Abstract
During 2006 through 2012, spontaneous group B Streptococcus infections were reported in 22 female KK-Ay mice, an animal model of type 2 diabetes. The affected mice were 5 to 27 wk old, and the cases involved various body sites, including cases of submandibular, caudal, and lumbar abscesses (n =18) or led to torticollis (n = 2), hydrocephalus (n = 1), or moribund clinical signs (n = 1). At necropsy, the mouse with hydrocephalus also demonstrated retained exudate in the uterus, and the moribund mouse showed renal inflammation. Streptococcus agalactiae was isolated in pure culture from all except 2 cases: the facial abscess also yielded Klebsiella pneumoniae, and the uterine exudate was coinfected with Staphylococcus aureus. In addition, S. agalactiae was isolated from the oral cavity and feces of normal KK-Ay mice. S. agalactiae potentially can cause a clinically significant spontaneous infection in a mouse model of diabetes.
KK-Ay mice, also known as yellow KK obese mice, are widely used as models for type 2 diabetes. KK mice develop moderate diabetes with mild obesity, but KK-Ay mice develop a mature-onset obesity and diabetes syndrome resulting from the antagonism of hypothalamic melanocortin receptor 4 due to ectopic expression of the agouti protein.2,4,10 KK-Ay mice show severe obesity, hypertriglyceridemia, hyperglycemia, hyperinsulinemia, and glucose intolerance by 8 wk of age.8
Group B Streptococcus, also known as Streptococcus agalactiae, is a major cause of neonatal sepsis and infection in pregnant women.12 S. agalactiae–related illness in healthy women is extremely uncommon and is almost always associated with underlying abnormalities, such as diabetes mellitus and immune compromise.6 Animal models of diabetes have provided evidence that hyperglycemia is associated with decreased bacterial clearance of S. agalactiae, possibly contributing to increased mortality among diabetic animals in sepsis experiments.3 Here we report a series of spontaneous S. agalactiae infections that occurred in KK-Ay mice and describe the associated clinical, microbiologic, and histologic findings.
Case Report
Female SPF yellow Kuo Kondo (KK)-Ay mice (age, 5 to 8 wk; CLEA Japan, Tokyo, Japan) were under controlled conditions. The mice were confirmed to be free of Citrobacter rodentium, Clostridium piliforme, Corynebacterium kutscheri, Mycoplasma pulmonis, Salmonella spp., Pasteurella pneumotropica, Pseudomonas aeruginosa, Helicobacter hepaticus, H. bilis, Filobacterium rodentium (formerly known as cilia-associated respiratory bacillus), mouse hepatitis virus, Sendai virus, ectromelia virus, mouse adenovirus, lymphocytic choriomeningitis virus, hantavirus, epizootic diarrhea of infant mice virus, pneumonia virus of mice, minute virus of mice, mouse cytomegalovirus, mouse encephalomyelitis virus, and reovirus type 3. Room conditions were: temperature, 20 to 26 °C; relative humidity, 55% ± 10%; and 12:12-h light:dark photocycle. The mice received a standard diet (FR-2, Funabashi Farm, Chiba, Japan) and water without restriction. They were housed in aluminum cages (20 × 30 × 10.7 cm; 4 or 5 mice per cage) containing sterilized wood shavings. This strain was used for many experiments from 2006 to 2012, and the number of female KK-Ay mice purchased varied from 1000 to 4000 annually. All judgments regarding the laboratory animals were performed in accordance with Japanese standards relating to the care and management of laboratory animals and relief of pain.11 The clinical signs that we describe in the current report became apparent before the mice were used in any experiments.
Fresh tissues or samples taken by using cotton swabs were inoculated on heart infusion agar (Difco Laboratories, Detroit, MI) and heart infusion agar containing 5% defibrinated horse blood (Nippon Bio-Test Laboratories, Tokyo, Japan) for isolation and identification of Streptococcus agalactiae. These plates were incubated aerobically at 37 °C for 18 to 24 h. According to colony morphology on horse blood agar (gray to white colonies with or without β-hemolysis) and gram-staining characteristics, presumptive Streptococcus colonies were selected and subcultured on horse blood agar. Christie–Atkins–Munch–Peterson (CAMP) and rapid hippurate hydrolysis testing were performed to establish whether group B Streptococcus organisms were present.5,7 To further verify these results, Lancefield group determination using Seroiden Strepto-kits (Eiken Chemical, Tokyo, Japan), API 20-Strep (bioMérieux, Marcy-l’Étoile, France) kits, and serotype determination by using latex agglutination testing (Denka Seiken, Tokyo, Japan) were performed. Other bacteria isolated were identified by using other kits such as EB20 (Nissui Pharmaceutical, Tokyo, Japan) for Enterobacteriaceae and SP18 (Nissui Pharmaceutical) for staphylococci.
Samples of the oral cavity were collected from 16 mice (age, 6 wk) by using cotton swabs soaked in sterile saline. These samples and fresh fecal samples were plated on custom S. agalactiae–selective agar: Columbia blood agar base (Oxoid Limited, Hampshire, United Kingdom), 39 g/L; starch, 10 g/L; 5% defibrinated horse blood; cinoxacin, 10 mg/L; and neomycin, 5 mg/L. These plates were incubated aerobically at 37 °C for 24 to 48 h.
Clinically ill mice were euthanized by CO2 exposure. At necropsy, the abscesses and liver were swabbed for bacterial culture. Kidney tissue was fixed in 10% neutral buffered formalin and embedded in paraffin wax; 5-μm sections were stained with hematoxylin and eosin for histology.
The number of animals with clinical signs in 2006, 2007, 2010, 2011, and 2012 was 2, 4, 7, 8, and 1, respectively, and comprised 11 cases of submandibular abscesses, 2 cases of caudal abscesses, 5 cases of other abscesses besides face and tail, 2 cases of torticollis, 1 case of hydrocephalus, and 1 case of moribund behavior (Table 1). Figure 1 shows a 25-wk-old mouse with a right submandibular abscess; submandibular abscesses affected mice older than 15 wk. Caudal abscesses were apparent as early as the time of arrival at our animal facility (5 wk of age). Except for submandibular and caudal abscesses, the remaining abscesses affected 6- to 14-wk-old mice. At necropsy, the mouse with hydrocephalus was revealed to have uterine enlargement, with retention of exudative fluid. S. agalactiae was isolated from the abscess sites and the liver and urine of the moribund mouse. The serotypes of the strains tested were all nontypeable. Several mice demonstrated submandibular abscesses that were coinfected with Klebsiella pneumoniae, and the case of uterine enlargement was positive for Staphylococcus aureus also. Among the 16 normal KK-Ay mice we tested, 11 had S. agalactiae in the oral cavity, and 15 were positive for the organism their feces.
Table 1.
Clinical signs in 22 research-naïve female KK-Aymice
| Clinical sign | Isolation site | No. of cases | Age (wk) at onset |
| Facial abscess | Left submandibular region | 6 | 16, 21, 21, 25, 25, 27 |
| Right submandibular region | 5 | 18, 20, 22, 25, 25a | |
| Tail abscess | Tail | 2 | 5, 5 |
| Abscesses at other sites | Left lumbar region | 2 | 7, 8 |
| Left abdominal region | 1 | 8 | |
| Right shoulder | 1 | 6 | |
| Right hindleg | 1 | 14 | |
| Torticollis | Middle and inner ear | 2 | 14, 15 |
| Hydrocephalus | Uterus | 1 | 9b |
| Moribund | Kidney | 1 | 8 |
S. agalactiae was isolated from all isolation sites.
Klebsiella pneumoniae was isolated also.
Staphylococcus aureus was isolated also.
Figure 1.
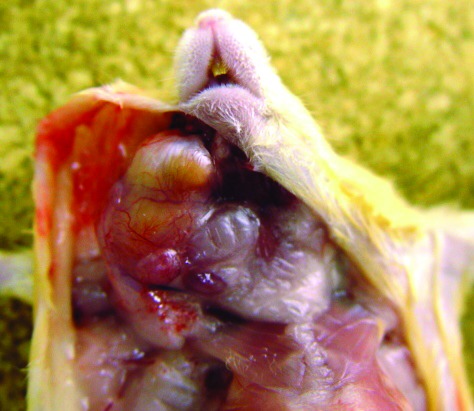
Submandibular abscess due to S. agalactiae in a 25-wk-old mouse. A pure culture of S. agalactiae was isolated from the abscess.
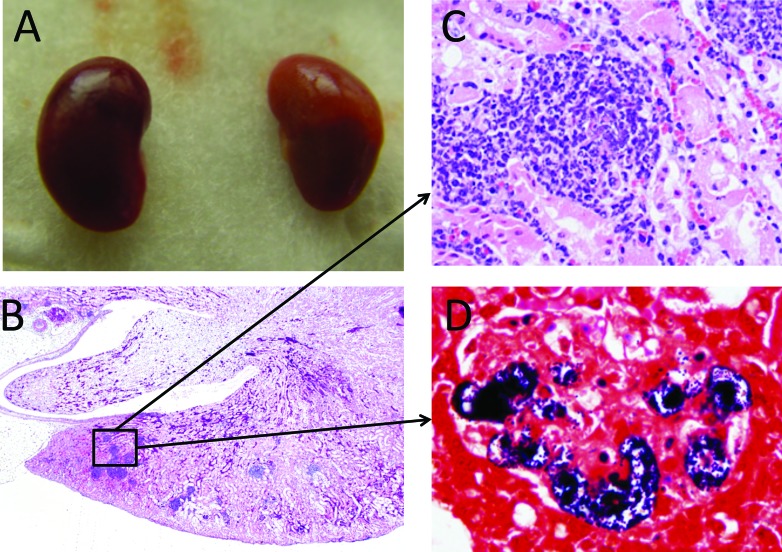
The kidneys of the moribund mouse (age, 8 wk) were pale and inflamed (Figure 2 A). Severe chronic nephritis was present in the cortex (Figure 2 B), and inflammatory infiltrates, including lymphocytes, neutrophils and plasma cells, were evident (Figure 2 C). The inflammation was more severe in the cortex than in the renal medulla. Inflammatory foci contained numerous gram-positive cocci (Figure 2 D).
Figure 2.
(A) Gross morphology of kidneys. (B) Photomicrograph of a kidney; magnification, 20×. (C) Photomicrograph showing glomerular nephritis; magnification, 400×. (D) Photomicrograph showing gram-positive cocci in the glomerulus. Gram stain; magnification, 1000×. The mouse was 8 wk old.
Discussion
Human diabetic patients reportedly have an increased susceptibility to group B Streptococcus bacteremia for several reasons, including hyperglycemia and related effects on phagocytic function and cell-mediated immunity.6 Similarly, streptozotocin-treated mice, are more susceptible to group B Streptococcus infection than control mice.3,13 Although disseminated infection due to S. agalactiae in a model of type II diabetes (such as KK-Ay mice) has not been reported previously, the isolation of S. agalactiae in both pure and mixed cultures from both abscesses and organs, as we report here, is strongly indicative of the clinical significance of this isolate in KK-Ay mice.
We here described 22 KK Ay mice that demonstrated clinical signs associated with infection by S. agalactiae. We did not perform any histopathology to confirm the diagnoses of abscess and torticollis, which were based solely on the clinical signs observed and their similar progression in all animals. The various abscesses might be attributed to bite wounds from fighting before or during transport and associated translocation of bacteria from the oral cavity; no bite wounds were noted in any of the mice. An interesting finding was that facial abscesses were more prevalent in mice older than 15 wk, perhaps due to the timing of the development of hyperglycemia in this strain. The colonization of group B Streptococcus in pregnant women and its transmission to their children is common, and S. agalactiae has been isolated from the ear canal of neonates of colonized mothers.16 We surmise that the torticollis in our mice was related to middle or inner ear infection by S. agalactiae through the upper respiratory tract, nasal cavity, or hematogenous route. The systemic infection observed in the moribund mouse was correlated with the isolation of bacteria from liver. Furthermore, S. agalactiae can cause urinary tract infections in humans.1 Given that the renal inflammation was more severe in the cortex than in the medulla (Figure 2 B) of this mouse, S. agalactiae most likely was disseminated hematogenously in this case.
The incidences of S. aureus and K. pneumoniae infections are also more prevalent in people with diabetes mellitus than in people without diabetes mellitus,9 and these 2 bacterial species were found concurrent bacterial isolates with S. agalactiae.14 in this report. KK-Ay mice might be also more susceptible to these pathogens.
Given the prevalence of S. agalactiae, we tested other diabetic mouse models that were purchased from commercial vendors. Groups of 16 BKS.Cg-Dock7m+/+Leprdb/J (Charles River Laboratories Japan, Kanagawa, Japan), BKS.Cg-+ Leprdb/+ Leprdb/Jcl (CLEA), and B6:V-Lepob/J (Charles River Laboratories Japan) mice (age, 5 wk) were all negative for S. agalactiae. Although S. agalactiae was reported as an oral bacterial isolate in animals from 2 BALB/c suppliers, no clinical signs of infection were apparent.15 At our animal facility, S. agalactiae has been isolated from the oral cavity and feces of BALB/c mice also (data not shown). Because clinical signs with abscesses as a prominent symptom have seldom occurred in either BALB/c mice or alternative mouse models of diabetes at our facility, S. agalactiae might be a risk factor for these clinical signs in diabetic mice. Our case report suggests that S. agalactiae should be included in the list of possible etiologic agents of disease in diabetic mice with clinical signs.
Our results are supported by clinical evidence indicating a correlation between people with diabetes mellitus and their propensity to acquire S. agalactiae infections. However, the effect of hyperglycemia on the susceptibility to S. agalactiae infection has not been fully elucidated. KK-Ay mice might be useful for evaluating the adaptation of S. agalactiae to hyperglycemic environments.
References
- 1.Bronsema DA, Adams JR, Pallares R, Wenzel RP. 1993. Secular trends in rates and etiology of nosocomial urinary tract infections at a university hospital. J Urol 150:414–416. [DOI] [PubMed] [Google Scholar]
- 2.Castle CK, Colca JR, Melchior GW. 1993. Lipoprotein profile characterization of the KK A(y) mouse, a rodent model of type II diabetes, before and after treatment with the insulin-sensitizing agent pioglitazone. Arterioscler Thromb 13:302–309. [DOI] [PubMed] [Google Scholar]
- 3.Edwards MS, Fuselier PA. 1983. Enhanced susceptibility of mice with streptozotocin-induced diabetes to type II group B streptococcal infection. Infect Immun 39:580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. 1997. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385:165–168. [DOI] [PubMed] [Google Scholar]
- 5.Flores AE, Ferrieri P. 1983. Biochemical markers of the penicillin-induced L phase of a group B, type III Streptococcus sp. J Clin Microbiol 18:961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang PY, Lee MH, Yang CC, Leu HS. 2006. Group B streptococcal bacteremia in nonpregnant adults. J Microbiol Immunol Infect 39:237–241. [PubMed] [Google Scholar]
- 7.Hwang MN, Ederer GM. 1975. Rapid hippurate hydrolysis method for presumptive identification of group B streptococci. J Clin Microbiol 1:114–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwatsuka H, Shino A, Suzuoki Z. 1970. General survey of diabetic features of yellow KK mice. Endocrinol Jpn 17: 23–35. [DOI] [PubMed] [Google Scholar]
- 9.Leibovici L, Yehezkelli Y, Porter A, Regev A, Krauze I, Harell D. 1996. Influence of diabetes mellitus and glycaemic control on the characteristics and outcome of common infections. Diabet Med 13:457–463. [DOI] [PubMed] [Google Scholar]
- 10.Miller MW, Duhl DM, Vrieling H, Cordes SP, Ollmann MM, Winkes BM, Barsg GS. 1993. Cloning of the mouse agouti gene predicts a secreted protein ubiquitously expressed in mice carrying the lethal yellow mutation. Genes Dev 7:454–467. [DOI] [PubMed] [Google Scholar]
- 11.Ministry of the Environment. [Internet] 2006. The care and management of laboratory animals and relief of pain. [Cited 28 April 2006] Available at: https://www.env.go.jp/nature/dobutsu/aigo/2_data/nt_h180428_88.html [In Japanese].
- 12.Nandyal RR. 2008. Update on group B streptococcal infections: perinatal and neonatal periods. J Perinat Neonatal Nurs 22:230–237. [DOI] [PubMed] [Google Scholar]
- 13.Puliti M, Bistoni F, Orefici G, Tissi L. 2006. Exacerbation of group B streptococcal sepsis and arthritis in diabetic mice. Microbes Infect 8:2376–2383. [DOI] [PubMed] [Google Scholar]
- 14.Raja NS. 2007. Microbiology of diabetic foot infections in a teaching hospital in Malaysia: a retrospective study of 194 cases. J Microbiol Immunol Infect 40:39–44. [PubMed] [Google Scholar]
- 15.Rodrigue L, Lavoie MC. 1996. Comparison of the proportions of oral bacterial species in BALB/c mice from different suppliers. Lab Anim 30:108–113. [DOI] [PubMed] [Google Scholar]
- 16.Shah D, Saxena S, Randhawa VS, Nangia S, Dutta R. 2013. Prospective analysis of risk factors associated with group B streptococcal colonisation in neonates born at a tertiary care centre in India. Paediatr Int Child Health 34:184–188. [DOI] [PubMed] [Google Scholar]