Abstract
The deletion of NFκB in epithelial tissues by using skin-specific promoters can cause both tumor formation and severe inflammatory dermatitis, indicating that this signaling pathway is important for the maintenance of immune homeostasis in epithelial tissues. In the present study, we crossed mice transgenic for loxP-Ikbk2 and human Gfap-cre to selectively delete IKK2 in CNS astrocytes. Unexpectedly, a subset of mice developed severe and progressive skin lesions marked by hyperplasia, hyperkeratosis, dysplasia, inflammation, and neoplasia with a subset of lesions diagnosed as squamous cell carcinoma (SCC). The development of lesions was monitored over a 3.5-y period and over 4 filial generations. Average age of onset of was 4 mo of age with 19.5% of mice affected with frequency increasing in progressive generations. Lesion development appeared to correlate not only with unintended IKK2 deletion in GFAP expressing cells of the epidermis, but also with increased expression of TNF in lesioned skin. The skins changes described in these animals are similar to those in transgenic mice with an epidermis-specific deletion of NFκB and thus represents another genetic mouse model that can be used to study the role of NFκB signaling in regulating the development of SCC.
Abbreviations: Cre, cyclic recombinase; F, floxed allele; GFAP, glial fibrillary acidic protein; Ikbk2, inhibitor of κB kinase 2; IKK, trimeric IκB kinase; SCC, squamous cell carcinoma
The NFκB pathway is ubiquitously expressed in cells and consists of a family of structurally related transcription factors that are key regulators in inflammation, embryogenesis, and apoptosis.4 These transcription factors are sequestered in the cytoplasm through interaction with the inhibitor of NFκB (IκB), until IκB is phosphorylated at serine 32/26 by the trimeric IκB kinase (IKK) complex, leading to polyubiquination, degradation, and nuclear translocation of p65/p50.6,16 The IKK complex comprises 2 catalytic subunits, IKK1/CHUK and IKKβ/IKK2, as well as a regulatory subunit (IKKγ). The catalytic subunit IKK2 is necessary for integrating multiple extracellular signals leading to activation of the canonical NFκB pathway and subsequent increases in transcription of immunomodulatory and apopototic genes.4,9
Although this pathway is vital in regulating gene expression in response to stress, abnormalities in NFκB regulation are involved in multiple pathologies, including inflammatory diseases, immune deficiencies, degenerative diseases, and carcinogenesis.21,28 Excessive activation of NFκB occurs in both inflammatory and neoplastic diseases of the skin, but modulation of this signaling factor in rodent models of epidermal diseases has shown mixed outcomes. Overexpression or deletion of NFκB can promote tumor formation, especially squamous cell carcinoma (SCC), or the development of severe psoriasis-like inflammatory conditions.21,28,29,32 The pathologic effects of loss of NFκB signaling in the skin thus merits further investigation, particularly for its connection to the development of epidermal neoplasia.
Cutaneous SCC is a locally aggressive, malignant neoplasm of the skin arising from epidermal keratinocytes,24 representing the second most common skin cancer in humans15 and accounting for 4% to 5% of all cutaneous cancers in dogs and 17.5% of all cutaneous cancers in cats.18 Although SCC has many diverse clinical manifestations, it is identifiable histologically by the presence of atypical keratinocytes extending through the epidermal layers, with the presence of hypertrophy, hyperkeratosis, acanthosis, and inflammatory infiltrate in the dermis.11,24 The overall metastatic potential of these tumors is quite low; however, tumor behavior is highly dependent on location and histologic subtype and when metastasis occurs, is associated with a poor prognosis.26
The molecular pathways that lead to SCC development are complex and not fully understood. The NFκB pathway has paradoxically been associated with both the progression of metastatic SCC and in protecting against sustained epidermal proliferation and tumor formation.30,32 Specifically, tumor development appears to depend on the expression of TNF, which is highly regulated by NFκB, given that inhibition of TNF signaling in mouse models can counteract tumor formation.22,29 In the present study, we noted the spontaneous development of SCC in transgenic mice with conditional deletion of IKK2 in cells expressing glial fibrillary acidic protein (GFAP). Although expression of the intermediate-filament protein GFAP has been considered specific to astrocytes of the CNS, recent studies have shown that nonneuronal cells, including epidermal and follicular keratinocytes and dermal fibroblasts, also express GFAP.7 Therefore, we postulated that SCC development resulted from unintended NFκB deletion in epidermal keratinocytes, leading to immunomodulatory alterations and resulting in increased TNF signaling and tumor development.
Materials and Methods
Animals.
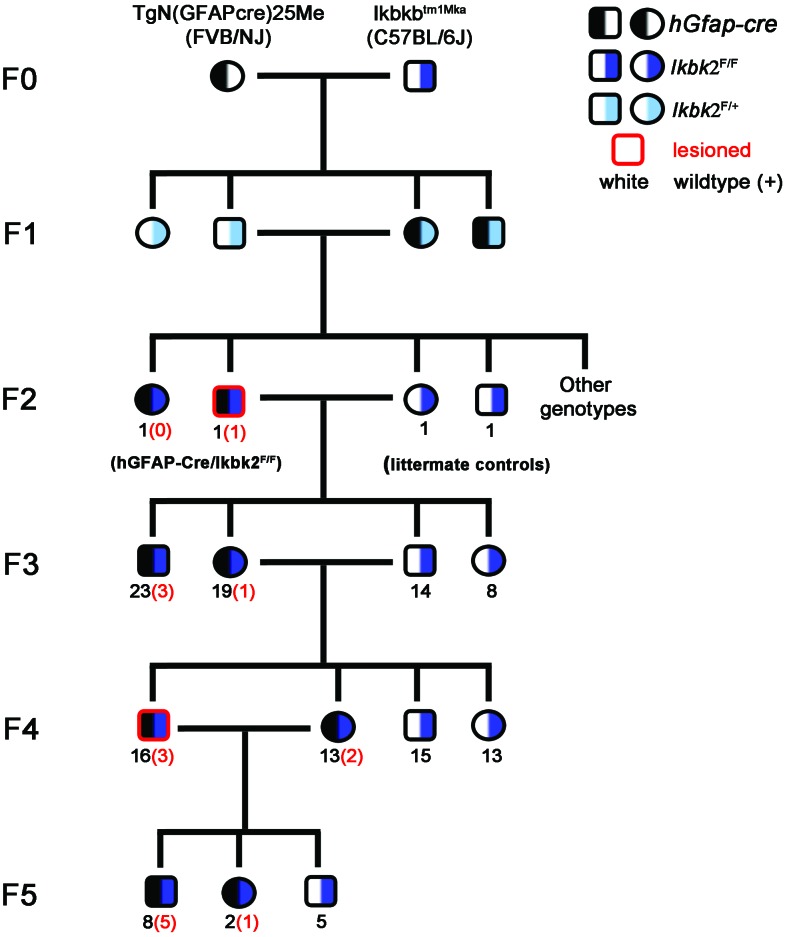
To generate mice with tissue-specific deletion of Ikbk2 in GFAP-expressing cells (hGfap-cre/Ikbk2F/F hereon), we crossed the Ikbkbtm1Mka transgenic mouse line, which contains 2 loxP sequences flanking exon 3 of Ikbk2 on a C57BL/6J substrain (kindly provided by Dr Michael Karin, University of California–San Diego), with FVB/NJ transgenic mice expressing cyclic recombinase (Cre) under the control of the human Gfap promoter (FVB-Tg[GFAP-cre]25Mes/J mice, stock no. 004600, Jackson Laboratory, Bar Harbor, ME). Mice were then bred over 5 generations to produce mixed-strain offspring with homozygous expression of the floxed Ikbk2 allele to study the involvement of astrocytic NFκB expression in modulating inflammatory responses to neurotoxicants (Figure 1). To account for genetic segregation in mixed-strain progeny, littermates of hGfap-cre/Ikbk2F/F mice lacking the hGfap-cre allele (known as Ikbk2F/F) were used as controls. Genotyping was performed on tissue from ear punches obtained at 21 d of age by using the primers 5′ GTC ATT TCC ACA GCC CTG TGA 3′ and 5′ CCT TGT CCT ATA GAA GCA CAA C 3′ to amplify the Ikbk2+ and Ikbk2F alleles as described previously20 and 5′ ACT CCT TCA TAA AGC CCT CG 3′ and 5′ ATC ACT CGT TGC ATC GAC CG 3′ to amplify the hGfap-cre allele.33
Figure 1.
Pedigree of hGfap-cre /Ikk2F/F mice over 6 filial generations (F0 through F5). Half black indicates presence of the hGfap-cre transgene, whereas half-navy blue or half-light blue indicates homozygozity or heterozygozity for the floxed Ikk2 allele, respectively. Black numerals indicate the number of mice of that genotype and sex in the indicated generation, whereas red numbers in parentheses indicate the number of lesioned hGfap-cre/Ikk2F/F mice of each sex. Red highlighted shapes indicate a lesioned mouse was used as a breeder for the subsequent generation.
Mice were maintained in IVC (Thoren Caging System, Hazelton, PA) in accordance with recommendations for housing density14 and provided Teklad Irradiated Diet 2918 (Harlan Laboratories, Madison, WI) and sterile-filtered drinking water without restriction. Early during monitoring, mice were housed at a density of 3 to 5 mice per cage; however, as the number of lesioned mice increased, housing density was limited to 1 to 3 animals per cage. All mice identified with lesions were individually housed at the time of lesion discovery. All mice were maintained under a 12:12-h light:dark cycle at temperatures of 21 to 24 °C. Cage changes occurred on a 10-d cycle with mice transferred by tail suspension.
Original founders mice from Jackson Laboratory and UC San Diego were quarantined at the time of purchase and deemed pathogen-free before entry into the general colony. Sentinel mice were used to monitor the health status of our experimental animals, with serology conducted on a quarterly basis, and all animals, including original founders, were confirmed to be free of murine pathogens including Sendai virus, pneumonia virus of mice, mouse hepatitis virus, minute virus of mice, mouse parvovirus, mouse norovirus, Theiler murine encephalitis virus, reovirus, rotavirus, lymphocytic choriomeningitis virus, ectromelia virus, mouse adenovirus, mouse cytomegalovirus, K virus, polyoma virus, Hantavirus, lactate dehydrogenase elevating virus, mouse thymic virus, Bordetella bronchiseptica, cilia-associated respiratory bacillus, Citrobacter rodentium, Corynebacterium kutcherii, Helicobacter spp., Klebsiella oxytoca, Mycoplasma pulmonis, Pasteurella pneumotropica, Salmonella spp., Streptobacillus moniliformis, Streptococcus pneumonia, Clostridium piliforme, pinworms, and ectoparasites. All procedures were performed in accordance with NIH guidelines for the care and use of laboratory animals, with approval by the IACUC of Colorado State University.
Lesion monitoring and analysis of frequency.
From September 2009 through March 2013, our colony comprised 139 mice, consisting of 82 hGfap-cre/Ikbk2F/F (that is, 3 F2, 42 F3, 29 F4, and 8 F5) mice and 57 Ikbk2F/F littermate controls. Lesions developed in 16 hGfap-cre/Ikbk2F/F (that is, 1 F2, 4 F3, 5 F4, and 6 F5) mice. No lesions were ever identified in Ikbk2F/F littermate controls. Cases of proliferative skin lesions were identified and monitored by facility care technicians and laboratory personnel during daily routine colony checks. When lesions were discovered, mice were marked for veterinary evaluation, housed individually, received daily nail trimming, and entered into the electronic animal medical record system used by the department of Laboratory Animal Resources at Colorado State University. Lesion size and animal condition was assessed biweekly after initial lesion discovery. In accordance with special IACUC approval, lesions were allowed to progress in size beyond the standard 3 cm for a single lesion, or 2 cm for multiple lesions, provided there was no ulceration, the body condition score was 3 or higher on a standard 5-point scale, and there were no health limitations to movement, eating, or drinking. Early cases often reached or exceeded standard lesion guidelines due to limited understanding of the mechanisms involved; however, as a progressive, nonhealing lesion pattern became more evident, euthanasia standards were adjusted. Late in the F3 generation and during the F4 and F5 generations, mice were euthanized when a single lesion exceeded 2 cm or multiple lesions arose (regardless of size); the body condition score dropped below 3, severe pruritus occurred, ulceration was greater than 4 mm, or mobility was limited. Interventional therapy, including nail trimming, individual housing, and oral and topical antibiotics, had no effect on the development or progression of lesions. The average age of onset was determined according to the time at which cases were reported through the animal medical record system or documented by the laboratory staff. All analysis was performed by using Prism software (version 6.0; GraphPad Software, San Diego, CA), with t testing used to assess sex-associated differences in age of onset, one-way ANOVA with Tukey posthoc testing to assess generation-associated differences in age of onset, and χ2 testing to compare frequencies between sexes and generations. A P value below 0.05 was deemed statically significant.
Necropsy and histopathology.
Mice were euthanized by using carbon dioxide narcosis and secondary cervical dislocation at specified study end points (that is, 6 mo of age) or when the severity of skin lesions required euthanasia as detailed earlier. Full necropsies were performed on 11 of the 16 cases to assess the presence of nondermatologic lesions, with the collection and preparation of multiple organ tissues, including tongue, esophagus, stomach, small intestine, large intestine, vagina, uterus, testes, heart, lungs, liver, kidney, brain, and peripheral lymph nodes. Skin samples encompassing the entire lesion from the epidermis to below the muscular layer and 4 mm of normal skin were collected from the scruff, pinna, and tail base of hGfap-cre/Ikbk2F/F mice (n = 11). Cross sections of 4 mm2 of grossly normal skin were obtained from hGfap-cre/Ikbk2F/F mice (n = 2) and Ikbk2F/F mice (n = 12). To standardize collection sites, skin from unaffected animals was obtained from both the scruff and tail base, the most common locations of lesions. Tissues were fixed in 10% neutral buffered formalin (VWR, West Chester, PA), paraffin-embedded, trimmed in 5-μm sections, and stained with hematoxylin and eosin for routine histopathologic analysis. A veterinary comparative medicine resident and laboratory personnel evaluated the histopathology of all skin lesions from hGfap-cre/Ikbk2F/F mice, and the skin lesions from 5 of the 11 cases necropsied were submitted for additional diagnostic review by a board-certified pathologist at Colorado State University.
Immunofluorescence.
Representative paraffin-embedded tissue sections of lesioned skin from hGfap-cre/Ikbk2F/F mice (n = 11) and the grossly normal skin of Ikbk2F/F mice (n = 12) were immunolabeled with GFAP and IKK2 to assess cell-specific loss of IKK2 in the layers of the skin as compared with littermate controls. Sections were deparaffinized and immunolabeled by using primary antibodies IKK2 (dilution, 1:100; Novus Biologicals, Littleton, CO), GFAP (1:100; Dako, Carpinteria, CA), or TNF (1:100; Abcam, Carlsbad, CA) as described previously and counter-labeled with Alexa Fluor 488- or 647-conjugated secondary antibodies (1:500; Molecular Probes, Carlsbad, CA).17 To confirm the specificity of fluorescent labeling, primary-only, secondary-only, and serum-substitution controls were performed (data not shown). Sections were mounted in media containing 4′,6- diamidino-2-phenylindole dihydrochloride to detect cell nuclei and cover slipped.
Fluorescence images were captured using 10× or 40× air plan apochromatic objectives on an inverted fluorescence microscope (Axiovert 200M, Carl Zeiss, Thornwood, NY) equipped with an ORCA-ER–cooled charge-coupled device camera (Hamamatsu Photonics, Hamamatsu City, Japan).
Results
Development of mice.
hGfap-cre/Ikbk2F/F mice originally were generated to investigate the role of NFκB in astrocytes as a key regulator of inflammatory gene expression in Parkinson disease pathogenesis.10 To establish conditional knockout of Ikbk2 in CNS astrocytes, mice expressing Cre under the control of the human Gfap promoter were bred to mice containing a conditional Ikbk2 allele, in which exon 3 is flanked by 2 loxP sites (Figure 1). Mice were bred back to homozygosity for the floxed allele over 4 generations, and littermates lacking Cre (known as Ikbk2F/F) were used as controls. Littermate-control mice (Ikbk2F/F) and heterozygotes expressing Cre and a single floxed allele (hGfap-cre/Ikbk2F/+) were entirely healthy, with no development of skin lesions.
Gross pathology.
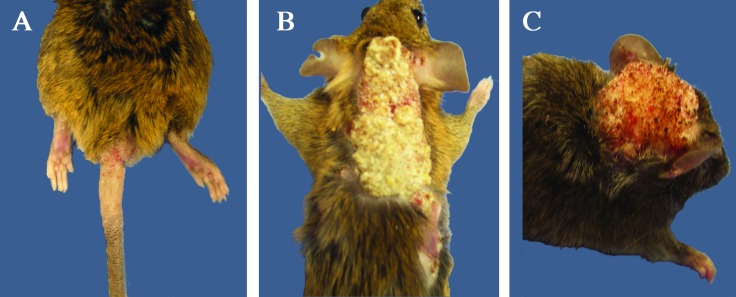
hGfap-cre/Ikbk2F/F mice (16 of 82) unexpectedly developed skin lesions (Figure 2) that progressed rapidly from focal areas of ulceration and thickening of the integument to extensive, proliferative growths. The lesions ranged from 0.5 to 5 cm in diameter, with variable incidence of pruritus, hypotrichosis, and ulceration. Skin of the tail base (Figure 2 A) and scruff (Figure 2 B) were affected most often, although the lesions often extended to incorporate the limbs, head (Figure 2 C), ears, prepuce, and trunk, with most mice having multiple lesions. Systemic disease was not apparent in any animal, and no lesions in other organs or epidermal surfaces were present. Severe lesions often resulted in apparent discomfort and reduced activity, grooming behavior, and overall body condition. In the majority of lesioned mice, euthanasia was deemed necessary within 1 mo of lesion development. To investigate the potential role of secondary or opportunistic bacterial infection in lesion progression, mice were treated with oral or topical antibiotics; however, this treatment was ineffective in curbing the progression of the skin lesions or increasing the comfort of the mice. In addition, to determine the possible role of fighting in the induction of lesions, beginning during F3, mice were isolated into individual caging at time of weaning; however, 4 of the 16 mice developed lesions despite isolation.
Figure 2.
Gross pathology of skin lesions in hGfap-cre/Ikbk2F/F mice. (A) Lesion on tail base and perianal region of with areas of hypotrichosis, hyperkeratosis, and ulceration. (B) Severe hyperkeratotic lesion with multifocal ulcerations at the periphery on scruff and lateral thorax. (C) Discrete, hyperkeratotic lesion on the dorsal head, causing displacement of the right pinna laterally. In all images, the background was changed from white to blue to enhance lesion visualization.
Histopathology.
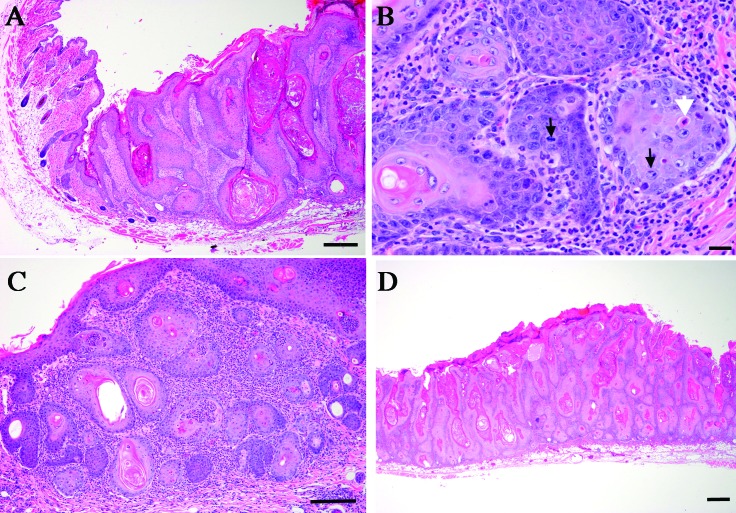
Skin lesions from 5 of the 16 hGfap-cre/Ikbk2F/F euthanized, lesioned mice from generations F3 through F5 were submitted for review to a board-certified veterinary pathologist. Several submitted mice had multiple lesions, with 8 individual lesions evaluated from 5 mice. Evaluation of epidermal changes included elements of hyperplasia, dysplasia, and neoplasia, and affected regions were moderately demarcated form more normal adjacent skin (Figure 3). In severely affected tissues, the epidermis was expanded as much as 10 times the normal thickness by coalescing nests of epithelial cells (Figure 3 A). Cells were cuboidal to polygonal with distinct cell boarders and abundant cytoplasm, round nuclei, and often-prominent nucleoli. Moderate anisocytosis and anisokaryosis were present. Mitotic figures were variable, most commonly noted in the basal layers, and ranged from 0 to 4 per high-power field (Figure 3 B). Nests of cells demonstrated central aggregation of disorganized keratin as well as cornification of individual cells in many areas. The superficial dermis was thickened by an inflammatory cell population composed predominantly of neutrophils with occasional multinucleated giant cells present in conjunction with free keratin (Figure 3 C). Overlying keratin was variably thickened, with mild to severe orthokeratotic hyperkeratotic changes that contributed to the thickening of the skin (Figure 3 D).
Figure 3.
Histopathology of skin lesions from hGfap-cre/Ikbk2F/F mice. (A) Hematoxylin and eosin staining shows the transition from unaffected skin to a region of increasing epidermal hyperplasia and dysplasia. Scale bar, 100 μm. (B) Hematoxylin and eosin staining reveals nests of neoplastic epithelial cells, with numerous mitotic figures (black arrows) and apoptosis of individual cells (white arrow). Scale bar, 20 μm. (C) Hematoxylin and eosin staining shows marked inflammatory cell infiltration with the tumor. Scale bar, 100 μm. (D) Hematoxylin and eosin staining reveals marked expansion of the epidermis by multifocal nests of epithelial cells. Scale bar, 100 μM.
The character of the lesions was highly variable between mice and within lesions on each mouse. Evaluation occurred only after the lesions had progressed severely and were compared with normal skin from nonlesioned hGfap-cre/Ikbk2F/F mice (n = 12) or littermate Ikbk2F/F mice (n = 2). Review of 8 lesions from 5 mice submitted to the board-certified pathologist determined that all 5 mice had at least one lesion positively identified as SCC (6 of 8 lesions), with no metastasis identified to regional lymph nodes. Most of the SCC were well-differentiated lesions, with one lesion noted to have SCC in situ. Other lesions (2 of 8) had severe hyperplasia, dysplasia, and inflammation but lacked the pleomorphic features or mitotic figures to warrant classification as SCC.
Lesion frequency and age of onset.
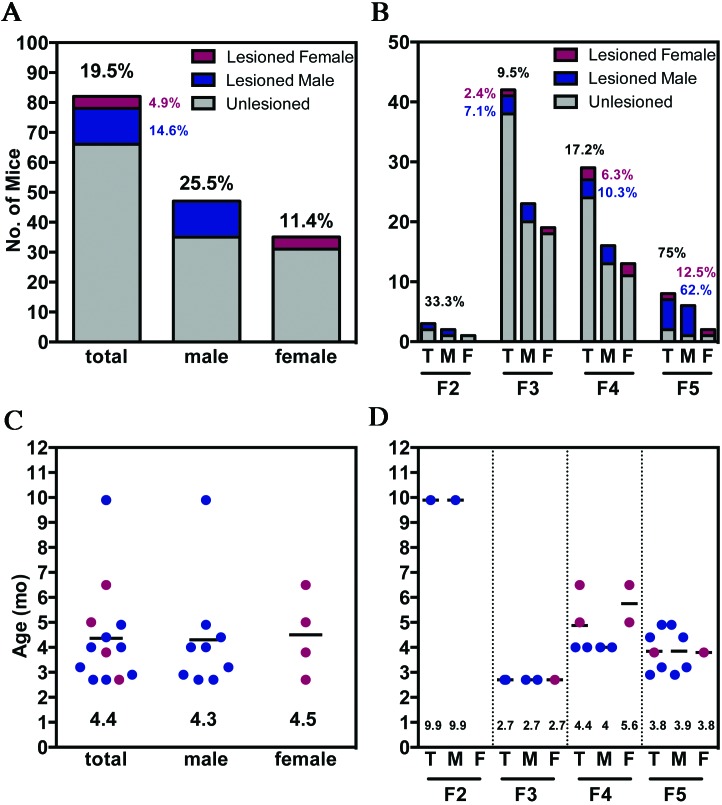
The development of gross skin lesions in the hGfap-cre/Ikbk2F/F colony was tracked through 4 filial generations (F2 through F5) over a 3.5-y period and included 82 total mice (47 male, 35 female). Of the 82 total hGfap-cre/Ikbk2F/F mice, 16 developed spontaneous and progressive skin lesions, for an overall colony frequency of 19.5% (Figure 4 A). The frequency was 25.5% (12 of 47) in male mice compared with 11.4% (4 of 35) in female mice. This trend of increased male frequency was preserved in each following generation but was not statistically significant (Fisher exact test, P = 0.16; Figure 4 B). Furthermore, after exclusion of the F2 generation, which comprised only 3 mice, the number of hGfap-cre/Ikbk2F/F mice that developed presumptive SCC lesions increased significantly (χ2 test, P = 0.02) in subsequent generations, from 9.5% overall (4 of 42 mice) in the F3 generation to 75% (6 of 8 mice) in F5.
Figure 4.
Frequency and age of onset of SCC in hGfap-cre/Ikbk2F/F mice. (A) Overall lesion development frequency of hGfap-cre/Ikbk2F/F total (T, black), male (M; blue) and female (F; red) mice, (B) with increasing percentage of mice affected through progressive generations. Gray indicates nononlesioned mic. Scatter plots depicting the age (m, months) of hGfap-cre/Ikbk2F/F total (T, black), male (M; blue) and female (F; red) mice at lesion development (C) overall and (D) by generation. Mean age is indicated by a black line and written at the bottom of the scatter plot.
In addition to frequency, the age of lesion development was tracked through biweekly inspection of the colony, beginning in the F3 generation. The average age of lesion onset over all generations was approximately 4.4 mo, regardless of sex (Figure 4 C). In each generation (Figure 4 D), the age of lesion onset did not differ significantly between sexes. The typical age of onset was 3 to 4 mo but varied significantly depending on the generation, from 9 mo in F2 (P < 0.001), 2.7 mo in F3 (P < 0.05), 4.4 mo in F4, and 3.8 mo in the F5 generation.
IKK2 and TNF expression in skin.
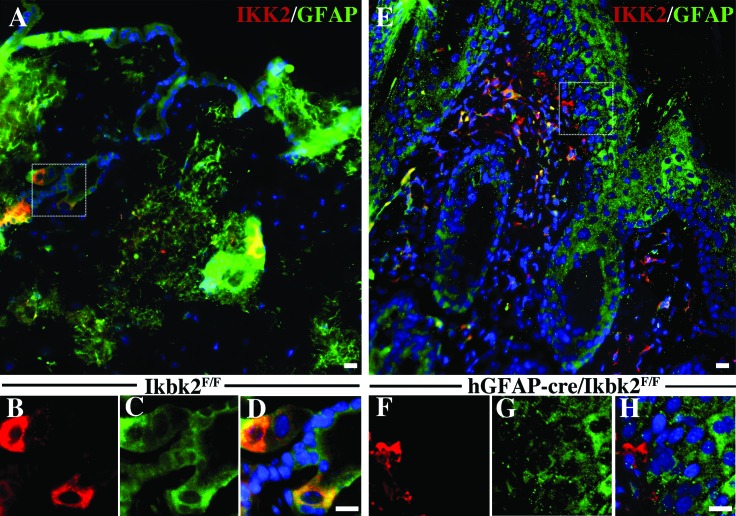
To investigate the etiology of SCC in hGfap-cre/Ikbk2F/F mice, matched skin sections from lesioned hGfap-cre/Ikbk2F/F(n = 11) and nonlesioned Ikbk2F/F(n = 12) littermate controls were immunolabeled for the presence of GFAP and IKK2 (Figure 5 A through H). In nonlesioned littermate controls, IKK2 was highly expressed in the fibroblasts of dermis, hair follicle keratinocytes, and sebaceous gland cells (Figure 5 A and 5 B). Lower levels of IKK2 expression were detected throughout all layers of the epidermis. GFAP expression in normal control skin was found in all keratinocytes of the epidermis, sebaceous gland cells, and fibroblasts of the dermis (Figure 5 A and 5 C). Assessment of colocalization of both proteins revealed that both GFAP-positive and GFAP-negative cells of the skin expressed IKK2 in Ikbk2F/F mice (Figure 5 A and D).
Figure 5.
Immunofluorescent analysis of IKK2 expression in Ikbk2F/F and hGfap-cre/Ikbk2F/F mice. (A) Normal skin from Ikbk2F/F mice (n = 12) has sporadic IKK2 (red) expression with colocalization found in GFAP- (green) expressing cells including keratinocytes, sebaceous glands, and dermal fibroblasts. Higher magnification images obtained from the dotted white line showing (B) IKK2 and (C) GFAP (D) colocalization in cells from an Ikbk2F/F mouse. (E) IKK2 (red) fluorescence is absent from GFAP- (green) positive cells in SCC lesions in hGfap-cre-Cre/Ikbk2F/F mice (n = 11). Higher magnification images obtained from the dotted white line show of (F) IKK2 and (G) GFAP (H) colocalization in cells from an hGfap-cre-Cre/Ikbk2F/F mouse. Nuclei are shown in blue. Scale bars, 20 μm.
In comparison, IKK2 levels were reduced in both lesioned (Figure 5 E and F) and nonlesioned (data not shown) skin of hGfap-cre/Ikbk2F/F mice. In addition, GFAP fluorescence intensity was reduced in lesioned areas despite similar labeling patterns (Figure 5 E and G). Loss of IKK2 occurred only in GFAP-expressing cells of the epidermis, whereas GFAP-positive fibroblasts of the dermis and nonGFAP-labeled cells retained IKK2 expression (Figure 5 E and 5 H). Most of the IKK2-associated fluorescence in lesioned skin was from infiltrating immune cells in both the dermis and epidermis.
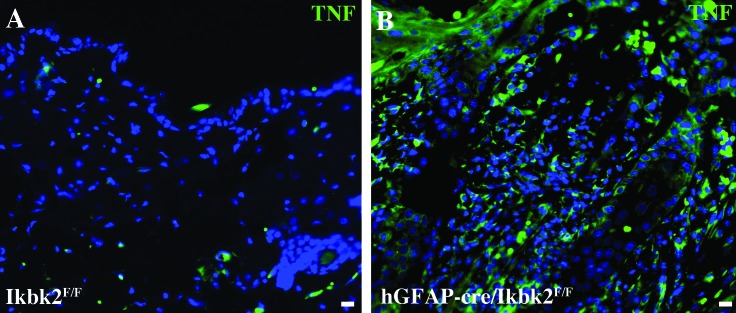
In light of the precedent of TNF dysimmunoregulation contributing to skin lesion formation in other conditional Ikbk2-knockout lines,21 we used immunofluorescence to label matched skin samples from lesioned hGfap-cre/Ikbk2F/F(n = 11) and nonlesioned Ikbk2F/F (n = 12) littermate controls for TNF (Figure 6). In normal skin obtained from littermate controls, TNF fluorescence intensity was low, with only occasional dermal fibroblasts showing positive expression (Figure 6 A). In contrast, lesioned skin and the surrounding normal skin in hGfap-cre/Ikbk2F/F mice showed markedly higher levels of TNF throughout all layers of the skin, including keratinocytes throughout the epidermis, cells within the dermis, and follicular keratinocytes (Figure 6 B), with most expression correlating with infiltrating immune cells in the epidermis and dermis. Nonlesioned skin from hGfap-cre/Ikbk2F/F mice showed similar TNF immunolabeling as their littermate controls (data not shown).
Figure 6.
Immunofluorescent analysis of TNF expression in Ikbk2F/F and hGfap-cre/Ikbk2F/F mice. (A) Normal skin from Ikbk2F/F mice (n = 12) has low to absent TNF fluorescence (green). (B) TNF (green) fluorescence is increased in all skin layers in SCC lesions and surrounding nonlesioned skin from hGfap-cre/Ikbk2F/F mice (n = 11). Nuclei are shown in blue. Scale bars, 20 μm.
Discussion
NFκB is a critical signaling pathway for genes regulating stress responses, immune function and apoptosis.21 Activation of NFκB is considered essential for inflammatory responses as well as for tumor development and progression.28 However, cell-specific deletion of NFκB can also promote severe inflammatory dermatitis and epidermal tumor development, most likely through upregulation of TNF by infiltrating immune cells, bringing into question the exact role of NFκB in epidermal disease.5,22,30 In the current study, we describe the spontaneous development of SCC in mice with site-directed deletion of IKK2, the essential kinase in activating the classic NFκB pathway.4 Mice originally were bred to achieve deletion of NFκB signaling in astrocytes of the CNS by using a hGfap-cre-directed promoter, which had the unexpected result of inducing formation of proliferative skin lesions at around 4 mo of age, affecting 19.5% of the colony and increasing in frequency over 4 generations. Further investigation into potential pathogenesis revealed deletion of IKK2 in GFAP-expressing keratinocytes and increased TNF expression in hGfap-cre/Ikbk2F/F mice.
Observation of the hGfap-cre/Ikbk2F/F mouse colony over a 3.5-y period revealed spontaneous development of skin lesions starting in the F2 generation. These skin lesions were evaluated histologically, and ultimately all 5 lesioned mice evaluated histologically by a board-certified pathologist were diagnosed with SCC. However, the majority of lesions were evaluated near end-stage, with 2 lesions of the evaluated mice deemed a proliferative, inflammatory lesion. Therefore, SCC may only represent the end-stage disease or only a small percentage of developed lesions. Previous studies that deleted NFκB by using keratinocyte-directed promoters such as keratin 5 and keratin 14 have resulted primarily in systemic and progressive dermatitis,5,21,27 with only one research group reporting development of spontaneous SCC.29,30 Lesions reported in these various studies ranged from focal lesions to widespread epidermal changes, with focal lesions correlating more commonly with neoplasia. The development of lesions in hGfap-cre/Ikbk2F/F mice followed a similar pattern, with pathologic skin changes tending to be focal rather than systemic. These focal changes showed more severe effects and eventually progressed toward neoplasia. Even the primary location of lesions on the scruff, head, and tail base is consistent with the previous report of spontaneous SCC in mice.30 This pattern of lesion location, with classic histopathologic changes of keratinocyte atypia and abnormal keratinization, further supports the diagnosis of SCC in this colony.
Over the course of our study, lesion development occurred in 19.5% of the mouse colony. The number of mice affected increased from generation F3 to F5, from an initial frequency of 9.5% to 75%. This pattern differs from other reported mouse models, where epidermal effects were fully penetrant, affecting the entire colony over time in a single generation.1,21,30 Ultimately, we most likely are underestimating the total frequency of lesions in hGfap-cre/Ikbk2F/F mice, because most nonlesioned mice were used by 6 mo of age in another research model. Other studies have seen variation in the time course of lesion development, and we even noted that F2 generation did not develop lesions until 9 mo of age. Even if the frequency were higher, it is unclear why it increased in subsequent generations. This difference might be due to either improved observation over time or to a factor of inheritance, given that lesioned mice were breeders in at least 2 generations and that SCC is known to be heritable in human populations.31 In addition, the difference in frequency could be a factor of creating a mixed strain consisting of both FVB/NJ and C57BL/6J, resulting in random genomic rearrangement that could greatly affect the observations seen. This possibility is especially important, given that FVB/N substrains show increased susceptibility to development of SCC in chemical induction models.13 The genetic segregation of FVB/N and C57BL/6 alleles might be important in determining which hGfap-cre/Ikbk2F/F mice developed lesions; however, genomic segregation likely is insufficient to explain induction of lesions, given that no littermate controls or mice with partial loss of IKK2 (hGfap-cre/Ikbk2F/+) developed lesions.
The average age of lesion onset was between 3 to 4 mo of age and varied significantly between generations but not between sexes. Generational differences in lesion onset mainly occurred in the F2 and F3 generations and most likely reflect the low number of lesioned animals. However, by the F4 and F5 generations, most mice developed lesions by 4 mo of age. This timing is similar to what has been reported in other epidermal mouse models with NFκB deletion, in which the development of lesions appears to be delayed, with the average age of onset occurring sometime in adulthood (2 to 6 mo of age).5,12,27,30 In addition, this delay in SCC development correlates with the height of Cre expression reported in hGfap-cre transgenic mice, where maximal expression occurs at approximately 3 mo of age.33
GFAP is an intermediate filament that, along with microtubules and microfilaments, comprises the cytoskeleton of most eukaryotic cells. Classically, GFAP was used as a specific protein marker for astrocytes; however, this specificity in the brain was due primarily to detection of only 1 of the 8 isoforms of this protein, and more recent research indicates that expression is more widespread in peripheral tissues then once thought.20 Despite the broad expression pattern of GFAP, only one known study has specifically investigated GFAP expression in cells of the skin,7 the results of which were reported years after the initial development and characterization of the FVB-Tg(GFAP-cre)25Mes/J mouse.33 In addition, a recent, more comprehensive characterization of the FVB-Tg(GFAP-cre)25Mes/J strain3 found positive Cre expression in follicles of the integument, but whether this effect was promoter-driven or ectopic was not determined. We therefore asked whether lesion development was related to epidermal expression of GFAP. Immunofluorescent studies examining GFAP in normal compared with pathologic skin of hGfap-cre /Ikbk2F/F and control mice confirmed the expression of GFAP in epidermal and follicular keratinocytes, sebaceous gland cells, and dermal fibroblasts. Coimmunolabeling with IKK2 revealed loss of IKK2 only in GFAP-positive cells of the epidermis but not in GFAP-negative cells or in the skin of control mice. These findings imply that IKK2 loss could partially explain the development of SCC in this mouse model and that IKK2 loss was most likely caused by GFAP-directed deletion and not by ectopic expression of Cre in transgenic mice.
Although IKK2 was deleted only in GFAP-positive cells of the skin, the loss of IKK2 was not specific to lesioned areas. Other models have reported similar discrepancies and have sought to determine the pathologic mechanisms involved, including investigating increased keratinocyte turnover,29 or increased interleukin-1 β expression.23 Despite discrepancies among these studies, one common feature of SCC is altered TNF signaling, because blocking TNF signaling or gene deletion of TNF is protective against disease in reported models.21 The lesions reported in hGfap-cre/Ikbk2F/F mice revealed changes in TNF expression with markedly increased TNF immunolabeling in lesioned—but not in normal—skin. TNF expression was evident in both IKK2-deficient (keratinocytes) and IKK2-containing cells (infiltrating immune cells), indicating alternate pathways such as AP1 and JAK–STAT, which are also involved in TNF regulation,2 This evidence supports the hypothesis that increased expression of TNF is associated with the development of skin lesions and, based on information in other studies, potentially a causative factor in tumor development. TNF is known to promote tumoriogenesis in multiple tissues, including the skin, through activation of the c-Jun terminal Kinase (JNK) pathway. In keratinocytes, JNK activation is usually inhibited by NFκB signaling through upregulation of NFκB-regulated antioxidant proteins.8,25 However, the trigger to induce inflammatory infiltration and increased TNF expression is unknown.10 Our use of antibiotics in lesioned mice to determine role of bacterial invasion was unsuccessful in mitigating lesion progression; however, prophylactic use was not assessed. Lesion development in isolation (mice housed individually) reduced the likelihood of aggression injury inciting lesion formation. Therefore, further study is required to determine what coinciting factor may play a role in upregulating TNF in the context of IKK2 loss.
Known risk factors for SCC in humans and in domestic animals include UV irradiation (most common), papilloma virus, chemical exposure, immunosuppressive drugs, injury, and chronic inflammation.18,31 NFκB signaling is known to promote tumor formation and induce inflammation28 but also to protect against tumor development in response to γ irradiation or TNF signaling.8,30 Due to the dual nature of this pathway in skin diseases, further investigation into possible coinciting factors is essential in understanding disease pathogenesis and the effect of any directed treatment. Although our study is not the first to document spontaneous SCC development after deletion of NFκB, it represents the first reported association of a Gfap-driven Cre promoter and is one of only a few genetic mouse models that spontaneously develops SCC. Because genetic models of SCC are rare and given that the role of GFAP in skin is largely unstudied, our model represents a potential means to better understand both the physiologic and pathologic roles of GFAP and NFκB in skin disease.
Acknowledgments
This work was funded by an NIH grant (ESO21656 to RBT).
References
- 1.Abel TW, Clark C, Bierie B, Chytil A, Aakre M, Gorska A, Moses HL. 2009. GFAP-Cre-mediated activation of oncogenic K-ras results in expansion of the subventricular zone and infiltrating glioma. Mol Cancer Res 7:645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banno T, Gazel A, Blumemberg M. 2005. Pathway-specific profiling identifies the NFκB-dependent tumor necrosis factor α-regulated genes in epidermal keratinocytes. J Biol Chem 280: 18973–18980. [DOI] [PubMed] [Google Scholar]
- 3.Blake JA, Eppig JT, Kadin JA, Richardson JE, Smith CL, Bult CJ. 2017. Mouse genome database (MGD)—2017: community knowledge resource for the laboratory mouse. Nucleic Acids Res 45:D723–D729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonizzi G, Karin M. 2004. The 2 NFκB activation pathways and their role in innate and adaptive immunity. Trends Immunol 25:280–288. [DOI] [PubMed] [Google Scholar]
- 5.Cornish GH, Tung SL, Marshall D, Ley S, Seddon BP. 2012. Tissue specific deletion of inhibitor of κB kinase 2 with OX40-Cre reveals the unanticipated expression from the OX40 locus in skin epidermis. PLoS ONE 7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costanzo A, Spallone G, Karin M. 2011. NFκB, IκB kinase, and interacting signal networks in squamous cell carcinomas, p 201–222, chapter 10. In: Glick AB, Waes CV. Signaling pathways in squamous cancer. New York (NY): Springer New York. [Google Scholar]
- 7.Danielyan L, Tolstonog G, Traub P, Salvetter J, Gleiter CH, Reisig D, Gebhardt R, Buniatian GH. 2007. Colocalization of glial fibrillary acidic protein, metallothionein, and MHC II in human, rat, NOD|[sol]|SCID, and nude mouse skin keratinocytes and fibroblasts. J Invest Dermatol 127:555–563. [DOI] [PubMed] [Google Scholar]
- 8.Faurschou A, Gniadecki R. 2008. TNFα stimulates Akt by a distinct aPKC-dependent pathway in premalignant keratinocytes. Exp Dermatol 17:992–997. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh S, Karin M. 2002. Missing pieces in the NFκB puzzle. Cell 109:S81–S96. [DOI] [PubMed] [Google Scholar]
- 10.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. 2010. Mechanisms underlying inflammation in neurodegeneration. Cell 140:918–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldenberg G, Golitz LE, Fitzpatrick J. 2010. Histopathology of skin cancer, p 17–35. In: Stockfleth E, Rosen T, Shumack S. Managing skin cancer, Heidelberg (Germany): Springer–Verlag. [Google Scholar]
- 12.Grinberg-Bleyer Y, Dainichi T, Oh H, Heise N, Klein U, Schmid RM, Hayden MS, Ghosh S. 2015. Cutting edge: NFκB p65 and c-Rel control epidermal development and immune homeostasis in the skin. The J Immunol 194:2472–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hennings H, Glick AB, Lowry DT, Krsmanovic LS, Sly LM, Yuspa SH. 1993. FVB/N mice: an inbred strain sensitive to the chemical induction of squamous cell carcinomas in the skin. Carcinogenesis 14:2353–2358. [DOI] [PubMed] [Google Scholar]
- 14.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 15.Karia PS, Han J, Schmults CD. 2013. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol 68:957–966. [DOI] [PubMed] [Google Scholar]
- 16.Karin M. 1999. How NFκB is activated: the role of the IkB kinase (IKK) complex. Oncogene 18:6867–6874. [DOI] [PubMed] [Google Scholar]
- 17.Kirkley KS, Madl JE, Duncan C, Gulland FM, Tjalkens RB. 2014. Domoic acid-induced seizures in California sea lions (Zalophus californianus) are associated with neuroinflammatory brain injury. Aquat Toxicol 156:259–268. [DOI] [PubMed] [Google Scholar]
- 18.Lascelles B, Parry AT, Stidworthy MF. 2000. Squamous cell carcinoma of the nasal planum in 17 dogs. Vet Rec 147:473–476. [DOI] [PubMed] [Google Scholar]
- 19.Li ZW, Omori SA, Labuda T, Karin M, Rickert RC. 2003. IKKβ is required for peripheral B cell survival and proliferation. J Immunol 170:4630–4637. [DOI] [PubMed] [Google Scholar]
- 20.Middeldorp J, Hol EM. 2011. GFAP in health and disease. Prog Neurobiol 93:421–443. [DOI] [PubMed] [Google Scholar]
- 21.Pasparakis M. 2009. Regulation of tissue homeostasis by NFκB signalling: implications for inflammatory diseases. Nat Rev Immunol 9:778–788. [DOI] [PubMed] [Google Scholar]
- 22.Pasparakis M, Courtois G, Hafner M, Schmidt-Supprian M, Nenci A, Toksoy A, Krampert M, Goebeler M, Gillitzer R, Israel A, Krieg T, Rajewsky K, Haase I. 2002. TNF mediated inflammatory skin disease in mice with epidermis-specific deletion of IKK2. Nature 417:861–866. [DOI] [PubMed] [Google Scholar]
- 23.Rebholz B, Haase I, Eckelt B, Paxian S, Flaig MJ, Ghoreschi K, Nedospasov SA, Mailhammer R, Debey-Pascher S, Schultze JL, Weindl G, Förster I, Huss R, Stratis A, Ruzicka T, Röcken M, Pfeffer K, Schmid RM, Rupec RA. 2007. Crosstalk between keratinocytes and adaptive immune cells in an IκBα protein-mediated inflammatory disease of the skin. Immunity 27:296–307. [DOI] [PubMed] [Google Scholar]
- 24.Reichrath J, Querings K. 2006. Histology of epithelial skin tumors, p 10–17.In: Reichrath J. Molecular mechanisms of basal cell and squamous cell carcinomas, New York (NY): Springer Science and Business Media. [Google Scholar]
- 25.Santos M, Perez P, Segrelles C, Ruiz S, Jorcano JL, Paramio JM. 2003. Impaired NFκB activation and increased production of tumor necrosis factor α in transgenic mice expressing keratin k10 in the basal layer of the epidermis. J Biol Chem 278: 13422–13430. [DOI] [PubMed] [Google Scholar]
- 26.Smoller BR. 2006. Squamous cell carcinoma: from precursor lesions to high-risk variants. Mod Pathol 19 Suppl 2:S88–S92. [DOI] [PubMed] [Google Scholar]
- 27.Stratis A, Pasparakis M, Markur D, Knaup R, Pofahl R, Metzger D, Chambon P, Krieg T, Haase I. 2006. Localized inflammatory skin disease following inducible ablation of IκB kinase 2 in murine epidermis. J Invest Dermatol 126:614–620. [DOI] [PubMed] [Google Scholar]
- 28.Sun XF, Zhang H. 2007. NFKB and NFKBI polymorphisms in relation to susceptibility of tumour and other diseases. Histol Histopathol 22:1387–1398. [DOI] [PubMed] [Google Scholar]
- 29.van Hogerlinden M, Rozell BL, Ährlund-Richter L, Toftgård R. 1999. Squamous cell carcinomas and increased apoptosis in skin with inhibited Rel/nuclear factor κB signaling. Cancer Res 59:3299–3303. [PubMed] [Google Scholar]
- 30.van Hogerlinden M, Rozell BL, Toftgard R, Sundberg JP. 2004. Characterization of the progressive skin disease and inflammatory cell infiltrate in mice with inhibited NFκB signaling. J Invest Dermatol 123:101–108. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Diepgen TL. 2006. The epidemiology of basal cell and squamous cell carcinoma, p 1–9. In: Reichrath J. Molecular mechanisms of basal cell and squamous cell carcinomas, New York (NY): Springer Science and Business Media. [Google Scholar]
- 32.Wullaert A, Bonnet MC, Pasparakis M. 2011. NFκB in the regulation of epithelial homeostasis and inflammation. Cell Res 21:146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhuo L, Theis M, Alvarez-Maya I, Brenner M, Willecke K, Messing A. 2001. hGFAP–Cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis 31:85 –94. [DOI] [PubMed] [Google Scholar]