Abstract
Cynomolgus monkeys are often used in preclinical transplantation research. Performing liver transplantation in cynomolgus monkeys is challenging because they poorly tolerate portal vein clamping during the anhepatic phase. Finding an alternative to portal vein clamping is necessary before preclinical liver transplant models can be performed with reliable outcomes. We used 3 different techniques to perform 5 liver transplants in male cynomolgus macaques (weight, 7.4–10.8 kg; mismatched for MHC I and II; matched for ABO). In procedure A, we clamped the portal vein briefly, as in human transplants, as well as the superior mesentery artery to minimize congestion at the expense of temporary ischemia (n = 2). In procedure B, we performed a temporary portocaval shunt with extracorporeal venovenous bypass (n = 1). For procedure C, we developed an H-shunt system (modified portocaval shunt) with extracorporeal bypass (n = 2). Postoperative immunosuppression comprised cyclosporine A, mycophenolate mofetil, and steroids. Recipients in procedure A developed hemodynamic instability and were euthanized within 2 d. The recipient that underwent procedure B was euthanized within 11 d due to inferior vena caval thrombosis. The H-shunt in procedure C led to minimal PV congestion during the anhepatic phase, and both recipients reached the 21-d survival endpoint with good graft function. Our novel H-shunt bypass system resulted in successful liver transplantation in cynomolgus macaques, with long-term posttransplant survival possible. This technical innovation makes possible the use of cynomolgus monkeys for preclinical liver transplant tolerance models.
Abbreviations: IRI, ischemia–reperfusion injury; IVC, inferior vena cava; PV, portal vein; SMA, superior mesenteric artery
The combination of bone marrow and kidney transplantation can produce allogeneic tolerance in cynomolgus macaques without permanent immunosuppression.3,5 This model was successfully adapted and translated to a clinical study in patients.4 We aim to investigate whether this protocol can be applied successfully to liver transplantation. Before tolerance protocols can be established in humans, a large animal model has to be tested and demonstrated to be safe. Because the cynomolgus macaque model has been used to develop protocols that lead to allogenic tolerance for organ transplantation, we decided to use this model for our liver transplant study.
The technique for human liver transplantation was developed in the 1960s through 1980s and is now well established. However, despite the frequent use of cynomolgus monkeys (Macaca fascicularis) in preclinical transplantation models for research, only a few facilities have reported successful liver transplants in these animals.2,6,8,9 The anatomy of the cynomolgus macaque liver has been clarified only recently,13 and one important feature in these animals is that the complete envelopment of the inferior vena cava (IVC) by hepatic parenchyma—coupled with numerous short hepatic veins between the IVC and liver—precludes the use of the ‘piggyback’ caval implantation technique, which would allow continued systemic venous blood return during transplantation. Therefore, a technical approach to liver transplantation in cynomolgus macaques must be established that compensates for the lack of both portal and systemic drainage during the procedure. We attempted 3 different techniques, which progressively decreased both bowel congestion during the anhepatic phase and the resulting ischemia–reperfusion injury (IRI) afterward.
Materials and Methods
Animals.
The study involved 10 male cynomolgus macaques (weight, 8.7 ± 1.1 kg [mean ± 1 SD]; range, 7.4 to 10.8 kg) were used. The animals’ exact ages were unknown because they were not bred in captivity, but all were past adolescence. All macaques were housed at the Institute of Comparative Medicine (Columbia University Medical Center, New York, NY). This facility holds a current USDA assurance and is an AAALAC-accredited institution. All animals were negative for B virus, simian T-lymphotropic virus, simian retrovirus, SIV, simian varicella virus, and malaria. Recipient and donor pairs were selected for compatible ABO blood type and mismatched cynomolgus leukocyte MHC antigens. Class I and II antigenic disparity was determined by MHC genotyping at University of Wisconsin.7,12 Donor–recipient pairs were chosen to ensure that the body weight of the recipient was heavier than that of the donor to prevent compression of the allografted liver by the recipient's smaller abdominal cavity. Postoperative care of animals was performed in accordance with NIH guidelines for the care and use of primates, and our protocols were approved by the IACUC of Columbia University.
Liver transplantation.
Macaques underwent orthotopic liver transplantation under general anesthesia. Ketamine (2.8 to 5.0 mg/kg IM) with dexmedetomidine hydrochloride (0.075 to 0.120 mg/kg IM) was used to induce anesthesia, with ketamine (3 to 10 mg/kg/h IV) and isoflurane (0.5% to 3% by endotracheal tube) for maintenance. Lidocaine (less than 4.5 mg/kg SC) mixed with bupivacaine (less than 1 mg/kg SC) was applied at the incision site just before both incision and closure of the incision to minimize postoperative pain. Before abdominal incision, central lines were placed in the left carotid artery and left internal jugular vein of all donors and recipients. We chose the left side to prevent cerebral infarction (air or a clot from a right carotid arterial line can flow into the left carotid artery); 2) to avoid slippage of the venous catheter into the superior vena cava (we can insert a longer catheter through the innominate vein when we insert it into the left jugular vein); and 3) to parallel the conditions used in recipients for procedures B and C, in which an additional venous line is placed into the left external jugular vein to reduce the outflow pressure during the bypass work. All recipient lines were connected to a swivel in a jacket-and-tether system (Lomir Biomedical, Malone, NY) for postoperative hemodynamic monitoring, blood sampling, and infusion.
During the donor surgery, the portal vein (PV), suprahepatic vena cava, and infrahepatic vena cava were dissected out. The hepatic artery was kept with the celiac trunk and abdominal aorta until the iliac bifurcation and aortic arch. The infrahepatic vena cava graft was dissected free for use in creating a portacaval shunt in the bypass cases (procedure C). Before perfusion, all donors’ blood was collected for transfusion to their recipients, and all recipients received a transfusion of donor blood during surgery; 1000 units of heparin were injected intravenously into all donors at 2 to 5 min before cross-clamping of the aorta. Then the diaphragm was incised and the suprahepatic vena cava transected. The liver was perfused in situ through the aorta and inferior mesenteric vein by using 300 to 500 mL cold CoStorSol (Preservation Solutions, Elkhorn, WI), after which the liver was removed.
The recipient surgery was performed by the same surgical team that performed the donor operation. Three technical approaches to managing portal vein drainage (procedures A through C) were tested during the anhepatic phase (Table 1). We started with the surgical technique described in procedure A, and subsequently revised our technique to that described as procedure B and then that of procedure C, so these procedures were not randomized.
Table 1.
Pairs, surgical procedures, and posttransplantation survival
| Donor animals |
Recipient animals |
|||||||||||||||||
| Surgical procedures |
||||||||||||||||||
| Type | ID no. | BW (kg) | Surgery time | Heparin (IU) | ID no. | BW (kg) | Surgery time | Heparin (IU) | WIT (min) | TIT (min) | SMA (+IMA) clamped (min) | On bypa ss (min) | H shunt | PC shunt | EC bypass | SMA clamp | IMA clamp | PST (d) |
| A | 37–238 | 8.7 | 5 h 49 min | 1000 | 36–119 | 9.1 | 10 h 17 min | 300 | 36 | 75 | 36 | 0 | – | – | – | + | – | 2 |
| A | 36–24 | 7.7 | 4 h 02 min | 1000 | 46–213 | 10.8 | 7 h 35 min | 500 | 40 | 90 | 40 | 0 | – | – | – | + | – | 0 |
| B | 90–55 | 7.7 | 2 h 58 min | 1000 | 90–52 | 7.6 | 7 h 46 min | 1300 | 37 | 225 | 19 (+12) | 48 | – | + | + | + | + | 11 |
| C | 36–178 | 9.0 | 3 h 17 min | 1000 | 36–160 | 8.9 | 7 h 06 min | 2000 | 35 | 263 | 0 | 37 | + | + | + | – | – | >21 |
| C | 90–42 | 7.4 | 4 h 26 min | 1000 | 90–44 | 7.7 | 9 h 15 min | 1300 | 46 | 231 | 0 | 61 | + | + | + | – | – | >21 |
BW, body weight; EC bypass, extracorporeal bypass; IMA, inferior mesenteric artery; PC shunt, portocaval shunt; PST, posttransplantation survival time; SMA, superior mesenteric artery; TIT, total ischemia time; WIT, warm ischemia time
All animals were male cynomolgus macaques (age unknown).
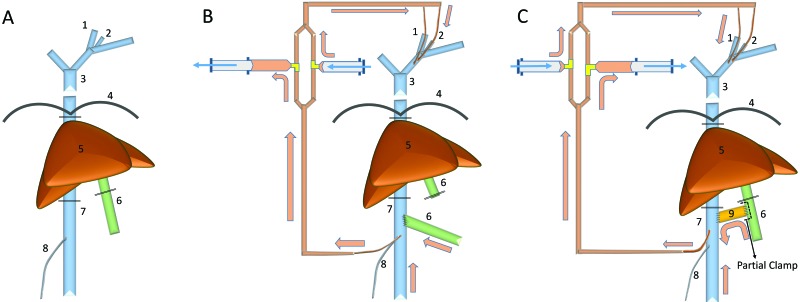
Procedure A: Superior mesenteric artery clamp (Figure 1 A).
Figure 1.
The evolution of liver transplantation procedure. (A) Procedure A (SMA clamp method). (B) Procedure B (SMA clamp + portocaval shunt method). (C) Procedure C (modified portocaval shunt [H-shunt] method). 1, Left internal jugular vein; 2, left external jugular vein; 3, superior vena cava; 4, diaphragm; 5, liver; 6, portal vein; 7, inferior vena cava; 8, right testicular vein; 9, donor IVC graft (H-shunt).
To prevent bowel congestion, the superior mesenteric artery (SMA) was clamped before clamping the PV and removing the liver; heparin (300 IU in macaque 36–119; 500 IU in 46–213) was administered intravenously prior to SMA clamping. After hepatectomy, the graft liver was placed into the hepatic fossa, and the suprahepatic vena cava, infrahepatic vena cava, and PV were anastomosed to those of the graft by using 7-0 Prolene (Ethicon, Somerville, NJ) suture in a running pattern. The graft was reperfused, and the SMA was unclamped. The graft aortic conduit was then anastomosed to the recipient aorta in an end-to-side fashion by using 7-0 Prolene running sutures. For biliary reconstruction, an end-to-end choledocho–choledochostomy was created by using interrupted gut sutures of chromic gut (Ethicon).
Procedure B: SMA clamp and portocaval shunt (Figure 1 B).
Before the liver was removed, a catheter for extracorporeal bypass was put into the IVC from the trunk of the right testicular vein, to prevent IVC stenosis and bleeding from the IVC. This catheter was connected to the extracorporeal bypass system, and the blood flow from the IVC was redirected into the superior vena cava through a dual parallel manual pumping bypass system line that was primed with normal saline. We intravenously injected 300 IU of heparin, and the SMA and inferior mesenteric artery were clamped during the construction of the portocaval shunt (19 min during the PV–IVC anastomosis), and the recipient PV was anastomosed to the recipient IVC in an end-to-side fashion. An additional 1000 IU of heparin was injected intravenously and manual bypass was initiated. After hepatectomy, the suprahepatic vena cava and infrahepatic vena cava anastomoses were performed by using running sutures of 7-0 Prolene (Ethicon). Prior to dismantling the portocaval shunt and performing the portal vein anastomosis, the SMA was clamped (12 min). Once the portal anastomosis was completed, the liver was reperfused, the SMA was unclamped and bypass was discontinued.
Procedure C: Modified portocaval shunt (H-shunt; Figure 1 C).
Procedure C involved a modified portocaval shunt (otherwise called an ‘H-shunt’), which connected the PV and IVC by using a donor IVC graft. A 2- to 3-cm vein graft from the infrahepatic vena cava of the donor liver graft was used to create the H-shunt. The proximal side of the donor IVC graft was anastomosed to the recipient IVC by using 7-0 Prolene (Ethicon) and a partial vessel clamp technique to maintain IVC blood flow and preserve blood pressure. After the donor IVC graft was clamped, the clamp from the recipient's IVC was removed. Then the duodenal side of the PV was partially clamped to preserve the portal vein flow and prevent ischemia due to the congestion of intestines. The distal side of the donor IVC graft was anastomosed to the recipient PV by using 7-0 Prolene (Ethicon) in a running pattern. Clamps on PV and the donor IVC graft were removed after completion of the anastomosis; the SMA and IMA were left unclamped during creation of the portocaval shunt. Finally, a catheter for extracorporeal bypass was put directly into the recipient's IVC and then secured with 2-0 silk ties, to prevent injury of the right testicular vein. This catheter was connected to the extracorporeal bypass with 2 syringe pumps on each parallel line, and the blood flow from the IVC and PV was redirected into the superior vena cava through this bypass system. By using this H-shunt system, continuous PV flow was maintained, and the SMA was not clamped at any time during the liver transplant procedure.
Macaques received 1000 IU (animal 36–160) or 300 IU (90–44) of heparin intravenously before creation of the H-shunt and then another 1000 IU before starting extracorporeal bypass. After hepatectomy, suprahepatic IVC, infrahepatic IVC, and then PV anastomoses were performed as described earlier, after which the liver was reperfused by removing the caval and PV clamps. The H-shunt was clamped, both ends were tied with 3-0 silk, and then the shunt was divided in the middle. The catheter for the extracorporeal bypass was removed from the IVC insertion site and the hole for the catheter was closed. The proximal end of the donor aorta (thoracic aorta) was anastomosed end-to-side to the recipient infrarenal aorta. The donor distal aorta was tied, and the bile ducts were anastomosed end-to-end, as described earlier.
Postoperative care.
After surgery, the recipients were observed continually until they moved easily without signs of respiratory distress. Once the macaques were fully awake, they were monitored hourly for the first 24 h. Each animal was assessed for signs of pain, respiratory rate, heart rate, blood pressure, and mobility. On postoperative day 2, animals were assessed every 3 h, with particular attention to pain, position, respiratory rate, heart rate, mobility, and appetite.
Continuous intravenous fluids (Ringer lactate solution or multiple-electrolyte injection, type 1; 0.5 to 15 mL/kg/h) were administered from a central venous line. A fentanyl transdermal patch (1 to 4 μg/kg/h) was placed for 72 h. Supplemental NSAID (flunixin meglumine, 1.0 to 2.2 mg/kg) were injected as needed; pain not relieved by NSAID was treated by additional administration of buprenorphine (0.01 mg/kg every 6 to 12 h) after day 3. Cefazolin sodium (25 mg/kg IV daily) was provided to the first case (macaque 36-119) on days 0 through 2; the other animals received ampicillin–sulbactam (300 mg/kg IV daily on days 0 through 2). Macaques were maintained on liquid diet on day 0; if the liquid diet was tolerated, the animals received regular monkey chow and enrichment items starting on postoperative day 1. Continuous intravenous fluids were reduced according to the urinary output and appetite of each animal. All macaques were provided environmental enrichment (toys, mirrors) as stipulated by our institutional protocol. To prevent disturbing the animals’ rest, lights were turned off for 12 h at night except during emergency. Preoperative blood draws were performed in a procedure room adjacent to the housing room to prevent agitating the other animals housed in the room. At no time were the animals housed individually; when necessary, we acquired a companion macaque to ensure the appropriate social environment. All cages had lattice windows so that the macaques could interact and touch each other at all times.
Immunosuppressive therapy.
All recipients underwent a conventional triple-drug immunosuppressive regimen consisting of cyclosporine A (Novartis, East Hanover, NJ), mycophenolate mofetil (Roche, Nutley, NJ), and methylprednisolone sodium succinate (Pfizer, New York, NY). Cyclosporine A was given at a starting dose of 15 mg/kg IM daily and was titrated to maintain a trough level of 300 to 500 ng/mL. Mycophenolate mofetil was given in food at a dose of 600 mg/m2 body surface area daily. Methylprednisolone sodium succinate was given intramuscularly or intravenously beginning at a dose of 10 mg/kg, with the dose tapered daily.
Results
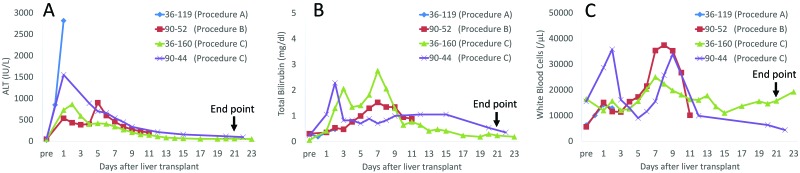
Table 1 summarizes the stepwise progression from procedure A to procedure C. In procedure A (n = 2), SMA clamping was performed during the anhepatic phase. Animal 36–119 required high-dose pressors (phenylephrine, 0.4 to 9.15 μg/kg/min; ephedrine, 0.03–0.1 mg/kg; vasopressin, 0.0003 to 0.002 units/kg/min) after reperfusion to manage ischemia–reperfusion injury (IRI) related to vasodilatation despite large-volume infusion of crystalloid solution. After surgery, anuria persisted from postoperative day 1 and led to euthanasia (pentobarbital sodium and phenytoin sodium, 1 mL per 3 kg body weight) on postoperative day 2. The ALT level in this macaque remained elevated until euthanasia (Figure 2 A).
Figure 2.
Liver function tests ([A] ALT and [B] serum total bilirubin levels) and (C) WBC counts after orthotopic liver transplantation.
In the next macaque (46–213) that underwent procedure A, we corrected intraoperative hypovolemia, anemia, acidosis (sodium bicarbonate, 1 mEq/kg IV), and hypothermia, but hypotension was profound after reperfusion of the allograft liver despite treatment with vasopressors (dopamine, 1 to 20 μg/kg/min; norepinephrine, 0.5 to 1.5 μg/kg/min; phenylephrine, 0.4 to 9.15 μg/kg/min; ephedrine, 0.03–0.1 mg/kg; epinephrine, 0.02 to 0.04 mg/kg; vasopressin, 0.0003 to 0.002 units/kg/min), and the animal was euthanized before abdominal closure. No other surgical complications were observed in these animals.
In procedure B (n = 1), the transplantation for macaque 90–52 involved a portocaval shunt. With this modification, we avoided significant hypotension during surgery, including during the anhepatic phase and after reperfusion of the allograft. Although ALT increased to approximately 900 IU/dL on postoperative day 5, it decreased after steroid pulse therapy and an increased dose of cyclosporine A. This animal developed venous congestion of the right testicle, likely related to cannulation and ligation of the right gonadal vein. He was euthanized on postoperative day 11 due to hemodynamic collapse resulting from a large IVC thrombosis. Normal renal function had been maintained throughout. No other surgical complications occurred intraoperatively or postoperatively in this animal.
In procedure C (n = 2), both animals (36–160 and 90–44) were transplanted by using the modified portocaval shunt (H-shunt), and the dual parallel manual extracorporeal bypass system remained normotensive throughout the liver transplantation surgery. Both animals achieved the end point of 21-d survival and were euthanized on postoperative days 22 and 23, respectively, without any major complications. Neither animal developed swelling of the testicle or an IVC thrombus; Figure 2 A and B shows the macaques’ ALT and bilirubin levels after liver transplantation. All liver function tests (AST, ALT, total bilirubin, and GGT) in procedure C animals were almost normal at the time of euthanasia. We removed all 3 catheters from macaque 90–44 (procedure C) on postoperative day 9, because the laboratory data (increased WBC count alone) suggested a central line infection (Figure 2 A through C). No other significant intraoperative or postoperative complications occurred in these animals.
There were no surgical complications in the donors.
Pathologic findings.
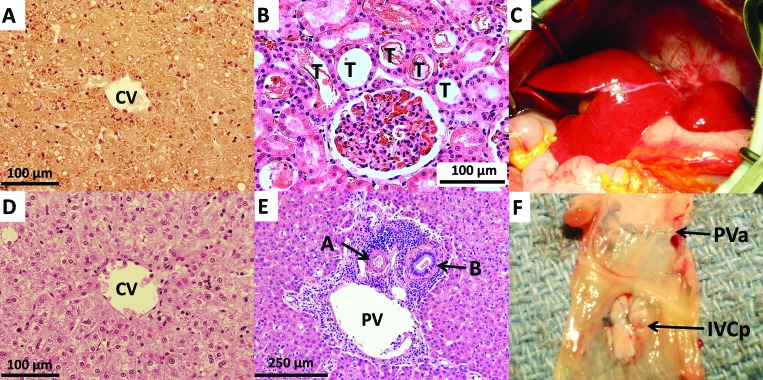
All recipients had necropsies. Gross findings of animal 36–119 (procedure A) showed that all anastomoses were patent, with no apparent bleeding. The small intestine and colon were slightly hyperemic. The liver was mildly congested but was not thrombosed; histology revealed moderate coagulative necrosis of hepatocytes in the allograft liver affecting centrilobular hepatocytes and extending to the lobular periphery, in some regions involving the entire lobular parenchyma to the edges of portal tracts and involving approximately 50% to 80% of the liver parenchyma (Figure 3 A). Renal histology showed cortical tubule dilation with loss of the brush border, compatible with moderate acute tubular necrosis involving approximately 30% of renal tubules (Figure 3 B). Some focal mild pancreatitis, possibly due to ischemia, was present also. Grossly, all anastomoses in macaque 46–213 (procedure A) were patent. Histology revealed moderate to marked coagulative necrosis in the allograft liver involving overall 50% to 80% of the liver parenchyma. In the kidney, tubular ectasia with proteinaceous casts were present, compatible with mild acute tubular necrosis.
Figure 3.
Gross and histopathologic findings. (A) Liver of animal 36–119 (procedure A) at necropsy on day 2 after transplantation shows marked coagulative necrosis on histology. (B) Severe acute tubular necrosis seen in the kidney of 36–119 on day 2. These findings are consistent with ischemia–reperfusion injury. (C) Normal gross appearance of transplanted allograft liver after reperfusion of animal 36–160 (procedure C). (D) Preserved liver without necrosis or ischemic injury around the central vein at necropsy of animal 36–160 on day 22. (E) Minimal rejection cellular infiltrate around the bile duct of animal 36–160 on day 22. (F) Portal vein of macaque 90–44 (procedure C) on day 23. No portal vein stenosis was seen after using the donor IVC patch. CV, Central vein; PV, portal vein; A, artery; B, bile duct; T, tubule; G, glomerulus.; PVa, portal vein anastomosis; IVCp, donor inferior vena caval patch.
Gross evaluation of macaque 90–52 (procedure B) disclosed a stricture of the IVC around the infrahepatic anastomosis area, which is also around the closing suture of the portocaval shunt. In addition, a white thrombus in the inferior vena cava was present, starting from the infrahepatic anastomosis to the caudal side. The pulmonary artery lacked white thrombi, indicating no pulmonary embolus occurred. Furthermore, the right testis was swollen due to congestion. Additional histologic findings were consistent with mild acute rejection and central lobular necrosis in liver due to hemodynamic collapse.
All anastomoses were patent grossly in macaques 36–160 and 90–44 (procedure C). No thrombi were seen in the IVC, iliac vein, femoral vein, or aorta, and no testicular abnormalities were seen. Histology confirmed the lack of IRI in the liver (Figure 3 D), kidney, pancreas, and intestines in both animals. Minimal to mild acute rejection was present in macaque 36–160 (Figure 3 E), and no rejection was seen in animal 90–44.
In summary, the pathologic findings of both animals that underwent procedure A showed moderate to marked coagulative necrosis in the allografted liver and mild to severe acute tubular necrosis in the native kidneys. These findings are compatible with IRI.10,11 The macaque that underwent procedure B demonstrated ischemia in the allografted liver due to hemodynamic collapse resulting from a large IVC thrombosis. Neither macaque with procedure C showed findings consistent with IRI at the time of autopsy (Figure 3 D and E). Despite triple immunosuppressive therapy, there was mild rejection of the allograft, manifested by cellular inflammation around the portal veins, in animal 36–160 (Figure 3 E).
Discussion
This study is the first to demonstrate that cynomolgus macaques can undergo orthotopic liver transplantation by using a portocaval shunt and extracorporeal bypass to avoid the severe complications of the anhepatic phase. The pathologic findings and the clinical course of the 2 animals that underwent procedure A were highly suggestive of IRI. This IRI was severe, and it was very difficult to maintain normal blood pressures during the anhepatic phase and after reperfusion of the allografted liver. SMA, PV, and IVC clamping resulted not only in intestinal ischemia but also congestion of the kidneys and lower body during the anhepatic phase; this congestion might have led to the severe IRI that occurred. This IRI is a likely cause of the severe acute renal tubular necrosis that led to anuria after surgery. In addition, cytokines released from the intestines might have drained into the allografted liver after reperfusion, consequently contributing to the marked coagulative necrosis seen on liver histology. Other centers have reported that liver transplantation can be performed in cynomolgus monkeys without using extracorporeal bypass8,9 and expressed the opinion that liver transplantation using venovenous bypass in cynomolgus macaques would be difficult because of the animal's small size.8 However, we observed severe IRI leading to early death in both of the cases we performed without using extracorporeal bypass, even though the warm ischemia time times were only 36 and 42 min. Another difference might be the warm ischemia times themselves, but these were not documented in the previous reports8,9 and were relatively brief (36 and 42 min) in our animals. During anhepatic phase in 15- to 20-kg dogs, the bypass system reportedly shunted the bloodflow from the infrahepatic IVC and PV into the suprahepatic IVC by using a mechanical pump, effectively preventing blood congestion in the superior vena cava, IVC, and PV systems.1 However, this canine method would be difficult to apply to 7- to 11-kg cynomolgus macaques due to their short suprahepatic IVC, which prevents effective cannulation, and to their smaller blood volume, which is insufficient for priming the venous reservoir for the mechanical bypass system used in the dog study.1 The bypass system we describe in the current report is optimal for the anhepatic phase of approximately 9-kg macaques. Nevertheless, the use of the extracorporal bypass allows for prolonged ischemic times during surgery without prohibitive intestinal ischemia and limits the IRI after reperfusion.
Two surgical methods could prevent IRI: a portocaval shunt with extracorporeal bypass or a portocaval shunt with a ‘piggyback’ technique for the vena caval anastomosis. A ‘piggyback’ technique uses a partial IVC clamp during IVC–hepatic vein anastomosis after dissecting off the liver but preserving the IVC, so that it can prevent the congestion of all infrahepatic organs when used with a portocaval shunt. Anatomically, the cynomolgus liver encircles the IVC, with numerous short hepatic veins entering the IVC, so that portocaval shunt with extracorporeal bypass is preferable to simplify the surgery. This strategy minimizes the congestion of the intestines and kidneys, because the IVC and lower body might function as a reservoir for blood during the anhepatic phase. In addition, oxygenation of the abdominal organs and lower legs could be preserved during the anhepatic phase by using this bypass technique.
To achieve the bypass, we needed to minimize the priming volume of the lines and pump. To minimize the priming volume, we used a manual pumping system instead of the mechanical pumping system used in neonatal–pediatric cardiac surgery. Mechanical pumping systems need a venous reservoir to prevent air embolism and requires significant volumes of blood for priming the venous reservoir. Compared with the mechanical pumping system, our manual bypass pumping system doesn't need a venous reservoir, and the priming volume for this system is around only 20 to 30 mL of crystalloid fluids. The other advantage of manual pumping is that we can control the inflow and outflow pressures by manual handling of the syringes, and we can see the flow volume during surgery. The inflow and outflow pressures cannot be manipulated with a mechanical system, which sometimes indicates an inaccurate flow rate when the venous pressure increases. We found the dual, parallel manual pumping bypass system we used maintained continuous bloodflow like a mechanical pumping system. In addition a bloodflow rate of 1 to 2 mL/s was sufficient to prevent IRI during the anhepatic phase. To prevent rupture of the outflow line due to high pressure, we used tubing designed for arterial line monitoring, which was successful.
Regarding the modified portocaval shunt (Figure 1 C), we were able to preserve PV flow through the shunt during anastomosis of the PV by using this system. During end-to-side anastomosis of the PV and IVC vein graft, we put a side-biting partial clamp on the PV, which allowed for partial flow through the PV during the creation of the portocaval shunt and prevented congestion of the intestines. During anastomosis of the PV to the allograft liver, we kept the PV flow to the extracorporeal bypass. Returning the blood to the heart by using the bypass well-maintained the blood pressure during the anhepatic phase and prevented IRI after reperfusion of the allograft. Another advantage of the modified PV shunt is that the H-shunt can be ligated and divided, leaving a portion of the donor IVC on the PV and IVC as a patch after bypass is discontinued. This practice prevents stenosis of the PV and IVC and prevents thrombus formation in those vessels (Figure 3 F).
Procedure B might have worked well if we could have prevented IVC stenosis. However, our procedure B animal experienced both IVC stenosis and IVC thrombus. To prevent the IVC stenosis, the donor IVC patch in procedure C worked well. Another advantage of procedure C is that we didn't need to clamp the PV completely during creation of the H-shunt. This advantage kept us from having to clamp the SMA and IMA as we did during the full PV clamping period for the PV–IVC anastomosis of procedure B. Therefore, we didn't have to be concerned about hypoxia of the recipient's intestines during the vascular anastomosis.
After some modifications, our novel manual bypass system resulted in successful liver transplantation in 2 cases of our cynomolgus monkey model, as evidenced by the fact that they both achieved the 21-d survival endpoint with good graft function. We will use this method to adapt an established combined bone marrow–kidney transplantation model3,5 to achieve liver allograft tolerance by using a similar conditioning protocol. The technical approach described here facilitates this and other preclinical liver transplantation studies using cynomolgus monkeys.
Acknowledgments
We acknowledge Mr. Michael and Mrs. Susan Kerr for their generous support of this work, and Drs. Masaru Kubota and Hiroyuki Tahara for excellent surgical contributions. Dr. Adam D. Griesemer received support from the American Association for the Study of Liver Diseases Career Development Award in Memory of the University of Michigan Transplant Team.
References
- 1.Chkhaidze Z, Khodeli NO, Pilishvili O, Partsakhashvili D, Jangavadze M, Kordzaia D. 2013. New model of veno–venous bypass for management of anhepatic phase in experimental study on dogs. Transplant Proc 45:1734–1738. [DOI] [PubMed] [Google Scholar]
- 2.Gotoh M, Monden M, Kanai T, Umeshita K, Wang K, Ukei T, Dono K, Tono T, Murata M, Tanimoto J, Hashimoto M, Okamura J, Mori T. 1991. Tolerance induction by liver grafting and FK506 treatment in nonhuman primates. Transplant Proc 23:3265–3268. [PubMed] [Google Scholar]
- 3.Kawai T, Cosimi AB, Colvin RB, Powelson J, Eason J, Kozlowski T, Sykes M, Monroy R, Tanaka M, Sachs DH. 1995. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation 59:256–262. [PubMed] [Google Scholar]
- 4.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, Shaffer J, Preffer FI, Ding R, Sharma V, Fishman JA, Dey B, Ko DS, Hertl M, Goes NB, Wong W, Williams WW, Jr, Colvin RB, Sykes M, Sachs DH. 2008. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med 358:353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawai T, Sogawa H, Boskovic S, Abrahamian G, Smith RN, Wee SL, Andrews D, Nadazdin O, Koyama I, Sykes M, Winn HJ, Colvin RB, Sachs DH, Cosimi AB. 2004. CD154 blockade for induction of mixed chimerism and prolonged renal allograft survival in nonhuman primates. Am J Transplant 4:1391–1398. [DOI] [PubMed] [Google Scholar]
- 6.Monden M, Gotoh M, Kanai T, Valdivia LA, Umeshita K, Endoh W, Nakano Y, Kawai M, Ohzato H, Ukei T, Dono K, Tono T, Murata M, Wang KS, Okamura J, Tanimoto Y, Hashimoto M, Mori T. 1990. A potent immunosuppressive effect of FK506 in orthotopic liver transplantation in primates. Transplant Proc 22:66–71. [PubMed] [Google Scholar]
- 7.O'Connor SL, Blasky AJ, Pendley CJ, Becker EA, Wiseman RW, Karl JA, Hughes AL, O'Connor DH. 2007. Comprehensive characterization of MHC class II haplotypes in Mauritian cynomolgus macaques. Immunogenetics 59:449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oura T, Yamashita K, Suzuki T, Fukumori D, Wantanabe M, Hirokata G, Wakayama K, Taniguchi M, Shimamura T, Miura T, Okimura K, Maeta K, Haga H, Kubota K, Shimizu A, Sakai F, Furukawa H, Todo S. 2012. Long-term hepatic allograft acceptance based on CD40 blockade by ASKP1240 in nonhuman primates. Am J Transplant 12:1740–1754. [DOI] [PubMed] [Google Scholar]
- 9.Oura T, Yamashita K, Suzuki T, Watanabe M, Hirokata G, Wakayama K, Taniguchi M, Shimamura T, Furukawa H, Todo S. 2014. A technique for orthotopic liver transplantation in cynomolgus monkeys. Transplantation 98:e58–e60. [DOI] [PubMed] [Google Scholar]
- 10.Park SW, Chen SW, Kim M, Brown KM, Kolls JK, D'Agati VD, Lee HT. 2010. Cytokines induce small intestine and liver injury after renal ischemia or nephrectomy. Lab Invest 91:63–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park SW, Kim M, Brown KM, D'Agati VD, Lee HT. 2011. Paneth cell-derived interleukin 17A causes multiorgan dysfunction after hepatic ischemia and reperfusion injury. Hepatology 53:1662–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pendley CJ, Becker EA, Karl JA, Blasky AJ, Wiseman RW, Hughes AL, O'Connor SL, O'Connor DH. 2008. MHC class I characterization of Indonesian cynomolgus macaques. Immunogenetics 60:339–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vons C, Beaudoin S, Helmy N, Dagher I, Weber A, Franco D. 2009. First description of the surgical anatomy of the cynomolgus monkey liver. Am J Primatol 71:400–408. [DOI] [PubMed] [Google Scholar]