-6420(86)33523-1. [DOI] [PubMed] [Google Scholar]
- 12.McLoone EM, O'Keefe M, McLoone SF, Lanigan BM. Long-term refractive and biometric outcomes following diode laser therapy for retinopathy of prematurity. J AAPOS. 2006;10:454–9. doi: 10.1016/j.jaapos.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Choi MY, Park IK, Yu YS. Long term refractive outcome in eyes of preterm infants with and without retinopathy of prematurity: Comparison of keratometric value, axial length, anterior chamber depth, and lens thickness. Br J Ophthalmol. 2000;84:138–43. doi: 10.1136/bjo.84.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YH, Chen SN, Lien RI, Shih CP, Chao AN, Chen KJ, et al. Refractive errors after the use of bevacizumab for the treatment of retinopathy of prematurity: 2-year outcomes. Eye (Lond) 2014;28:1080–6. doi: 10.1038/eye.2014.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinn GE, Dobson V, Davitt BV, Hardy RJ, Tung B, Pedroza C, et al. Progression of myopia and high myopia in the early treatment for retinopathy of prematurity study: Findings to 3 years of age. Ophthalmology. 2008;115:1058–64.e1. doi: 10.1016/j.ophtha.2007.07.028. [DOI] [PubMed] [Google Scholar]


