Abstract
Background
The uptake of intensified active TB case finding among HIV-infected patients using symptom screening is not well understood. We evaluated the rate and completeness of each interim step in the TB “diagnostic cascade” to understand real-world barriers to active TB case detection.
Methods
We conducted a cohort analysis of new, ART-naïve, HIV-infected patients who attended a large HIV clinic in Mbarara, Uganda (March 1, 2012 – September 30, 2013). We used medical records to extract the date of completion of each step in the diagnostic cascade: symptom screen, order, collection, processing, and result. Factors associated with lack of sputum order were evaluated using multivariate Poisson regression and a chart review of 50 screen positive patients.
Results
Of 2613 patients, 2439 (93%) were screened for TB and 682(28%) screened positive. Only 90(13.2%) had a sputum order. Of this group, 83% completed the diagnostic cascade, 13% were diagnosed with TB, and 50% had a sputum result within 1 day of their visit. Sputum ordering was associated with WHO Stage 3 or 4 HIV disease and greater number of symptoms. The main identifiable reasons for lack of sputum order in chart review were treatment for presumed malaria (51%) or bacterial infection (43%).
Conclusions
The majority of newly enrolled HIV-infected patients who screened positive for suspected TB did not have a sputum order, and those who did were more likely to have more advanced HIV disease and symptoms. Further evaluation of provider behavior in the management of screen positive patients could improve active TB case detection rates.
Introduction
Clinical studies have yielded a growing number of treatment strategies (i.e. early antiretroviral therapy (ART) initiation, isoniazid preventive therapy, etc), to address both active and latent tuberculosis in HIV infected patients that hold great promise for reducing TB related morbidity and mortality. The use of these strategies in real-world patient populations, however, depends on the ability of the health system to accurately and rapidly classify HIV infected patients as either having active tuberculosis or presumed latent infection. Screening algorithms based on simple clinical criteria (cough, fever, night sweats, weight loss) have high sensitivity for active TB [1, 2]. Yet, in real-world settings, the successful uptake of symptom screening for the diagnosis of active TB is not well understood, in part because HIV/TB services frequently straddle different agencies, occur in different clinics and laboratories and are therefore often poorly integrated and difficult to measure [3, 4].
In order to understand processes in the evaluation of HIV infected persons for TB, we mapped a pulmonary TB diagnostic care cascade. This cascade is composed of symptom screening and among those who screen positive, sputum order, sputum collection, sputum receipt in laboratory, sputum smear testing, and sputum result. We applied this framework to a large cohort of HIV positive patients in Mbarara, Uganda. Identification of gaps in this cascade is the first step in designing strategies to address them and thereby improve implementation of active TB screening among persons living with HIV (PLHIV).
Methods
Design and Study Population
We conducted a cohort analysis of newly enrolled, ART naïve, HIV-infected patients who attended the Immune Suppression Syndrome (ISS) clinic in Mbarara, Uganda between March 1, 2012 and September 30, 2013. The ISS clinic is a large volume clinic, serving approximately 20,000 patients and generating over 200 patient visits a day. The ISS clinic, which is part of the Mbarara University of Science and Technology, is a PEPFAR supported clinic and serves as one of the participating clinics in the East Africa International Epidemiologic Database to Evaluate AIDS (IeDEA) Consortium. At the ISS clinic, a diagnosis of TB is typically made via fluorescence microscopy on sputum specimens at an on-site mycobacteriology (TB) laboratory. Chest radiography (CXR) is available, though not routinely used. Gene-Xpert MTB/RIF© diagnostic testing for TB was not available during the study period.
Measurements
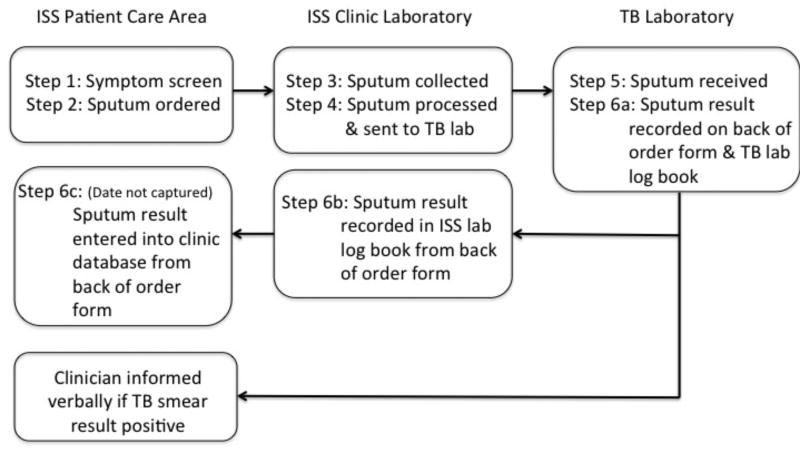
We established six key processes that we sought to measure as part of the pulmonary “TB diagnostic cascade”: (1) symptom screening, (2) sputum order, (3) sputum collection, (4) transport of specimen from ISS laboratory to TB laboratory for testing, (5) receipt of specimen at TB lab for testing, and (6) recording of TB smear results. The process flow of the various steps in the TB diagnostic cascade for the first sputum collected is summarized in Figure 1. We obtained the dates for each of these steps by using existing ISS clinic electronic or paper records and by reviewing ISS laboratory logbook and TB laboratory registers. Step 1 (symptom screening) was obtained by extracting from the ISS clinic electronic medical record the responses to the four symptom screening questions recommended in WHO’s 2011 Guidelines for intensified tuberculosis case-finding for PLHIV: cough of any duration, night sweats, fever, and weight loss. [5]. Step 2 (sputum order) was obtained from a paper sputum order/result form. Steps 3 (sputum collection) and 4 (transport of specimen to TB laboratory) were obtained from direct review of the ISS laboratory logbook and Step 5 (receipt of specimen at TB laboratory) was obtained from direct review of the TB laboratory register. Step 6 (reporting of TB smear results) was obtained by including any record of a sputum result in either the ISS laboratory logbook (6b) or the sputum order/result form (6c). These data were cross-checked against the TB laboratory register (6a) by research staff to ensure completeness of data capture.
Figure 1. Process flow for TB diagnostic cascade of first sputum sample1 at ISS Clinic.
1The diagram above focuses on procedures for collection of the first sputum sample. Procedure for collection of second sputum sample: While at the ISS clinic laboratory patients are given a specimen collection cup to collect a second early morning sputum sample and are instructed to bring the specimen directly to the ISS laboratory. Subsequent steps in the flow are the same as those for first sputum samples.
In addition, to verify completeness of data in Steps 1 and 2 and to identify reasons for not ordering a sputum, the charts of 50 patients were randomly selected from all symptom screen positive visits (either new or returning patient visits) in patients for whom there was no subsequent TB sputum test ordered. Presence or absence of WHO symptoms and sputum order were verified. Among patients with WHO symptoms but no sputum order, any attributable reason noted in the chart (i.e. alternate diagnosis, patient already on TB treatment, etc) was coded and recorded.
Data on TB treatment initiation were obtained from direct review of paper records at TB-HIV clinic and data on patient demographic characteristics and ART initiation were obtained from the ISS clinic electronic medical records.
Statistical Analysis
Patient demographic characteristics, time (in days) between each of the six processes, and time to complete the entire cascade were evaluated for each newly enrolled patient. Completion of the TB diagnostic care cascade is defined here as having a smear result (positive or negative) of the first sputum sample collected for a patient. This is a mechanistic definition and is not meant to serve as a clinical definition (which generally requires either one positive smear or two negative smears). Chi-square and Wilcoxon rank sum tests were used in unadjusted analyses of demographic characteristics. Poisson regression was used to derive risk ratios to evaluate factors associated with a sputum sample being ordered within 14 days of enrollment visit amongst patients with a positive symptom screen. We evaluated the association between the type and number of symptoms and probability of sputum order in two separate models because type and number of symptoms were collinear. For example, patients with cough were more likely to have three or more symptoms. All data were analyzed using Stata MP 13.1.
Results
Patient characteristics
There were 2,613 newly enrolled patients at the ISS clinic during the study period. The median age of all new patients was 30 years (IQR: 25 – 38) and 1034 (40%) were male (Table 1). The majority of patients (71%) entered the clinic after having been diagnosed with HIV via routine testing and 53% had WHO Stage I disease at the time of enrollment. Of the entire cohort of newly enrolled patients, 2439 (93%) were screened for symptoms of tuberculosis at their initial clinic visit and 682 (28%) had at least one of the following four symptoms: cough, night sweats, fever, or weight loss. The most common symptom among patients who screened positive was cough (70%). (Table 2) Sixty-six percent of those with any symptoms had only one positive symptom and 34% had at least two positive symptoms.
Table 1.
Demographic and clinical characteristics of newly enrolled patients at ISS clinic during March 1, 2013 to September 30, 20131 (N=2613)
| Characteristic | All new patients n (%) |
|---|---|
| Age in years, median (IQR) | 30 (25– 38) |
| Male sex | 1034 (40) |
| Pregnant | 232 (15) |
| Married | 1347 (52) |
| Education | |
| Primary | 1342(62) |
| Secondary | 600 (28) |
| Tertiary | 232 (11) |
| Monthly income (<100,000 Ugandan Shillings) | 1794 (72) |
| Entry point into care | |
| Routine testing and counseling | 1867 (71) |
| Outpatient | 188 (7.2) |
| PMTCT | 55 (2.1) |
| AIDS Information Center | 42 (1.6) |
| Private | 55 (2.1) |
| Self | 80 (3.1) |
| Other | 103 (3.9) |
| Unknown or not documented | 223 (8.5) |
| Time to reach clinic | |
| Less than 30 minutes | 602 (25) |
| 30 – 60 minutes | 1109 (45) |
| 1 – 2 hours | 467 (19) |
| 2 – 3 hours | 189 (7.7) |
| > 3 hours | 80 (3.3) |
| WHO Stage | |
| Stage 1 | 1164 (53) |
| Stage 2 | 725 (33) |
| Stage 3 | 59 (2.7) |
| Stage 4 | 251 (11) |
Denominators vary due to missing data
Table 2.
Comparison of patients with and without sputum ordered among those who screened positive using WHO TB symptom screening criteria (N=682)
| Screen positive (N=682) n(%) |
Screen positive & sputum collected1 (N=90) n(%) |
Screen positive & sputum NOT collected1 (N= 592) n(%) |
Unadjusted Risk Ratio (95 %CI) |
Adjusted Risk Ratio (95% CI) Type of Symptoms |
Adjusted Risk Ratio (95 %CI) Number of Symptoms |
|
|---|---|---|---|---|---|---|
| Median age (IQR) years | 30.9 (25.2 – 37.7) | 33.3 (27.6 – 40.5) | 30.3 (25.2 – 37.6) | 1.02 (1.00–1.04) | ||
| Male gender | 322 (47) | 56 (62) | 266 (45) | 1.84 (1.24 – 2.74) | ||
| Type of Symptom | ||||||
| Cough | 478 (70) | 87 (97) | 391 (66) | 12.37 (3.96 – 38.7) | 8.80 (2.2 – 35.9) | |
| Fever | 341 (50) | 62 (69) | 279 (47) | 2.21 (1.45 – 3.37) | ||
| Night sweats | 91 (13) | 48 (53) | 43 (7) | 7.42 (5.22 – 10.5) | 1.67 (1.2 – 2.3) | |
| Weight loss | 150 (22) | 29 (32) | 121 (20) | 1.69 (1.12 – 2.52) | ||
| Number of Symptoms | ||||||
| 1 | 449 (66) | 20 (22) | 429 (73) | Ref | Ref | |
| 2 | 124 (18) | 19 (21) | 105 (18) | 3.44 (1.90 – 6.24) | 1.35 (0.7 – 2.6) | |
| 3 | 73 (11) | 36 (40) | 37 (6) | 11.07 (6.80 – 18.0) | 2.28 (1.3– 4.1) | |
| 4 | 36 (5) | 15 (17) | 21 (4) | 9.35 (5.25 – 16.7) | 2.00 (0.98–4.1) | |
| WHO Disease Stage | ||||||
| 1 | 78 (13) | 1 (1) | 77 (15) | Ref | Ref | Ref |
| 2 | 298 (51) | 4 (5) | 294 (58) | 1.05 (0.12 – 9.25) | 1.02 (0.12 – 9.0) | 1.01 (0.11– 9.1) |
| 3 | 24 (41) | 3 (4) | 21 (4) | 9.75 (1.06 – 89.6) | 8.84 (1.01 – 77.5) | 6.99 (0.71– 68.4) |
| 4 | 190 (32) | 77 (91) | 113 (22) | 31.6 (4.47 – 223.7) | 20.6 (2.9 – 146.2) | 20.6 (2.8– 152.7) |
Within 14d of initial clinic visit
TB diagnostic cascade overview
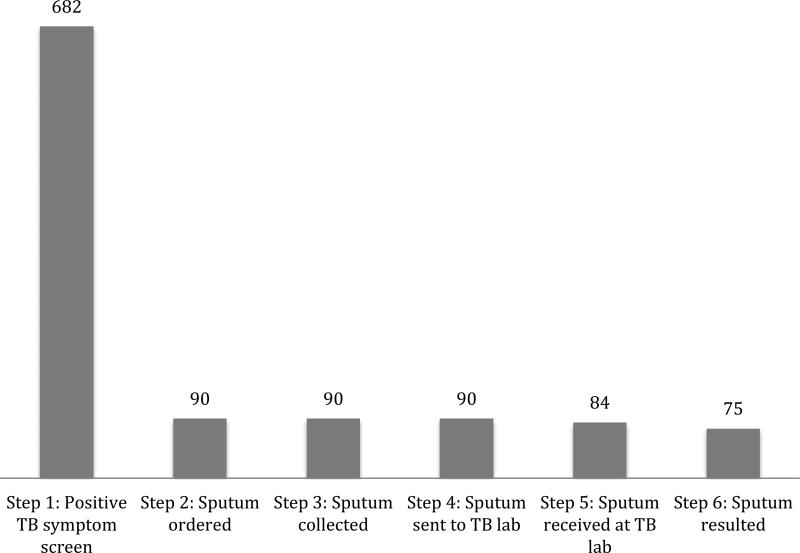
The TB diagnostic cascade (beginning with positive symptom screen and ending with TB smear microscopy results) for all newly enrolled patients at the ISS clinic is summarized in Figure 2. Of the 682 patients who screened positive, 90 (13.2%) had a sputum sample ordered. All patients with a sputum order had a sputum sample collected. Of the group with sputa collected, 75 (83%) completed the TB diagnostic cascade and 12 (13%) were diagnosed with TB based on sputum smear microscopy within 60 days of initial visit. Of the 90 patients who had a sputum collected, only 10 (11%) had a second sputum sample and 2 (2%) had a third sputum sample processed. No second or third sputum samples were TB smear positive.
Figure 2. TB diagnostic cascade of first sputum sample among newly enrolled patients at ISS clinic during March 1, 2012 to September 30, 20131.
1Step 1: same day as enrollment visit, Step 2: within 14 days of enrollment visit, Steps 3–6: no limitation of time horizon
Time to completion of TB diagnostic cascade
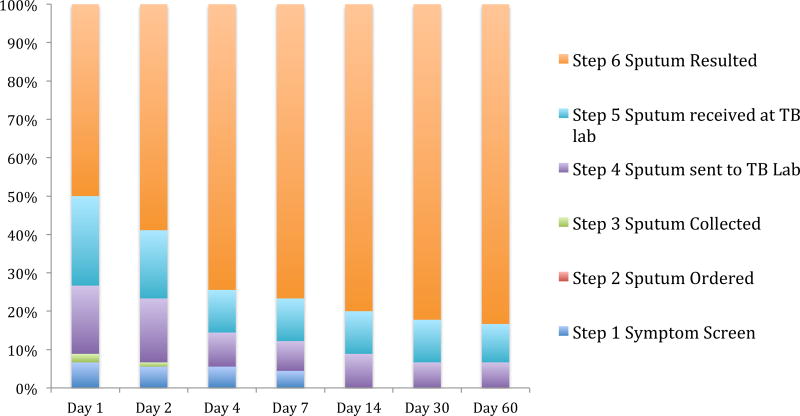
Figure 3 describes the completion of specific cascade steps over time. Of the 90 patients who had a sputum test ordered, 75 patients completed the diagnostic cascade. The cumulative incidence of cascade completion was 59% at two days and 74% at four days following positive symptom screening among those with a sputum order. The median time to complete the cascade was 1 day from initial clinic visit. Few patients completed the cascade after Day 4: 8/75 (11%) completed the cascade between Day 4 and Day 60. The fifteen (17%) patients who had not completed the cascade by 60 days post-screening visit never went on to complete the cascade.
Figure 3. Completion of TB diagnostic cascade over time of first sputum sample among newly enrolled patients at ISS Mbarara Clinic between March 1 2012 and Sept 30 2013 (n=90)1.
1Step 1: same day as enrollment visit, Step 2: within 14 days of enrollment visit, Steps 3–6: no limitation of time horizon
Factors associated with sputum order among symptom screen-positive patients
Cough was reported in nearly all patients (97%) with symptoms who had a sputum ordered within 14 days of enrollment visit compared to 66% of patients with symptoms who did not have sputum ordered. (Table 2) Other factors found to be associated with sputum collection in univariate analyses included: male sex, greater number of symptoms present, presence of night sweats, and higher WHO HIV disease stage. In a multivariate regression model including specific TB screening symptoms (and excluding number of symptoms), cough (RR (risk ratio): 8.80, 95% CI: 2.2– 35.9), night sweats (RR: 1.67, 95% CI: 1.2 – 2.3), and WHO Stage 3 (RR: 8.84, 95% CI:1.0 – 77.5) or Stage 4 (RR: 20.6, 95% CI: 2.9 – 146.2) HIV disease compared to WHO Stage 1 remained statistically significant. In a multivariate model including number of symptoms (and excluding specific TB screening symptoms), having three symptoms (RR: 2.28, 95% CI: 1.3– 4.1) compared to having only one symptom and WHO Stage 4 HIV disease (RR: 20.6, 95% CI: 2.8– 152.7) as compared to WHO Stage 1 HIV disease remained statistically significant. There was evidence for linear trend between sputum order and number of WHO symptoms (p=0.02).
Chart review of symptom screen-positive medical records from new and existing patients
There were 2067 new or follow-up HIV clinical visits during the study period in which a patient had a positive symptom screen but no sputum order was sent. Fifty charts were randomly selected from this group for additional in-depth review, of which 43 were able to be located for review. Of these 43 charts, 6 (14%) provided no indication for why a sputum had not been ordered. Of those charts where a reason for lack of sputum order could be identified (n=37, 86 %), the majority were due to presumptive treatment of an alternate diagnosis: malaria (19 (51%) (CI: 34%–68%) or bacterial infection 16 (43%) CI: 27%–61%). One (3%) patient had an active diagnosis of tuberculosis at the time of initial clinic visit.
TB Treatment Initiation
Of all the 12 smear positive patients in our cascade, 8 were initiated on TB treatment (median: 4 days from enrolment, range: 1–8 days) and ART (median: 51 days from enrollment; range: 21–139 days). Of the four patients not initiated on TB treatment, three were referred to TB clinic but were lost to follow up (LTFU) before registration at TB clinic and one was LTFU after registration.
Discussion
In a large HIV clinic in Uganda, we found uptake of symptom screening for TB to be high among patients enrolling in care. However, the majority of those who screened positive did not have a sputum order (87%). Factors associated with ordering a sputum test included more advanced HIV disease (by WHO stage) and a greater number of symptoms. In a random sample of charts reviewed for a deeper understanding of reasons sputum tests were not ordered, the presence of a presumed alternative diagnosis such as malaria or bacterial infections was common (81%). Only 50% of patients with a sputum order had a sputum test result within one day of their visit, however the majority (83%) of patients with a sputum order did complete the diagnostic cascade for the first sputum collected and did so within four days of their clinic visit. Few patients had a second sputum test.
While our study is comparable to previous studies of PLHIV that identified a drop-off between symptom screening and either sputum collection [6] or sputum result [7], we additionally evaluated the interim steps along the entire TB diagnostic cascade to more specifically pinpoint the location of these gaps. Our data show that the major drop-off in the TB diagnostic cascade was provider driven (rather than patient or laboratory driven) and occurred between symptom screening and sputum ordering. TB symptom screening at initial clinic visit was relatively complete (>90%) but a sputum test was not ordered for the vast majority of symptom screen positive patients (87%). Two studies among non-HIV populations, using alternate symptom screening strategies (cough > 2 weeks duration and an eight item checklist, respectively), similarly identified provider lack of ordering as the main gap in their diagnostic cascades [8, 9].
Our study uniquely evaluated the rate of completion of each step in the diagnostic cascade. Although many studies have measured the contribution of health system delays to the diagnosis of TB (median of 24 days (IQR: 8–36) in a systematic review), most report this delay as a broad category without identifying how each step in the TB diagnostic cascade contributes to overall health system delay [10, 11]. We found that the greatest time delays occurred in receipt and subsequent testing of the sputum specimen at the TB laboratory, with only 50% of sputum results obtained within one day. Few patients in our analysis had a second sputum test. Given clinic policy during the study period to request patients return for a second early morning sputum sample, the low frequency of second sputum collection is not surprising. Barriers for patient return to clinic for additional testing and/or results and inability to contact patients with a positive smear result have been cited as key drivers of delays in TB diagnosis and treatment initiation [12]. As a result, in 2011, WHO endorsed a recommendation for same-day diagnosis with two sputum specimens collected and examined on the same day of patient evaluation [13]. Thus, achieving same day “test and treat” appears to be hindered here both by specimen collection practices and testing delays.
The reasons for lack of provider sputum ordering are not clear and need to be evaluated further. Because provider lack of sputum ordering was a major gap identified in our study, we evaluated predictors of sputum ordering and conducted a medical record review among a subset of patients who did not have a sputum order. We found that patients who had a sputum order were more likely to be “sick” (WHO Stage 3 or 4 HIV disease and greater number of positive symptoms). Not only is TB incidence highest in WHO Stage 3 or 4 disease [14], pulmonary and extrapulmonary TB are also WHO stage disease-defining conditions. Therefore, limiting sputum ordering to this group would be rational only if the reason for selective sputum ordering is due to concern for resource limitations or if sputum testing is being used only to confirm empirical TB diagnoses. However, limiting the more intensified active TB case finding efforts to this group excludes Stage 1 and 2 patients who are still at high risk of TB. Additionally, prioritizing patients based on number of positive symptoms is problematic given that many studies do not show an increase in positive predictive value (PPV) with increasing number of positive symptoms. In other words, it does not make sense to prioritize testing among those with a greater number of symptoms as these patients are not necessarily more likely to have TB disease [2, 15]. In evaluating reasons for lack of sputum order, our medical record review showed that suspicion for malaria or bacterial infections were the most commonly identified reasons for not ordering a sputum. Whether these treatment decisions were appropriate or not is difficult to ascertain without knowing the smear status of all symptom screen positive patients in the study cohort. However, lack of clinical suspicion for TB and treatment with antibiotics has been associated with diagnostic delays in several other studies [10, 16, 17]. Previous qualitative work in Uganda suggests that in addition to lack of clinical suspicion for TB, barriers to diagnostic testing include lack of knowledge/training as well as lack of motivation due to limited health care worker time and discomfort working with TB patients and handling sputum specimens [18]. Further qualitative work is needed within our local context to understand provider behavior and reasons for lack of sputum ordering.
With improved knowledge of evidence-practice gaps and their determinants, targeted interventions can be designed for maximal impact. Authors of the afore-mentioned study propose several potential interventions including (a) facilitated workshops and on-going training on TB evaluation guidelines to target lack of knowledge, (b) peer coaching, opinion leaders, incentives, and feedback to target low motivation, (c) visual job aides in all workspaces to target self efficacy, and (d) task shifting to target lack of human resources. One relatively simple intervention would be to modify the existing initial clinical encounter form and explicitly note that WHO guidelines suggest that affirmative answer to any of the four symptom screening questions merits testing of sputum. Visual prompting may improve providers’ psychological capability to order sputa. Additionally modifications to clinic policies and processes to allow for same-day second sputum collection are also likely to have high impact in addressing the evidence-practice gap. Further evaluation within our local context is necessary to aid in selecting and prioritizing which interventions are likely to have the greatest impact.
Our study had several limitations. First, this is a single center study and while the approach to process evaluation is widely applicable, the specific results are not necessarily generalizable to other settings. In addition, the data on the utilization of chest radiography for evaluation of symptom screen positive patients were not available. We only evaluated completion of diagnostic activities at the patient’s enrollment clinic and we cannot exclude the possibility that some patients transferred care and completed their workup elsewhere. Finally, data on empiric TB treatment initiation in our cohort was not available.
We identified that the major gap in the TB diagnostic cascade at a large HIV clinic in Uganda was lack of provider ordering of sputum tests among screen positive patients. We were also able to highlight other key challenges to implementing a WHO recommended screening strategy including lack of same day testing and results. Process evaluations can provide important information about the magnitude and type of barriers encountered during implementation of evidence-based guidance and thereby allow for more directed interventions to improve quality of care. Further evaluation of provider behavior towards sputum ordering could improve active TB case detection rates.
Acknowledgments
Sources of support: NIH (P30 AI027763 and U01 AI069911)
Footnotes
Conflicts of interest: None
References
- 1.Getahun H, et al. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studies. PLoS Med. 2011;8(1):e1000391. doi: 10.1371/journal.pmed.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cain KP, et al. An algorithm for tuberculosis screening and diagnosis in people with HIV. N Engl J Med. 2010;362(8):707–16. doi: 10.1056/NEJMoa0907488. [DOI] [PubMed] [Google Scholar]
- 3.Getahun H, et al. Implementation of isoniazid preventive therapy for people living with HIV worldwide: barriers and solutions. Aids. 2010;24(Suppl 5):S57–65. doi: 10.1097/01.aids.0000391023.03037.1f. [DOI] [PubMed] [Google Scholar]
- 4.Lester R, et al. Barriers to implementation of isoniazid preventive therapy in HIV clinics: a qualitative study. AIDS. 2010;24(Suppl 5):S45–8. doi: 10.1097/01.aids.0000391021.18284.12. [DOI] [PubMed] [Google Scholar]
- 5.WHO. Guidelines on intensified tuberculosis case-finding and isoniazid preventive therapy for people with HIV in rsource constrained settings. WHO; Geneva, Switzerland: 2011. [Google Scholar]
- 6.Van Rie A, et al. High uptake of systematic HIV counseling and testing and TB symptom screening at a primary care clinic in South Africa. PLoS One. 2014;9(9):e105428. doi: 10.1371/journal.pone.0105428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burmen B MS, Cavanaugh JS, et al. 19th International AIDS Conference. Washington DC: 2012. Tuberculosis screening and management of HIV-positive patients with TB, Nyanza Province, Kenya, July 2009 – August 2010. [Google Scholar]
- 8.Bloss E, et al. Lessons learned during tuberculosis screening in public medical clinics in Francistown, Botswana. Int J Tuberc Lung Dis. 2012;16(8):1030–2. doi: 10.5588/ijtld.11.0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis J, et al. Evaluating tuberculosis case detection via real-time monitoring of tuberculosis diagnostic services. Am J Respir Crit Care Med. 2011;184(3):362–7. doi: 10.1164/rccm.201012-1984OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Storla DG, Yimer S, Bjune GA. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health. 2008;8:15. doi: 10.1186/1471-2458-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finnie RK, et al. Factors associated with patient and health care system delay in diagnosis and treatment for TB in sub-Saharan African countries with high burdens of TB and HIV. Trop Med Int Health. 2011;16(4):394–411. doi: 10.1111/j.1365-3156.2010.02718.x. [DOI] [PubMed] [Google Scholar]
- 12.Davis JL, et al. Test and treat: a new standard for smear-positive tuberculosis. J Acquir Immune Defic Syndr. 2012;61(1):e6–8. doi: 10.1097/QAI.0b013e3182614bc5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO. Same-day diagnosis of tuberculosis by microscopy: policy statement. WHO; Geneva, Switzerland: 2011. [PubMed] [Google Scholar]
- 14.Lawn SD, Badri M, Wood R. Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a South African cohort. AIDS. 2005;19(18):2109–16. doi: 10.1097/01.aids.0000194808.20035.c1. [DOI] [PubMed] [Google Scholar]
- 15.Corbett EL, et al. Provider-initiated symptom screening for tuberculosis in Zimbabwe: diagnostic value and the effect of HIV status. Bull World Health Organ. 2010;88(1):13–21. doi: 10.2471/BLT.08.055467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salaniponi FM, et al. Care seeking behaviour and diagnostic processes in patients with smear-positive pulmonary tuberculosis in Malawi. Int J Tuberc Lung Dis. 2000;4(4):327–32. [PubMed] [Google Scholar]
- 17.Harries AD, et al. Defining and assessing the maximum number of visits patients should make to a health facility to obtain a diagnosis of pulmonary tuberculosis. Int J Tuberc Lung Dis. 2003;7(10):953–8. [PubMed] [Google Scholar]
- 18.Cattamanchi A, et al. Health worker perspectives on barriers to delivery of routine tuberculosis diagnostic evaluation services in Uganda: a qualitative study to guide clinic-based interventions. BMC Health Serv Res. 2015;15:10. doi: 10.1186/s12913-014-0668-0. [DOI] [PMC free article] [PubMed] [Google Scholar]