SUMMARY
Pairing and synapsis of homologous chromosomes during meiosis is crucial for producing genetically normal gametes, and is dependent upon repair of SPO11-induced double stranded breaks (DSBs) by homologous recombination. To prevent transmission of genetic defects, diverse organisms have evolved mechanisms to eliminate meiocytes containing unrepaired DSBs or unsynapsed chromosomes. Here, we show that the CHK2 (CHEK2)-dependent DNA damage checkpoint culls not only recombination-defective mouse oocytes, but also SPO11-deficient oocytes that are severely defective in homolog synapsis. The checkpoint is triggered in those oocytes that accumulate a threshold level spontaneous DSBs (~10) in late Prophase I, the repair of which is inhibited by presence of HORMAD1/2 on unsynapsed chromosome axes. Furthermore, Hormad2 deletion rescued fertility of oocytes containing a synapsis-proficient, DSB repair-defective mutation in a gene (Trip13) required for removal of HORMADs from synapsed chromosomes, suggesting that many meiotic DSBs are normally repaired by intersister recombination in mice.
INTRODUCTION
Genome maintenance in germ cells is critical for fertility, prevention of birth defects, and the genetic stability of species. Throughout mammalian germ lineage development, from primordial germ cells (PGCs) through completion of meiosis, there are mechanisms that prevent transmission of gametes with genetic defects. Indeed, mutation rates in germ cells are far lower than in somatic cells (Conrad et al., 2011; Murphey et al., 2013; Stambrook and Tichy, 2010). This is reflected by the exquisite sensitivity of PGCs to mutations in certain DNA repair genes (Agoulnik et al., 2002; Luo et al., 2014; Nadler and Braun, 2000) (Watanabe et al., 2013), resting oocytes to clastogens such as radiation and chemotherapeutics (Maltaris et al., 2007; Perez et al., 1997; Suh et al., 2006), and developing prophase I meiocytes to genetic anomalies including a modicum of DNA damage (Meirow and Nugent, 2001; Suh et al., 2006) or the presence of a single asynapsed chromosome or even a chromosomal subregion (Burgoyne and Baker, 1985; Homolka et al., 2012).
Genetic and developmental analyses of mouse mutants have suggested there are at least two distinct checkpoints during meiotic prophase I in oocytes, one that monitors DSB repair, and another that monitors synapsis. Oocytes defective for either synapsis or DSB repair are eliminated with different dynamics and severity. Females with mutations causing pervasive asynapsis alone (e.g. Spo11−/−) are born with a grossly reduced oocyte pool. The surviving oocytes undergo folliculogenesis but are reproductively inviable, becoming exhausted within a few weeks by atresia and ovulation (Di Giacomo et al., 2005). Oocytes defective in DSB repair alone (Trip13Gt/Gt), or defective in both synapsis and meiotic DSB repair (e.g. Dmc1−/−; Msh5−/−), are virtually completely eliminated between late gestation and wean age by the action of a DNA damage checkpoint (Di Giacomo et al., 2005; Li and Schimenti, 2007). Furthermore, genetic ablation of meiotic DSB formation confers a Spo11−/− -like phenotype to such DSB repair mutants, consistent with the existence of separate DNA damage and synapsis checkpoints (Di Giacomo et al., 2005; Finsterbusch et al., 2016; Li and Schimenti, 2007; Reinholdt and Schimenti, 2005). For DSB repair, CHK2 (checkpoint kinase 2) signaling to TRP53/TAp63 is crucial for eliminating Trip13Gt/Gt mutant oocytes that exhibit full chromosome synapsis but have unrepaired SPO11-induced DSBs (Bolcun-Filas et al., 2014). Interestingly, Chk2 deficiency imparted a Spo11 null-like phenotype upon Dmc1−/− ovaries, consistent with separate, sequentially-acting checkpoints (Bolcun-Filas et al., 2014). Genetic evidence for a distinct synapsis checkpoint came from studies of mice lacking HORMAD1 or HORMAD2, proteins which load onto axes of meiotic chromosomes throughout early prophase I, but are removed upon synapsis (Wojtasz et al., 2009). Ablation of either in mice prevented loss of SPO11-deficient oocytes, resulting in the persistence of a [nonfertile] primordial follicle reserve in adults (Daniel et al., 2011; Kogo et al., 2012a; Wojtasz et al., 2012). These data suggested that the HORMADs are components of a synapsis checkpoint pathway. Another mechanism for elimination of oocytes is related to the phenomenon of MSUC (meiotic silencing of unsynapsed chromatin). Though not formally a checkpoint, the transcriptional inactivation of a chromosome containing genes essential for oocyte survival and development can block progression past diplonema (Cloutier et al., 2015).
Whereas these lines of evidence support the existence of separate checkpoints monitoring DNA damage and synapsis, studies in non-mammalian organisms indicate that the “pachytene checkpoint” – a term referring to delayed progression of meiosis or death of meiocytes triggered by genetic aberrations present in late pachynema – is more complex, consisting of both distinct and overlapping signaling pathways that also impact DNA repair modalities such as choice of recombination partner for the repair of meiotic DSBs (e.g. sister chromatid vs. homolog) (Joshi et al., 2015; MacQueen and Hochwagen, 2011; Roeder and Bailis, 2000; Subramanian and Hochwagen, 2014). Here, we report the results of a series of experiments designed to discriminate whether the pachytene checkpoint in mouse oocytes indeed consists of distinct pathways responding to different signals, or if the responses are integrated into a single checkpoint pathway. Using a variety of mouse mutants, we show that most oocytes which are highly defective for chromosome synapsis accumulate spontaneous DSBs at a level that can trigger the CHK2-dependent DNA damage signaling pathway, leading to their elimination. Additionally, we present evidence that the reason asynaptic Spo11−/− oocytes can be rescued by HORMAD1/2 deficiency is that their absence disrupts the so-called barrier to sister chromatid recombination (BSCR), enabling intersister (IS) repair of those spontaneous DSBs. Taken together, we propose that the “pachytene checkpoint” consists primarily of a canonical DNA damage signaling pathway, and that extensive asynapsis leads to oocyte loss by inhibiting HR repair rather than triggering a distinct “synapsis checkpoint.”
RESULTS
CHK2 is involved in the elimination of Spo11−/− oocytes
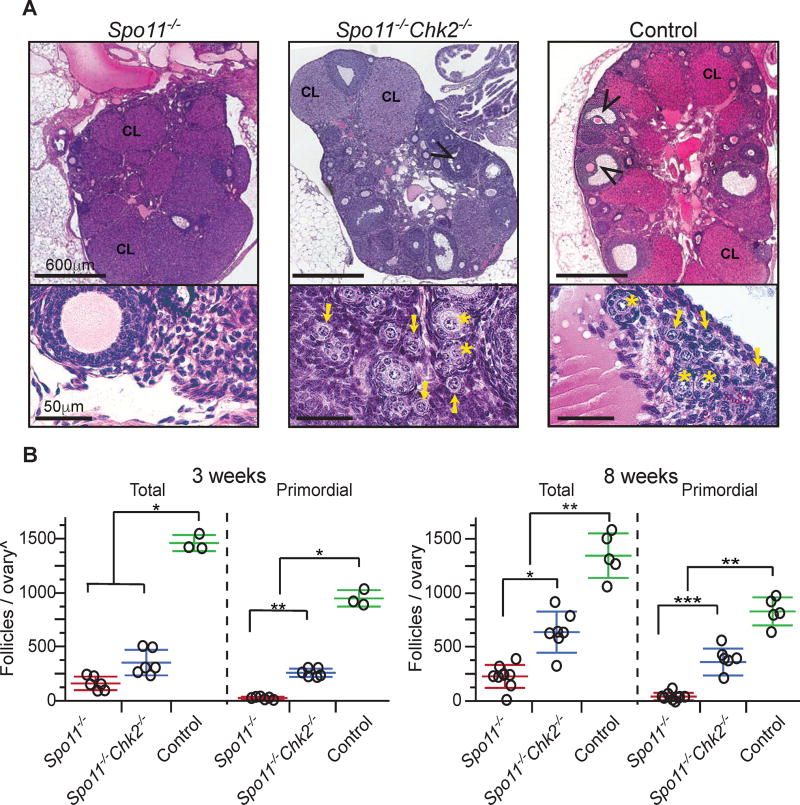
To investigate potential overlap in the meiotic DSB repair and synapsis checkpoint pathways in mice, we tested whether CHK2, a well-defined DSB signal transducer, contributes to the elimination of Spo11−/− oocytes that are asynaptic due to lack of programmed meiotic DSBs needed for recombination-driven homolog pairing. Consistent with prior reports (Baudat et al., 2000; Di Giacomo et al., 2005), we observed a greatly reduced number of total follicles in 3 week postpartum (pp) Spo11−/− ovaries compared to WT, and in particular, the oocyte reserve (pool of primordial resting follicles) was almost completely exhausted by 8 weeks of age (Fig. 1). Surprisingly, Chk2 deletion rescued the oocyte reserve (Fig. 1A,B), albeit not to WT levels. The rescued follicles in double mutant females persisted robustly at least until 6 months pp (in one case, 554 total in a single ovary).
Figure 1. CHK2 is required for efficient elimination of asynaptic Spo11−/− mouse oocytes.
(A) H&E stained histological sections of 8 weeks old ovaries. Black arrowheads indicate antral follicles. CL= Corpus Luteum; the presence of corpora lutea are indicative of prior rounds of ovulation. The lower portion of each panel contains a higher magnification image of an ovarian cortical region, where primordial follicles reside. Yellow arrows and stars indicate primordial and primary follicles, respectively.
(B) Follicle counts from ovaries of indicated genotypes at 3 and 8 weeks postpartum, respectively. Each data point is from a single ovary, each being from a different animal. Total = all follicle types. Horizontal hashes denote mean and standard deviation. Littermate controls included animals with the following genotypes: Spo11+/+Chk2+/+, Spo11+/− Chk2+/− and Spo11+/+Chk2+/−. (^) The values obtained for the 3 weeks follicles/ovaries counts are not comparable to the 8 weeks (see methods). Asterisks indicate p-values: (*) 0.005 ≤ p-values ≤ 0.05, (**)0.001 ≤ p-values ≤ 0.005 and (***) p-values ≤ 0.001 derived from a non-parametric, one-way ANOVA test (Kruskal-Wallis).
HORMAD2 deficiency prevents elimination of Trip13 mutant oocytes that have complete synapsis but unrepaired meiotic DSBs, restoring female fertility
Taken alone, the rescue of Spo11−/− oocytes by Chk2 deletion suggests that severe asynapsis leads to CHK2 activation and signaling to mediate oocyte elimination. This led us to postulate that either: 1) CHK2 is a common component of otherwise distinct synapsis and DNA damage checkpoints, or 2) that there is a single linear checkpoint pathway that responds to both asynapsis and DNA damage, and that DNA damage activates the checkpoint pathway more robustly or sooner in prophase I (thus accounting for the different patterns of oocyte elimination in asynaptic vs. DSB repair-deficient oocytes mentioned above (Di Giacomo et al., 2005)).
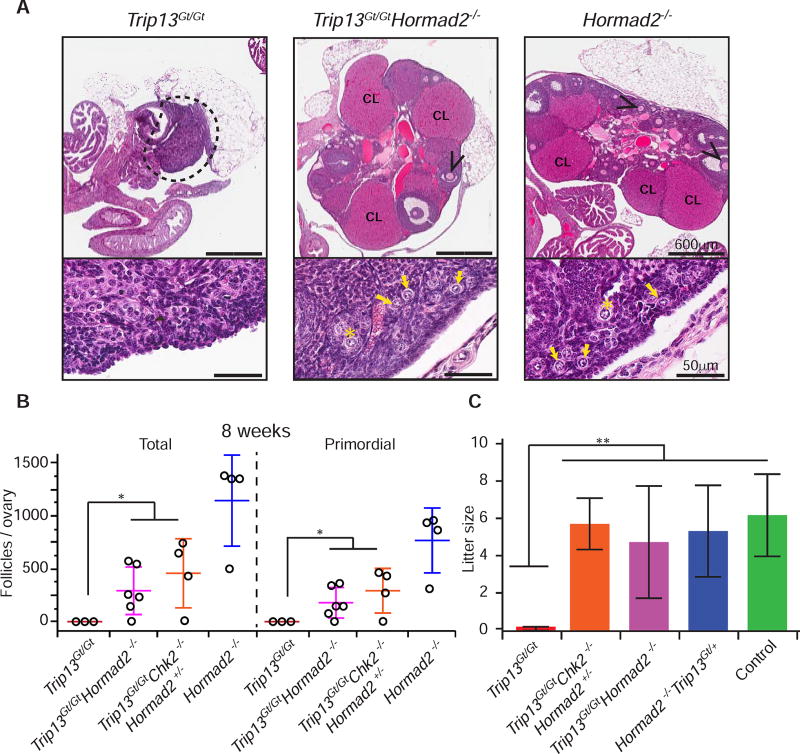
We reasoned that if there is a single linear checkpoint pathway, then putative synapsis checkpoint genes required to eliminate Spo11−/− oocytes would also be required to eliminate Trip13Gt/Gt oocytes. Trip13Gt/Gt meiocytes have synapsed chromosomes and persistent SPO11-dependent DSBs, which leads to neonatal depletion of follicles in a CHK2>TRP53/TAp63 pathway-dependent manner (Fig. 2A)(Bolcun-Filas et al., 2014; Li and Schimenti, 2007). To test this, we determined whether deficiency of HORMAD2, a putative synapsis checkpoint protein, could rescue Trip13Gt/Gt oocytes. HORMAD2 and its paralog HORMAD1 are “HORMA” (Hop1, Rev7 and Mad2) domain-containing proteins orthologous to the Saccharomyces cerevisiae synaptonemal complex (SC) axial element protein Hop1p, and deletion of either prevents elimination of Spo11−/− oocytes (Daniel et al., 2011; Kogo et al., 2012a; Wojtasz et al., 2012). We used a mutant of Hormad2 rather than Hormad1, because deletion of the latter disrupts recombination and homolog synapsis (Daniel et al., 2011; Kogo et al., 2012b; Shin et al., 2010). Remarkably, not only did ovaries of 2 month old Trip13Gt/Gt Hormad2−/− mice retain a substantial primordial follicle pool (Fig. 2A,B), but also these females were fertile (Fig. 2C). The rescued fertility of these oocytes suggested either that these DSBs were compatible with further oocyte maturation, or that they were eventually repaired as in the case of Trip13Gt/Gt females whose fertility was restored by Chk2 ablation (Bolcun-Filas et al., 2014). The dynamics of DSB repair are addressed below.
Figure 2. Synapsis-competent Trip13Gt/Gt oocytes are eliminated in a HORMAD2-dependent manner.
(A) H&E stained histological sections of 8 week old ovaries of indicated genotypes. Black arrowheads indicate antral follicles. CL= Corpus Luteum. The lower half of each panel shows a higher magnification of cortical regions of ovaries. Yellow arrows and stars indicate primordial and primary follicles, respectively.
(B) Follicle quantification of 8 week old ovaries. Each data point is from a single ovary, each being from a different animal. “Total” = all follicle types. Horizontal hashes denote mean and standard deviation. The statistic used was Kruskal-Wallis. * indicates p-value = 0.002.
(C) Graphed are mean litter sizes. N ≥ 3 females tested for fertility per genotypic group. Control matings were between mice with the genotypes Trip13Gt/+ and Trip13Gt/+ Hormad2+/−. Error bars represent standard deviation and ** indicates p-value ≤ 0.005 derived from the Kruskal-Wallis test.
Since TRIP13 is required for removal of the HORMADs from chromosome axes upon synapsis (Wojtasz et al., 2009), and persistence of HORMADs on unsynapsed chromosomes correlates with MSUC-mediated silencing of essential genes (Cloutier et al., 2015; Wojtasz et al., 2012), the question arises as to whether Trip13Gt/Gt oocytes are eliminated not because of unrepaired DSBs, but rather by transcriptional silencing. However, this is unlikely for the following reasons. First, Trip13Gt/Gt oocytes are depleted with a temporal pattern and degree consistent with mutants defective in DSB repair, not asynapsis (Di Giacomo et al., 2005; Li and Schimenti, 2007). Second, Spo11 is epistatic to Trip13, in that Trip13Gt/Gt Spo11−/− ovaries resemble Spo11 single mutants in their pattern of oocyte elimination (Li and Schimenti, 2007), demonstrating that unrepaired meiotic DSBs drive early culling of Trip13 mutant oocytes. Third, HORMAD persistence on synapsed Trip13Gt/Gt or unsynapsed Spo11−/− meiotic chromosome axes is not affected by Chk2 deletion (Fig. S1), which might be predicted if CHK2 was rescuing either mutant class by disrupting the ability of HORMADs to signal asynapsis. The latter is further supported by the fact that CHK2 depletion does not interfere with MSCI (meiotic sex chromosome inactivation, which is mechanistically similar or identical to MSUC) in males (Pacheco et al., 2015), and that Chk2−/− mice are fertile unlike Hormad1−/− animals (Daniel et al., 2011; Kogo et al., 2012b; Shin et al., 2013).
HORMAD2 inhibits DSB repair in prophase I oocytes
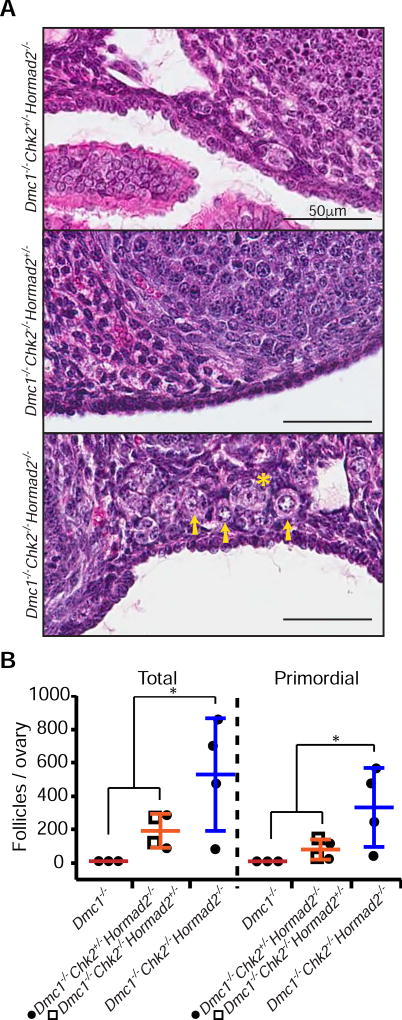
That HORMAD2 deficiency could rescue both Trip13Gt/Gt and Spo11−/− oocytes is consistent with a single checkpoint capable of detecting both damaged DNA and asynapsed chromosomes. If there is indeed a single checkpoint pathway, then combined deficiency for CHK2 and HORMAD2 should rescue asynaptic and DSB repair-defective Dmc1−/− oocytes to the same degree as deficiency for either one alone. However, Dmc1−/− Chk2−/− Hormad2−/− females had ≥3 fold increase in primordial and total follicles compared to Dmc1−/− Hormad2−/− or Dmc1−/− Chk2−/− ovaries (Fig. 3A,B, and Fig. S2). This lack of epistasis indicates that HORMAD2 and CHK2 are not functioning solely as members of a single linear checkpoint pathway sensing either or both asynapsis and DNA damage.
Figure 3. HORMAD2 and CHK2 are not in the same checkpoint pathway.
(A) H&E stained histological sections of cortical regions of 8 week old mutant mouse ovaries, where primordial follicles are concentrated. Histology of whole ovaries of these genotypes are represented in Fig. S2. Primordial follicles, which constitute the oocyte reserve, are indicated by yellow arrows, and a primary follicle by a star. Residual Dmc1−/− ovaries are not represented because they are completely devoid of oocytes (Pittman et al., 1998).
(B) Follicle counts from ovaries of indicated genotypes at 8 weeks of age. “Total” = all types of follicles. Data points represent follicle counts derived from one ovary, each ovary originating from a different animal. Asterisk indicates p-value ≤ 0.05 (Kruskal-Wallis test).
We therefore considered two alternative explanations for why Hormad2 deficiency rescues Trip13Gt/Gt oocytes: 1) it reduces the number of SPO11-induced DSBs to a level sufficient for synapsis, but below the threshold for checkpoint activation; and/or 2) it facilitates DSB repair. Studies of related proteins support both explanations. Absence of the budding yeast ortholog Hop1p not only decreases meiotic DSB formation, but also increases use of the sister chromatid as a template for HR repair (Carballo et al., 2008; Lam and Keeney, 2014; Latypov et al., 2010; Mao-Draayer et al., 1996; Niu et al., 2005; Schwacha and Kleckner, 1997). Mouse HORMAD1 is required for loading HORMAD2 onto unsynapsed axes, proper SC formation (Daniel et al., 2011), and normal levels of meiotic DSBs (Daniel et al., 2011; Stanzione et al., 2016). Whereas Dmc1−/− Hormad1−/− or irradiated Hormad1−/− oocytes exhibit fewer DSB markers than oocytes containing HORMAD1 (Daniel et al., 2011; Shin et al., 2010), this can be attributable largely to enhanced repair (Shin et al., 2013). Intersister (IS) HR repair of DSBs in S. cerevisiae is substantial and it increases in hop1 mutants (Goldfarb and Lichten, 2010). Moreover, disruption of SC axes in mice (deletion of Sycp2 or Sycp3) appears to alter recombination partner choice in favor of the sister chromatid, decreasing persistent DSBs in Trip13Gt/Gt oocytes to a degree that diminishes their elimination in a RAD54-dependent manner (Li et al., 2011). These data led us to hypothesize that the rescue of Trip13 mutant oocytes by Hormad2 deficiency was due to increased DSB repair, possibly by diminishing the BSCR.
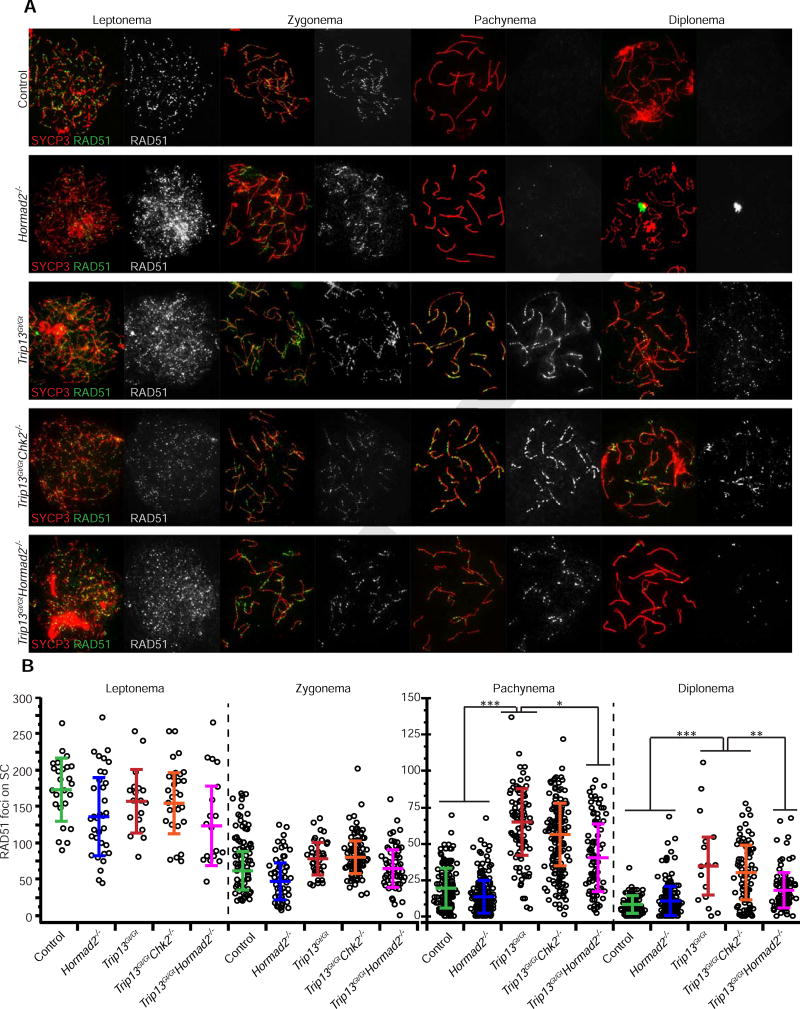
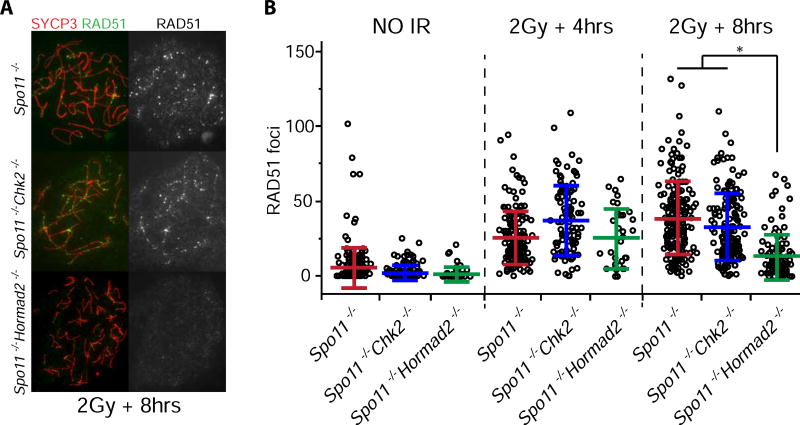
To test this, we quantified levels and rates of meiotic DSB repair in various genotypes of prophase I oocytes. Whereas the number of leptotene and zygotene stage RAD51 foci was not significantly different in Trip13Gt/Gt Hormad2−/− oocytes compared to Trip13Gt/Gt or other control and mutant genotypes (Fig. 4A,B; Table S1), there were significantly fewer compared to Trip13Gt/Gt by pachynema and diplonema (p = 0.02 and 0.03, respectively, using Tukey HSD in a mixed model). RAD51 levels in Trip13Gt/Gt and Trip13Gt/Gt Chk2−/− newborn oocytes remained high in diplonema compared to all other genotypes (Fig. 4A,B; Table S1), presumably reflecting a relative deficiency in DSB repair. Furthermore, we found that RAD51 foci induced by 2Gy of ionizing radiation (IR) disappeared more rapidly in Spo11−/− Hormad2−/− oocytes than either Spo11−/− or Spo11−/− Chk2−/− oocytes, as assessed 8 hours after treatment (Fig. 5; Table S2). Overall, the data suggest that HORMAD2 on the axes of either asynapsed (Spo11−/−) or synapsed (Trip13Gt/Gt) (Wojtasz et al., 2009) meiotic chromosomes inhibits IS recombination-mediated DSB repair.
Figure 4. Depletion of HORMAD2 accelerates DSB-repair during early stages of meiotic prophase I.
(A) Representative images of meiotic chromosome spreads from oocytes at different substages of meiotic prophase I, probed with antibodies for SYCP3 (SC axis protein) and the DSB marker RAD51. Oocytes were isolated from female embryos ranging from 15.5 dpc to newborns. See Figure S1 for HORMAD2 localization in meiotic mutants.
(B) Numbers of RAD51 foci in specified meiotic prophase I substage of indicated mutants. Only RAD51 foci present on SYCP3 stained axes were scored. Each data point represents one cell. In each genotypic group, at each stage, the counts are derived from at least three animals. Horizontal hashes in summary statistic plots denote mean and standard deviation. Values of the mixed model calculation can be found in Table S1. Colors correspond to genotypes. Asterisks indicate statistical significant differences between groups in terms of the least square means of RAD51 foci. p-values: *** p ≤ 0.001; ** p ≤ 0.005; * p ≤ 0.05 (Tukey HSD). See Table S1 for raw data and statistical calculations associated with (B).
Figure 5. Depletion of HORMAD2 accelerates repair of induced DSBs in oocytes.
(A) Immunolabeling of surface spread chromosomes from oocytes after exposure to ionizing radiation (IR). Fetal ovaries were collected at 15.5 dpc, cultured 24 hours, exposed to 2 Gy of IR, then cultured for an additional 4–8 hours. Shown are those recovered 8 hours after IR. See Figure S4 for single Hormad2−/− single mutant results.
(B) Quantification of RAD51 foci. Each data point represents one oocyte. The graphs include mean and standard deviation, and are color coded according to genotypic group. The 4 and 8 hrs unirradiated samples were combined. Data were derived from at least two different animals per condition. See Table S2 for raw data and statistical calculations associated with (B).
Evidence that CHK2-mediated elimination of asynaptic oocytes is driven by accumulation of SPO11-independent DSBs
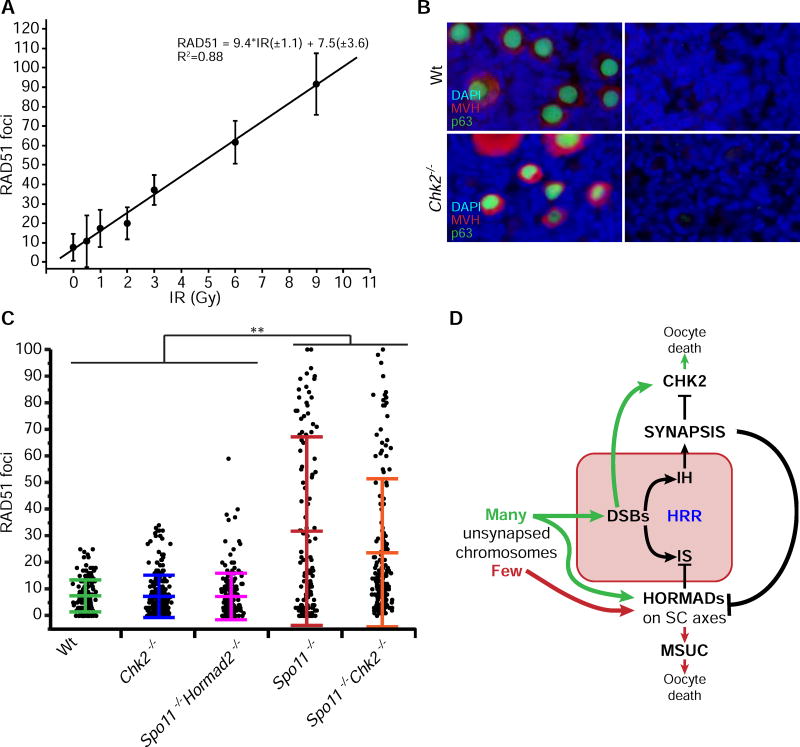
If indeed Hormad2 deletion rescues DSB-containing oocytes by weakening or eliminating the BSCR, this raises the question as to why HORMAD2 deficiency rescues Spo11−/− oocytes that don’t make meiotic DSBs. A clue comes from the surprising observation that Spo11−/− oocytes sustain DSBs of unknown origin (but possibly from LINE-1 retrotransposon activation) during early pachynema (Malki et al., 2014), (Carofiglio et al., 2013). We hypothesized that these DSBs occur at levels sufficient to trigger the CHK2-dependent checkpoint in Spo11−/− oocytes, but that in the absence of SC axis-bound HORMAD2, there is sufficient DSB repair to prevent checkpoint activation. To test this, we determined the threshold number of DSBs that kills WT and Chk2−/− oocytes by exposing explanted newborn ovaries to a range of IR. RAD51 foci on chromosome axes accumulated roughly linearly in oocytes exposed to 0.5 – 9Gy (Fig. 6A; Fig. S5), and Chk2−/− oocytes withstood up to 7Gy (Fig. 6B), a dosage that induces 73.3 RAD51 foci (Fig. 6A). In contrast, as little as 0.3Gy (10.3 foci by linear regression) abolished the entire primordial follicle pool of WT ovaries. Consistent with our hypothesis that HORMAD2 prevents DSB repair, the SC axes of Spo11−/− zygotene/pachytene-like chromosomes in newborn oocytes contained far more discrete RAD51 foci (raw average of 39.8; likely an underestimate, see Fig. S3) than in Spo11−/− Hormad2−/− oocytes (avg. 7.3 foci), the latter being almost identical to WT or Chk2−/− oocytes (7.5 and 7.3 respectively; Fig. 6C, Table S3) in which HORMAD2 has been removed from synapsed chromosomes. These data indicate that the majority of Spo11−/− oocytes (60.8%) bear a level of DSBs (>10.3 foci) sufficient to trigger their elimination by the CHK2-dependent DNA damage checkpoint, while most WT oocytes (71%) are below this threshold (Table S3).
Figure 6. DNA damage threshold required to trigger oocyte death, and evidence for HORMAD-mediated inhibition of IS repair.
(A) Linear regression for conversion of radiation dosages to RAD51 focus counts. Meiotic surface spreads were made from WT neonatal ovaries 2.5 hrs after IR. Plotted are means with standard deviations. Each IR dose has focus counts from ~25 oocytes derived from a total of 18 animals. See Figure S5 for single cell foci counts and numerical values.
(B) Chk2−/− oocytes are highly IR resistant. Shown are immunofluorescence images of ovarian sections labeled with nuclear and cytoplasmic germ cell markers (p63 and MVH, respectively).
(C) RAD51 focus counts from newborn oocyte spreads. Only oocytes with discrete patterns of RAD51 foci were scored, as defined in Fig. S3. Data points represent individual oocytes, derived from at least five different animals from each genotypic group. Horizontal hashes denote means and standard deviations calculated using a mixed model (see methods). Asterisks indicate statistically significant differences between groups with p-values: *** p ≤ 0.001; ** p ≤ 0.005; * p ≤ 0.05 (Tukey HSD). See Table S3 for raw data and statistical calculations.
(D) Model for pachytene checkpoint activation in mouse oocytes. Oocytes with many unsynapsed chromosomes (green) ultimately accumulate DSBs, which cannot be repaired due to block to IS recombination imposed by HORMADs on asynapsed axes. Failure of DSB repair leads to activation of CHK2 and downstream effector proteins (p53/TAp63) that trigger apoptosis. Few asynapsed chromosomes (red) lead to inactivation of essential genes by MSUC thereby causing oocyte death.
HRR - Homologous Recombination Repair; IH - Interhomolog; IS - Intersister; MSUC - Meiotic Silencing of Unsynapsed Chromatin.
DISCUSSION
Meiocytes have genetic quality control mechanisms that respond to their unique developmental circumstances, chromosome biology and cell cycle. For example, the pachytene/prophase I checkpoint is active only at a point in prophase I at which DSBs have normally been repaired, but not during the time between programmed DSB formation and HR repair. While the oocyte "pachytene checkpoint" is distinct with respect to its cell cycle timing and its ability to monitor an event (chromosome synapsis) unique to meiosis, our current and prior (Bolcun-Filas et al., 2014) work indicate that for circumstances involving extensive asynapsis and DNA damage, this checkpoint in oocytes involves a DNA damage response (DDR) common to somatic cells. Our surprising finding that the DDR is involved in culling of Spo11−/− oocytes raises the question of how SPO11-independent DSBs - first reported by Carofiglio et al (Carofiglio et al., 2013) and confirmed here - arise on unsynapsed chromosomes. One possible source is LINE-1 retrotransposon activation, which has been correlated with natural oocyte attrition (Malki et al., 2014). However, transposon expression normally occurs only transiently at the onset of meiosis before epigenetic silencing (van der Heijden and Bortvin, 2009). It is possible that the extensive asynapsis in Spo11−/− oocytes per se, or disruption of the meiotic program including the normal course of DSB induction and repair, interferes with transposon silencing. Another possibility is that unsynapsed chromosomes are more susceptible to spontaneous breakage. These outcomes could be exacerbated by extended retention of HORMADs on unsynapsed axes, inhibiting repair of these breaks. An intriguing question is whether the production of these SPO11-independent DSBs, whatever their origin, evolved as a contributory mechanism for genetic quality control. It is also conceivable that the extended presence of HORMADs themselves contributes to spontaneous DSB formation, possibly as a "last ditch" mechanism to drive pairing or synapsis in chromosomes devoid of sufficient interhomolog recombination events.
The late appearance and highly variable number (Fig. 6C) of SPO11-independent DSBs in Spo11−/− oocytes may explain the differences in timing and extent of oocyte elimination in exclusively asynaptic vs. DSB repair-deficient (e.g. Dmc1, Trip13) mutants. As reported by Di Giacomo and colleagues (Di Giacomo et al., 2005), whereas Dmc1−/− oocytes were completely eliminated before dictyate arrest and follicle formation, Spo11−/− ovaries contained ~15–20% of WT numbers of follicles (including 27 fold less primordial follicles by 4 days pp); this reduced oocyte reserve was depleted by 2–3 months of age by subsequent cycles of recruitment and maturation. Additionally, Dmc1−/− oocytes degenerate before Spo11−/− oocytes, suggesting that an earlier-acting mechanism was triggering Dmc1−/− oocyte death. These distinctions, in conjunction with epistasis analysis of mutants doubly deficient for Spo11 and DSB repair mutations, led to the conclusion that there are DSB-dependent and -independent mechanisms to eliminate defective oocytes. We suggest that the difference in timing of oocyte elimination, at least in part, may be related to the DSB load. The abundant SPO11 DSBs formed early in prophase I may trigger the checkpoint sooner and more uniformly in recombination mutants that fail to reduce DSB levels in a timely manner. According to this scenario, spontaneous DSBs that don’t arise until latter stages of [abnormal] prophase I in Spo11−/− oocytes would trigger the DNA damage checkpoint at a later point. Based on our data (Fig. 6A; Fig S5), we suggest that those oocytes with below-threshold DSB levels escape the DNA damage checkpoint, and are either eliminated by other mechanisms (see below) or survive to constitute the reduced follicular reserve in Spo11 mutants.
While the CHK2-dependent checkpoint is of central importance to genetic quality control in oocytes, our observations that Chk2 deletion does not fully restore oocyte numbers to WT levels in mutants indicates that it is not absolutely required for eliminating all oocytes with unrepaired DSBs. Rather, the fraction of oocytes rescued is inversely related to the burden of unrepaired meiotic DSBs. For example, whereas Chk2 deficiency rescued nearly 1/3 of Trip13Gt/Gt oocytes (which are partially proficient for DSB repair and which harbor 35±4 and 63±4.7 persistent RAD51 foci in diplonema and pachynema, respectively; Fig. 4B), it rescued only a small fraction (~5%) of profoundly recombination-deficient Dmc1−/− oocytes (harboring an average of ~150 RAD51 foci (Li et al., 2011)). We posit that the oocytes that fail to be rescued in these mutants are eliminated either by a separate or a complementary checkpoint pathway (for example, ATR-CHK1 (Smith et al., 2010)), or succumb from catastrophic levels of DNA damage. It is informative that deletion of Hormad1, but not Hormad2, rescues Dmc1−/− oocytes to a greater extent than Chk2 deletion. As discussed earlier, the rescued Dmc1−/− Hormad1−/− oocytes had a marked reduction in DSBs (Bolcun-Filas et al., 2014; Shin et al., 2013; Wojtasz et al., 2012). Since HORMAD1 is needed to load HORMAD2 onto unsynapsed chromosome axes (not vice versa), then the impact of Hormad1 deletion upon IS recombination constitutes the combined roles of both HORMAD proteins. However, when Hormad2 alone is deleted, the continued presence of chromosomally-bound HORMAD1 may provide a less-effective, but still substantive, BSCR. The lower level of residual DSBs in Spo11 and Trip13 mutant oocytes (compared to Dmc1−/−) may render them responsive to a weaker BSCR such as when Hormad2 is deleted. We postulate that because of its involvement in stimulating SPO11 activity (Daniel et al., 2011), Hormad1 deletion is very effective in rescuing a DSB repair mutant like Dmc1 because not only are fewer DSBs formed, but also IS recombination is more active.
Our results add to increasing evidence that IS recombination is important in mammalian meiosis. As discussed in the text, the HORMADs and SC axial element structure appear to inhibit IS repair of meiotic DSBs preferentially, thus allowing IH recombination to drive homolog pairing and synapsis. However, as synapsis progresses and the SC is formed, the HORMADs are removed and presumably both IS and IH recombination can occur readily as in yeast (Subramanian et al., 2016). Since not all RAD51 foci disappear by pachynema when synapsis is complete (for example, see Fig. 4B), it is possible that a substantial fraction of these DSBs are normally repaired by IS recombination. We speculate that the persistent unrepaired DSBs on synapsed chromosomes of Trip13 mutants, which retain HORMADs on their SCs, may actually constitute a substantial fraction of SPO11-induced DSBs (an average of ~65/oocyte nucleus of the 200–300 induced; Fig. 4) that would normally be repaired by IS recombination. However, we cannot rule out the possibility that the “persistent” DSBs on synapsed Trip13Gt/Gt chromosomes actually arise from continued SPO11 cleavage signaled by continued presence of SC-bound HORMADs (Kauppi et al., 2013).
In trying to decipher the quality-control mechanisms functioning during meiosis, it is important to recognize that experimental studies such as those performed here employ mutants with pervasive, non-physiological levels of defects. Meiocytes in wild-type individuals would have less extreme genetic defects. In oocytes bearing a small number (1–3) of unsynapsed chromosomes, the unsynapsed chromosomes underwent transcriptional silencing (MSUC) during pachynema, causing elimination at the diplotene stage (Cloutier et al., 2015; Kouznetsova et al., 2009) from lack of essential gene products encoded by these chromosomes (Cloutier et al., 2015). However, oocytes with more than 2–3 unsynapsed chromosomes impairs MSUC, presumably due to a limiting amount of BRCA1 (Kouznetsova et al., 2009). Nevertheless, Spo11−/− meiocytes typically exhibit “pseudo sex bodies,” named as such because they resemble the XY (sex) body, involving a small number of asynapsed autosomes (Bellani et al., 2005). Formation of pseudo sex bodies in Spo11−/− oocytes is dependent upon HORMADs (Daniel et al., 2011; Kogo et al., 2012b), leading to the proposal that these are responsible for oocyte elimination (Kogo et al., 2012a). This may be the case in a subset of oocytes where the pseudo sex body impacts either a chromosomal region containing haploinsufficient loci, or both alleles of a locus needed for meiotic progression or oocyte survival. Since CHK2 deficiency can rescue Spo11−/− oocytes while not abolishing HORMAD localization (Fig. S1) or pseudo sex body formation (not shown), yet does not rescue all Spo11 oocytes, it is likely that neither MSUC nor CHK2 alone is entirely responsible for elimination of all oocytes with pervasive asynapsis. Finally, because MSUC involves many components of the DNA damage response (Fernandez-Capetillo et al., 2003; Ichijima et al., 2011; Turner et al., 2004), it is conceivable that asynapsis leading to MSUC would activate effector elements of the DNA damage checkpoint pathway, including CHK2. However, this does not appear to be the case, because silenced supernumerary chromosomes do not eliminate oocytes (Cloutier et al., 2015), MSCI (meiotic sex chromosome inactivation) does not kill spermatocytes, and asynaptic oocytes are not eliminated in a pattern typical of DNA repair mutants.
The “pachytene checkpoint” has commonly been thought to consist of separate DNA damage and synapsis checkpoints in multiple organisms. However, the finding that MSUC can cause death of oocytes led to the suggestion that there is only 1 formal cell cycle checkpoint in mouse oocytes - the DNA damage checkpoint (Cloutier et al., 2015) - and our data provides mechanistic evidence consistent with this idea. Current information supports a model (Fig. 6D) for two major mechanisms by which oocytes with synapsis defects are eliminated: 1) MSUC, for oocytes with a small number of asynapsed chromosomes that do not accumulate unrepaired DSBs above a threshold, and in which both homologs chromosomes bearing essential genes for meiotic progression are silenced (Cloutier et al., 2015); and 2) the DNA damage checkpoint, for oocytes with multiple asynapsed chromosomes that accumulate a sufficient number of DSBs to trigger the DNA damage checkpoint (Fig. 6D). These disparate mechanisms may have distinct purposes. Because oocytes with only 1 or 2 unsynapsed chromosomes may not efficiently trigger the spindle assembly checkpoint (SAC) (LeMaire-Adkins et al., 1997), the MSUC pathway would safeguard against aneuploidy. Superficially, it would seem that because oocytes with extensive asynapsis would effectively trigger the SAC, that the DNA damage checkpoint mechanism is redundant. However, it is likely advantageous reproductively to eliminate such defective oocytes before they enter dictyate as constituents of the ovarian reserve, otherwise the fraction of unproductive ovulations (those terminated by the SAC) would increase, thus compromising fecundity.
STAR METHODS
KEY RESOURCES TABLE
(attached)
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for reagents may be directed to and will be fulfilled by the Lead Contact, John Schimenti (jcs92@cornell.edu)
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Experiments were performed on female mice, and of course male mice were used for matings to produce desired genotypes. Samples for histological analysis were from eight week old animals. The alleles used have been previously described and were the following: Trip13Gt(RRB047)Byg (referred to as Trip13Gt in the manuscript) (Li and Schimenti, 2007); Dmc1tm1Jcs (Pittman et al., 1998); Chk2tm1Mak (Hirao et al., 2002); Spo11tm1Mjn (Baudat et al., 2000); and Hormad2 (Kogo et al., 2012a). All mice were in a mixed genetic background of strains C57Bl/6J and C3H/HeJ. The Cornell’s Animal Care and Use Committee approved all animal usage, under protocol 2004-0038 to JCS.
The embryonic age of pre-term animals was counted using the morning in which copulation plug was detected as being the 0.5 days post coitus (dpc).
METHODS DETAILS
Organ Culture and Irradiation
Embryonic and postpartum explanted ovaries were cultured under conditions as we previously detailed (Rinaldi et al., 2017). Ovaries were irradiated in a 137cesium irradiator with a rotating turntable. Immediately after irradiation, the media was replaced, and ovaries were cultured for indicated periods of time prior to tissue processing.
Histology and Immunostaining
Ovaries were dissected and incubated in Bouin’s fixative overnight at room temperature. Afterwards, tissues were washed in 70% ethanol prior to being embedded in paraffin for serial sectioning at 6µm thickness. Ovaries were stained with Harris Hematoxylin and Eosin (H&E) and follicles counted in every fifth section except for the three-week counts reported in Figure 1B, in which every 12th section was counted. There was no correction factor applied to the values reported. Only one ovary per animal was used.
Cultured ovaries, used for histological sections followed by immunostaining, were fixed in 4% paraformaldehyde/PBS over night at 4°C. After 70% ethanol washes, ovaries were embedded in paraffin and serially sectioned at 5µm. These ovaries were immunostained using standard methods. Briefly, slides were deparaffinized and re-hydrated prior to antigen retrieval using sodium citrate buffer. Slides were blocked with 5% goat serum (PBS/Tween 20) and incubated at 4°C overnight with primary antibodies: mouse anti-p63 (1:500, 4A4, Novus Biologicals); and rabbit anti-MVH (1:1000, Abcam). Afterwards, sections were incubated with Alexa Fluor® secondary antibodies for one hour and Hoechst dye for 5 minutes. Slides were mounted with ProLong Anti-fade (Thermo-Fisher) and imaged.
Histological images were obtained from slides digitized using a Leica Scanscope CS2.
Immunofluorescence of meiotic chromosome surface spreads
Meiotic surface spreads of prophase I female meiocytes were prepared using an adaptation (Reinholdt et al., 2004) of a drying-down technique (Peters et al., 1997) that was described in great detail in the former reference. Meiotic stages (leptonema-diakinesis) were determined based on SYCP3 staining patterns (Gray and Cohen, 2016). Slides were stored at −80°C until immunostained. For staining, slides were brought to room temperature (RT) and washed once with PBS+0.1% Tween-20 (PBS-T). Slides were blocked for 40 minutes at RT with PBS-T containing 5% normal goat serum (5%GS-PBS-T). Primary antibodies were diluted into 5%GS-PBS-T and incubated overnight at RT in a humidified chamber. Antibodies and dilutions used included: rabbit anti-RAD51 (1:250 Abcam 176458), mouse anti-SYCP3 (1:600 Abcam) and guinea pig anti-HORMAD2 antibody (1:1000, kind gift from Attila Toth). Secondary antibodies used were diluted 1:1000 in in 5%GS-PBS-T and included goat anti-rabbit Alexa 488/594, goat anti-mouse Alexa 488/594 and goat anti-guinea pig Alexa 488/594. Images were taken using an Olympus microscope with 40× lens or 100× immersion oil lens and CCD camera.
Focus Quantification
Foci were quantified both manually, through the visualization and annotation of individual foci, and also semi-automatically using Fiji-ImageJ (Schindelin et al., 2012). Semi-automated counts were performed using binary images obtained from the RAD51-labeled channel, with the threshold set above background level. The count was obtained after performing “Watershed”, by the “Analyze Particles” functionality with size set for 1.5 to infinity. Cell counts that displayed discrepancy of more than 20% between manual and semi-automated counts were discarded.
Fertility Test
To test if HORMAD2 deficiency was able to rescue the Trip13Gt/Gt sterility phenotype, three double mutant females were mated to wild type C3H/HeJ males proven to be fertile through previous matings. Each female provided more than 4 consecutive litters up to the time of preparation of this manuscript. All three females originated from different litters. Trip13Gt/Gt littermates were housed with fertile males and used as negative controls.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical analysis
Comparisons between compound mutants and controls were done using littermates or related animals. Unless otherwise noted, all experiments used at least three mice per experimental group. All statistical analyses were done using JMP Pro12 software (SAS Inc., Cary, NC-USA, version 12.0.1). Comparisons of fertility and follicle counts between genotypic groups were tested using both the Tukey honest significance different (HSD) and the non-parametric, one-way ANOVA test (Kruskal -Wallis). Both tests provided concordant results. RAD51 focus counts were analyzed using a mixed model with animal ID as random effect and genotype as fixed effect. Least square means (LSMeans) differences were tested using Tukey HSD. The residuals from the mixed model were normally distributed.
DATA AND SOFTWARE AVAILABILITY
Raw data of RAD51 foci counts are in supplementary tables (Table S1, Table S2, and Table S3). The raw image files can be downloaded at Mendeley data: https://data.mendeley.com/datasets/3n2yfpk4vh/draft?a=52519ac3-7b00-4178-8418-3b9fee9b23d0.
Supplementary Material
Supplemental Table S1: Related to Figure 4
Supplemental Table S2: Related to Figure 5
Highlights.
Late meiosis I oocytes bearing >10 DSBs are killed by the DNA damage checkpoint
CHK2 is responsible for eliminating many asynaptic, SPO11-deficient mouse oocytes
Spo11−/− oocytes acquire spontaneous DSBs that often exceed the 10 DSB threshold
HORMAD2 on pachytene chromosomes prevents DSB repair via intersister recombination
Acknowledgments
This work was supported by a grant from the National Institutes of Health (R01 GM45415 to JCS), and contract CO29155 from the NY State Stem Cell Program (NYSTEM). The authors would like to thank R. Munroe and C. Abratte for generating chimeric mice, Stephen Parry from the Cornell Statistical Consulting Unit (CSCU) for help with the statistical analysis, Dr. Atilla Toth for the HORMAD2 antibody, and M.A. Handel for feedback on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
V.D.R. and E.B-F. performed the experiments and contributed to the writing of the paper. H.K. and H.K. provided the Hormad2 mutant ESCs and provided feedback on the manuscript. J.C.S. supervised the work and wrote most of the paper.
References
- Agoulnik AI, Lu B, Zhu Q, Truong C, s Ty MT, Arango N, Chada KK, Bishop CE. A novel gene, Pog, is necessary for primordial germ cell proliferation in the mouse and underlies the germ cell deficient mutation, gcd. Hum Mol Genet. 2002;11:3047–3053. doi: 10.1093/hmg/11.24.3047. [DOI] [PubMed] [Google Scholar]
- Baudat F, Manova K, Yuen JP, Jasin M, Keeney S. Chromosome synapsis defects and sexually dimorphic meiotic progression in mice lacking Spo11. Mol Cell. 2000;6:989–998. doi: 10.1016/s1097-2765(00)00098-8. [DOI] [PubMed] [Google Scholar]
- Bellani MA, Romanienko PJ, Cairatti DA, Camerini-Otero RD. SPO11 is required for sex-body formation, and Spo11 heterozygosity rescues the prophase arrest of Atm−/− spermatocytes. J Cell Sci. 2005;118:3233–3245. doi: 10.1242/jcs.02466. [DOI] [PubMed] [Google Scholar]
- Bolcun-Filas E, Rinaldi VD, White ME, Schimenti JC. Reversal of female infertility by Chk2 ablation reveals the oocyte DNA damage checkpoint pathway. Science. 2014;343:533–536. doi: 10.1126/science.1247671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne PS, Baker TG. Perinatal oocyte loss in XO mice and its implications for the aetiology of gonadal dysgenesis in XO women. J Reprod Fertil. 1985;75:633–645. doi: 10.1530/jrf.0.0750633. [DOI] [PubMed] [Google Scholar]
- Carballo J, Johnson A, Sedgwick S, Cha R. Phosphorylation of the axial element protein Hop1 by Mec1/Tel1 Ensures meiotic interhomolog recombination. Cell. 2008;132:758–770. doi: 10.1016/j.cell.2008.01.035. [DOI] [PubMed] [Google Scholar]
- Carofiglio F, Inagaki A, de Vries S, Wassenaar E, Schoenmakers S, Vermeulen C, van Cappellen WA, Sleddens-Linkels E, Grootegoed JA, Te Riele HP, et al. SPO11-independent DNA repair foci and their role in meiotic silencing. PLoS Genet. 2013;9:e1003538. doi: 10.1371/journal.pgen.1003538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier JM, Mahadevaiah SK, ElInati E, Nussenzweig A, Toth A, Turner JM. Histone H2AFX Links Meiotic Chromosome Asynapsis to Prophase I Oocyte Loss in Mammals. PLoS Genet. 2015;11:e1005462. doi: 10.1371/journal.pgen.1005462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad DF, Keebler JE, DePristo MA, Lindsay SJ, Zhang Y, Casals F, Idaghdour Y, Hartl CL, Torroja C, Garimella KV, et al. Variation in genome-wide mutation rates within and between human families. Nat Genet. 2011;43:712–714. doi: 10.1038/ng.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel K, Lange J, Hached K, Fu J, Anastassiadis K, Roig I, Cooke HJ, Stewart AF, Wassmann K, Jasin M, et al. Meiotic homologue alignment and its quality surveillance are controlled by mouse HORMAD1. Nat Cell Biol. 2011;13:599–610. doi: 10.1038/ncb2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giacomo M, Barchi M, Baudat F, Edelmann W, Keeney S, Jasin M. Distinct DNA-damage-dependent and -independent responses drive the loss of oocytes in recombination-defective mouse mutants. Proc Nat Acad Sci USA. 2005;102:737–742. doi: 10.1073/pnas.0406212102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Capetillo O, Celeste A, Nussenzweig A. Focusing on foci: H2AX and the recruitment of DNA-damage response factors. Cell Cycle. 2003;2:426–427. [PubMed] [Google Scholar]
- Finsterbusch F, Ravindranathan R, Dereli I, Stanzione M, Trankner D, Toth A. Alignment of Homologous Chromosomes and Effective Repair of Programmed DNA Double-Strand Breaks during Mouse Meiosis Require the Minichromosome Maintenance Domain Containing 2 (MCMDC2) Protein. PLoS Genet. 2016;12:e1006393. doi: 10.1371/journal.pgen.1006393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb T, Lichten M. Frequent and efficient use of the sister chromatid for DNA double-strand break repair during budding yeast meiosis. PLoS Biol. 2010;8:e1000520. doi: 10.1371/journal.pbio.1000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray S, Cohen PE. Control of Meiotic Crossovers: From Double-Strand Break Formation to Designation. Annu Rev Genet. 2016;50:175–210. doi: 10.1146/annurev-genet-120215-035111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirao A, Cheung A, Duncan G, Girard PM, Elia AJ, Wakeham A, Okada H, Sarkissian T, Wong JA, Sakai T, et al. Chk2 is a tumor suppressor that regulates apoptosis in both an ataxia telangiectasia mutated (ATM)-dependent and an ATM-independent manner. Mol Cell Biol. 2002;22:6521–6532. doi: 10.1128/MCB.22.18.6521-6532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homolka D, Jansa P, Forejt J. Genetically enhanced asynapsis of autosomal chromatin promotes transcriptional dysregulation and meiotic failure. Chromosoma. 2012;121:91–104. doi: 10.1007/s00412-011-0346-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichijima Y, Ichijima M, Lou Z, Nussenzweig A, Camerini-Otero RD, Chen J, Andreassen PR, Namekawa SH. MDC1 directs chromosome-wide silencing of the sex chromosomes in male germ cells. Genes Dev. 2011;25:959–971. doi: 10.1101/gad.2030811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi N, Brown MS, Bishop DK, Borner GV. Gradual implementation of the meiotic recombination program via checkpoint pathways controlled by global DSB levels. Mol Cell. 2015;57:797–811. doi: 10.1016/j.molcel.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauppi L, Barchi M, Lange J, Baudat F, Jasin M, Keeney S. Numerical constraints and feedback control of double-strand breaks in mouse meiosis. Genes Dev. 2013;27:873–886. doi: 10.1101/gad.213652.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogo H, Tsutsumi M, Inagaki H, Ohye T, Kiyonari H, Kurahashi H. HORMAD2 is essential for synapsis surveillance during meiotic prophase via the recruitment of ATR activity. Genes Cells. 2012a;17:897–912. doi: 10.1111/gtc.12005. [DOI] [PubMed] [Google Scholar]
- Kogo H, Tsutsumi M, Ohye T, Inagaki H, Abe T, Kurahashi H. HORMAD1-dependent checkpoint/surveillance mechanism eliminates asynaptic oocytes. Genes Cells. 2012b;17:439–454. doi: 10.1111/j.1365-2443.2012.01600.x. [DOI] [PubMed] [Google Scholar]
- Kouznetsova A, Wang H, Bellani M, Camerini-Otero RD, Jessberger R, Hoog C. BRCA1-mediated chromatin silencing is limited to oocytes with a small number of asynapsed chromosomes. J Cell Sci. 2009;122:2446–2452. doi: 10.1242/jcs.049353. [DOI] [PubMed] [Google Scholar]
- Lam I, Keeney S. Mechanism and regulation of meiotic recombination initiation. Cold Spring Harbor perspectives in biology. 2014;7:a016634. doi: 10.1101/cshperspect.a016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latypov V, Rothenberg M, Lorenz A, Octobre G, Csutak O, Lehmann E, Loidl J, Kohli J. Roles of Hop1 and Mek1 in meiotic chromosome pairing and recombination partner choice in Schizosaccharomyces pombe. Mol Cell Biol. 2010;30:1570–1581. doi: 10.1128/MCB.00919-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMaire-Adkins R, Radke K, Hunt PA. Lack of checkpoint control at the metaphase/anaphase transition: a mechanism of meiotic nondisjunction in mammalian females. J Cell Biol. 1997;139:1611–1619. doi: 10.1083/jcb.139.7.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XC, Bolcun-Filas E, Schimenti JC. Genetic evidence that synaptonemal complex axial elements govern recombination pathway choice in mice. Genetics. 2011;189:71–82. doi: 10.1534/genetics.111.130674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XC, Schimenti JC. Mouse pachytene checkpoint 2 (Trip13) is required for completing meiotic recombination but not synapsis. PLoS Genet. 2007;3:e130. doi: 10.1371/journal.pgen.0030130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Hartford SA, Zeng R, Southard TL, Shima N, Schimenti JC. Hypersensitivity of primordial germ cells to compromised replication-associated DNA repair involves ATM-p53-p21 signaling. PLoS Genet. 2014;10:e1004471. doi: 10.1371/journal.pgen.1004471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen AJ, Hochwagen A. Checkpoint mechanisms: the puppet masters of meiotic prophase. Trends Cell Biol. 2011;21:393–400. doi: 10.1016/j.tcb.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Malki S, van der Heijden GW, O'Donnell KA, Martin SL, Bortvin A. A role for retrotransposon LINE-1 in fetal oocyte attrition in mice. Dev Cell. 2014;29:521–533. doi: 10.1016/j.devcel.2014.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltaris T, Seufert R, Fischl F, Schaffrath M, Pollow K, Koelbl H, Dittrich R. The effect of cancer treatment on female fertility and strategies for preserving fertility. Eur J Obstet Gynecol Reprod Biol. 2007;130:148–155. doi: 10.1016/j.ejogrb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Mao-Draayer Y, Galbraith AM, Pittman DL, Cool M, Malone RE. Analysis of meiotic recombination pathways in the yeast, Saccharomyces cerevisiae. Genetics. 1996;144:71–86. doi: 10.1093/genetics/144.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meirow D, Nugent D. The effects of radiotherapy and chemotherapy on female reproduction. Human reproduction update. 2001;7:535–543. doi: 10.1093/humupd/7.6.535. [DOI] [PubMed] [Google Scholar]
- Murphey P, McLean DJ, McMahan CA, Walter CA, McCarrey JR. Enhanced genetic integrity in mouse germ cells. Biol Reprod. 2013;88:6. doi: 10.1095/biolreprod.112.103481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler JJ, Braun RE. Fanconi anemia complementation group C is required for proliferation of murine primordial germ cells. Genesis. 2000;27:117–123. doi: 10.1002/1526-968x(200007)27:3<117::aid-gene40>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Niu H, Wan L, Baumgartner B, Schaefer D, Loidl J, Hollingsworth NM. Partner choice during meiosis is regulated by Hop1-promoted dimerization of Mek1. Mol Biol Cell. 2005;16:5804–5818. doi: 10.1091/mbc.E05-05-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco S, Marcet-Ortega M, Lange J, Jasin M, Keeney S, Roig I. The ATM signaling cascade promotes recombination-dependent pachytene arrest in mouse spermatocytes. PLoS Genet. 2015;11:e1005017. doi: 10.1371/journal.pgen.1005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez GI, Knudson CM, Leykin L, Korsmeyer SJ, Tilly JL. Apoptosis-associated signaling pathways are required for chemotherapy-mediated female germ cell destruction. Nat Med. 1997;3:1228–1232. doi: 10.1038/nm1197-1228. [DOI] [PubMed] [Google Scholar]
- Peters AH, Plug AW, van Vugt MJ, de Boer P. A drying-down technique for the spreading of mammalian meiocytes from the male and female germline. Chromosome research : an international journal on the molecular, supramolecular and evolutionary aspects of chromosome biology. 1997;5:66–68. doi: 10.1023/a:1018445520117. [DOI] [PubMed] [Google Scholar]
- Pittman D, Cobb J, Schimenti K, Wilson L, Cooper D, Brignull E, Handel MA, Schimenti J. Meiotic prophase arrest with failure of chromosome pairing and synapsis in mice deficient for Dmc1, a germline-specific RecA homolog. Mol Cell. 1998;1:697–705. doi: 10.1016/s1097-2765(00)80069-6. [DOI] [PubMed] [Google Scholar]
- Reinholdt L, Ashley T, Schimenti J, Shima N. Forward genetic screens for meiotic and mitotic recombination-defective mutants in mice. Methods Mol Biol. 2004;262:87–107. doi: 10.1385/1-59259-761-0:087. [DOI] [PubMed] [Google Scholar]
- Reinholdt LG, Schimenti JC. Mei1 is epistatic to Dmc1 during mouse meiosis. Chromosoma. 2005;114:127–134. doi: 10.1007/s00412-005-0346-4. [DOI] [PubMed] [Google Scholar]
- Rinaldi VD, Hsieh K, Munroe R, Bolcun-Filas EM, Schimenti JC. Pharmacological Inhibition of the DNA Damage Checkpoint Prevents Radiation-Induced Oocyte Death. Genetics. 2017 doi: 10.1534/genetics.117.203455. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder GS, Bailis JM. The pachytene checkpoint. Trends Genet. 2000;16:395–403. doi: 10.1016/s0168-9525(00)02080-1. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwacha A, Kleckner N. Interhomolog bias during meiotic recombination: meiotic functions promote a highly differentiated interhomolog-only pathway. Cell. 1997;90:1123–1135. doi: 10.1016/s0092-8674(00)80378-5. [DOI] [PubMed] [Google Scholar]
- Shin YH, Choi Y, Erdin SU, Yatsenko SA, Kloc M, Yang F, Wang PJ, Meistrich ML, Rajkovic A. Hormad1 mutation disrupts synaptonemal complex formation, recombination, and chromosome segregation in mammalian meiosis. PLoS Genet. 2010;6:e1001190. doi: 10.1371/journal.pgen.1001190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin YH, McGuire MM, Rajkovic A. Mouse HORMAD1 is a meiosis i checkpoint protein that modulates DNA double-strand break repair during female meiosis. Biol Reprod. 2013;89:29. doi: 10.1095/biolreprod.112.106773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J, Tho LM, Xu N, Gillespie DA. The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Adv Cancer Res. 2010;108:73–112. doi: 10.1016/B978-0-12-380888-2.00003-0. [DOI] [PubMed] [Google Scholar]
- Stambrook PJ, Tichy ED. Preservation of genomic integrity in mouse embryonic stem cells. Adv Exp Medi Biol. 2010;695:59–75. doi: 10.1007/978-1-4419-7037-4_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanzione M, Baumann M, Papanikos F, Dereli I, Lange J, Ramlal A, Trankner D, Shibuya H, de Massy B, Watanabe Y, et al. Meiotic DNA break formation requires the unsynapsed chromosome axis-binding protein IHO1 (CCDC36) in mice. Nat Cell Biol. 2016;18:1208–1220. doi: 10.1038/ncb3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian VV, Hochwagen A. The meiotic checkpoint network: step-by-step through meiotic prophase. Cold Spring Harbor perspectives in biology. 2014;6:a016675. doi: 10.1101/cshperspect.a016675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian VV, MacQueen AJ, Vader G, Shinohara M, Sanchez A, Borde V, Shinohara A, Hochwagen A. Chromosome Synapsis Alleviates Mek1-Dependent Suppression of Meiotic DNA Repair. PLoS Biol. 2016;14:e1002369. doi: 10.1371/journal.pbio.1002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh EK, Yang A, Kettenbach A, Bamberger C, Michaelis AH, Zhu Z, Elvin JA, Bronson RT, Crum CP, McKeon F. p63 protects the female germ line during meiotic arrest. Nature. 2006;444:624–628. doi: 10.1038/nature05337. [DOI] [PubMed] [Google Scholar]
- Turner JM, Aprelikova O, Xu X, Wang R, Kim S, Chandramouli GV, Barrett JC, Burgoyne PS, Deng CX. BRCA1, histone H2AX phosphorylation, and male meiotic sex chromosome inactivation. Curr Biol. 2004;14:2135–2142. doi: 10.1016/j.cub.2004.11.032. [DOI] [PubMed] [Google Scholar]
- van der Heijden GW, Bortvin A. Transient relaxation of transposon silencing at the onset of mammalian meiosis. Epigenetics. 2009;4:76–79. doi: 10.4161/epi.4.2.7783. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Mii S, Asai N, Asai M, Niimi K, Ushida K, Kato T, Enomoto A, Ishii H, Takahashi M, et al. The REV7 subunit of DNA polymerase zeta is essential for primordial germ cell maintenance in the mouse. J Biol Chem. 2013;288:10459–10471. doi: 10.1074/jbc.M112.421966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtasz L, Cloutier JM, Baumann M, Daniel K, Varga J, Fu J, Anastassiadis K, Stewart AF, Remenyi A, Turner JM, et al. Meiotic DNA double-strand breaks and chromosome asynapsis in mice are monitored by distinct HORMAD2-independent and -dependent mechanisms. Genes Dev. 2012;26:958–973. doi: 10.1101/gad.187559.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtasz L, Daniel K, Roig I, Bolcun-Filas E, Xu H, Boonsanay V, Eckmann CR, Cooke HJ, Jasin M, Keeney S, et al. Mouse HORMAD1 and HORMAD2, two conserved meiotic chromosomal proteins, are depleted from synapsed chromosome axes with the help of TRIP13 AAA-ATPase. PLoS Genet. 2009;5:e1000702. doi: 10.1371/journal.pgen.1000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1: Related to Figure 4
Supplemental Table S2: Related to Figure 5