Abstract
Coccidioides spp. are saprophytic, dimorphic fungi that are endemic to arid climates, are capable of infecting many species, and result in diverse clinical presentations. An indoor-housed laboratory rhesus macaque presented with weight loss and decreased activity and appetite. During the diagnostic evaluation, a bronchiolar–alveolar pattern in the cranial lung lobes, consistent with bronchopneumonia, was noted on radiographs. Given the poor prognosis, the macaque was euthanized. Confirming the radiographic assessment, gross necropsy findings included multifocal to coalescing areas of consolidation in the right and left cranial lung lobes. Microscopically, the consolidated regions were consistent with a pyogranulomatous bronchopneumonia and contained round, nonbudding, fungal yeast structures considered to be morphologically consistent with Coccidioides immitis. Culture and colony morphology results were confirmed through additional diagnostic testing. Sequencing of the D1–D2 domain of the 28S large ribosomal subunit positively matched with a known sequence specific to C. immitis. Serology for Coccidioides spp. by both latex agglutination (IgM) and immunodiffusion (IgG) was positive. In this rhesus macaque, the concordant results from histology, culture, DNA sequencing, and serology were collectively used to confirm the diagnosis of coccidioidomycosis. This animal likely acquired a latent pulmonary infection with Coccidioides months prior to arrival, when housed outdoors in a Coccidioides-endemic area. The nonspecific clinical presentation in this macaque, coupled with the recent history of indoor housing and lag between clinical presentation and outdoor housing, can make similar diagnostic cases challenging and highlights the need for awareness regarding animal source when making an accurate diagnosis in an institutional laboratory setting.
Coccidioides spp are saprophytic, dimorphic fungi that are endemic to arid climates such as the southwestern United States, Mexico, and South America. Many mammalian species are susceptible to infection, with or without development of clinically evident disease, the presentation of which is dependent on the involved organ. Diagnosis can be made by visualization of the organism in histologic sections and by cytology, serology for antibody in complement fixation or tube precipitin tests, latex agglutination, immunodiffusion, or culture, with or without multiple cavitating radiographic lesions.8,13,14 Coccidioides spp. infections have been previously reported in the pulmonary form in rhesus macaques4,5 and a bonnet macaque3 and in the disseminated form in a cynomolgus macaque,14 Japanese macaque,9 and rhesus macaques.3-5 Here we describe the diagnosis of respiratory coccidioidomycosis in an indoor-housed laboratory rhesus macaque. This case emphasizes the need for awareness of latent infection, and coccidioidomycosis should be considered a relevant differential diagnosis even in indoor-housed laboratory settings, particularly when NHP have originated from sources in endemic regions.
Case Report
All described procedures were conducted in accordance with the GlaxoSmithKline Policy on the Care, Welfare, and Treatment of Laboratory Animals and were performed in an AAALAC-accredited facility. The macaque was included in an IACUC-approved protocol and was housed and cared for according to the Animal Welfare Act,1 Animal Welfare Regulations,2 Public Health Service Policy,16 and the Guide for the Care and Use of Laboratory Animals.11
A 4-y-old, male rhesus macaque (Macaca mulatta) of Indian origin and obtained from Covance Research Products (Alice, TX), presented for veterinary physical examination after demonstrating decreased levels of activity and appetite and an approximately 10% weight loss over 2 mo. The macaque was reported to have eaten all of the rations offered but often took several hours to finish feeding. At the time of clinical presentation, the macaque had been pair-housed with other rhesus monkeys for approximately 9 mo in our indoor-only facility, maintained on a 12:12-h light:dark cycle with unrestricted access to water, and fed a standard primate diet (no. 5048, Certified Primate Diet, LabDiet, St Louis, MO), with additional foods (that is, fruits, vegetables) provided as enrichment. Care staff observe all animals twice daily. The macaque's study history was limited to a single pharmacokinetic study that occurred approximately 6 mo earlier, with no adverse effects and with no measurable drug levels at 2 wk after dosing. Prior to placement on study, the macaque underwent a battery of routine diagnostic procedures, including intradermal skin testing for tuberculosis, fecal float, direct fecal smear, PCR testing for SRV, and CBC and clinical biochemical analyses. All results for screened infectious agents were negative, and all hematologic and clinical pathology parameters were within normal limits.
During cage approach for the clinical examination, the macaque initially was recumbent on the cage flooring, which had not been observed previously in this animal, but he rose quickly and displayed the expected, typical responsive behaviors toward care staff. The macaque was then removed from the cage, chaired, and examined. During the exam, other than having a thin body condition (score of 2/5), the animal was bright, alert, responsive, and well-hydrated, with pink mucous membranes and no palpable abnormalities. A series of diagnostic tests was requested, and after the physical examination, the macaque was placed on daily observation of food consumption, fecal and urine output, and overall activity.
Diagnostic testing requested after clinical examination included intradermal tuberculin testing and rectal culture (both negative). The macaque was sedated with 10 mg/kg ketamine (100 mg/mL; Ketaved, Vedco, St Joseph, MO) for these tests. Comparison with published reference values,13 CBC analysis (ADVIA 2120i, Siemens, Tarrytown, NY) revealed leukocytosis (13.8 × 103 cells/µL) with neutrophilia (11.8 × 103 cells /µL) and lymphopenia (1.28 × 103 cells/µL) and chemistry panel results (AU680, Beckman Coulter, Brea, CA) demonstrated mildly low electrolyte levels for K+ (3.2 mEq/L) and Cl– (107 mEq/L). Serum creatinine (0.52 mg/dL), albumin (3.6 g/dL), AST (14 U/L), ALT (10 U/L), cholesterol (87 mg/dL), and Ca2+ (9.6 mg/dL) were also low, whereas ALP (555 U/L) was mildly elevated and globulins (4.3 g/dL) were markedly elevated. Together, the neutrophilia, lymphopenia, hypoalbuminemia, and hyperglobulinemia were interpreted as an inflammatory leukogram with a possible stress component. We suspected an underlying chronic infection of presumptive enteric origin, in light of the animal's weight loss and relatively minor alterations in bloodwork. The macaque was provided high-calorie food supplementation and administered enrofloxacin.
After 2 wk of empirical treatment, no clinical improvement was noted, and the macaque's weight had decreased from 5.6 kg to 5.3 kg, despite caloric supplementation. Care staff described infrequent episodes of gagging or coughing, possibly related to feeding. Given this observation and the animal's continued decline, additional diagnostic testing was conducted, including lateral and ventrodorsal thoracic radiographs that were performed under sedation. Radiographs of the lungs revealed a bronchiolar–alveolar pattern in the cranial lobes, consistent with bronchopneumonia (Figure 1). At this time, the list of differential diagnoses included aspiration pneumonia, bronchopneumonia due to bacterial infection (for example, Mycobacterium tuberculosis, M. bovis, Streptococcus pneumoniae, Nocardiaspp. ), fungal infection (for example, coccidioidomycosis, pneumocystosis, histoplasmosis, blastomycosis), parasitic infection (toxoplasmosis), neoplasia, pulmonary hemorrhage, pulmonary edema, and atelectasis.13,18 In light of the poor prognosis and prolonged treatment necessary for a modest chance of improvement,9 the macaque was euthanized, and a postmortem examination was subsequently conducted, followed by sampling for diagnostic tests.
Figure 1.

Lateral thoracic radiograph showing an alveolar pattern with consolidation (arrows) in the cranial lung lobes.
Gross necropsy findings included multifocal to coalescing areas of consolidation in the right and left cranial lung lobes (Figure 2). Purulent material was readily expressible on cut surface of more severely affected areas, which subsequently were cultured. The pericardial sac and tricuspid valves were thickened. Both tracheobronchial lymph nodes were moderately enlarged. The right and left cranial lung lobes, heart, and tracheobronchial lymph nodes were collected in neutral buffered formalin for histopathology evaluation. Microscopically, tissue sections of lung at low magnification contained multifocal to coalescing lesions, with partial to complete alveolar and bronchial consolidation (Figure 3). The consolidated regions at higher magnification were consistent with pyogranulomatous bronchopneumonia, characterized by mixed leukocellular infiltrates composed of varying populations of degenerate neutrophils, epithelioid macrophages, and multinucleated giant cells and fewer lymphocytes (Figure 4). Staining with hematoxylin and eosin and processing according to routine methods for histologic evaluation, revealed round, nonbudding, fungal yeast structures, ranging from 15 to 80 µm, with a nonstaining, translucent double wall and containing numerous 2- to 5-µm basophilic internal structures (endospores) scattered throughout the infiltrates. Application of periodic acid–Schiff staining with fast green counterstaining demonstrated preferential selectivity for high polysaccharide moieties abundant in cell walls of living fungi, the various magenta-staining components were prominently highlighted and considered to be morphologically consistent with cell walls and granule-containing endospores carrying Coccidioides immitis (Figure 4, inset).
Figure 2.
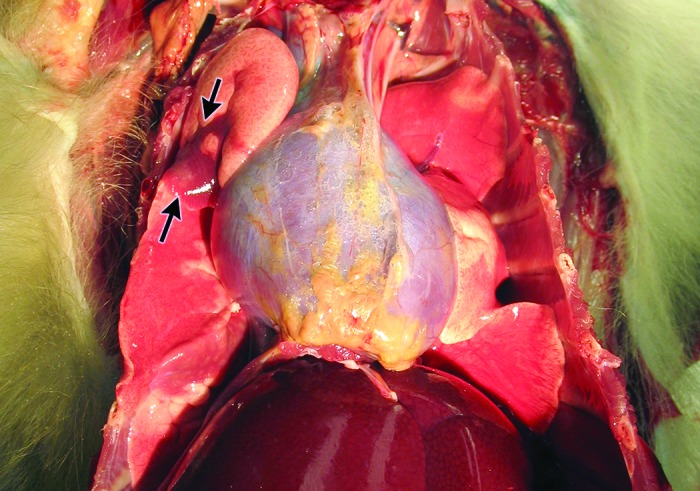
Macroscopic ventral–dorsal view of thorax of rhesus macaque. From this angle, distinct areas in the right cranial lung lobe show dark red-discolored and depressed regions (arrows) that sharply contrast with the adjacent, pink lung tissue.
Figure 3.
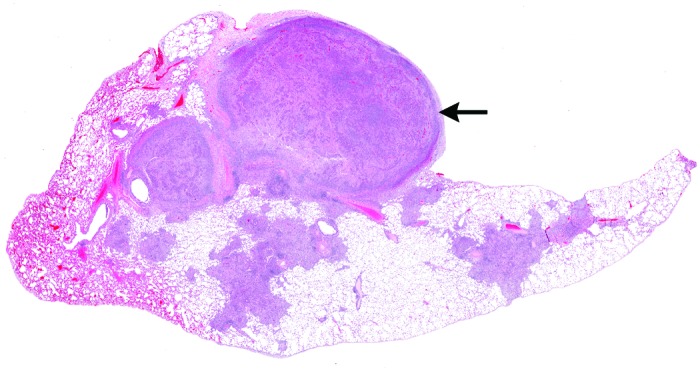
Histologic section of macaque lung, demonstrating multifocal to coalescing areas of consolidation throughout the section. Note the severely affected nodular area (top) that is protrusively distorting the pleura. Hematoxylin and eosin stain; magnification, 3×.
Figure 4.
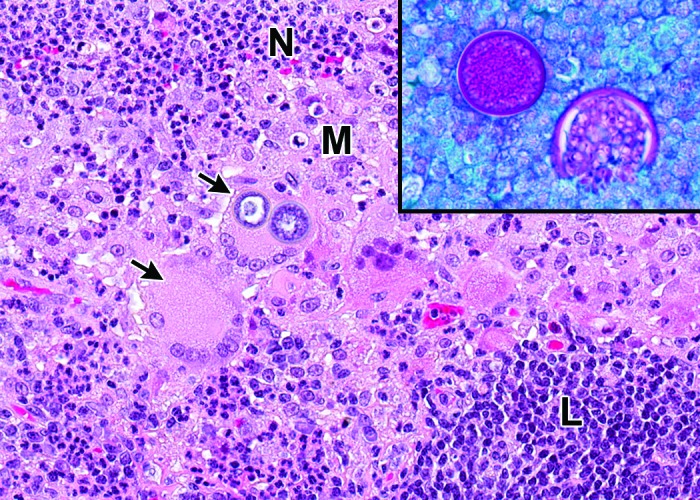
Histologic section of macaque lung with pyogranulomatous inflammation. Inflammation is characterized by a mixed population of degenerate neutrophils (N), epithelioid macrophages (M), multinucleated giant cells (arrows), and few lymphocytes (L). One of the giant cells contains phagocytized yeast structures. Hematoxylin and eosin stain; magnification, 200×. The inset demonstrates spherules containing abundant, magenta-stained, internal endospores consistent with Coccidioides immitis. Periodic acid–Schiff stain with fast green counterstain; magnification, 400×.
At necropsy, blood was collected for repeat hematology, clinical chemistry, and diagnostic workup for routine infectious agents, with results similar to the antemortem screen observed. Additional postmortem diagnostic tests to identify infectious agents were also conducted, which included PCR analysis of EDTA-treated whole blood (SIV, SRV and Mycobacterium spp.) and serum ELISA (SIV, B virus antibody, measles antibody, SRV), all of which were performed by VRL Laboratories (San Antonio, TX). All results were negative. Lung abscess, trachea, and urine cultures were submitted. Samples plated on sheep blood agar and MacConkey agar (Remel, Lenexa, KS) were incubated at 36 °C in CO2 and ambient air, respectively. Additional samples plated on sheep blood agar were incubated at 36 °C under anaerobic conditions. Samples were plated on Sabouraud dextrose agar (Edge Biologicals, Memphis, TN) and incubated at 30 °C in an ambient air incubator. The culture of the lung abscess was positive for Streptococcus salivarius and moderate fungal growth. The tracheal culture yielded fungal growth with similar colony morphology. Specifically, flat, velvety, slightly fuzzy colonies were present on sheep blood agar plate and on a sealed Sabouraud dextrose agar plate by day 3.
A touch tape preparation for light microscopic examination, using methylene blue stain, was performed from a colony on the sheep blood agar plate yielded only small, delicate, septated hyphae uncharacteristic of Coccidioides. The procedure for this method is to touch fungal growth with the sticky side of clear tape, lift the tape with attached fungal elements from the colony, and lower it onto a drop of methylene blue stain (ready to use) on a glass slide. Young cultures of Coccidioides produce characteristic structures termed ‘racquet hyphae,’ which are swollen areas situated at the end or middle of a hyphal strand and which are morphologically similar in appearance to a tennis racquet.17 These characteristic structures were not obtained from our primary culture; furthermore, repeats of the tape preparation procedure over the next 48 h did not provide any additional microscopic morphologic clues, such as conidia, sporangia, and rhizoids.
Due to the inability to identify the putative pathogen by culture morphology, the fungus was subcultured to a Sabouraud dextrose agar plate for preservation, and the original Sabouraud dextrose agar plate was submitted to the New York State Animal Health Diagnostic Laboratory (Ithaca, NY) for identification. However by day 6, an additional touch tape preparation from the original sheep blood agar plate made by our inhouse diagnostic personnel contained large, round, rectangular and barrel-shaped arthroconidia, alternating with empty cells, characteristic of Coccidioides, were obtained after culture maturation (Figure 5). Similar culture and morphology results were obtained by the New York State Animal Health Diagnostic Laboratory, leading to DNA sequencing of the D1–D2 domain of the 28S large ribosomal subunit. The sequence obtained positively matched to a known sequence specific to C. immitis, differentiating it from C. posadasii.
Figure 5.
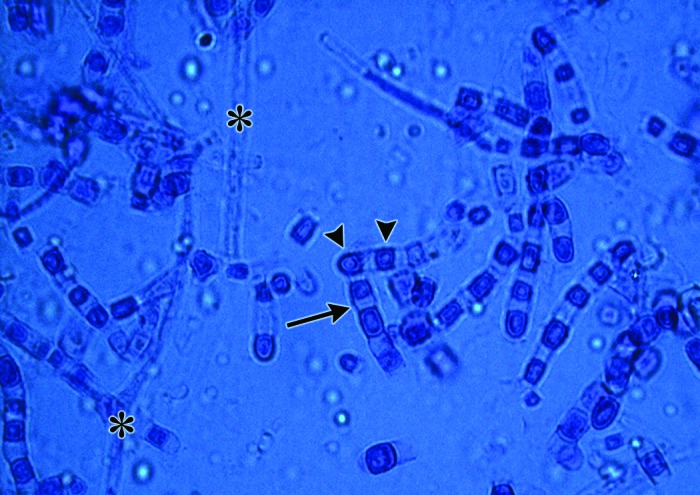
Micrograph of touch-tape preparation from mature fungal culture, demonstrating immature hyphal structures (*). In time, with fungal colony maturation, arthrocondia (arrowheads) separated by disjunctor cells appearing as intervening dead spaces (arrow) arise. Methylene blue stain; magnification, 40×.
Serology (Immuno-Mycologics, Norman, OK) was performed on a serum sample that had been collected from the macaque prior to necropsy and was positive for Coccidioides spp. by both latex agglutination (IgM) and immunodiffusion (IgG), a strong indication that these 2 assays are appropriate for NHP samples. Given that the results from all diagnostic tests collectively confirmed Coccidioides spp., fungal cultures were performed on bronchoalveolar lavage samples from all remaining animals housed in the same room as the affected macaque. Coccidioides spp. was not isolated from any other animal. Serologic latex agglutination and immunodiffusion assays performed on serum from the remaining animals (acquired from the same source) were all negative.
Discussion
Coccidioidomycosis primarily manifests as a respiratory disease, with inhalation of even a single spore from disturbed soil as the typical mode of infection.7,10 In mature Coccidioides soil colonies, arthroconidia are separated by a dead space, called a disjunctor cell, creating alternate arthroconidial spores. When these conidia break apart, part of the disjunctor cell in front or behind the arthrocondium provides the spores with wing-like lift, allowing spores to persist for extended periods in the air.17 Once inhaled, the fungus changes from the environmental arthroconidial spore form to yeast spherules within infected tissue(s). Although primarily a respiratory illness, the disease can progress to widespread dissemination.8,10 Experimental infections in rhesus macaques led to arthrospores within the bronchioles and alveoli within 3 d, clinical response at 7 d, and disseminated infection as soon as 30 d after exposure, although many other infected animals recovered clinically but still had active infections at necropsy at day 378.4 Numerous species, including cats, dogs, cattle, sheep, pigs, and NHP are susceptible to infection, with variable degrees of disease and clinical signs.15 In humans, respiratory disease as a result of infection with coccidioidomycosis is referred to as ‘Valley Fever.’ Similar to that in animals, human infection most often occurs by inhalation of the arthroconidia, which are readily aerosolized when disturbed.10 Most cases follow exposure to high quantities of dust in endemic areas. These situations have ranged from occupational exposure through agricultural, construction, or archeological work to weather-associated events, such as dust storms, landslides, and earthquakes.6,7,12
Because coccidioidomycosis is generally not contagious between animals, no quarantine procedures were instituted for the remaining animals from the same room and source. However, extreme caution was used when handling diagnostic or postmortem material from any animal in that room, to avoid parenteral exposure4 and aerosolization possibly leading to inhalation. Cases involving disseminated infection are more likely with immunodeficiency.7,12
Differential diagnoses for gross pathologic findings of lung consolidation, enlarged tracheobronchial lymph nodes, and pleural adhesions in NHP include various infectious pneumonias or neoplasia.8,13 In the rhesus macaque we presented, the concordance between the histologic, culture, DNA sequencing, and serologic results, was used to confirm the diagnosis of coccidioidomycosis. Other clinical signs that can be associated with this disease but were not present in this animal include vertebral infection with lameness, joint pain, and skin rashes.6,7,13 The described macaque likely acquired a latent pulmonary infection with Coccidioides months prior to arrival, when it was housed in an outside facility in the southwestern United States, where Coccidioides is endemic. We failed to identify any likely causes or evidence of immunosuppression that might have contributed to the emergence of Coccidioidomycosis. The vague and subtle clinical presentation in this animal, coupled with the lengthy history of indoor housing and the latency of the infection, can make similar cases challenging to diagnose and highlight the need for awareness regarding an animal's source when making an accurate diagnosis in an institutional laboratory setting.
Acknowledgments
We thank Kathleen Morasco for her help in processing diagnostic samples and Beverly Maleeff for her technical expertise with image preparation for publishing.
References
- 1.Animal Welfare Act as Amended 2008. 7 USC §2131–2156. [Google Scholar]
- 2.Animal Welfare Regulations 2008. 9 CRF § 3.129. [Google Scholar]
- 3.Beaman L, Holmberg C, Henrickson R, Osburn B. 1980. The incidence of coccidioidomycosis among nonhuman primates housed outdoors at the California Primate Research Center. J Med Primatol 9:254–261. [DOI] [PubMed] [Google Scholar]
- 4.Blundell GP, Castleberry MW, Lowe EP, Converse JL. 1961. The pathology of coccidioides immitis in the Macaca mulatta. Am J Pathol 39:613–630. [PMC free article] [PubMed] [Google Scholar]
- 5.Breznock AW, Hendrickson RV, Silverman S, Schwartz LW. 1975. Coccidioidomycosis in a rhesus monkey. J Am Vet Med Assoc 167:657–661. [PubMed] [Google Scholar]
- 6.Castleman WL, Anderson J, Holmberg CA. 1980. Posterior paralysis and spinal osteomyelitis in a rhesus monkey with coccidioidomycosis. J Am Vet Med Assoc 177:933–934. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC). [Internet] 2009. Biosafety in Microbiological and Biomedical Laboratories, 5th ed [Cited 31 August 2016]. Available at: www.cdc.gov/biosafety/publications/bmbl5/BMBL5_sect_VIII_b.pdf.
- 8.Gibson SV. 1998. Bacterial and mycotic diseases, p 59–110. In: Bennett BT, Abee CR, Henrickson R. Nonhuman primates in biomedial research: Diseases. San Diego (CA): Academic Press. [Google Scholar]
- 9.Graybill JR, Griffith L, Sun SH. 1990. Fluconazole therapy for coccidioidomycosis in Japanese macaques. Rev Infect Dis 12 Suppl 3:S286–S290. [DOI] [PubMed] [Google Scholar]
- 10.Hector RF, Laniado-Laborin R. 2005. Coccidioidomycosis— a fungal disease of the Americas. PLoS Med 2:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 12.Kirkland TN, Fierer J. 1996. Coccidioidomycosis: a reemerging infectious disease. Emerg Infect Dis 2:192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magden ER, Mansfield KG, Simmons JH, Abee CR. 2015. Nonhuman primates, p 771–930. In: Anderson LC, Otto G, Pritchett-Corning KR, Whary MT, Fox JG. Laboratory animal medicine, 3rd ed Amsterdam (Netherlands): Elsevier. [Google Scholar]
- 14.Mense MG, Batey KL, Estep S, Armstrong K, Fleurie G, Suttie AW. 2013. Disseminated coccidioidomycosis in a cynomolgus monkey (Macaca fascicularis). J Primatol 2:111. [Google Scholar]
- 15.Merck Veterinary Manual. [Internet] 2014. Coccidioidomycosis (Valley Fever). [Cited 31 August 2016]. Available at: http://www.merckvetmanual.com/mvm/generalized_conditions/fungal_infections/coccidioidomycosis.html
- 16.National Institute of Health. [Internet] 2015. Office of Laboratory Animal Welfare – Public health service policy on humane care and use of laboratory animals. [Cited 31 August 2016]. Available at: https://grants.nih.gov/grants/olaw/references/phspol.htm# PublicHealthServicePolicyonHumaneCareandUseofLaboratory
- 17.Roberts GD, [Internet] 2012. Hot Topic— Dimorphic Fungi: Coccidioidomycosis. [Cited 6 September 2016]. Available at: http://www.mayomedicallaboratories.com/articles/hottopics/2012/12-dimorphic/cocci.html.
- 18.Wilkinson LM, Wallace JM, Cline JM. 1999. Disseminated blastomycosis in a rhesus monkey (Macaca mulatta). Vet Pathol 36:460–462. [DOI] [PubMed] [Google Scholar]


