Abstract
Key points
The release probability of the odorant receptor neuron (ORN) is reportedly one of the highest in the brain and is predicted to impose a transient temporal filter on postsynaptic cells.
Mitral cells responded to high frequency ORN stimulation with sustained transmission, whereas external tufted cells responded transiently.
The release probability of ORNs (0.7) was equivalent across mitral and external tufted cells and could be explained by a single pool of slowly recycling vesicles.
The sustained response in mitral cells resulted from dendrodendritic amplification in mitral cells, which was blocked by NMDA and mGluR1 receptor antagonists, converting mitral cell responses to transient response profiles.
Our results suggest that although the afferent ORN synapse shows strong synaptic depression, dendrodendritic circuitry in mitral cells produces robust amplification of brief afferent input, and thus the relative strength of axodendritic and dendrodendritic input determines the postsynaptic response profile.
Abstract
Short‐term synaptic plasticity is a critical regulator of neural circuits, and largely determines how information is temporally processed. In the olfactory bulb, afferent olfactory receptor neurons respond to increasing concentrations of odorants with barrages of action potentials, and their terminals have an extraordinarily high release probability. These features suggest that during naturalistic stimuli, afferent input to the olfactory bulb is subject to strong synaptic depression, presumably truncating the postsynaptic response to afferent stimuli. To examine this issue, we used single glomerular stimulation in mouse olfactory bulb slices to measure the synaptic dynamics of afferent‐evoked input at physiological stimulus frequencies. In cell‐attached recordings, mitral cells responded to high frequency stimulation with sustained responses, whereas external tufted cells responded transiently. Consistent with previous reports, olfactory nerve terminals onto both cell types had a high release probability (0.7), from a single pool of slowly recycling vesicles, indicating that the distinct responses of mitral and external tufted cells to high frequency stimulation did not originate presyaptically. Rather, distinct temporal response profiles in mitral cells and external tufted cells could be attributed to slow dendrodendritic responses in mitral cells, as blocking this slow current in mitral cells converted mitral cell responses to a transient response profile, typical of external tufted cells. Our results suggest that despite strong axodendritic synaptic depression, the balance of axodendritic and dendrodendritic circuitry in external tufted cells and mitral cells, respectively, tunes the postsynaptic responses to high frequency, naturalistic stimulation.
Keywords: Release probability, Presynaptic terminal, Olfactory bulb, mitral cell, external tufted cell, olfactory receptor neuron
Key points
The release probability of the odorant receptor neuron (ORN) is reportedly one of the highest in the brain and is predicted to impose a transient temporal filter on postsynaptic cells.
Mitral cells responded to high frequency ORN stimulation with sustained transmission, whereas external tufted cells responded transiently.
The release probability of ORNs (0.7) was equivalent across mitral and external tufted cells and could be explained by a single pool of slowly recycling vesicles.
The sustained response in mitral cells resulted from dendrodendritic amplification in mitral cells, which was blocked by NMDA and mGluR1 receptor antagonists, converting mitral cell responses to transient response profiles.
Our results suggest that although the afferent ORN synapse shows strong synaptic depression, dendrodendritic circuitry in mitral cells produces robust amplification of brief afferent input, and thus the relative strength of axodendritic and dendrodendritic input determines the postsynaptic response profile.
Abbreviation
- ORN
odorant receptor neuron
Introduction
The computational capacity of neural circuits is largely determined by the short‐term synaptic dynamics within the circuit (Abbott & Regehr, 2004), as determined by pre‐ and postsynaptic mechanisms. Short‐term synaptic depression, which generally occurs at high release probability synapses, results in a net decrease in postsynaptic responses with repeated stimulation, and is often attributed to depletion of the readily releasable pool of synaptic vesicles (Liley & North, 1953; Betz, 1970; von Gersdorff & Borst, 2002; Regehr, 2012). However, at some synapses, multiple pools of synaptic vesicles with distinct release probabilities can protect the circuit from synaptic depression during high frequency stimulation (Lu & Trussell, 2016; Taschenberger et al. 2016; Turecek et al. 2016).
In the olfactory bulb, principal neurons receive monosynaptic input from olfactory receptor neuron afferents (Gire & Schoppa, 2009; Najac et al. 2011; Gire et al. 2012; Vaaga & Westbrook, 2016). Odorant receptor neurons (ORNs) respond to increasing odorant concentrations with monotonic increases in firing frequency up to 100 Hz (Sicard, 1986; Duchamp‐Viret et al. 1999; Rospars et al. 2003; Tan et al. 2010). Furthermore, the release probability of the afferent synapse between the ORN and its postsynaptic targets is one of the highest reported in the brain (ca 0.8–0.9; Murphy et al. 2004). Together, these features suggest that the transmission between ORNs and principal neurons is subject to robust short‐term depression. However, in vivo, mitral cells respond to olfactory input with sustained responses (Giraudet et al. 2002; Nagayama et al. 2004; Leng et al. 2014), suggesting either that release probability during trains is not as high as has been reported, or that other circuit mechanisms maintain sustained transmission.
To examine the synaptic dynamics between ORN afferents and principal neurons in response to physiologically relevant stimulation frequencies, we recorded the postsynaptic responses of mitral cells and external tufted cells during high frequency afferent stimulation. Our results suggest that the high release probability and slow vesicle dynamics within the ORN are optimized for faithful transmission, but dendrodendritic amplification in mitral cells compensates for the strong synaptic depression and strongly amplifies afferent input.
Methods
Animals
We used adult (> p24) male and female C57Bl6/J as well as Tg(Thy1‐YFP) GJrs heterozygous mice. The Oregon Health and Science University Institutional Animal Care and Use Committee approved all animal procedures.
Slice preparation
Olfactory bulb slices were prepared as described previously (Schoppa & Westbrook, 2001). Mice were given an intraperitoneal injection of 2% 2,2,2‐tribromoethanol (0.7–0.8 ml) and monitored until fully anaesthetized, then transcardially perfused with oxygenated 4°C modified artificial cerebrospinal fluid (ACSF), which contained (in mm): 83 NaCl, 2.5 KCl, 1 NaH2PO4, 26.2 NaHCO3, 22 dextrose, 72 sucrose, 0.5 CaCl2, 3.3 MgSO4 (300–310 mosmol l−1, pH 7.3). The brain was quickly removed and coronally blocked at the level of the striatum. Horizontal sections (300 μm) through the olfactory bulb were made using a Leica 1200S vibrating blade microtome. Slices were recovered in warm (32–36°C) ACSF for 30 min and then were stored at room temperature until transfer to the recording chamber. Unless otherwise noted, the ACSF contained (in mm): 125 NaCl, 25 NaHCO3, 1.25 NaH2PO4, 3 KCl, 2.5 dextrose, 2 CaCl2, 1 MgCl2 (300–310 mosmol l−1, pH 7.3).
Electrophysiology
Whole cell voltage clamp and current clamp recordings were made from mitral cells and external tufted cells under differential interference contrast (DIC) optics. Mitral cells and external tufted cells were identified as described previously (Hayar et al. 2005; Vaaga & Westbrook, 2016). Briefly, mitral cells were identified by their soma position within the mitral cell layer and external tufted cells were identified by their relatively large soma positioned within the outer two‐thirds of the glomerular layer. Patch pipettes (3–5 MΩ) contained (in mm): 120 potassium gluconate, 20 KCl, 10 HEPES, 0.1 EGTA, 4 Mg‐ATP, 0.3 Na‐GTP, 0.05 Alexa‐594 hydrazide and 5 QX‐314. We made no correction for the liquid junction potential (−7 mV). During cell‐attached recordings, the membrane patch was held at −70 mV after achieving a gigaohm seal. Data were acquired using a Multiclamp 700b amplifier (Molecular Devices, Sunnyvale, CA, USA) and AxographX acquisition software. Data were digitized at 10 kHz and low pass Bessel filtered at 4 kHz. For cell‐attached recordings, the data were filtered post hoc at 1 kHz. During whole‐cell recordings the series resistance was continually monitored with a −10 mV hyperpolarizing step. Series resistance was generally < 25 MΩ and was not compensated. Cells with greater than 30% change in series resistance during the recording were excluded from analysis. All recordings were made at 34‐36°C.
EPSCs were elicited using single glomerulus theta stimulation, as described previously (Vaaga & Westbrook, 2016). Stimulation was provided by a constant current stimulator (100 μs, 3.2–32 mA) in conjunction with a small bore theta electrode (2 μm) placed directly in the axon bundle entering the target glomerulus. All recordings were made along the medial aspect of the olfactory bulb, and recordings were only made if the ORN bundle entering the target glomerulus was clearly identifiable under DIC optics. Stimulation trains (10, 25 and 50 Hz, 20 pulses) were chosen to represent the approximate firing rate of ORNs in response to odorant presentation (Sicard, 1986; Duchamp‐Viret et al. 1999; Carey et al. 2009; Tan et al. 2010). ORN stimulation was repeated at 60 s intervals, to prevent rundown. All drugs were prepared from stock solutions according to manufacturer specifications and applied via a gravity fed perfusion system. The drugs used included 2 mm kynurenic acid, 500 nm sulpiride, 200 nm CGP55845, 10 μm 3‐((R)‐2‐carboxypiperazin‐4‐yl)‐propyl‐1‐phosphonic acid (CPP) and 20 μm CPCCOEt. All drugs were purchased from Abcam Biochemical (Cambridge, MA, USA) or Tocris Biosciences (Ellisville, MO, USA).
Data analysis
Electrophysiology data were analysed using AxographX (www.axograph.com) and IGOR Pro (version 6.22A, Wavemetrics, Lake Oswego, OR, USA). Spike waveforms in cell‐attached recordings were detected using a threshold detection criterion in AxographX, which was used to calculate the total spike number and to generate raster plots. Voltage clamp traces represent the average of 5–10 sweeps after baseline subtraction. Fast EPSC amplitude measurements were made foot‐to‐peak, to eliminate any contribution of the slow current. To directly measure the slow current we recorded the EPSC amplitude just prior to each stimulus within the train. The total charge transfer (0–2.5 s after stimulus onset) was measured using a built‐in AxographX routine. Data were normalized to the first fast peak EPSC amplitude, unless otherwise noted.
To estimate release probability, we used two methods to calculate the size of the readily releasable pool, each of which utilizes different assumptions (Neher, 2015; Thanawala & Regehr, 2016). In the Schneggenburger, Meyer and Neher (SMN) method, the cumulative fast EPSC amplitude (at 50 Hz stimulation) was plotted as a function of stimulus number and a linear fit was made using the last five responses in the train. The readily releasable pool size was estimated as the y‐intercept of the linear fit (Schneggenburger et al. 1999, 2002), and release probability was calculated by dividing the initial EPSC amplitude by the size of the readily releasable pool. In the Elmqvist–Quastal (EQ) method (Elmqvist & Quastel, 1965), the fast EPSC amplitude was plotted as a function of the cumulative EPSC amplitude. A linear fit to the first three EPSCs was used to calculate the size of the readily releasable pool (x‐intercept). Release probability was then calculated as in the SMN method.
Statistics
All data are reported as means ± SEM unless otherwise indicated. Statistical analysis was performed in Prism6 (GraphPad Software, La Jolla, CA, USA). One‐way and two‐way repeated measure experiments were analysed using ANOVA with Holm–Sidak post hoc pairwise comparisons as indicated in the text. To compare the exponential fit across data sets, an extra sum of squares F test was performed to compare lines of best fit. In agreement with previous electrophysiological studies, data were assumed to be normally distributed, and were thus analysed using parametric statistics. Student's t test for paired or unpaired data was used as appropriate. Sample sizes were chosen to detect an effect size of 20%, based on prior, similar experiments, with a power of 0.8. In all experiments, the initial value for α was set to P < 0.05, and was adjusted for multiple comparisons as appropriate.
Results
Different temporal response profiles in mitral and external tufted cells
To examine the synaptic dynamics of principal neuron activity in response to high frequency afferent stimulation, we first measured the spiking of mitral and external tufted cells using cell‐attached recordings. Both cell types responded to 50 Hz ORN stimulation with spikes throughout the stimulus train (Fig. 1 A–D). Mitral cells and external tufted cells produced similar numbers of spikes early in the train; however, action potentials in external tufted cells gradually decreased, such that by the seventh stimulus, mitral cells produced more action potentials per successive stimulus than external tufted cells (two‐way ANOVA; P < 0.01; n = 7 mitral cells, 8 external tufted cells; Fig. 1 E). Likewise, mitral cells continued to spike well after the end of the stimulus train, contributing to the higher total number of spikes produced (mitral cells: 161.8 ± 27.2 spikes per trial, n = 7 cells; external tufted cells: 45.2 ± 9.0 spikes per trial, n = 8 cells; 2.5 s window, unpaired Student's t test: P = 0.009; Fig. 1 F). In fact, in mitral cells, 87.1 ± 15.3 spikes were produced after the stimulus (n = 7 cells), whereas only 9.2 ± 3.6 spikes were produced after the stimulus in external tufted cells (2 s window; n = 7 cells; unpaired Student's t test: P = 0.003). Similar response patterns were observed when the ORN was stimulated with shorter, high frequency bursts (5 pulses at 50 Hz), which may more accurately represent the time course of ORN firing in vivo (Carey & Wachowiak, 2011). In response to short bursts, mitral cells produced 80.1 ± 18.1 spikes (n = 7 cells) whereas external tufted cells produced 17.6 ± 6.0 spikes (n = 7 cells; unpaired Student's t test: P = 0.007). Furthermore, mitral cells produced significantly more spikes after the stimulus train (mitral cells: 56.3 ± 13.8 spikes after the train, n = 7 cells; external tufted cells: 9.7 ± 4.6 spikes after the train; unpaired Student's t test: P = 0.008), consistent with the sustained firing of mitral cells.
Figure 1. Sustained transmission in mitral and external tufted cells.
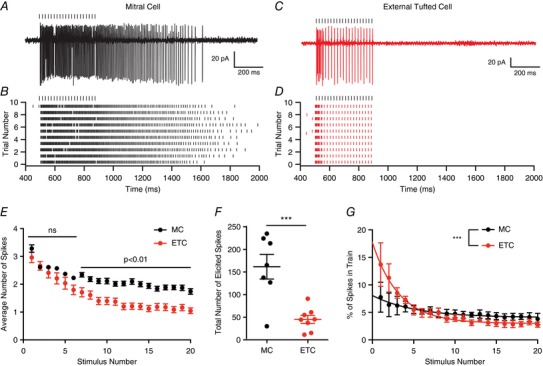
A, cell attached recording from mitral cell in response to 50 Hz ORN stimulation. B, raster plot of mitral cell response. Mitral cells responded to ORN stimulation with sustained responses, which outlasted the stimulus. C and D, cell attached recording (C) and associated raster plot (D) of external tufted cell response to 50 Hz ORN stimulation. External tufted cells produced much more transient response profiles. E, plot of the average number of action potentials produced following each stimulus in the train. Mitral cells and external tufted cells produce similar numbers of action potentials at the beginning of the train. By the end of the train, however, mitral cells produce approximately twice as many action potentials as external tufted cells. F, the total number of spikes produced (within 2.5 s) in mitral cells is significantly higher than in external tufted cells. G, plot of the fraction of total spikes in the train as a function of stimulus number. Mitral cells have a more shallow relationship, consistent with sustained transmission. External tufted cells have a significantly steeper relationship, indicative of transient response profiles. [Color figure can be viewed at wileyonlinelibrary.com]
In order to quantify the temporal filter in mitral cells and external tufted cells, we calculated the percentage of the total spikes that occurred within 20 ms of each stimulus in the 50 Hz train. Using this metric, a steep input–output curve is indicative of a transient temporal filter. In both mitral cells and external tufted cells, the input–output curve was fit by a single exponential decay. In mitral cells, this relationship was relatively shallow (τ = 5.2 stimuli), consistent with the observed sustained transmission. On average, mitral cells produced 7.8 ± 2.7% of total spikes immediately after the first stimulus and 3.8 ± 1.0% of spikes following the final stimulus (n = 7 cells). In contrast, external tufted cells had a much steeper input–output relationship (τ = 3.2 stimuli), producing 13.7 ± 4.0% of total spikes after the first stimulus and 2.8 ± 0.47% following the final stimulus (extra sum of squares F test: P < 0.0001, n = 8 cells). Thus the two cell types have distinct response properties with mitral cells responding to high frequency stimulation with a sustained response, whereas external tufted cells respond transiently.
Despite the distinct temporal responses across the stimulus train, the relative timing of spikes elicited at the beginning of the stimulus was remarkably stereotyped between mitral cells and external tufted cells. There was no difference in the first spike latency (mitral cells: 3.7 ± 1.07 ms, n = 7 cells; external tufted cells: 3.4 ± 0.90 ms, n = 8 cells; unpaired Student's t test: P = 0.83), consistent with monosynaptic afferent input (Vaaga & Westbrook, 2016). Furthermore, the standard deviation (jitter) of each successive spike elicited within the first 20 ms of the stimulus was not significantly different for the first four spikes (1st spike: external tufted cell: 0.6 ± 0.4 ms, mitral cell: 0.6 ± 0.3 ms, P = 0.98; 2nd spike: external tufted cell: 0.9 ± 0.2 ms, mitral cell: 1.0 ± 0.2 ms, P = 0.72; 3rd spike: external tufted cell: 1.0 ± 0.3 ms; mitral cell: 1.4 ± 0.3 ms, P = 0.36; 4th spike: external tufted cell: 0.8 ± 0.2 ms; mitral cell: 1.7 ± 0.6 ms, P = 0.25; n = 7 mitral cells, 8 external tufted cells, unpaired Student's t test).
Responses to repeated high frequency stimulation
Although mitral cells and external tufted cells have distinct temporal response profiles to single bursts of high frequency stimulation, we also wanted to examine the responses of both cells to repeated high frequency bursts, which more accurately mimic active sniffing events. It is possible that the sustained response of mitral cells makes them less effective at following high frequency stimulation. To test this, we stimulated the ORN at 50 Hz (5 pulses), repeating the stimulation 5 times with an inter‐burst interval of 200 ms, representing an approximate sniff frequency of 5 Hz (Welker, 1964; Youngentob et al. 1987). In mitral cells, repeated high frequency stimulation elicited a sustained barrage of action potentials that persisted throughout the stimulus train and beyond (6 of 7 cells; Fig. 2 A and B). In addition to the sustained spiking response, the majority of cells (5 of 7 cell) also had transient increases in firing rate associated with each high frequency ORN burst (45.9 ± 4.4% of total spikes during ORN bursts; n = 7 cells; Fig. 2 A and B), indicating that mitral cells are, in fact, well positioned to follow repeated stimulation. Conversely, in external tufted cells, spiking was phase locked to ORN stimulation, with little sustained spiking (85.6 ± 7.8% of total spikes during ORN bursts; n = 7 cells; unpaired Student's t test: P = 0.0008; Fig. 2 C and D). In some cells (3 of 7) phase locked responses were limited to the first two ORN bursts. Together these data indicate that in response to repeated high frequency stimulation, both cell types have transient ORN‐driven responses, but mitral cells have additional sustained spiking between each ORN burst.
Figure 2. Distinct temporal responses to repeated high frequency stimulation.
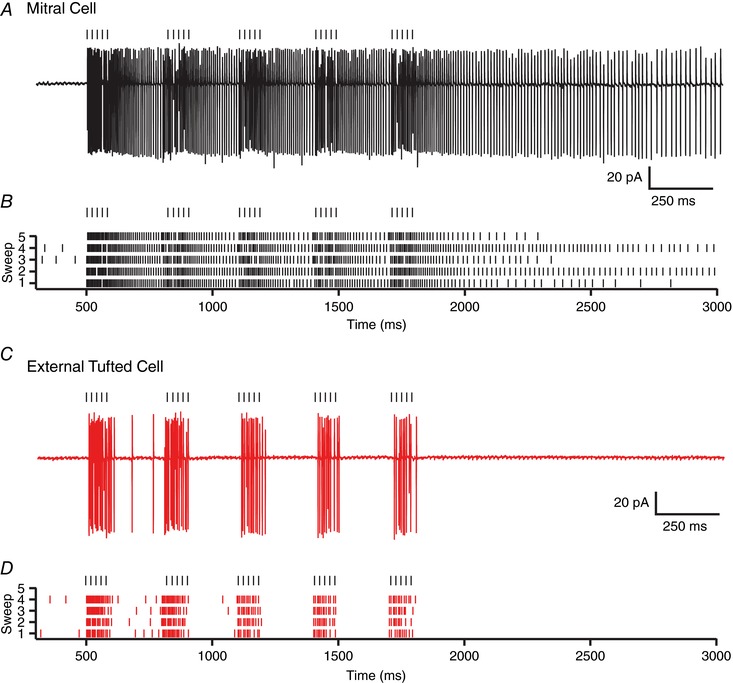
A, cell attached recording from a mitral cell in response to repeated high frequency stimulation mimicking active sniffing. B, raster plot of mitral cell spiking response showing both transient and sustained responses to repeated high frequency stimulation. C, cell attached recording from an external tufted cell. D, raster plot demonstrating transient response profile of external tufted cells. [Color figure can be viewed at wileyonlinelibrary.com]
High release probability from a single pool of synaptic vesicles
Differences in release probability of ORN terminals could underlie the distinct responses of mitral cells and external tufted cells, as release probability has only been examined in tufted cells (Murphy et al. 2004). To determine the release probability, we stimulated at high frequencies to estimate the size of the readily releasable pool using two analytical approaches as described in Methods (Elmqvist & Quastel, 1965; Schneggenburger et al. 1999; Neher, 2015; Thanawala & Regehr, 2016). Consistent with a high release probability synapse, 50 Hz trains of stimuli elicited robust depression of the phasic EPSC amplitude in mitral cells (Fig. 3 A) and external tufted cells (Fig. 3 B). Both the SMN method (Fig. 3 C, E) and EQ method (Fig. 3 D, E) yielded similar estimates of the size of the readily releasable pool in mitral cells and external tufted cells. Accordingly, there was no difference in the release probability between cell types (SMN: mitral cells: 0.67 ± 0.02, n = 7 cells, external tufted cells: 0.71 ± 0.06, n = 8 cells, P = 0.51; EQ: mitral cells: 0.66 ± 0.02, external tufted cells: 0.73 ± 0.03, P = 0.14; Fig. 3 E). These results indicate that the release probability of ORNs is high, but somewhat lower than previous estimates in tufted cells (Murphy et al. 2004), which likely reflects the activation of presynaptic D2 and GABAB receptors in our experiments (Nickell et al. 1994; Aroniadou‐Anderjaska et al. 2000; Ennis et al. 2001; Wachowiak et al. 2005; Maher & Westbrook, 2008; Shao et al. 2009; Vaaga et al. 2017). Consistent with this hypothesis, measurements of the release probability in D2 and GABAB receptor antagonists (500 nm sulpiride and 200 nm CGP55845, respectively) increased the release probability to 0.95 ± 0.06 (SMN method, n = 4 cells, unpaired Student's t test: P = 0.008). Furthermore, 2 mm kynurenic acid, which blocks receptor saturation and desensitization (Trussell et al. 1993; Wadiche & Jahr, 2001; Foster et al. 2002; Wong et al. 2003; Chanda & Xu‐Friedman, 2010) did not affect the paired pulse ratio (control: 0.24 ± 0.05; 2 mm kynurenic acid: 0.25 ± 0.05, n = 5 cells, paired Student's t test: 0.70; Fig. 3 F and G), suggesting that at the ORN afferent synapse, synaptic depression is primarily mediated by presynaptic factors, and is consistent with univesicular release (Murphy et al. 2004; Taschenberger et al. 2016).
Figure 3. Olfactory receptor neurons have a high release probability.
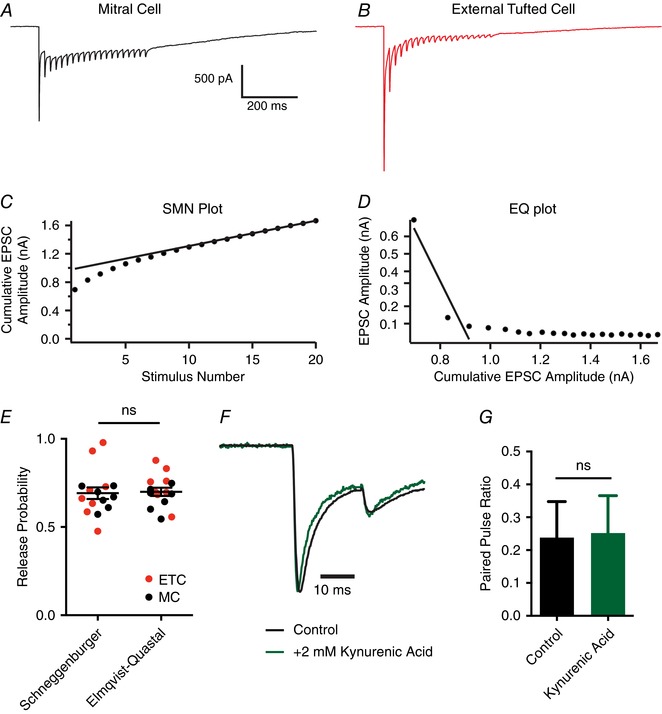
A and B, representative whole‐cell voltage clamp responses to 50 Hz stimulation in mitral cells (A) and external tufted cells (B). C and D, estimate of the release probability using the SMN method (C) and EQ method (D) for the mitral cell shown in A. E, estimates of release probability do not differ between the SMN and EQ methods, and there was no difference in the release probability between mitral cells and external tufted cells. F, paired pulse ratio in external tufted cells before and after addition of 2 mM Kynurenic acid to prevent receptor saturation and desensitization. The response in Kynurenic acid has been scaled to the control. G, summary of the paired pulse ratio in external tufted cell before and after 2 mM Kynurenic acid, suggesting postsynaptic saturation and desensitization do not contribute to synaptic depression. [Color figure can be viewed at wileyonlinelibrary.com]
In other circuits (Mennerick & Matthews, 1996; Sakaba & Neher, 2001; Lu & Trussell, 2016; Turecek et al. 2016), multiple pools of synaptic vesicles have heterogeneous release probabilities, which, if present, could obscure our measurements of release probability and support sustained transmission at high stimulation frequencies (Neher, 2015; Turecek et al. 2016). To test for the presence of multiple pools of synaptic vesicles, we stimulated at 10 Hz (20 pulses) to deplete the high release probability pool then switched to 50 Hz stimulation (20 pulses), a protocol that has been used to reveal a transient facilitation resulting from the low release probability of a separate pool of vesicles (Lu & Trussell, 2016; Turecek et al. 2016). In both mitral cells and external tufted cells this stimulation protocol failed to elicit facilitation (Fig. 4 A and B); rather, switching to high frequency stimulation elicited further depression of the ORN‐evoked phasic EPSC (external tufted cell: EPSC21: 25.3 ± 0.4% of control, EPSC22: 14.7 ± 0.2% of control, n = 7 cells; mitral cell: EPSC21: 14.7 ± 1.6% of control, EPSC22: 8.5 ± 1.4% of control, n = 4 cells; Fig. 4 B), suggesting a single pool of synaptic vesicles in both cells. Likewise, the decay of the phasic EPSC amplitude as a function of stimulus number was best fit with a single exponential function in both cell types (external tufted cell: τ: 0.68; extra sum of squares F test: P = 0.49; mitral cell: τ: 0.78; extra sum of squares F test: P = 0.18; Fig. 4 C). Together, these data indicate that a single pool of high release probability vesicles is sufficient to explain release from afferent olfactory nerve terminals.
Figure 4. Single pool of slowly recycling vesicles.
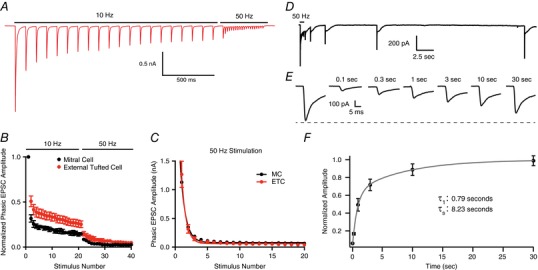
A, representative external tufted cell recording showing 10 Hz stimulation followed by 50 Hz stimulation. B, group data showing immediate depression following 10 Hz stimulation, suggesting a single pool of synaptic vesicles in both mitral cells and external tufted cells. C, in both cell types, the phasic EPSC amplitude plotted as a function of stimulus number is fit by a single exponential decay. D and E, recovery of phasic EPSC amplitude following 50 Hz stimulation suggests that vesicle replenishment is slow. F, recovery time course (pooled data from both mitral and external tufted cells) is best fit by a double exponential. [Color figure can be viewed at wileyonlinelibrary.com]
Sustained responses in some cases can be maintained despite high release probability as a result of fast vesicle replenishment (Wang & Kaczmarek, 1998; Saviane & Silver, 2006). However, the phasic EPSC amplitude recovered surprisingly slowly, following a double exponential time course (data pooled from both cell types; τ1: 0.79 s; τ2: 8.23 s; Fig. 4 D–F), suggesting that fast vesicle replenishment does not contribute to the sustained responses in mitral cells.
Dendrodendritic excitation maintains sustained transmission
Our results suggest that properties of the afferent presynaptic terminal alone cannot explain the sustained transmission observed in mitral cells. To determine what mechanisms support sustained transmission, we examined the responses of mitral cells and external tufted cells in voltage clamp following stimulation across a range of stimulus frequencies (10 Hz, 25 Hz, 50 Hz; Fig. 5 A and B). Across stimulus frequencies, the phasic EPSC showed robust depression (Fig. 5 D and E). Surprisingly, even relatively low stimulus frequencies (10 Hz) elicited strong depression in mitral and external tufted cells, consistent with the slow vesicle replenishment rates and unusually high release probability. In both cell types, there was a significant effect of stimulus frequency on the degree of phasic EPSC depression (one‐way ANOVA: mitral cell: P = 0.0003; external tufted cell: P < 0.0001). In both cells, the depression increased from 10 Hz to 25 Hz (mitral cells: 10 Hz: 16.4 ± 1.3% of EPSC1, n = 6 cells; 25 Hz: 9.4 ± 2.1% of EPSC1 n = 5 cells, Holm–Sidak post hoc comparison: P < 0.05; external tufted cells: 10 Hz: 14.8 ± 2.0, n = 7 cells; 25 Hz: 5.8 ± 0.9% of control, n = 7 cells, Holm–Sidak post hoc comparison: P < 0.001), but was not significantly different between 25 Hz and 50 Hz (mitral cell: 25 Hz: 9.4 ± 2.1% of EPSC1 n = 5 cells, 50 Hz: 5.4 ± 0.9% of EPSC1, n = 6 cells, Holm–Sidak post hoc comparison: P > 0.05; external tufted cell: 25 Hz: 5.8 ± 0.9% of EPSC1, n = 7 cells, 50 Hz: 4.4 ± 0.6% of EPSC1, n = 8 cells, Holm–Sidak post hoc comparison: P > 0.05). There was no difference in the total degree of phasic depression between mitral cells and external tufted cells at any stimulus frequency tested (Fig. 5 G), consistent with similar presynaptic properties of the incoming afferents.
Figure 5. Differential modulation of phasic and slow currents in mitral and external tufted cells.
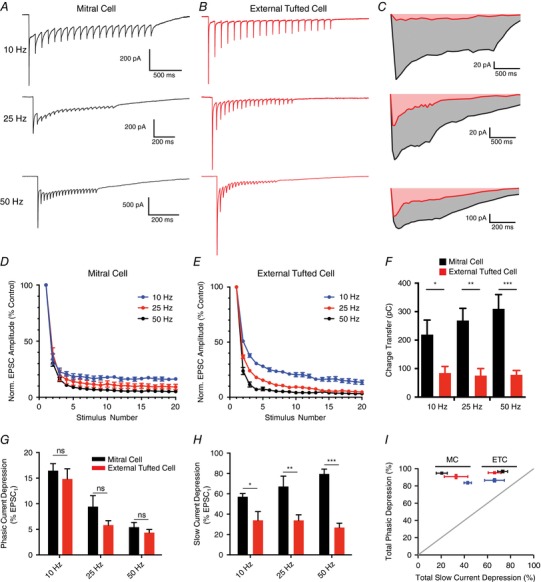
A and B, whole‐cell voltage clamp responses of mitral cells (A) and external tufted cells (B) to stimulation at various frequencies (10, 25, 50 Hz). C, comparison of the slow, envelope current measured in mitral cells and external tufted cells at each stimulus frequency. Mitral cells had consistently larger envelope currents. D, depression of the phasic EPSC amplitude as a function of stimulus number in mitral cells across stimulation frequencies (10 Hz, 25 Hz, 50 Hz). E, depression of phasic EPSC amplitude as a function of stimulus number in external tufted cells. F, the total charge transfer (measured 2.5 s after stimulus onset) was significantly larger in mitral cells than external tufted cells across all stimulation frequencies. There was no significant difference across stimulus frequencies within either cell type. G, total phasic depression in mitral cells and external tufted cells across stimulation frequencies. There was no significant difference between cell types at any frequency tested. H, total slow current depression in mitral cells and external tufted cells across stimulation frequencies. Mitral cells had significantly less slow current depression at all stimulus frequencies tested. I, plot showing a direct comparison of phasic depression and tonic depression across cell types and frequencies (10 Hz, 25 Hz, 50 Hz). Although the phasic depression was similar between cell types and frequencies, the slow current was differentially regulated in mitral cells and external tufted cells. [Color figure can be viewed at wileyonlinelibrary.com]
However in mitral cells, phasic EPSCs were superimposed on a large, slow envelope current at all stimulus frequencies, reflecting the much larger dendrodendritic currents in mitral cells compared to external tufted cells (Fig. 5 C; Vaaga & Westbrook, 2016). In mitral cells, the decay kinetics, but not the rising phase, of the fast EPSC became slightly slower during high frequency stimulation (data not shown), likely reflecting AMPA receptor‐dependent dendrodendritic glutamate release (Fig. 5 A). Such kinetic changes in the EPSC decay were not observed in external tufted cells (Fig. 5 B), consistent with the lack of secondary dendrodendritic activation in external tufted cells (Vaaga and Westbrook, 2016). The total charge transfer was nearly 3 times larger in mitral cells (10 Hz: mitral cell: 219.9 ± 50.6 pC, n = 6 cells, external tufted cell: 84.4 ± 23.25 pC, n = 6 cells, Holm–Sidak post hoc comparison: P < 0.05; 25 Hz: mitral cell: 268.0 ± 43.4 pC, n = 5 cells, external tufted cell: 75.5 ± 24.7 pC, n = 7 cells, Holm–Sidak post hoc comparison: P < 0.01; 50 Hz: mitral cell: 309.9 ± 50.1 pC, n = 7 cells, external tufted cell: 78.3 ± 12.3 pC, n = 7 cells; Holm–Sidak post hoc comparison: P < 0.001; Fig. 5 F). Interestingly, the charge transfer was not sensitive to stimulation frequency (mitral cell: one‐way ANOVA: P = 0.43; external tufted cell: one‐way ANOVA: P = 0.96; Fig. 5 F), consistent with an all‐or‐none dendrodendritic slow EPSC (Carlson et al. 2000; De Saint Jan et al. 2009; Gire & Schoppa, 2009). Unlike the phasic responses, the degree of depression of the slow envelope current within the stimulus train was significantly different between mitral cells and external tufted cells (10 Hz: mitral cell: 57.1 ± 3.2% of EPSC1, external tufted cell 34.0 ± 8.4% of EPSC1, Holm–Sidak post hoc comparison: P < 0.05; 25 Hz: mitral cell: 67.2 ± 10.2% of EPSC1, external tufted cell: 33.9 ± 5.5% of EPSC1, Holm–Sidak post hoc comparison: P < 0.01; 50 Hz: mitral cell: 79.5 ± 4.8% of EPSC1, external tufted cell: 26.8 ± 4% of EPSC1, Holm–Sidak post hoc comparison: P < 0.0001; Fig. 5 H).
In both mitral cells and external tufted cells, the depression of the phasic component was significantly larger than the depression of the slow, envelope current, and therefore all the data points fell above the unity line in a plot of phasic EPSC depression as a function of slow EPSC depression (Fig. 5 I). Furthermore, the similarity of phasic depression and distinct slow current depression across cell types produced two identifiable clusters when the phasic and slow current depression were directly compared (Fig. 5 I). Together these data suggest that a robust slow current supports sustained transmission in mitral cells, which is relatively insensitive to short‐term depression and stimulus frequency.
The mitral cell slow current is responsible for sustained transmission
To explicitly test the role of the slow current in generating the sustained transmission in mitral cells, we blocked NMDA and mGluR1 receptors (10 μm CPP and 20 μm CPCCOEt, respectively), which effectively blocks the slow current in mitral cells (De Saint Jan & Westbrook, 2007; Vaaga & Westbrook, 2016). As expected, bath application of NMDA and mGluR1 antagonists reduced the total charge transfer in mitral cells (mitral cell: 309.9 ± 50.1 pC, n = 7 cells; mitral cell + CPP/CPCCOEt: 42.3 ± 8.5 pC, n = 6 cells, Holm–Sidak post hoc comparison: P < 0.0001; Fig. 6 A and B), to levels comparable to the charge transfer in external tufted cells (external tufted cell: 78.27 ± 12.26, n = 7 cells, Holm–Sidak post hoc comparison: P > 0.05; Fig. 6 B). Thus, blocking the slow current converts the mitral cell response pattern to an external tufted cell pattern.
Figure 6. Blocking the slow current converts mitral cell responses into external tufted cell responses.
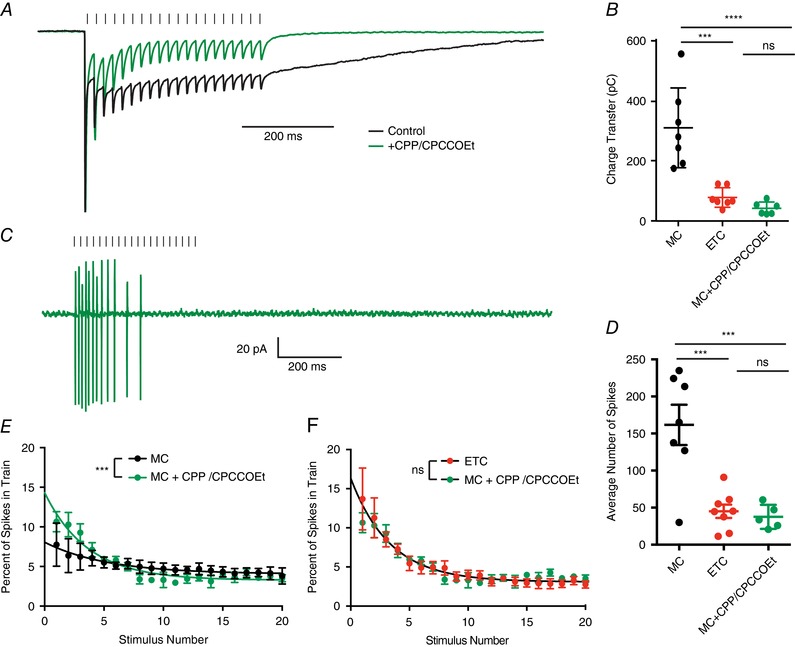
A, peak scaled comparison of the whole cell voltage clamp recordings from mitral cells in control and 10 μm CPP/20 μm CPCCOEt in response to 50 Hz ORN stimulation. As expected, CPP/CPCCOEt blocked a significant portion of the slow envelope current. B, comparison of the total charge transfer in mitral cells, external tufted cells and mitral cells with CPP/CPCCOEt shows that blocking the NMDA/mGluR1 receptor‐dependent current significantly reduces the total charge transfer to levels comparable to external tufted cells. C, cell‐attached recording from mitral cell in response to 50 Hz ORN stimulation shows transient spiking profile of mitral cells when NMDA and mGluR1 receptors are blocked. D, the total number of action potentials produced in mitral cells with NMDA and mGluR1 receptors are similar to external tufted cell responses. E, comparison of the temporal profile of mitral cell spiking in control and with CPP/CPCCOEt. Block of NMDA and mGluR1 receptors reveals transient response profile of mitral cells. F, with NMDA and mGluR1 receptors blocked, the temporal profile of mitral cell spiking is not significantly different from the responses of external tufted cells. [Color figure can be viewed at wileyonlinelibrary.com]
In cell attached recordings of mitral cells, blocking NMDA and mGluR1 receptors also caused a 4‐fold reduction in the total number of spikes produced following 50 Hz stimulation (mitral cell: 161.8 ± 27.2 spikes, n = 7 cells; mitral cell + CPP/CPCCOEt: 37.62 ± 7.3 spikes, n = 5 cells, Holm–Sidak post hoc comparison: P < 0.001; external tufted cell: 45.2 ± 9.0 spikes, n = 8 cells; Holm–Sidak post hoc comparison: P > 0.05; Fig. 6 D). Furthermore, bath application of NMDA and mGluR1 receptor antagonists also altered the temporal patterning of spikes, converting the sustained responses of mitral cells to more transient responses (extra sum of squares F test: P < 0.001; Fig. 6 E), which were not significantly different from the responses in external tufted cells (extra sum of squares F test: P > 0.05; Fig. 6 F). Together these data suggest that differences in the amplitude of the slow current between mitral cells and external tufted cells are responsible for the sustained transmission in mitral cells, and produce their distinct temporal spiking patterns.
Discussion
In the glomerular microcircuit, the interplay of axodendritic and dendrodendritic synapses is critical to postsynaptic processing of afferent input. Although the primary function of the glomerulus is to enhance the signal‐to‐noise ratio (Chen & Shepherd, 2005), the synaptic dynamics in response to high frequency ORN stimulation have not previously been examined. Here we demonstrate that mitral cells and external tufted cells respond to high frequency afferent input with distinct temporal filters. Mitral cells produce sustained responses, requiring dendrodendritic amplification, whereas the lack of dendrodendritic amplification in external tufted cells results in transient responses. During repeated high frequency stimulation, which mimics active sniffing, mitral cell responses contained both a sustained and phase‐locked component, whereas external tufted cell responses were primarily phase‐locked. Together, our results indicate that axodendritic and dendrodendritic circuits are functionally separable such that the relative balance of the two circuits determines the temporal filter of the postsynaptic cell.
Comparison with other synapses
Previous steady state measurements of ORN release probability were near 1 (Murphy et al. 2004). Our results using high frequency trains of stimuli match those estimates when presynaptic D2 and GABAB receptors are blocked. However, tonic and/or afferent‐evoked activation of presynaptic D2 and GABAB receptors during high frequency trains reduced the release probability by approximately 30%. Although many synapses, such as the climbing fibre synapse in the cerebellum, have a high release probability (Silver et al. 1998; Dittman et al. 2000), such terminals generally show multi‐vesicular release (Wadiche & Jahr, 2001; Rudolph et al. 2015). However, the similar paired pulse ratio in control and low affinity antagonists suggest that ORN synapses operate using univesicular release (Murphy et al. 2004; Taschenberger et al. 2016). Although a uniquantal, high release probability synapse occurs in barrel cortex between layer 4 and layer 2/3 neurons (Silver et al. 2003), the presynaptic neuron generally fires only one to two action potentials in response to whisker stimulation in vivo (Brecht & Sakmann, 2002). Thus synaptic depression resulting from a high release probability is unlikely to impact the postsynaptic response. The univesicular, high release probability of the ORN, therefore, is unusual because individual ORNs sustain firing at high frequencies (ca 50 Hz) in response to odorants (Sicard, 1986; Duchamp‐Viret et al. 1999; Carey et al. 2009; Tan et al. 2010).
To maintain transmission under these conditions, the high initial release probability of the ORN could be compensated by fast vesicle recycling (Kushmerick et al. 2006; Saviane & Silver, 2006) or a second pool of low release probability vesicles (Lu & Trussell, 2016; Taschenberger et al. 2016; Turecek et al. 2016). These properties, however, are not present in ORNs. In fact, the recovery of the fast EPSC following depletion was approximately 10‐fold slower than at the calyx of Held (Kushmerick et al. 2006), suggesting that individual ORNs may only transiently contribute to the postsynaptic response, thereby providing a rationale for the massive convergence of unimodal ORNs onto single glomeruli.
Distinct roles of axodendritic and dendrodendritic input
One advantage of such a high initial release probability is that odorant binding events in the periphery are faithfully transmitted to the olfactory bulb in a nearly all‐or‐none manner. The olfactory system is exquisitely sensitive, capable of detecting odorants at concentrations as low as 1 part per 1015 molecules (Julius & Katz, 2004). In the periphery, this high sensitivity is achieved through biochemical amplification downstream of G‐protein‐coupled odorant receptors, such that a single odorant receptor‐binding event can elicit an action potential in the ORN (Lynch & Barry, 1989). The high release probability of ORNs maintains the high sensitivity of the olfactory system, by ensuring that ORN activity is faithfully converted to a postsynaptic response. However, this circuit design comes at a cost in that individual nerve terminals can only transiently contribute to postsynaptic activation, thereby requiring an ensemble of functionally redundant channels to accurately convey information with high fidelity.
However, the high release probability of axodendritic input comes at a cost: the ‘noisy’ olfactory environment dramatically increases the total number of activated glomeruli in response to ambient air. Thus, multiple mechanisms, including dendrodendritic amplification, boost the signal‐to‐noise ratio within the glomerulus (Carlson et al. 2000; Chen & Shepherd, 2005; De Saint Jan & Westbrook, 2007; Vaaga & Westbrook, 2016). Our results demonstrate that the robust increase in synaptic charge associated with the slow dendrodendritic current, selectively expressed in mitral cells, effectively converts the transient axodendritic input into a sustained response.
Our results indicate that both mitral cells and external tufted cells are capable of following repeated ORN stimulations, which mimic active sniff frequencies (Welker, 1964; Youngentob et al. 1987). These data are consistent with in vivo recordings from mitral cells, which show distinct ORN‐evoked transients during active sniffing (Carey & Wachowiak, 2011). In our experiments, mitral cells and external tufted cells differ in the sustained firing rate during high frequency stimulation, as external tufted cell responses were primarily phase locked to ORN stimulation. These results suggest that in response to active sniffing, mitral cells and external tufted cells convey temporally distinct information, resulting from different degrees of dendrodendritic amplification.
Parallel input paths convey temporally distinct information
Mitral and external tufted cells represent parallel input pathways. For example, in vivo, tufted cells respond to lower odorant concentrations, have concentration‐invariant responses, and respond to odorants earlier in the sniff cycle (Nagayama et al. 2004; Fukunaga et al. 2012; Igarashi et al. 2012; Kikuta et al. 2013). Mitral cells, on the other hand, are more narrowly tuned than tufted cells, and shift their responses relative to the sniff cycle in response to increasing odorant concentrations (Nagayama et al. 2004; Kikuta et al. 2013). These in vivo results are consistent with the view that tufted cell responses maintain the sensitivity of the ORN, via strong afferent‐evoked responses. On the other hand, mitral cells, while still responsive to stimuli at sniff frequencies as shown in our experiments, provide robust amplification, via strong dendrodendritic circuitry.
Within piriform cortex, the concentration‐invariant network of activated pyramidal cells encodes odorant identity whereas concentration is encoded by the temporal response profiles of pyramidal cells (Bolding & Franks, 2017). The spiking patterns of these pyramidal cells have two distinct peaks, one with a short latency and one with a longer latency. As concentration increases, the lag between these peaks shortens (Bolding & Franks, 2017). Mechanistically, this may result from the integration of olfactory bulb projection neurons with distinct temporal spiking profiles. Such an activation scheme would require overlapping projection patterns in piriform cortex, but single axon tracing studies suggest that mitral cells and tufted cells project to largely non‐overlapping regions of olfactory cortex (Igarahsi et al. 2012). Resolving the exact projection patterns and mechanisms behind generating distinct timing signals in piriform cortex is critical to understanding the encoding of concentration within the olfactory system. Our results, however, demonstrate that the balance of axodendritic and dendrodendritic synaptic strength likely contributes to the unique computations within these parallel input pathways, by imposing unique temporal filters in each cell type.
Additional information
Competing interests
The authors declare no competing interests, financial or otherwise.
Author contributions
The work was carried out in the Westbrook Laboratory at the Vollum Institute at Oregon Health and Science University. C.E.V. and G.L.W. designed and conceived of the work. C.E.V. performed the experiments and analysed the data. C.E.V. and G.L.W. drafted and revised the manuscript. Both C.E.V. and G.L.W. approve the final version of the manuscript, and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all persons that qualify for authorship are listed.
Funding
This work was supported by NS26494 (G.L.W.; NIH, NINDS), National Science Foundation Graduate Research Fellowship DGE 0925180 (C.E.V.), and a LaCroute Neurobiology of Disease fellowship (C.E.V.).
Acknowledgements
We thank Dr Henrique von Gersdorff and members of the Westbrook lab for helpful comments on this manuscript.
The manuscript was first published as a preprint: Vaaga CE and Westbrook GL (2017). Distinct temporal filters in mitral cells and external tufted cells of the olfactory bulb. bioRxiv https://doi.org/10.1101/135541
References
- Abbott LF & Regehr WG (2004). Synaptic computation. Nature 431, 796–803. [DOI] [PubMed] [Google Scholar]
- Aroniadou‐Anderjaska V, Zhou F‐M, Priest CA, Ennis M & Shipley MT (2000). Tonic and synaptically evoked presynaptic inhibition of sensory input to the rat olfactory bulb via GABAB heteroreceptors. J Neurophysiol 84, 1194–1203. [DOI] [PubMed] [Google Scholar]
- Betz WJ (1970). Depression of transmitter release at the neuromuscular junction of the frog. J Physiol 206, 629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolding KA & Franks KM (2017). Complementary codes for odor identity and intensity in olfactory cortex. Elife 6, e22630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht M & Sakmann B (2002). Dynamic representation of whisker deflection by synaptic potentials in spiny stellate and pyramidal cells in the barrels and septa of layer 4 rat somatosensory cortex. J Physiol 543, 49–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey RM, Verhagen JV, Wesson DW, Pírez N & Wachowiak M (2009). Temporal structure of receptor neuron input to the olfactory bulb imaged in behaving rats. J Neurophysiol 101, 1073–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey RM & Wachowiak M (2011). Effect of sniffing on the temporal structure of mitral/tufted cell output from the olfactory bulb. J Neurosci 31, 10615–10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson GC, Shipley MT & Keller A (2000). Long‐lasting depolarizations in mitral cells of the rat olfactory bulb. J Neurosci 20, 2011–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda S & Xu‐Friedman MA (2010). A low‐affinity antagonist reveals saturation and desensitization in mature synapses in the auditory brain stem. J Neurophysiol 103, 1915–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WR & Shepherd GM (2005). The olfactory glomerulus: a cortical module with specific functions. J Neurocytol 34, 353–360. [DOI] [PubMed] [Google Scholar]
- De Saint Jan D, Hirnet D, Westbrook GL & Charpak S (2009). External tufted cells drive the output of olfactory bulb glomeruli. J Neurosci 29, 2043–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Saint Jan D & Westbrook GL (2007). Disynaptic amplification of metabotropic glutamate receptor 1 responses in the olfactory bulb. J Neurosci 27, 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittman JS, Kreitzer AC & Regehr WG (2000). Interplay between facilitation, depression, and residual calcium at three presynaptic terminals. J Neurosci 20, 1374–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchamp‐Viret P, Chaput MA & Duchamp A (1999). Odor response properties of rat olfactory receptor neurons. Science 284, 2171–2174. [DOI] [PubMed] [Google Scholar]
- Elmqvist D & Quastel DM (1965). A quantitative study of end‐plate potentials in isolated human muscle. J Physiol 178, 505–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis M, Zhou F‐M, Ciombor KJ, Aroniadou‐Anderjaska V, Hayar A, Borrelli E, Zimmer LA, Margolis F & Shipley MT (2001). Dopamine D2 receptor‐mediated presynaptic inhibition of olfactory nerve terminals. J Neurophysiol 86, 2986–2997. [DOI] [PubMed] [Google Scholar]
- Foster KA, Kreitzer AC & Regehr WG (2002). Interaction of postsynaptic receptor saturation with presynaptic mechanisms produces a reliable synapse. Neuron 36, 1115–1126. [DOI] [PubMed] [Google Scholar]
- Fukunaga I, Berning M, Kollo M, Schmaltz A & Schaefer AT (2012). Two distinct channels of olfactory bulb output. Neuron 75, 320–329. [DOI] [PubMed] [Google Scholar]
- Giraudet P, Berthommier F & Chaput M (2002). Mitral cell temporal response patterns evoked by odor mixtures in the rat olfactory bulb. J Neurophysiol 88, 829–838. [DOI] [PubMed] [Google Scholar]
- Gire DH, Franks KM, Zak JD, Tanaka KF, Whitesell JD, Mulligan AA, Hen R & Schoppa NE (2012). Mitral cells in the olfactory bulb are mainly excited through a multistep signaling path. J Neurosci 32, 2964–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gire DH & Schoppa NE (2009). Control of on/off glomerular signaling by a local GABAergic microcircuit in the olfactory bulb. J Neurosci 29, 13454–13464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Shipley MT & Ennis M (2005). Olfactory bulb external tufted cells are synchronized by multiple intraglomerular mechanisms. J Neurosci 25, 8197–8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi KM, Ieki N, An M, Yamaguchi Y, Nagayama S, Kobayakawa K, Kobayakawa R, Tanifuji M, Sakano H, Chen WR & Mori K (2012). Parallel mitral and tufted cell pathways route distinct odor information to different targets in the olfactory cortex. J Neurosci 32, 7970–7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D & Katz LC (2004). A Nobel for smell. Cell 119, 747–752. [DOI] [PubMed] [Google Scholar]
- Kikuta S, Fletcher ML, Homma R, Yamasoba T & Nagayama S (2013). Odorant response properties of individual neurons in an olfactory glomerular module. Neuron 77, 1122–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmerick C, Renden R & von Gersdorff H (2006). Physiological temperatures reduce the rate of vesicle pool depletion and short‐term depression via an acceleration of vesicle recruitment. J Neurosci 26, 1366–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G, Hashimoto H, Tsuji C, Sabatier N & Ludwig M (2014). Discharge patterning in rat olfactory bulb mitral cells in vivo. Physiol Rep 2, e12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liley AW & North KA (1953). An electrical investigation of effects of repetitive stimulation on mammalian neuromuscular junction. J Neurophysiol 16, 509–527. [DOI] [PubMed] [Google Scholar]
- Lu H‐W & Trussell LO (2016). Spontaneous activity defines effective convergence ratios in an inhibitory circuit. J Neurosci 36, 3268–3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JW & Barry PH (1989). Action potentials initiated by single channels opening in a small neuron (rat olfactory receptor). Biophys J 55, 755–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher BJ & Westbrook GL (2008). Co‐transmission of dopamine and GABA in periglomerular cells. J Neurophysiol 99, 1559–1564. [DOI] [PubMed] [Google Scholar]
- Mennerick S & Matthews G (1996). Ultrafast exocytosis elicited by calcium current in synaptic terminals of retinal bipolar neurons. Neuron 17, 1241–1249. [DOI] [PubMed] [Google Scholar]
- Murphy GJ, Glickfeld LL, Balsen Z & Isaacson JS (2004). Sensory neuron signaling to the brain: properties of transmitter release from olfactory nerve terminals. J Neurosci 24, 3023–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama S, Takahashi YK, Yoshihara Y & Mori K (2004). Mitral and tufted cells differ in the decoding manner of odor maps in the rat olfactory bulb. J Neurophysiol 91, 2532–2540. [DOI] [PubMed] [Google Scholar]
- Najac M, De Saint Jan D, Reguero L, Grandes P & Charpak S (2011). Monosynaptic and polysynaptic feed‐forward inputs to mitral cells from olfactory sensory neurons. J Neurosci 31, 8722–8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E (2015). Merits and limitations of vesicle pool models in view of heterogeneous populations of synaptic vesicles. Neuron 87, 1131–1142. [DOI] [PubMed] [Google Scholar]
- Nickell WT, Behbehani MM & Shipley MT (1994). Evidence for GABAB‐mediated inhibition of transmission from the olfactory nerve to mitral cells in the rat olfactory bulb. Brain Res Bull 35, 119–123. [DOI] [PubMed] [Google Scholar]
- Regehr WG (2012). Short‐term presynaptic plasticity. Cold Spring Harb Perspect Biol 4, a005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rospars J‐P, Lánský P, Duchamp A & Duchamp‐Viret P (2003). Relation between stimulus and response in frog olfactory receptor neurons in vivo. Eur J Neurosci 18, 1135–1154. [DOI] [PubMed] [Google Scholar]
- Rudolph S, Tsai MC, von Gersdorff H, Wadiche JI (2015). The ubiquitous nature of multivesicular release. Trends Neurosci 38,428–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaba T & Neher E (2001). Calmodulin mediates rapid recruitment of fast‐releasing synaptic vesicles at a calyx‐type synapse. Neuron 32, 1119–1131. [DOI] [PubMed] [Google Scholar]
- Saviane C & Silver RA (2006). Fast vesicle reloading and a large pool sustain high bandwidth transmission at a central synapse. Nature 439, 983–987. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Meyer AC & Neher E (1999). Released fraction and total size of a pool of immediately available transmitter quanta at a calyx synapse. Neuron 23, 399–409. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Sakaba T & Neher E (2002). Vesicle pools and short‐term synaptic depression: lessons from a large synapse. Trends Neurosci 25, 206–212. [DOI] [PubMed] [Google Scholar]
- Schoppa NE & Westbrook GL (2001). Glomerulus‐specific synchronization of mitral cells in the olfactory bulb. Neuron 31, 639–651. [DOI] [PubMed] [Google Scholar]
- Shao Z, Puche AC, Kiyokage E, Szabo G & Shipley MT (2009). Two GABAergic intraglomerular circuits differentially regulate tonic and phasic presynaptic inhibition of olfactory nerve terminals. J Neurophysiol 101, 1988–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard G (1986). Electrophysiological recordings from olfactory receptor cells in adult mice. Brain Res 397, 405–408. [DOI] [PubMed] [Google Scholar]
- Silver RA, Lubke J, Sakmann B & Feldmeyer D (2003). High‐probability uniquantal transmission at excitatory synapses in barrel cortex. Science 302, 1981–1984. [DOI] [PubMed] [Google Scholar]
- Silver RA, Momiyama A & Cull‐Candy SG (1998). Locus of frequency‐dependent depression identified with multiple‐probability fluctuation analysis at rat climbing fibre‐Purkinje cell synapses. J Physiol 510, 881–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Savigner A, Ma M & Luo M (2010). Odor information processing by the olfactory bulb analyzed in gene‐targeted mice. Neuron 65, 912–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschenberger H, Woehler A & Neher E (2016). Superpriming of synaptic vesicles as a common basis for intersynapse variability and modulation of synaptic strength. Proc Natl Acad Sci USA 113, E4548–E4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanawala MS & Regehr WG (2016). Determining synaptic parameters using high‐frequency activation. J Neurosci Methods 264, 136–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell LO, Zhang S & Raman IM (1993). Desensitization of AMPA receptors upon multiquantal neurotransmitter release. Neuron 10, 1185–1196. [DOI] [PubMed] [Google Scholar]
- Turecek J, Jackman SL & Regehr WG (2016). Synaptic specializations support frequency‐independent Purkinje cell output from the cerebellar cortex. Cell Rep 17, 3256–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaaga CE & Westbrook GL (2016). Parallel processing of afferent olfactory sensory information. J Physiol 594, 6715–6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaaga CE, Yorgason JT, Williams JT & Westbrook GL (2017). Presynaptic gain control by endogenous cotransmission of dopamine and GABA in the olfactory bulb. J Neurophysiol 117, 1163–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gersdorff H & Borst JGG (2002). Short‐term plasticity at the calyx of Held. Nat Rev Neurosci 3, 53–64. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, McGann JP, Heyward PM, Shao Z, Puche AC & Shipley MT (2005). Inhibition of olfactory receptor neuron input to olfactory bulb glomeruli mediated by suppression of presynaptic calcium influx. J Neurophysiol 94, 2700–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadiche JI & Jahr CE (2001). Multivesicular release at climbing fiber‐Purkinje cell synapses. Neuron 32, 301–313. [DOI] [PubMed] [Google Scholar]
- Wang LY & Kaczmarek LK (1998). High‐frequency firing helps replenish the readily releasable pool of synaptic vesicles. Nature 394, 384–388. [DOI] [PubMed] [Google Scholar]
- Welker WI (1964). Analysis of sniffing in the albino rat. Behavior 22, 223–244. [Google Scholar]
- Wong AYC, Graham BP, Billups B & Forsythe ID (2003). Distinguishing between presynaptic and postsynaptic mechanisms of short‐term depression during action potential trains. J Neurosci 23, 4868–4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngentob SL, Mozell MM, Sheehe PR & Hornung DE (1987). A quantitative analysis of sniffing strategies in rats performing odor discrimination tasks. Physiol Behav 41, 59–69. [DOI] [PubMed] [Google Scholar]


