Abstract
BACKGROUND/OBJECTIVES
Several studies have reported that consumption of Salvia Hispanica L.,commonly known as chia seed, may exert beneficial effects on health outcomes. The main purpose of this study was to examine the influence of chia seed consumption as a mid-morning snack on short-term satiety.
SUBJECTS/METHODS
Subjects (n = 24) were tested using a randomized, cross-over design consisting of three mid-morning snacks. Yogurt with no chia seed, yogurt with 7 g chia seed, and yogurt with 14 g chia seed were given to subjects on different test days. After subjects were asked to report visual analog scale (VAS) scores on sensory outcomes, ad libitum lunch was served, and energy intake of individuals was measured.
RESULTS
VAS scores indicated that participants reported significantly lower scores for hunger (P = 0.033), prospective food consumption (P = 0.031), amounts of food that could be consumed (P = 0.017), desire for sugary foods (P = 0.015), and higher scores for satiety (P = 0.031) on the test days with 7 g and 14 g chia seed. Energy intake of individuals during ad libitum lunch was significantly lower when they consumed yogurt with 7 g or 14 g chia seed (P = 0.037).
CONCLUSIONS
The study demonstrated that chia seed consumption as a mid-morning snack may induce short-term satiety in healthy individuals.
Keywords: Seeds, salvia, satiety response, energy intake, obesity
INTRODUCTION
In recent years, research into nutritional strategies aiming to prevent excess energy intake has increased in response to rising levels of obesity and obesity-related diseases [1]. Since drug treatments or supplements of bioactive molecules are ineffective or exhibit adverse side effects, focus has shifted towards natural dietary components with potential impact upon appetite and satiety [2]. In this context, several studies have reported that specific foods with higher contents of fibre can increase satiety and reduce hunger between meals [3,4]. These efficient strategies, which help to reduce total caloric intake, are required to improve and regulate healthy eating habits [5].
Salvia hispanica L., commonly known as chia seed, was used in central America and Mexico during pre-Columbian times as an important dietary component [6,7]. This seed is a natural source of omega-3 fatty acids, fibre, and protein [8]. Recently, chia seed has become a popular functional food due to its α-linolenic acid, vitamin, mineral, and phytochemical contents [9]. Chia oil consists of 60% α-linolenic acid, which makes it the most abundant plant source of omega-3 fatty acids [10]. Additionally, a protein content of 19–23% with a non-limiting amino acid profile and fibre content above 30% of total weight has increased the usability of chia seed for treating numerous health-related conditions [11]. Chia extract contains 8.8% total phenolic content in dry matter, which suggests strong antioxidant activity [12]. Considering its healthy nutritional composition, chia seed can be incorporated as a functional component in several food products. Indeed, studies that have assessed the effects of consumption of chia seed on nutritional profile, bioactive compound contents, and texture-related properties reported improved outcomes related to better formulations [10,13].
To date, several studies have reported the beneficial and protective effects of chia seed consumption on cardiovascular diseases and diabetes [14], hypertension [15], and other disorders [16]. The omega-3 fatty acids content of chia seed appears to have a positive impact upon the mechanisms of these chronic diseases [17]. However, evidence has shown that these outcomes are linked to reductions in post-prandial glycaemia, which can be attributed to the high fibre content of chia seed [18,19]. In addition to reductions in post-prandial glycaemia, appetite ratings were shown to be reduced in one study [19]. The existence of higher amounts of fibre in the diet may help to slow down digestion and thus release of glucose [20]. Given the importance of consuming whole grain foods such as chia seed, there is increasing interest in their effects on appetite and satiety measures.
Several studies have demonstrated that supplementation of common dietary products with high fibre foods or foods with beneficial compounds exerts positive effects on short-term appetite regulation and satiety [21,22]. The effects of chia seed supplementation to regular diet on short-term satiety and energy intake are largely unknown. In addition, one study reported that chia seed consumption may not affect overweight or obesity parameters in the long term [23]. Therefore, the main objective of the present study was to compare three different amounts of chia seed added to yogurt as a mid-morning snack on satiety and energy intake during subsequent meals.
Foods can induce an emotional response when eaten [24,25], but the effects of specific foods such as chia seed on this response are unclear. Recently, examination of emotions in response to food consumption was carried out using several methods [26,27]. One of these methods is the profile of mood states (POMS), which has been used to measure the effects of experimental manipulations on various emotional responses [28]. Meal intake using different compositions was shown to affect the POMS [29]. Hence, the second aim of this study was to determine whether or not different amounts of chia seed consumption influence mood states in participants.
SUBJECTS AND METHODS
Subjects
Twenty-four female subjects were recruited from Hacettepe University and the surrounding community through an announcement and advertisement. A questionnaire examining general health and nutritional habits applied to all volunteers. Inclusion criteria of the study were as follows: women in good health between the ages of 19–25 years who were non-smokers, not dieting, not diagnosed with any metabolic disease, not professional athletes, not pregnant or lactating, not using drugs, and possessing no food allergies or extreme dislikes for specific foods. None of the participants were taking medications known to affect appetite or weight regulation. Regular breakfast, snack, and lunch consumption was an additional inclusion criterion for participants. Subjects with extreme dislikes for specific foods were also excluded. Subjects were excluded if they scored > 9 on the Beck depression scale and had a measured Body Mass Index (BMI) < 18 or > 25 kg/m2. Participants' body weight, height, and body compositions were measured by Jawon XScan Plus 950 (Jawon Medical, Gyeongsan, Korea). Since the menstrual cycle may affect appetite ratings, experiment days were arranged at 1 week before menstruation for all participants. Each participant signed an informed consent document before the study. Ethical approval was confirmed by the Clinical Researches Ethics Board of Hacettepe University, number GO15/25-02.
Study design and experimental protocol
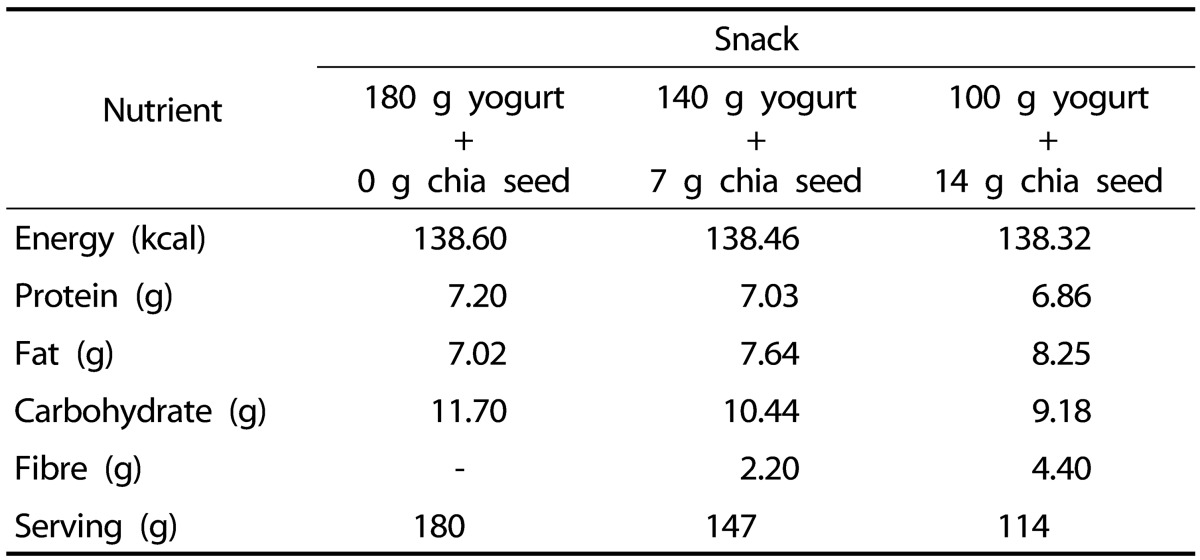
This was a cross-over study conducted at the Nutrition Laboratory in the Faculty of Health Sciences, Department of Nutrition and Dietetics, Hacettepe University, Ankara, Turkey. The experimental protocol of this study used the European consensus on postprandial studies evaluating appetite measures and eating behaviours [30]. The study was carried out on 3 separate days, with a 1-week washout period between each study day. On experiment days, participants arrived at 08.00 h after fasting for 12 h and left at 14.00 h. On the previous evening, participants ate dinner consisting of a bowl of tomato soup (200 mL), grilled meat (60 g), salad (200 g), and white bread (50 g) (total 602 kcal) and breakfast consisting of two thin slices of white bread (50 g), a slice of cheese (30 g), and a cup of tea served at 08.30 h (total 250 kcal). Participants were asked to consume the full breakfast within 15 min. Further food or beverage intake was not allowed until the mid-morning snack was served. Then, subjects were tested using three mid-morning snacks closely matched for energy content at 11.00 h. On each test day, participants were randomly assigned to eat 180 g yogurt without chia seed, 140 g yogurt with 7 g chia seed, or 100 g yogurt with 14 g chia seed. Organic chia seed, Salvia Hispanica L., was purchased from ASEL Foods, Ankara, Turkey. The nutritional composition of 100 g chia seed was as follows: energy 438 kcal, fat 31.1 g, omega-3 18.5 g, omega-6 5.7 g, fibre 31.4 g, and protein 20.4 g. Table 1 shows the nutritional compositions of the mid-morning snacks. After 2 hours, ad libitum lunch was served. Lunch consisted of pasta and a soft drink. The serving table was a separate dining table at a moderately close distance where the subjects sat. On each test day, the same amounts and types of foods were served, and the buffet items were identical. Same portion sizes, serving cutlery, and serving bowls were used on the test days. The subjects were instructed to eat lunch until satisfied and allowed to refill their plate whenever they wanted. Throughout the study, participants were in the same room and allowed to read or use laptops throughout the experiment. Physical activity and social interaction were limited. Subjects were not allowed to see how much other subjects consumed. Energy and macronutrient intakes of subjects were measured by weighing the amounts of food and drinks consumed and converting these values into energy (kcal) and macronutrients based on the manufacturer's labelling. Participants were informed that the topic of the research was to examine their energy intakes on different test days and were not given any detailed information about the compositions of the mid-morning snacks.
Table 1. Nutritional composition of the mid-morning snacks containing 0 g, 7 g, or 14 g chia seed.
Visual analogue scales
Visual analogue scales (VAS) was used to assess hunger, satiety, prospective food consumption, amounts of food they could consume, and desire for sugary foods throughout the study period [31]. Appetite ratings were recorded on 100 mm VAS with words anchored at each end describing the extremes of a unipolar question (for instance, for hunger: “I am not hungry at all”/“I have never been more hungry”, for satiety: “I am not sated at all”/“I have never been more sated”, for prospective food consumption: “I cannot consume any food at all”/“I have never wanted to consume food that much”, for desire for sugary snack: “I do not want to consume a sugary snack at all”/“I have never wanted to consume a sugary snack that much”, for amount of food: “I can only have a small amount of food”/“I can eat a large amount of food”). VAS scores were measured before breakfast and until the end of the study for a total of 23 times. Before the study period, subjects were informed on how to fill out VAS forms.
POMS
POMS is commonly used to measure the effects of experimental manipulations on various approaches [24]. The validated form of POMS was used in the current study [32]. POMS measures six identifiable mood or affective factors: “tension-anxiety”, “depression-dejection”, “anger-hostility”, “vigor-activity”, “fatigueinertia”, and “confusion-bewilderment”. The score for each factor was calculated by adding the responses that defined the mood factor. The response numbers defining each mood were as follows: “0 = not at all”, “1 = a little”, “2 =moderately”, “3 = quite a bit”, and “4 = extremely”.
Statistical analyses
Data were analysed using the Statistical Package for the Social Sciences (SPSS) version 22 (SPSS Inc., Chicago, IL, USA). The primary outcome of this trial was to assess the effects of different amounts of chia seed consumption with yogurt as a mid-morning snack on energy and macronutrient intakes during an ad libitum lunch. For the primary outcome, data were analysed using a general linear model, ANOVA. Where ANOVA was significant, Bonferroni post-hoc analysis was used for comparisons between conditions. The secondary outcome variables were subjects' VAS and POMS scores. For the secondary outcome, data were analysed using a repeated measures ANCOVA with baseline measurement as the covariate. Subjects and test day were included in the procedure, in addition to the mid-morning snack/time interaction. Data on the area under the curve (AUC) for VAS were obtained using GraphPad Prism version 6 (Graphpad Software Inc., La Jolla, CA, USA). Baseline values were added as covariates in the AUC data. Data were given as mean ± standard error of the mean unless otherwise stated. Power analysis indicated that 24 subjects per condition were required in order to estimate a minimum effect size of 18.35% (for energy intake difference) for comparisons between treatment arms (yielding a power of 0.80 and alpha 0.05) [33]. P < 0.05 was considered statistically significant.
RESULTS
Subjects
All participants completed the study successfully, and all subject data were analysed for each test meal. Participants were 23.12 ± 1.92 (mean ± standard error) years of age, weighed 56.71 ± 7.11 kg (mean ± standard error) with a BMI of 21.53 ± 2.31 kg/m2 (mean ± standard error), and had a waist circumference of 74.06 ± 7.94 cm (mean ± standard error).
VAS and POMS scores
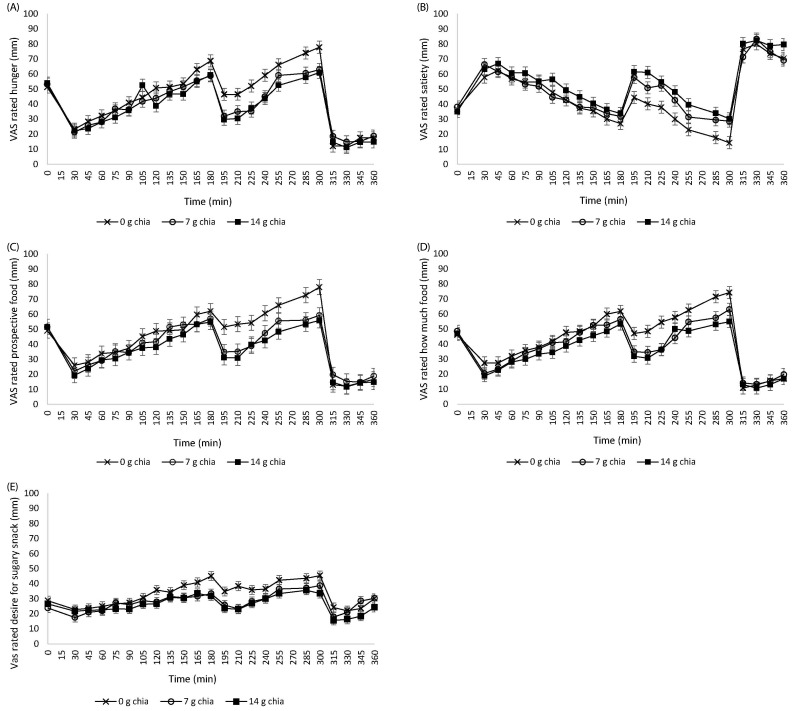
Fig. 1 shows VAS-rated hunger (a), satiety (b), prospective food consumption (c), amounts of food that could be consumed (d), and desire for sugary foods (e). Baseline values did not differ between test days (P > 0.05). VAS scores were significantly influenced by consumption of different amounts of chia seed with yogurt as a mid-morning snack. Participants reported significantly lower scores for hunger (P = 0.033), prospective food consumption (P = 0.031), amounts of food that could be consumed (P = 0.017), desire for sugary foods (P = 0.015), and higher scores for satiety (P = 0.031) on the test days with 7 g and 14 g chia seed when compared to 0 g chia seed. These differences were pronounced at 210, 225, 285,, and 300 minutes. Furthermore, AUC data of VAS scores indicated that hunger was significantly reduced (P = 0.048), and satiety was significantly increased (P = 0.017) on the test days with 7 g and 14 g chia seed when compared to 0 g chia seed (Table 2). AUC data on prospective food consumption, amounts of food that could be consumed, and desire for sugary foods were not different between the study days (P > 0.05).
Fig. 1. Mean VAS scores (± SEM) during the 3 test days, n = 24.
(A) VAS-rated hunger, (B) VAS-rated satiety, (C) VAS-rated prospective food consumption, (D) VAS-rated food amount, and (E) VAS-rated desire for sugary snack. A light breakfast was served at 08.30 h, immediately after recording baseline VAS scores. Lunch was served at 13.00 h. Repeated measures indicated that VAS scores were significantly influenced on the test days with 7 g chia seed and 14 g chia seed when compared to 0 g chia seed (main effect of treatment, P < 0.05). Post-hoc analysis did not indicate a significant difference between the groups (7 g chia seed and 14 g chia seed). VAS, visual analog scale; SEM, standard error of the mean.
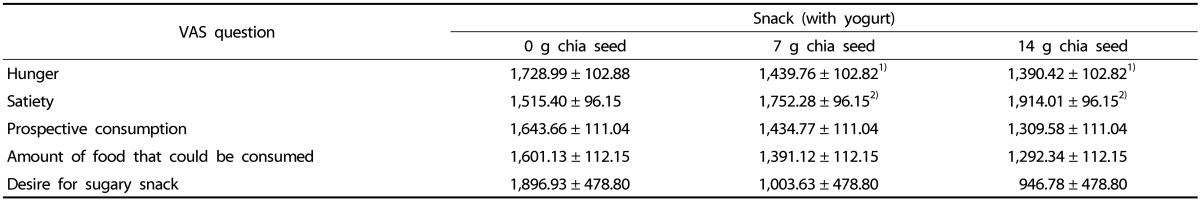
Table 2. AUC data of VAS scores during the 3 test days.
AUC, area under the curve; VAS, visual analog scale; SEM, standard error of the mean.
Mean VAS scores (± SEM) during the 3 test days, n = 24.
1 )ANOVA indicated that AUC data of VAS score of hunger was significantly reduced on the test days with 7 g chia seed and 14 g chia seed compared to 0 g chia seed (main effect of treatment, P = 0.048).
2)ANOVA indicated that AUC data of VAS score of satiety significantly increased on the test days with 7 g chia seed and 14 g chia seed compared to 0 g chia seed (main effect of treatment, P = 0.017). Post-hoc analysis did not indicate a significant difference between the groups (7 g chia seed and 14 g chia seed).
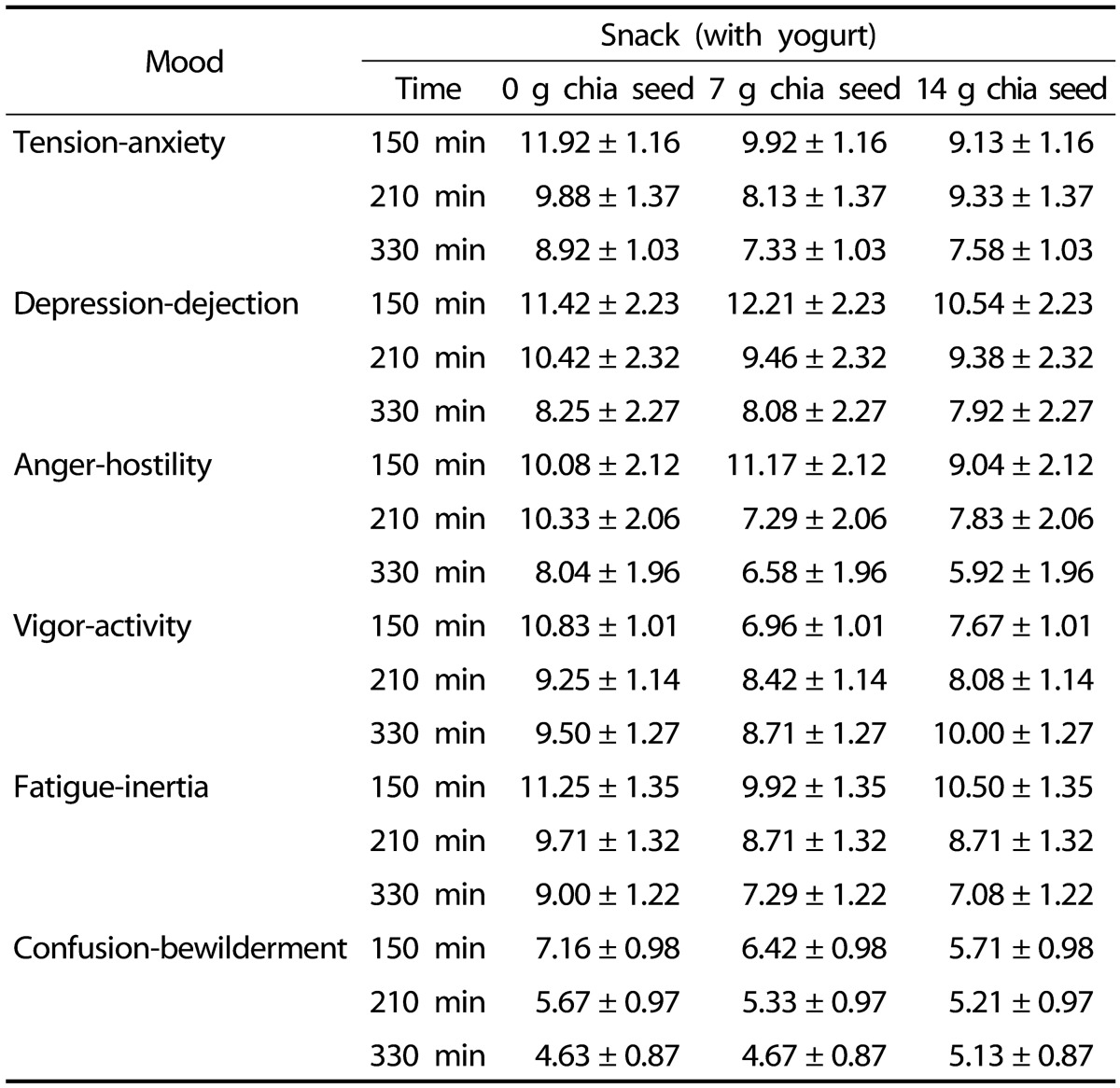
Table 3 shows POMS scores that were recorded at three different time points throughout the study. Mood states of tension-anxiety, depression-dejection, anger-hostility, vigor-activity, fatigues-inertia, and confusion-bewilderment did not exhibit different scores between the test days and time of measurements (P > 0.05).
Table 3. POMS data of the participants recorded at 150 min, 210 min, and 330 min during the 3 test days.
POMS, profile of mood states; SEM, standard error of the mean.
Mean POMS scores (± SEM) recorded at 150 min, 210 min, and 330 min during the 3 test days, n = 24. Repeated measures indicate that POMS scores were not influenced by snack consumption or time (P > 0.05).
Energy intakes
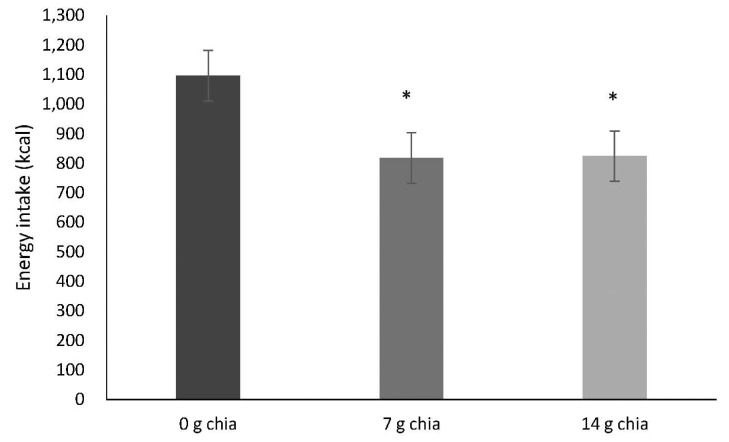
Fig. 2 indicates the mean energy intakes during ad libitum lunch on the test days. When the participants consumed yogurt with 7 g chia seed or 14 g chia seed, energy intakes from the following ad libitum meal were reduced by 25.39% and 24.75%, respectively (0 g chia seed: 1,095.78 ± 85.34 kcal, 7 g chia seed: 817.54 ± 85.34 kcal, 14 g chia seed: 824.10 ± 85.35 kcal, P = 0.037). No significant difference was detected between 7 g chia seed and 14 g chia seed consumption (Fig. 2).
Fig. 2. Mean energy intake (kcal) (± SEM) during the ad libitum lunch on 3 test days, n = 24.
* ANOVA found that energy intake was significantly reduced on the test days with 7 g and 14 g chia seed compared to 0 g chia seed (main effect of treatment, P = 0.037). Post-hoc analysis did not indicate a significant difference between the groups (7 g chia seed and 14 g chia seed).
DISCUSSION
The present study examined the acute effects of two different amounts of chia seed supplementation on satiety outcomes, energy intake, and POMS for the first time. Both 7 g and 14 g chia seed with plain yogurt induced an increased feeling of satiety and reduced feeling of hunger in participants. Moreover, total energy intake during the prospective ad libitum lunch was nearly 25% lower on these experiment days, and mood profile was not influenced by these factors. These results suggest that chia seed consumption can be a useful dietary strategy in the prevention of overweight and obesity status in healthy individuals.
The amounts of chia seed used in the present study, 7 g and 14 g, were chosen to match the European Union decision, which approved unprocessed chia seed as a novel food ingredient to be sold as pre-packed chia seed with a recommended daily intake of up to 15 g [34]. One study that previously examined the effects of different amounts of chia seed consumption on post-prandial glycaemia indicated a strong dose-response effect, which led us to assess a minimal dose of 7 g chia seed in the current study [18]. However, neither satiety-related measurements nor mood state scores showed a significant difference between 7 g and 14 g chia seed consumption in the current study. This outcome can be attributed to the threshold for satiating effectiveness of chia seed, as shown using different dietary components in other studies [35,36]. Based on this result, it can be suggested that the threshold for energy intake reduction by chia seed is 7 g, although further studies are needed to clarify this outcome.
The ability of chia seed to provide a sense of higher satiety observed in the current study appears to be consistent across various study designs within an acute period [18,19,36]. Due to the evidence of reduced postprandial glycaemia and increased satiety [18], this acute effect of chia seed can be associated with its high fibre content. It is well-established that dietary fibre can prolong satiety through various mechanisms such as delaying gastric emptying, slowing the glycemic response, and increasing gastric distension [37]. Hence, ingestion of chia seed may induce the sense of fullness, and this effect may continue until the following meal. In addition to fibre, an abundance of omega-3 fatty acids in chia seed may play a role in appetite suppression. Although there are very limited data about the acute effects of omega-3 fatty acids on the satiety response, few studies have reported the potential effects of long-chain poly unsaturated fatty acids on food intake modulation and gut peptides [38,39]. In the current study, the nutritional compositions of the mid-morning snacks consumed on test days were closely matched by modifying their yogurt contents. Despite slight differences in macronutrients, energy values were identical. Therefore, the outcomes of this study can be linked to chia seeds' nutrient contents, although the volume of test meals was also shown to be an important factor when examining satiety in such studies [40]. Although the test meals used in the current study had different volumes, our results indicate that the lower volume of the test meals with chia seed was adequate to induce satiety and reduce hunger.
Studies that examined the addition of chia seed to several food products reported positive outcomes on physical and rheological measurements [10]. In addition to physical and rheological properties, consumer acceptance is an important parameter in such studies. The palatability response or liking of mid-morning snacks was not measured in the current study. Regarding liking or disliking, palatability and pleasantness of foods can interact with appetite and satiation, and the amount of eaten food may vary according to these parameters [41]. Most studies that assessed chia seed as an additive to traditional recipes obtained acceptable sensory parameters [42,43,44]. Despite a lack of data about participants' acceptance of the midmorning snacks in the current study, none of the participants reported an unpleasantness regarding the taste of yogurts on any of the test days. Due to evidence that colour, visual texture, and odour of foods are influential on the amount of food ingestion [45], consumer acceptance and pleasantness of the test meals should be evaluated in future studies using chia seed with yogurt.
The effects of acute chia seed consumption on POMS scores were explored in the current study to determine whether or not chia seed supplementation exerts any undesirable sense or mood state. The link between these parameters originated from a different study, which reported that ingestion of snacks with different nutritional compositions is associated with better mood aspects [46]. Chia seed consumption did not alter mood states in this study. This result is similar to another study, which examined the long-term effects of consumption of 50 g/day chia seed for 12 weeks on symptoms for pain, allergies, stress, focus/concentration, and overall well-being in overweight subjects [23].
The effects of long-term consumption of chia seed on overweight- or obesity-related parameters remain controversial unlike acute-term studies [14,23,47]. Significant reductions in body weight were observed after 6 months of chia seed consumption in one study [47], whereas no change in body weight was reported following 12 weeks of consumption in another study [23]. Difficulties in standardization of study designs and metabolic and nutritional differences in study populations may have contributed to these results [14,15]. For this reason, translation of the current evidence on the satiating effects of chia seed in acute-term studies into long-term assumptions related to weight loss is still premature. Although numerous studies have reported that ingestion of foods with healthy nutritional compositions may induce satiety, reduce hunger, and lower energy intake in prospective meals, further clinical trials are needed to identify the precise effects of chia seed consumption on these parameters [18,19].
Finally, there are a few limitations in the current study. Firstly, the outcomes of this study were obtained from female subjects in order to eliminate the confounding effects of sex differences on eating behaviours [48]. Secondly, participants were tested in a nutrition laboratory where their habitual eating parameters might be affected due to the different environment. Therefore, future studies should focus on conducting research in more natural settings including male subjects as well as assessing biochemical parameters related with appetite. In addition, it was shown that nutritional behaviours can be altered in subjects who are overweight or obese due to motivational and emotional drives related to food intake [49]. Hence, testing a similar hypothesis in different clinical groups may help to clarify the exact mechanisms underlying the satiating effects of chia seed.
In conclusion, the present study showed that consumption of 7 g or 14 g of chia seed with plain yogurt as a mid-morning snack induced an increased senses of fullness. This outcome was reflected in energy intake of participants during the following meal. In addition, chia seed consumption did not influence mood states. The basis for the current study was the hypothesis that supplementation of regular diet with chia seed may induce beneficial effects on sensory outcomes related to acute-term satiety and mood states. Since several biochemical and physical mechanisms underlie these principal aims of the current study, future studies will continue to examine related indicators.
Footnotes
CONFLICT OF INTEREST: The authors declare no potential conflicts of interests.
References
- 1.Sofer S, Stark AH, Madar Z. Nutrition targeting by food timing: time-related dietary approaches to combat obesity and metabolic syndrome. Adv Nutr. 2015;6:214–223. doi: 10.3945/an.114.007518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jung HS, Lim Y, Kim EK. Therapeutic phytogenic compounds for obesity and diabetes. Int J Mol Sci. 2014;15:21505–21537. doi: 10.3390/ijms151121505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aoe S, Ikenaga T, Noguchi H, Kohashi C, Kakumoto K, Kohda N. Effect of cooked white rice with high β-glucan barley on appetite and energy intake in healthy Japanese subjects: a randomized controlled trial. Plant Foods Hum Nutr. 2014;69:325–330. doi: 10.1007/s11130-014-0437-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perrigue MM, Monsivais P, Drewnowski A. Added soluble fiber enhances the satiating power of low-energy-density liquid yogurts. J Am Diet Assoc. 2009;109:1862–1868. doi: 10.1016/j.jada.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Ewart-Pierce E, Mejía Ruiz MJ, Gittelsohn J. “Whole-of-Community” obesity prevention: a review of challenges and opportunities in multilevel, multicomponent interventions. Curr Obes Rep. 2016;5:361–374. doi: 10.1007/s13679-016-0226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ullah R, Nadeem M, Khalique A, Imran M, Mehmood S, Javid A, Hussain J. Nutritional and therapeutic perspectives of chia (Salvia hispanica L.): a review. J Food Sci Technol. 2016;53:1750–1758. doi: 10.1007/s13197-015-1967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayerza R, Coates W. Composition of chia (Salvia hispanica) grown in six tropical and subtropical ecosystems of South America. Trop Sci. 2004;44:131–135. [Google Scholar]
- 8.Mohd Ali N, Yeap SK, Ho WY, Beh BK, Tan SW, Tan SG. The promising future of chia, Salvia hispanica L. J Biomed Biotechnol. 2012;2012:171956. doi: 10.1155/2012/171956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valdivia-López MÁ, Tecante A. Chia (Salvia hispanica): a review of native mexican seed and its nutritional and functional properties. Adv Food Nutr Res. 2015;75:53–75. doi: 10.1016/bs.afnr.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Julio LM, Ixtaina VY, Fernández M, Torres Sánchez RM, Nolasco SM, Tomás MC. Development and characterization of functional O/W emulsions with chia seed (Salvia hispanica L.) by-products. J Food Sci Technol. 2016;53:3206–3214. doi: 10.1007/s13197-016-2295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandoval-Oliveros MR, Paredes-López O. Isolation and characterization of proteins from chia seeds (Salvia hispanica L.) J Agric Food Chem. 2013;61:193–201. doi: 10.1021/jf3034978. [DOI] [PubMed] [Google Scholar]
- 12.Reyes-Caudillo E, Tecante A, Valdivia-López MA. Dietary fibre content and antioxidant activity of phenolic compounds present in Mexican chia (Salvia hispanica L.) seeds. Food Chem. 2008;107:656–663. [Google Scholar]
- 13.Menga V, Amato M, Phillips TD, Angelino D, Morreale F, Fares C. Gluten-free pasta incorporating chia (Salvia hispanica L.) as thickening agent: an approach to naturally improve the nutritional profile and the in vitro carbohydrate digestibility. Food Chem. 2017;221:1954–1961. doi: 10.1016/j.foodchem.2016.11.151. [DOI] [PubMed] [Google Scholar]
- 14.Vuksan V, Whitham D, Sievenpiper JL, Jenkins AL, Rogovik AL, Bazinet RP, Vidgen E, Hanna A. Supplementation of conventional therapy with the novel grain Salba (Salvia hispanica L.) improves major and emerging cardiovascular risk factors in type 2 diabetes: results of a randomized controlled trial. Diabetes Care. 2007;30:2804–2810. doi: 10.2337/dc07-1144. [DOI] [PubMed] [Google Scholar]
- 15.Toscano LT, da Silva CS, Toscano LT, de Almeida AE, Santos Ada C, Silva AS. Chia flour supplementation reduces blood pressure in hypertensive subjects. Plant Foods Hum Nutr. 2014;69:392–398. doi: 10.1007/s11130-014-0452-7. [DOI] [PubMed] [Google Scholar]
- 16.Valenzuela R, Bascuñán K, Chamorro R, Barrera C, Sandoval J, Puigrredon C, Parraguez G, Orellana P, Gonzalez V, Valenzuela A. Modification of docosahexaenoic acid composition of milk from nursing women who received alpha linolenic acid from chia oil during gestation and nursing. Nutrients. 2015;7:6405–6424. doi: 10.3390/nu7085289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin F, Nieman DC, Sha W, Xie G, Qiu Y, Jia W. Supplementation of milled chia seeds increases plasma ALA and EPA in postmenopausal women. Plant Foods Hum Nutr. 2012;67:105–110. doi: 10.1007/s11130-012-0286-0. [DOI] [PubMed] [Google Scholar]
- 18.Ho H, Lee AS, Jovanovski E, Jenkins AL, Desouza R, Vuksan V. Effect of whole and ground Salba seeds (Salvia Hispanica L.) on postprandial glycemia in healthy volunteers: a randomized controlled, dose-response trial. Eur J Clin Nutr. 2013;67:786–788. doi: 10.1038/ejcn.2013.103. [DOI] [PubMed] [Google Scholar]
- 19.Vuksan V, Jenkins AL, Dias AG, Lee AS, Jovanovski E, Rogovik AL, Hanna A. Reduction in postprandial glucose excursion and prolongation of satiety: possible explanation of the long-term effects of whole grain Salba (Salvia Hispanica L.) Eur J Clin Nutr. 2010;64:436–438. doi: 10.1038/ejcn.2009.159. [DOI] [PubMed] [Google Scholar]
- 20.Razzaq HA, Sutton KH, Motoi L. Modifying glucose release from high carbohydrate foods with natural polymers extracted from cereals. J Sci Food Agric. 2011;91:2621–2627. doi: 10.1002/jsfa.4500. [DOI] [PubMed] [Google Scholar]
- 21.Doyon CY, Tremblay A, Rioux LE, Rhéaume C, Cianflone K, Poursharifi P, Turgeon SL. Acute effects of protein composition and fibre enrichment of yogurt consumed as snacks on appetite sensations and subsequent ad libitum energy intake in healthy men. Appl Physiol Nutr Metab. 2015;40:980–989. doi: 10.1139/apnm-2014-0403. [DOI] [PubMed] [Google Scholar]
- 22.Arshad MU, Ishtiaq S, Anjum FM, Saeed F, Chatha SA, Imran A. Acute effects of different dietary polysaccharides added in milk on food intake, postprandial appetite and glycemic responses in healthy young females. Int J Food Sci Nutr. 2016;67:715–722. doi: 10.1080/09637486.2016.1191446. [DOI] [PubMed] [Google Scholar]
- 23.Nieman DC, Cayea EJ, Austin MD, Henson DA, McAnulty SR, Jin F. Chia seed does not promote weight loss or alter disease risk factors in overweight adults. Nutr Res. 2009;29:414–418. doi: 10.1016/j.nutres.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Lieberman HR, Wurtman JJ, Chew B. Changes in mood after carbohydrate consumption among obese individuals. Am J Clin Nutr. 1986;44:772–778. doi: 10.1093/ajcn/44.6.772. [DOI] [PubMed] [Google Scholar]
- 25.Osdoba KE, Mann T, Redden JP, Vickers Z. Using food to reduce stress: effects of choosing meal components and preparing a meal. Food Qual Prefer. 2015;39:241–250. [Google Scholar]
- 26.Cardello AV, Meiselman HL, Schutz HG, Craig C, Given Z, Lesher LL, Eicher S. Measuring emotional responses to foods and food names using questionnaires. Food Qual Prefer. 2012;24:243–250. [Google Scholar]
- 27.King SC, Meiselman HL, Carr BT. Measuring emotions associated with foods in consumer testing. Food Qual Prefer. 2010;21:1114–1116. [Google Scholar]
- 28.Mento C, Le Donne M, Crisafulli S, Rizzo A, Settineri S. BMI at early puerperium: body image, eating attitudes and mood states. J Obstet Gynaecol. 2017;37:428–434. doi: 10.1080/01443615.2016.1250727. [DOI] [PubMed] [Google Scholar]
- 29.Kien CL, Bunn JY, Tompkins CL, Dumas JA, Crain KI, Ebenstein DB, Koves TR, Muoio DM. Substituting dietary monounsaturated fat for saturated fat is associated with increased daily physical activity and resting energy expenditure and with changes in mood. Am J Clin Nutr. 2013;97:689–697. doi: 10.3945/ajcn.112.051730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blundell J, de Graaf C, Hulshof T, Jebb S, Livingstone B, Lluch A, Mela D, Salah S, Schuring E, van der Knaap H, Westerterp M. Appetite control: methodological aspects of the evaluation of foods. Obes Rev. 2010;11:251–270. doi: 10.1111/j.1467-789X.2010.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 32.Selvi Y, Gulec M, Aydin A, Besiroglu L. Psychometric evaluation of the Turkish language version of the Profile of Mood States (POMS) J Mood Disord. 2011;1:152–161. [Google Scholar]
- 33.Tabachnick BG, Fidell LS. Using Multivariate Statistics. 3rd ed. New York (NY): HarperCollins College Publishers; 1996. [Google Scholar]
- 34.European Commission. Commission Implementing Decision of 22 January 2013: authorising an extension of use of chia (Salvia hispanica) seed as a novel food ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council (2013/50/EU) Brussels: Official Journal of the European Union; 2013. [Google Scholar]
- 35.Veldhorst MA, Nieuwenhuizen AG, Hochstenbach-Waelen A, van Vught AJ, Westerterp KR, Engelen MP, Brummer RJ, Deutz NE, Westerterp-Plantenga MS. Dose-dependent satiating effect of whey relative to casein or soy. Physiol Behav. 2009;96:675–682. doi: 10.1016/j.physbeh.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Vuksan V, Choleva L, Jovanovski E, Jenkins AL, Au-Yeung F, Dias AG, Ho HV, Zurbau A, Duvnjak L. Comparison of flax (Linum usitatissimum) and Salba-chia (Salvia hispanica L.) seeds on postprandial glycemia and satiety in healthy individuals: a randomized, controlled, crossover study. Eur J Clin Nutr. 2017;71:234–238. doi: 10.1038/ejcn.2016.148. [DOI] [PubMed] [Google Scholar]
- 37.Benini L, Castellani G, Brighenti F, Heaton KW, Brentegani MT, Casiraghi MC, Sembenini C, Pellegrini N, Fioretta A, Minniti G, Porrini M, Testolin G, Vantini I. Gastric emptying of a solid meal is accelerated by the removal of dietary fibre naturally present in food. Gut. 1995;36:825–830. doi: 10.1136/gut.36.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buckley JD, Howe PR. Long-chain omega-3 polyunsaturated fatty acids may be beneficial for reducing obesity-a review. Nutrients. 2010;2:1212–1230. doi: 10.3390/nu2121212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harden CJ, Dible VA, Russell JM, Garaiova I, Plummer SF, Barker ME, Corfe BM. Long-chain polyunsaturated fatty acid supplementation had no effect on body weight but reduced energy intake in overweight and obese women. Nutr Res. 2014;34:17–24. doi: 10.1016/j.nutres.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Kral TV, Roe LS, Rolls BJ. Combined effects of energy density and portion size on energy intake in women. Am J Clin Nutr. 2004;79:962–968. doi: 10.1093/ajcn/79.6.962. [DOI] [PubMed] [Google Scholar]
- 41.De Graaf C, De Jong LS, Lambers AC. Palatability affects satiation but not satiety. Physiol Behav. 1999;66:681–688. doi: 10.1016/s0031-9384(98)00335-7. [DOI] [PubMed] [Google Scholar]
- 42.Borneo R, Aguirre A, León AE. Chia (Salvia hispanica L) gel can be used as egg or oil replacer in cake formulations. J Am Diet Assoc. 2010;110:946–949. doi: 10.1016/j.jada.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 43.Rodrigues JB, Paixão JA, Cruz AG, Bolini HM. Chocolate milk with chia oil: ideal sweetness, sweeteners equivalence, and dynamic sensory evaluation using a time-intensity methodology. J Food Sci. 2015;80:S2944–S2949. doi: 10.1111/1750-3841.13120. [DOI] [PubMed] [Google Scholar]
- 44.Luna Pizarro P, Almeida EL, Coelho AS, Sammán NC, Hubinger MD, Chang YK. Functional bread with n-3 alpha linolenic acid from whole chia (Salvia hispanica L.) flour. J Food Sci Technol. 2015;52:4475–4482. doi: 10.1007/s13197-014-1477-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li XE, Jervis SM, Drake MA. Examining extrinsic factors that influence product acceptance: a review. J Food Sci. 2015;80:R901–R909. doi: 10.1111/1750-3841.12852. [DOI] [PubMed] [Google Scholar]
- 46.Leidy HJ, Todd CB, Zino AZ, Immel JE, Mukherjea R, Shafer RS, Ortinau LC, Braun M. Consuming high-protein soy snacks affects appetite control, satiety, and diet quality in young people and influences select aspects of mood and cognition. J Nutr. 2015;145:1614–1622. doi: 10.3945/jn.115.212092. [DOI] [PubMed] [Google Scholar]
- 47.Vuksan V, Jenkins AL, Brissette C, Choleva L, Jovanovski E, Gibbs AL, Bazinet RP, Au-Yeung F, Zurbau A, Ho HV, Duvnjak L, Sievenpiper JL, Josse RG, Hanna A. Salba-chia (Salvia hispanica L.) in the treatment of overweight and obese patients with type 2 diabetes: a double-blind randomized controlled trial. Nutr Metab Cardiovasc Dis. 2017;27:138–146. doi: 10.1016/j.numecd.2016.11.124. [DOI] [PubMed] [Google Scholar]
- 48.Asarian L, Geary N. Sex differences in the physiology of eating. Am J Physiol Regul Integr Comp Physiol. 2013;305:R1215–R1267. doi: 10.1152/ajpregu.00446.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Provencher V, Jacob R. Impact of perceived healthiness of food on food choices and intake. Curr Obes Rep. 2016;5:65–71. doi: 10.1007/s13679-016-0192-0. [DOI] [PubMed] [Google Scholar]