Abstract
Background
Imprime PGG is an innate immune cell modulator that primes neutrophils and monocytes/macrophages to exert anti-tumor activity against complement opsonized tumor cells. In patients with KRAS-mutant colorectal cancer (CRC), cetuximab alone is ineffective; however, it can bind to tumor cells and induce opsonization for recognition by Imprime PGG-bound innate immune cells. Primary objective of this study was to determine the antitumor activity of Imprime PGG plus cetuximab in patients with KRAS-mutant metastatic CRC.
Patients and Methods
The study had a two-stage Simon’s optimal design with 80% power to detect a target objective response rate (ORR) of ≥10% at a 10% significance level. Patients received weekly Imprime PGG (4 mg/kg) and cetuximab (loading dose 400 mg/m2, then 250 mg/m2) intravenously. The primary endpoint was ORR; secondary endpoints included duration of response (DOR), time to progression (TTP), overall survival (OS), disease control rate (DCR), progression free survival (PFS) and safety. Stage 1 of the study was to enroll 17 evaluable patients.
Results
One partial response (5.6%) was observed among 18 patients enrolled into Stage 1. Median DOR was 4.2 months, TTP 2.7 months, and OS 6.6 months. Overall, observed toxicity was as expected from cetuximab alone. The most common (≥20%) adverse events related to Imprime PGG were fatigue (38.9%), infusion reaction (22.2%), and headache (22.2%). There was no grade 4 toxicity nor treatment-related deaths.
Conclusion
Imprime PGG combined with cetuximab in patients with KRAS-mutant CRC showed compelling, albeit modest, clinical activity. This study provides proof of principle that Imprime PGG, in combination with complement-activating Antibodies (Abs), is associated with clinical activity.
Keywords: Beta-glucan, immune, antibody, epidermal growth factor receptor, complement
INTRODUCTION
Colorectal cancer (CRC) accounts for approximately 143,000 new cases and 50,000 deaths per year in the United States.1 The treatment of CRC depends on the stage of the disease. For patients with metastatic CRC, treatment includes systemic chemotherapy with 5-fluorouracil, capecitabine, irinotecan, oxaliplatin, and recently regorafenib and ziv-aflibercept. In addition, three monoclonal antibodies (MAbs), bevacizumab, that inhibits Vascular Endothelial Growth Factor (VEGF), and cetuximab and panitumumab, that inhibit the Epidermal Growth Factor Receptor (EGFR) pathway, are used for treatment of CRC. While cetuximab and panitumumab are effective in patients whose tumors have wild type RAS, there is no clinical benefit from either anti-EGFR MAb in patients whose tumors harbor mutant RAS, including KRAS which defines the sub-population in this study that comprise 30–45% of CRC.2,3,4
In contrast to cytotoxic chemotherapies and inhibitors of either the VEGF or EGFR pathways, novel strategies under development are being designed to induce the immune system to target CRC. Anti-tumor immunity classically consists of adaptive immunity, including T-cells, and innate immunity, including neutrophils, monocytes/ macrophages and natural killer (NK) cells. Imprime PGG is an innate immune modulator being developed for the treatment of cancer in combination with complement-activating, tumor-targeting MAb therapy.
Imprime PGG is a soluble, beta-1,3/1,6 glucan isolated from the cell wall of a proprietary strain of yeast (Saccharomyces cerevisiae). Beta-1,3/1,6 glucan is a fungal pathogen-associated molecular pattern (PAMP), and as such, its recognition by innate immune cells is central to antifungal immunity. 5 Beta-1,3/1,6 glucan has been shown to bind complement receptor 3 (CR3) on innate immune cells, priming them to exert cytotoxic activity against opsonized yeast;6 cytotoxic activity of beta-1,3/1–6 glucan-bound innate immune cells has also been demonstrated against opsonized mammalian cell targets, including tumor cells.7,8 These effects have been shown to be mediated via innate immune cells (neutrophils and monocytes/macrophages) and a mechanism involving CR3 and complement. Anti-tumor effects are not observed 1) in mice depleted of innate myeloid cells, including neutrophils9, 2) in knock-out mice that do not express CR3 on their innate immune cells10, and 3) in knock-out mice deficient in complement (C3).9 Co-administration of the complement-activating, tumor-targeting MAb has been shown to be important not only for inducing iC3b opsonization of the tumor, but also for facilitating the production of C5a which attracts innate immune cells to the tumor microenvironment.11 Thus, it is proposed that Imprime PGG-bound innate immune cells migrate to the tumor microenvironment as a result of a chemo-attractant gradient, and once there, engage iC3b opsonized tumor cells and exert cytotoxic activity. Additionally, recent data support the potential of Imprime PGG to not only affect the innate immune system, but also to orchestrate a coordinated anti-tumor response involving the adaptive immune system. In vitro studies with human cells have shown that Imprime PGG also modulates polarization of monocyte-derived macrophages and enhances maturation of dendritic cells leading to increased antigen presentation to adaptive immune cells with expansion of CD4+ and CD8+ T cells, increased production of the potent anti-tumor cytokine interferon gamma (IFN-γ), and upregulation of PDL1 on tumor cells.12,13
Cetuximab is an IgG1 MAb directed at blocking EGFR signaling. However, it is also capable of activating complement, resulting in iC3b deposition on the surface of EGFR-expressing tumor cells as well as local release of chemo-attractants, such as C5a.14 Thus, although cetuximab would be ineffective at inhibiting EGFR-mediated signal transduction in RAS-mutant tumors, it should still be capable of binding EGFR to activate complement. There is also no correlation between RAS mutation status and EGFR expression. 15 Based on this, and the mechanism of action of Imprime PGG, this proof of concept study was designed to test an immunological approach to the treatment of CRC.
MATERIALS AND METHODS
Eligibility Criteria
Eligible patients were adults with histologically or cytologically confirmed metastatic colon or rectal cancer with known KRAS mutation and measurable disease, who failed previous irinotecan- and oxaliplatin-containing regimens in either adjuvant or metastatic settings or were intolerant to irinotecan-based therapies. Eligible patients had an Eastern Cooperative Oncology Group (ECOG) performance status of ≤1 and had adequate bone marrow, renal, and hepatic function. This study was conducted according to the Declaration of Helsinki and with approval from Institutional Review Boards of each participating study site. All participants provided written informed consent before participating.
Study Design
The clinical trial was a Simon optimal two-stage, open-label, single arm study.16 Seventeen patients were planned to be enrolled in Stage 1. If no objective tumor responses (partial response [PR] or complete response [CR]) were observed in the first 17 treated patients, then patient enrollment would be terminated. If at least one objective tumor response was observed in the first 17 treated patients, after these patients had completed at least one cycle of therapy, then the study was to be expanded to enroll a total of 56 treated patients. At the end of the study, if 2 or less objective tumor responses were observed, then the hypothesis of true response rate of 10% would be rejected. If at the end of the study ≥ 3 objective tumor responses were observed, then further investigation of the study drug in this patient population would be considered.
All patients received Imprime PGG weekly at 4 mg/kg followed by cetuximab weekly via intravenous (i.v.) infusion. Imprime PGG was dosed first, followed by cetuximab, so any safety events that were associated with dosing could be captured. The initial dose of cetuximab was 400 mg/m2 on Cycle 1/Day 1 and subsequent doses of cetuximab were 250 mg/m2 in accordance with the Cetuximab prescribing information. An individual cycle of therapy was defined as a 6-week period; treatment was administered on Day 1 of each week. Patients were dosed until disease progression or discontinuation from the study for other reasons (e.g., safety, non-compliance).
Assessments
Safety
Safety assessments included history and physical examinations, vital signs, ECOG performance status, adverse events (AEs), blood chemistry, complete blood counts with differential, and urinalysis. Safety assessments were performed at screening, and then weekly at each visit. AE severity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), version 3.0. Relationships of adverse events to cetuximab or Imprime PGG (definitely, probably, possibly, unlikely, or unrelated) were assessed by the Principal Investigator at each site.
Serum samples were collected and banked. Markers such as cytokines, chemokines, components of the complement pathway or other relevant biologically active markers that may be identified as new information becomes available through preclinical research and published studies will be studied.
Efficacy
Tumor response was assessed using RECIST version 1.0 every 6-weeks.
Statistical Analysis
All patients enrolled were included in clinical outcome and safety analyses. Both efficacy and safety analyses included all subjects who received at least one dose of study drug.
Descriptive statistics were used to summarize demographic characteristics, efficacy response, and safety data. Categorical data were presented by N and % for each category and continuous data were presented by mean, standard deviation, median, minimum, and maximum. Kaplan-Meier estimates were utilized for time-to-event analysis. The ORR was calculated as the proportion of patients demonstrating a confirmed CR or PR at any time on study. The DCR was calculated as the proportion of patients demonstrating CR, PR, or stable disease (SD). DOR, duration of SD, TTP, PFS, and OS were computed using Kaplan-Meier methodology and 95% confidence intervals (CI) were provided. Duration of disease control was defined as the time at which criteria were met for CR, PR or SD (whichever status was recorded first) until the first date on which recurrence or PD was objectively documented, taking as reference for PD the smallest measurements recorded previously (per RECIST). Duration of SD was defined as the time from the date of the first dose of study drug to the date the criteria for disease progression were met, with a minimum time interval for the duration of SD being 8 weeks. Subjects who did not progress were censored at their last tumor assessment. TTP was defined as the time from the date of the first dose of study drug to the date of documented tumor progression and was censored on the date of death from any cause for patients who died without documented tumor progression, and at the last known contact date for patients who were lost to follow-up or who were alive at the time of analysis. PFS was defined as the time from the date of the first dose of study drug until the first date on which recurrence or progression, or death due to any cause was documented, and was censored at the date of the last contact for patients who were lost to follow-up or who were alive at the time of analysis. OS was defined as the time from the date of the first dose of study drug until the date of death of the patient due to any cause, and was censored at the date of the last contact for patients who were alive or lost to follow-up at the time of analysis. For safety analyses, the proportion of patients experiencing adverse events, serious adverse events and drug toxicities were summarized by system organ class (SOC) using the Medical Dictionary for Regulatory Activities (MedDRA) version 12.0.
RESULTS
Patients
A total of 18 patients were enrolled in Stage 1 of the study from June to August 2009. Enrollment was 18 instead of the initially planned 17 because in retrospect, a patient classified as non-evaluable was subsequently re-adjudicated to be evaluable. All 18 patients were included in the analyses. The majority of patients had ECOG performance status 1. All patients had previously failed two or more prior chemotherapeutic regimens. A summary of patient demographics is shown in Table 1. All patients were started at the planned dose and schedule. The primary reason for study discontinuation was radiographic disease progression (n=14, 77.8%). Four (4; 22.2%) patients discontinued due to clinical disease progression, and were analyzed using date of death as no date of progression was available. No patients discontinued due to treatment-related adverse events. The mean (range) number of treatment cycles was 2.2 (1 – 11) and the mean (range) treatment duration was 2.8 months (0.3 –16 months). Stage 2 of the study was not initiated.
Table 1.
Patient Demographics
| Demographic category | n=18 | |
|---|---|---|
| Age, mean (range) | 58.2 (39–78) | |
| Gender: Male, n (%) | 11 (61.1) | |
| Primary: Colon Cancer, n (%) | 13 (72.2) | |
| Rectal Cancer, n (%) | 5 (27.8) | |
| Prior CRC regimens, median (range) | 2 (2–3) | |
| ECOG PS, n (%) | 0 | 5 (27.8) |
| 1 | 13 (72.2) |
ECOG PS= Eastern Cooperative Oncology Group Performance Status
Safety and Tolerability
Imprime PGG-related AEs of any grade that occurred in more than 10% of patients are summarized in Table 2. Grade 3 Imprime PGG-related AEs that occurred in any number of patients are summarized in Table 3. No grade 3 AEs were observed in more than 1 patient and no grade 4 AEs were observed in any patient. No patients had AEs that led to death during the study and no deaths were attributed to Imprime PGG. Cetuximab-related AEs were consistent with those expected from cetuximab alone. The most common adverse events related to Imprime PGG were fatigue (38.9%), infusion related reaction (22.2%), and headache (22.2%). Infusion reactions were noted early within the Imprime PGG program that were temporally related to Imprime PGG dosing. Dosing instructions were then modified to add recommended pre-medication with antihistamines and steroids, decreasing the frequency and severity of reactions. Cetuximab dose adjustments occurred in 4 (22.2%) patients due to fingernail bed desquamation in 1 (5.6%) patient and weight decrease in 3 (16.7%) patients. No patients discontinued due to treatment-related AEs. No clinically significant trends were noted in laboratory values, vital signs, or physical examinations over time. All patients discontinued therapy due to disease progression.
Table 2.
Any grade Imprime PGG-related adverse events occurring in ≥10% patients
| Adverse events | n (%) |
|---|---|
| Fatigue | 7 (38.9) |
| Headache | 4 (22.2) |
| Infusion related reaction | 4 (22.2) |
| Chills | 3 (16.7) |
| Diarrhea | 3 (16.7) |
| Hypomagnesaemia | 3 (16.7) |
| Abdominal pain | 2 (11.1) |
| Elevated alanine aminotransferase | 2 (11.1) |
| Elevated aspartate aminotransferase | 2 (11.1) |
| Asthenia | 2 (11.1) |
| Mucosal inflammation | 2 (11.1) |
| Nausea | 2 (11.1) |
| Stomatitis | 2 (11.1) |
| Tachycardia | 2 (11.1) |
| Vomiting | 2 (11.1) |
AEs reported by the investigator as possibly, probably, or definitely related to Imprime PGG are included.
Table 3.
Grade 3 Imprime PGG-related adverse events
| Adverse event | n (%) |
|---|---|
| Elevated alkaline phosphatase | 1 (5.6) |
| Hypophosphatemia | 1 (5.6) |
| Infection (respiratory) | 1 (5.6) |
| Exfoliative rash | 1 (5.6) |
| Aseptic meningitis | 1 (5.6) |
AEs possibly, probably, or definitely-related are included.
Antitumor activity
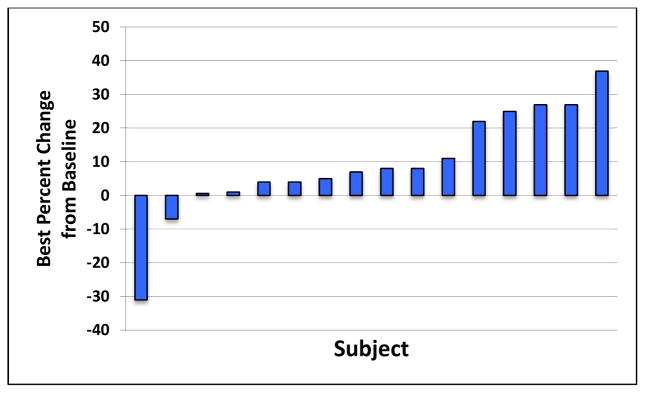
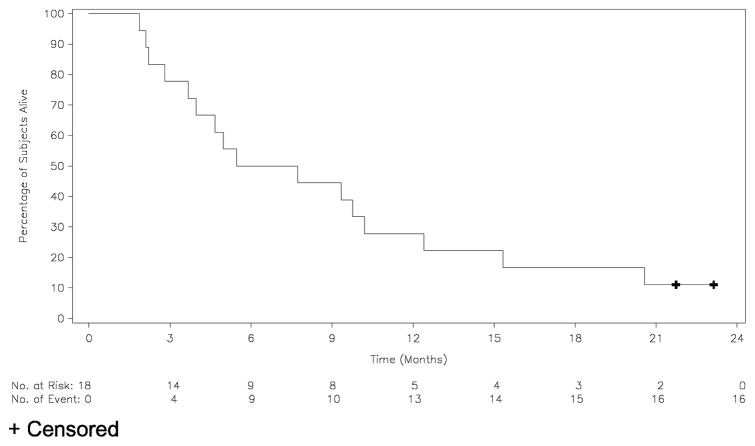
A waterfall plot representing change from baseline in the sum of the longest diameters of identified target lesions is shown in Figure 1. One confirmed partial response (PR) was observed (5.6%) in a patient with G12S KRAS mutation. Nine patients (50%) demonstrated stable disease (SD) as best response. Ten patients (55.6%) experienced disease control (CR, PR, or SD). The duration of tumor response for the patient with PR was 4.2 months. The median duration of SD was 2.8 months (0.4 – 7.2 months). The median duration of disease control was 1.4 months (0.0 – 14.9 months). The median PFS and TTP were 2.2 months (1.2 – 16.2 months) and 2.7 months (1.2 – 16.2 months), respectively. The median duration of overall survival was 6.6 months (1.9 – 23.1 months) (Table 4), with two patients still surviving at study closeout (22.9 and 21.5 months, respectively). Survival is presented in the Kaplan-Meier survival curve shown in Figure 2. The 1-year overall survival rate was 27.8%.
Figure 1. Waterfall plot of best fractional change in tumor size relative to baseline.
The best tumor size percentage change from baseline is defined as the maximal reduction or minimal increase in sum of longest dimensions of target lesions relative to pretreatment assessment. No post baseline data available for two patients.
Table 4.
Efficacy data summary
| Efficacy endpoint | Patients (n=18) |
|---|---|
| ORR, % (95% CI) | 5.6 (0.1, 27.3) |
| DCR, % (95% CI) | 55.6 (30.8, 78.5) |
| Best response, n (%) | |
| CR | 0 (0) |
| PR | 1 (5.6) |
| SD | 9 (50.0) |
| Duration of tumor response, mo | 4.2 |
| Duration of SD, mo (range) (95% CI) | 2.8 (0.4, 7.2) (2.7–5.8) |
| Median TTP, mo (range) (95% CI) | 2.7 (1.2 – 16.2) (1.8, 7.2) |
| Median PFS, mo (range) (95% CI) | 2.2 (1.2 – 16.2) (1.8, 2.8) |
| Median OS, mo (range) (95% CI) | 6.6 (1.9 – 23.1) (4.0, 10.2) |
| Survival at 1 Year (%) | 27.8 |
ORR=overall response rate; DCR=disease control rate; CR=complete response; PR=partial response; SD=stable disease; TTP=time to tumor progression; PFS=progression-free survival; OS=overall survival; mo=months; CI=confidence interval
Figure 2.
Kaplan-Meier Survival Curve
DISCUSSION
The primary objective of the study was to assess clinical efficacy of the combination of Imprime PGG with cetuximab in patients with Stage IV KRAS-mutant metastatic colorectal cancer. In the previous CO.17 study of cetuximab monotherapy in CRC that included KRAS-mutant patients, the median PFS in the KRAS-mutant group was 1.8 months, and the median overall survival was 4.5 months 17. Monotherapy studies of the anti-EGFR antibody panitumumab in CRC included KRAS-mutant patients, and the reported median PFS and OS in the KRAS-mutant group was 7.4 weeks and 22.2 weeks, respectively, in one study 18 and, 7.4 weeks and 4.5 months, respectively, in a second study. 19 Panitumumab and cetuximab have comparable single agent efficacy, 20 therefore, these results were combined for the purposes of this discussion. Recognizing the limitations of these cross study comparisons, our study compared favorably across clinical endpoints. Treatment with Imprime PGG and cetuximab resulted in a median PFS of 2.2 months (27% increase vs. historical reference) and median overall survival of 6.6 months (40% increase vs. historical reference).
Furthermore, treatment with the combination of Imprime PGG weekly at 4 mg/kg in combination with standard-dose cetuximab resulted in a confirmed partial response in 1 patient that was not attributed to cetuximab due the presence of a codon 12 KRAS (Gly12Ser) mutation (and specifically not the KRAS G13D mutation). 21 This observation of a response was proof of principle of the potential activity of this approach.
Antibody-dependent cell-mediated cytotoxicity (ADCC) mediated via the innate immune system is effected via two sequential mechanisms; 1) NK induced target cell cytolysis via release of perforin and granzyme followed by 2) death receptor ligand induced apoptosis.22 Although CD95L mediated ADCC is blocked by mutant KRAS, the former mechanism is KRAS independent. Therefore, combining Imprime PGG with enhancement of perforin/granzyme production (e.g., CD137 agonists23) is a reasonable area of exploration.
A further objective of this study was to assess safety of the combination of cetuximab plus Imprime PGG. As anticipated, the combination was well tolerated with no clinically significant safety concerns. Infusion-related reactions observed early in the Imprime PGG administration were addressed via a protocol amendment, which clarified premedication recommendations with antihistamines and steroids. The typical cetuximab-related AEs were observed as expected. Grade 3 Imprime PGG-related AEs were rare and none occurred in more than one patient. There were no grade 4 AEs.
Given the observed clinical benefit in PFS and OS compared to historical controls in this clinical trial and based on data from another phase 2 clinical trial in colorectal cancer, a decision was made to proceed with a Phase 3 study in patients with KRAS-wild type metastatic CRC (NCT01309126; PRIMUS), in which patients are randomized to receive either Imprime PGG and cetuximab or cetuximab alone. This study is currently ongoing.
CONCLUSION
The objective response of a CRC patient with a KRAS codon 12 mutation to the combination of cetuximab plus Imprime PGG and the encouraging survival data in this Phase 2 study provide evidence that CRC can be targeted by the immune system and support further development of Imprime PGG plus complement-activating antibodies in the treatment of cancer.
Clinical practice points.
Imprime PGG, a yeast-derived beta-glucan polymer, induces complement receptor 3-dependent cell-mediated anti-tumor activity when administered with a complement-activating antibody, such as cetuximab.
This is the first clinical study to combine Imprime PGG and cetuximab in KRAS-mutant metastatic CRC.
The combination is safe and demonstrated activity in a patient with a KRAS codon 12 mutation.
This study shows that the immune system can be harnessed to target CRC and is proof of principle that Imprime PGG, with complement-activating Abs, can result in clinical activity.
Further studies of Imprime PGG plus cetuximab in CRC are ongoing.
Acknowledgments
This trial was sponsored by Biothera.
Footnotes
- Segal NH, Gada P, and Senzer N: No financial disclosures.
- Saltz LB: Since study completion is an uncompensated member of sponsor’s Clinical Advisory Board.
- Patchen ML and Gargano MA: Employees of the study sponsor and have Biothera stock options and/or grants.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: a cancer journal for clinicians. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Cutsem EV, Lang I, Folprecht G, et al. Cetuximab plus FOLFIRI in the treatment of metastatic colorectal cancer (mCRC): The influence of KRAS and BRAF biomarkers on outcome: Updated data from the CRYSTAL trial. ASCO Gastrointestinal Cancers Symposium; 2010. [Google Scholar]
- 3.De Roock W, De Schutter J, De Hertogh G, et al. KRAS mutations preclude tumor shrinkage of colorectal cancers treated with cetuximab. J Clin Oncol (Meeting Abstracts) 2007;25:4132. [Google Scholar]
- 4.Bokemeyer C, Kohne CH, Ciardiello F, et al. FOLFOX4 plus cetuximab treatment and RAS mutations in colorectal cancer. Eur J Cancer. 2015;51:1243–52. doi: 10.1016/j.ejca.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown GD, Gordon S. Immune recognition of fungal beta-glucans. Cellular microbiology. 2005;7:471–9. doi: 10.1111/j.1462-5822.2005.00505.x. [DOI] [PubMed] [Google Scholar]
- 6.Cain JA, Newman SL, Ross GD. Role of complement receptor type three and serum opsonins in the neutrophil response to yeast. Complement. 1987;4:75–86. doi: 10.1159/000463011. [DOI] [PubMed] [Google Scholar]
- 7.Vetvicka V, Thornton BP, Ross GD. Soluble beta-glucan polysaccharide binding to the lectin site of neutrophil or natural killer cell complement receptor type 3 (CD11b/CD18) generates a primed state of the receptor capable of mediating cytotoxicity of iC3b-opsonized target cells. The Journal of clinical investigation. 1996;98:50–61. doi: 10.1172/JCI118777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xia Y, Vetvicka V, Yan J, Hanikyrova M, Mayadas T, Ross GD. The beta-glucan-binding lectin site of mouse CR3 (CD11b/CD18) and its function in generating a primed state of the receptor that mediates cytotoxic activation in response to iC3b-opsonized target cells. J Immunol. 1999;162:2281–90. [PubMed] [Google Scholar]
- 9.Qi C, Cai Y, Gunn L, et al. Differential pathways regulating innate and adaptive antitumor immune responses by particulate and soluble yeast-derived beta-glucans. Blood. 2011;117:6825–36. doi: 10.1182/blood-2011-02-339812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li B, Allendorf DJ, Hansen R, et al. Yeast beta-glucan amplifies phagocyte killing of iC3bopsonized tumor cells via complement receptor 3-Syk-phosphatidylinositol 3-kinase pathway. J Immunol. 2006;177:1661–9. doi: 10.4049/jimmunol.177.3.1661. [DOI] [PubMed] [Google Scholar]
- 11.Li B, Allendorf DJ, Hansen R, et al. Combined yeast {beta}-glucan and antitumor monoclonal antibody therapy requires C5a-mediated neutrophil chemotaxis via regulation of decay-accelerating factor CD55. Cancer research. 2007;67:7421–30. doi: 10.1158/0008-5472.CAN-07-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan AS, Qiu X, Jonas A, Kangas T, Ottoson NR, Bose N. Imprime PGG modulates the function of monocyte-derived M2 macrophages and dendritic cells to drive T-cell expansion. Annual Meeting of the American Association of Cancer Research; 2015; Philadelphia. [Google Scholar]
- 13.Bose N, Chan AS, Jonas A, Qiu X, Ottoson NR. Imprime PGG treatment elicits a coordinated antitumor immune response that triggers enhanced expression of PD-L1 on tumor cells as well as monocyte-derived macrophages and dendritic cells. Annual Meeting of the American Association of Cancer Research; 2015; Philadelphia, PA. [Google Scholar]
- 14.Hsu YF, Ajona D, Corrales L, et al. Complement activation mediates cetuximab inhibition of non-small cell lung cancer tumor growth in vivo. Molecular cancer. 2010;9:139. doi: 10.1186/1476-4598-9-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milano G, Etienne-Grimaldi MC, Dahan L, et al. Epidermal growth factor receptor (EGFR) status and K-Ras mutations in colorectal cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2008;19:2033–8. doi: 10.1093/annonc/mdn416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen TT, Ng TH. Optimal flexible designs in phase II clinical trials. Statistics in medicine. 1998;17:2301–12. doi: 10.1002/(sici)1097-0258(19981030)17:20<2301::aid-sim927>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 17.Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. The New England journal of medicine. 2008;359:1757–65. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 18.Freeman DJ, Juan T, Reiner M, et al. Association of K-ras Mutational Status and Clinical Outcomes in Patients with Metastatic Colorectal Cancer Receiving Panitumumab Alone. Clinical Colorectal Cancer. 2008;7:184–90. doi: 10.3816/CCC.2008.n.024. [DOI] [PubMed] [Google Scholar]
- 19.Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:1626–34. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 20.Price TJ, Peeters M, Kim T, Li J, Cascinu S, Ruff P. ASPECCT: a randomized, multicenter, open-label, phase 3 study of panitumumab (pmab) vs cetuximab (cmab) for previously treated wild-type (WT) KRAS metastatic colorectal cancer (mCRC). European Cancer Congress; 2013; Amsterdam, The Netherlands. [Google Scholar]
- 21.Mao C, Huang YF, Yang ZY, Zheng DY, Chen JZ, Tang JL. KRAS p.G13D mutation and codon 12 mutations are not created equal in predicting clinical outcomes of cetuximab in metastatic colorectal cancer: a systematic review and meta-analysis. Cancer. 2013;119:714–21. doi: 10.1002/cncr.27804. [DOI] [PubMed] [Google Scholar]
- 22.Nakadate Y, Kodera Y, Kitamura Y, et al. KRAS mutation confers resistance to antibody-dependent cellular cytotoxicity of cetuximab against human colorectal cancer cells. Int J Cancer. 2013;134:2146–2155. doi: 10.1002/ijc.28550. [DOI] [PubMed] [Google Scholar]
- 23.Kohrt HE, Colevas AD, Houot R, et al. CD137 Activation Augments the Efficacy of EGFR-Targeted Therapy. J Clin Invest. 2014;124:2668–82. doi: 10.1172/JCI73014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]