Abstract
Background
The number of individuals who reach extreme age is quickly increasing. Much of the current literature focuses on impaired cognition in extreme age, and debate continues regarding what constitutes “normal” cognition in extreme age. This study aimed to provide oldest-old normative data and to compare cognitive performances of cognitively-intact elderly individuals from the Framingham Heart Study.
Methods
1302 individuals ages 65+ years old from the Framingham Heart Study were separated into five-year age bands and compared on cognitive tests. Multivariate linear regression analyses were conducted, adjusting for gender, the WRAT-III Reading score, and cohort. Analyses also included comparisons between 418 individuals ages 80+ and 884 individuals ages 65–79, and comparisons within oldest-old age bands.
Results
Normative data for all participants are presented. Significant differences were found on most tests between age groups in the overall analysis between young-old and oldest-old, and analysis of oldest-old age bands also revealed select significant differences (all p’s <.05).
Conclusion
As aging increases, significant cognitive differences and increased variability in performances are evident. These results support the use of age appropriate normative data for oldest-old individuals.
INTRODUCTION
The number of individuals who reach extreme age (85+ years) is quickly increasing, and the oldest-old are now the world’s fastest growing population (Kinsella & He, 2008). While much biomedical interest is now being turned to this group, a paucity of literature remains in the study of cognition in these very elderly, with some research extending the definition to include those as young as 80 years old (Katsumata et al., 2012; Lucca et al., 2012). Additional study of neuropsychological test performance is needed for further examination of several compelling questions in this field, the most pressing of which are: Is cognition distinct in the non-demented oldest-old as compared to young-old individuals? and What constitutes “normal” cognition in extreme age? Recent literature suggests that some cognitive domains, such as episodic memory, attentional capacity and processing speed, evidence decline in the oldest-old, while others (e.g., memory strategies, aspects of language and executive functions) are preserved (Slavin, Brodaty, & Sachdev, 2013) though disparate results have been reported in part due to methodological difficulties such as small sample sizes and restricted educational range. The answer to these questions will help to focus health care resources and public health policy for this elderly group, including guidelines for decision-making capacity, independent living, and driving. Additionally, cognitive measures have been shown to better predict mental health than physical measures (Poon et al., 2010), pointing toward a need for more accurate characterization of cognition for maximization of quality of life.
Much of the current cognitive aging research focuses on dementia (Yaffe et al., 2011), while less is known about individuals who reach oldest-old levels without significant cognitive impairment. Much of the research on non-demented cognitive aging in the oldest old focuses on the extreme end of the aging spectrum, which are the centenarians. Previous estimates suggest that anywhere from 11–54% of centenarians exhibit intact cognition (Kravitz, Schmeidler, & Beeri, 2012; Slavin et al., 2013). A recent imaging study of adults 18–94 found that most brain structures do not follow a consistent path life and that different structures display a mix of trajectories. The authors suggest that accelerated decline in extreme age is not the norm of healthy brain aging (Fjell et al., 2013). Another imaging study by Harrison and colleagues (2012) suggested that “Superagers,” defined as individuals ages 80+ with memory performances equivalent to normative values for 50–65 year-olds, have thicker cerebral cortex than similar-aged peers and do not exhibit atrophy when compared to a 50–65 year-old control group. They questioned whether Superagers are born with thicker cortex or resist change over time (Harrison, Weintraub, Mesulam, & Rogalski, 2012). Cognitive studies including normative studies for this group are limited, and while use of normative data from younger samples can result in misclassification of cognitive status so can use of centenarian data on oldest old samples below the age of 100.
Some studies have begun to publish normative data on the cognitively intact oldest-old, though many are limited by small sample sizes and high levels of education. The widely cited Mayo’s Older Americans Normative Studies (MOANS) provided age- and age and IQ-specific normative data for individuals ages 56–94 in Olmsted County, Minnesota on a number of cognitive tests for samples of Caucasian and later, African-American participants (Ivnik et al., 1992a, 1992b; Lucas et al., 2005; Steinberg, Bieliauskas, Smith, & Ivnik, 2005). Participants for the MOANS studies were recruited based on visits to one of the two primary care clinics within the Mayo system rather than a truly random sampling from the community, and the number of participants ages 80 and above in the MOANS studies was typically small across studies. As the MOANS data was collected in the late 1980s and early 1990s and other studies have provided evidence of differences in cognition between birth cohorts (Au et al., 2004; Hulur, Infurna, Ram, & Gerstorf, 2012), use of these data may become less relevant as time from data collection passes.
Ravdin et al. published normative data based on age groups of older adults. In this study only 35 individuals were over the age of 85 and most were women. While age was significant on tests of letter and semantic fluency, education was not (Ravdin, Katzen, Agrawal, & Relkin, 2003). Participants were excluded if they scored two standard deviations below the mean on any test of a previous neuropsychological evaluation. Educational level of excluded participants was not described. This exclusion may not, however, have been warranted. One review discussed the increase in within-subjects variability in cognitive performance in the oldest-old compared to young-old individuals (Calvert, Hollander-Rodriguez, Kaye, & Leahy, 2006; Perls, 2004), so eliminating those based solely on poor performance may have disqualified individuals who either had low premorbid but intact cognitive function or reflect the broad range of normal variability previously demonstrated in this age group.
Beeri and colleagues published normative data for the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) battery in a group of oldest-old individuals. Beeri and coauthors found that age and education were important influencing factors in cognitive performance, highlighting the importance of normative data for the oldest-old. Participants in this study had an average of 15 years of education, and while the authors fitted regression models for hypothetical individuals including those with lower education, this assumes a linear trajectory that may not be accurate for such individuals (Beeri et al., 2006).
A population-based study of the oldest-old including 244 centenarians pointed out that at extreme age, visual, sensory and motor impairment can limit the types of neuropsychological tests individuals are able to complete (Miller et al., 2010). The authors noted that their method of assigning scores of 0 to participants who were unable to complete the tasks (Mini-mental State Examination (MMSE), Severe Impairment Battery (SIB), and Behavioral Dyscontrol Scale (BDS)) due to these impairments may limit the ability to generalize their results to studies with other methods of handling this issue. Additionally, screening for dementia was not a part of this study and creating norms by combining cognitive performance in individuals with and without dementia is problematic.
Davey and coauthors provided normative data by gender, race, education and residential status on additional neuropsychological and physical measures (Finger Tapping Test (FTT), WAIS Similarities, MMSE, BDS, Controlled Oral Word Association Test (COWAT), SIB, the Global Deterioration Scale, the Field Object Memory Exam (FOME)) and physical measures for the same sample as described by Miller and colleagues (Davey et al., 2010). They found that cognitive and physical performances were better for men than women, whites than African Americans, community than facility residents, and those with more than high school education than those with less than high school education. Further analyses suggested that the associations with gender and race were likely attributable to differences in residential status or education. Davey and coauthors noted that education is not a reliable indicator of premorbid status and suggested that reading level may be more valuable, particularly when considering race.
Elias and colleagues provided age- and education-based normative data for 397 individuals ages 70–98 years old on a number of cognitive measures (WAIS Information, Similarities, Digit Span, Block Design subtests, the Trail Making Test, WMS Logical Memory and Visual Reproduction subtests, the Auditory Verbal Learning Test, the COWAT, FTT, and MMSE (Elias et al., 2011). They also provided normative data by age, education and gender, but cautioned against over-interpretation of these data due to small sample sizes. While they found that education was significantly related to cognitive performance, this effect was inconsistent across tests. The authors cautioned that adjusting for education may mask important age-cohort differences due to the inverse relationship between age and education in elderly individuals. Only 14 individuals in their study were over the age of 90.
Welsh-Bohmer and coauthors published normative data using a larger sample of oldest-old individuals (N=507) for Animal Fluency, the 15-item Boston Naming Test, the MMSE, CERAD word list, the Trail Making Test, a praxis test, WMS Logical Memory, the Benton Visual Retention Test, the COWAT, the Symbol-Digit Modalities test, and the Shipley Institute of Living Scale. Results highlighted visuomotor deficits in the oldest-old regardless of educational status, and effects of age and education across most measures (Welsh-Bohmer et al., 2009). These authors note that the amount of variance explained by their demographic variables was quite limited for some tests and hypothesized that other factors such as “native intelligence” and genetics may be more involved. Further studies similar to this are necessary to clarify which other variables are related to cognition in the oldest-old.
The goal of this paper extends previous literature by providing normative data on the cognitive performance of individuals ages 65–90+ from the community-based Framingham Heart Study (FHS) on widely used tests in cognitive research for members of the oldest-old. Additionally, we compare cognitive performance of young-old and oldest-old individuals defined as at least 80 years of age. While some recent studies of the oldest-old use age 85 or 90 as the lowest age for this group (Beeri et al., 2006), we have included individuals starting at age 80 as has been reported in other studies (Ravdin et al., 2003). Further comparisons of cognitive performance among age bands within the oldest old group (80–84, 85–89, 90+) are included allowing for the identification of potential differences within this sample of interest.
METHODS
Participants
The FHS began prospective examination of the community-based Original Cohort in 1948 (Dawber, Meadors, & Moore, 1951). The Offspring Cohort was recruited in 1971 and includes the children of Original Cohort members and their spouses. Since 1999, all surviving members of the Original and Offspring Cohorts were invited to participate in a larger study of brain imaging and cognition. The present investigation includes 1302 participants from both cohorts who completed a neuropsychological assessment between 1999 and 2009. Only one assessment per individual (the individual’s initial assessment) was used for analysis. Individuals from both cohorts were included to allow for the comparisons of a range of young-old (ages 65–79) and oldest-old (ages 80+) individuals. Participants with documented clinical stroke, clinical dementia or other neurological disorders were excluded (N=243; 159 from the Original Cohort and 84 from the Offspring Cohort) as described in previous publications (Seshadri et al., 2006). Additionally, participants whose sensory impairment did not allow them to complete a test were excluded. All but eight young-old participants (N=876) were from the Offspring Cohort. Within the oldest-old group (N= 418), 40 were from the Offspring Cohort and 378 were from the Original Cohort. All participants provided written signed consent and the study was approved by the Boston University School of Medicine Institutional Review Board.
Measures
The neuropsychological test battery consisted of tests sampling across several major cognitive domains, including premorbid level of cognitive functioning (Wide Range Achievement Test-Third Edition (WRAT-III) (Wilkinson, 1993)), verbal memory (Wechsler Memory Scale (WMS) Logical Memory Immediate (LMI) and Delay (LMD) conditions (Wechsler, 1945)), attention and executive function (Trail Making Test (Reitan, 1958)), verbal fluency (Controlled Oral Word Association Test – FAS version (COWAT) (Borkowski, Benton, & Spreen, 1967)), visuospatial perception and organization (Hooper Visual Organizational Test (HVOT) (Hooper, 1983)), confrontation naming (Boston Naming Test – 30 Item Version (even items) (BNT) (Williams, Mack, & Henderson, 1989)), and abstract reasoning (WAIS Similarities (Wechsler, 1955)) as described in previous FHS publications (Au et al., 2004; Elias, Elias, D’Agostino, Silbershatz, & Wolf, 1997). Table 1 lists the tests administered and score ranges used. We used the WRAT-III Reading Score as a measure of premorbid intelligence and a surrogate for education attainment, as it has been suggested to be a more accurate assessment of pre-morbid cognitive functioning than years of education (Manly, Jacobs, Touradji, Small, & Stern, 2002; O’Bryant, Schrimsher, & O’Jile, 2005). Many oldest-old individuals, particularly women, ended their formal education after high school, reflected in the US Census Bureau’s report that as of 2003, 67.6% of individuals age 75+ completed 12 or fewer years of formal education (He, Sengupta, Velkoff, & DeBarros, 2005). FHS continues to utilize test versions that were used during the initial phases of the study (e.g., original versions of the WMS and WAIS) for the purposes of longitudinal analysis. Table 2 lists the age, gender, education and WRAT-III Reading Scores for all participants in this study.
Table 1.
Neuropsychological battery
| Neuropsychological Test Measure | Cognitive Domain | Range |
|---|---|---|
| Wide Range Achievement Test 3 Reading Subtest (WRAT-III Reading) | Premorbid Cognitive Functioning | 0–57 |
| Hooper Visual Organizational Test (HVOT) | Visuospatial Perception and Organization | 0–30 |
| WMS – Logical Memory – Immediate Recall (LMI) | Verbal Memory | 0–24 |
| WMS – Logical Memory –Delayed Recall (LMD) | Verbal Memory | 0–24 |
| Boston Naming Test – 30 Item Version (Even Items) (BNT) | Naming | 0–30 |
| Controlled Word Association Test (FAS Version) (COWAT) | Verbal Fluency | 0–60+ |
| Trail Making Test A completion time (TMA) | Attention and Executive Function | 0–420 |
| Trail Making Test B completion time (TMB) | Executive Function | 0–600 |
| Trail Making Test B completion time – Trail Making Test A completion time (TMB-TMA) | Executive Function | Dependent on TMB and TMA |
| WAIS – Similarities subtest (SIM) | Abstract Reasoning | 0–26 |
Table 2.
Distribution of age, gender, education and WRAT-III Reading scores
| Age | N | % women | Education levels | WRAT-III Reading (Average ±SD) |
|---|---|---|---|---|
| 65–69 | 381 | 207 (54.3%) | No HS: 14 (3.7%) HS: 128 (33.6%) SC: 121 (31.7%) CD: 118 (31%) |
41.5±4.9 45.1±4.3 48.2±4.0 51.5±3.9 |
| 70–74 | 321 | 163 (50.8%) | No HS: 32 (10%) HS: 116 (36.1%) SC: 71 (22.1%) CD: 102 (31.8%) |
41.6±4.3 45.7±4.6 48.1±4.3 50.9±3.8 |
| 75–79 | 182 | 99 (54.4%) | No HS: 12 (6.6%) HS: 72 (39.5%) SC: 54 (29.7%) CD: 44 (24.2%) |
39.2±5.5 45.5±4.2 47.9±4.5 50.8±3.9 |
| 80–84 | 252 | 156 (61.9%) | No HS: 42 (16.7%) HS: 123 (48.8%) SC: 49 (19.4%) CD: 38 (15.1%) |
41.6±5.2 46.2±4.7 49.0±3.6 51.4±3.9 |
| 85–89 | 109 | 69 (63.9%) | No HS: 28 (25.9%) HS: 40 (37.1%) SC: 23 (21.3%) CD: 17 (15.7%) |
41.3±6.7 46.1±5.5 48.6±4.5 52.5±2.9 |
| 90+ | 57 | 43 (75.4%) | No HS: 25 (43.9%) HS: 10 (17.5%) SC: 12 (21.1%) CD: 10 (17.5%) |
41.6±5.0 49.4±5.3 47.7±6.3 52.6±2.5 |
HS= high school, SC= some college, CD= college degree
Procedures
Subjects were administered a neuropsychological test battery using standard test administration protocols given by trained technicians. With the exception of the Trail Making Tests A and B, both timed tests, higher scores indicate better performance. For the two Trails tests, lower scores were considered better. Also Trail Making A (Trails A) was subtracted from the raw Trail Making B (Trails B) score to compute an executive function score corrected for simple attention, as supported by the literature (Corrigan & Hinkeldey, 1987; Sanchez-Cubillo et al., 2009). For the normative tables, Trail Making B was included as a separate variable for clinical utility.
Statistical analyses
The oldest-old age band from the two cohorts with group size sufficient for analysis (80–84 year-olds) was compared on demographic (age, gender, education, WRAT-III Reading Score) and cognitive variables, as cohort differences in age, education, social and economic factors and cognitive functions have been demonstrated in a wider age range of FHS participants (Au et al., 2004). Table 3 lists the results. There were significant differences between cohorts in age (p<.001), education (p<.05), and on one cognitive measure (HVOT p<.05). Despite the fact that all oldest-old individuals grew up in the same era, some cohort differences were evident. Therefore, though cohorts were combined for analyses in this study, cohort was included as a covariate.
Table 3.
Cohort Differences on Demographic and Cognitive Variables
| 80–84 | |||
|---|---|---|---|
| Original | Offspring | p-value | |
| N= 216 | N= 36 | ||
| Age | 82.7±1.3 | 81.6±1.4 | <0.001 |
| Male, N (%) | 80 (37.4%) | 16 (44.4%) | ns |
| Education, N (%) | <HS: 40(18.5%) HS: 107(49.5%) SC: 38(17.6%) CD: 31(14.4%) |
<HS: 2(5.6%) HS: 16(44.4%) SC: 11(30.6%) CD: 7(19.4%) |
0.031 |
| WRAT* | 48[43,51] | 48.5[45,51] | ns+ |
| LMI | 9.1±3.8 | 8.9±4.3 | ns+ |
| LMD | 7.8±4.0 | 8.2±4.1 | ns+ |
| TMB-TMA* | 1.6[1.1,3.0] | 1.5[0.8,2.2] | ns+ |
| SIM | 13.3±4.8 | 14.7±4.1 | ns+ |
| COWAT | 32.5±13.4 | 38.4±11.6 | ns+ |
| HVOT* | 20.5[16.5,23] | 23.5[18.5,25] | 0.014+ |
| BNT* | 25[21,27] | 26[22,28] | ns+ |
HS: high school; SC: some college; CD: college degree; LMI: WMS-R Logical Memory Immediate Recall; LMD: WMS-R Logical Memory Delayed Recall; TMB-TMA: Trail Making Test B-Trail Making Test A; SIM: WAIS-R Similarities Subtest; COWAT: Controlled Word Association Test; HVOT: Hooper Visual Organizational Test; BNT: Boston Naming Test.
All values are mean±SD unless otherwise noted
values are Median [Q1, Q3]
age-adjusted p-value
Normative tables show the distribution of test scores for specific age in oldest-old (80–84, 85–89, 90+) and young-old (65–69, 70–74, 75–79); we present the average score, standard deviation, median score, 95th percentile, upper (75%) and lower (25%) quartiles as well as the 5th percentile scores. We included for the normative tables only those measures that are currently widely used (e.g., omitted original WMS and WAIS scores). Normative data based on age and level of education (< high school or > high school) are provided in the supplemental materials (Supplemental Table 4.1 and 5.1).
Data on all cognitive tests for all age bands (65–90+ in five-year increments) were compared using multivariate linear regression analysis, adjusting for gender, the WRAT-III Reading score, and cohort, to determine whether statistical significance exists between age bands. WRAT-III Reading scores, HVOT scores, BNT scores, and Trails B scores were log transformed to correct for skewness. Results are presented using betas and standard errors, which can be interpreted as differences in means. Unstandardized coefficients are provided in the supplemental materials (Supplemental Tables 6.1, 7.1 and 8.1).
Data for young-old (65–79) and oldest-old (80+) groups were also compared using multivariate linear regression analysis, adjusting for gender, the WRAT-III Reading score, and cohort, to determine whether cognitive performance in the oldest-old is distinct from that of the young-old. Cognitive performance in the two most aged five-year age bands within the oldest-old were compared to the 80–84 age band, again using multivariate linear regression adjusting for gender, WRAT-III Reading score and cohort; post-hoc pair-wise comparisons were made among the sub-groups using Tukey’s procedure.
Finally, we examined possible effect modification by gender with tests of interaction, as there are purported cognitive differences between men and women at extreme age (Whittle et al., 2007). For all analyses, age (group) was used as a categorical variable. Significance level was set at 0.05 for all comparisons except for tests for interactions, for which the significance levels were set at 0.10. All analyses were performed with SAS 9.3 (SAS Institute Inc, Cary, NC).
RESULTS
Age Based Norms
We report performance data based on the Original and Offspring Cohorts combined. Tables 4 and 5 present norms by age for the oldest-old and young-old, respectively. Normative data based on age and level of education (<high school or >high school) are provided in the supplemental materials (Supplemental Table 4.1 and 5.1).
Table 4.
Normative data by age for Oldest Old
| HVOT | BNT | COWAT | TMA | TMB | TMB-TMA | |
|---|---|---|---|---|---|---|
| 80–84 | ||||||
| Mean ± SD | 20.0±4.5 | 23.6±5.2 | 32.7±13.4 | 54.1±25.7 | 198.5±147.3 | 145.2±139.7 |
| 95% | 26.0 | 30.0 | 57.0 | 29.0 | 67.0 | 29.0 |
| 75% | 24.0 | 27.0 | 40.0 | 38.0 | 107.0 | 63.0 |
| Median | 20.8 | 25.0 | 31.0 | 48.0 | 145.0 | 94.0 |
| 25% | 17.0 | 21.0 | 22.0 | 63.0 | 225.0 | 172.0 |
| 5% | 12.0 | 16.0 | 15.0 | 106.0 | 600.0 | 546.0 |
| 85–89 | ||||||
| Mean ± SD | 18.3±4.7 | 22.7±6.3 | 30.0±12.8 | 62.0±43.2 | 217.1±162.6 | 159.6±143.1 |
| 95% | 25.0 | 29.0 | 52.0 | 27.0 | 69.0 | 34.0 |
| 75% | 22.0 | 27.0 | 40.0 | 37.0 | 112.0 | 68.0 |
| Median | 19.0 | 25.0 | 29.0 | 49.0 | 146.5 | 104.0 |
| 25% | 16.0 | 20.0 | 21.0 | 74.0 | 239.0 | 189.5 |
| 5% | 9.5 | 12.0 | 13.0 | 124.0 | 600.0 | 499.0 |
| 90+ | ||||||
| Mean ± SD | 15.9±4.9 | 21.1±5.6 | 29.0±14.7 | 68.1±47.0 | 264.4±171.9 | 196.3±153.0 |
| 95% | 23.5 | 27.0 | 52.0 | 35.0 | 99.0 | 61.0 |
| 75% | 19.5 | 25.0 | 43.0 | 49.0 | 125.0 | 70.0 |
| Median | 16.0 | 22.0 | 29.0 | 58.0 | 184.0 | 118.0 |
| 25% | 13.5 | 19.0 | 18.0 | 70.0 | 382.0 | 304.0 |
| 5% | 6.0 | 12.0 | 8.0 | 124.0 | 600.0 | 530.0 |
HVOT: Hooper Visual Organizational Test; BNT: Boston Naming Test; COWAT: Controlled Word Association Test; TMA: Trail Making Test A; TMB: Trail Making Test B; TMB-TMA: Trail Making Test B-Trail Making Test A.
Table 5.
Normative data by age for Young-Old
| HVOT | BNT | COWAT | TMA | TMB | TMB-TMA | |
|---|---|---|---|---|---|---|
| 65–69 | ||||||
| Mean ± SD | 24.6±2.9 | 26.8±2.6 | 33.2±10.2 | 34.6±12.1 | 87.1±43.8 | 52.8±41.8 |
| 95% | 28.5 | 30.0 | 52.0 | 20.0 | 48.0 | 16.0 |
| 75% | 26.5 | 29.0 | 40.0 | 26.0 | 62.0 | 31.0 |
| Median | 25.0 | 27.0 | 35.0 | 32.0 | 77.0 | 43.5 |
| 25% | 23.0 | 25.0 | 26.0 | 40.0 | 100.0 | 64.0 |
| 5% | 19.0 | 22.0 | 13.0 | 54.0 | 155.0 | 120.0 |
| 70–74 | ||||||
| Mean ± SD | 23.7±3.3 | 26.6±2.9 | 30.2±15.4 | 39.3±15.2 | 111.0±81.0 | 71.7±77.4 |
| 95% | 28.0 | 30.0 | 60.0 | 21.5 | 50.0 | 20.0 |
| 75% | 26.0 | 29.0 | 40.0 | 29.0 | 70.0 | 36.0 |
| Median | 24.5 | 27.0 | 26.0 | 36.0 | 93.0 | 54.0 |
| 25% | 22.0 | 25.0 | 21.0 | 46.0 | 122.0 | 81.0 |
| 5% | 17.3 | 21.0 | 7.0 | 68.0 | 204.0 | 155.0 |
| 75–79 | ||||||
| Mean ± SD | 22.1±3.9 | 25.4±4.6 | 33.4±11.0 | 44.0±23.0 | 135.0±107.1 | 91.6±102.3 |
| 95% | 27.0 | 30.0 | 48.0 | 24.0 | 56.0 | 19.0 |
| 75% | 25.0 | 28.0 | 43.0 | 31.0 | 79.0 | 42.0 |
| Median | 23.0 | 26.0 | 34.0 | 37.0 | 98.0 | 59.5 |
| 25% | 20.0 | 24.0 | 25.0 | 51.0 | 148.0 | 97.0 |
| 5% | 14.5 | 19.0 | 16.0 | 77.0 | 324.0 | 258.0 |
HVOT: Hooper Visual Organizational Test; BNT: Boston Naming Test; COWAT: Controlled Word Association Test; TMA: Trail Making Test A; TMB: Trail Making Test B; TMB-TMA: Trail Making Test B-Trail Making Test A.
Comparison of All Age Bands
For all comparisons, the 65–69 age band was used as a reference and all scores were adjusted for gender, the logarithm of WRAT-III Reading score, and cohort. As shown in Table 6, there were no significant differences in performance across age bands on COWAT. There were significant differences between each age band and the referent group on the Trails B-A and HVOT (see Figure 1). Additionally, there were significant differences between the referent group and all other age bands except the 70–74 year-old age band on WMS LMI, WMS LMD, and BNT. Finally, there were significant differences between the referent group and all other age bands except the 85–89 year-old age band on the WAIS Similarities subtest (see Figure 2).
Table 6.
Comparison of Cognitive Data of Age Bands Compared to Referent Group (65–69 year-olds; n=381)
| Age Band | 70–74 | 75–79 | 80–84 | 85–89 | 90+ |
|---|---|---|---|---|---|
|
| |||||
| N | 321 | 182 | 252 | 109 | 57 |
| Logical Memory Immediate | −0.11±0.07 | −0.22±0.08** | −0.57±0.15*** | −0.71±0.18*** | −1.06±0.21*** |
| Logical Memory Delayed | −0.03±0.07 | −0.23±0.08** | −0.45±0.15** | −0.59±0.18*** | −0.82±0.21*** |
| Trail Making Tests (B–A)† | −0.21±0.07*** | −0.39±0.08*** | −0.58±0.14*** | −0.70±0.17*** | −1.05±0.21*** |
| Similarities | −0.14±0.06* | −0.23±0.07** | −0.28±0.13* | −0.24±0.16 | −0.75±0.19*** |
| Controlled Word Association Test | −0.06±0.29 | 0.07±0.29 | 0.39±0.33 | 0.10±0.35 | 0.11±0.37 |
| Hooper Visual Organizational Test† | −0.23±0.06*** | −0.59±0.08*** | −0.76±0.13*** | −1.02±0.16*** | −1.34±0.20*** |
| Boston Naming Test† | −0.01±0.06 | −0.20±0.07** | −0.31±0.13* | −0.37±0.15* | −0.71±0.19*** |
Natural log transformed
Results are β±SE [in standard deviation units based on the entire sample (65–90+)].
Models adjusted for gender, natural log of WRAT and the cohort effect
p<.05;
p≤.01,
p≤.001
Figure 1.
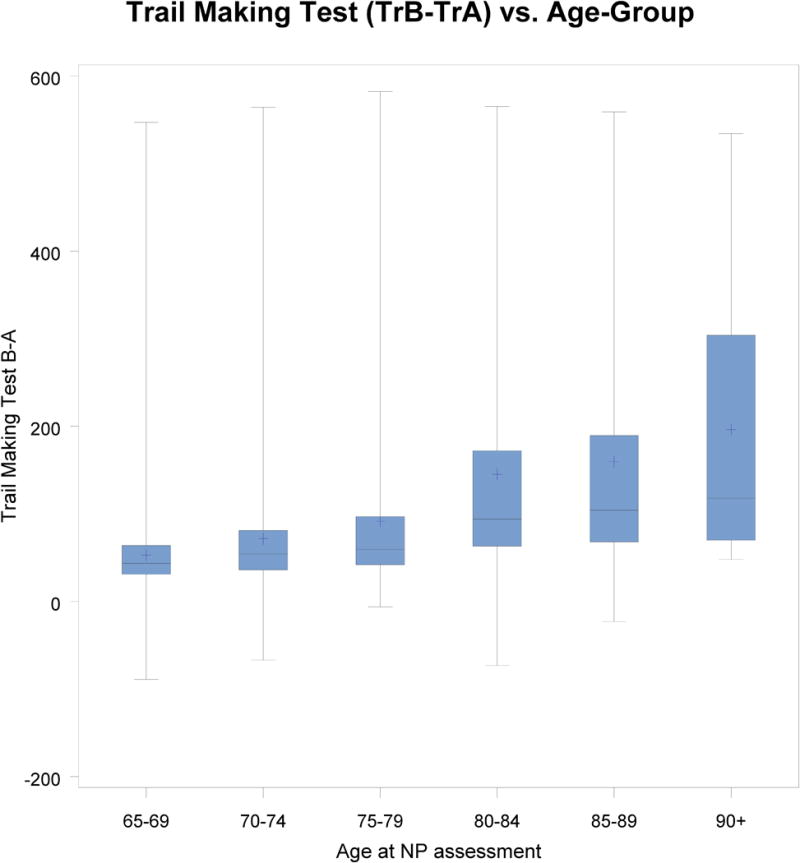
Trail Making Test Data of Age Subgroups for All Participants (65–90+ year-olds)
Figure 2.
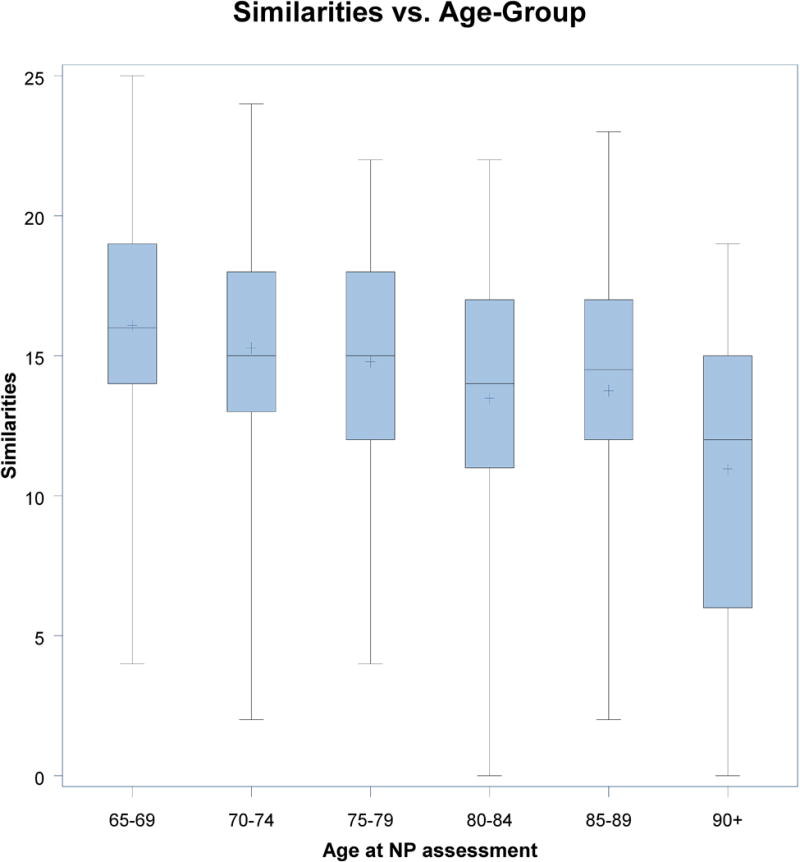
WAIS Similarities Data of Age Subgroups for All Participants (65–90+ year-olds)
Comparison of Young-old to Oldest-old
As shown in Table 7, we found differences in cognitive performance between individuals in the young-old (aged 65–79) and oldest-old age group (80+) on all cognitive measures evaluated except for SIM and COWAT. For all comparisons, the 65–79 age band was used as a reference, and all scores were adjusted for gender, the logarithm of WRAT-III Reading score, and cohort as above. Performances by oldest-old individuals on LMI, LMD, TMB-TMA, HVOT, and BNT were significantly worse than young-old performance.
Table 7.
Comparison of Oldest-Old (80+) Cognitive Data to Young-Old (65–79; n = 884)
| N | 418 |
|---|---|
| Logical Memory Immediate | −0.49 ± 0.14*** |
| Logical Memory Delayed | −0.40 ± 0.14** |
| Trail Making Tests (B–A)† | −0.41 ± 0.13** |
| Similarities | −0.17 ± 0.12 |
| Controlled Word Association Test | 0.29 ± 0.26 |
| Hooper Visual Organizational Test† | −0.54±0.13*** |
| Boston Naming Test† | −0.26±0.12* |
Natural log transformed
Results are β±SE [in standard deviation units based on the entire sample (65–90+)].
Models adjusted for gender, natural log of WRAT and the cohort effect
p<.05,
p≤.01,
p≤.001
Comparison of Age Bands within the Oldest-old
For all comparisons, the 80–84 age band was used as a reference and all scores were adjusted for gender, the logarithm of WRAT-III Reading score, and cohort as above. As shown in Table 8, there were significant differences in performance between the 85–89 age band and the referent group on COWAT and HVOT. There were significant differences between the 90+ age band and the referent group on all tests but COWAT. Post-hoc pairwise comparisons using the Tukey procedure revealed a significant difference between the 85–89 and 90+ age bands for SIM (p<0.05).
Table 8.
Comparison of Cognitive Data of Three Oldest-Old Age Bands Compared to Referent Group (80–84 year-olds; n=252)
| Age Band | 85–89 | 90+ |
|---|---|---|
|
| ||
| N | 109 | 57 |
| Logical Memory Immediate | −0.14 ± 0.12 | −0.49 ± 0.17** |
| Logical Memory Delayed | −0.15 ± 0.12 | −0.38 ± 0.17* |
| Trail Making Tests (B–A)† | −0.16 ± 0.15 | −0.50 ± 0.21* |
| Similarities | 0.03 ± 0.12 | −0.49 ± 0.17 ** |
| Controlled Word Association Test | −0.28 ± 0.12* | −0.28 ± 0.16 |
| Hooper Visual Organizational Test† | −0.26 ± 0.10** | −0.58 ± 0.14*** |
| Boston Naming Test† | −0.05 ± 0.10 | −0.40 ± 0.14** |
Natural log transformed
Results are β±SE [in standard deviation units based on the entire sample (65–95+)].
Models adjusted for gender, natural log of WRAT and the cohort effect
p<.05;
p≤.01,
p≤.001
Effect Modification by Gender
There was a significant interaction between gender and age when comparing the young-old and oldest-old groups on the LMI; F(1,1209)=3.12, MSE=0.847, p<0.10. Stratified analysis by gender revealed a difference between young-old and oldest-old only among women; β±se: −0.53±0.18 (p<0.01), There was also a significant interaction between gender and age when comparing young-old and oldest-old groups on SIM; F(1,1207) = 5.03, MSE=0.666, p<0.05 and COWAT F(1,346) = 3.25, MSE = 0.779, p<0.10. Stratified analysis by gender revealed no significant differences between young-old and oldest-old among either gender for these measures. We did not find significant effect modification by gender on any other cognitive measure for the young-old—oldest-old comparison, nor when comparing age age bands within the oldest-old.
DISCUSSION
In the present study, normative data were provided and cognitive performance was investigated among elderly individuals from the Original and Offspring Cohorts of FHS with a particular focus on cognitive performance of oldest-old individuals. Accordingly, comparisons between participants ages 65–69 and five-year age bands up to 90+, between young-old and oldest-old age groups, and between five-year age bands within the oldest-old were examined for potential cognitive differences. Results from the initial analysis reinforce previous literature in showing that significant differences exist between elderly individuals and that as age increases, variability in test performance increases. The findings that cognitive performance in the oldest-old was significantly different from that of young-old individuals on five of seven measures compared support previous literature highlighting the need for normative data that is specific to oldest-old individuals. Performance on these tests, which have been related to activities of daily living for elderly individuals (Reppermund et al., 2011; Royall, Palmer, Chiodo, & Polk, 2005), encourage further study to elucidate which abilities are most sensitive to change as individuals reach extreme age, with implications for daily life function. Continued use of normative data that treat aging as a homogenous group (i.e. 75+) may result in inappropriate expectations for cognitive function and misclassification of cognitive deficit for oldest-old individuals. Using age bands for normative data such as what is presented in the current study will provide much more accurate and specific information about the abilities of individuals in extreme age.
Additionally, within the oldest-old age group, there were significant differences between age bands, particularly between the youngest (80–84) and oldest (90+) age bands, on tests of memory, abstract reasoning, executive functioning, visuospatial perception and confrontation naming, but not verbal (phonemic) fluency. Results concerning the 90+ age band should be considered cautiously as there were only 57 participants in this age band; this information was included despite this limitation because it highlights the range of ages within the FHS sample. The number of individuals in this group is likely to grow and will be included in future studies; it is of interest that though this data is preliminary, cognition does appear to continue to change at this advanced age, which supports recent studies of cognitive changes even among centenarians (Davey et al., 2013; Davey et al., 2010; Miller et al., 2010). These results are generally similar to those reported by Welsh-Bohmer and colleagues and Elias and co-authors (Elias et al., 2011; Welsh-Bohmer et al., 2009). Both groups found that oldest-old participants (85+ and 90–98, respectively) performed less well than younger participants on most tests of cognitive functioning except phonemic fluency. Direct comparisons between normative studies of the oldest-old are difficult due to differences across studies in sample (e.g., exclusion criteria for participants), methodology (we used WRAT Reading-adjusted scores for analysis – please see rationale for this type of analysis later in the Discussion), versions of neuropsychological tests (e.g., FAS verbal fluency versus CFL, BNT-15 versus BNT-30), reporting of age bands (e.g., five-year versus 10-year age bands) and levels of education (two levels versus three levels of education). These results provide further evidence that oldest-old individuals are a distinct and heterogeneous group.
Comparisons of demographic variables and of specific cognitive tests were investigated for the Original and Offspring Cohorts because of previous findings of cohort differences (Au et al., 2004; Hulur et al., 2012). The Offspring Cohort was used for young-old to oldest-old comparisons because for this period of data collection (1999–2009), just eight individuals from the Original Cohort were of young-old age. In the present study, the Original Cohort had greater numbers of oldest-old participants than the Offspring Cohort. Importantly, age-group matched comparisons between the Original and Offspring Cohorts still found differences in age, education and in visuospatial perception, suggesting that cohort differences should continue to be considered in future, longitudinal studies of cognitive aging. As more individuals in the Offspring Cohort reach extreme age, it will be important to examine cohort effects in the oldest-old, to see how enhancements in well-being due to generational differences affect cognitive functioning.
There was a significant interaction between age and gender on an immediate memory task, with stratified analysis by gender displaying a difference between young-old and oldest-old only among women, and on abstract reasoning and verbal fluency tasks, though stratified analysis by gender for these tasks revealed no differences between young-old and oldest-old individuals for either gender. Gender differences in verbal fluency and visuospatial skills have been supported previously with women generally outperforming men (fluency) and men generally outperforming women (visuospatial skills). While gender differences in extreme aging have been suggested in the literature, particularly that men who do reach extreme age fare better physically and cognitively than women of the same age (Andersen, Sebastiani, Dworkis, Feldman, & Perls, 2012; Xie, Matthews, Jagger, Bond, & Brayne, 2008) (Davey et al., 2010), multiple studies have found that gender effects may be attributable to effects of education and age (Beeri et al., 2006; Davey et al., 2010; Dore, Elias, Robbins, Elias, & Brennan, 2007; Elias et al., 2011; Kenny et al., 2013; Welsh-Bohmer et al., 2009). Indeed, in the current study, there are large educational differences between age bands (e.g., 16–43% of individuals in the oldest-old age bands, where the proportion of women is higher, with less than high school education compared to 3–10% of young-old individuals, where the proportion of women to men is nearly equivalent, with less than high school education), although the interaction reported was not a result of educational differences. Other studies of cognition in the extremely elderly have not found an effect of gender (Coluccia, Gamboz, & Brandimonte, 2011; Davey et al., 2013; Stein et al., 2012). The lack of other effect modifications by gender in the present study is consistent with literature suggesting that gender may not be the most significant contributor to cognition among the oldest-old, though additional evidence about the specific nature of the interaction on these measures is a subject for future study.
The present study provides substantial evidence for the use of normative data distinct to cognitively intact oldest-old individuals and encourages a breakdown of scores even within this age group. Compared to previous studies of cognition in the oldest-old, the present study provides updated information for a longitudinally well-characterized community-based sample. We used WRAT-III reading scores as a measure of premorbid intelligence and a surrogate for education attainment, and we encourage future studies to consider this variable as well, as it has been suggested to be a more accurate assessment of pre-morbid cognitive functioning than years of education, particularly for this age range. For example, Ahl and coauthors (2013) found that in the Framingham Offspring cohort, MCI as defined by WRAT-adjusted as compared to education-adjusted norms was more strongly associated with incident dementia for all cognitive tests examined (Ahl et al., 2013). Their suggestion of using reading level as a more accurate surrogate than years of formal education has been supported by other studies of the oldest-old (Davey et al., 2013; Davey et al., 2010; Elias et al., 2011). The influence of reading level on cognitive performance has been found in other populations as well. Mokri and colleagues (2012) compared Mexican literate and nonliterate participants without formal education and found that those with reading and writing skills performed significantly better on most tests, and suggested that literacy skills should be considered as an influential factor to cognitive performance (Mokri et al., 2012). In a sample of HIV+ and HIV− women, Manly and coauthors (2011) found that reading level as defined by the WRAT-III had the strongest relationship to cognitive performance, compared to HIV status, education, and in some cases, age (Manly et al., 2011). However, the majority of studies of cognition in the oldest-old do use education as a marker for premorbid ability and it is often found to be a significant predictor of cognitive performance (Beeri et al., 2006; Coluccia et al., 2011; Davey et al., 2010; Dore et al., 2007; Kenny et al., 2013; Miller et al., 2010; Stein et al., 2012; Welsh-Bohmer et al., 2009), though a study of verbal fluency performance in elderly individuals found no significant differences based on reading ability or education (Ravdin et al., 2003). We do provide age- and education-based normative data in the supplementary materials as we know that clinicians more often have access to years of education than WRAT Reading performance.
Results from the current study provide a jumping off point from which future investigations will be able to further elucidate the complex nature and relations among multiple variables in the oldest-old. For example, clinicopathological studies of the oldest-old have found poor concordance between functional and pathological brain status, and point out that there are likely additional, as yet unknown variables to account for different types of cognitive trajectories in this extreme age group (Balasubramanian, Kawas, Peltz, Brookmeyer, & Corrada, 2012; Corrada, Berlau, & Kawas, 2012). As the population continues to age, it will be important to consider enhancements in variables contributing to quality of life to set expectations for cognition in extreme age.
There are multiple limitations of this study that should be addressed. The population in this study is Caucasian, which reflects the oldest-old population in this community setting in 1948, the date of initial recruitment. Also, due to the nature of participating in this multigenerational study, FHS participants are carefully monitored and may be more highly attuned to changes in health and cognitive status. In addition, participants with sensory impairments sufficient to preclude completion of any test were excluded. Therefore, generalizations to a wider population should be undertaken with caution. Our study was cross-sectional; longitudinal studies of cognitively intact aging individuals from a variety of ethnic backgrounds will be imperative in order to predict which individuals are likely to reach extreme age and what factors, including potentially modifiable factors, increase the likelihood of successful aging. Additionally, the use of older versions of some cognitive tests (e.g., original WMS and WAIS), while important for longitudinal study that includes use of these measures from 1976 to present, is a limitation as most clinicians today use updated versions of these tests.
Supplementary Material
Contributor Information
Ivy N. Miller, Department of Psychology, Minneapolis VA Healthcare System, Minneapolis, MN, 55417, USA, 612-467-3036
Jayandra J. Himali, Framingham Heart Study; Department of Neurology, Boston University School of Medicine;, 72 E. Concord Street, B-6, Boston, MA 02215, USA, 617-638 5139
Alexa S. Beiser, Framingham Heart Study; Department of Neurology, Boston University School of Medicine; Department of Biostatistics, Boston University School of Public Health, 72 E. Concord Street, B-6, Boston, MA 02215, USA, 617-638-5450
Joanne M. Murabito, Framingham Heart Study; Section of General Internal Medicine, Department of Medicine, Boston University School of Medicine, 72 E. Concord Street, B-6, Boston, MA 02215, USA, 508-935-3461
Sudha Seshadri, Framingham Heart Study; Department of Neurology, Boston University School of Medicine, 72 E. Concord Street, B-6, Boston, MA 02215, USA, 617-414-1337
Philip A. Wolf, Framingham Heart Study; Department of Neurology, Boston University School of Medicine, 72 E. Concord Street, B-6, Boston, MA 02215, USA, 617-638-5450
Rhoda Au, Framingham Heart Study; Department of Neurology, Boston University School of Medicine, 72 E. Concord Street, B-6, Boston, MA 02215, USA, 617-638-5450
References
- Ahl RE, Beiser A, Seshadri S, Auerbach S, Wolf PA, Au R. Defining MCI in the Framingham Heart Study Offspring: education versus WRAT-based norms. Alzheimer Disease and Associated Disorders. 2013;27(4):330–336. doi: 10.1097/WAD.0b013e31827bde32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Sebastiani P, Dworkis DA, Feldman L, Perls TT. Health span approximates life span among many supercentenarians: compression of morbidity at the approximate limit of life span. Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2012;67(4):395–405. doi: 10.1093/gerona/glr223. doi: glr223 [pii] 10.1093/gerona/glr223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au R, Seshadri S, Wolf PA, Elias M, Elias P, Sullivan L, Beiser A, D’Agostino RB. New norms for a new generation: cognitive performance in the framingham offspring cohort. Experimental Aging Research. 2004;30(4):333–358. doi: 10.1080/03610730490484380. doi: 10.1080/03610730490484380 N7BE2BY76G270MLK [pii] [DOI] [PubMed] [Google Scholar]
- Balasubramanian AB, Kawas CH, Peltz CB, Brookmeyer R, Corrada MM. Alzheimer disease pathology and longitudinal cognitive performance in the oldest-old with no dementia. Neurology. 2012;79(9):915–921. doi: 10.1212/WNL.0b013e318266fc77. doi: WNL.0b013e318266fc77 [pii] 10.1212/WNL.0b013e318266fc77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeri MS, Schmeidler J, Sano M, Wang J, Lally R, Grossman H, Silverman JM. Age, gender, and education norms on the CERAD neuropsychological battery in the oldest old. Neurology. 2006;67(6):1006–1010. doi: 10.1212/01.wnl.0000237548.15734.cd. doi: 67/6/1006 [pii] 10.1212/01.wnl.0000237548.15734.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkowski JG, Benton AL, Spreen O. Word fluency and brain damage. Neuropsychologia. 1967;5:135–40. [Google Scholar]
- Calvert JF, Jr, Hollander-Rodriguez J, Kaye J, Leahy M. Dementia-free survival among centenarians: an evidence-based review. Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2006;61(9):951–956. doi: 10.1093/gerona/61.9.951. [DOI] [PubMed] [Google Scholar]
- Coluccia E, Gamboz N, Brandimonte MA. Normative data for a battery of free recall, cued recall and recognition tests in the elderly Italian population. Neurol Sci. 2011;32(6):1103–1114. doi: 10.1007/s10072-011-0747-5. [DOI] [PubMed] [Google Scholar]
- Corrada MM, Berlau DJ, Kawas CH. A population-based clinicopathological study in the oldest-old: the 90+ study. Current Alzheimer Research. 2012;9(6):709–717. doi: 10.2174/156720512801322537. doi: CAR-EPUB-20120402-004 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan JD, Hinkeldey NS. Relationships between parts A and B of the Trail Making Test. Journal of Clinical Psychology. 1987;43(4):402–409. doi: 10.1002/1097-4679(198707)43:4<402::aid-jclp2270430411>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Davey A, Dai T, Woodard JL, Miller LS, Gondo Y, Johnson MA, Hausman DB, Martin P, Green RC, Allen RH, Stabler SP, Poon LW. Profiles of cognitive functioning in a population-based sample of centenarians using factor mixture analysis. Experimental Aging Research. 2013;39(2):125–144. doi: 10.1080/0361073X.2013.761869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey A, Elias MF, Siegler IC, Lele U, Martin P, Johnson MA, Hausman DB, Poon LW. Cognitive function, physical performance, health, and disease: norms from the georgia centenarian study. Experimental Aging Research. 2010;36(4):394–425. doi: 10.1080/0361073X.2010.509010. doi: 926880122 [pii] 10.1080/0361073X.2010.509010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. American Journal of Public Health and the Nations Health. 1951;41(3):279–281. doi: 10.2105/AJPH.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore GA, Elias MF, Robbins MA, Elias PK, Brennan SL. Cognitive performance and age: norms from the Maine-Syracuse Study. Experimental Aging Research. 2007;33(3):205–271. doi: 10.1080/03610730701319087. doi: 778476658 [pii] 10.1080/03610730701319087. [DOI] [PubMed] [Google Scholar]
- Elias MF, Dore GA, Goodell AL, Davey A, Zilioli MK, Brennan S, Robbins MA. Normative data for elderly adults: the Maine-Syracuse study. Experimental Aging Research. 2011;37(2):142–178. doi: 10.1080/0361073X.2011.554511. doi: 935083324 [pii] 10.1080/0361073X.2011.554511. [DOI] [PubMed] [Google Scholar]
- Elias MF, Elias PK, D’Agostino RB, Silbershatz H, Wolf PA. Role of age, education, and gender on cognitive performance in the Framingham Heart Study: community-based norms. Experimental Aging Research. 1997;23(3):201–235. doi: 10.1080/03610739708254281. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Grydeland H, Amlien I, Espeseth T, Reinvang I, Raz N, Holland D, Dale AM, Walhovd KB. Critical ages in the life course of the adult brain: nonlinear subcortical aging. Neurobiology of Aging. 2013;34(10):2239–2247. doi: 10.1016/j.neurobiolaging.2013.04.006. doi: S0197-4580(13)00159-0 [pii] 10.1016/j.neurobiolaging.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison TM, Weintraub S, Mesulam MM, Rogalski E. Superior memory and higher cortical volumes in unusually successful cognitive aging. Journal of the International Neuropsychological Society. 2012;18(6):1081–1085. doi: 10.1017/S1355617712000847. doi: S1355617712000847 [pii] 10.1017/S1355617712000847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Sengupta M, Velkoff VA, DeBarros KA. 65+ in the United States: 2005. U.S. Census Bureau; 2005. [Google Scholar]
- Hooper HE. Hooper Visual Organization Test (VOT) Los Angeles: Western Psychological Services; 1983. [Google Scholar]
- Hulur G, Infurna FJ, Ram N, Gerstorf D. Cohorts Based on Decade of Death: No Evidence for Secular Trends Favoring Later Cohorts in Cognitive Aging and Terminal Decline in the AHEAD Study. Psychology and Aging. 2012 doi: 10.1037/a0029965. doi: 2012-27245-001 [pii] 10.1037/a0029965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivnik RJ, Malec JF, Smith GE, Tangalos E, Petersen RC, Kokmen E, Kurland LT. Mayo’s older americans normative studies: WAIS-R norms for ages 56 to 97. The Clinical Neuropsychologist. 1992a;6(Supplement 001) [Google Scholar]
- Ivnik RJ, Malec JF, Smith GE, Tangalos E, Petersen RC, Kokmen E, Kurland LT. Mayo’s older americans normative studies: WMS-R norms for ages 56 to 94. The Clinical Neuropsychologist. 1992b;6(Supplement 001):49–82. doi: 10.1080/13854049208401879. [DOI] [Google Scholar]
- Katsumata Y, Todoriki H, Higashiuesato Y, Yasura S, Willcox DC, Ohya Y, Willcox BJ, Dodge HH. Metabolic syndrome and cognitive decline among the oldest old in Okinawa: in search of a mechanism. The KOCOA Project. Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2012;67(2):126–134. doi: 10.1093/gerona/glr189. doi: glr189 [pii] 10.1093/gerona/glr189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny RA, Coen RF, Frewen J, Donoghue OA, Cronin H, Savva GM. Normative values of cognitive and physical function in older adults: findings from the Irish Longitudinal Study on Ageing. Journal of the American Geriatrics Society. 2013;61(Suppl 2):S279–290. doi: 10.1111/jgs.12195. [DOI] [PubMed] [Google Scholar]
- Kinsella K, He W. An Aging World: 2008 International Population Reports, P95/09-1. Washington, DC: U.S. Government Printing Office; 2008. [Google Scholar]
- Kravitz E, Schmeidler J, Beeri MS. Cognitive decline and dementia in the oldest-old. Rambam Maimonides Med J. 2012;3(4):e0026. doi: 10.5041/RMMJ.10092. doi: 10.5041/RMMJ.10092 rmmj-3-4-e0026 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JA, Ivnik RJ, Smith GE, Ferman TJ, Willis FB, Petersen RC, Graff-Radford NR. Mayo’s Older African Americans Normative Studies: norms for Boston Naming Test, Controlled Oral Word Association, Category Fluency, Animal Naming, Token Test, WRAT-3 Reading, Trail Making Test, Stroop Test, and Judgment of Line Orientation. The Clinical Neuropsychologist. 2005;19(2):243–269. doi: 10.1080/13854040590945337. doi: W1715133L6336200 [pii] 10.1080/13854040590945337. [DOI] [PubMed] [Google Scholar]
- Lucca U, Garri M, Recchia A, Logroscino G, Tiraboschi P, Franceschi M, Bertinotti C, Biotti A, Gargantini E, Maragna M, Nobili A, Pasina L, Franchi C, Riva E, Tettamanti M. A Population-based study of dementia in the oldest old: the Monzino 80-plus study. BioMedCentral Neurology. 2012;11:54. doi: 10.1186/1471-2377-11-54. doi: 1471-2377-11-54 [pii] 10.1186/1471-2377-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly JJ, Jacobs DM, Touradji P, Small SA, Stern Y. Reading level attenuates differences in neuropsychological test performance between African American and White elders. Journal of the International Neuropsychological Society. 2002;8(3):341–348. doi: 10.1017/S1355617702813157. [DOI] [PubMed] [Google Scholar]
- Manly JJ, Smith C, Crystal HA, Richardson J, Golub ET, Greenblatt R, Robison E, Martin EM, Young M. Relationship of ethnicity, age, education, and reading level to speed and executive function among HIV+ and HIV− women: the Women’s Interagency HIV Study (WIHS) Neurocognitive Substudy. Journal of Clinical and Experimental Neuropsychology. 2011;33(8):853–863. doi: 10.1080/13803395.2010.547662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LS, Mitchell MB, Woodard JL, Davey A, Martin P, Poon LW, Jazwinski SM, Green RC, Gearing M, Markesbery WR, Johnson MA, Tenover JS, Rodgers WL, Hausman DB, Arnold J, Siegler IC. Cognitive performance in centenarians and the oldest old: norms from the Georgia Centenarian Study. Aging Neuropsychol Cogn. 2010;17(5):575–590. doi: 10.1080/13825585.2010.481355. doi: 922692907 [pii] 10.1080/13825585.2010.481355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokri H, Avila-Funes JA, Le Goff L, Ruiz-Arregui L, Gutierrez Robledo LM, Amieva H. Self-reported reading and writing skills in elderly who never attended school influence cognitive performances: results from the Coyoacan cohort study. The Journal of Nutrition, Health & Aging. 2012;16(7):621–624. doi: 10.1007/s12603-012-0070-8. [DOI] [PubMed] [Google Scholar]
- O’Bryant SE, Schrimsher GW, O’Jile JR. Discrepancies between self-reported years of education and estimated reading level: potential implications for neuropsychologists. Applied Neuropsychology. 2005;12(1):5–11. doi: 10.1207/s15324826an1201_2. [DOI] [PubMed] [Google Scholar]
- Perls T. Dementia-free centenarians. Experimental Gerontology. 2004;39(11–12):1587–1593. doi: 10.1016/j.exger.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Poon LW, Martin P, Bishop A, Cho J, da Rosa G, Deshpande N, Hensley R, Macdonald M, Margrett J, Randall GK, Woodard JL, Miller LS. Understanding centenarians’ psychosocial dynamics and their contributions to health and quality of life. Current Gerontology and Geriatrics Research. 2010 doi: 10.1155/2010/680657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravdin LD, Katzen HL, Agrawal P, Relkin NR. Letter and semantic fluency in older adults: effects of mild depressive symptoms and age-stratified normative data. The Clinical Neuropsychologist. 2003;17(2):195–202. doi: 10.1076/clin.17.2.195.16500. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]
- Reppermund S, Sachdev PS, Crawford J, Kochan NA, Slavin MJ, Kang K, Trollor JN, Draper B, Brodaty H. The relationship of neuropsychological function to instrumental activities of daily living in mild cognitive impairment. International Journal of Geriatric Psychiatry. 2011;26(8):843–852. doi: 10.1002/gps.2612. [DOI] [PubMed] [Google Scholar]
- Royall DR, Palmer R, Chiodo LK, Polk MJ. Normal rates of cognitive change in successful aging: the freedom house study. Journal of the International Neuropsychological Society. 2005;11(7):899–909. doi: 10.1017/s135561770505109x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Cubillo I, Perianez JA, Adrover-Roig D, Rodriguez-Sanchez JM, Rios-Lago M, Tirapu J, Barcelo F. Construct validity of the Trail Making Test: role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. Journal of the International Neuropsychological Society. 2009;15(3):438–450. doi: 10.1017/S1355617709090626. doi: S1355617709090626 [pii] 10.1017/S1355617709090626. [DOI] [PubMed] [Google Scholar]
- Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB, Wolf PA. The lifetime risk of stroke: estimates from the Framingham Study. Stroke. 2006;37(2):345–350. doi: 10.1161/01.STR.0000199613.38911.b2. doi: 01.STR.0000199613.38911.b2 [pii] 10.1161/01.STR.0000199613.38911.b2. [DOI] [PubMed] [Google Scholar]
- Slavin MJ, Brodaty H, Sachdev PS. Challenges of diagnosing dementia in the oldest old population. Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2013;68(9):1103–1111. doi: 10.1093/gerona/glt051. doi: glt051 [pii]10.1093/gerona/glt051. [DOI] [PubMed] [Google Scholar]
- Stein J, Luppa M, Maier W, Tebarth F, Heser K, Scherer M, Zimmermann T, Eisele M, Bickel H, Mosch E, Weyerer S, Werle J, Pentzek M, Fuchs A, Wiese B, Prokein J, Konig HH, Leicht H, Riedel-Heller SG. The assessment of changes in cognitive functioning in the elderly: age- and education-specific reliable change indices for the SIDAM. Dementia and Geriatric Cognitive Disorders. 2012;33(2–3):73–83. doi: 10.1159/000336864. doi: 000336864 [pii] 10.1159/000336864. [DOI] [PubMed] [Google Scholar]
- Steinberg BA, Bieliauskas LA, Smith GE, Ivnik RJ. Mayo’s Older Americans Normative Studies: Age- and IQ-Adjusted Norms for the Trail-Making Test, the Stroop Test, and MAE Controlled Oral Word Association Test. The Clinical Neuropsychologist. 2005;19(3–4):329–377. doi: 10.1080/13854040590945210. doi: V54M885551447434 [pii] 10.1080/13854040590945210. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale: Manual. San Antonio, TX: Psychological Corporation; 1945. [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale. New York: The Psychological Corporation; 1955. [Google Scholar]
- Welsh-Bohmer KA, Ostbye T, Sanders L, Pieper CF, Hayden KM, Tschanz JT, Norton MC. Neuropsychological performance in advanced age: influences of demographic factors and Apolipoprotein E: findings from the Cache County Memory Study. The Clinical Neuropsychologist. 2009;23(1):77–99. doi: 10.1080/13854040801894730. doi: 794002676 [pii] 10.1080/13854040801894730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle C, Corrada MM, Dick M, Ziegler R, Kahle-Wrobleski K, Paganini-Hill A, Kawas C. Neuropsychological data in nondemented oldest old: the 90+ Study. Journal of Clinical and Experimental Neuropsychology. 2007;29(3):290–299. doi: 10.1080/13803390600678038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS. The Wide Range Achievement Test: Manual. 3rd. Wilmington, DE: Wide Range; 1993. Vol. [Google Scholar]
- Williams BW, Mack W, Henderson VW. Boston naming test in Alzheimer’s disease. Neuropsychologia. 1989;27(8):1073–1079. doi: 10.1016/0028-3932(89)90186-3. [DOI] [PubMed] [Google Scholar]
- Xie J, Matthews FE, Jagger C, Bond J, Brayne C. The oldest old in England and Wales: a descriptive analysis based on the MRC Cognitive Function and Ageing Study. Age and Ageing. 2008;37(4):396–402. doi: 10.1093/ageing/afn061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Middleton LE, Lui LY, Spira AP, Stone K, Racine C, Ensrud KE, Kramer JH. Mild cognitive impairment, dementia, and their subtypes in oldest old women. Archives of Neurology. 2011;68(5):631–636. doi: 10.1001/archneurol.2011.82. doi: 68/5/631 [pii] 10.1001/archneurol.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


