Abstract
BACKGROUND/OBJECTIVES
This study was conducted to investigate the effect of a corn silk extract on improving benign prostatic hyperplasia (BPH).
MATERIALS/METHODS
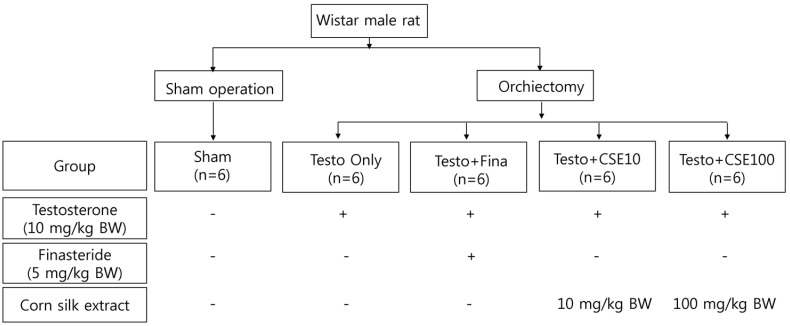
The experimental animals, 6-week-old male Wistar rats, were divided into sham-operated control (Sham) and experimental groups. The experimental group, which underwent orchiectomy and received subcutaneous injection of 10 mg/kg of testosterone propionate to induce BPH, was divided into a Testo Only group that received only testosterone, a Testo+Fina group that received testosterone and 5 mg/kg finasteride, a Testo+CSE10 group that received testosterone and 10 mg/kg of corn silk extract, and a Testo+CSE100 group that received testosterone and 100 mg/kg of corn silk extract. Prostate weight and concentrations of dihydrotestosterone (DHT), 5α-reductase 2 (5α-R2), and prostate specific antigen (PSA) in serum or prostate tissue were determined. The mRNA expressions of 5α-R2 and proliferating cell nuclear antigen (PCNA) in prostate tissue were also measured.
RESULTS
Compared to the Sham group, prostate weight was significantly higher in the Testo Only group and decreased significantly in the Testo+Fina, Testo+CSE10, and Testo+CSE100 groups (P < 0.05), results that were consistent with those for serum DHT concentrations. The concentrations of 5α-R2 in serum and prostate as well as the mRNA expression of 5α-R2 in prostate were significantly lower in the Testo+Fina, Testo+CSE10, and Testo+CSE100 groups than that in the Testo Only group (P < 0.05). Similarly, the concentrations of PSA in serum and prostate were significantly lower in the Testo+Fina, Testo+CSE10, and Testo+CSE100 groups (P < 0.05) than in the Testo Only group. The mRNA expression of PCNA in prostate dose-independently decreased in the Testo+CSE-treated groups (P < 0.05).
CONCLUSIONS
BPH was induced through injection of testosterone, and corn silk extract treatment improved BPH symptoms by inhibiting the mRNA expression of 5α-R2 and decreasing the amount of 5α-R2, DHT, and PSA in serum and prostate tissue.
Keywords: Prostate, testosterone, Zea mays, finasteride, maysin
INTRODUCTION
Corn (Zea mays L.) is an annual herbaceous plant that originated in South America. The edible parts of corn have been widely used as food sources for both human and animal, but, until recently, the corn silk byproduct has been discarded [1]. Corn silk is a yellow- to light-brown-colored ingredient known for its effectiveness in the treatment of urinary infection and related diseases [2]. In Vietnam, China, and South America, it has been widely used as a treatment for diseases such as urinary stones, nephritis, and diabetes and as a diuretic [1,2,3]. The Korean Rural Development Administration has successfully developed a technological approach for separating physiologically active substances, such as maysin, from corn silk, and has conducted physiological activity screening and efficacy assays on maysin [4]. Maysin (C-glycosyl flavone) is synthesized along a branch of the flavonoid pathway in corn silk and is a host-plant resistance factor against corn earworm [5].
The incidence rate of urological diseases is increasing, especially in middle-aged men, and the prevalence of such diseases increases with age; an estimated 50% of men exhibit histologic evidence of benign prostatic hyperplasia (BPH) by age 50, increasing to 80% at age 80 [6,7]. BPH is a complex urinary disease that results in abnormalities due to prostate enlargement, which causes obstruction of the bladder and lower urinary tract [8]. Obstruction of urination normally leads to symptoms such as urinary frequency, urination disorder, and bladder occlusion, which may be symptomatic or asymptomatic depending on the patient [6,7,8]. Although the mechanism underlying BPH pathogenesis has not been fully elucidated, the most prevalent theory is that testosterone and dihydrotestosterone (DHT) are involved in the proliferation of prostate cells. The most potent endogenous androgen, DHT, has substantially greater affinity for the androgen receptor (AR) than does testosterone, indicating that DHT is the primary factor in prostate cell proliferation [9]. Two 5α-reductase (5α-R) isoenzymes, 5α-R1 and 5α-R2, have been reported, and 5α-R2 induces testosterone in the prostate to convert irreversibly to DHT [10]. Finasteride, a widely used medicine for BPH treatment, is a 5α-R2 inhibitor that decreases the DHT concentration in the prostate. However, it must be taken for a minimum of 4 to 5 months for an improvement in the BPH symptoms [11]. There are reports indicating that if such medication stops, not only can urination disorders recur, but also side effects of the drug, such as impotence, loss of libido, hypotension, and tachycardia, can arise [11,12]. Thus, many researchers have reported that natural plants, such as extracts of tomato and black raspberry, are useful for prevention and treatment of BPH through their capacity to regulate expressions of DHT, 5α-R2, AR, and prostate-specific antigen (PSA) [13,14,15,16].
In vivo and in vitro studies on the beneficial effects of corn silk such as its anti-obesity, lipid metabolism, immunity enhancement, antioxidant, and hepatoprotective activities have been reported [3,17,18,19,20,21,22]. However, studies related to corn silk and prostate are limited [17,18,19,22]. There are no previous reports describing the effect of a high-maysin corn silk extract (CSE) on improving prostate enlargement and the mechanism behind such an effect. The purposes of this study were to describe the effect of a high-maysin CSE on prostate hypertrophy in rat and elucidate the mechanism behind that effect.
MATERIALS AND METHODS
Animals and study design
Six-week-old male Wistar rats, used as experimental animals, were purchased from Dae Han Bio Link (Daejeon, Korea). The control group consisted of 6 rats that underwent a sham operation (i.e., suture after laparotomy; Sham group), and the experimental group consisted of 24 rats whose testes were excised after laparotomy. The 24 rats in the experimental group were then divided into 4 groups of 6 rats each by applying a randomized block design. To induce BPH, all 4 groups were injected, using a 0.45 µm hypodermic syringe, with 10 mg/kg body weight of testosterone propionate (Wako, Osaka, Japan) dissolved in a 9:1 mixture of filtered and sterilized corn oil and ethanol. The Sham group only received the 9:1 corn oil/ethanol solution in order to achieve similar subcutaneous injection conditions in all groups. Dosage of the administered testosterone commonly differs among studies, ranging from 1-20 mg/kg [24,25]. For this experiment, a dosage of 10 mg/kg was selected based on preliminary experimental results (data not shown).
To investigate the effect of CSE on BPH, the four experimental (BPH test) groups were administered differently: 1) BPH test group injected with testosterone (Testo Only group); 2) as a positive control group, BPH test group injected with testosterone and orally administered with 5 mg/kg body weight of finasteride (Sigma-Aldrich, St. Louis, MO, USA) dissolved in distilled and filtered ethanol (Testo+Fina group); 3) BPH test group injected with testosterone and orally administered with 10 mg/kg body weight of CSE (Testo+CSE10 group); and 4) BPH test group injected with testosterone and orally administered with 100 mg/kg body weight of CSE (Testo+CSE100 group). The Sham and Testo Only groups were orally administered distilled water to provide similar oral administration condition in all groups.
The experimental design is summarized in Fig. 1. Previous studies reported no toxicity in rats fed up to 100 mg/kg body weight of high-maysin CSE for 4 weeks [20,26]. Thus, in this study, rats were administered 10 mg/kg or 100 mg/kg of CSE to observe the effect of CSE on testosterone-induced BPH in rat. The extraction procedure used to obtain a high-maysin CSE has been previously described [4,20]. In brief, unpollinated corn silk was extracted by using prethanol A (C2H5OH). After the chlorophyll in the corn silk sample was removed by applying methylene chloride (CH2Cl2), the sample was concentrated under reduced pressure. After the sample was completely dried in a vacuum concentrator, the dried sample was dissolved in ethanol and injected into a preparative C18 column (Büchi, Newcastle, DE, USA) to obtain the desired high-maysin CSE. From 1 kg of corn silk, 3.5 g of high maysin CSE was obtained with a concentration of 2,783 mg of maysin per 100 g corn silk.
Fig. 1. Experimental design.
The length of the experimental period was 6 weeks. During the experiment, animals were freely fed the AIN-93G diet [27] and were provided with distilled water ad libitum. Each rat was raised in a separate stainless cage that was kept at a temperature of 24 ± 1℃ with a relative humidity of 50% ± 10% and an alternating 12 h day and 12 h night environment. Dietary intake was measured three times a week, and body weight was measured twice a week at a designated time. All experiments met the guidelines of the animal-testing ethics committee of Dankook University (Approval Code: 16-023).
Sample Preparation
At the end of the experimental period, the animals were fasted for 12 h and then anesthetized. Laparotomy was performed to collect blood samples from the heart. After blood sampling, the liver, spleen, epididymal fat pad, and prostate were removed, washed with 0.9% NaCl solution, and weighed. Blood samples were centrifuged at 3,000 rpm/min for 15 min at 4℃ and serum was collected. All samples were stored at -70℃ until needed.
Measurement of serum aspartate transaminase (AST) and alanine transaminase (ALT) activity
The AST and ALT activity were measured by using assay kits (Asan Pharm, Seoul, Korea) according to the protocols presented in the assay kit. After measuring absorbance at a 505 nm wavelength, the value was converted to Karmen units.
Histopathological changes of the prostate
A part of tissue from the prostate was fixed in a 10% buffered formalin solution (Sigma-Aldrich, St. Louis, MO, USA), immersed in ethanol (Duksan, Iansan, Korea), and dehydrated to be formed into a paraffin block. Each prostate sample was cut to a thickness of 4 µm with a microtome, and the slice attached to a gelatin-coated slide. Tissue fragments were immersed in xylene to remove paraffin for staining and the samples were then rehydrated in ethanol and distilled water. Rehydrated tissues were stained with hematoxylin-eosin (H&E) and histologically observed by using an optical microscope (Olympus, BX 51, Japan).
Serum dihydrotestosterone (DHT) concentration
The serum concentration of DHT was determined by using a rat DHT ELISA kit (Alpco, NH, USA). Sample absorbance was determined at 450 nm by using a microplate reader (Tecan, Port Melbourne, Australia).
Prostate-specific antigen (PSA) concentration in serum and prostate
Prostate tissue was homogenized by the method described in the rat PSA ELISA kit (Cusabio Biotech, Newark, DE, USA) and using the protocol included in the kit. Briefly, prostate tissue (100 mg) was rinsed with PBS, homogenized, and stored overnight at -20°C. Two freeze-thaw cycles were performed to break the cell membranes, after which the homogenates were centrifuged, the supernatant was removed and aliquots were stored at -20℃ until needed. The concentration of PSA in serum and prostate tissue were determined at 450 nm (Tecan, Port Melbourne, Australia).
Concentration of 5α-reductase 2 (5α-R2) in serum and prostate
The concentration of 5α-R2 in serum and prostate tissue were determined by using a rat 5α-R2 ELISA kit (Cusabio Biotech, Newark, DE, USA). Prostate tissue homogenates were obtained in the same manner as in the PSA assay.
RNA isolation and reverse transcription (RT) of prostate tissue
Total RNA in prostate tissue samples (0.2 g) was isolated by adding Easy-blue (Intron Biotechnology, Sungnam, Korea), a total RNA extraction reagent, followed by extraction by adding chloroform (Sigma-Aldrich, St. Louis, MO, USA), isopropanol (Samchun, Pyeongtaek, Korea), and 75% ethanol (Duksan, Iansan, Korea). To determine the concentration of the extracted RNA sample, the purity of RNA was calculated from the ratio of absorbances at 260 nm and 280 nm by using microplate reader, after which, the concentration of RNA was calculated the using the absorbance at 260 nm. A 5 µg sample of RNA was added to a tube along with 1 µL of Oligo DT; DEPC water was then added to form a 12 µL solution. After incubation, 7 µL of 2× reaction mixture (10× RT buffer 4 µL, 0.1 M DTT 2 µL, 10 mM NTP 1 µL) and 0.5 µL of Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA, USA) were added and incubation continued. After adding 0.5 µL of RNase H and 80 µL of DEPC water, it was further incubated and the supernatant was stored at -20℃ until needed.
Real-time PCR
SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA; 10 µL), 1 µL of forward and reverse primer (Table 1), and 6 µL of nuclease-free water (Biosesang, Seongnam, Korea) were added to a prepared cDNA sample, making a total sample of 20 µL. The real-time PCR was carried out by using an Applied Biosystems StepOne system (Applied Biosystems, Foster City, CA, USA) with software version 2.1 with sequences of 95℃ for 10 min, 95℃ for 15 min, 60℃ for 1 min, 95° for 15 min, 60°C for 1 min, 95℃ for 15 min repeated for 40 cycles. Data were calculated by using the ΔΔCT method within a program embedded in Applied Biosystems StepOne software v2.1. Primers sequences used in the real-time PCR analyses included 5α-R2 (forward, 5′-GCA AAG TTT CTG TGGAGG A-3′; reverse, 5′-AAG CAA CTG GAA TAA CAA AG A-3′), PCNA (forward, 5′-GAG CAA CTT GGA ATC CCA GAA CAG G-3′; reverse, 5′-CCA AGC TCC CCA CTC GCA GAA AAC T-3′), and GAPDH (forward, 5′-CGG GAA ACC CAT CAC CAT CT-3′; reverse, 5′-CAC AAA CAT GGG GGC ATC AG-3′).
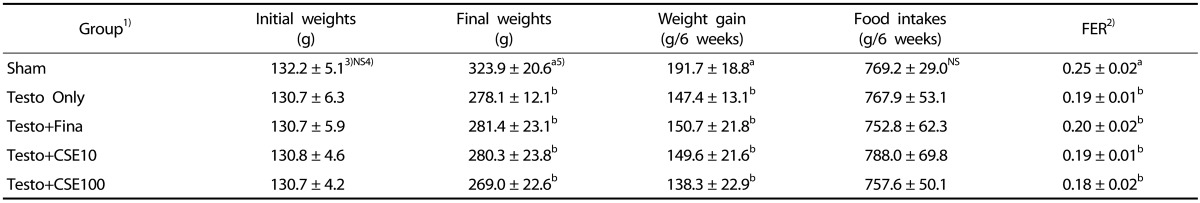
Table 1. Body weights, weight gain, and food efficiency ratio of experimental rats.
1)Sham: corn oil + ethanol injection and distilled water oral intake; Testo Only: testosterone injection (10 mg/kg) and distilled water oral intake; Testo+Fina: testosterone injection (10 mg/kg) and finasteride (5 mg/kg) oral intake; Testo+CSE10: testosterone injection (10 mg/kg) and corn silk extract (10 mg/kg) oral intake; Testo+CSE100: testosterone injection (10 mg/kg) and corn silk extract (100 mg/kg) oral intake 2)FER (food efficiency ratio) = Body weight gain (g) /Diet intake (g). 3)Mean ± SD 4)NS: Not significant 5)a>b: Different letters indicate significant differences among group at α = 0.05 as determined by Duncan's multiple range test.
Statistical analysis
Statistical analysis was performed by using the Statistical Package for the Social Sciences software (SPSS Institute, Armonk, NY, USA). Data are expressed as mean and standard deviation values, and statistically significant differences among groups were evaluated by using one-way-ANOVA (analysis of variance). Statistically significant differences among group means were tested at α = 0.05 by using Duncan's multiple range tests.
RESULTS
Effects of CSE on body weights and food intakes
The mean body weight, food intake, and food efficiency ratio (FER) of all groups are shown in Table 1. The initial weights ranged from 130-132 g, and there were no significant differences among the groups. The final weight of the Sham group after the 6-week experimental period was 323.9 g, which was significantly higher than those in the testosterone-treated groups (P < 0.05). There were no significant differences in the change in body weight among the groups injected with testosterone after orchiectomy, regardless of additional treatments. Food intakes also showed no significant differences among the groups. The FER of the Sham group was significantly higher than those of the testosterone-treated groups (P < 0.05), however, among the groups injected with testosterone after orchiectomy, there were no significant differences in FER, regardless of additional treatments.
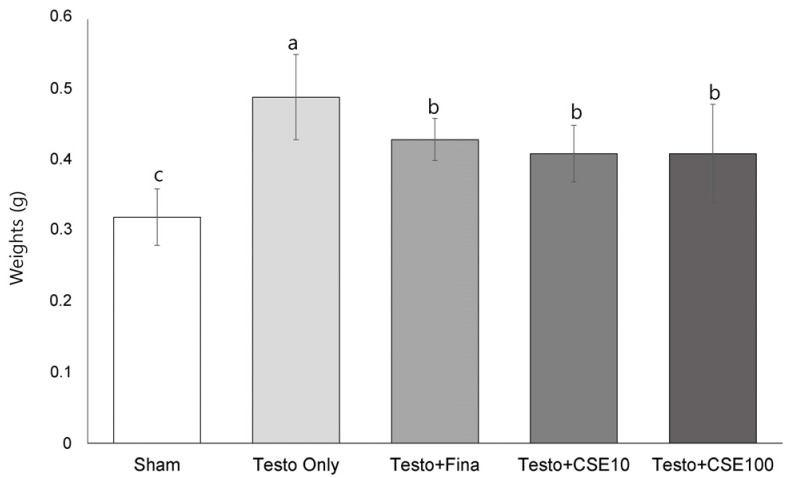
Effects of CSE on prostate weights
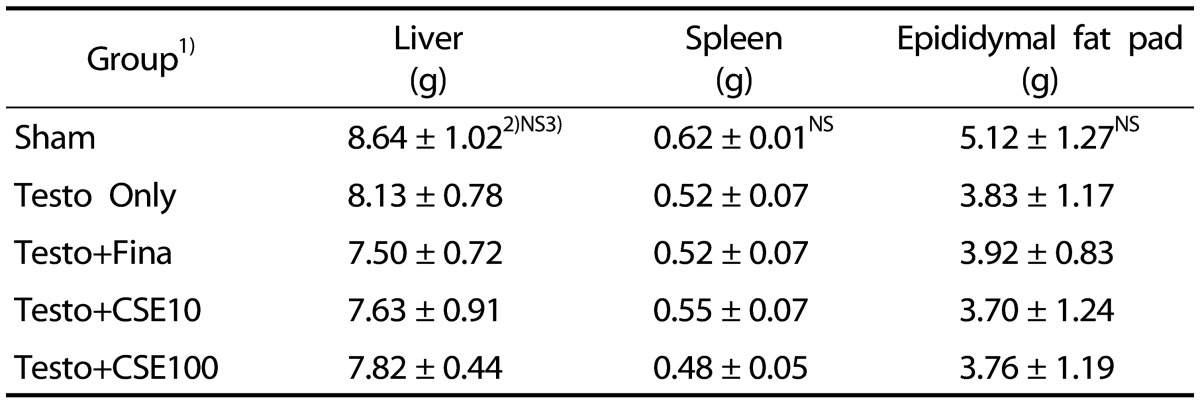
There were no significant differences in the weights of liver, spleen, or epididymal fat pad among the groups (Table 2). Prostate weight was the lowest in the Sham group. Among the groups treated with testosterone, the Testo Only group showed the highest prostate weight (P < 0.05). There were no significant differences among the Testo+Fina, Testo+CSE10, and Testo+ CSE100 groups (Fig. 2). In the testosterone-treated groups, the prostate weight was increased, indicating that testosteroneinduced BPH. The prostate weight in the finasteride treatment group (Testo+Fina) as well as in the CSE treatment groups (Testo+ CSE10 or Testo+CSE100) were significantly lower than that of the Testo Only group, but no dose-dependent effects of corn silk treatment were detected.
Table 2. Organ weights of experimental rats.
1)Sham: corn oil + ethanol injection and distilled water oral intake; Testo Only: testosterone injection (10 mg/kg) and distilled water oral intake; Testo+Fina: testosterone injection (10 mg/kg) and finasteride (5 mg/kg) oral intake; Testo+CSE10: testosterone injection (10 mg/kg) and corn silk extract (10 mg/kg) oral intake; Testo+CSE100: testosterone injection (10 mg/kg) and corn silk extract (100 mg/kg) oral intake
2)Mean ± SD
3)NS: Not significant
Fig. 2. Effects of corn silk extract on prostate weights.
Sham: corn oil + ethanol injection and distilled water oral intake; Testo Only: testosterone injection (10 mg/kg) and distilled water oral intake; Testo+Fina: testosterone injection (10 mg/kg) and finasteride (5 mg/kg) oral intake; Testo+CSE10: testosterone injection (10 mg/kg) and corn silk extract (10 mg/kg) oral intake; Testo+CSE100: testosterone injection (10 mg/kg) and corn silk extract (100 mg/kg) oral intake. Different letters indicate significant differences among group at α = 0.05 as determined by Duncan's multiple range test.
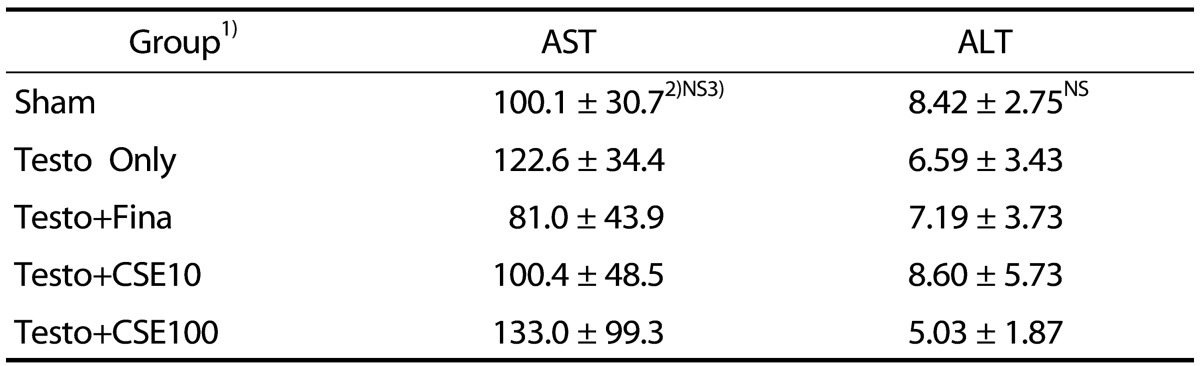
Effects of CSE on activity of AST and ALT
To determine whether intake of CSE produces a toxic effect, AST and ALT activities were measured. The results show that the intake of CSE in this study was safe as there were no significant differences in AST and ALT activities among all of the groups (Table 3).
Table 3. Activity of aspartate transaminase (AST) and alanine transaminase (ALT) (Karmen/mL).
1)Sham: corn oil + ethanol injection and distilled water oral intake; Testo Only: testosterone injection (10 mg/kg) and distilled water oral intake; Testo+Fina: testosterone injection (10 mg/kg) and finasteride (5 mg/kg) oral intake; Testo+ CSE10: testosterone injection (10 mg/kg) and corn silk extract (10 mg/kg) oral intake; Testo+CSE100: testosterone injection (10 mg/kg) and corn silk extract (100 mg/kg) oral intake
2)Mean ± SD
3)NS: Not significant
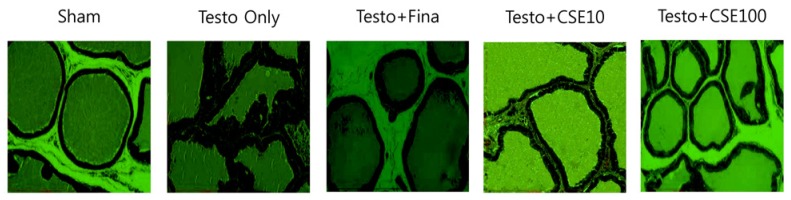
Effects of CSE on histopathological prostate change
The effects of CSE on morphological changes in rat prostate are shown in Fig. 3. Compared to the Sham group, the Testo Only group exhibited darker prostate epithelium, decreased prostate tissue lumen area, and infolded epithelium in many parts of the prostate. Testosterone treatments with finasteride (Testo+Fina) and CSE (Testo+CSE10 or Testo+CSE100) attenuated the histopathological alterations induced by testosterone.
Fig. 3. Hematoxylin-eosin (H&E) staining of prostate.
Sham: corn oil + ethanol injection and distilled water oral intake; Testo Only: testosterone injection (10 mg/kg) and distilled water oral intake; Testo+Fina: testosterone injection (10 mg/kg) and finasteride (5 mg/kg) oral intake; Testo+CSE10: testosterone injection (10 mg/kg) and corn silk extract (10 mg/kg) oral intake; Testo+CSE100: testosterone injection (10 mg/kg) and corn silk extract (100 mg/kg) oral intake. (×20)
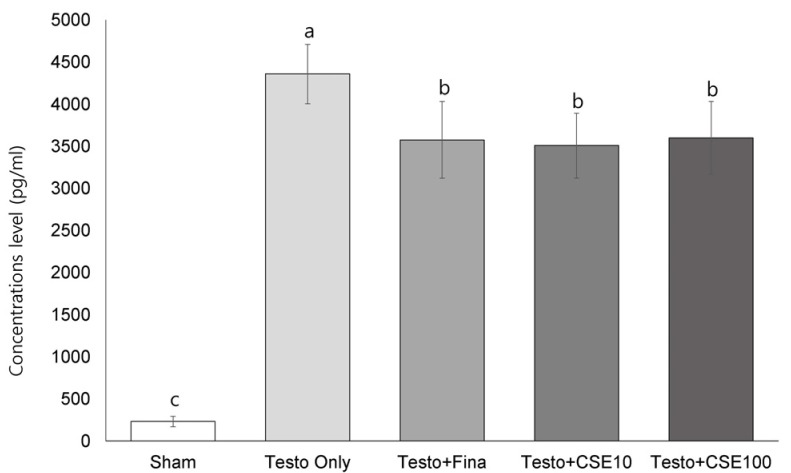
Effects of CSE on dihydrotestosterone (DHT) concentration
Serum DHT concentration was significantly higher in the Testo Only group (P < 0.05) than in the Sham group (Fig. 4). In the Testo+Fina, Testo+CSE10, and Testo+CSE100 groups, serum DHT concentration were significantly lower than that of the Testo Only group (P < 0.05). The results indicate that CSE is effective in decreasing the DHT concentration in serum; however, there was no dose-dependent difference between the two CSE groups.
Fig. 4. Effect of corn silk extract on serum dihydrotestosterone (DHT) concentration.
Sham: corn oil + ethanol injection and distilled water oral intake; Testo Only: testosterone injection (10 mg/kg) and distilled water oral intake; Testo+Fina: testosterone injection (10 mg/kg) and finasteride (5 mg/kg) oral intake; Testo+CSE10: testosterone injection (10 mg/kg) and corn silk extract (10 mg/kg) oral intake; Testo+CSE100: testosterone injection (10 mg/kg) and corn silk extract (100 mg/kg) oral intake. Different letters indicate significant differences among group at α = 0.05 as determined by Duncan's multiple range test.
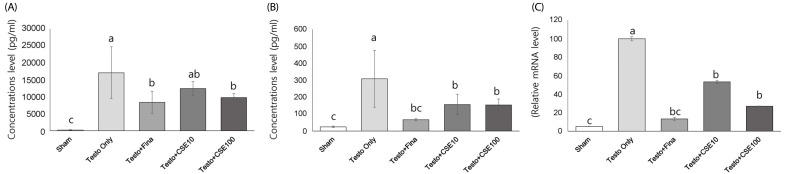
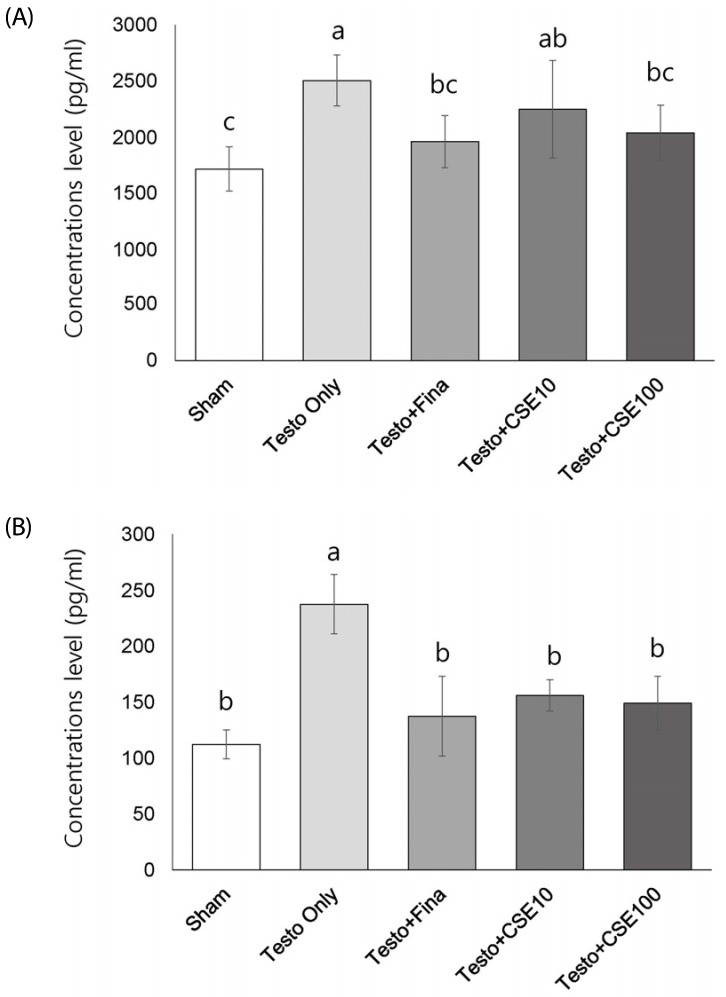
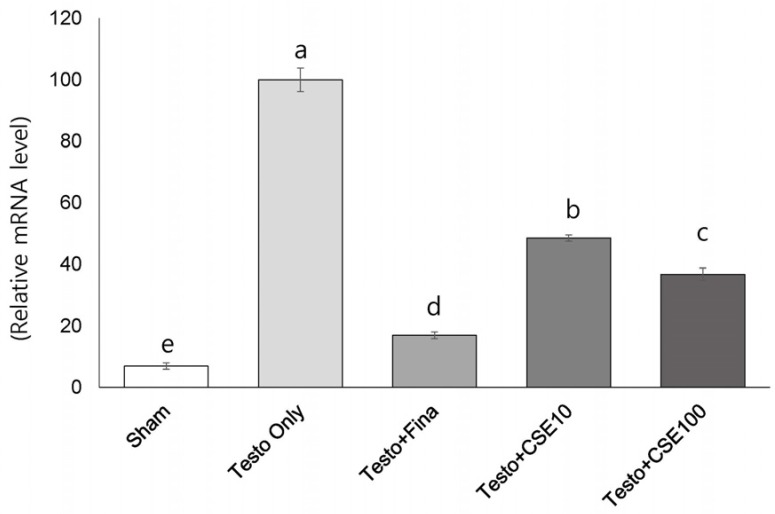
Effects of CSE on 5α-reductase 2 (5α-R2) concentration and mRNA expression
The serum concentration of 5α-R2 significantly was significantly higher in the Testo Only group (P < 0.05) than in the Sham group (Fig. 5A). The 5α-R2 concentrations in the Testo+Fina and Testo+CSE100 groups were significantly lower than that in the Testo Only group. The 5α-R2 concentrations in prostate tissue showed similar tendencies to those in serum, but in prostate, the Testo+Fina, Testo+CSE10, and Testo+CSE100 groups all had significantly lower 5α-R2 concentrations that that in the Testo Only group (Fig. 5B). The mRNA expression of 5α-R2 in the Testo Only group was significantly higher (P < 0.05) than that in the Sham group (Fig. 5C). The mRNA expressions of 5α-R2 in the Testo+Fina, Testo+ CSE10, and Testo+CSE 100 groups were significantly lower (P < 0.05) than that in the Testo Only group. There was no CSE dose dependency observed in serum, prostate, or mRNA expressions of 5α-R2.
Fig. 5. Effect of corn silk extract on 5α-reductase 2 (5α-R2) concentrations and mRNA expression.
Sham: corn oil + ethanol injection and distilled water oral intake; Testo Only: testosterone injection (10 mg/kg) and distilled water oral intake; Testo+Fina: testosterone injection (10 mg/kg) and finasteride (5 mg/kg) oral intake; Testo+CSE10: testosterone injection (10 mg/kg) and corn silk extract (10 mg/kg) oral intake; Testo+CSE100: testosterone injection (10 mg/kg) and corn silk extract (100 mg/kg) oral intake. A: 5α-R2 concentration in prostate in serum, B: 5α-R2 concentration in prostate, C: 5α-R2 mRNA expression in prostate. Different letters indicate significant differences among group at α = 0.05 as determined by Duncan's multiple range test.
Effects of CSE on prostate-specific antigen (PSA) concentration
The concentrations of PSA in serum and prostate are shown in Fig. 6. Serum PSA concentration was significantly higher in the Testo Only group (P < 0.05) than in the Sham group (Fig. 6A). Serum PSA concentration in the Testo+Fina and Testo+ CSE100 groups were significantly lower than that in the Testo Only group (P < 0.05) and were not significantly different from that in the Sham group. The PSA concentration in prostate tissue showed the same tendency as that in serum (Fig. 6B); however, in prostate, even the Testo+CSE10 group PSA level was significantly lower than that in the Testo Only group. Moreover, the PSA level in all three groups (Testo+Fina, Testo+CSE10, and Testo+CSE 100) were not significantly different from that in the Sham group.
Fig. 6. Effect of corn silk extract on prostate-specific antigen (PSA) concentrations in serum and prostate.
Sham: corn oil + ethanol injection and distilled water oral intake; Testo Only: testosterone injection (10 mg/kg) and distilled water oral intake; Testo+Fina: testosterone injection (10 mg/kg) and finasteride (5 mg/kg) oral intake; Testo+CSE10: testosterone injection (10 mg/kg) and corn silk extract (10 mg/kg) oral intake; Testo+CSE100: testosterone injection (10 mg/kg) and corn silk extract (100 mg/kg) oral intake. A: serum, B: prostate. Different letters indicate significant differences among group at α = 0.05 as determined by Duncan's multiple range test.
Effects of CSE on mRNA expression of proliferating cell nuclear antigen (PCNA)
The mRNA expression of PCNA was significantly higher in the Testo Only group (P < 0.05) than in the Sham group (Fig. 7). The PCNA expression levels in the Testo+Fina, Testo+CSE10, and Testo+CSE100 groups were significantly lower (P < 0.05) than that in the Testo Only group. The effect of CSE on mRNA expression of PCNA was dose dependent.
Fig. 7. Effect of corn silk extract on mRNA expression of proliferating cell nuclear antigen (PCNA) in prostate.
Sham: corn oil + ethanol injection and distilled water oral intake; Testo Only: testosterone injection (10 mg/kg) and distilled water oral intake; Testo+Fina: testosterone injection (10 mg/kg) and finasteride (5 mg/kg) oral intake; Testo+CSE10: testosterone injection (10 mg/kg) and corn silk extract (10 mg/kg) oral intake; Testo+CSE100: testosterone injection (10 mg/kg) and corn silk extract (100 mg/kg) oral intake. Different letters indicate significant differences among group at α = 0.05 as determined by Duncan's multiple range test.
DISCUSSION
Proliferation and death of prostate cells are regulated by androgen, and abnormal cell proliferation of prostate cells can result in dysuria [6]. Although BPH is not an obstacle to life, it does reduce quality of life, and, therefore, research attention has focused on its prevention and treatment [7]. The most common BPH model in animal study involves injection of artificial hormones [8,12,15,23,24,25,26]. Periodic injection of testosterone into rats whose testes are excised promotes prostate cell proliferation and further proliferation of connective tissue and fibrosis. Finasteride is a drug commonly used for BPH treatment and is often used as a positive control in BPH-related animal study [29]. Therefore, in this study, to investigate the effect of CSE on BPH, rats received subcutaneous injection of 10 mg/kg of testosterone propionate to induce BPH, and finasteride (5α-R2 inhibitor) treatment was used as a positive control.
After BPH inducement and CSE treatment, we determined the enzyme activities of AST and ALT to clarify the safety of the CSE administered (10 or 100 mg/kg body weight) in this study. The serum enzyme activities of ALT and AST are widely used as indicators of tissue injury, and the concentrations of these enzymes may increase markedly after injury to a specific tissue or organ, such as hepatic injury. No significant increases in ALT and AST activity were observed in the CSE-treated rats in this study, indicating that the CSE intake levels were safe.
Similar to the effect of finasteride treatment, CSE treatment resulted in a reduction of prostate weight in this study. The prostate weight reduction following CSE treatment was not caused by changes in food intake or body weight gain because no such statistical differences were observed among the Testo Only, Testo+Fina, and Testo+CSE groups. Observations of H&E-stained prostate tissue revealed that the color of prostate epithelium was darkened in the Testo Only group. The other BPH test groups treated with either finasteride or CSE exhibited less darkening; moreover, those groups had similar prostate shapes as that in the control group, suggesting that the high-maysin CSE used in this study is capable of controlling prostate weight and the thickness of the prostate tissue.
DHT has an important role in the development of BPH. Testosterone, the precursor of DHT, is synthesized in the testes and adrenal glands, and is converted to DHT via the action of the enzyme 5α-R2, which is mainly present in prostate, epididymis, hair follicle, and liver tissue. DHT has substantially greater affinity for AR than testosterone does, and binding of DHT to AR in the prostate results in the production of proteins such as PSA as well as regulatory proteins that induce cell proliferation, resulting in BPH [29].
In this study, compared to DHT concentration in the Testo Only group, intake of CSE or finasteride decreased the concentration of DHT in serum. CSE treatment also resulted in significant reduction of 5α-R2 levels both in serum and in prostate, and in mRNA expression levels of 5α-R2. These results were consistent with our observations showing lower prostate weight and attenuation of the histopathological alterations induced by testosterone in the CSE-treated groups. Therefore, the study results suggest that administration of a high-maysin CSE can reduce prostate weight and inhibit prostate tissue hypertrophy, and these results are associated with the decreases in DHT and 5α-R2 levels in serum and prostate.
The PSA protein is increased in the blood during the onset of BPH due to immune-related problems in prostate, and PSA level is mainly used as an indicator of the status of prostate cancer [13]. In this study, compared to the Testo Only group, the intake of CSE decreased the concentration of PSA in prostate. PSA is produced through the phosphorylation of DHT within prostatic stromal cells [30]. Considering all of the above results, it can be concluded that the decrease in PSA concentration due to CSE ingestion may have a synergistic effect on decreases in the levels of DHT and 5α-R2 resulting in prostate weight reduction and inhibition of prostate tissue hypertrophy.
Studies using natural plants have reported that changes in 5α-R2, DHT, and PSA can be used as indicators of BPH status. For example, DHT concentration was significantly decreased in an experimental group treated with black raspberry infusion compared to that of the study's BPH control group [15]. In addition, the concentration of 5α-R2 was decreased in experimental groups treated with a Schisandrae Fructus extract although the decrease was not dose-dependent [16]. Furthermore, in an experiment to see the effects of Paljeong-san Pharmacopuncture on BPH, DHT concentrations were significantly decreased without dose dependency in rats with induced BPH compared to the results of the study's BPH control group [31].
The PCNA protein has a role in fostering DNA replication by acting as a cofactor for DNA polymerase δ, which is required for DNA synthesis [32]. PCNA is involved in body cell proliferation and is usually observed during the G1 and S cell-cycle phases. An increase in PCNA indicates cell proliferation due to DNA replication, and thus it is referred to as a cell proliferation marker [33]. In this study, the mRNA expressions of PCNA were significantly higher in the Testo Only group than in the Sham group. Both the Testo+Fina group and the two CSE groups showed lower PCNA mRNA expressions than that in the Testo Only group. Moreover, the CSE groups responded to CSE treatment in a dose-dependent manner, thereby suggesting that CSE may inhibit prostate enlargement via a mechanism that inhibits prostate cell proliferation. Previous studies have shown that the PCNA expression level is significantly increased in animal models with induced BPH, and that excessive cell proliferation in stroma or at the epithelial surface has a critical role in the development of BPH [29,30,31,32,33,34]. The present in vivo study is the first to demonstrate the effect of CSE treatment on mRNA expression of PCNA. Considering the results showing prostate weight reduction and improvement in the histopathological alterations in prostates of BPH rats, we suggest that treatment with high-maysin CSE could inhibit prostate cell proliferation in prostate cells of BPH rats by regulating the mRNA expression of PCNA and the mRNA expression of 5α-R2.
The effect of CSE treatment on rats with induced BPH did not consistently occur in a dose-dependent manner. Compared with Testo Only group, treatment groups with 10 or 100 mg/kg CSE treatment showed significantly lower DHT, 5α-R2, and PSA levels, but there was no significant difference between the two dose levels. The lack of differences may be largely due to individual rat's variation in response to the CSE treatment. Therefore, if the number of animals per experiment group is increased or the experiment period is prolonged, a dose-dependence effect of CSE treatment may be revealed.
In conclusion, this study has shown that CSE treatment of rats with testosterone-induced BPH has a similar efficacy to finasteride treatment. Thus, it is suggested that high-maysin CSE, by inhibiting the mRNA expression of 5α-R2 and PCNA and decreasing the concentrations of 5α-R2, DHT, and PSA, may be a potential natural compound for BPH treatment.
Footnotes
This work was carried out with the support of the “Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ0113052016)” Rural Development Administration, Korea.
CONFLICT OF INTEREST: The authors declare no potential conflicts of interest.
References
- 1.Hasanudin K, Hashim P, Mustafa S. Corn silk (Stigma maydis) in healthcare: a phytochemical and pharmacological review. Molecules. 2012;17:9697–9715. doi: 10.3390/molecules17089697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grases F, March JG, Ramis M, Costa-Bauzá A. The influence of Zea mays on urinary risk factors for kidney stones in rats. Phytother Res. 1993;7:146–149. [Google Scholar]
- 3.Min OJ, Sharma BR, Park CM, Rhyu DY. Effect of myadis stigma water extract on adipogenesis and blood glucose in 3T3-L1 adipocytes and db/db mice. Korean J Pharmacogn. 2011;42:201–208. [Google Scholar]
- 4.Kim SL, Kim MJ, Lee YY, Jung GH, Son BY, Lee JS, Kwon YU, Park YI. Isolation and identification of flavonoids from corn silk. Korean J Crop Sci. 2014;59:435–444. [Google Scholar]
- 5.Byrne PF, McMullen MD, Snook ME, Musket TA, Theuri JM, Widstrom NW, Wiseman BR, Coe EH. Quantitative trait loci and metabolic pathways: genetic control of the concentration of maysin, a corn earworm resistance factor, in maize silks. Proc Natl Acad Sci U S A. 1996;93:8820–8825. doi: 10.1073/pnas.93.17.8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee YJ, Lee JW, Park J, Seo SI, Chung JI, Yoo TK, Son H. Nationwide incidence and treatment pattern of benign prostatic hyperplasia in Korea. Investig Clin Urol. 2016;57:424–430. doi: 10.4111/icu.2016.57.6.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Unnikrishnan R, Almassi N, Fareed K. Benign prostatic hyperplasia: evaluation and medical management in primary care. Cleve Clin J Med. 2017;84:53–64. doi: 10.3949/ccjm.84a.16008. [DOI] [PubMed] [Google Scholar]
- 8.Cho SH, Han YH, Kim YS. Effects of bee venom herbal acupuncture on experimental rat model of benign prostatic hyperplasia. J Korean Orient Intern Med. 2010;31:166–176. [Google Scholar]
- 9.Roehrborn CG. Pathology of benign prostatic hyperplasia. Int J Impot Res. 2008;20(Suppl 3):S11–S18. doi: 10.1038/ijir.2008.55. [DOI] [PubMed] [Google Scholar]
- 10.Bartsch G, Rittmaster RS, Klocker H. Dihydrotestosterone and the concept of 5alpha-reductase inhibition in human benign prostatic hyperplasia. World J Urol. 2002;19:413–425. doi: 10.1007/s00345-002-0248-5. [DOI] [PubMed] [Google Scholar]
- 11.Hirshburg JM, Kelsey PA, Therrien CA, Gavino AC, Reichenberg JS. Adverse effects and safety of 5-alpha reductase inhibitors (finasteride, dutasteride): a systematic review. J Clin Aesthet Dermatol. 2016;9:56–62. [PMC free article] [PubMed] [Google Scholar]
- 12.Guo M, Heran B, Flannigan R, Kezouh A, Etminan M. Persistent sexual dysfunction with finasteride 1 mg taken for hair loss. Pharmacotherapy. 2016;36:1180–1184. doi: 10.1002/phar.1837. [DOI] [PubMed] [Google Scholar]
- 13.Kang HS, Kim GY, Jung I, Oh SD, Kim CH, Shim BS, Park KH, Oh SJ. The effect of the compound of tomato extract to the prostatic cancer cell and the prostate of the rat model of benign prostatic hyperplasia. Korean J Pharmacogn. 2007;38:197–203. [Google Scholar]
- 14.Mobley D, Feibus A, Baum N. Benign prostatic hyperplasia and urinary symptoms: evaluation and treatment. Postgrad Med. 2015;127:301–307. doi: 10.1080/00325481.2015.1018799. [DOI] [PubMed] [Google Scholar]
- 15.Lee SJ, Choi HR, Lee JH, Kwon JW, Lee HK, Jeong JT, Lee TB. Effects of unripe black raspberry extracts on prostate cancer cell line and rat model of benign prostatic hyperplasia. J Korean Soc Food Sci Nutr. 2014;43:507–515. [Google Scholar]
- 16.Moon JM, Seok GH, Cho SI. Antiproliferative effect of schisandrae fructus extract on PC-3 human prostate cancer cells. Korean J Herbol. 2012;27:17–23. [Google Scholar]
- 17.Singh NK, Sahu AN, Singh SK. Free radical scavenging and hepatoprotective activities of standardized methanolic Extract of Maydis Stigma. Pharmacologyonline. 2009;2:440–449. [Google Scholar]
- 18.Liu J, Wang C, Wang Z, Zhang C, Lu S, Liu J. The antioxidant and free-radical scavenging activities of extract and fractions from corn silk (Zea mays L.) and related flavone glycosides. Food Chem. 2011;126:261–269. [Google Scholar]
- 19.Al-Ali M, Wahbi S, Twaij H, Al-Badr A. Tribulus terrestris: preliminary study of its diuretic and contractile effects and comparison with Zea mays. J Ethnopharmacol. 2003;85:257–260. doi: 10.1016/s0378-8741(03)00014-x. [DOI] [PubMed] [Google Scholar]
- 20.Lee EY, Kim SL, Kang HJ, Kim MH, Ha AW, Kim WK. High maysin corn silk extract reduces body weight and fat deposition in C57BL/6J mice fed high-fat diets. Nutr Res Pract. 2016;10:575–582. doi: 10.4162/nrp.2016.10.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J, Kim SL, Lee S, Chung MJ, Park YI. Immunostimulating activity of maysin isolated from corn silk in murine RAW 264.7 macrophages. BMB Rep. 2014;47:382–387. doi: 10.5483/BMBRep.2014.47.7.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J, Lee S, Kim SL, Choi JW, Seo JY, Choi DJ, Park YI. Corn silk maysin induces apoptotic cell death in PC-3 prostate cancer cells via mitochondria-dependent pathway. Life Sci. 2014;119:47–55. doi: 10.1016/j.lfs.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Lee DH, Lee JS, Kim YS. The effects of lygodium japonicum on experimental rat model of benign prostatic hyperplasia. J Korean Orient Intern Med. 2010;31:457–466. [Google Scholar]
- 24.Park DJ, Kang SH, Cho YH. The antihyperplastic effect of oral catechin ingestion in a rat model of benign prostatic hyperplasia. Korean J Urol. 2006;47:1289–1293. [Google Scholar]
- 25.Wu X, Gu Y, Li L. The anti-hyperplasia, anti-oxidative and anti-inflammatory properties of Qing Ye Dan and swertiamarin in testosterone-induced benign prostatic hyperplasia in rats. Toxicol Lett. 2017;265:9–16. doi: 10.1016/j.toxlet.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Cha JH, Kim SR, Kang HJ, Kim MH, Ha AW, Kim WK. Corn silk extract improves cholesterol metabolism in C57BL/6J mouse fed high-fat diets. Nutr Res Pract. 2016;10:501–506. doi: 10.4162/nrp.2016.10.5.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr. 1997;127:838S–841S. doi: 10.1093/jn/127.5.838S. [DOI] [PubMed] [Google Scholar]
- 28.Park JS, Moon JH, Huh JS, Kong MH, Kim HJ. Comparison of correlation between prostate volume and obesity indices. Korean J Obes. 2015;24:95–100. [Google Scholar]
- 29.Bruchovsky N, Lesser B, Van Doorn E, Craven S. Hormonal effects on cell proliferation in rat prostate. Vitam Horm. 1975;33:61–102. doi: 10.1016/s0083-6729(08)60951-6. [DOI] [PubMed] [Google Scholar]
- 30.Afriyie DK, Asare GA, Bugyei K, Adjei S, Lin JM, Peng J, Hong ZF. Treatment of benign prostatic hyperplasia with Croton membranaceus in an experimental animal model. J Ethnopharmacol. 2014;157:90–98. doi: 10.1016/j.jep.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Kim CW, Lee KH. Effects of paljeong-san pharmacopuncture on experimental rat model of benign prostatic hyperplasia. J Korean Acupunct Moxibustion Soc. 2014;31:95–103. [Google Scholar]
- 32.Jaskulski D, Gatti C, Travali S, Calabretta B, Baserga R. Regulation of the proliferating cell nuclear antigen cyclin and thymidine kinase mRNA levels by growth factors. J Biol Chem. 1988;263:10175–10179. [PubMed] [Google Scholar]
- 33.Zhong W, Peng J, He H, Wu D, Han Z, Bi X, Dai Q. Ki-67 and PCNA expression in prostate cancer and benign prostatic hyperplasia. Clin Invest Med. 2008;31:E8–E15. doi: 10.25011/cim.v31i1.3136. [DOI] [PubMed] [Google Scholar]
- 34.Zhong X, Lin J, Zhou J, Xu W, Hong Z. Anti-proliferative effects of qianliening capsules on prostatic hyperplasia in vitro and in vivo. Mol Med Rep. 2015;12:1699–1708. doi: 10.3892/mmr.2015.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]