Abstract
Context
Detectable levels of cardiac troponin T (cTnT) are strongly associated with structural heart disease and increased risk of death and adverse cardiovascular events; however, cTnT is rarely detectable in the general population using standard assays.
Objectives
To determine the prevalence and determinants of detectable cTnT in the population using a new highly sensitive assay and to assess whether cTnT levels measured with the new assay associate with pathological cardiac phenotypes and subsequent mortality.
Design, Setting, and Participants
Cardiac troponin T levels were measured using both the standard and the highly sensitive assays in 3546 individuals aged 30 to 65 years enrolled between 2000 and 2002 in the Dallas Heart Study, a multiethnic, population-based cohort study. Mortality follow-up was complete through 2007. Participants were placed into 5 categories based on cTnT levels.
Main Outcome Measures
Magnetic resonance imaging measurements of cardiac structure and function and mortality through a median of 6.4 (interquartile range, 6.0–6.8) years of follow-up.
Results
In Dallas County, the prevalence of detectable cTnT (≥0.003 ng/mL) was 25.0% (95% confidence interval [CI], 22.7%−27.4%) with the highly sensitive assay vs 0.7% (95% CI, 0.3%−1.1%) with the standard assay. Prevalence was 37.1% (95% CI, 33.3%−41.0%) in men vs 12.9% (95%CI,10.6%−15.2%) in women and 14.0% (95% CI, 11.2%−16.9%) in participants younger than 40 years vs 57.6% (95% CI,47.0%−68.2%) in those 60 years and older. Prevalence of left ventricular hypertrophy increased from 7.5% (95% CI, 6.4%−8.8%) in the lowest cTnT category (<0.003 ng/mL) to 48.1% (95% CI, 36.7%−59.6%) in the highest (≥0.014 ng/mL) (P < .001); prevalence of left ventricular systolic dysfunction and chronic kidney disease also increased across categories (P < .001 for each). During a median follow-up of 6.4 years, there were 151 total deaths, including 62 cardiovascular disease deaths. All-cause mortality increased from 1.9% (95% CI, 1.5%−2.6%) to 28.4% (95% CI, 21.0%−37.8%) across higher cTnT categories (P < .001). After adjustment for traditional risk factors, C-reactive protein level, chronic kidney disease, and N-terminal pro-brain-type natriuretic peptide level, cTnT category remained independently associated with all-cause mortality (adjusted hazard ratio, 2.8 [95% CI, 1.4–5.2] in the highest category). Adding cTnT categories to the fully adjusted mortality model modestly improved model fit (P = .02) and the integrated discrimination index (0.010 [95% CI, 0.002–0.018]; P = .01).
Conclusion
In this population-based cohort, cTnT detected with a highly sensitive assay was associated with structural heart disease and subsequent risk for all-cause mortality.
Cardiac troponins T (cTnT) and I are the preferred biomarkers for the diagnosis of acute myocardial infarction (MI).1,2 Increasingly it has been recognized that elevated troponin levels may be detected in other clinical scenarios in which acute myocardial injury may occur3,4 as well as in several chronic disease states, including coronary artery disease (CAD), heart failure, and chronic kidney disease (CKD).5–7 Troponins T and I are also occasionally detectable in individuals from the general population using standard assays.8–11 Although the prevalence in the population is low, detectable troponin associates strongly with structural heart disease8 and an increased risk of death and adverse cardiovascular events.9–11 These findings suggest that troponin may be useful for detecting subclinical cardiovascular disease and assessing cardiovascular disease risk in the general population; however, the low prevalence of detection with standard assays would limit the utility of troponin measurement for these clinical applications.8
Recently, a highly sensitive assay for cTnT has been developed that detects levels approximately 10-fold lower than those detectable with the standard assay. In patients with suspected acute coronary syndromes, this assay improves accuracy for the diagnosis of MI compared with the standard cTnT assay.12 In patients with chronic heart failure13 and chronic CAD,14 circulating cTnT is detectable in almost all individuals with the highly sensitive assay, and higher levels correlate strongly with increased cardiovascular mortality. Here, we report an evaluation of the highly sensitive assay in a general population cohort with detailed cardiovascular phenotyping and long-term follow-up for mortality.
METHODS
Study Population
The Dallas Heart Study (DHS) is a multiethnic, population-based cohort study of Dallas County residents. The study was approved by the University of Texas Southwestern institutional review board, and all participants provided written informed consent. Details of the study design and participant selection have been described previously.15
The random probability sample included intentional oversampling of black individuals to comprise 50% of the cohort. Enrollment occurred between July 2000 and September 2002, and data collection was performed in 3 phases, beginning with an initial in-home visit (n = 6101) to collect demographic information, medical history, blood pressure, and anthropometric measurements. Participants aged 30 to 65 years were asked to participate in a second in-home visit (n = 3557) to collect fasting blood and urine samples and then a final visit at University of Texas Southwestern Medical Center (n = 2971) where dual-energy x-ray absorptiometry (DEXA) scanning for body composition, detailed cardiovascular phenotyping by electron-beam computed tomography (EBCT), and cardiac and aortic magnetic resonance imaging (MRI) were performed.
Sampling weights were calculated for each participant to reflect differential probabilities of selection and attrition of participants between visits. These sampling weights permit the generation of unbiased estimates of population frequencies in Dallas County (additional methods presented in the eSupplement available at http://www.jama.com).15 The present study includes all 3546 individuals with cTnT levels measured using the highly sensitive assay, including 2501 with cardiac MRI and 2770 with EBCT.
Biomarker Assays
Blood was collected into EDTA tubes, refrigerated at 4°C for 4 hours or less, centrifuged, and the plasma removed and stored at −70°C. Brain-type natriuretic peptide,16 N-terminal pro-brain-type natriuretic peptide (NT-proBNP),16 and high-sensitivity C-reactive protein (CRP)17 were measured as previously described. Levels of cTnT were measured previously using a standard assay with a lower limit of detection of 0.01 ng/mL (Elecysys-2010 Troponin T; Roche Diagnostics, Indianapolis, Indiana).8 For the present study, cTnT levels were measured using a highly sensitive assay on an automated platform (Elecsys-2010 Troponin T hs STAT, Roche Diagnostics), with a lower detection limit of 0.003 ng/mL and a reported 99th percentile value in apparently healthy individuals of 0.014 ng/mL.18
Imaging Procedures
Detailed methods for MRI, EBCT, and DEXA scans in the DHS have been reported.19–21 EBCT was performed in duplicate on a single scanner (Imatron Inc, San Bruno, California) to assess coronary artery calcium (CAC), with results averaged. Scans were scored by the Agatston method and classified as 0 to ≤10, >10 to ≤100, >100 to ≤400, and >400 Agatston units.19 DEXA was used to estimate body composition, dividing total body mass into bone, fat, and lean mass components.19 Cardiac and aortic MRI was performed using a 1.5-Tesla system (Intera; Philips Medical Systems, Best, the Netherlands). Left ventricular mass, wall thickness, end diastolic volume (LVEDV), and ejection fraction (LVEF) were calculated from short-axis sequences. Left ventricular hypertrophy (LVH) was defined as left ventricular mass greater than 89 g/m2 in women and greater than 112 g/m2 in men, based on a phenotypically normal subpopulation of the DHS cohort.20 Details of aortic MRI and compliance methods are presented in the eSupplement.
Variable Definitions
Race/ethnicity was self-reported. Coronary heart disease (CHD) was defined as self-reported prior MI or revascularization. Cardiovascular disease was defined as CHD or self-reported history of heart failure or stroke. For purposes of categorizing baseline risk, Framingham Risk Score (FRS) estimates of 10-year CHD events were determined using published algorithms22 with a correction for statin treatment,23 and participants were categorized into low-risk (<10%), intermediate-risk (10%−20%), and high-risk (>20%) categories; participants with diabetes or CHD were categorized into the high-risk group. Other variable definitions are provided in the eSupplement.
Mortality Follow-up
The National Death Index was queried to determine participant mortality through December 31, 2007. Deaths were classified as cardiovascular if they included International Statistical Classification of Diseases, 10th Revision codes I00-I99.24
Statistical Analyses
Continuous variables are reported as median (interquartile range) and categorical variables as proportions. The pre-specified primary analyses assessed cTnT level as an ordered categorical variable with secondary analyses as a dichotomous variable (detectable vs undetectable levels). For the analyses of cTnT as a categorical variable, participants were divided into 5 a priori–determined categories based on cTnT levels determined using the highly sensitive assay: those with undetectable cTnT were placed in the first category; those with cTnT levels greater than or equal to the previously reported 99th percentile value (≥0.014 ng/mL) were placed in the fifth category, and those with cTnT levels between 0.003 and 0.014 ng/mL were divided into tertiles for categories 2 through 4. Demographic and clinical variables and cardiovascular phenotypes were compared across cTnT categories using the Jonckheere-Terpstra trend test, which is a nonparametric trend test for ordered classes.25 Sensitivity analyses were performed restricted to participants without prevalent cardiovascular disease and those in the lowest FRS category.
Logistic regression was used to identify variables independently associated with detectable cTnT. Candidate variables tested included age, sex, race/ethnicity, diabetes, hypertension, prior MI, heart failure or angina, hospitalization within the past year, estimated glomerular filtration rate, CAC, LVEF, left ventricular mass, lean mass by DEXA, and body surface area. Left ventricular mass was replaced with LVEDV and left ventricular wall thickness in a second model.
All-cause and cardiovascular disease mortality were estimated using the Nelson-Aalen estimator,26 and cumulative incidence curves were compared across cTnT categories using the log-rank test. Multivariable Cox proportional hazards models were used to determine associations of cTnT categories with mortality after serially adjusting for traditional risk factors plus high-sensitivity CRP level, estimated glomerular filtration rate, and NT-proBNP level. Sensitivity analyses were performed replacing cTnT categories with cTnT as a log-transformed continuous variable, with undetectable values assigned a value just below the lower detection limit of the assay (2.99 ng/mL). Time-dependent C statistics27 were calculated for models with and without cTnT and compared using bootstrap resampling, with model fit assessed by the likelihood ratio test and calibration by the Hosmer-Lemeshow statistic. The integrated discrimination index (IDI) was calculated for the addition of cTnT to each model.28 The IDI represents the improvement in average sensitivity minus any increase in 1 –specificity when a variable is added to a model.
Sample weighting was used for determining the prevalence of detectable cTnT in Dallas County and in subsets of the population (eSupplement). For all other analyses evaluating associations within the DHS cohort, no sample weighting was used. All P values are 2-sided; P < .05 was considered statistically significant. No adjustment was made for multiple comparisons. Statistical analyses were performed using SAS version 9.2 (SAS Institute Inc, Cary, North Carolina).
RESULTS
Prevalence of Detectable cTnT
The sample-weight–adjusted prevalence of detectable cTnT in Dallas County adults was 25.0% (95% confidence interval [CI], 22.7%−27.4%) using the highly sensitive cTnT assay and 0.7% (95% CI, 0.3%−1.1%) using the standard assay. Among individuals without cardiovascular disease, CKD, subclinical heart disease, diabetes, or hypertension, the prevalence of detectable cTnT with the highly sensitive assay was 16.2% (95% CI, 13.3%−19.1%) (Table 1). The prevalence of a cTnT concentration of 0.014 ng/mL or greater (the 99th percentile cutpoint for diagnosis of MI) was 2.0% (95% CI, 1.5%−2.6%) in Dallas County adults and 1.9% (95% CI, 1.0%−2.0%) in those without prior cardiovascular disease (Table 1).
Table 1.
Prevalence of Detectable Cardiac Troponin T (≥0.003 ng/mL) and Levels Greater Than or Equal to the 99th Percentile Value (≥0.014 ng/mL) in the Dallas County Population and in Selected Subgroups
| Group | Sample Size, No. |
cTnT Level, ng/mL
|
|||
|---|---|---|---|---|---|
| ≥0.003
|
≥0.014
|
||||
| No. (%) | Sample Weight–Adjusted Prevalence, % (95% CI) |
No. (%) | Sample Weight–Adjusted Prevalence, % (95% CI) |
||
| Overall population | 3546 | 957 (27.0) | 25.0 (22.7–27.4) | 122 (3.4) | 2.0 (1.5–2.6) |
|
| |||||
| Restricted population | |||||
| Without CHD | 3428 | 891 (26.0) | 24.2 (21.8–26.5) | 103 (3.0) | 1.8 (1.2–2.4) |
|
| |||||
| Without cardiovascular disease | 3277 | 813 (24.8) | 23.7 (21.3–26.1) | 82 (2.5) | 1.9 (1.0–2.0) |
|
| |||||
| Without cardiovascular disease or CKDa | 3222 | 773 (24.0) | 23.1 (20.7–25.5) | 65 (2.3) | 1.2 (0.8–1.7) |
|
| |||||
| Without cardiovascular disease, CKD, or subclinical heart disease | 2554 | 510 (20.0) | 19.3 (16.8–21.8) | 43 (1.7) | 1.1 (0.6–1.7) |
|
| |||||
| Without cardiovascular disease, CKD, subclinical heart disease, diabetes, or hypertensionb | 1854 | 292 (15.7) | 16.2 (13.3–19.1) | 16 (0.9) | 0.6 (0.1–1.0) |
|
| |||||
| Age, yc | |||||
| 30-<40 | 1156 | 172 (14.9) | 14.0 (11.2–16.9) | 20 (1.7) | 1.0 (0.4–1.7) |
|
| |||||
| 40-<50 | 1152 | 279 (24.2) | 22.1 (18.1–26.2) | 24 (2.1) | 0.8 (0.3–1.3) |
|
| |||||
| 50-<60 | 846 | 343 (40.5) | 37.4 (32.4–42.3) | 56 (6.6) | 4.6 (2.6–6.6) |
|
| |||||
| 60–65 | 247 | 138 (55.9) | 57.6 (47.0–68.2) | 22 (8.9) | 5.2 (2.2–8.2) |
|
| |||||
| Sexd | |||||
| Men | 1565 | 670 (42.8) | 37.1 (33.3–41.0) | 85 (5.4) | 2.8 (1.9–3.7) |
|
| |||||
| Women | 1981 | 287 (14.5) | 12.9 (10.6–15.2) | 37 (1.9) | 1.3 (0.6–2.0) |
|
| |||||
| Self-reported race/ethnicitye | |||||
| Black | 1828 | 599 (32.8) | 34.4 (30.6–38.3) | 94 (5.1) | 4.7 (3.2–6.3) |
|
| |||||
| White | 1042 | 248 (23.8) | 25.4 (21.8–29.0) | 21 (2.0) | 1.8 (0.9–2.7) |
|
| |||||
| Hispanic | 601 | 101 (16.8) | 19.0 (14.5–23.5) | 7 (1.2) | 0.7 (0.1–1.3) |
|
| |||||
| Other | 75 | 9 (12.0) | 8.7 (2.0–15.5) | 0 | 0 |
Abbreviations: CI, confidence interval; CHD, coronary heart disease; CKD, chronic kidney disease; cTnT, cardiac troponin T.
Chronic kidney disease defined as an estimated glomerular filtration rate less than 60 mL/min per 1.73 m2.
Subclinical heart disease defined as left ventricular hypertrophy, left ventricular ejection fraction less than 55%, or a coronary artery calcium score greater than 10.
P < .001 for trend across age categories for the prevalence of detectable cTnT.
P < .001 for comparisons of the prevalence of detectable cTnT by sex.
P < .001 for comparison of the prevalence of detectable cTnT across racial/ethnic subgroups.
The prevalence of detectable cTnT varied markedly with age, ranging from 14.0% (95% CI, 11.2%−16.9%) in participants aged 40 to 50 years to 57.6% (95% CI, 47.0%−68.2%) in those aged 60 to 65 years (P < .001) (Table 1). Large differences in prevalence were also seen according to sex and race/ethnicity: men were 3-fold more likely to have detectable levels than women (37.1% [95% CI, 33.3%−41.0%] vs 12.9% [95% CI, 10.6%−15.2%], P < .001), and black participants had a significantly higher prevalence of detectable cTnT than Hispanic or white participants (P < .001) (Table 1). The prevalence of detectable cTnT stratified by sex and race/ethnicity is shown in eFigure 1.
Univariable Associations of cTnT Levels With Risk Factors and Cardiovascular Phenotypes
Two-thirds of participants in the highest cTnT category had undetectable cTnT levels with the standard assay (Table 2). The prevalence of hypertension increased from 27.2% (95% CI, 25.5%−28.9%) to 70.9% (95% CI, 61.8%−79.0%) and prevalence of diabetes from 7.7% (95% CI, 6.7%−8.8%) to 41.0% (95% CI, 32.2%−50.3%) across categories of increasing cTnT levels. Estimated glomerular filtration rate decreased from 99.5 mL/min per 1.73 m2 (95% CI, 87.1–114.4) to 88.0 (95% CI, 62.7–107.9) mL/min per 1.73 m2 (P < .001 for trend) across categories of higher cTnT levels, but smoking status and levels of low-density lipoprotein cholesterol did not change. Although higher body mass index was modestly associated with increasing cTnT levels, DEXA measurements of body composition demonstrated discordant associations with lean and fat mass: cTnT was positively associated with lean mass but inversely (and weakly) associated with fat mass (Table 2).
Table 2.
Demographic Characteristics, Cardiovascular Risk Factors, and Cardiac Phenotypes Across Increasing Categories of Cardiac Troponin T Level
| Variable | cTnT Category, ng/mLa
|
P for Trend |
||||
|---|---|---|---|---|---|---|
| <0.003 (n = 2589) |
0.003–0.00440 (n = 278) |
0.00441–0.00657 (n = 279) |
0.0066-<0.0014 (n = 278) |
≥0.0014 (n = 122) |
||
| cTnT ≥0.01 ng/mL with standard assay, No./total (%) | 0/2589 | 0/278 | 0/279 | 1/277 (0.4) | 40/120 (33.3) | <.001 |
|
| ||||||
| Age, median (IQR), y | 41 (35–49) | 47 (39–55) | 49 (41–55) | 52 (45–58) | 53 (44–58) | <.001 |
|
| ||||||
| Men, No./total (%) | 895/2589 (34.6) | 175/278 (62.9) | 196/279 (70.3) | 214/278 (77.0) | 85/122 (69.7) | <.001 |
|
| ||||||
| Race/ethnicity, No./total (%) | ||||||
| Black | 1229/2589 (47.5) | 150/278 (54.0) | 173/279 (62.0) | 182/278 (65.5) | 94/122 (77.0) | <.001 |
|
| ||||||
| White | 794/2589 (30.7) | 88/278 (31.7) | 78/279 (28.0) | 61/278 (21.9) | 21/122 (17.2) | <.001 |
|
| ||||||
| Hispanic | 500/2589 (19.3) | 37/278 (13.3) | 27/279 (9.7) | 30/278 (10.8) | 7/122 (5.7) | <.001 |
|
| ||||||
| Other | 66/2589 (2.5) | 3/278 (1.1) | 1/279 (0.4) | 5/278 (1.8) | 0/278 | .008 |
|
| ||||||
| Hypertension, No./total (%) | 694/2554 (27.2) | 116/277 (41.9) | 125/271 (46.1) | 170/274 (62.0) | 83/117 (70.9) | <.001 |
|
| ||||||
| Blood pressure, median (IQR), mm Hg | ||||||
| Systolic | 119.7 | 127.0 | 126.0 | 134.3 | 136.7 | <.001 |
| (110.0–130.7) | (117.3–137.7) | (117.0–140.7) | (121.7–153.0) | (121.3–161.0) | ||
|
| ||||||
| Diastolic | 76.3 (70.7–83.0) | 79.3 (73.0–85.0) | 79.0 (72.7–86.0) | 83.0 (75.3–89.0) | 81.7 (74.0–89.3) | <.001 |
|
| ||||||
| Diabetes, No./total (%) | 200/2588 (7.7) | 38/278 (13.7) | 43/279 (15.4) | 79/278 (28.4) | 50/122 (41.0) | <.001 |
|
| ||||||
| Fasting glucose, median (IQR), mg/dL | 92 (84–100) | 94 (85–103) | 94 (87–106) | 99 (87–122) | 99.5 (86–152) | <.001 |
|
| ||||||
| Metabolic syndrome, No./total (%)b | 787/2589 (30.4) | 96/278 (34.5) | 107/279 (38.4) | 136/278 (48.9) | 63/122 (51.6) | <.001 |
|
| ||||||
| Hypercholesterolemia, No./total (%) | 282/2587 (10.9) | 52/278 (18.7) | 38/279 (13.6) | 57/278 (20.5) | 29/122 (23.8) | <.001 |
|
| ||||||
| Lipids, median (IQR), mg/dL | ||||||
| Total cholesterol | 176 (153–202) | 180.5 (156–207) | 179 (157–202) | 176.5 (154–205) | 175.5 (148–195) | .50 |
|
| ||||||
| LDL-C | 103 (82–125) | 107.5 (84–129) | 108 (84–130) | 104.5 (82–129) | 96.5 (74–120) | .66 |
|
| ||||||
| HDL-C | 48 (40–58) | 45 (38–55) | 46 (38–54) | 45 (38–52) | 44.5 (36–57) | <.001 |
|
| ||||||
| Triglycerides | 92 (66–142) | 107 (72–158) | 102 (72–153) | 106.5 (74–170) | 106 (74–170) | <.001 |
|
| ||||||
| Current smoking, No./total (%) | 787/2586 (30.4) | 62/277 (22.4) | 56/279 (20.1) | 92/277 (33.2) | 37/122 (30.3) | .31 |
|
| ||||||
| Cocaine use, No./total (%) | 355/2589 (13.7) | 28/278 (10.1) | 37/279 (13.3) | 40/278 (14.4) | 13/122 (10.7) | .55 |
|
| ||||||
| Prior heart failure, No./total (%) | 56/2589 (2.2) | 12/278 (4.3) | 16/279 (5.7) | 22/278 (7.9) | 23/122 (18.9) | <.001 |
|
| ||||||
| Prior CHD, No./total (%) | 52/2589 (2.0) | 11/278 (4.0) | 15/279 (5.4) | 21/278 (7.6) | 19/122 (15.6) | <.001 |
|
| ||||||
| Prior CVD, No./total (%) | 125/2589 (4.8) | 25/278 (9.0) | 30/279 (10.8) | 49/278 (17.6) | 40/122 (32.8) | <.001 |
|
| ||||||
| Framingham risk score–estimated 10-y risk for CHD, No./total (%)b | ||||||
| <10% | 2140/2537 (84.4) | 196/275 (71.3) | 183/269 (68.0) | 118/273 (43.2) | 43/117 (36.8) | <.001 |
|
| ||||||
| 10%−20% | 121/2537 (4.8) | 23/275 (8.4) | 25/269 (9.3) | 40/273 (14.7) | 8/117 (6.8) | <.001 |
|
| ||||||
| >20% | 276/2537 (10.9) | 56/275 (20.4) | 61/269 (22.7) | 115/273 (42.1) | 66/117 (56.4) | <.001 |
|
| ||||||
| Body composition, median (IQR) | ||||||
| BMIc | 29.2 (25.1–34.4) | 29.4 (26.0–33.9) | 29.7 (26.5–35.0) | 30.6 (27.0–35.0) | 30.1 (25.4–37.7) | <.001 |
|
| ||||||
| Lean mass, kg | 52.4 (44.4–61.7) | 58.8 (50.4–67.6) | 62.9 (55.3–70.0) | 63.3 (54.6–70.6) | 60.8 (53.1–71.5) | <.001 |
|
| ||||||
| Fat mass, kg | 26.0 (19.0–35.0) | 25.6 (18.1–32.9) | 24.7 (18.8–32.7) | 25.8 (18.0–32.5) | 24.2 (16.7–33.5) | .01 |
|
| ||||||
| Estimated GFR, median (IQR), mL/min per 1.73 m2 | 99.5 | 93.0 | 93.4 | 87.3 | 88.0 | <.001 |
| (87.1–114.4) | (82.44–110.1) | (80.8–109.2) | (77.5–104.9) | (62.7–107.9) | ||
|
| ||||||
| CKD stage, No./total (%) | ||||||
| 1 | 1804/2588 (69.7) | 158/278 (56.8) | 161/279 (57.7) | 124/278 (44.6) | 58/122 (47.5) | <.001 |
|
| ||||||
| 2 | 767/2588 (29.6) | 113/278 (40.6) | 110/279 (39.4) | 135/278 (48.6) | 33/122 (27.0) | <.001 |
|
| ||||||
| 3 | 15/2588 (0.6) | 6/278 (2.2) | 6/279 (2.2) | 18/278 (6.5) | 16/122 (13.1) | <.001 |
|
| ||||||
| 4 | 0/2588 | 1/278 (0.4) | 0/279 | 0/278 | 1/122 (0.8) | .02 |
|
| ||||||
| 5 | 2/2588 (0.1) | 0/278 | 2/279 (0.7) | 1/278 (0.4) | 14/122 (11.5) | <.001 |
|
| ||||||
| Left ventricular mass, median (IQR), g | 149.1 | 171.5 | 183.7 | 191.7 | 219.6 | <.001 |
| (125.1–177.5) | (146.1–198.5) | (156.3–212.3) | (159.0–227.3) | (176.9–266.4) | ||
|
| ||||||
| Left ventricular mass/BSA, median (IQR), g/m2 | 77.4 (68.3–88.5) | 86.3 (74.6–96.9) | 89.2 (77.3–103.2) | 92.1 (80.1–106.8) | 103.8 (88.6–131.1) | <.001 |
|
| ||||||
| LVH, No./total (%) | 154/2043 (7.5) | 22/213 (10.3) | 40/232 (17.2) | 60/232 (25.9) | 38/79 (48.1) | <.001 |
|
| ||||||
| LVEDV, median (IQR), mL | 96.8 (83.7–112.1) | 102.1 (85.7–118.7) | 108.5 (94.5–122.8) | 104.4 (86.8–130.1) | 112.6 (92.4–143.7) | <.001 |
|
| ||||||
| LVEDV/BSA, median (IQR), mL/m2 | 50.7 (44.8–57.5) | 50.8 (44.1–58) | 52.6 (46.3–61.4) | 51.8 (43.9–60.7) | 54.5 (47.6–67.2) | .001 |
|
| ||||||
| LV wall thickness, median (IQR), mm | 11.1 (10.1–12.3) | 12.2 (11.1–13.4) | 12.2 (11.2–13.4) | 12.8 (11.7–14.5) | 13.4 (12.0–15.3) | <.001 |
|
| ||||||
| LVEF, median (IQR), % | 73.3 (68.6–77.6) | 72.8 (67.7–77.2) | 71.8 (67.0–76.1) | 70.1 (64.5–76.5) | 72.6 (60.8–77.6) | <.001 |
|
| ||||||
| LVEF <40%, No./total (%) | 1/2044 (0.05) | 0/213 | 3/232 (1.3) | 6/232 (2.6) | 4/79 (5.1) | <.001 |
|
| ||||||
| Coronary artery calcium score, Agatston units, No./total (%) | ||||||
| ≤10 | 1712/2034 (84.2) | 152/213 (71.4) | 154/227 (67.8) | 122/223 (54.7) | 40/73 (54.8) | <.001 |
|
| ||||||
| >10–100 | 212/2034 (10.4) | 29/213 (13.6) | 32/227 (14.1) | 46/223 (20.6) | 5/73 (6.8) | .001 |
|
| ||||||
| >100–400 | 77/2034 (3.8) | 24/213 (11.3) | 27/227 (11.9) | 30/223 (13.5) | 16/73 (21.9) | <.001 |
|
| ||||||
| >400 | 33/2034 (1.6) | 8/213 (3.8) | 14/227 (6.2) | 25/223 (11.2) | 12/73 (16.4) | <.001 |
|
| ||||||
| Aortic wall thickness, median (IQR), mm | 1.62 (1.46–1.79) | 1.71 (1.54–1.97) | 1.77 (1.61–1.92) | 1.84 (1.65–2.15) | 1.85 (1.60–2.07) | <.001 |
|
| ||||||
| Aortic compliance, median (IQR), mL/mm Hg | 25.1 (17.6–33.6) | 21.8 (14.6–28.4) | 19.9 (14.1–28.2) | 17.6 (12.3–24.2) | 15.6 (9.8–22.3) | <.001 |
|
| ||||||
| High-sensitivity CRP, median (IQR), mg/L | 2.8 (1.1–7.1) | 2.8 (1.2–6.2) | 2.3 (1.0–6.0) | 3.4 (1.3–7.1) | 3.8 (1.7–9.1) | .48 |
|
| ||||||
| NT-proBNP, median (IQR), ng/L | 28.2 (13.2–55) | 27.4 (10.6–61.2) | 24.3 (12.8–56.5) | 39.2 (15.7–104.4) | 104.9 (42.9–557.4) | <.001 |
|
| ||||||
| BNP, median (IQR), pg/mL | 2.8 (0–12.1) | 3.1 (0–15) | 3.1 (0–14.7) | 3.9 (0–17.7) | 17.5 (2.9–75.3) | <.001 |
Abbreviations: BMI, body mass index; BNP, brain-type natriuretic peptide; BSA, body surface area; CHD, coronary heart disease; CKD, chronic kidney disease; CRP, C-reactive protein; cTnT, cardiac troponin T; CVD, cardiovascular disease; GFR, glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; NT-proBNP, N-terminal pro-brain-type natriuretic peptide.
SI conversion factors: To convert total cholesterol, LDL-C, and HDL-C values to mmol/L, multiply by 0.0259; to convert triglyceride values to mmol/L, multiply by 0.0113; to convert glucose values to mmol/L, multiply by 0.0555.
Denominator values reflect the number of participants with data available for the specific variable. Variation in the denominator reflects absent data for that variable.
Defined according to the National Cholesterol Education Program Adult Treatment Panel III criteria (see eSupplement for detailed definition).
Calculated as weight in kilograms divided by height in meters squared.
Across cTnT categories, left ventricular mass increased markedly, as did left ventricular wall thickness, and the proportion of individuals classified as having LVH increased from 7.5% (95% CI, 6.4%−8.8%) to 48.1% (95% CI, 36.7%−59.6%) (P < .001 for trend). Modest increases in LVEDV and decreases in LVEF were also seen with higher cTnT levels. CAC score and abdominal aortic wall thickness increased while aortic compliance decreased across higher cTnT categories (Table 2). Strong graded associations were also evident between increasing severity of selected pathologic phenotypes and the prevalence of detectable cTnT, including indexed left ventricular mass, LVEF, CAC score, and CKD (eFigure 2).
Self-reported heart failure, CHD, and cardiovascular disease were more frequent with higher cTnT levels (Table 2). In sensitivity analyses excluding participants with cardiovascular disease (eTable 1), similar associations were observed between cTnT categories and demographic variables, risk factors, and cardiac phenotypes. Additionally, when these analyses were restricted to individuals predicted by the FRS to be at low risk (estimated 10-year risk of CHD <10% [n = 2680]), cTnT remained associated with multiple pathological cardiac phenotypes (eTable 2).
Independent Determinants of Detectable cTnT in the Population
In a multivariable logistic regression model with detectable cTnT as the dependent variable, male sex, older age, black race, history of heart failure, lower estimated glomerular filtration rate, and higher left ventricular mass independently associated with detectable cTnT, whereas prior MI or angina, LVEF, CAC score, lean mass, and body surface area did not (C statistic of model, 0.81) (eTable 3). When left ventricular mass was replaced with left ventricular wall thickness and LVEDV, the model performed similarly and both wall thickness and LVEDV remained associated with detectable cTnT.
Association of cTnT With All-Cause and Cardiovascular Mortality
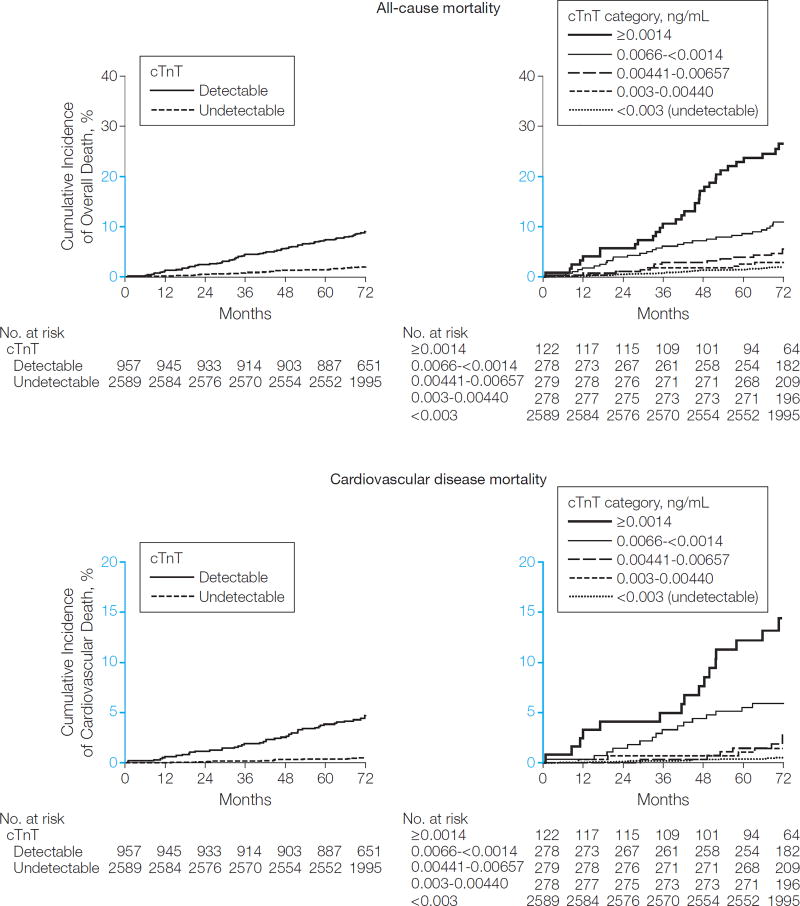
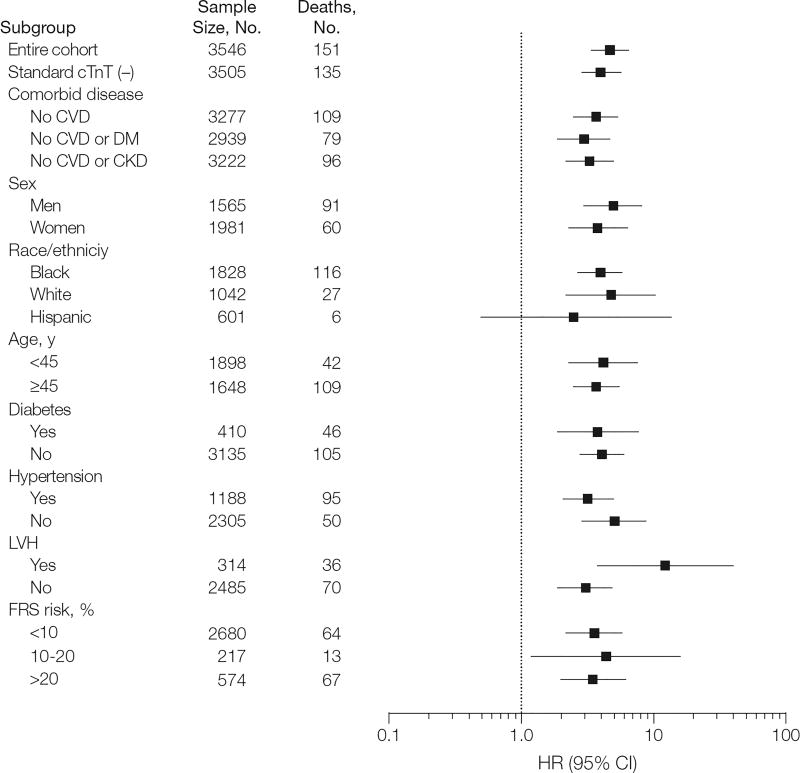
Over a median follow-up period of 6.4 years (interquartile range, 6.0–6.8 years), 151 total deaths and 62 cardiovascular disease deaths occurred. Unadjusted all-cause mortality occurred in 1.9% (95% CI, 1.5%−2.6%) of participants with undetectable cTnT vs 9.1% (95% CI, 7.4%−11.1%) of those with detectable cTnT (P < .001) and increased from 1.9% (95% CI, 1.5%−2.6%) to 28.4% (95% CI, 21.0%−37.8%) across higher cTnT categories (P < .001) (Figure 1). Similar trends were observed for cardiovascular disease mortality (Figure 1). Detectable cTnT and increasing cTnT categories remained associated with mortality after excluding participants with cTnT detected by the standard assay, as well as those with prevalent CHD or cardiovascular disease. Associations of cTnT level with all-cause and cardiovascular disease mortality were consistent in subgroups defined by sex, race/ethnicity, age, hypertension, and diabetes (Figure 2, eFigure 3, eTable 4, and eTable 5).
Figure 1.
Unadjusted Nelson-Aalen Curves for All-Cause and Cardiovascular Mortality
P< .001 for all between-group comparisons by the log-rank test. Detectable cardiac troponin T (cTnT) levels are 0.003 ng/mL or greater by the highly sensitive assay. Y-axes shown in blue indicate the range from 0% to 20%.
Figure 2.
Unadjusted Hazard Ratios for All-Cause Mortality Associated With Detectable Cardiac Troponin T (≥0.003 ng/mL) in Selected Subgroups
CKD indicates chronic kidney disease; cTnT, cardiac troponin T; CVD, cardiovascular disease; DM, diabetes mellitus; FRS, Framingham Risk Score; LVH, left ventricular hypertrophy.
In a series of Cox proportional hazards models adjusting for traditional risk factors (model 1) and further adjusting for high-sensitivity CRP level (model 2), higher cTnT categories demonstrated a graded association with all-cause and cardiovascular disease mortality. The addition of cTnT to the baseline models significantly improved the C statistic (0.818 vs 0.793; P = .001), IDI (0.044 [95% CI, 0.025–0.063]; P < .001), and model fit (P< .001) (Table 3). Only modest attenuation of the hazards was seen with further adjustment for CKD categories (model 3). Although more significant attenuation was seen with additional adjustment for NT-proBNP level (model 4), cTnT in the fourth category (hazard ratio [HR], 2.0 [95% CI, 1.2–3.4]) and fifth category (HR, 2.8 [95% CI, 1.4–5.2]) remained independently associated with all-cause mortality in the fully adjusted models, and the addition of cTnT improved model fit (P=.02) and the IDI (0.010 [95% CI, 0.002–0.018]; P=.01) but not the C statistic (0.827 [95% CI, 0.789–0.864] vs 0.821 [95% CI, 0.783–0.859]); P=.12) (Table 3). In contrast, in the same fully adjusted model, cTnT level measured with the standard assay did not associate with all-cause mortality and did not improve model fit, the IDI, or the C statistic (P>.15 for each). NT-proBNP level was associated with all-cause (P< .001) and cardiovascular disease (P < .001) mortality in the fully adjusted model, but high-sensitivity CRP level was not (P = .44 for all-cause mortality and P = .28 for cardiovascular disease mortality). Sensitivity analyses using log-transformed cTnT as a continuous variable yielded qualitatively similar results, including in the fully adjusted all-cause mortality model (HR per log unit change, 1.5 [95% CI, 1.1–1.9]; P=.006).
Table 3.
Multivariable-Adjusted Associations Between Cardiac Troponin T Categories and All-Cause and Cardiovascular Mortality
| Events, No. |
Event Rate at 6 y, % (95% CI) |
HR (95% CI)a
|
||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |||
| All-cause mortality | ||||||
| cTnT category, ng/mL | ||||||
| <0.003 | 52 | 1.9 (1.5 to 2.6) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
|
| ||||||
| 0.003–0.00440 | 9 | 2.9 (1.5 to 5.8) | 1.2 (0.6 to 2.4) | 1.2 (0.6 to 2.4) | 1.1 (0.5 to 2.3) | 1.0 (0.5 to 2.1) |
|
| ||||||
| 0.00441–0.00657 | 17 | 5.3 (3.2 to 8.9) | 2.0 (1.1 to 3.5) | 2.0 (1.1 to 3.6) | 1.9 (1.1 to 3.4) | 1.7 (1.0 to 3.1) |
|
| ||||||
| 0.0066-<0.0014 | 31 | 11.5 (8.1 to 16.0) | 2.7 (1.7 to 4.5) | 2.8 (1.7 to 4.6) | 2.5 (1.5 to 4.1) | 2.0 (1.2 to 3.4) |
|
| ||||||
| ≥0.0014 | 34 | 28.4 (21.0 to 37.8) | 6.7 (4.0 to 11.3) | 6.7 (4.0 to 11.2) | 4.8 (2.7 to 8.7) | 2.8 (1.4 to 5.2) |
|
| ||||||
| C statistic for models | ||||||
| Without highly sensitive cTnT assay | 0.793 | 0.793 | 0.806 | 0.821 | ||
| (0.757 to 0.829) | (0.757 to 0.829) | (0.769 to 0.842) | (0.783 to 0.859) | |||
|
| ||||||
| With highly sensitive cTnT assay | 0.818 | 0.818 | 0.823 | 0.827 | ||
| (0.782 to 0.854) | (0.782 to 0.853) | (0.788 to 0.858) | (0.789 to 0.864) | |||
|
| ||||||
| P value, bootstrap | .001 | .001 | .02 | .12 | ||
|
| ||||||
| P value, likelihood ratio test | <.001 | <.001 | <.001 | .02 | ||
|
| ||||||
| IDI | 0.044 | 0.044 | 0.024 | 0.010 | ||
| (0.025 to 0.063) | (0.026 to 0.062) | (0.011 to 0.040) | (0.002 to 0.018) | |||
|
| ||||||
| P value for IDI | <.001 | <.001 | <.001 | .01 | ||
|
| ||||||
| Cardiovascular mortality | ||||||
| cTnT category, ng/mL | ||||||
| <0.003 | 16 | 0.5 (0.3 to 0.9) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
|
| ||||||
| 0.003–0.00440 | 4 | 1.5 (0.6 to 3.9) | 1.6 (0.5 to 4.9) | 1.6 (0.5 to 4.9) | 1.4 (0.5 to 4.3) | 1.2 (0.4 to 3.8) |
|
| ||||||
| 0.00441–0.00657 | 7 | 2.8 (1.4 to 5.9) | 2.4 (0.9 to 6.1) | 2.5 (1.0 to 6.3) | 2.1 (0.8 to 5.3) | 1.7 (0.7 to 4.4) |
|
| ||||||
| 0.0066-<0.0014 | 17 | 6.0 (3.7 to 9.7) | 4.6 (2.1 to 10.0) | 4.7 (2.1 to 10.2) | 3.5 (1.6 to 7.7) | 2.4 (1.1 to 5.5) |
|
| ||||||
| ≥0.0014 | 15 | 14.4 (8.9 to 22.8) | 8.5 (3.7 to 19.4) | 8.4 (3.7 to 19.2) | 4.5 (1.7 to 11.8) | 1.7 (0.6 to 5.1) |
|
| ||||||
| C statistic for model | ||||||
| Without highly sensitive cTnT assay | 0.832 | 0.833 | 0.865 | 0.889 | ||
| (0.789 to 0.874) | (0.791 to 0.876) | (0.820 to 0.910) | (0.844 to 0.932) | |||
|
| ||||||
| With highly sensitive cTnT assay | 0.868 | 0.869 | 0.882 | 0.893 | ||
| (0.826 to 0.909) | (0.828 to 0.909) | (0.841 to 0.923) | (0.849 to 0.936) | |||
|
| ||||||
| P value, bootstrap | .004 | .005 | .05 | .28 | ||
|
| ||||||
| P value, likelihood ratio test | <.001 | <.001 | .02 | .37 | ||
|
| ||||||
| IDI | 0.027 | 0.029 | 0.010 | 0.005 | ||
| (0.012 to 0.042) | (0.014 to 0.044) | (−0.003 to 0.021) | (−0.003 to 0.013) | |||
|
| ||||||
| P value for IDI | <.001 | <.001 | .10 | .24 | ||
Abbreviations: CI, confidence interval; cTnT, cardiac troponin T; HR, hazard ratio; IDI, integrated discrimination index.
Models include participants with complete data for all variables (n = 3459). Model 1 adjusted for age, race/ethnicity, sex, diabetes, hypertension, hypercholesterolemia, low high-density lipoprotein cholesterol level, and current smoking. Model 2 adjusted for variables in model 1 plus log-transformed values of high-sensitivity C-reactive protein. Model 3 adjusted for variables in model 2 plus chronic kidney disease categories. Model 4 adjusted for variables in model 3 plus log-transformed values of N-terminal pro-brain-type natriuretic peptide.
COMMENT
Circulating cardiac troponin T is detectable in approximately 25% of adults aged 30 to 65 years in the general population using a novel highly sensitive assay, with wide variation in its prevalence according to age, sex, and race/ethnicity. Higher levels of cTnT measured with the highly sensitive assay, well below the detection range of standard assays, are associated with cardiac structural abnormalities including LVH (both left ventricular wall thickening and dilation) and left ventricular systolic dysfunction. These associations were consistent in lower-risk subgroups defined by the absence of known cardiovascular disease or a low FRS category. Moreover, higher cTnT levels demonstrate a graded association with all-cause and cardiovascular disease mortality independent of traditional risk factors, renal function, and levels of other biomarkers such as high-sensitivity CRP and NT-proBNP.
The important contributions of chronic sources of myocardial injury to troponin release have become increasingly evident as troponin measurements have been applied to ambulatory patient populations, particularly with highly sensitive assays. For example, nearly 100% of clinical trial participants with stable chronic heart failure and CAD had detectable cTnT levels using a highly sensitive assay.13,14
We applied the highly sensitive assay to a randomly sampled general population cohort with a large proportion of women and ethnic minorities and found that cTnT was commonly detectable in adults at levels well below the detection limit of a standard assay. Although several prior studies have measured levels of troponins in general population cohorts, they have used standard troponin assays that are much less sensitive than the assay used in the present study.8–11,29 For instance, the prevalence of detectable cTnT with a standard assay in this same young DHS cohort was only 0.7%.8,29 The finding that male sex, older age, and black race are associated with higher troponin levels has been suggested previously, but prior studies used less-sensitive assays and had a low rate of detectable troponins, precluding adequately powered multivariable analyses.8,9,29,30
In univariable analyses, cTnT was associated with multiple cardiac risk factors, measures of atherosclerosis burden, and cardiac structural phenotypes, most notably left ventricular mass and wall thickness. In multivariable analyses, diabetes, hypertension, worse renal function, and increased left ventricular mass, wall thickness and chamber dilation, but not CAC score, remained independent determinants of detectable cTnT. These findings should be interpreted in the context of prior studies investigating standard troponin assays in elderly individuals from the general population29 as well as in patients with chronic CAD,31 which suggest that chronic elevation of troponin levels is mediated to a greater extent by indices of heart failure (such as higher left ventricular mass, lower LVEF, or increased NT-proBNP levels) than indices of atherosclerosis or ischemia. In the Prevention of Events with Angiotensin Converting Enzyme Inhibition (PEACE) trial, which applied a highly sensitive assay to patients with chronic CAD, cTnT was associated with death and heart failure but not MI.14 In contrast, in patients with acute coronary syndromes, low levels of troponin correlate with angiographic evidence of greater lesion severity and complexity32 and identify patients at greater risk for ischemic complications than for death or heart failure.33,34 Taken together, these findings suggest important differences in the pathophysiology of troponin release in the chronic compared with the acute setting.
Prior studies have described associations between increased troponin levels detected with standard assays and future risk for mortality.9–11 Here, we report that these associations extend to much lower troponin levels not detected with assays in current clinical use. Indeed, only one-third of the participants in the highest cTnT category had cTnT levels measurable with the standard-generation cTnT assay. Higher cTnT levels associated strongly with future risk for total and cardiovascular disease mortality, and consistent associations were observed in individuals without prior cardiovascular disease and those who would be deemed at low risk based on the FRS. Associations with mortality were only modestly attenuated after adjustment for traditional risk factors, high-sensitivity CRP level, and CKD. Further adjustment for levels of NT-proBNP, which was also associated with total and cardiovascular disease death in this cohort, resulted in more substantial attenuation of the HRs, suggesting that NT-proBNP and cTnT provide partly overlapping information concerning cardiac structural and functional abnormalities. However, even after full adjustment, cTnT in the highest 2 categories remained independently associated with all-cause mortality, with adjusted HRs in a range that would suggest potential clinical utility.35 The addition of cTnT to a variety of risk prediction models increased model fit and the IDI, a metric of discrimination.
The present findings suggest that future studies should be performed to assess whether measurement of cTnT levels with a highly sensitive assay adds value to traditional cardiovascular risk factors as well as measures of renal function and NT-proBNP levels for risk assessment in the general population. In the fully adjusted model, which included NT-proBNP levels and renal function, improvements in the C statistic and the IDI with cTnT were small, and the clinical significance of changes of this magnitude are not clear. Future studies will need to evaluate clinical performance metrics, such as net reclassification improvement, in data sets with long-term follow-up for nonfatal end points, to fully evaluate the incremental value of measurement of cTnT levels. In addition, appropriate diagnostic and therapeutic responses would need to be defined before population-based screening could be recommended. However, our data showing associations with LVH and other cardiac structural abnormalities as well as with mortality among individuals classified at low risk using the FRS suggest that low levels of cTnT may identify subclinical structural heart disease and contributors to cardiovascular disease risk not fully captured by current risk-assessment tools. The independent and additive associations of cTnT and NT-proBNP suggest that combinations of these 2 markers may perform better than either marker alone.
Our finding of a high prevalence of detectable cTnT in the general population has important, and complex, implications for the use of highly sensitive troponin assays for diagnosing acute MI in the hospital setting. Among patients with clinical presentations suspicious for MI, higher-sensitivity assays improve diagnostic sensitivity, particularly early after presentation, but reduce specificity.12,36 When applied to patients with a high clinical suspicion for MI, the net result is improved accuracy. However, if applied to individuals with a lower likelihood of MI, but with factors associated with higher cTnT levels such as older age, male sex, black race, hypertension, diabetes, CKD, and LVH, the results of the highly sensitive troponin assays will have lower specificity, and false-positive diagnoses of MI will be more common. Because current guidelines recommend more intensive treatment for patients with suspected acute coronary syndromes who have elevated troponin levels,1,2 it is possible that widespread application of highly sensitive assays without integrating new approaches to discriminate acute injury37 will expose some patients to unnecessary risk and expense. Additional investigation is needed before the full clinical implications of highly sensitivity troponin assays can be determined.
This study has several strengths. The sampling strategy used for the DHS permits generation of unbiased population estimates of the prevalence of detectable cTnT among Dallas County adults. The availability of cardiac MRI and EBCT data in this large population-based cohort allows detailed cardiac phenotypes to be assessed for their associations with cTnT. The measurement of cTnT by standard as well as highly sensitive assays allows demonstration of the incremental value of the novel assay.
This study also has several limitations that merit comment. First, not all participants completed cardiac imaging studies. Second, adjudicated nonfatal outcome data are not yet available for the DHS; thus, full evaluation of the incremental value of measuring cTnT levels for risk prediction over currently available risk assessment algorithms such as the FRS could not be performed. Third, only a single baseline measurement of cTnT level and other covariates was performed. Finally, the number of cardiovascular deaths was relatively small, limiting statistical power for this outcome.
CONCLUSIONS
Using a highly sensitivity assay, cTnT was detectable in approximately 25% of adults in the general population and was associated with structural heart disease and risk of subsequent all-cause mortality. Higher cTnT levels, below the detection range of currently available assays, may be considered a marker of “end organ” cardiovascular damage from a variety of risk factors and pathological cardiac and vascular processes.
Supplementary Material
Acknowledgments
Dr de Lemos reported receiving research grants from Roche Diagnostics and Biosite/Inverness and receiving consulting fees and/or lecture honoraria from Tethys Biomedical, Johnson & Johnson, Roche Diagnostics, Biosite/Inverness, Siemens, AstraZeneca, Pfizer, BMS/sanofi-aventis, and Merck/Schering. Dr Drazner reported receiving consulting fees from Biosite/Inverness and lecture honoraria from GlaxoSmithKline. Dr Omland reported receiving research support and lecture honoraria from Roche Diagnostics and Abbott Laboratories. Dr Morrow reported receiving honoraria for educational presentations from Beckman-Coulter, CV Therapeutics, and Eli Lilly and receiving consulting fees from Beckman-Coulter, Boerhinger Ingelheim, Instrumentation Laboratories, Menarini, Merck, sanofi-aventis, Servier, Roche Diagnostics, Siemens, and AstraZeneca. The TIMI Study Group, which supports Dr Morrow’s salary, has received significant research grant support from Accumetrics, Amgen, AstraZeneca, Beckman Coulter, Bristol-Myers Squibb, CV Therapeutics, Daiichi Sankyo Co Ltd, Eli Lilly and Co, GlaxoSmithKline, Integrated Therapeutics, Merck and Co, Merck-Schering Plough Joint Venture, Nanosphere, Novartis Pharmaceuticals, Nuvelo, Ortho-Clinical Diagnostics, Pfizer, Roche Diagnostics, sanofi-aventis, Siemens, and Singulex. Dr McGuire reported receiving grant support from GlaxoSmithKline; receiving consulting fees from Tethys Bioscience, Biosite, F. Hoffmann LaRoche/Genentech, Cardiovascular Therapeutics, AstraZeneca, Novo Nordisk, Daiichi-Sankyo, Mitsubishi Tanabe Pharma, Johnson & Johnson, and sanofi-aventis; and receiving lecture honoraria from Pfizer and Takeda.
Funding/Support: Grant support for the Dallas Heart Study was provided by the Donald W. Reynolds Foundation and by US Public Health Service General Clinical Research Center grant M01-RR00633 from National Institutes of Health (NIH)/NCRR-CR. This study was supported in part by the North and Central Texas Clinical and Translational Science Initiative (NIH grant UL1 RR024982). Financial and materials support for measurements of cardiac troponin T (cTnT) levels in the present study were provided by Roche Diagnostics (Indianapolis, Indiana). Roche Diagnostics also previously provided materials and support for measurement of levels of cTnT, N-terminal pro-brain-type natriuretic peptide, and C-reactive protein in the Dallas Heart Study.
Role of the Sponsor: Roche Diagnostics and the NIH had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Additional Contributions: We thank Jerry Ashmore, MT, and Barbara Morgan, MT, BS, MEd, for performing the highly sensitive cardiac troponin T assays and Helen Hobbs, MD, Teresa Eversole, BS, and Kathleen Wilkinson, MS, for their leadership of the Dallas Heart Study. The affiliation for all additional contributors listed is University of Texas Southwestern Medical Center, Dallas. Partial salary support was provided to Barbara Morgan and Jerry Ashmore.
Footnotes
Author Contributions: Dr de Lemos had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: de Lemos, McGuire.
Acquisition of data: de Lemos, Khera, Hashim, McGuire.
Analysis and interpretation of data: de Lemos, Drazner, Omland, Ayers, Khera, Rohatgi, Berry, Das, Morrow, McGuire.
Drafting of the manuscript: de Lemos.
Critical revision of the manuscript for important intellectual content: de Lemos, Drazner, Omland, Ayers, Khera, Rohatgi, Hashim, Berry, Das, Morrow, McGuire.
Statistical analysis: Ayers.
Obtained funding: de Lemos, McGuire.
Administrative, technical, or material support: Hashim.
Study supervision: de Lemos.
Financial Disclosures: No other authors reported disclosures.
Online-Only Material: The eSupplement, eFigures 1– 3, and eTables 1– 5 are available at http://www.jama.com.
References
- 1.Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction. J Am Coll Cardiol. 2007;50(7):e1–e157. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Morrow DA, Cannon CP, Jesse RL, et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Clin Chem. 2007;53(4):552–574. doi: 10.1373/clinchem.2006.084194. [DOI] [PubMed] [Google Scholar]
- 3.Konstantinides S, Geibel A, Olschewski M, et al. Importance of cardiac troponins I and T in risk stratification of patients with acute pulmonary embolism. Circulation. 2002;106(10):1263–1268. doi: 10.1161/01.cir.0000028422.51668.a2. [DOI] [PubMed] [Google Scholar]
- 4.Peacock WFIV, De Marco T, Fonarow GC, et al. Cardiac troponin and outcome in acute heart failure. N Engl J Med. 2008;358(20):2117–2126. doi: 10.1056/NEJMoa0706824. [DOI] [PubMed] [Google Scholar]
- 5.Eggers KM, Lagerqvist B, Venge P, et al. Persistent cardiac troponin I elevation in stabilized patients after an episode of acute coronary syndrome predicts long-term mortality. Circulation. 2007;116(17):1907–1914. doi: 10.1161/CIRCULATIONAHA.107.708529. [DOI] [PubMed] [Google Scholar]
- 6.Horwich TB, Patel J, MacLellan WR, Fonarow GC. Cardiac troponin I is associated with impaired hemodynamics, progressive left ventricular dysfunction, and increased mortality rates in advanced heart failure. Circulation. 2003;108(7):833–838. doi: 10.1161/01.CIR.0000084543.79097.34. [DOI] [PubMed] [Google Scholar]
- 7.Apple FS, Murakami MM, Pearce LA, Herzog CA. Predictive value of cardiac troponin I and T for subsequent death in end-stage renal disease. Circulation. 2002;106(23):2941–2945. doi: 10.1161/01.cir.0000041254.30637.34. [DOI] [PubMed] [Google Scholar]
- 8.Wallace TW, Abdullah SM, Drazner MH, et al. Prevalence and determinants of troponin T elevation in the general population. Circulation. 2006;113(16):1958–1965. doi: 10.1161/CIRCULATIONAHA.105.609974. [DOI] [PubMed] [Google Scholar]
- 9.Daniels LB, Laughlin GA, Clopton P, et al. Minimally elevated cardiac troponin T and elevated N-terminal pro-B-type natriuretic peptide predict mortality in older adults. J Am Coll Cardiol. 2008;52(6):450–459. doi: 10.1016/j.jacc.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zethelius B, Johnston N, Venge P. Troponin I as a predictor of coronary heart disease and mortality in 70-year-old men. Circulation. 2006;113(8):1071–1078. doi: 10.1161/CIRCULATIONAHA.105.570762. [DOI] [PubMed] [Google Scholar]
- 11.Blankenberg S, Zeller T, Saarela O, et al. Contribution of 30 biomarkers to 10-year cardiovascular risk estimation in 2 population cohorts. Circulation. 2010;121(22):2388–2397. doi: 10.1161/CIRCULATIONAHA.109.901413. [DOI] [PubMed] [Google Scholar]
- 12.Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361(9):858–867. doi: 10.1056/NEJMoa0900428. [DOI] [PubMed] [Google Scholar]
- 13.Latini R, Masson S, Anand IS, et al. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation. 2007;116(11):1242–1249. doi: 10.1161/CIRCULATIONAHA.106.655076. [DOI] [PubMed] [Google Scholar]
- 14.Omland T, de Lemos JA, Sabatine MS, et al. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009;361(26):2538–2547. doi: 10.1056/NEJMoa0805299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Victor RG, Haley RW, Willett DL, et al. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93(12):1473–1480. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 16.de Lemos JA, McGuire DK, Khera A, et al. Screening the population for left ventricular hypertrophy and left ventricular systolic dysfunction using natriuretic peptides. Am Heart J. 2009;157(4):746–753. doi: 10.1016/j.ahj.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 17.Khera A, McGuire DK, Murphy SA, et al. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005;46(3):464–469. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 18.Giannitsis E, Kurz K, Hallermayer K, et al. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56(2):254–261. doi: 10.1373/clinchem.2009.132654. [DOI] [PubMed] [Google Scholar]
- 19.Das SR, Drazner MH, Dries DL, et al. Impact of body mass and body composition on circulating levels of natriuretic peptides: results from the Dallas Heart Study. Circulation. 2005;112(14):2163–2168. doi: 10.1161/CIRCULATIONAHA.105.555573. [DOI] [PubMed] [Google Scholar]
- 20.Drazner MH, Dries DL, Peshock RM, et al. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: the Dallas Heart Study. Hypertension. 2005;46(1):124–129. doi: 10.1161/01.HYP.0000169972.96201.8e. [DOI] [PubMed] [Google Scholar]
- 21.Abdullah SM, Khera A, Das SR, et al. Relation of coronary atherosclerosis determined by electron beam computed tomography and plasma levels of N-terminal pro-brain natriuretic peptide in a multiethnic population-based sample (the Dallas Heart Study) Am J Cardiol. 2005;96(9):1284–1289. doi: 10.1016/j.amjcard.2005.06.073. [DOI] [PubMed] [Google Scholar]
- 22.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 23.See R, Lindsey JB, Patel MJ, et al. Application of the screening for Heart Attack Prevention and Education Task Force recommendations to an urban population. Arch Intern Med. 2008;168(10):1055–1062. doi: 10.1001/archinte.168.10.1055. [DOI] [PubMed] [Google Scholar]
- 24.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease stroke statistics—2010 update: a report from the American Heart Association [published correction appears in Circulation. 2010;121(12):e260] Circulation. 2010;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 25.Jonckheere AR, Bower GH. Non-parametric trend tests for learning data. Br J Math Stat Psychol. 1967;20(2):163–186. doi: 10.1111/j.2044-8317.1967.tb00385.x. [DOI] [PubMed] [Google Scholar]
- 26.Colosimo E, Ferreira F, Oliveira M, Sousa C. Empirical comparisons between Kaplan-Meier and Nelson-Aalen survival function estimators. J Stat Computation Computing. 2002;72(4):299–308. [Google Scholar]
- 27.Antolini L, Boracchi P, Biganzoli E. A time-dependent discrimination index for survival data. Stat Med. 2005;24(24):3927–3944. doi: 10.1002/sim.2427. [DOI] [PubMed] [Google Scholar]
- 28.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker. Stat Med. 2008;27(2):157–172. 207–212. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 29.Eggers KM, Lind L, Ahlström H, et al. Prevalence and pathophysiological mechanisms of elevated cardiac troponin I levels in a population-based sample of elderly subjects. Eur Heart J. 2008;29(18):2252–2258. doi: 10.1093/eurheartj/ehn327. [DOI] [PubMed] [Google Scholar]
- 30.Apple FS, Quist HE, Doyle PJ, et al. Plasma 99th percentile reference limits for cardiac troponin and creatine kinase MB mass for use with European Society of Cardiology/American College of Cardiology consensus recommendations. Clin Chem. 2003;49(8):1331–1336. doi: 10.1373/49.8.1331. [DOI] [PubMed] [Google Scholar]
- 31.Eggers KM, Lagerqvist B, Oldgren J, Venge P, Wallentin L, Lindahl B. Pathophysiologic mechanisms of persistent cardiac troponin I elevation in stabilized patients after an episode of acute coronary syndrome. Am Heart J. 2008;156(3):588–594. doi: 10.1016/j.ahj.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 32.Wong GC, Morrow DA, Murphy S, et al. Elevations in troponin T and I are associated with abnormal tissue level perfusion: a TACTICS-TIMI 18 substudy. Circulation. 2002;106(2):202–207. doi: 10.1161/01.cir.0000021921.14653.28. [DOI] [PubMed] [Google Scholar]
- 33.Morrow DA, Cannon CP, Rifai N, et al. Ability of minor elevations of troponins I and T to predict benefit from an early invasive strategy in patients with unstable angina and non-ST elevation myocardial infarction. JAMA. 2001;286(19):2405–2412. doi: 10.1001/jama.286.19.2405. [DOI] [PubMed] [Google Scholar]
- 34.Lindahl B, Diderholm E, Lagerqvist B, Venge P, Wallentin L. Mechanisms behind the prognostic value of troponin T in unstable coronary artery disease: a FRISC II substudy. J Am Coll Cardiol. 2001;38(4):979–986. doi: 10.1016/s0735-1097(01)01501-7. [DOI] [PubMed] [Google Scholar]
- 35.Morrow DA, de Lemos JA. Benchmarks for the assessment of novel cardiovascular biomarkers. Circulation. 2007;115(8):949–952. doi: 10.1161/CIRCULATIONAHA.106.683110. [DOI] [PubMed] [Google Scholar]
- 36.Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361(9):868–877. doi: 10.1056/NEJMoa0903515. [DOI] [PubMed] [Google Scholar]
- 37.Morrow DA, Antman EM. Evaluation of high-sensitivity assays for cardiac troponin. Clin Chem. 2009;55(1):5–8. doi: 10.1373/clinchem.2008.117218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.