Abstract
Background:
The rational design and optimization of tissue engineering strategies for cell-based therapy requires a baseline understanding of the concentration and prevalence of osteogenic progenitor cell populations in the source tissues. The aim of this study was to (1) define the efficiency of, and variation among individuals in, bone marrow aspiration as a means of osteogenic connective tissue progenitor (CTP-O) harvest compared with harvest from iliac cancellous bone, and (2) determine the location of CTP-Os within native cancellous bone and their distribution between the marrow-space and trabecular-surface tissue compartments.
Methods:
Eight 2-mL bone marrow aspiration (BMA) samples and one 7-mm transcortical biopsy sample were obtained from the anterior iliac crest of 33 human subjects. Two cell populations were obtained from the iliac cancellous bone (ICB) sample. The ICB sample was placed into αMEM (alpha-minimal essential medium) with antibiotic-antimycotic and minced into small pieces (1 to 2 mm in diameter) with a sharp osteotome. Cells that could be mechanically disassociated from the ICB sample were defined as marrow-space (IC-MS) cells, and cells that were disassociated only after enzymatic digestion were defined as trabecular-surface (IC-TS) cells. The 3 sources of bone and marrow-derived cells were compared on the basis of cellularity and the concentration and prevalence of CTP-Os through colony-forming unit (CFU) analysis.
Results:
Large variation was seen among patients with respect to cell and CTP-O yield from the IC-MS, IC-TS, and BMA samples and in the relative distribution of CTP-Os between the IC-MS and IC-TS fractions. The CTP-O prevalence was highest in the IC-TS fraction, which was 11.4-fold greater than in the IC-MS fraction (p < 0.0001) and 1.7-fold greater than in the BMA fraction. However, the median concentration of CTP-Os in the ICB (combining MS and TS fractions) was only 3.04 ± 1.1-fold greater than that in BMA (4,265 compared with 1,402 CTP/mL; p = 0.00004).
Conclusions:
Bone marrow aspiration of a 2-mL volume at a given needle site is an effective means of harvesting CTP-Os, albeit diluted with peripheral blood. However, the median concentration of CTP-Os is 3-fold less than from native iliac cancellous bone. The distribution of CTP-Os between the IC-MS and IC-TS fractions varies widely among patients.
Clinical Relevance:
Bone marrow aspiration is an effective means of harvesting CTP-Os but is associated with dilution with peripheral blood. Overall, we found that 63.5% of all CTP-Os within iliac cancellous bone resided on the trabecular surface; however, 48% of the patients had more CTP-Os contributed by the IC-MS than the IC-TS fraction.
Tissue regeneration and effective remodeling in all settings requires the effective recruitment and activation of stem cells and progenitor cells, whose progeny are capable of generating the new tissue or tissues that are required1,2. As a result, the rational development of cell-based therapy strategies that target or use progenitor cell populations requires an understanding of the concentration, prevalence, biological potential, heterogeneity, and distribution of stem cells and progenitor cells that may be of use3,4. The term connective tissue progenitor (CTP) has been defined and used in this effort. CTPs are defined as the heterogeneous population of “tissue-resident” stem and progenitor cells that are capable of being activated to proliferate and generate progeny that can differentiate to express ≥1 connective-tissue phenotype5-10. CTPs can be assayed on the basis of colony formation. CTPs that give rise to colonies that exhibit osteoblastic differentiation can be defined as osteogenic CTPs (CTP-Os). Thus, the term CTP-O includes all potential sources of native bone-forming stem cells and progenitor cells that are clinically available today for orthopaedic tissue engineering.
Note that a colony-founding CTP in native tissues (i.e., a tissue-resident cell) whose progeny can differentiate into bone is distinct from the definition of mesenchymal stem cells (MSCs). In contrast to the heterogeneous population of tissue-resident CTPs, MSCs are defined as purified homogeneous, culture-expanded cells that retain pluripotency (i.e., the capacity to differentiate into bone, cartilage, and fat) and that also express a defined set of cell surface markers11.
The presence of colony-founding CTP-Os in cancellous bone has been well documented12, making autogenous bone a reliable clinical source of osteogenic progenitors12-19. CTP-Os can also be readily harvested by bone marrow aspiration. The iliac crest is the most anatomically accessible site for the harvest of a large volume of bone or bone marrow9,10,20-22. Aspiration techniques that limit dilution with peripheral blood, using multiple small-volume aspirations, have been defined and advocated to optimize the concentration and yield of CTP-Os6,7,23. CTPs can also be isolated by the in vitro digestion of explanted tissues, such as fat24,25 and muscle26-29, although the CTP populations in other tissues demonstrate different patterns of differentiation and media requirements in vitro30.
All of these cell sources represent potential tools for tissue engineering of bone and other musculoskeletal tissues, although the biological potential of CTPs may vary by tissue source. Substantial variation has been reported from subject to subject and sample to sample. Some of this variation is related to age and sex8 and some, to surgical site and technique9,10. The extent to which the intrinsic histological or metabolic properties of local tissues are a source of variation remains to be determined.
The rational design and optimization of cell-based approaches to bone regeneration requires a baseline understanding of the concentration and prevalence of CTP-Os in specific source tissues and the sources and magnitude of variation among the tissues of individual patients. The aim of this study was to (1) define the efficiency of, and variation among individuals in, bone marrow aspiration as a means of CTP-O harvest compared with harvest from iliac cancellous bone, and (2) determine the location of CTP-Os within native cancellous bone and their distribution between the marrow space and trabecular surface tissue compartments.
Materials and Methods
Bone Marrow Aspiration and Processing
This study received institutional board approval, and the patients provided informed consent. Eight samples from bone marrow aspiration and 1 transcortical biopsy sample (7 mm in diameter) were obtained from the anterior iliac crest in 33 patients (21 female and 12 male) who were undergoing elective hip arthroplasty procedures. The median patient age was 61 years (range, 44 to 81 years). The mean body mass index (and standard deviation) was 31.6 ± 9.4 kg/m2 (range, 22.1 to 48.2 kg/m2). The inclusion and exclusion criteria are shown in the Appendix.
Samples were harvested from the ipsilateral anterior iliac crest. A 1-mm stab incision was made parallel to the Langer lines in the skin. A bone marrow aspiration needle (Lee-Lok; Lee Medical) was advanced with a lateral technique. The needle tip was advanced through the lateral cortex of the pelvis into the intramedullary cavity. The obturator was removed, and a 10-mL syringe containing 1 mL of heparin (1,000 units of Na-heparin/mL) was attached. Eight bone marrow aspirations—which, as recommended from prior work, were limited to a 2-mL volume at a given needle site to limit dilution with peripheral blood—were performed6. The 8 samples were then pooled (the BMA [bone marrow aspiration] fraction). The BMA fraction then was suspended in 48 mL of alpha-minimum essential medium (α-MEM; GIBCO) containing 20 U/mL of Na-heparin and then centrifuged at 400 × g for 10 minutes6,8,23. The nucleated cell fraction (buffy coat) was isolated and resuspended in osteogenic media: α-MEM containing 10% fetal bovine serum (BioWhittaker), 50 μg/mL of sodium ascorbate (Sigma), and 1% penicillin-streptomycin (Life Technologies).
Transcortical Iliac Crest Biopsy and Cancellous-Bone Processing
After bone marrow aspirate harvest, a customized, 7-mm core biopsy needle (modified Mayo needle or Sims needle) was used to harvest a transcortical sample from the iliac crest31 (Fig. 1). This needle was advanced using a trocar and sleeve to a site approximately 2 cm posterior to the anterior superior iliac spine, i.e., 1 cm anterior to the region of aspiration. The core sample was taken using a manual technique. Each core sample was measured to calculate the iliac cancellous bone (ICB) volume, and the cortical bone ends were removed sharply (Fig. 1). The ICB sample was then placed into αMEM with 1% penicillin-streptomycin (Life Technologies) and minced into small pieces (1 to 2 mm in diameter) with a sharp osteotome. Cells that were mechanically liberated by the mincing process and agitation were collected and defined as the iliac crest marrow space (IC-MS) fraction32. Cells that remained adherent to the trabecular surface (IC-TS fraction) were then recovered by collagenase type-I digestion (Sigma), 100 U/mL for 90 minutes at 37°C. The number of cells in each fraction was counted using a hemocytometer with the nucleated cell concentration (cellularity) presented as nucleated cells × 106 per mL of aspirate or per g of tissue. To determine the prevalence of CTP-Os in each sample, an established CTP-O colony-forming unit (CFU) assay was used33-37. Cells from the BMA and IC-MS fractions were plated at a density of 5 × 105 nucleated cells in each of two 4.2-cm2 wells on a glass chamber slide (Lab-Tek; Nalge Nunc International) in osteogenic medium (αMEM containing 10% fetal bovine serum, 10−8M dexamethasone, 25 mg/mL ascorbate, and penicillin-streptomycin). Cells from the IC-TS fraction were plated at a density of 1 × 105 nucleated cells per 4.2-cm2 chamber slide, to avoid overcrowding the CTP-O colonies because of the generally larger prevalence of CTP-Os in the IC-TS fraction, and they were cultured in the same condition as the BMA and IC-MS fractions. Cultures were maintained at 37°C in a humidified atmosphere of 5% CO2 in air. The medium was first changed after 48 hours. Cultures were harvested and fixed on day 6 with acetone-methanol (1:1). DAPI (4ʹ,6-diamidino-2-phenylindole; Vector Laboratories), a fluorescent stain that binds strongly to AT-rich regions in DNA, was used to identify nuclei. Alkaline phosphatase staining was used as a marker of early osteoblastic differentiation6,8,23,33,37.
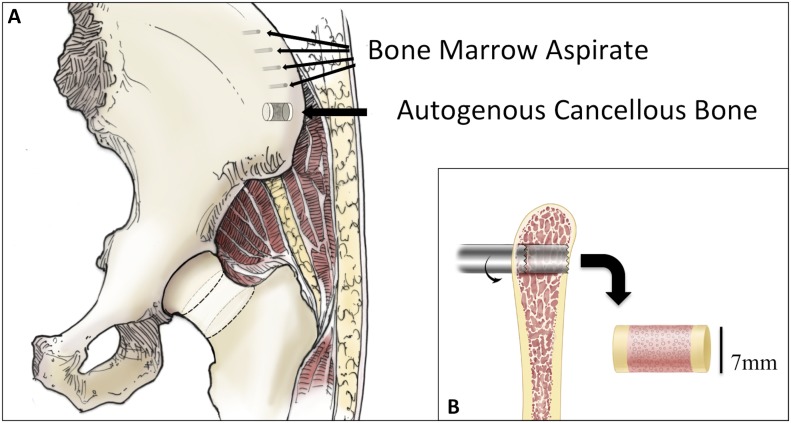
Fig. 1.
Fig. 1-A Anatomical representation of the human iliac crest, with the sites of bone marrow aspiration and autogenous cancellous bone harvest indicated. Fig. 1-B Sagittal view of the iliac crest depicting the transcortical bone core sample (7 mm in diameter) harvested with a customized biopsy needle.
Image Acquisition and CFU Analysis
The fluorescent image of the entire chamber area (2.05 × 2.05 cm) of each sample was acquired with a 2,048 × 3,072 Quantix K6303E 12-bit digital camera (Roper Scientific) attached to a Leica DMXRA motorized fluorescent microscope equipped with a motorized x-y stage. The automated imaging system acquired 540 individual images (20 columns × 27 rows), covering the entire chamber surface. These 512 × 768, 8-bit gray-level images were acquired using a ×10 objective (pixel size = 1.78 μm). Nuclei stained with DAPI allowed discrete localization of CTP colonies and the overlay of anteroposterior images (Fig. 2).
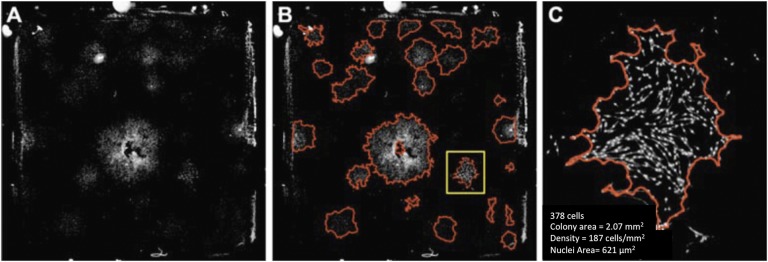
Fig. 2.
Fig. 2-A Large field-of-view images (480 image tiles stitched together) of CTPs seeded on 20.5 × 20.5-mm chamber slides and stained with DAPI. The images shown here have been background-corrected (flattening illumination for each tile) and processed for the removal of artifacts. Fig. 2-B Images processed for colony segmentation (red outline) using an automated algorithm. Fig. 2-C Magnified colony (delineated by yellow in Fig. 2-B) indicating quantitative parameters that may be extracted. (Reprinted, with permission, from ASTM F2944-12 Standard Test Method for Automated Colony Forming Unit (CFU) Assays—Image Acquisition and Analysis Method for Enumerating and Characterizing Cells and Colonies in Culture, copyright ASTM International, 100 Barr Harbor Drive, West Conshohocken, PA 19428. A copy of the complete standard may be obtained from ASTM, www.astm.org.)
The quantitative characterization of each colony (cell number, colony surface area, cell density, and alkaline phosphatase area) was performed using a custom software suite (Colonyze; Cleveland Clinic)37. A colony was defined as a group of ≥8 cells at day 6, based on proximity suggesting a common founding cell (i.e., CTP)33-36. CTP-O prevalence was defined as the number of colonies that formed per million cells plated37. To compare the in vitro performance of the progeny of the founding CTP, we measured and compared 3 colony-level metrics: cells per colony, cell density within each colony (cells per mm2), and the area of alkaline phosphatase expression per cell (mean area of alkaline phosphatase-positive pixels/cell presented as μm2/cell). These provided metrics of relative proliferation, migration, and early osteoblastic differentiation23.
Statistical Analysis
The primary outcomes for each sample fraction (BMA, IC-MS, and IC-TS) were cellularity, prevalence of CTP-Os, and CTP-O concentration. The total content of the ICB samples (IC-Total) was calculated as the sum of cells or CTP-Os from the IC-MS and IC-TS fractions for each subject. An analysis of variance (ANOVA) was used to compare the fractions. The patient effect was included as a random factor and Tukey tests were used to compare the means. Because of the skewed distributions, a log transformation was used to carry out the analyses of the 3 outcomes. Back transformations were used to allow results to be reported as geometric means and corresponding 95% confidence intervals (CIs). All analyses were performed using JMP 8.0 (SAS Institute).
Results
Nucleated Cell Concentration in BMA and ICB Samples
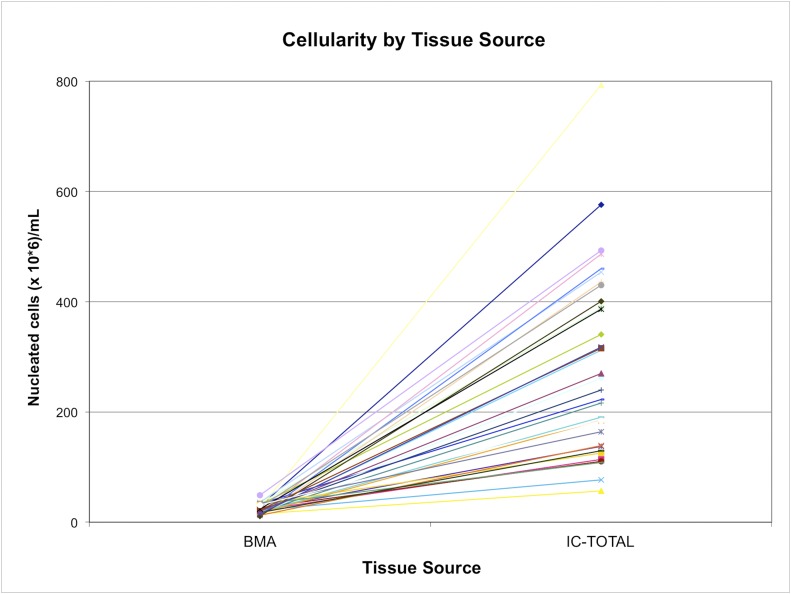
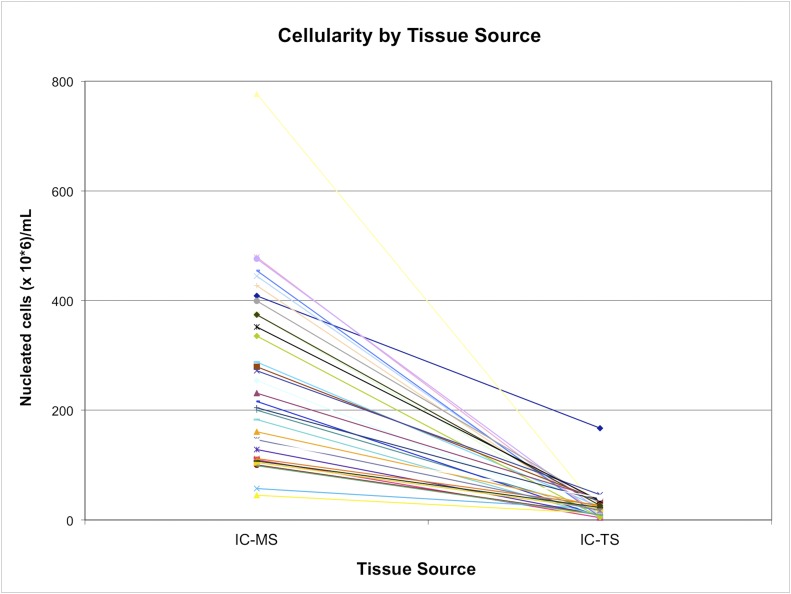
The geometric mean nucleated cell concentration in the IC-MS fraction was 256 ± 196 × 106 nucleated cells/mL, which was significantly higher than in the BMA fraction (21.9 ± 8.9 × 106 nucleated cells/mL) and IC-TS fraction (18.1 ± 18.9 × 106 nucleated cells/mL) (p < 0.001) (Table I). The nucleated cell concentration was highly variable among subjects, particularly with respect to the IC-MS fraction. The IC-MS fraction, therefore, dominated the variation in the calculation of IC-Total nucleated cell concentration (Figs. 3 and 4; see Appendix).
TABLE I.
Comparison of Bone Marrow Aspiration (BMA), Iliac Crest Marrow Space (IC-MS), and Iliac Crest Trabecular Surface (IC-TS) Fractions
| Outcome | BMA* | IC-MS* | IC-TS* | P Value† |
| Cellularity (nucleated cells × 106 per mL of aspirate or per g of tissue) | 21.9 (19.0-25.2)‡ | 256 (202-324) | 18.1 (14.2-23.0)‡ | <0.0001 |
| CTP-O prevalence (CTP-O per 106 nucleated cells) | 38.1 (25.7-56.6)‡ | 4.28 (2.9-6.4) | 58.9 (39.3-88.6)‡ | <0.0001 |
| CTP-O concentration (CTP-O per mL of aspirate or per g of tissue) | 835 (506-1,375) | 1,180 (712-1,977) | 1,155 (689-1,936) | 0.73 |
| No. of cells per colony (a metric of proliferation) | 58.29‡ | 35.5§ | 38.2‡§ | 0.013 |
| Cell density (a metric of cell migration) (cells per mm2) | 153 (135-173) | 125 (111-142) | 150 (133-170) | 0.051 |
| Area of alkaline phosphatase activity per cell (a metric of differentiation) (μm2/cell) | 28.2 (17.0-46.7) | 39.6 (23.9-65.6) | 67.7 (40.8-112) | 0.064 |
The values are given as the geometric mean, with the 95% confidence interval in parentheses.
ANOVA.
No significant difference between the indicated groups according to Tukey test, with alpha = 0.05.
No significant difference between the indicated groups according to Tukey test, with alpha = 0.05.
Fig. 3.
The yield of nucleated cells (cellularity) from bone marrow aspiration (BMA) and iliac crest bone, with values for each subject linked by a colored line. IC-TOTAL represents the combined total from the iliac crest marrow space (IC-MS) and trabecular surface (IC-TS) samples. The values are given as millions of nucleated cells per mL of sample.
Fig. 4.
The yield of nucleated cells (cellularity) from the marrow space (IC-MS) and trabecular surface (IC-TS) fractions obtained from the iliac crest bone, with values for each subject linked by a colored line. The values are given as millions of nucleated cells per mL of sample.
CTP-O Prevalence Among BMA, IC-MS, and IC-TS Fractions
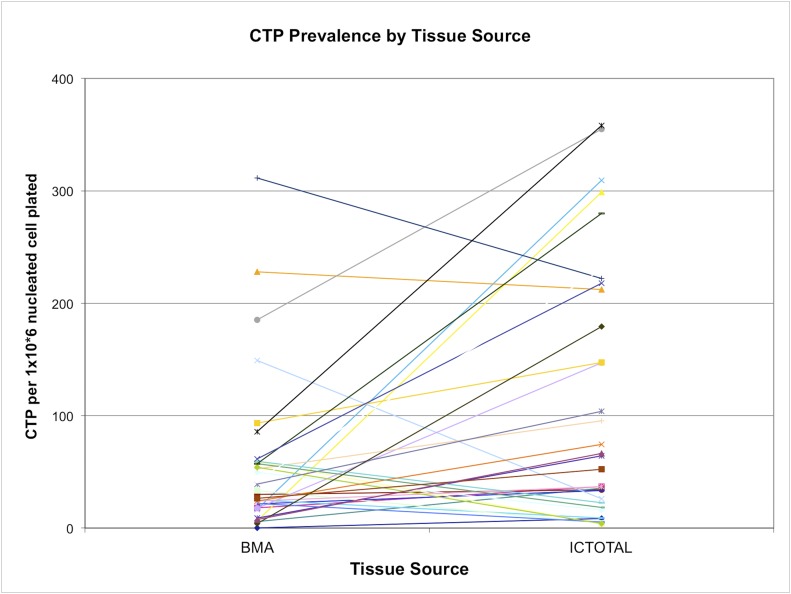
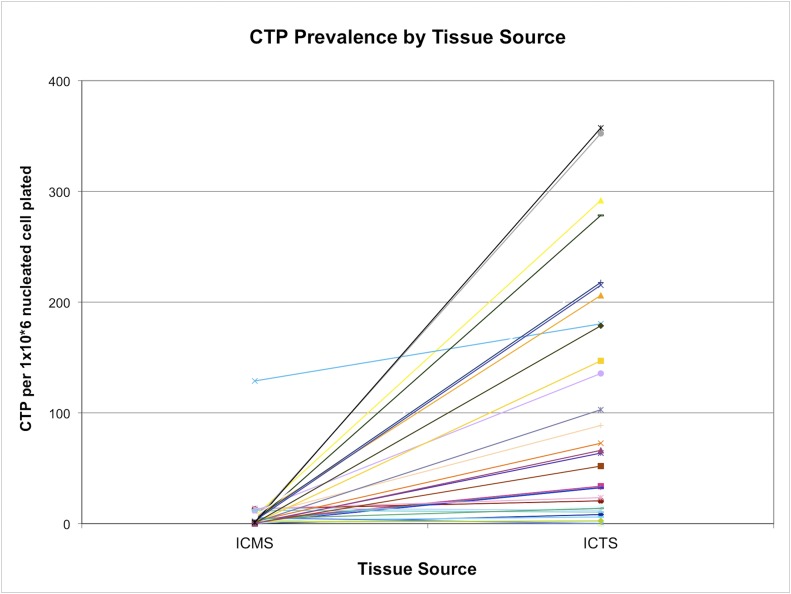
The CTP-O prevalence in the BMA and IC-TS fractions was highly variable. By contrast, the IC-MS fraction showed less variation (Figs. 5 and 6). Absolute CTP-O prevalence was much lower in the IC-MS fraction (geometric mean, 4.28 ± 22.6 CTP-Os per 106 nucleated cells plated) compared with that in the BMA fraction (38.1 ± 69.0 CTP-Os per 106 nucleated cells plated) and the IC-TS fraction (58.9 ± 105.6 CTP-Os per 106 nucleated cells plated) (p < 0.001). The CTP-O prevalence did not differ significantly between the BMA and IC-TS fractions (see Appendix). The CTP-O prevalence was 11.4-fold greater in the IC-TS fraction than in the IC-MS fraction (p < 0.0001) and 1.7-fold greater than in the BMA fraction.
Fig. 5.
The prevalence of CTP-Os from bone marrow aspiration (BMA) and iliac crest bone core, with values for each subject linked by a colored line. IC-TOTAL represents the combined total from the iliac crest marrow space (IC-MS) and trabecular surface (IC-TS) samples. The values are given as the number of CTP-Os per million nucleated cells plated.
Fig. 6.
The prevalence of CTP-Os in the marrow space (IC-MS) and trabecular surface (IC-TS) fractions obtained from the iliac crest bone, with values for each subject linked by a colored line. The values are given as the number of CTPs per million nucleated cells plated.
CTP-O Concentration in BMA and ICB Samples
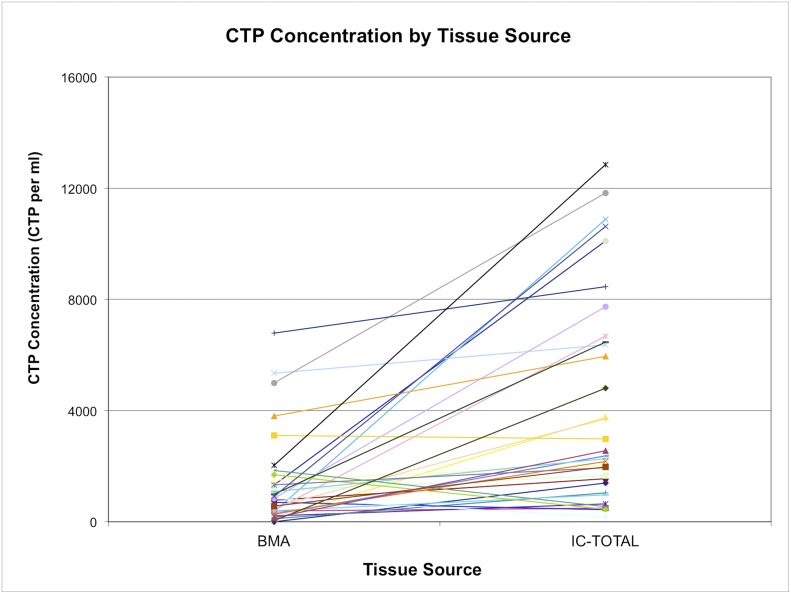
The CTP-O concentration in each fraction was calculated as the product of the nucleated cell concentration (106 of nucleated cells per mL or per g of sample) times the CTP-O prevalence (CTP-Os per 106 cells). Figure 7 illustrates that there was significant variation in the CTP-O concentration among bone marrow aspiration samples as well as for the IC-Total. The geometric mean CTP-O concentration in the IC-Total of 2,335 CTP-Os per mL (95% CI, 1,429 to 5,001 CTP-Os per mL) was significantly greater than that for the BMA fraction (835 CTP-Os per mL [95% CI, 506 to 1,375 CTP-Os per mL]) (p < 0.0001) (Table I). The CTP-O concentration did not differ significantly among the BMA, IC-MS, and IC-TS fractions (p = 0.73).
Fig. 7.
The concentration of CTP-Os from bone marrow aspiration (BMA) compared with iliac crest bone core, with values for each subject linked by a colored line. IC-TOTAL represents the combined total from the iliac crest marrow space (IC-MS) and trabecular surface (IC-TS) samples. The values are given as the number of CTP-Os per mL of tissue.
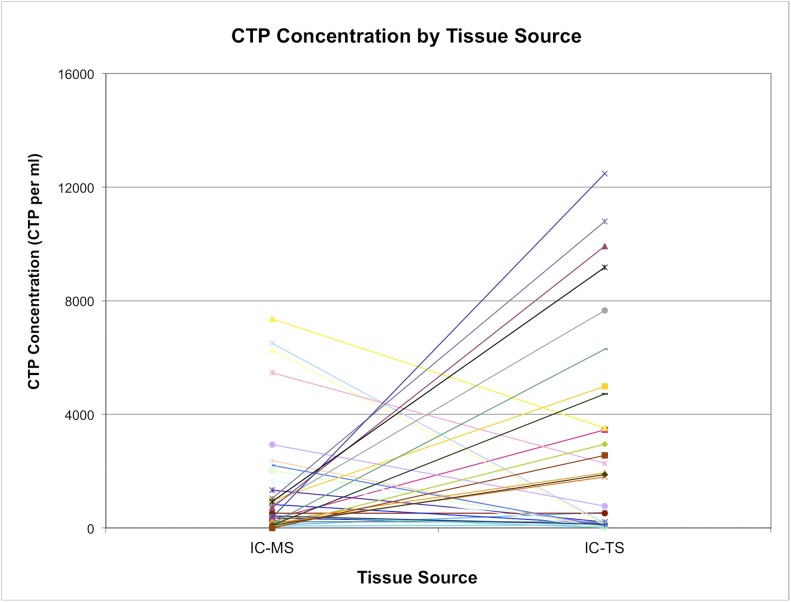
Overall, the mean contribution of IC-TS-derived CTP-Os to the IC-Total CTP-Os was 0.8 (95% CI, 0.50 to 1.1). However, this ratio was highly variable among subjects. Seventeen (52%) of the subjects had more CTP-Os contributed by TS cells, and 16 (48%) had more CTP-Os contributed by MS cells (Fig. 8; see Appendix). The median concentration of CTP-Os in the ICB (combining MS and TS fractions) was only 3.04 ± 1.1-fold greater than that in BMA (4,265 compared with 1,402 CTP/mL; p = 0.00004).
Fig. 8.
The concentration of CTP-Os in the marrow space (IC-MS) and trabecular surface (IC-TS) fractions obtained from the iliac crest bone, with values for each subject linked by a colored line. The values are given as the number of CTP-Os per mL of tissue.
Comparison of CTP Colony-Level Metrics Among BMA, IC-MS, and IC-TS Fractions
As shown in Table I, colonies founded by CTP-Os obtained by bone marrow aspiration were more proliferative than were colonies founded by CTP-Os obtained from the IC-MS fraction or the IC-TS fraction (58.3 cells per colony compared with 35.5 and 38.2 cells per colony, respectively; p = 0.013). There were no significant differences in cell density or alkaline phosphatase activity among the 3 fractions. However, there was a strong trend toward lower cell density in the IC-MS-derived colonies (p = 0.051) and greater alkaline phosphatase activity in the IC-TS-derived colonies (p = 0.064).
Discussion
This study was designed with 2 aims, namely, to (1) define the current efficiency of, and variation among individuals in, bone marrow aspiration as a means of CTP-O harvest compared with harvest from iliac cancellous bone, and (2) determine the location of CTP-Os within native cancellous bone and their distribution between the marrow-space and trabecular-surface tissue compartments, so as to inform the design and selection of CTP-O harvest and processing methods to enhance CTP-O concentration and prevalence in cell populations available for clinical use.
Regarding aim 1 (Figs. 3, 5, and 7), this study allowed the direct comparison of the yield of cells and CTP-Os using bone marrow aspiration versus open harvest of native iliac crest cancellous bone tissue. These data demonstrate wide variation in the concentration of cells and CTP-Os among individuals. Bone marrow aspiration efficacy and dilution can be calculated from CTP-O concentration data. Since blood contains virtually no colony-forming cells38, all of the colonies detected in bone marrow aspiration with this assay should represent CTPs with various capacities for osteogenic differentiation that are derived from ICB (either the MS or TS fraction). Because the geometric mean CTP-O concentration for the entire ICB across all subjects was 3,215 CTP-Os per mL and the mean for the BMA fraction was 835 CTP-Os per mL, the BMA fraction therefore contained a number of CTP-Os that represent roughly 26% of the CTP-Os present in the ICB. The remaining volume from bone marrow aspiration, a mean of 740 μL per mL of aspirate, would then be blood. This calculation provides an estimate of a roughly 4-fold dilution with peripheral blood. In fact, because of the skewing in the data, the most accurate calculation of mean dilution is derived from the geometric mean data for total CTP-O concentration for the BMA fraction compared with CTP-O concentration for the IC-Total, which yields an actual geometric mean dilution of 3.04 ± 1.1-fold.
Regarding aim 2 (Figs. 4, 6, and 8), we found that the location and distribution of cells and CTP-Os within these 2 tissue compartments varied widely from subject to subject. Overall, >90% of the nucleated cells in ICB can be collected by mincing and agitation (the IC-MS fraction), yielding a mean of 256 million cells per mL of ICB in this 33-patient cohort. Following the harvest of cells by mechanical means, an additional fraction of cells (<10% of the cells in ICB) can be released by enzymatic digestion to liberate them from the trabecular surface (IC-TS fraction), yielding a mean of 18 million cells per mL of ICB. Because of the >10-fold higher prevalence of CTP-Os in the IC-TS fraction, across all subjects, a mean of 63.5% of CTP-Os were derived from the IC-TS fraction, a geometric mean of 1,155 CTPs per mL of ICB. This is comparable with some reports involving rodent bone comparing yields of femoral irrigation and digestion39-41. These data indicate that, in general, more than half of all CTPs were resident on, or entrapped in, tissue near the trabecular surface. However, few patients overall fit this neat picture. In this study, 16 (48%) of the patients derived more CTP-Os from the IC-MS fraction than from the IC-TS fraction, while 85% of those had more than two-thirds of CTP-Os from the IC-MS fraction. This variation has 2 important implications for tissue-engineering strategies involving the harvest and processing of cells and CTP-Os from iliac cancellous bone. First, neither the IC-MS nor the IC-TS fraction can be neglected. Harvest and processing methods should include strategies for capture of CTP-Os from both tissue fractions, removing the possibility of discarding a patient’s primary CTP-O reservoir by selecting one or the other. Second, further work needs to be conducted to understand the physiological mechanism of this variation in the distribution of CTP-Os within iliac cancellous bone. The distribution or dominant location of CTP-Os may have clinical implications and diagnostic or predictive value.
We did not demonstrate definitive differences between CTP-Os derived from the BMA, MS, or TS tissue sources. Secondary analysis of the biological performance of CTP-Os derived from BMA, MS, or TS fractions indicated more rapid proliferation among the clonal progeny of BMA-derived CTP-Os. Trends were seen suggesting decreased cell density among the clonal progeny of MS-derived CTP-Os and an increase in alkaline phosphatase expression among clonal progeny of TS-derived CTP-Os. These findings could suggest that TS-derived CTP-Os could either be more osteogenic or more rapidly express osteogenic differentiation than MS-derived CTP-Os. Analysis of a larger dataset and in vitro assessment over longer time periods will be needed to determine if these trends are generalizable. Moreover, differences between MS and TS-derived CTPs may represent underlying differences in the biological potential among the colony-founding CTP-Os in these sites. It is also possible for differences in performance to arise as a result of exposure to hematopoietic cells in the MS fraction (either secreted factors, cell-cell contact, or degradation products). Differences could also arise due to secondary effects of the enzymatic digestion to which TS cells are exposed. Similarly, BMA-derived CTP-Os and their progeny, which demonstrated larger colony size than either the MS or TS-derived colonies, may be influenced by the trauma of bone marrow aspiration and/or greater exposure to serum, platelets, and mature circulating cells (monocytes, macrophages, lymphocytes, platelets, or erythrocytes) in the early culture environment, prior to media change at 48 hours. Therefore, at present, we consider the biological potential of CTP-Os derived from BMA, MS, or TS fractions to be comparable, until proven otherwise.
In conclusion, our data suggest that bone marrow aspiration, limiting aspiration volume to 2 mL per needle position, is an effective method for the harvest of CTP-Os from the iliac crest. However, even using this optimized method, bone marrow aspiration is still associated with a 3 to 4-fold dilution of CTP-Os with peripheral blood. CTP-Os can be found among cells in the marrow space that are easily separated by agitation and among cells on the trabecular surface that must be removed by enzymatic digestion. Because individuals vary widely with respect to the predominant location of CTP-Os, neither the MS nor TS fractions in cancellous bone can be neglected as a potential source of CTP-Os in current or future clinical applications. These data quantify the magnitude of opportunity that exists to improve on the efficiency of current bone marrow aspirate harvest methods.
Appendix
A table listing the inclusion and exclusion criteria and figures showing cellularity, CTP-O prevalence, and CTP-O concentration across tissue sources are available with the online version of this article as a data supplement at jbjs.org (http://links.lww.com/JBJS/E345).
Acknowledgments
Note: The authors thank Ingrid Baumann for skilled technical assistance.
Footnotes
Investigation performed at the Cleveland Clinic, Cleveland, Ohio
Disclosure: This work was performed through the support of NIH AR049687, Epidemiology of Connective Tissue Progenitor Cells (09/26/2002 to 08/31/2007), and NIH 1R01AR063733-01, Epidemiology of Human Chondrogenic Progenitor Cells (01/1/14 to 12/30/17). On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one or more of the authors checked “yes” to indicate that the author had other relationships or activities that could be perceived to influence, or have the potential to influence, what was written in this work (http://links.lww.com/JBJS/E344).
References
- 1.Muschler GF, Midura RJ, Nakamoto C. Practical modeling concepts for connective tissue stem cell and progenitor compartment kinetics. J Biomed Biotechnol. 2003;2003(3):170-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muschler GF, Midura RJ. Connective tissue progenitors: practical concepts for clinical applications. Clin Orthop Relat Res. 2002. February;395:66-80. [DOI] [PubMed] [Google Scholar]
- 3.Chahla J, Piuzzi NS, Mitchell JJ, Dean CS, Pascual-Garrido C, LaPrade RF, Muschler GF. Intra-articular cellular therapy for osteoarthritis and focal cartilage defects of the knee: a systematic review of the literature and study quality analysis. J Bone Joint Surg Am. 2016. September 21;98(18):1511-21. [DOI] [PubMed] [Google Scholar]
- 4.Marcucio RS, Nauth A, Giannoudis PV, Bahney C, Piuzzi NS, Muschler G, Miclau T 3rd. Stem cell therapies in orthopaedic trauma. J Orthop Trauma. 2015. December;29(12)(Suppl 12):S24-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLain RF, Fleming JE, Boehm CA, Muschler GF. Aspiration of osteoprogenitor cells for augmenting spinal fusion: comparison of progenitor cell concentrations from the vertebral body and iliac crest. J Bone Joint Surg Am. 2005. December;87(12):2655-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muschler GF, Boehm C, Easley K. Aspiration to obtain osteoblast progenitor cells from human bone marrow: the influence of aspiration volume. J Bone Joint Surg Am. 1997. November;79(11):1699-709. [DOI] [PubMed] [Google Scholar]
- 7.Muschler GF, Nakamoto C, Griffith LG. Engineering principles of clinical cell-based tissue engineering. J Bone Joint Surg Am. 2004. July;86(7):1541-58. [DOI] [PubMed] [Google Scholar]
- 8.Muschler GF, Nitto H, Boehm CA, Easley KA. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res. 2001. January;19(1):117-25. [DOI] [PubMed] [Google Scholar]
- 9.Hyer CF, Berlet GC, Bussewitz BW, Hankins T, Ziegler HL, Philbin TM. Quantitative assessment of the yield of osteoblastic connective tissue progenitors in bone marrow aspirate from the iliac crest, tibia, and calcaneus. J Bone Joint Surg Am. 2013. July 17;95(14):1312-6. [DOI] [PubMed] [Google Scholar]
- 10.Pierini M, Di Bella C, Dozza B, Frisoni T, Martella E, Bellotti C, Remondini D, Lucarelli E, Giannini S, Donati D. The posterior iliac crest outperforms the anterior iliac crest when obtaining mesenchymal stem cells from bone marrow. J Bone Joint Surg Am. 2013. June 19;95(12):1101-7. [DOI] [PubMed] [Google Scholar]
- 11.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-7. [DOI] [PubMed] [Google Scholar]
- 12.Tuli R, Tuli S, Nandi S, Wang ML, Alexander PG, Haleem-Smith H, Hozack WJ, Manner PA, Danielson KG, Tuan RS. Characterization of multipotential mesenchymal progenitor cells derived from human trabecular bone. Stem Cells. 2003;21(6):681-93. [DOI] [PubMed] [Google Scholar]
- 13.Sakaguchi Y, Sekiya I, Yagishita K, Ichinose S, Shinomiya K, Muneta T. Suspended cells from trabecular bone by collagenase digestion become virtually identical to mesenchymal stem cells obtained from marrow aspirates. Blood. 2004. November 1;104(9):2728-35. Epub 2004 Jul 8. [DOI] [PubMed] [Google Scholar]
- 14.Nuttall ME, Patton AJ, Olivera DL, Nadeau DP, Gowen M. Human trabecular bone cells are able to express both osteoblastic and adipocytic phenotype: implications for osteopenic disorders. J Bone Miner Res. 1998. March;13(3):371-82. [DOI] [PubMed] [Google Scholar]
- 15.Nöth U, Osyczka AM, Tuli R, Hickok NJ, Danielson KG, Tuan RS. Multilineage mesenchymal differentiation potential of human trabecular bone-derived cells. J Orthop Res. 2002. September;20(5):1060-9. [DOI] [PubMed] [Google Scholar]
- 16.Sottile V, Halleux C, Bassilana F, Keller H, Seuwen K. Stem cell characteristics of human trabecular bone-derived cells. Bone. 2002. May;30(5):699-704. [DOI] [PubMed] [Google Scholar]
- 17.Robey PG, Termine JD. Human bone cells in vitro. Calcif Tissue Int. 1985. September;37(5):453-60. [PubMed] [Google Scholar]
- 18.Robey PG. Collagenase-treated trabecular bone fragments: a reproducible source of cells in the osteoblastic lineage. Calcif Tissue Int. 1995;56(Suppl 1):S11-2. [Google Scholar]
- 19.Majeska RJ. Culture of osteoblastic cells. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of bone biology. San Diego: Academic Press; 1996. p 1229-37. [Google Scholar]
- 20.Sandhu HS, Grewal HS, Parvataneni H. Bone grafting for spinal fusion. Orthop Clin North Am. 1999. October;30(4):685-98. [DOI] [PubMed] [Google Scholar]
- 21.Savolainen S, Usenius JP, Hernesniemi J. Iliac crest versus artificial bone grafts in 250 cervical fusions. Acta Neurochir (Wien). 1994;129(1-2):54-7. [DOI] [PubMed] [Google Scholar]
- 22.Mirovsky Y, Neuwirth MG. Comparison between the outer table and intracortical methods of obtaining autogenous bone graft from the iliac crest. Spine. 2000. July 1;25(13):1722-5. [DOI] [PubMed] [Google Scholar]
- 23.Majors AK, Boehm CA, Nitto H, Midura RJ, Muschler GF. Characterization of human bone marrow stromal cells with respect to osteoblastic differentiation. J Orthop Res. 1997. July;15(4):546-57. [DOI] [PubMed] [Google Scholar]
- 24.Mizuno H. Adipose-derived stem cells for tissue repair and regeneration: ten years of research and a literature review. J Nippon Med Sch. 2009;76(2):56-66. [DOI] [PubMed] [Google Scholar]
- 25.de Villiers JA, Houreld N, Abrahamse H. Adipose derived stem cells and smooth muscle cells: implications for regenerative medicine. Stem Cell Rev. 2009. September;5(3):256-65. Epub 2009 Aug 11. [DOI] [PubMed] [Google Scholar]
- 26.Buckingham M, Montarras D. Skeletal muscle stem cells. Curr Opin Genet Dev. 2008. August;18(4):330-6. Epub 2008 Jul 30. [DOI] [PubMed] [Google Scholar]
- 27.Quintero AJ, Wright VJ, Fu FH, Huard J. Stem cells for the treatment of skeletal muscle injury. Clin Sports Med. 2009. January;28(1):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cerletti M, Shadrach JL, Jurga S, Sherwood R, Wagers AJ. Regulation and function of skeletal muscle stem cells. Cold Spring Harb Symp Quant Biol. 2008;73:317-22. Epub 2009 Feb 9. [DOI] [PubMed] [Google Scholar]
- 29.Schindeler A, Liu R, Little DG. The contribution of different cell lineages to bone repair: exploring a role for muscle stem cells. Differentiation. 2009. January;77(1):12-8. Epub 2008 Oct 22. [DOI] [PubMed] [Google Scholar]
- 30.Qadan MA. Sourcing and modulation of the fate of connective tissue progenitors [PhD dissertation]. Kent, OH: Kent State University; 2016. [Google Scholar]
- 31.Lundy MW, Stauffer M, Wergedal JE, Baylink DJ, Featherstone JD, Hodgson SF, Riggs BL. Histomorphometric analysis of iliac crest bone biopsies in placebo-treated versus fluoride-treated subjects. Osteoporos Int. 1995. March;5(2):115-29. [DOI] [PubMed] [Google Scholar]
- 32.Jonsson KB, Frost A, Nilsson O, Ljunghall S, Ljunggren O. Three isolation techniques for primary culture of human osteoblast-like cells: a comparison. Acta Orthop Scand. 1999. August;70(4):365-73. [DOI] [PubMed] [Google Scholar]
- 33.ASTM. Standard test method for automated colony forming unit (CFU) assays — image acquisition and analysis method for enumerating and characterizing cells and colonies in culture. ASTM Int. 2012:1-11. [Google Scholar]
- 34.Villarruel SM, Boehm CA, Pennington M, Bryan JA, Powell KA, Muschler GF. The effect of oxygen tension on the in vitro assay of human osteoblastic connective tissue progenitor cells. J Orthop Res. 2008. October;26(10):1390-7. [DOI] [PubMed] [Google Scholar]
- 35.Caralla T, Joshi P, Fleury S, Luangphakdy V, Shinohara K, Pan H, Boehm C, Vasanji A, Hefferan TE, Walker E, Yaszemski M, Hascall V, Zborowski M, Muschler GF. In vivo transplantation of autogenous marrow-derived cells following rapid intraoperative magnetic separation based on hyaluronan to augment bone regeneration. Tissue Eng Part A. 2013. January;19(1-2):125-34. Epub 2012 Oct 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heylman CM, Caralla TN, Boehm CA, Patterson TE, Muschler GF. Slowing the onset of hypoxia increases colony forming efficiency of connective tissue progenitor cells in vitro. J Regen Med Tissue Eng. 2013. September 26:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Powell KA, Nakamoto C, Villarruel S, Boehm C, Muschler G. Quantitative image analysis of connective tissue progenitors. Anal Quant Cytol Histol. 2007. April;29(2):112-21. [PubMed] [Google Scholar]
- 38.Wan C, He Q, McCaigue M, Marsh D, Li G. Characteristics of circulating mesenchymal stem cells in fracture non-union patients. In: Proceedings of the 27th American Society for Bone and Mineral Research. Vol 20, Suppl. Nashville, TN: American Society for Bone and Mineral Research; 2005. p S366.
- 39.Patt HM, Maloney MA. Relationship of bone marrow cellularity and proliferative activity: a local regulatory mechanism. Cell Tissue Kinet. 1972. July;5(4):303-9. [DOI] [PubMed] [Google Scholar]
- 40.Patt HM, Maloney MA. The bgJ/bgJ: W/WV bone marrow chimera. A model for studying stem cell regulation. Blood Cells. 1978;4(1-2):27-35. [PubMed] [Google Scholar]
- 41.Maloney MA, Lamela RA, Patt HM. The question of bone marrow stromal fibroblast traffic. Ann N Y Acad Sci. 1985;459:190-7. [DOI] [PubMed] [Google Scholar]