Abstract
Specifically designed restraint chairs are the preferred method of restraint for research studies that require NHP to sit in place for sustained periods of time. In light of increasing emphasis on refinement of restraint to improve animal wellbeing, it is important to have a better understanding of this potentially stressful procedure. Although chair restraint is used internationally, very little published information is available on this subject. We developed a survey to obtain an overview of equipment, procedures, and plans for improvement regarding chair restraint. We received 101 responses from people working in academic, government, contract research, and pharmaceutical laboratories within the Americas, Europe and Asia. Findings indicate that the majority of laboratories using restraint chairs work with macaque species. Restraint chairs are used for a wide range of procedures, including cognitive testing, recording neuronal activity, functional MRI, intravenous infusion, and blood sampling. Approximately 2/3 of laboratories use an enclosed ‘box chair,’ which the animal is trained to enter and then to extend its head through an opening on the top of the chair; the remaining one third of laboratories use an ‘open chair’ design, in which manual handling or the pole-and-collar system is used to transfer and secure the animal into the chair. Respondents reported that when selecting the type of chair to use, they considered comfort for the animal, ease of use, and the ability to adjust fit between animals of different sizes. Various training methods and timeframes are used to prepare macaques for restraint chair procedures. Several laboratories are incorporating greater use of positive reinforcement training. The community that uses these restraint procedures needs to work together to define best practice; our survey results can help in that effort.
Abbreviations: NC3Rs, National Centre for Replacement, Refinement, and Reduction of Animals in Research; NRT, negative reinforcement training; PRT, positive reinforcement training
The use of specifically designed restraint chairs is the most common method of restraint for various research studies that require conscious NHP to ‘sit’ in place for sustained periods of time. For decades, restraint chairs have been used in the laboratory environment to facilitate studies that include electrophysiology, eye tracking, neuroimaging, computer testing, dosing and biologic sampling procedures.3,10,14,22,25,32,38 An important goal for investigators using restraint is to train the NHP to exit the home cage, enter the chair, accept restraint, and perform reliably, in the shortest possible time and without undue stress.
All restraint chairs typically secure the animal at a ‘tether point’ (usually at the neck), but chairs vary in their design. ‘Open’ chair designs allow access to all of the animal's body. The NHP is fitted with a collar around the neck, to which a pole can be attached and used to lead the animal to the chair, where the collar is then secured into place on the chair, or the monkey may be handled and manually placed into the chair. The open design differs from ‘closed’ chair designs, in which the NHP is enclosed in an acrylic box. The animal is trained to enter through a door and to lift its head out of an opening on top of the box; the neck is then secured by a yoke that is slid into place, preventing the animal from pulling its head back into the box. The closed chair can be modified so that research staff can access arms, legs, and other parts of the animal's body for various procedures.
The methods used to transfer monkeys from their home enclosure to the chair vary also. Anecdotally, some laboratories use a single pole and one trainer, 2 poles and 2 trainers, a leash, and so forth. In Europe, use of the pole-and-collar system is not considered good practice and is not a recommended method of restraint.20 Some research groups use a combination of positive and negative reinforcement, but others emphasize positive techniques and elicit voluntary cooperation from the animal.36 For a procedure that is used fairly commonly throughout the NHP research community, there seems to be little discussion about the differences in equipment and methodologies used to prepare animals for research studies requiring chair restraint.
The Guide for the Care and Use of Laboratory Animals is the standard for animal care and use in the United States and many other countries outside of Europe.31 The 2011 version of the Guide has expanded the guidance regarding the use of animal training in the context of restraint and specifically recommends the use of positive reinforcement training (PRT) when animals are placed in restraint devices, to help them adapt to the equipment and personnel; this version of the Guide also makes other recommendations to minimize the effect of restraint on animal wellbeing (p 29).31 With PRT, the animal receives rewards for desired behavioral responses and is reinforced for cooperating with individual steps in the behavior sequence until the end-goal behavior is reached; undesired behavior is usually ignored. Similar recommendations are made in Appendix A to the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes ETS 123, which states that NHP “dislike being handled and are stressed by it” and that “training animals to cooperate should be encouraged, as this will reduce the stress otherwise caused by handling.”13 Because neither set of guidelines provides detailed instruction on animal training, it is important for staff involved in these procedures to be familiar with any relevant literature that may help guide them (for example, reference 24).
As more emphasis is placed on the links between good animal management, welfare, and the quality of science derived from laboratory animals, researchers are sharing new techniques in scientific procedures and research methods.34 The use of PRT techniques in the overall management of NHP in the laboratory environment seems to be increasing.2 Training NHP to participate in various husbandry, veterinary, and research procedures by using PRT has many benefits, including reduced animal distress,11,35 an increase in cooperation and flexibility with captive management,6,7,47 and reduced use of anesthetic agents;12,18,41 all of these advantages may ultimately contribute toward improving the quality of the science.
Publications that compare modern refinements with more traditional methods used to prepare NHP for chair restraint have increased. Anderson and Houghton first described the pole-and-collar method used to transfer NHP from their home enclosure to a restraint chair, and this report is still used and cited in recent literature.1 In this method, the animal is confined to a small portion of its cage to attach the pole to its collar. If it resists, it is held firmly with the pole until resistance stops. The animal is then transferred to a restraint chair and secured in place before being provided with a food reward. This method relies primarily on force and aspects of negative reinforcement training (NRT). With NRT, the removal of an aversive stimulus immediately after a desired behavior increases the frequency of the behavior occurring over time (for example, using a cage ‘squeeze back’ mechanism to move a NHP into position for a procedure at the front of the cage and releasing the squeeze-back as soon as the desired movement toward the cage front is performed). NRT can be effective in achieving the desired behavior, but potentially at a cost to the animal's wellbeing. Other authors recently demonstrated the feasibility of training rhesus macaques (Macaca mulatta) by using predominantly PRT to gain cooperation with the pole-and-collar process, with fewer elements of negative reinforcement (that is, the cage squeeze-back mechanism), thus refining traditional pole-and-collar methods.24
Other scientists have had success preparing animals for chair restraint that were deemed untrainable for the pole and collar process or suffered from a behavioral pathology. Cooperative restraint training prepares rhesus macaques for restraint by using a closed chair.5 The macaque is transferred to a box chair and is trained to position its head through an opening on top of the chair, by using combinations of PRT, desensitization, and NRT. This method also proved useful for “challenging” animals previously deemed unsuited to traditional restraint techniques. These authors have built on this work by evaluating individual differences in behavior, which are then used to structure individual training programs to accommodate variation in animal temperament.4 Understanding how individual differences influence animal learning can be used by the trainer to set learning expectations on an animal-by-animal basis. Other colleagues developed a ‘smart chair’ that responds to the NHP, training it to enter the chair in the trainer's absence.33 This allowed the authors to keep rhesus macaques within a research program that might have otherwise been excluded due to their significant behavioral pathologies. The animals responded more positively to the initial stages of training with the automated training system than they did with a human trainer and were able to achieve a good training foundation.
Information regarding approaches used internationally for preparing and training monkeys for chair restraint has not yet been compiled nor have the perspectives of those doing this work regarding the effectiveness and desirability of various aspects of such training. Given the increasing emphasis on refinement of restraint procedures to improve animal wellbeing, it is important to have a better understanding of this procedure, which can be stressful for NHP.8,15-17,19,21,23,26,27,39 Therefore, we conducted a survey to document current practices and to identify opportunities for refinement. Sharing this information with the research community that uses chair restraint for NHP, including investigators, research technicians, those who train the NHP, animal welfare officers, and IACUC members, may be helpful as they strive to improve animal wellbeing, no matter where in the world they are working.
Materials and Methods
A qualitative survey was conducted among laboratories using chair restraint procedures with NHP. Between 27 January and 25 February 2016, survey invitations were emailed to 124 targeted employees working in laboratories using NHP in research. Recipients were identified through the primate welfare network of the National Centre for Replacement, Refinement and Reduction of Animals in Research (NC3Rs), the UK Network for Named Animal Care and Welfare Officers working with NHP, the UK Expert Group for NHP Neuroscience Research, and publications in the field. In addition, an open invitation to participate was sent to the Behavioral Management Consortium of the US National Primate Research Centers, the Society for Neuroscience Committee on Animals in Research, and the Association of Primate Veterinarians. Furthermore, there was an open call on social media through the LinkedIn groups Society for Neuroscience, Animal Models in Neuroscience, European Primate Veterinarians, and NHP Toxicology. Participants were asked to limit responses to one per laboratory or research group to decrease the chance of repetition. Participation was voluntary, and responses were anonymous. Research funded by the NC3Rs involving regulated procedures or the potential to cause harm to animals or people is reviewed by the local ethics committee, and all legal permissions must be in place before the research commences.
The survey was constructed and administered through SurveyMonkey44 and comprised 39 questions organized around 4 key themes: background; equipment; preparing the animal for restraint procedures; and personnel. Comment boxes were provided for some questions to allow the submission of additional information as free text. The data acquired were managed according to a standard data management plan for NC3Rs office-led data sharing projects. Some respondents did not answer all of the questions. The number of responses to each question is therefore unique and is reported in absolute terms. Only anonymized data are reported (any free-text responses that could identify individual facilities have been redacted). The χ2 test was used to test for associations between responses to dichotomous (yes/no) questions and categorical variables, such as chair type and training method.
Results
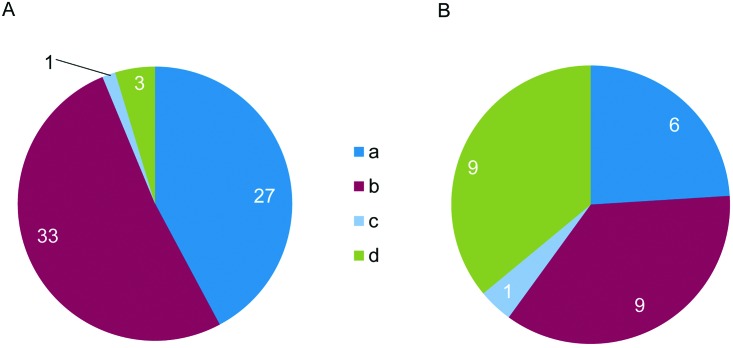
A total of 101 responses were returned for analysis, from laboratories in the Americas (62), Europe (33), and Asia (6; Figure 1). The overall response rate cannot be determined because some of the invitations to participate were open calls, such that the potential number of laboratories that could respond is unknown, and responses were anonymous. Responding laboratories were based in the following sectors: academic (66), contract research (15), pharmaceutical industry (10), government (9), and other (nonprofit, 1). Due to similarities in the procedures for which restraint chairs were used, the responses from academic and government sectors have been combined in the figures presented here, as have those from contract research, pharmaceutical and other sectors.
Figure 1.
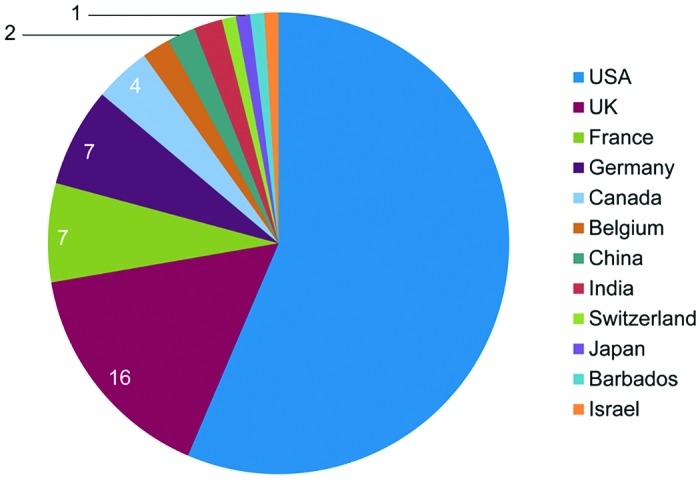
Survey respondents by country. Responses to question 2: “In which country do you work?” (n = 101).
Respondents worked with mainly rhesus macaques (M. mulatta; 85 of 101 respondents) and cynomolgus macaques (M. fasicularis; 39 of 101). Seven laboratories worked with other macaque species (bonnet macaques [M. radiata], Japanese macaques [M. fuscata]), 6 with marmosets (Callithrix spp.), 4 with vervet monkeys (Chlorocebus pygerythrus or C. sabaeus), 4 with baboons (Papio spp.), and 2 with squirrel monkeys (Saimiri spp.).
Background.
Respondents were asked to focus their answers to the majority of questions (nos. 4 through 39) on the species they work with most frequently. Rhesus macaques were predominantly used in the academic and government sectors (68 of 75 respondents) and cynomolgus macaques in the contract research and pharmaceutical sectors (25 of 26). There was a fairly even distribution of age and sex of NHP used: juvenile or adolescent males (71 of 101); juvenile or adolescent females (53 of 101); mature or adult males (86 of 101); and mature or adult females (62 of 101). Two laboratories used elderly or geriatric animals.
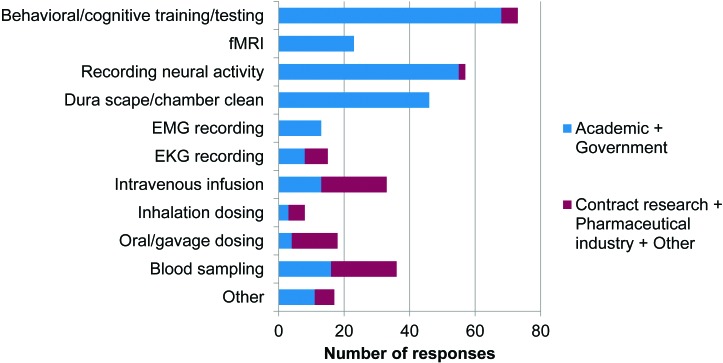
Within the academic and government sectors, monkeys were chaired mostly for neuroscience procedures, such as behavioral or cognitive testing (68 of 75 respondents), functional MRI (23 of 75), recording neural activity (55 of 75), dura scrape or chamber cleaning (46 of 75), and EMG recording (13 of 75; Figure 2). Within the contract research and pharmaceutical industry sectors, NHP were chaired mostly for toxicology and safety pharmacology procedures, such as dosing of test compounds by intravenous infusion (20 of 26) or oral gavage (14 of 26), blood sampling (20 of 26), and ECG recording (7 of 26).
Figure 2.
Procedures involving chair restraint. Responses to question 6: “What procedures are being conducted with the monkeys when restrained in the chair? (tick all that apply)” (n = 101).
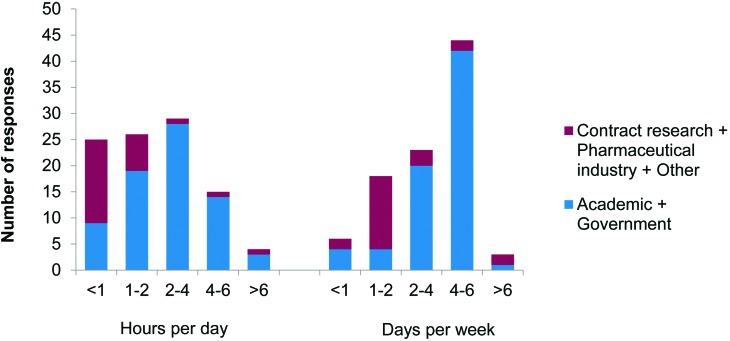
The median duration for which NHP were restrained in the chair was 2 to 4 h per day (28 of 73 respondents) on 4 to 6 d per week (42 of 71) in the academic and government sectors; and less than 1 h per day (16 of 26) on 1 or 2 d per week (14 of 23) in the contract research and pharmaceutical sectors (Figure 3). The length of time monkeys were actively involved in restraint procedures was highly variable, ranging from 1 mo to 8 y in the academic and government sectors and less than 1 mo to 2 y in the contract research and pharmaceutical sectors (Figure 3). Where rest periods (that is periods of time where no chair restraint was conducted) were provided, they were typically 1 to 2 d in duration in the academic and government sectors (38 of 74) and 2 to 7 d in the contract research and pharmaceutical sectors (6 of 21). Nine laboratories did not provide rest periods. A variety of additional restraints were used with the chair; restraint of the head (56 of 101) and arm or wrist (17 of 101) was used in the academic and government sectors, and restraint of the upper and lower limbs (30 of 46) in the contract research and pharmaceutical sectors.
Figure 3.
Duration of chair restraint. Responses to question 7: “What is the typical duration of time the monkeys you work with are restrained in a chair for research purposes? (for awake, behaving electrophysiology studies, give the duration for the testing session)” (hours per day, n = 99; days per week, n = 94).
Equipment.
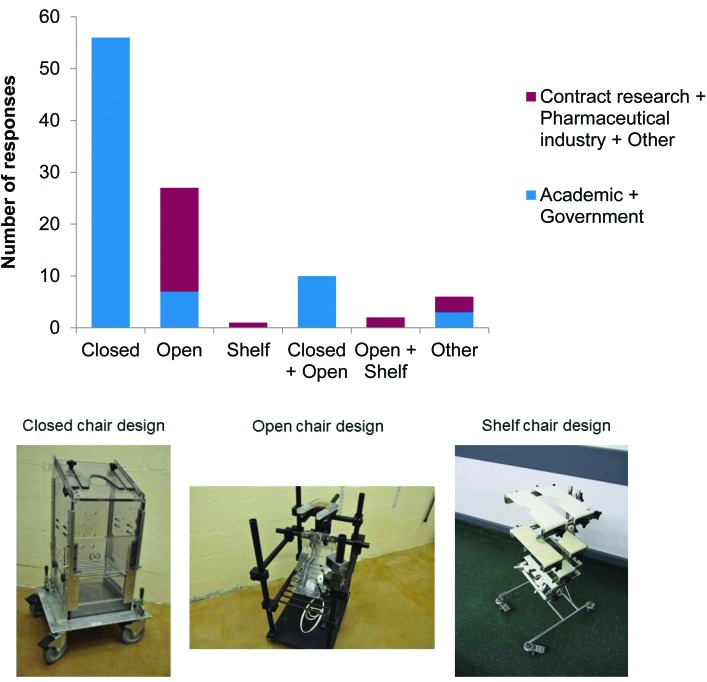
The most frequently used type of chair was the closed-chair design (66 of 100 respondents), which was used exclusively in academic and government sectors, followed by the open-chair design (39 of 100), which was popular in the contract research and pharmaceutical sectors, probably because this chair design allows easy access to the animal's limbs for dosing and sampling procedures (Figure 4). The shelf-chair design was used in only 4 laboratories; this chair is similar to the open chair in that it allows good access to the animal and enables restraint of the neck and waist, but the shelves provide points at which the animal's wrists and ankles can be secured. ‘Other’ designs included chairs that did not fit in either category and the typically were manufactured or modified for a specific purpose; for example, chairs for use in horizontal-bore magnets (MRI) and inhalation studies (closed chair incorporating a helmet and seat; 6 of 100).
Figure 4.
Types of chairs. Responses to question 11: “What type of chair do you use in your lab? (tick all that apply; see pictures below)” (n = 100).
Chairs were most often obtained from a commercial supplier (56 of 100 respondents) or made on site (49 of 100); 27 laboratories customized commercially available chairs. A variety of factors were considered in the choice of chair, with the animal's comfort rated as the most important (Figure 5).
Figure 5.
Choice of chair. Responses to question 13: “When deciding which type of chair to use in your research (or during the development of your chair) what are the most important factors to consider? (tick your top five factors)” (n = 100).
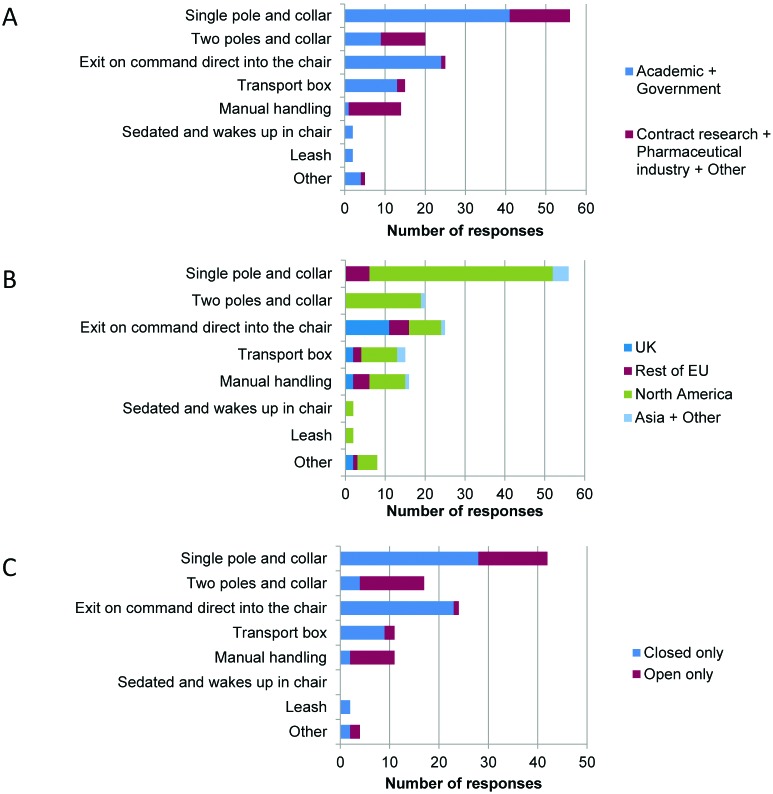
There was a great deal of variation between laboratories in the methods used to transfer the NHP, once fully trained, from the home environment to the chair (Figure 6). Single pole and collar (41 of 96 respondents), 2 poles and collar (9 of 96), exit on command directly into the chair (24 of 96), and transport box (13 of 96) were the methods used most frequently in the academic and government sectors. Pole and collar (15 of 43), manual handling (13 of 43), and 2 poles and collar (11 of 43) were the most common methods in the contract research and pharmaceutical sectors. Regional differences were present as well: pole and collar methods were not used in the United Kingdom, where the most frequent method of transfer was exiting from the monkey's home enclosure on command and directly into the chair (11 of 17); this method was used almost exclusively with closed-chair designs. Open-chair designs were used with one or 2 poles and collar or manual handling.
Figure 6.
Transfer to the chair. Responses to question 14: “Once fully trained, how is the monkey transferred from the home environment to the chair? (tick all that apply)” (n = 100). Responses grouped according to (A) sector, (B) region, and (C) chair type.
Preparing the animal for restraint procedures.
A total of 74 of 89 laboratories reported having a standard operating procedure or other guideline for preparing monkeys for chair restraint, whereas 58 of 89 laboratories had a standard operating procedure or other guideline for monitoring monkeys while they are in the restraint chair. A description of the training procedures to be used was required in submitted IACUC protocols or project licenses in 72 of 89 laboratories.
The training methods used to prepare NHP for restraint procedures in the academic and government sectors were nearly always based on active, stepwise training using positive reinforcement or a combination of positive and negative reinforcement (Figure 7). Within the contract research and pharmaceutical sectors, however, approximately 1/3 of laboratories did not use stepwise training and instead placed the monkey in the chair and relied on it becoming habituated to the chair-restraint procedure over time, with very little active training.
Figure 7.
Training methods. Responses to question 18: “What type of training procedures best describe the methods used to prepare your monkeys for restraint procedures? (tick one from a to d) (a) Monkey is trained in a stepwise fashion, and is allowed to progress through the steps at his/her own pace. Positive reinforcement (for example, food treat, verbal praise) is provided for cooperating with each of the steps. No negative reinforcement (for example, use of the squeeze-back mechanism, tugging on the pole to encourage the monkey to move) is used. (b) Monkey is trained in a stepwise fashion, but if not progressing as expected, the use of a squeeze-back mechanism, second pole, or other negative reinforcer is used to speed up the process. Positive reinforcement is provided throughout the process. (c) The use of a squeeze-back mechanism or other similar negative reinforcer is introduced at the beginning of the training phase and is paired with a positive reinforcer. The monkey goes through the training steps at a predetermined pace. (d) Monkey is placed in the chair and rewards are provided once secured. Struggling is observed in the beginning, but over time the monkey appears calm and cooperates with the procedure.” (A) Responses from academic and government sectors (n = 64). (B) Responses from contract research and pharmaceutical sectors (n = 25).
The use of food or fluid control (or both) was common in the academic and government sectors (52 of 65 respondents) but relatively rare in the contract research and pharmaceutical sectors (7 of 25). Twelve academic laboratories in North America and one in the United Kingdom did not use food or fluid control; 10 of these were conducting behavioral or cognitive testing of the monkeys, of which 4 also involved neural recording. The 7 contract research or pharmaceutical laboratories using fluid or food control were conducting similar procedures to the laboratories in these sectors that did not use food or fluid control, namely, intravenous infusion, oral or gavage dosing, blood sampling, and ECG recording.
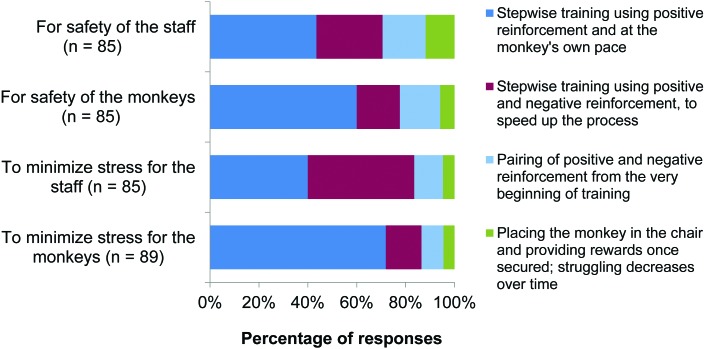
The majority of respondents considered stepwise training using positive reinforcement to be the best approach for minimizing stress for the NHP and for the safety of the animals and staff (Figure 8). Approximately equal numbers of respondents considered positive reinforcement, or a combination of positive and negative reinforcement, to be the best approach to minimize stress for staff.
Figure 8.
Best approaches for safety and minimizing stress. Responses to question 20: “Which is the best approach? (tick one in each column)” (n = 89).
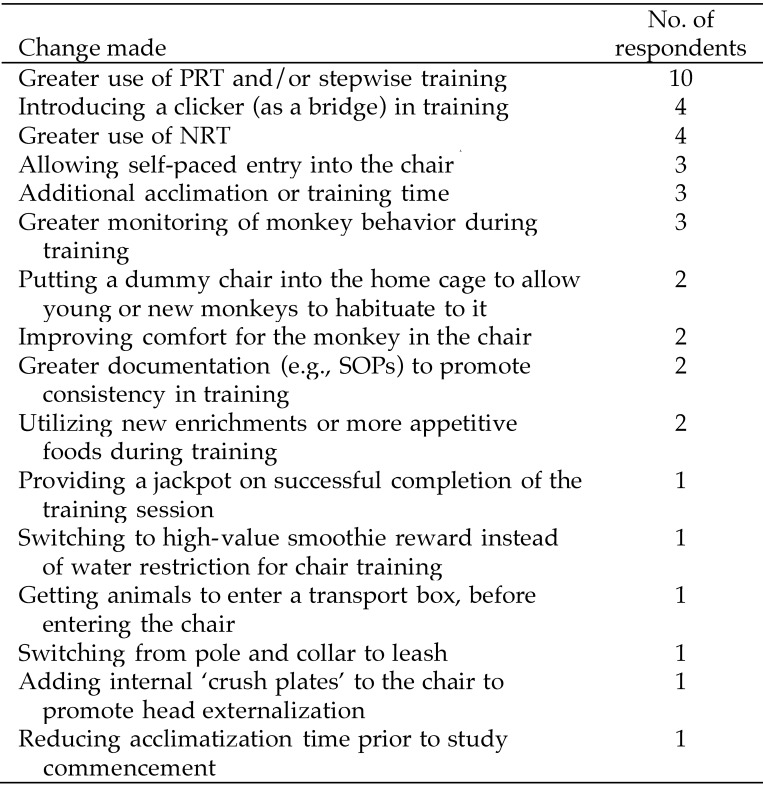
Thirty-six laboratories reported having changed their chair training methods in recent years (Figure 9). The majority of the changes would be expected to improve animal wellbeing. The most frequent change was greater use of positive reinforcement and stepwise training methods. The reported reasons for this change were that the animals were less aggressive or agitated and more cooperative, making staff–animal interactions easier and safer. Four laboratories reported greater use of negative reinforcement. The reason given for this change was failure of the animals to progress sufficiently quickly through the training schedule.
Figure 9.
Changes to chair-training methods. Responses to question 21: “Have you made changes to your chair-training methods in the last few years, and if so, why? (please give details)” (n = 36).
The average length of time used to prepare monkeys for chair restraint equipment was most often 2 to 8 wk in the academic and government sectors (44 of 59) and 1 to 2 wk in the contract research and pharmaceutical sectors (13 of 25). A total of 38 of 89 laboratories considered that their monkeys would benefit from additional preparation time for restraint procedures. A greater proportion of respondents in the contract research and pharmaceutical sectors shared this view (18 of 25) than did those in the academic and government sectors (20 of 64; χ2 = 12.201, P < 0.05). Similarly, a greater proportion of those using only open chairs shared this view (18 of 25) than did those using only closed chairs (14 of 49; χ2 = 12.721, P < 0.05). There was no relationship between the proportion of respondents that considered additional training time would be beneficial and the training method used (χ2 = 0.771, P > 0.05) or length of time used to prepare monkeys for chair restraint (χ2 = 0.4.913, P > 0.05).
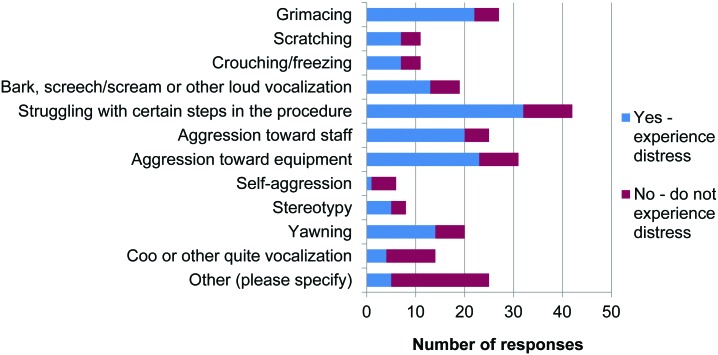
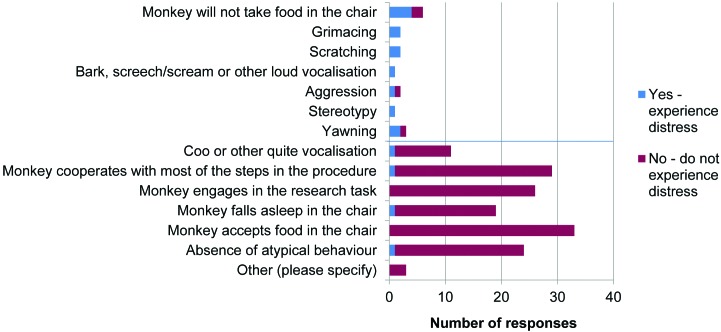
Respondents were asked whether they thought that monkeys experience distress during initial training procedures for chair restraint. Distress was defined as “an aversive, negative state in which coping and adaptation processes in response to stressors fail to return [the animal] to physiological and/or psychological homeostasis; occurs when stress is severe, prolonged, or both.”28 Among the 89 laboratories that responded, 42 replied ‘yes’ and 47 replied ‘no.’ There was no relationship between training method used and response to this question (χ2 = 1.542, P > 0.05). Respondents then were asked what behaviors or responses by the animals led to their conclusion; a list of behavioral signs of fear and anxiety were presented along with one sign of positive welfare (coo or other quiet vocalization). A total of 34 of 89 laboratories considered that their monkeys did not experience distress (‘no’) but incorrectly assigned at least one behavioral sign of distress in support of this conclusion (Figure 10).
Figure 10.
Behaviors during initial training. Responses to question 25: “What behaviors or responses lead you to your conclusion? (tick all that apply)” (n = 89).
Respondents were then asked whether they thought that NHP experience distress once well trained; 7 of 89 replied ‘yes’ and 82 of 89 replied ‘no.’ Respondents were again asked what behaviors or responses by the monkeys led to their conclusion; a list of behavioral signs of fear and anxiety were presented, along with signs of acceptance of the restraint procedure. Most of these respondents (79 of 89) correctly identified signs indicating distress or lack of distress (Figure 11).
Figure 11.
Behaviors once well trained. Responses to question 27: “What behaviors or responses lead you to your conclusion? (tick all that apply)” (n = 89).
When a fully trained monkey begins to regress (that is, resists or no longer cooperates with restraint procedures), this lapse was most often addressed in the academic and government sectors by giving the monkey a break in participation and providing additional time to train through the regression (33 of 78) or by continuing to use the monkey but incorporating additional time for training outside of research or testing (24 of 78). These approaches also were used in the contract research and pharmaceutical sectors, but in those settings the problem was more likely to be addressed by removing the monkey from the study (12 of 35). Eleven laboratories reported that they made the monkey participate with the restraint procedures.
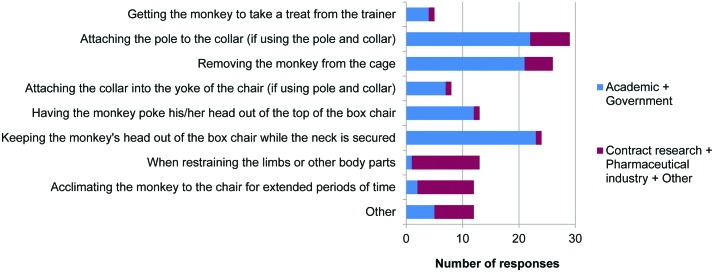
The most challenging steps in preparing a naïve monkey for chair restraint within the academic and government sectors were considered to be attaching the pole to the collar, removing the monkey from the cage, and having the monkey poke its head out of the top of the box chair and keep it there (Figure 12). Within the contract research and pharmaceutical sectors, the most challenging steps were restraining the limbs or other body parts and acclimating the animal to the chair for extended periods. A total of 39 of 89 laboratories had experienced a monkey that did not acclimate to chairing procedures. This situation was most often dealt with by providing additional training before the research began (28 of 89 respondents) or removing the monkey from the study (24 of 89); some laboratories assigned a new trainer to the animal (8 of 89).
Figure 12.
Challenging steps. Responses to question 29: “What is the most challenging step in preparing a naïve monkey for chair restraint? (tick all that apply)” (n = 89).
For industry studies (in which NHP typically do not perform any behavioral or cognitive tasks in the chair), environmental enrichment is usually provided while the monkey is in the chair (23 of 24 respondents). This enrichment was most often food items (11 of 24), television (3/24), human interaction (3 of 24), or a combination of these items, sometimes including toys (6/24).
Personnel.
Trainers conducting the initial training of monkeys for chair restraint in the academic and government sectors were most often research assistants (43 of 139 respondents), doctoral students (29 of 139), animal technicians (21 of 139), and principal investigators (19 of 139). In the contract research and pharmaceuticals sectors, trainers were most often animal technicians (19 of 42). The use of dedicated animal trainers was relatively rare (10 of 139 for the academic and government sectors; 7 of 42 for the contract research and pharmaceuticals sectors). The trainer who conducted initial training was often the person who chaired the NHP during the data collection period (60 of 79). Trainers were most often themselves trained by someone within their laboratory (63 of 79) or by an animal trainer from within their institution (28 of 79); very few (3 of 79) were trained by an animal trainer from an external organization or consultancy.
When those training a NHP for chair restraint considered the animal to be fully trained, someone outside of the laboratory assessed the animal's level of training in only 13 of 54 laboratories in the academic sector and 6 of 25 laboratories in the pharmaceutical sector. The person who deemed the NHP fully trained was most often the trainer themselves (61 of 110 respondents), the principal investigator (25 of 76 in the academic sector), or animal behavior specialist (4 of 34 in the industry sector).
Almost half of respondents felt that the person conducting the chair restraint training experienced distress during the process (34 of 79 laboratories). Respondents who held this view were not more likely to consider that the animals also experienced distress during initial training (χ2 = 0.202, P > 0.05). However, these respondents were more likely to consider that the NHP would benefit from additional preparation time for restraint procedures (χ2 = 0.069, P < 0.05). There was no relationship between respondents experiencing distress and training method used (χ2 = 1.542, P > 0.05) or the average length of time taken to prepare monkeys for chair restraint equipment before research procedures begin (χ2 = 5.587, P > 0.05). In all sectors, the best approach for chair training was considered to be a combination of positive and negative reinforcement (40 of 79) or solely positive reinforcement (29 of 79).
The final survey question asked “If future studies were conducted investigating chair restraint, what questions would you want answered?” The overarching theme for all responses to this question was identifying principles underlying best practice for chair restraint procedures. Respondents were keen to have answers to questions such as: How do we make the chairing procedure a more positive experience for the animals? Can multipurpose chairs be designed, that offer additional restraint if necessary in a safe manner and that allow animals more control in moving from their home enclosure to the restraint chair? Can we pool our knowledge to write a standard protocol or description of chair-restraint procedures that incorporates a variety of training techniques appropriate for the time available, as well as tips and strategies for those animals that may be more challenging to train (that is, animals anxious or fearful at the start of training, those that don't want to keep their head out of the top of the box chair when the neck plate is being put into place)? There was also interest in examples of animal behaviors to look for that would indicate when NHP are ready to move from one training step to the next (that is, a greater understanding of macaque behavior).
Discussion
The survey has 2 limitations: first, the results may not be fully representative of the broader NHP research community because of an unknown response rate, and, second, response bias might be present due to concerns about how the surveyed information might be reported or used. Although the total number of laboratories using chair restraint with NHP is unknown, based on our professional knowledge of the NHP research landscape and the geographic distribution of facilities, we consider that the 101 responses received is sufficiently representative to draw valid conclusions. Response bias is a limitation of every survey-based research project and might have influenced our findings, but there is no other practical way to obtain this information.
NHP in the academic and government sectors, in particular, spend a large part of their day, most days a week, in a restraint chair. It is important therefore to ensure that the restraint process is as fully refined as possible. Doing so will reduce any potential stress and keep discomfort to a minimum, ultimately facilitating good performance from the animal and high-quality scientific data.
Fit and comfort in the chair were found to be the most important factors when laboratories are deciding what type of restraint chair to use, but whether research groups are considering all of the pros and cons of the various designs available is unclear.27 Purchasing a chair is a major investment, and we suggest that laboratories new to chair restraint consult with someone familiar with different styles of chairs and chairing procedures and review any recent refinements that might benefit the animals’ welfare and the outcome of the research before making this purchase. We found that a considerable number of laboratories manufacture their chairs inhouse or buy commercially available chairs and customize them to suit their needs. It is sometimes thought that NHP fitted with head implants cannot go into a closed chair, because of the risk of damaging the implant when entering the box or elevating the head through the opening; however, in fact, with appropriate modifications, many laboratories successfully use this type of chair with implanted animals. Open chairs give easy access to the animal's limbs, but, in our experience, users of open chairs often seem to be unaware of modifications to the closed-chair design that can yield similar results and allow similar procedures to be conducted with the animals. Researchers using restraint procedures may have more success preparing their animals in a shorter time frame by using the closed box chair.
The method of transfer from the home environment into the chair is another important consideration in improving the experience of the NHP. One disadvantage of the open chair is that it requires the animal to be guided by the trainer, using the pole-and-collar method or manual handling. This process decreases the animal's control, and control and choice are considered to be important for the wellbeing of captive NHP.9,40 With the closed chair, animals can be trained to perform each of the steps necessary to transfer from the home cage to the chair and to lift its head out of the opening of the chair without the use of pole and collar. Therefore, we were surprised at the number of laboratories using the pole-and-collar method with closed chairs. Macaques can be successfully trained by using a combination of PRT and NRT to voluntarily exit the home cage and go directly into the closed chair or to enter a transport box first.4,5 Not only is this method of transfer likely to allow the animal greater perceived control, but, judging from comments submitted as part of the survey, it may also be less stressful for staff than is the use of the pole and collar. As a research community, we should move toward a more positive training experience for the monkeys used in restraint procedures, no matter what the design of the chair or the method of transfer.4,24 Therefore researchers and animal care staff should stay current with published refinement techniques and make preferential use of the most humane training methods whenever possible.
The survey results indicated that many laboratories have standard operating procedures in place to increase consistency in animal training and monitoring, which we consider to be good practice. We found that most academic and government laboratories use chair-training procedures based on PRT techniques or a combination of PRT and NRT. These techniques were generally recognized to be the best training methods for the safety of the animals and staff and for minimizing stress, which also is encouraging. Incorporating NRT is considered by our respondents to expedite training and reduce stress for staff, but there is an appreciation that NRT may not be the best approach for preparing monkeys for chair restraint. Approximately 1/3 of laboratories in the contract research and pharmaceutical industry sectors do not actively train the monkeys for cooperation with chair restraint and instead rely on the animals gradually becoming accustomed to the procedures through repeated exposure, although some groups may also desensitize by using food rewards. We recommend more prestudy training based on PRT techniques, wherever possible.
Use of food or fluid control during research procedures involving restraint was common in the academic and government sectors, probably to motivate extended sequences of responses on behavioral tasks. Food and fluid restriction should not be necessary for chair restraint training, and have the potential to compromise animal wellbeing; however, refinement measures have been reported.37 Food and fluid control is intended to increase motivation in animals, but some have argued that similar performance can be achieved with the use of conditioned reinforcers or variable-ratio schedules of reinforcement.46 Why a minority of laboratories in the contract research and pharmaceuticals sectors use of fluid or food control is unclear. Understanding more about their practices may provide opportunity for refinement.
Nearly half of respondents, especially those in the contract research and pharmaceutical sectors, considered that their NHP would benefit from additional preparation time for restraint procedures. This belief probably reflects the pressures of a busy contract research sector with a high turnover of studies and limited time for preparing animals before studies commence. We recommend increased efforts to provide sufficient time for training based on PRT, because this modification will likely minimize the effects of some variables (for example, by avoiding fear responses and ceiling or floor effects in physiologic variables such as heart rate and blood pressure), thereby enabling greater accuracy in identifying drug-induced changes.18,21,45 Consideration should be given to introducing basic training techniques while NHP are at the breeding or supplying establishment (that is, before animals arrive at the research facility) or during quarantine periods prior to starting research procedures.
Within the academic and government sectors, there was large variation in the length of time used to prepare monkeys for chair restraint equipment before research procedures began. Delays with initial chair training are undesirable, because they delay the collection of data and delivery of the science within the period of grant funding. Experience has shown that macaques can be trained for cooperation with chair restraint by using predominantly PRT techniques relatively quickly when a closed chair is used.4 Although switching chair designs can be expensive, the decrease in personnel time needed to prepare animals for restraint procedures and the accelerated pace of the science likely will more than recoup the expense of purchasing new chairs.
The majority of respondents considered that initial chair-restraint training procedures caused the NHP to experience distress but that the animals did not experience distress once fully trained. Although this may be true, evidence suggests that reduced behavioral agitation may not necessarily reflect psychologic habituation to restraint procedures.8,39,43 Laboratories should move toward best practice to avoid or minimize distress during training, in line with the refinement principle of the 3Rs, and to ensure staff members have a good working knowledge of macaque behavior. The responses received indicate some confusion about behavioral signs of distress, and this confusion is a concern from animal wellbeing and scientific perspectives. We recommend that those involved in chair restraint training refresh their knowledge of macaque behavior, for example, by using the NC3Rs Macaque Website,29 attending relevant workshops or conferences, taking a class in NHP behavior, or reading published literature.
We were encouraged to see that the majority of our respondents dealt with regression (that is, resisting and no longer cooperating with restraint procedures) in their animals by providing additional time to train through the regression, sometimes even providing breaks in research participation. However, some laboratories in multiple sectors instead continue to require the animal to participate in the restraint procedures. This approach is likely to negatively affect the animal's wellbeing and potentially any resulting data. In this situation, we recommend dialogue with other laboratories to learn how they approach this scenario.4,33 Some laboratories felt it best to remove a difficult to train monkey from the study, despite the initial time and resources invested, which could require the use of more animals overall.
When questioned about the most challenging step in preparing a naïve monkey for chair restraint, attaching the pole to the collar and removing the monkey from the home cage were reported. If not conducted properly, this process can cause fear and resistance to the procedure (for example, pushing or biting at the pole, resisting exiting the cage, aggression toward the trainer), thereby increasing the risk of injury to all involved and decreasing the likelihood of gaining cooperation from the animal with future procedures in the chair (for example, struggling or grabbing when additional restraints are used for blood draws). Having the NHP extend and keep its head out of the top of the box chair is clearly an additional challenge in laboratories using this design. The number of contract research and pharmaceutical laboratories reporting challenges with restraining limbs or other body parts and with acclimating the animal to the chair for extended periods of time suggests that the NHP might not be acclimated to the restraint chair in the first place, despite the almost universal provision of rewards. Again, a new set of eyes on a training scenario may be able to provide a novel approach, and we therefore recommend consulting with experts from outside of the facility. Taking advantage of learning opportunities, such as exchange visits and workshops (for example, the one we held at the 2016 AALAS national meeting; to be repeated at the 2017 meeting) may also be helpful in addressing some of these challenges.
Overall, the changes that laboratories reported making or considered making to their chair-training methods are incredibly positive. Laboratories are incorporating more PRT procedures, including the use of a conditioned reinforcer (clicker), more desensitization to equipment, and more reinforcement provided throughout the procedure, including jackpots (a larger than usual reinforcer, often unexpected, which is delivered contingent on reaching a particular training criterion or after an exceptional effort on the part of the animal). There is also fairly widespread use of written standard operating procedures, guidelines, and documentation of training efforts. In addition, equipment changes have been made that provide animals more choice and focus on comfort.42 Some laboratories reported having moved away from force training and the use of fluid restriction and increased the time provided to animals when preparing them for the chair. Some researchers are focusing more on individual animal behavior and training plans, some of which do include a combination of positive and negative reinforcement training. Respondents indicated that these changes are being made because of “cultural changes” within their facilities; to increase consistency or ‘harmonize’ training between individual trainers; to have calmer and more cooperative NHP that are both comfortable and confident in working with research staff, more willing to participate and less “agitated and aggressive” for certain procedures; and to provide animals with more choice and control (quotation marks denote direct quotes from the survey responses). Refinements such as these may be made as new research findings become available and regulations change and require more emphasis on animal wellbeing, thus supporting why it is so important to share new information.
Within the academic and government sectors, the survey revealed that diverse job roles are involved with the initial training of monkeys for chair restraint. Principal investigators often delegate this responsibility to research assistants and doctoral students; there is a view that gaining experience training animals is a necessary part of the training of a doctoral student in NHP behavioral neuroscience. These trainers learn their techniques from another laboratory member and most often are the ones who decide when a NHP is fully trained and ready to begin participating in research. We believe that this apprenticeship approach to training of staff can risk perpetuation of poor and outdated animal training practice (for example, supervisor to student, or principal investigator to research assistant). Almost half of respondents felt that the person conducting the chair-restraint training experiences distress during the process (that is, fear of getting scratched of bitten or of not being strong enough to handle the animal; observing the animal struggle; feeling pressure from study time constraints, especially when animals progress slowly through the steps), and this state was correlated with distress for the animals during the initial training period. Training an animal for restraint procedures is a challenging task and not always one in which students are interested. If this distress in personnel can be avoided, job satisfaction for the handler or trainer consequently might increase.
Relatively few facilities, especially in the academic and government sectors, have dedicated animal trainers who assume the major responsibility for all of the training of the NHP or for training all staff who work directly with the monkeys. Providing such personnel can be a very powerful means of ensuring consistent and effective training practice, which can improve the research outcomes. There is a general move in this direction in the United States2 and (anecdotally) in the United Kingdom, and the costs of staff for animal training can be included in research grant applications. We recommend that laboratories give serious consideration to this approach. In addition, few facilities use external consultants, a resource that can provide tailored advice on best practices specific to their laboratory, the equipment used, the protocol followed, the animals’ temperaments, and so forth. Some facilities reported that an animal behavior specialist was the person to decide when a NHP is fully trained. Such a specialist is likely to give the most robust assessment from the perspective of animal wellbeing.
Information sharing is important and should be increased. We hope these survey results will provide an opportunity for laboratories to benchmark their practices and determine how they might be modified to improve animal wellbeing and scientific quality. Incorporating a brief description of the chair design and methods used to prepare animals for restraint into manuscripts can be a good mechanism for information sharing. We intend to follow the publication of the survey results with more detailed guidance on training protocols and tips for dealing with challenging situations. Surveys of current practice such as the current one are important within the broader framework of the 3Rs, because they provide a baseline and ideas for progressing with a policy of continuous improvement in animal wellbeing and for publicly demonstrating the positive changes being made by the scientific community.
Finally, many gaps in research evaluating training for chair restraint remain. We suggest that studies of the following topics would be useful: formal comparisons of the effectiveness of PRT compared with NRT compared with a combination of both; identification of approaches most suitable for animals of a given temperament; assessment of automated approaches to animal training; and objective measures of stress or distress and how these states might affect the data being collected. The NC3Rs has 3Rs research funding schemes open to researchers in the United Kingdom,30 which might facilitate such projects.
Acknowledgments
We are grateful to Anna Mitchell and Maria Martinez (University of Oxford) for initial testing of, and comments on, the questionnaire. We also thank all of the respondents for the generous donation of their time and information.
References
- 1.Anderson JH, Houghton P. 1983. The pole and collar system. A technique for handling and training nonhuman primates. Lab Anim 12:47–49. [Google Scholar]
- 2.Baker KC. 2016. Survey of 2014 behavioral management programs for laboratory primates in the United States. Am J Primatol 78:780–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balin H, Israel SL. 1963. Rhesus monkey restraint chair for the experimental study of ovulation. J Appl Physiol 18:1270–1271. [DOI] [PubMed] [Google Scholar]
- 4.Bliss-Moreau E, Moadab G. 2016. Variation in behavioral reactivity is associated with cooperative restraint training efficiency. J Am Assoc Lab Anim Sci 55:41–49. [PMC free article] [PubMed] [Google Scholar]
- 5.Bliss-Moreau E, Theil JH, Moadab G. 2013. Efficient cooperative restraint training with rhesus macaques. J Appl Anim Welf Sci 16:98–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bloomsmith MA, Laule GE, Alford PL, Thurston RH. 1994. Using training to moderate chimpanzee aggression during feeding. Zoo Biol 13:557–566. [Google Scholar]
- 7.Bloomsmith MA, Stone AM, Laule GE. 1998. Positive reinforcement training to enhance the voluntary movement of group-housed chimpanzees within their enclosures. Zoo Biol 17:333–341. [Google Scholar]
- 8.Bouyer JJ, Dedet L, Debray O, Rougeul A. 1978. Restraint in primate chair may cause unusual behaviour in baboons; electrocorticographic correlates and corrective effects of diazepam. Electroencephalogr Clin Neurophysiol 44:562–567. [DOI] [PubMed] [Google Scholar]
- 9.Buchanan-Smith HM, Badihi I. 2012. The psychology of control: effects of control over supplementary light on welfare of marmosets. Appl Anim Behav Sci 137:166–174. [Google Scholar]
- 10.Carlson KR. 1972. A temporary restraint chair for monkeys. Physiol Behav 9:493–494. [DOI] [PubMed] [Google Scholar]
- 11.Clay AW, Bloomsmith MA, Marr JM, Maple TL. 2009. Habituation and desensitization as methods for reducing fearful behavior in singly housed rhesus macaques. Am J Primatol 71:30–39. [DOI] [PubMed] [Google Scholar]
- 12.Coleman K, Pranger L, Maier A, Lambeth SP, Perlman JE, Thiele E, Schapiro SJ. 2008. Training rhesus macaques for venipuncture using positive reinforcement techniques: a comparison with chimpanzees. J Am Assoc Lab Anim Sci 47:37–41. [PMC free article] [PubMed] [Google Scholar]
- 13.Council of Europe 2006. Appendix A of the European convention for the protection of vertebrate animals used for experimental and other scientific purposes (ETS no. 123): Guidelines for accommodation and care of animals (article 5 of the convention). Approved by the multilateral consultation. Strasbourg: Council of Europe. [Cited 4 January 2017]. Available at: https://rm.coe.int/ CoERMPublicCommonSearchServices/DisplayDCTMContent? documentId=090000168007a445
- 14.Foeller P, Tychsen L. 2002. Eye movement training and recording in alert macaque monkeys. 1. Operant visual conditioning. 2. Magnetic search coil and head restraint surgical implantation. 3. Calibration and recording. Strabismus 10:5–22. [DOI] [PubMed] [Google Scholar]
- 15.Gauquelin-Koch G, Blanquie JP, Viso M, Florence G, Milhaud C, Gharib C. 1996. Hormonal response to restraint in rhesus monkeys. J Med Primatol 25:387–396. [DOI] [PubMed] [Google Scholar]
- 16.Golub MS, Anderson JH. 1986. Adaptation of pregnant rhesus monkeys to short-term chair restraint. Lab Anim Sci 36:507–511. [PubMed] [Google Scholar]
- 17.Goosen DJ, Davies JH, Maree M, Dormehl IC. 1984. The influence of physical and chemical restraint on the physiology of the chacma baboon (Papio ursinus). J Med Primatol 13:339–351. [PubMed] [Google Scholar]
- 18.Graham ML, Rieke EF, Mutch LA, Zolondek EK, Faig AW, Dufour TA, Munson JW, Kittredge JA, Schuurman HJ. 2012. Successful implementation of cooperative handling eliminates the need for restraint in a complex nonhuman primate disease model. J Med Primatol 41:89–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hassimoto M, Harada T. 2003. Use of a telemetry system to examine recovery of the cardiovascular system after excitement induced by handling stress in a conscious cynomolgus monkey (Macaca fascicularis). J Med Primatol 32:346–352. [DOI] [PubMed] [Google Scholar]
- 20.Jennings M, Prescott MJ, Members of the Joint Working Group on Refinement (Primates) Buchanan-Smith HM, Gamble MR, Gore M, Hawkins P, Hubrecht R, Hudson S, Jennings M, Keeley JR, Morris K, Morton DB, Owen S, Pearce PC, Prescott MJ, Robb D, Rumble RJ, Wolfensohn S, Buist D. 2009 Refinements in husbandry, care and common procedures for nonhuman primates: 9th report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Lab Anim 43 Suppl 1:1–47. [DOI] [PubMed] [Google Scholar]
- 21.Lee JI, Shin JS, Lee JE, Jung WY, Lee G, Kim MS, Park CG, Kim SJ. 2013. Changes of N:L ratio and cortisol levels associated with experimental training in untrained rhesus macaques. J Med Primatol 42:10–14. [DOI] [PubMed] [Google Scholar]
- 22.Lennox MS, Taylor RG. 1983. A restraint chair for primates. Lab Anim 17:225–226. [DOI] [PubMed] [Google Scholar]
- 23.Maninger N, Capitanio JP, Mason WA, Ruys JD, Mendoza SP. 2010. Acute and chronic stress increase DHEAS concentrations in rhesus monkeys. Psychoneuroendocrinology 35: 1055–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMillan JL, Perlman JE, Galvan A, Wichmann T, Bloomsmith MA. 2014. Refining the pole-and-collar method of restraint: emphasizing the use of positive training techniques with rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci 53:61–68. [PMC free article] [PubMed] [Google Scholar]
- 25.Milhaud CL, Klein MJ, Merkel MC. 1980. A new restraining chair for rhesus monkeys (Macaca mulatta). J Med Primatol 9:62–70. [DOI] [PubMed] [Google Scholar]
- 26.Morrow-Tesch JL, McGlone JJ, Norman RL. 1993. Consequences of restraint stress on natural killer cell activity, behavior, and hormone levels in rhesus macaques (Macaca mulatta). Psychoneuroendocrinology 18:383–395. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura RK, Coates R, Crawford H, Friedman D. 1982. A flexible restraint chair for the cynomolgus monkey (Macaca fascicularis). J Med Primatol 11:178–185. [PubMed] [Google Scholar]
- 28.National Research Council 2008. Recognition and alleviation of distress in laboratory animals. Washington (DC): National Academy Press. [PubMed] [Google Scholar]
- 29.NC3Rs. [Internet] 2017. The macaque website. [Cited 19 July 2017]. Available at: http://www.nc3rs.org.uk/macaques/
- 30.NC3Rs. [Internet] 2017. NC3Rs 3Rs research funding. [Cited 19 July 2017]. Available at: http://www.nc3rs.org.uk/funding
- 31.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals. Washington (DC): National Academy Press. [Google Scholar]
- 32.O'Byrne KT, Morris KD. 1988. A restraint system for the common marmoset (Callithrix jacchus). Lab Anim 22:148–150. [DOI] [PubMed] [Google Scholar]
- 33.Ponce CR, Genecin MP, Perez-Melera G, Livingstone MS. 2016. Automated chair-training of rhesus macaques. J Neurosci Methods 263:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prescott MJ. 2016. Online resources for improving the care and use of nonhuman primates in research. Primate Biology 3:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prescott MJ, Buchanan-Smith HM. 2003. Training nonhuman primates using positive reinforcement techniques: Guest editors’ introduction. J Appl Anim Welf Sci 6:157–161. [DOI] [PubMed] [Google Scholar]
- 36.Prescott MJ, Buchanan-Smith HM. 2007. Training laboratory-housed nonhuman primates, part 1: a UK survey. Anim Welf 16:21–26. [Google Scholar]
- 37.Prescott MJ, Brown VJ, Flecknell PA, Gaffan D, Garrod K, Lemon RN, Parker AJ, Ryder K, Schultz W, Scott L, Watson J, Whitfield L. 2010. Refinement of the use of food and fluid control as motivational tools for macaques used in behavioural neuroscience research: report of a working group of the NC3Rs. J Neurosci Methods 193:167–188. [DOI] [PubMed] [Google Scholar]
- 38.Robbins DO, Zwick H, Leedy M, Stearns G. 1986. Acute restraint device for rhesus monkeys. Lab Anim Sci 36:68–70. [PubMed] [Google Scholar]
- 39.Ruys JD, Mendoza SP, Capitanio JP, Mason WA. 2004. Behavioral and physiological adaptation to repeated chair restraint in rhesus macaques. Physiol Behav 82:205–213. [DOI] [PubMed] [Google Scholar]
- 40.Schapiro SJ, Lambeth SP. 2007. Control, choice, and assessments of the value of behavioral management to nonhuman primates in captivity. J Appl Anim Welf Sci 10:39–47. [DOI] [PubMed] [Google Scholar]
- 41.Schapiro SJ, Perlman JE, Thiele E, Lambeth S. 2005. Training nonhuman primates to perform behaviors useful in biomedical research. Lab Anim (NY) 34:37–42. [DOI] [PubMed] [Google Scholar]
- 42.Schultz-Darken NJ, Pape RM, Tannenbaum PL, Saltzman W, Abbott DH. 2004. Novel restraint system for neuroendocrine studies of socially living common marmoset monkeys. Lab Anim 38:393–405. [DOI] [PubMed] [Google Scholar]
- 43.Shirasaki Y, Yoshioka N, Kanazawa K, Maekawa T, Horikawa T, Hayashi T. 2013. Effect of physical restraint on glucose tolerance in cynomolgus monkeys. J Med Primatol 42:165–168. [DOI] [PubMed] [Google Scholar]
- 44.SurveyMonkey. [Internet] 2017. Survey services. [Cited 19 July 2017]. Available at: https://www.surveymonkey.com/
- 45.Tasker L, [Internet] 2012. Linking welfare and quality of scientific output in cynomolgus macaques (Macaca fascicularis) used for regulatory toxicology. PhD thesis, University of Stirling; [Cited 4 January 2017]. Available at: https://dspace.stir.ac.uk/bitstream/1893/9801/1/Lou%20Tasker%20Thesis%202012.pdf [Google Scholar]
- 46.Westlund K. 2012. Can conditioned reinforcers and variable-ratio schedules make food and fluid control redundant? A comment on the NC3Rs working group's report. J Neurosci Methods 204:202–205. [DOI] [PubMed] [Google Scholar]
- 47.Veeder CL, Bloomsmith MA, McMillan JL, Perlman JE, Martin AL. 2009. Positive reinforcement training to enhance the voluntary movement of group-housed sooty mangabeys (Cercocebus atys atys). J Am Assoc Lab Anim Sci 48:192–195. [PMC free article] [PubMed] [Google Scholar]