Abstract
Vitamin-D insufficiency and sarcoidosis are more common and severe in African Americans (AA) than Caucasians. In sarcoidosis, substrate-dependent extrarenal 1,25-dihydroxyvitamin-D (1,25-(OH)2D) production is thought to contribute to hypercalciuria and hypercalcemia, and vitamin-D repletion is often avoided. However, the anti-inflammatory properties of vitamin-D may also be beneficial. We prospectively examined serum vitamin-D levels, calcium balance, and the effects of vitamin-D repletion in 86 AA and Caucasian patients with biopsy-proven active sarcoidosis from the USA (US) and Italy (IT) in university-affiliated outpatient clinics. Clinical features, pulmonary function, and calciotropic hormones were measured. 16 patients with vitamin-D deficiency and normal serum ionized calcium (Ca2+) were treated with oral ergocalciferol (50,000 IU/week) for 12 weeks. Baseline mineral parameters were similar in US (93% AA) and IT (95% Caucasian) patients irrespective of glucocorticoid treatment. Pulmonary dysfunction was less pronounced in IT patients. Nephrolithiasis (in 11% US, 17% IT patients) was associated with higher urinary calcium excretion. Vitamin-D deficiency was not more prevalent in patients compared to the respective general populations. As serum 25-hydroxyvitamin-D (25-OHD) rose postrepletion, serum 1,25-(OH)2D, γ-globulins, and the previously elevated angiotensin converting enzyme (ACE) levels declined. Asymptomatic reversible increases in Ca2+ or urinary calcium/creatinine (Ca/Cr) developed in three patients during repletion. In conclusion, Caucasian and AA patients show similar calcium and vitamin D profiles. The higher prevalence of hypercalciuria and nephrolithiasis in sarcoidosis is unrelated to endogenous vitamin-D levels. Vitamin-D repletion in sarcoidosis is generally safe, although calcium balance should be monitored. A hypothesis that 25-OHD repletion suppresses granulomatous immune activity is provided.
INTRODUCTION
Sarcoidosis is a multisystem disorder of undetermined etiology characterised by widespread non-caseating granulomatous inflammation.1 Patients most commonly present with bilateral hilar lymphadenopathy, pulmonary infiltrates, and skin and ocular lesions,2 although all organs may be involved. Activated macrophages and lymphocytes within the granuloma mediate extrarenal hydroxylation of 25-hydroxyvitamin D (25-OHD) to its active metabolite 1,25-dihydroxyvitamin D (1,25-(OH)2D) independent of parathyroid hormone (PTH) or calcium regulation3 but dependent on substrate (25-OHD) concentration.4 Increased 1,25-(OH)2D, in turn, stimulates gastrointestinal calcium absorption and renal tubular calcium reabsorption, suppresses PTH production and osteoclast bone resorption, and promotes osteoblast bone mineralisation, thereby predisposing to the development of hypercalcemia, hypercalciuria and the formation of kidney stones.5–9 Some have suggested that in sarcoidosis, vitamin D supplementation may induce hypercalcemia and hypercalciuria at doses normally insufficient to cause these alterations in healthy subjects.10–13 On the other hand, vitamin D also possesses antimicrobial and anti-inflammatory properties, and is known to modulate innate and adaptive immunity and mitigate granulomatous inflammation.14,15 In this context, low endogenous vitamin D levels may potentially aggravate granulomatous inflammation in sarcoidosis while repletion of vitamin D levels may serve a beneficial adaptive role. The relative importance of these divergent mechanisms remains to be established.
Both sarcoidosis16 and vitamin D deficiency17 18 are more common and more severe in African Americans (AA) than in Caucasians. It is plausible that the risk of calcium and vitamin D disturbance (hypercalcemia, hypercalciuria, and nephrolithiasis) and the response to vitamin D supplementation may also differ between AA and Caucasian patients with sarcoidosis. To clarify this issue, we compared the calcium and vitamin D profiles between predominantly AA sarcoidosis patients in USA (US) and predominantly Caucasian sarcoidosis patients in Italy (IT), and examined whether vitamin D repletion raises the risk of hypercalcemia and hypercalciuria in these two cohorts.
SUBJECTS AND METHODS
Subjects
A total of 44 patients were recruited from the Sarcoidosis Clinic at the Parkland Health & Hospital System, University of Texas Southwestern Medical Center in Dallas, Texas, USA. A total of 42 patients were recruited from the outpatient clinics of the Second University of Naples and the University Federico II of Naples in Naples, and the University of Padova in Padova, Italy. All patients had biopsy-proven active granulomatous disease consistent with the clinical diagnosis of sarcoidosis. Exclusion criteria included a history of malignancy, creatinine clearance <70 mL/min, and primary hyperparathyroidism.
Data from a separate multiethnic population-based study (Dallas Heart Study, DHS19) consisting of 3447 Dallas residents without sarcoidosis including 1766 AA adults served as historical control for the assessment of vitamin D status and comparison with US patients with sarcoidosis. Another population-based cohort, the Progetto Veneto Anziani study (Pro.V.A)20 consisting of 1927 Italian Caucasians without sarcoidosis served as historical control for the assessment of vitamin D status and comparison with IT patients with sarcoidosis. The Institutional Review Board of the University of Texas Southwestern Medical Center and the Ethics Committee of the University Hospital of the Second University of Naples approved the protocol. Written informed consent was obtained from all subjects.
Measurements
Demographic information, as well as clinical and laboratory profiles and treatment regimens, was reviewed. Fasting venous blood was drawn for serum chemistry, 25-OHD, 1,25-(OH)2D, and PTH, and a 24 h urine sample was collected for the measurement of the total volume, creatinine, and calcium. None of the patients was taking a pharmacological repletion dose of vitamin D. A few were taking over-the-counter vitamin D and or calcium supplements, which were stopped at least 1 week prior to undergoing study. Following baseline studies, 16 US patients with reduced serum 25-OHD (<75 nmol/L) who consented to undergo repletion therapy were prescribed ergocalciferol (50,000 IU) orally once a week for 12 weeks. Serum and 24 h urinary calcium levels were measured every 4 weeks. Adherence was assured by telephone calls, monthly office visits and by pill count at the end of the study.
Analytical procedures
Serum calcium, phosphorus, and creatinine were analyzed as part of a systematic multichannel analysis (SYNCHRON CX9 ALX system; Beckman Coulter Fullerton, California, USA). Serum 1,25-(OH)2D and PTH were measured by ELISA (IDS Immunodiagnostic Systems, Gaithersburg, Maryland, USA). Serum 25-OHD was measured by ELISA (IDS Immunodiagnostic Systems, Gaithersburg, Maryland, USA) or direct competitive chemiluminescence immunoassay (Liaison, DiaSorin, Turin, Italy). γ-Globulins were quantified on the basis of serum protein electrophoresis and immunofixation. Serum angiotensin converting enzyme (ACE) level was quantified on the basis of spectrophotometric quantification (absorbance at 340 nm) by the Mayo Laboratory. Urinary calcium was analyzed by atomic absorption spectrophotometry.
Statistical analysis
Normal distribution of the variables was established using histograms and the Wilk-Shapiro normality test. All results were reported as mean±SD. Two-sample t-tests were used to evaluate statistical significance among groups. The paired t test was used to compare results before and after ergocalciferol supplementation. Pearson correlation was used to analyze the relationship between variables. Statistical significance was set at two-tailed α=0.05. Microsoft Excel and SAS V.9.4 (SAS Institute, Cary, North Carolina, USA) were used to perform statistical analysis.
RESULTS
Demographics
Patient characteristics are shown in table 1. Ninety five per cent of IT patients were Caucasian while 93% of US patients were of African descent. Both populations had a preponderance of middle-aged females. Multisystem organ involvement was similar in both populations.
Table 1.
Demographic, pulmonary functions, organ involvement, and serum 25-OHD levels in US & IT patients, and control cohorts
| Dallas, USA |
Naples/Padova, Italy |
p Value | |
|---|---|---|---|
| Patients (n) | 44 | 42 | |
| Gender (male/female) | 5/39 | 16/26 | 0.004 |
| Race (Caucasians/AA) | 3/41 | 40/2 | <0.0001 |
| Age (years) | 49.5±10.2 | 54.0±13.0 | 0.07 |
| Height (m) | 1.64±0.09 | 1.62±0.09 | 0.46 |
| Weight (kg) | 83.0±18.6 | 74.5±12.5 | 0.02 |
| BMI (kg/m2) | 30.9±7.5 | 28.2±4.4 | 0.05 |
| Prednisone use (%) | 48 | 74 | 0.004 |
| Kidney stones (%) | 11 | 17 | 0.42 |
| Pulmonary functions | |||
| FVC (L) | 2.46±0.65 | 3.00±1.00 | 0.004 |
| FVC (% predicted) | 76.8±15.4 | 94.1±17.2 | <0.0001 |
| FEV1 (L) | 1.88±0.56 | 2.72±0.95 | <0.0001 |
| FEV1 (% predicted) | 71.8±16.7 | 92.8±19.3 | <0.0001 |
| FEV1/FVC ratio | 0.76±0.08 | 0.86±0.18 | 0.01 |
| FEV1/FVC ratio (% predicted) | 0.94±0.10 | 0.98±0.09 | <0.0001 |
| DLCO (mL/mm Hg/min) | 14.7±4.2 | 18.8±7.3 | 0.01 |
| DLCO (% predicted) | 61.4±15.1 | 76.1±13.8 | 0.0002 |
| VA (L. BTPS) | 3.77±0.91 | 3.71±2.08 | 0.91 |
| DLCO/VA | 3.90±0.69 | 3.69±1.98 | 0.71 |
| Number of active organs involved | |||
| 1 (Lung only) | 14 | 18 | 0.29 |
| 2 (Lung+1 other) | 14 | 19 | 0.20 |
| 3 (Lung+2 other) | 11 | 4 | 0.15 |
| 4 (Lung+3 other) | 3 | 1 | 0.33 |
| 5 (Lung+4 other) | 2 | 0 | 0.16 |
|
| |||
|
Serum 25-OHD range, nmol/L |
Dallas, US Patients |
DHS | p Value |
|
| |||
| <50 (deficiency) | 63.60% | 80.60% | 0.005 |
| 50–75 (insufficiency) | 22.70% | 16.60% | 0.28 |
| <75 (deficiency/insufficiency) | 86.30% | 97.20% | <0.0001 |
| >75 (sufficiency) | 13.70% | 2.80% | <0.0001 |
|
| |||
|
Serum 25-OHD range, nmol/L |
Naples/Padova, Italy Patients |
Pro.V.A | p Value |
|
| |||
| <50 (deficiency) | 50.00% | 49.70% | 0.97 |
| 50–75 (insufficiency) | 23.80% | 22.50% | 0.84 |
| <75 (deficiency/insufficiency) | 73.80% | 72.20% | 0.82 |
| >75 (sufficiency) | 26.20% | 27.80% | 0.82 |
Results are expressed as mean±SD.
25-OHD, 25-hydroxyvitamin-D; AA, African Americans; BMI, body mass index; BTPS, body temperature and pressure, saturated with water vapor; DLCO, diffusing capacity for carbon monoxide; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; VA, alveolar volume; IT, Italy; US, USA.
Pulmonary functions
Lung involvement, defined by clinical, radiological and/or PFT abnormalities, was present in all patients. US patients have lower forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), FEV1/FVC ratio, and lung diffusing capacity for carbon monoxide (DLCO) than IT patients (table 1).
Clinical, blood, and urinary profiles
Twelve of the 86 patients had a documented history of nephrolithiasis (table 2). Five Italian patients had a history of nephrolithiasis and two developed nephrolithiasis during the study period, for a total of seven stone formers. Five US patients were either diagnosed with nephrolithiasis during the study period or found to have asymptomatic stones on abdominal CT. There were no significant differences in age, weight, body mass index (BMI), serum biochemistry, or calciotropic hormones in kidney stone formers and non-kidney stone formers. Stone formers had a higher urinary calcium excretion (7.61±3.19 vs 5.04 ±3.99 mmol/24 h; p=0.03) and urinary calcium/creatinine (Ca/Cr) ratio (0.36±0.27 vs 0.59±0.24 mmol/mmol, p=0.11) than non-stone formers while 24 h urinary volume was not different (table 2). There was no significant relationship between serum 25-OHD or 1,25-(OH)2D and 24 h urinary calcium excretion (figure 1) in stone formers or non-stone formers (p=0.90 and p=0.33, respectively).
Table 2.
Nephrolithiasis profiles in US and IT patients
| Dallas, USA
| ||||
|---|---|---|---|---|
| Dallas, US patients | Stone | No stone | p Value | Total |
| Number | 5 | 39 | 44 | |
| Age (years) | 42.0±8.9* | 50.5±10.1 | 0.1 | 49.5±10.2 |
| Height (m) | 1.72±0.06 | 1.63±0.08 | 0.03 | 1.64±0.09 |
| Weight (kg) | 98.9±30.0 | 81.0±16.1* | 0.26 | 83.0±18.6 |
| BMI (kg/m2) | 34.1±12.6 | 30.4±6.7 | 0.55 | 30.9±7.5 |
| Blood | ||||
| Creatinine (μmol/L) | 115±44 | 80±27 | 0.17 | 88±35 |
| Total calcium (mmol/L) | 2.33±0.13 | 2.38±0.08 | 0.46 | 2.35±0.08 |
| Phosphorus (mmol/L) | 1.26±0.29 | 1.1±0.23 | 0.28 | 1.1±0.23 |
| Albumin (μmol/L) | 5.4±1.0 | 5.7±0.6 | 0.48 | 5.7±0.6 |
| PTH (pmol/L) | 3.0±2.1 | 6.1±3.7 | 0.03 | 5.6±3.7 |
| 25-OHD (nmol/L) | 35±15 | 47±22 | 0.43 | 45±22 |
| 1.25-(OH)2D (pmol/L) | 120±31* | 104±44* | 0.43 | 107±44* |
| 24 h Urine | ||||
| Volume (L/24 h) | 1.96±0.75 | 1.70±0.77 | 0.56 | 1.73±0.76 |
| Calcium (mmol/24 h) | 6.24±1.95 | 3.14±2.15* | 0.02 | 3.59±2.37* |
| Creatinine (mmol/24 h) | 13.30±2.24* | 10.84±4.23* | 0.08 | 11.21±4.07* |
| FE-Ca (%) | 2.59±1.37 | 1.11±0.81* | 0.07 | 1.34±1.03* |
| Ca/Cr ratio (mmol/mmol) | 0.49±0.20 | 0.31±0.20 | 0.11 | 0.33±0.21 |
|
| ||||
| Naples/Padova, Italy patients | Stone | No Stone | p Value | Total |
|
| ||||
| Number | 7 | 35 | 42 | |
| Age (years) | 57.0±5.8* | 53.6±14.0 | 0.37 | 54.1±13.0 |
| Height (m) | 1.64±0.11 | 1.62±0.08 | 0.69 | 1.62±0.09 |
| Weight (kg) | 81.3±9.4 | 73.1±12.7* | 0.07 | 74.5±12.5 |
| BMI (kg/m2) | 30.4±4.7 | 27.7±4.3 | 0.2 | 28.2±4.4 |
| Blood | ||||
| Creatinine (μmol/L) | 88±9 | 80±27 | 0.74 | 80±18 |
| Total calcium (mmol/L) | 2.50±0.08 | 2.38±0.10 | 0.67 | 2.38±0.10 |
| Phosphorus (mmol/L) | 0.97±0.10 | 1.23±0.32 | 0.02 | 1.13±0.29 |
| Albumin (μmol/L) | 5.8±1.0 | 5.4±0.9 | 0.22 | 5.4±0.9 |
| PTH (pmol/L) | 6.1±4.0 | 4.6±2.5 | 0.37 | 5.0±3.0 |
| 25-OHD (nmol/L) | 55±20 | 52±27 | 0.82 | 52±27 |
| 1.25-(OH)2D (pmol/L) | 62±18* | 65±31* | 0.91 | 65±29* |
| 24 h urine | ||||
| Volume (L/24 h) | 2.00±0.79 | 1.77±0.46 | 0.71 | 1.79±0.53 |
| Calcium (mmol/24 h) | 8.58±3.66 | 6.89±4.48* | 0.31 | 7.19±4.34* |
| Creatinine (mmol/24 h) | 11.98±4.66* | 8.87±2.23* | 0.18 | 10.30±3.76* |
| FE-Ca (%) | 2.30±0.97 | 2.37±1.53* | 0.02 | 2.33±1.18* |
| Ca/Cr ratio (mmol/mmol) | 0.68±0.25 | 0.63±0.37 | 0.83 | 0.66±0.31 |
|
| ||||
| All patients (IT & US) | Stone | No Stone | p Value | Total |
|
| ||||
| Number | 12 | 74 | 86 | |
| Age (years) | 50.5±10.2 | 52.0±12.1 | 0.66 | 51.8±11.8 |
| Height (m) | 1.67±0.10 | 1.63±0.08 | 0.16 | 1.63±0.09 |
| Weight (kg) | 88.6±21.3 | 77.4±15.1 | 0.11 | 79.0±16.4 |
| BMI (kg/m2) | 32.0±8.6 | 29.2±5.9 | 0.3 | 29.6±6.4 |
| Blood | ||||
| Creatinine (μmol/L) | 95±34 | 80±25 | 0.17 | 82±27 |
| Total calcium (mmol/L) | 2.36±0.10 | 2.37±0.09 | 0.81 | 2.37±0.09 |
| Phosphorus (mmol/L) | 1.11±0.27 | 1.12±0.25 | 0.93 | 1.11±0.25 |
| Albumin (μmol/L) | 5.7±1.0 | 5.5±0.7 | 0.68 | 5.5±0.8 |
| PTH (pmol/L) | 4.8±3.6 | 5.4±3.3 | 0.6 | 5.3±3.3 |
| 25-OHD (nmol/L) | 81±39 | 85±43 | 0.84 | 81±43 |
| 1.25-(OH)2D (pmol/L) | 94±39 | 96±44 | 0.82 | 96±44 |
| 24 h urine | ||||
| Volume (L/24 h) | 1.91±0.74 | 1.73±0.63 | 0.46 | 1.76±0.65 |
| Calcium (mmol/24 h) | 7.61±3.19 | 5.04±3.99 | 0.03 | 5.49±3.97 |
| Creatinine (mmol/24 h) | 10.44±3.97 | 12.58±3.65 | 0.11 | 10.95±3.96 |
| FE-Ca (%) | 2.43±1.11 | 1.30±1.02 | 0.01 | 1.59±1.15 |
| Ca/Cr ratio (mmol/mmol) | 0.36±0.27 | 0.59±0.24 | 0.02 | 0.42±0.28 |
Mean±SD; p value indicates stone versus no stone.
p<0.05 between corresponding IT & US groups (eg, IT stone former vs US stone former).
1,25-(OH)2D, 1,25-dihydroxyvitamin D; BMI, body mass index; Ca/Cr, calcium to creatine ratio; FE-Ca, fractional excretion of calcium; IT, Italy; OHD, hydroxyvitamin-D; PTH, parathyroid hormone; US, USA.
Figure 1.
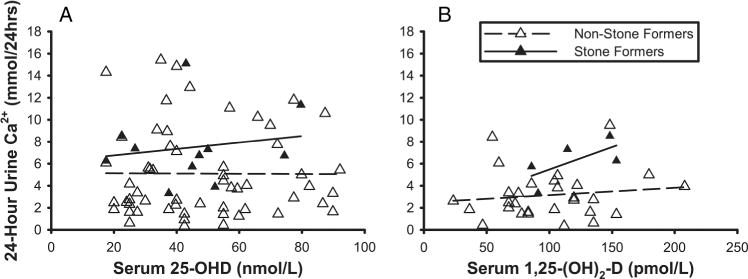
Relationship of 24 h urinary calcium excretion to serum 25-OHD (panel a) and 1,25-(OH)2D (panel b) in patients with sarcoidosis (US and IT combined). There was no significant difference between those with or without a history of nephrolithiasis (p=0.901 and 0.332, respectively). 1,25-(OH)2D, 1,25-dihydroxyvitamin D; 25-OHD, 25-hydroxyvitamin-D; IT, Italy; US, USA.
Nephrolithiasis
None of the stone forming patients exhibited hypercalcemia. IT kidney stone formers had a lower serum phosphorus level than those without kidney stone (0.97±0.10 vs 1.23±0.32 mmol/L, p=0.02). Urinary calcium excretion was higher in IT stone formers versus IT non-stone formers, although the difference did not reach statistical significance, while urinary calcium excretion was statistically higher in US stone formers versus non-stone formers (6.24±1.95 vs 3.14±2.15 mmol/24 h, p=0.02) in accordance with the corresponding fractional excretion of calcium (FE-Ca) (table 2). Urinary calcium (IT: 7.19±4.34 vs US: 3.59 ±2.37 mmol/24 h, p=0.0001) and Ca/Cr ratio (IT: 0.66 ±0.31 vs US: 0.33±0.21 mmol/mmol, p=0.02) were considerably higher in IT patients than US patients (table 2). Serum 1,25-(OH)2D was considerably higher in US patients than IT patients (IT: 65±29 vs US: 107±44 pmol/L, p=0.0002), regardless of stone forming conditions, although 25-OHD levels were not significantly different between IT patients and US patients (IT: 52±27 vs US: 45 ±22 nmol/L, p=0.20) (table 2). Other laboratory indices of sarcoidosis activity did not differ between stone formers versus non-stone formers within each cohort.
Glucocorticoid therapy
Forty-eight per cent of US patients and 74% of IT patients were taking prednisone. There were no statistically significant differences in baseline parameters between glucocorticoid-treated and untreated US patients (table 3). Baseline FEV1 was significantly higher in glucocorticoid-treated patients than untreated IT patients (2.95±0.96 vs 1.96±0.26 L, p=0.0001). Glucocorticoid-treated IT patients had slightly lower endogenous serum 25-OHD than untreated patients (47±20 vs 62±37 nmol/L, respectively) and lower serum 1,25-(OH)2D (57±23 vs 104 ±16 pmol/L, respectively), although the differences were not statistically significant. Glucocorticoid-treated IT patients had significantly higher urinary calcium excretion (8.13±4.29 vs 4.72±3.64 mmol/24 h, p<0.05).
Table 3.
Glucocorticoid treatment profiles in US and IT patients
| Dallas, USA
|
Naples/Padova, Italy
|
|||||
|---|---|---|---|---|---|---|
| Prednisone | No prednisone | p Value | Prednisone | No prednisone | p Value | |
| Number | 21 | 23 | 31 | 11 | ||
| Age (years) | 47.3±9.3 | 51.5±10.9* | 0.18 | 52.0±13.2 | 60.0±10.5* | 0.06 |
| Disease duration (months) | 83±72 | 89±92 | 0.83 | NA | NA | |
| Height (m) | 1.63±0.10 | 1.65±0.07 | 0.65 | 1.64±0.08 | 1.60±0.09 | 0.23 |
| Weight (kg) | 79.8±18.6 | 85.9±18.4* | 0.29 | 75.8±12.55 | 71.2±12.11* | 0.3 |
| BMI (kg/m2) | 30.2±8.0 | 31.5±7.1 | 0.58 | 28.4±4.8 | 27.9±3.6 | 0.73 |
| Pulmonary function | ||||||
| FVC (L) | 2.43±0.71* | 2.49±0.60 | 0.78 | 3.27±1.07* | 2.51±0.48 | 0.007 |
| FVC (% predicted) | 76±17* | 77±14* | 0.77 | 93±19* | 98±11* | 0.36 |
| FEV1 (L) | 1.87±0.64* | 1.89±0.52 | 0.89 | 2.95±0.96* | 1.96±0.26 | 0.0001 |
| FEV1 (% predicted) | 71±19* | 73±14* | 0.78 | 93±22* | 92±10* | 0.89 |
| FEV1/FVC | 0.76±0.09* | 0.76±0.07 | 0.99 | 0.88±0.21* | 0.78±0.02 | 0.04 |
| FEV1/FVC (% predicted) | 0.93±0.12* | 0.94±0.08 | 0.75 | 0.99±0.10* | 0.94±0.03 | 0.04 |
| DLCO (mL/mm Hg/min) | 13.9±3.4* | 15.4±4.8 | 0.27 | 19.9±8.2* | 15.7±1.9 | 0.02 |
| DLCO (% predicted) | 60±14* | 63±16 | 0.49 | 77±14* | 73±13 | 0.44 |
| VA (L. BTPS) | 3.54±0.69 | 3.98±1.04 | 0.14 | 4.25±1.86 | 1.91±2.05 | 0.17 |
| DLCO/VA | 3.92±0.70 | 3.89±0.70 | 0.89 | 3.41±1.52 | 4.61±3.37 | 0.60 |
| Blood | ||||||
| Creatinine (μmol/L) | 80±27 | 88±27 | 0.48 | 80±27 | 71±9 | 0.16 |
| Total calcium (mmol/L) | 2.35±0.08 | 2.35±0.10 | 0.87 | 2.38±0.10 | 2.38±0.10 | 0.98 |
| Phosphorus (mmol/L) | 1.03±0.23 | 1.16±0.23 | 0.09 | 1.13±0.32 | 1.16±0.26 | 0.88 |
| Albumin (μmol/L) | 5.7±0.6 | 5.7±0.6 | 0.68 | 5.4±0.7 | 5.4±1.3 | 0.93 |
| PTH (pmol/L) | 6.6±3.5 | 4.6±3.7 | 0.11 | 5.0±3.1 | 5.0±2.6 | 0.99 |
| 25-OHD (nmol/L) | 45±20 | 45±25* | 0.91 | 47±20 | 62±37* | 0.22 |
| 1.25-(OH)2D (pmol/L) | 104±39* | 109±47 | 0.81 | 57±23* | 104±16 | 0.09 |
| 24 h urine | ||||||
| Volume (L/24 h) | 1.62±0.58* | 1.86±0.93* | 0.39 | 1.79±0.55* | 1.79±0.50* | 0.98 |
| Calcium (mmol/24 h) | 3.44±1.77* | 3.77±2.89 | 0.68 | 8.13±4.29* | 4.72±3.64 | 0.03 |
| Creatinine (mmol/24 h) | 10.88±4.39 | 11.52±3.84* | 0.66 | 10.78±4.15 | 8.72±1.57* | 0.23 |
| FE-Ca (%) | 1.27±0.69* | 1.40±1.30* | 0.71 | 2.53±0.91* | 1.82±1.88* | 0.59 |
| Ca/Cr ratio (mmol/mmol) | 0.34±0.17* | 0.33±0.25 | 0.85 | 0.70±0.24* | 0.51±0.50 | 0.58 |
Mean±SD. p Value indicates prednisone versus no prednisone.
Indicates p<0.05 between corresponding IT & US groups (eg, IT patient with prednisone vs US patient with prednisone). FE-Ca: Fractional excretion of calcium.
1,25-(OH)2D, 1,25-dihydroxyvitamin D; 25-OHD, 25-hydroxyvitamin-D; BMI, body mass index; BTPS, body temperature and pressure, saturated with water vapor; DLCO, diffusing capacity for carbon monoxide; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; VA, alveolar volume; IT, Italy; US, USA.
Serum vitamin D levels
As expected, vitamin D deficiency (25-OHD <50 nmol/L) was more prevalent in AA patients than in Caucasian patients. Insufficient 25-OHD levels (<75 nmol/L) were observed in 67 of 86 patients (73.8% of IT; 86.3% of US). A similar percentage (23.8% of IT patients and 22.7% of US patients) had 25-OHD levels between 50 and 75 nmol/L, while 50.0% of IT patients and 63.6% of US patients had 25-OHD levels <50 nmol/L (table 1).
Comparison to ethnic populations
From the DHS database,19 1766 AA subjects without sarcoidosis served as controls for US patients. The average serum 25-OHD level in US patients with sarcoidosis was higher than in DHS AA subjects (45±22 vs 37±17 nmol/L, p=0.04). The percentage of US patients with deficient 25-OHD levels (<50 nmol/L) was lower than that of DHS AA subjects (63.6% vs 80.6%, p=0.005), and the percentage with optimal (>75 nmol/L) 25-OHD levels was higher than that of DHS AA subjects (13.7% vs 2.8%, p<0.0001) (table 1). From the Pro.V.A database,20 1927 Caucasian subjects without sarcoidosis served as controls for IT patients. Although Pro.V.A subjects were older than IT patients (73.9±6.7 vs 54.1±13.0 years old, p<0.05), the distribution among clinically defined 25-OHD ranges was nearly identical (table 1). Results showed a comparable or lower prevalence of reduced endogenous vitamin D levels compared with that in the corresponding general populations, that is, the prevalence of vitamin D insufficiency or deficiency was not increased among patients with sarcoidosis.
Effect of vitamin D repletion
Sixteen US patients with reduced baseline 25-OHD levels (<75 nmol/L) agreed to be treated with ergocalciferol for 12 weeks. Baseline serum calcium, 25-OHD, urinary Ca/Cr ratio and 1,25-(OH)2D were compared with that obtained at the end of therapy (figure 2 and table 4). Following repletion, the serum 25-OHD level rose in 15 of the 16 patients (average from 42±12 to 82±25 nmol/L, p<0.0001) while remaining essentially unchanged in 1 patient (table 4). The mean ionized serum calcium level was unchanged (from 1.33±0.05 to 1.33±0.09 mmol/L, p=0.67), although 2 patients developed mildly and transiently increased ionized serum calcium postrepletion above normal range (from 1.38 to 1.50 mmol/L and from 1.30 to 1.48 mmol/L). In one of these 2 patients, postrepletion serum total calcium (2.40–2.78 mmol/L), urinary calcium (2.47 to 11.85 mmol/24 h), and urinary Ca/Cr ratio (0.24 to 0.77 mmol/mmol) also mildly exceeded normal limits. In the other patient with elevated postrepletion serum ionized Ca2+ level, serum total calcium remained normal (from 2.38 to 2.55 mmol/L) while 24 h urinary Ca/Cr ratio was abnormally elevated both pre-repletion and postrepletion (from 0.73 to 0.79 mmol/mmol). In a third patient who showed significant postrepletion increases in serum 25-OHD (from 25 to 150 nmol/L), an increase in urinary calcium (from 2.74 to 6.06 mmol/24 h) and a large decrease in urinary creatinine (from 8.09 to 2.87 mmol/24 h) led to a significant increase in urinary Ca/Cr ratio (from 0.34 to 2.11 mmol/mmol) while serum total and ionized calcium remained within normal ranges (from 2.40 to 2.23 mmol/L and 1.25 to 1.33 mmol/L, respectively). Thus, in total, 3 patients developed increased blood and/or urine calcium indices as a result of vitamin D repletion; the increases were mild, asymptomatic, and reversible.
Figure 2.
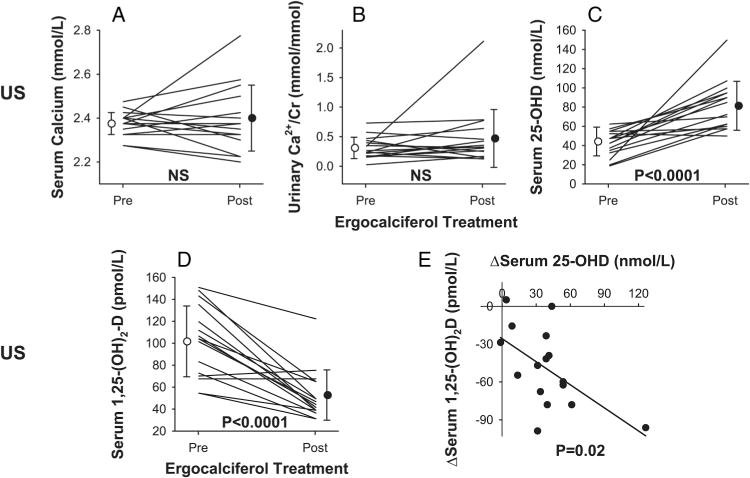
Effects of 12 weeks of ergocalciferol treatment in US patients with sarcoidosis and reduced serum vitamin D levels. (A) Serum total calcium (p=0.58 pre vs post). (B) 24 h urine Ca/Cr (p=0.18 pre vs post). (C) Serum 25-OHD (p<0.0001 pre vs post). (D) Serum 1,25-(OH)2D (p<0.0001 pre vs post). Individual data points and group mean±SD are shown. (E) Relationship between (post–pre) changes (Δ) in serum 1,25-(OH)2D and 25-OHD concentrations following ergocalciferol treatment (y=−0.6×—10.1, R2=0.33, p=0.02). 1,25-(OH)2D, 1,25-dihydroxyvitamin D; 25-OHD, 25-hydroxyvitamin-D; US, USA.
Table 4.
Pre- and post-vitamin D repletion in US patients
| Serum
|
24 h Urine
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| US Patients | 25-OHD (nmol/l) |
1,25-(OH)2D (pmol/L) |
Total Calcium (mmol/L) |
Ionized Ca2+ (mmol/L) |
PTH (pmol/L) |
ACE (nKat/L) | γ-globulins (g/dL) |
Calcium (mmol/24 h) |
Creatinine (mmol/24 h) |
Ca/Cr (mmol/mmol) |
FE-Ca (%) |
| 1 | 47→85 | 73→31 | 2.33→2.38 | 1.33→1.33 | 8.1→5.0 | 101→168 | 0.6→0.7 | 2.37→0.70 | 5.98→2.79 | 0.40→0.25 | 1.45→0.80 |
| 2 | 35→72 | 55→31 | 2.28→2.23 | 1.33→1.25 | 3.4→5.1 | 2412→1508* | 2.2→2.0 | 6.99→0.62 | 16.07→4.28 | 0.44→0.15 | 1.81→0.61 |
| 3 | 57→90 | 107→39 | 2.40→2.35 | 1.30→1.38 | 11.2→7.7 | 0.8→0.7 | 3.82→2.84 | 21.70→22.46 | 0.18→0.13 | 0.90→0.74 | |
| 4 | 55→95 | 104→65 | 2.35→2.43 | 3.1→4.1 | 1926→1273* | 1.7→1.5 | 4.44→4.77 | 7.74→10.45 | 0.57→0.46 | 2.20→1.72 | |
| 5 | 47→107 | 143→65 | 2.48→2.58 | 1.28→1.40 | 4.1→5.8 | 1.5→1.4 | 5.79→8.81 | 12.35→13.81 | 0.47→0.64 | 1.43→1.84 | |
| 6 | 55→57 | 70→75 | 2.38→2.38 | 5.4→7.9 | 1106→905* | 1.8→1.4 | 2.94→3.54 | 13.42→9.99 | 0.22→0.36 | 0.72→1.14 | |
| 7 | 37→90 | 104→44 | 2.28→2.20 | 1.28→1.20 | 6.6→6.3 | 369→402 | 0.8→0.8 | 1.85→2.97 | 11.74→6.99 | 0.16→0.43 | 0.41→1.37 |
| 8 | 32→62 | 83→36 | 2.33→2.33 | 1.30→1.28 | 12.0→8.3 | 553→854 | 1.7→1.2 | 1.45→3.12 | 9.42→10.11 | 0.15→0.31 | 0.98→1.61 |
| 9 | 52→50 | 151→122 | 2.40→2.50 | 1.33→1.35 | 4.7→5.4 | 1374→1072* | 2.8→2.7 | 2.67→1.92 | 7.64→6.07 | 0.35→0.32 | 0.66→0.71 |
| 10 | 42→100 | 148→49 | 2.38→2.55 | 1.38→1.50 | 2.4→1.3 | 972→838* | 1.8→1.7 | 9.51→9.03 | 13.05→11.52 | 0.73→0.79 | 2.36→2.26 |
| 11 | 42→95 | 112→49 | 2.33→2.33 | 1.33→1.30 | 9.1→10.9 | 1424→1340* | 2.0→1.8 | 0.35→2.89 | 13.23→17.29 | 0.03→0.17 | 0.08→0.49 |
| 12 | 62→70 | 55→39 | 2.43→2.40 | 1.40→1.35 | 5.3→3.9 | 1072→1022* | 1.6→1.5 | 1.62→2.00 | 9.55→7.60 | 0.17→0.26 | 0.48→0.85 |
| 13 | 25→150 | 135→39 | 2.40→2.23 | 1.25→1.33 | 2.3→5.9 | 754→620 | 1.9→1.6 | 2.74→6.06 | 8.09→2.87 | 0.34→2.11 | 0.93→7.39 |
| 14 | 47→60 | 101→47 | 2.45→2.30 | 1.28→1.23 | 7.4→6.6 | 1106→1307* | 2.4→2.1 | 1.82→0.90 | 7.66→7.25 | 0.24→0.12 | 0.55→0.34 |
| 15 | 20→62 | 68→68 | 2.40→2.78 | 1.30→1.48 | 2.9→0.3 | 2328→1960* | 2.4→2.6 | 2.47→11.85 | 10.53→15.35 | 0.24→0.77 | 1.17→4.58 |
| 16 | 19→57 | 120→42 | 2.38→2.33 | 1.40→1.25 | 5.3→3.2 | 720→754 | 2.0→1.8 | 3.77→5.36 | 14.25→16.49 | 0.26→0.33 | 0.77→1.09 |
| Mean | 42→82 | 101→52 | 2.38→2.40 | 1.33→1.33 | 5.8→5.5 | 1106→1005 | 1.8→1.6 | 3.41→4.21 | 11.40→10.33 | 0.31→0.47 | 1.06→1.72 |
| SD | 12→25 | 31→23 | 0.05→0.15 | 0.05→0.09 | 3.1→2.6 | 687→469 | 0.6→0.6 | 2.33→3.28 | 3.98→5.58 | 0.18→0.49 | 0.65→1.82 |
| p Value pre vs post | 0.0001 | <0.0001 | 0.58 | 0.67 | 0.54 | 0.09 | 0.002 | 0.34 | 0.32 | 0.18 | 0.17 |
Mean±SD, Pre → Post-repletion.
Indicates patients with elevated baseline angiotensin converting enzyme (ACE). In this subgroup, ACE levels dropped from 1524±553 pre-repletion to 1240±352 nKat/L (p=0.04) postrepletion.
1,25-(OH)2D, 1,25-dihydroxyvitamin D; 25-OHD, 25-hydroxyvitamin-D; Ca/Cr, calcium to creatinine ratio; FE-Ca, fractional excretion of calcium; PTH, parathyroid hormone.
Unexpectedly, average serum 1,25-(OH)2D levels of 16 US patients declined significantly postrepletion from 101 ±31 to 52±23 pmol/L (p<0.0001). An absolute decrease was observed in 14 of the 16 US patients, whereas serum level did not change in 2 of the 16 patients (figure 2).
There was no difference in the pre-repletion to postrepletion change in serum calcium (p=0.81), 25-OHD (p=0.68), or 1,25-(OH)2D (p=0.86) levels between patients who were taking prednisone (n=6) and those not taking prednisone (n=10).
Serum ACE and γ-globulin levels were also affected following vitamin D repletion. Taking all treated patients together, the serum ACE level declined slightly but not significantly from pre-repletion to postrepletion (1106±687 to 1005±469 nKat/L, p=0.09). However, in the subgroup of nine US patients with elevated baseline ACE levels, their ACE levels declined significantly from pre-repletion to postrepletion (1524±553 to 1240±352 nKat/L, p=0.04). Serum γ-globulins also decreased slightly but significantly from pre-repletion to postrepletion (1.8±0.6 to 1.6±0.6 g/dL, p=0.002) (table 4). Bone-specific alkaline phosphatase was not significantly affected by repletion (from 93±44 to 92±39 μg/L, p=0.81).
Pulmonary function changed variably from pre-vitamin D to post-vitamin D repletion. There were no significant changes in FVC (2.41±0.72 vs 2.50±0.75 L, p=0.32, % predicted FVC (76±18 vs 73±16%, p=0.34), FEV1 (1.80±0.62 vs 1.91±0.57 L, p=0.10), or % predicted FEV1 (70±19 vs 74±17%, p=0.24). There were small though statistically significant increases in pre-repletion versus postrepletion FEV1/FVC (0.74±0.09 vs 0.77±0.08, p=0.01) and % predicted FEV1/FVC (93±13 vs 102±11%, p<0.01). There was a modest and proportional decrease in prerepletion versus postrepletion DLCO (13.33±3.82 vs 11.86±3.33 mL.(mm Hg.min)−1, p<0.01) and alveolar volume (VA) (3.40±0.88 vs 3.05±0.86 L, p<0.01) so that DLCO/VA remained unchanged (3.93±0.56 vs 3.94±0.64, p=0.89).
DISCUSSION
This prospective study compared calcium and vitamin D homeostasis in two ethnically and geographically distinct populations with sarcoidosis. The US patients were mostly AA (93%) and female (89%), compared to Italian subjects (95% Caucasians, 62% female). Fewer US patients were on prednisone treatment than IT patients, and US patients had worse FVC, FEV1 and DLCO than IT patients. There are several salient discoveries: First, patients with sarcoidosis with vastly different ethnic and lifestyle backgrounds appear to be similar in clinical and biochemical parameters. Second, nephrolithiasis is common in patients with sarcoidosis and is largely driven by hypercalciuria, but the urinary calcium excretion rate is not significantly associated with serum vitamin D levels. Third, calcium & vitamin D parameters do not differ between patients taking glucocorticoids versus those not taking glucocorticoids, except that glucocorticoid-treated IT patients had higher urinary calcium excretion. Fourth, the prevalence of vitamin D insufficiency is lower in AA patients than in the general AA population and not different between IT patients with sarcoidosis and the general IT population. Fifth, there is an inverse relationship between the changes in 25-OHD levels and changes in 1,25-(OH)2D following repletion of vitamin D levels. Sixth, repletion of vitamin D stores in sarcoidosis is generally safe and effective; mild asymptomatic reversible hypercalcemia and/or hypercalciuria infrequently develop during treatment.
Prevalence of nephrolithiasis in sarcoidosis in the literature ranges between 1.3 and 14%9,21–25 and hypercalciuria (>5.0 mmol/24 h) has been demonstrated in more than half of the patients with sarcoidosis on an average calcium intake of 10.0 mmol/24 h.21 In our study, 7/42 Italian (17%) and 5/44 US (11%) patients had kidney stones compared to the general Italian and US population prevalence of 7.5% and 8%, respectively.26,27 Basal levels of serum 25-OHD did not differ between these two groups. Urinary calcium excretion was higher in kidney stone formers than non-kidney stone formers, but did not bear any significant relationship to serum 25-OHD and 1,25-(OH)2D. This vitamin D-independent hypercalciuria in the sarcoidosis population may reflect an impairment in renal tubular calcium reabsorption,9 and possibly renal tubular interstitial involvement by sarcoidosis.28–31
In IT patients, lower endogenous 25-OHD and 1,25-(OH)2D levels were observed in glucocorticoid-treated patients, accompanied by higher urinary calcium excretion, suggesting possible bone loss. In US patients, there were no differences in calcium & vitamin D parameters between glucocorticoid-treated patients and non-glucocorticoid-treated patients. However, US patients had lower FVC, FEV1 and DLCO (% predicted), and glucocorticoid-treated US patients had lower FVC, FEV1 and DLCO (% predicted) compared to the corresponding IT patients, although DLCO per unit alveolar volume (DLCO/VA) was not different between US and IT patients, table 1). On the other hand, glucocorticoid-treated US patients had significantly higher 1,25-(OH)2D than glucocorticoid-treated IT patients (table 3). These observations suggest that US patients had more severe restriction of lung volume and greater extrarenal 1,25-(OH)2D conversion than IT patients. A higher BMI may explain the lower lung volume in US patients. Lung function parameters (as % predicted) were not different between glucocorticoid-treated and non-glucocorticoid-treated patients within either the US or IT subgroups (table 3).
As expected, the prevalence of reduced 25-OHD levels was higher among US AA patients than among IT Caucasian patients; the difference mirrors that in the respective general populations (table 1). The prevalence of vitamin D deficiency was lower in US patients with sarcoidosis than in the general AA population.
Our prospective data indicate that vitamin D supplementation for 3 months in vitamin D-deficient patients with sarcoidosis is effective in repletion of vitamin D stores and generally safe, although mild elevation of serum and urinary calcium levels may develop; thus, calcium balance should be monitored in all patients during vitamin D supplementation. Our results are consistent with the findings of a retrospective study of 301 patients with sarcoidosis that calcium (500 mg daily) and vitamin D (400 IU daily) supplementation did not raise serum calcium concentration.15 In a short-term prospective study of four patients taking ergocalciferol 10,000 IU daily for 12 days, urinary calcium rose by 21%, from 3.42 to 4.14 mmol/24 h, 6 which was similar to our observations over 3 months. The variable and modest postrepletion changes we observed in lung function measures at rest are inconclusive. Spirometry indices were unchanged or slightly improved. The reductions in DLCO and VA with a stable DLCO/VA suggest reduced inspired lung volume. A larger cohort with longer treatment duration and measurement of lung volume by plethysmography will be required to draw definitive conclusions regarding lung function changes.
Vitamin D plays a major role in skeletal health,32,33 as well as in immune-modulation mediating adaptive immunity34 and suppressing granulomatous inflammation. The serum 25-OHD level is inversely related to sarcoidosis disease activity.15 An important observation in our study is that serum 1,25-(OH)2D decreased markedly by ~50% in 14/16 patients after 3 months of 25-OHD supplementation (from 101±31 to 52±23 pmol/L, p<0.0001) while remaining unchanged in 2/16 patients. In contrast, an earlier study involving four patients treated with vitamin D2 daily for 12 days reported a mild increase (14%) in plasma 1,25-(OH)2D from 75 to 86 pmol/L.6 In our US patients, baseline serum 1,25-(OH)2D was 65% higher (table 2) while measures of lung function (FVC, FEV1, FEV1/FVC and DLCO) were 12–30% lower (table 1) than in IT patients, consistent with more severe granulomatous inflammation in the predominantly African American cohort. The marked (average 50%) decrease in serum 1,25-(OH)2D level following 25-OHD supplementation may be related to the anti-inflammatory action of vitamin D, which has been shown to enhance innate immunity (eg, promote anti-bactericidal activity) and suppress adaptive immunity (eg, promote anti-inflammation), facilitate antigen elimination, and improve the outcome of granulomatous diseases such as mycobacterial infection through changes in γ interferon and cathelicidin concentrations.35–37 The high prevalence of vitamin D deficiency in AA38 has been reported to predispose to more aggressive M. tuberculosis infection,35 and vitamin D supplementation has shown significant benefit in treating tuberculosis in vitamin D deficient patients.35,38–41 In this context, extrarenal production of 1,25-(OH)2D in sarcoidosis, particularly in patients with vitamin D deficiency, may be an adaptive response by immune-active cells in an attempt to mitigate antigen-stimulated granulomatous inflammation. In our patients with sarcoidosis, repletion of endogenous vitamin D stores reduces the need for extrarenal 1,25-(OH)2D production, and could explain the fall in serum 1,25-(OH)2D level. This putative mechanism, while consistent with current understanding of vitamin D homeostasis, will require further studies for validation.
The key strengths of this study include a carefully designed cross-sectional comparison of two ethnically diverse patients’ cohorts with their respective ethnic general populations as control and with a subgroup intervention arm (vitamin D supplementation). The key limitations of the study include the small number of patients given vitamin D supplementation. Also, dietary calcium intake was not included in our analysis.
In conclusion, our two populations of patients with sarcoidosis with a diverse genetic background and lifestyle show similar calcium and vitamin D parameters. Compared to the ethnically matched healthy controls, patients with sarcoidosis are not more likely to be vitamin D deficient. The prevalence of nephrolithiasis is higher in patients with sarcoidosis than in the general population. The sole identifiable risk factor for nephrolithiasis, hypercalciuria, is unrelated to the status of vitamin D levels. Repletion of 25-OHD stores in patients with sarcoidosis and vitamin D insufficiency is generally safe, although in a minority of patients’ serum and/or urinary calcium levels it may increase above normal; thus, the calcium balance should be monitored during treatment. In patients with an elevated basal serum ACE level, repletion of 25-OHD stores is associated with a consistent decline in ACE level, suggesting suppression of granulomatous immune activity. Repletion of 25-OHD stores is also associated with an unexpected reduction in serum 1,25-(OH)2D level, suggesting suppression of autonomous ectopic 1,25-(OH)2D production by granuloma-associated immune cells. Whether repletion of 25-OHD mitigates the clinical course of granulomatous inflammation remains to be determined.
Significance of study.
What is already known about this subject?
-
►
Both sarcoidosis and vitamin D deficiency are more common in African Americans than in Caucasians.
-
►
The risk of calcium and vitamin D disturbance (hypercalcemia, hypercalciuria, and nephrolithiasis) may also differ between these two sarcoidosis patient populations.
-
►
Physicians are ambivalent about giving vitamin D supplementation to patients with sarcoidosis for fear of mineral side effects.
What are the new findings?
-
►
Sarcoidosis patients from two distinct ethnic and lifestyle backgrounds are largely similar in clinical, biochemical and mineral parameters.
-
►
Nephrolithiasis is common in patients with sarcoidosis and largely driven by hypercalciuria, but the urinary calcium excretion rate is not significantly associated with serum vitamin D levels.
-
►
The prevalence of vitamin D insufficiency is not higher in patients with sarcoidosis compared to the general racially matched population.
-
►
Repletion of vitamin D stores in active sarcoidosis is generally safe and effective. Repletion is associated with consistent decreases in serum 1,25-(OH)2D levels and in the previously elevated serum ACE levels, suggesting suppression of granulomatous immune activity.
How might these results change the focus of research or clinical practice?
-
►
Physicians can be informed that active sarcoidosis does not predispose to vitamin D deficiency, and race per se does not influence the calcium and vitamin D parameters of these patients.
-
►
While repletion of vitamin D stores in active sarcoidosis is generally safe, calcium balance should be monitored during repletion.
-
►
Repletion of vitamin D stores in active sarcoidosis may suppress granulomatous immune activity. This novel observation should be confirmed by further research.
Acknowledgments
The authors thank Elaine Isaminger and Madhuri Poduri for technical assistance, and Susan Wu for assistance with manuscript preparation. The study was partly supported by Grant Number UL1RR024982 as part of the North and Central Texas Clinical and Translational Science Initiative from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH.
Funding: This project was supported in part by the ‘North and Central Texas Clinical and Translational Science Initiative’ Grant (UL1RR024982) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research. The Dallas Heart Study (DHS) is supported in part by grant UL1TR001105 from the National Center for Advancing Translational Sciences, NIH. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the granting agencies.
Footnotes
Contributors: CCWH and KS conceived and designed the study.
G Capolongo, MA, AS, AAS, AC, CA, MZ, CCWH and KS recruited patients and performed or supervised the measurements. G Capolongo, LHRX, BH and CCWH analyzed the data and wrote the manuscript. G Capasso, NMM, and OWM contributed to data analysis and interpretation and provided advice on the manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Provenance and peer review: Not commissioned; externally peer reviewed.
Informed consent: ‘Informed consent was obtained from all individual participants included in the study’.
References
- 1.Costabel U, Hunninghake GW. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J. 1999;14:735–7. doi: 10.1034/j.1399-3003.1999.14d02.x. [DOI] [PubMed] [Google Scholar]
- 2.Dastoori M, Fedele S, Leao JC, et al. Sarcoidosis—a clinically orientated review. J Oral Pathol Med. 2013;42:281–9. doi: 10.1111/j.1600-0714.2012.01198.x. [DOI] [PubMed] [Google Scholar]
- 3.Reichel H, Koeffler HP, Barbers R, et al. Regulation of 1,25-dihydroxyvitamin D3 production by cultured alveolar macrophages from normal human donors and from patients with pulmonary sarcoidosis. J Clin Endocrinol Metab. 1987;65:1201–9. doi: 10.1210/jcem-65-6-1201. [DOI] [PubMed] [Google Scholar]
- 4.Adams JS, Gacad MA, Anders A, et al. Biochemical indicators of disordered vitamin D and calcium homeostasis in sarcoidosis. Sarcoidosis. 1986;3:1–6. [PubMed] [Google Scholar]
- 5.Rizzato G, Colombo P. Nephrolithiasis as a presenting feature of chronic sarcoidosis: a prospective study. Sarcoidosis Vasc Diffuse Lung Dis. 1996;13:167–72. [PubMed] [Google Scholar]
- 6.Bell NH, Stern PH, Pantzer E, et al. Evidence that increased circulating 1 alpha, 25-dihydroxyvitamin D is the probable cause for abnormal calcium metabolism in sarcoidosis. J Clin Invest. 1979;64:218–25. doi: 10.1172/JCI109442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams JS, Gacad MA, Singer FR, et al. Production of 1,25-dihydroxyvitamin D3 by pulmonary alveolar macrophages from patients with sarcoidosis. Ann N Y Acad Sci. 1986;465:587–94. doi: 10.1111/j.1749-6632.1986.tb18535.x. [DOI] [PubMed] [Google Scholar]
- 8.Papapoulos SE, Clemens TL, Fraher LJ, et al. 1, 25-dihydroxycholecalciferol in the pathogenesis of the hypercalcaemia of sarcoidosis. Lancet. 1979;1:627–30. doi: 10.1016/s0140-6736(79)91076-6. [DOI] [PubMed] [Google Scholar]
- 9.Rizzato G, Fraioli P, Montemurro L. Nephrolithiasis as a presenting feature of chronic sarcoidosis. Thorax. 1995;50:555–9. doi: 10.1136/thx.50.5.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bell NH, Gill JR, Jr, Bartter FC. On the abnormal calcium absorption in sarcoidosis. Evidence for increased sensitivity to vitamin D. Am J Med. 1964;36:500–13. doi: 10.1016/0002-9343(64)90099-3. [DOI] [PubMed] [Google Scholar]
- 11.Bell NH, Bartter FC. Transient reversal of hyperabsorption of calcium and of abnormal sensitivity to vitamin D in a patient with sarcoidosis during episode of nephritis. Ann Intern Med. 1964;61:702–10. doi: 10.7326/0003-4819-61-4-702. [DOI] [PubMed] [Google Scholar]
- 12.Mather G. Calcium metabolism and bone changes in sarcoidosis. Br Med J. 1957;1:248–53. doi: 10.1136/bmj.1.5013.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson G, Care AD, Anderson CK. The effect of cortisone on vitamin D2-induced nephrocalcinosis in the rat. Clin Sci. 1957;16:181–5. [PubMed] [Google Scholar]
- 14.Holick MF, Chen TC, Lu Z, et al. Vitamin D and skin physiology: a D-lightful story. J Bone Miner Res. 2007;22(Suppl 2):V28–33. doi: 10.1359/jbmr.07s211. [DOI] [PubMed] [Google Scholar]
- 15.Kamphuis LS, Bonte-Mineur F, van Laar JA, et al. Calcium and vitamin D in sarcoidosis: is supplementation safe? J Bone Miner Res. 2014;29:2498–503. doi: 10.1002/jbmr.2262. [DOI] [PubMed] [Google Scholar]
- 16.Judson MA, Boan AD, Lackland DT. The clinical course of sarcoidosis: presentation, diagnosis, and treatment in a large White and black cohort in the United States. Sarcoidosis Vasc Diffuse Lung Dis. 2012;29:119–27. [PubMed] [Google Scholar]
- 17.Zadshir A, Tareen N, Pan D, et al. The prevalence of hypovitaminosis D among US adults: data from the NHANES III. Ethn Dis. 2005;15:S5-97–101. [PubMed] [Google Scholar]
- 18.Gallagher JC, Sai AJ. Vitamin D insufficiency, deficiency, and bone health. J Clin Endocrinol Metab. 2010;95:2630–3. doi: 10.1210/jc.2010-0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Victor RG, Haley RW, Willett DL, et al. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93:1473–80. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 20.Toffanello ED, Coin A, Perissinotto E, et al. Vitamin D deficiency predicts cognitive decline in older men and women: The Pro.V.A. Study. Neurology. 2014;83:2292–8. doi: 10.1212/WNL.0000000000001080. [DOI] [PubMed] [Google Scholar]
- 21.Lebacq E, Desmet V, Verhaegen H. Renal involvement in sarcoidosis. Postgrad Med J. 1970;46:526–9. doi: 10.1136/pgmj.46.538.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longcope WT, Freiman DG. A study of sarcoidosis; based on a combined investigation of 160 cases including 30 autopsies from The Johns Hopkins Hospital and Massachusetts General Hospital. Medicine (Baltimore) 1952;31:1–132. [PubMed] [Google Scholar]
- 23.Murphy GP, Schirmer HK. Nephrocalcinosis, urolithiasis and renal insufficiency sarcoidosis. J Urol. 1961;86:702–6. doi: 10.1016/S0022-5347(17)65251-0. [DOI] [PubMed] [Google Scholar]
- 24.Muther RS, McCarron DA, Bennett WM. Renal manifestations of sarcoidosis. Arch Intern Med. 1981;141:643–5. [PubMed] [Google Scholar]
- 25.Rizzato G. Extrapulmonary presentation of sarcoidosis. Curr Opin Pulm Med. 2001;7:295–297. doi: 10.1097/00063198-200109000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Croppi E, Ferraro PM, Taddei L, et al. Prevalence of renal stones in an Italian urban population: a general practice-based study. Urol Res. 2012;40:517–22. doi: 10.1007/s00240-012-0477-z. [DOI] [PubMed] [Google Scholar]
- 27.Scales CD, Jr, Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160–5. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta R, Beaudet L, Moore J, et al. Treatment of sarcoid granulomatous interstitial nephritis with adalimumab. NDT plus. 2009;2:139–42. doi: 10.1093/ndtplus/sfn200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajakariar R, Sharples EJ, Raftery MJ, et al. Sarcoid tubulo-interstitial nephritis: long-term outcome and response to corticosteroid therapy. Kidney Int. 2006;70:165–9. doi: 10.1038/sj.ki.5001512. [DOI] [PubMed] [Google Scholar]
- 30.Robson MG, Banerjee D, Hopster D, et al. Seven cases of granulomatous interstitial nephritis in the absence of extrarenal sarcoid. Nephrol Dial Transplan. 2003;18:280–4. doi: 10.1093/ndt/18.2.280. [DOI] [PubMed] [Google Scholar]
- 31.Joss N, Morris S, Young B, et al. Granulomatous interstitial nephritis. Clin J Am Soc Nephrol. 2007;2:222–30. doi: 10.2215/CJN.01790506. [DOI] [PubMed] [Google Scholar]
- 32.Thomas MK, Lloyd-Jones DM, Thadhani RI, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338:777–83. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 33.Cauley JA, Lacroix AZ, Wu L, et al. Serum 25-hydroxyvitamin D concentrations and risk for hip fractures. Ann Intern Med. 2008;149:242–50. doi: 10.7326/0003-4819-149-4-200808190-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemire JM. Immunomodulatory role of 1,25-dihydroxyvitamin D3. J Cell Biochem. 1992;49:26–31. doi: 10.1002/jcb.240490106. [DOI] [PubMed] [Google Scholar]
- 35.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 36.Reichel H, Koeffler HP, Tobler A, et al. 1 alpha,25-Dihydroxyvitamin D3 inhibits gamma-interferon synthesis by normal human peripheral blood lymphocytes. Proc Natl Acad Sci USA. 1987;84:3385–9. doi: 10.1073/pnas.84.10.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zasloff M. Antimicrobial peptides, innate immunity, and the normally sterile urinary tract. J Am Soc Nephrol. 2007;18:2810–6. doi: 10.1681/ASN.2007050611. [DOI] [PubMed] [Google Scholar]
- 38.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 39.Martineau AR, Wilkinson RJ, Wilkinson KA, et al. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med. 2007;176:208–13. doi: 10.1164/rccm.200701-007OC. [DOI] [PubMed] [Google Scholar]
- 40.Martineau AR, Wilkinson KA, Newton SM, et al. IFN-gamma- and TNF-independent vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. J Immunol. 2007;178:7190–8. doi: 10.4049/jimmunol.178.11.7190. [DOI] [PubMed] [Google Scholar]
- 41.Martineau AR, Honecker FU, Wilkinson RJ, et al. Vitamin D in the treatment of pulmonary tuberculosis. J Steroid Biochem Mol Biol. 2007;103:793–8. doi: 10.1016/j.jsbmb.2006.12.052. [DOI] [PubMed] [Google Scholar]


