Abstract:
On the outer surface of a human cell there is a dense layer of complex carbohydrates called glycocalyx, also referred to as glycans or the sugar coating on the cell surface, which is composed of a complex array of oligosaccharide and polysaccharide glucose chains that are covalently bonded to proteoglycans and lipids bound to the cell membrane surface. Studies of an intact endothelial glycocalyx layer (EGL) have revealed a number of critical functions that relate the importance of this protective layer to vascular integrity and permeability. These functions include the following: stabilization and maintenance of the vascular endothelium, an active reservoir of essential plasma proteins (i.e., albumin, antithrombin, heparan sulfate, and antioxidants), a buffer zone between the blood (formed elements) and the surface of the endothelium, and a mechanotransducer to detect changes in shear stress that facilitate vascular tone. There have been numerous review articles about the structure and function of endothelial glycocalyx over the past two decades, yet there still remains a significant knowledge gap in the perfusion literature around the importance of EGL. Perioperative fluid management and gaseous microemboli can both contribute to the damage/degradation of endothelial glycocalyx. A damaged EGL can result in systemic and myocardial edema, platelet and leukocyte adhesion, fluid extravasation, and contributes to microvascular perfusion heterogeneity. Knowledge of the importance of endothelial glycocalyx will enable clinicians to have a better understanding of the impact of gaseous microbubbles, hyperoxia, and ischemic reperfusion injury during cardiac surgery. The purpose of this article is to provide an in depth review of the EGL and how this protective barrier impacts the microcirculation, fluid homeostasis, inflammation, and edema during cardiac surgery.
Keywords: glycocalyx, endothelial glycocalyx, cardiopulmonary bypass, gaseous, icroemboli, microbubbles, hemodilution, hyperoxia, volume loading, syndecan, eparan, CPB
In 1896, a physiologist by the name of Ernest Starling presented his hypothesis to explain the movement of fluid between tissues that he based on two known forces of intravascular fluid movement: hydrostatic pressure and colloid oncotic pressure (COP) (1). This hypothesis resulted in the Starling equation, which has been used for the past 119 years by clinicians to help explain the reasons behind fluid shifts between the intravascular and the extravascular spaces. However, physiologists believed that hydrostatic and colloidal pressure gradients never seemed to fully explain transcapillary fluid movement (2,3). Even as far back as 75 years ago, several physiologists speculated that there might be some force or thin endocapillary layer of material that influenced fluid movement between the intravascular and extravascular spaces (4,5). In 1966, researchers identified the endothelial glycocalyx layer (EGL) through the use of electron microscopy (6). Since the time of that discovery, researchers have demonstrated that an intact EGL plays a major role in maintaining the vascular integrity and fluid homeostasis (7). In fact, physiologists now consider the EGL to be the primary structure that provides ionic and colloid osmotic gradients within the vasculature and prevents tissue edema (8–12). These findings have led to a major revision of the Starling equation (2,9,13), which is described in detail by Levick (12).
The revision of the Starling equation and the current knowledge around the EGL has led to current suggestions that clinicians revise their thinking on fluid prescription and fluid administration to positively impact transcapillary fluid movement and improve patient outcomes (14–16), as will be described later in this review. Even though glycocalyx has been recognized for more than five decades, its importance in medicine and surgery is only beginning to become recognized. In the past, investigations into the importance of glycans in medicine have received little attention from researchers, but in 2012, the National Academy of Sciences advocated a need for this to change (17). Interest in the presence of the EGL has grown dramatically in only the past 16 years. At the time of writing this review, a simple search of PubMed revealed the presence of the word “glycocalyx” in over 2,724 abstracts since 1958, with 1,570 (58%) of these abstracts appearing since the year 2000. Using the same method to search for the words “endothelial glycocalyx” resulted in the presence of 903 abstracts, with 758 (85%) of these abstracts being published during this same period of time.
GLYCOCALYX
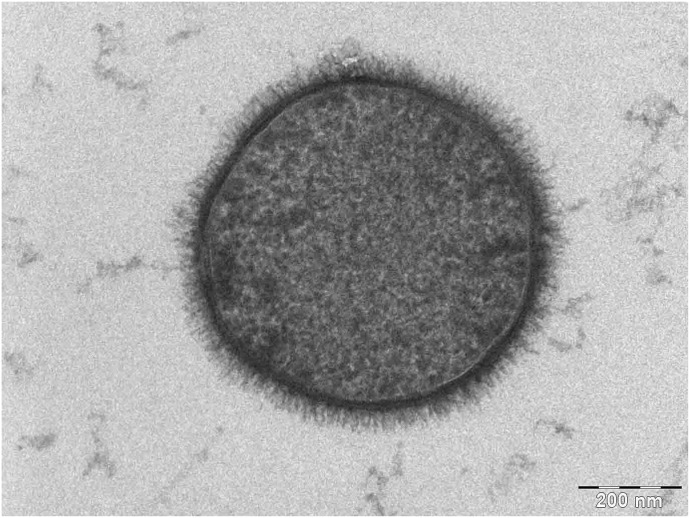
Glycocalyx is a word used to describe a sugar coating found on all mammalian cells, including cancer and bacterial cells (Figure 1). These complex carbohydrates are believed to be covalently bonded to proteins on the cell membrane surface layer. Information on polysaccharides and glycans goes back as far as the early 1900s, with the 1st mention of the word glycocalyx appearing in the literature in 1958 (18). This protein bound glycocalyx covering maintains several functions for the cell, such as cell to cell recognition, intracellular adhesion, and maintenance of the entire cells’ plasma wall homeostasis (19). A simple way to appreciate the feel of the glycocalyx is to hold a fish and feel the slimy surface on its scales. This slimy surface is also called glycocalyx and functions to protect the fish from bacteria and aid its movement in the water.
Figure 1.
Glycocalyx on the bacterium Bacillus subtilis. Weiner A: The Weizmann Institute of Science 2006: Rehovot, Israel (https://commons.wikimedia.org/wiki/File:Bacillus_subtilis.jpg).
ENDOTHELIAL GLYCOCALYX
Structure/Function: Exclusion Zone
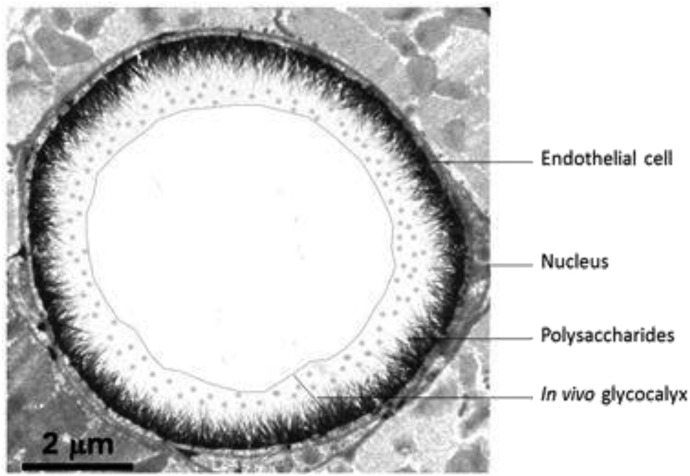
Endothelial glycocalyx is a membrane bound covering of the endothelial cells that is found on the intraluminal surfaces of all blood vessels and organs and is composed of glycoproteins that hold between 700 and 1,000 mL of non- circulating plasma volume. This intraluminal layer maintains its own COP because of its plasma protein content (primarily albumin) that is trapped within the EGL. Subsequently, it also has a higher COP than that of circulating plasma because of its retention of plasma proteins (20,21). For this reason, the EGL is thought to contribute to approximately 60% of the intravascular COP (22). Structurally, the EGL is a negatively charged gel-like layer that is composed of an intricate array of oligosaccharide and polysaccharide chains called glycosaminoglycans (e.g., heparan sulfate, hyaluronic acid, chondroitin sulfate, dermatan sulfate, and keratan sulfate), which are covalently bonded to glycosylated membrane proteins called proteoglycans (antithrombin III, integrins, and selectins) and membrane bound proteoglycans such as syndecans, glypicans and perlecans, and other plasma proteins (Figure 2). The EGL ranges in thickness between .1 and 4.5 µm, depending on the location/size of the vessel, and maintains an active reservoir of proteins and polysaccharides, such as antithrombin III and heparan sulfate (23–25).
Figure 2.
Intact endothelial glycocalyx layer (EGL). Courtesy of Professor H. Vink. From the website Glycocalyx; http://glycocalyx.nl/background.php.
An intact EGL maintains a separation between circulating plasma and vascular endothelial cells (VECs), creating an “exclusion zone” which keeps the formed elements of the blood (red cells, white cells, and platelets) from actually contacting the surface of the endothelial cells (Figure 2). In the presence of an intact EGL, water and electrolytes will pass freely across this layer and then beyond the endothelial cells through the intercellular clefts. With the exception of albumin, this exclusion zone also prevents high molecular weight colloids that are >70 kDa from contacting the endothelial cells. Albumin is the only major plasma protein that moves easily between the plasma and the EGL because of the selectively permeability nature of the EGL to natural colloids with molecular weights <70 kDa (16,26–28).
An intact EGL functions to maintain the intravascular plasma COP, bind anticoagulation factors, modulate leukocyte interactions with the endothelium, prevent direct or consequential damage to the intraluminal endothelial cells, and contribute to the vascular tone of the microcirculation (29). In the presence of high transendothelial pressures and a damaged or degraded EGL, colloids will move freely from the intravascular space to the extravascular space (regardless of plasma COP) just as easily as crystalloids (3,14,20). However, there is evidence in animals that one of the ways to protect and preserve endothelial glycocalyx is by maintaining high levels of plasma and natural colloids within the intravascular space (11,30,31). This plasma protective effect is further supported by in vitro evidence in animals during hemorrhagic shock and may play a role in the preservation of syndecan-1 (32).
Mechanotransduction
Because of the location of the EGL between the blood and the surface of VECs, one function attributed to the EGL is that of a mechanotransducer. As a mechanotransducer, it is able to mediate changes in luminal shear stress by initiating the release of nitric oxide (NO) (27). Fluid shear stress generated by flowing blood through the microcirculation is transmitted through the EGL structure to the underlying endothelial cells through glycosaminoglycan side chains. This information is then transmitted to specific membrane bound core proteins (syndecans) linked to the endothelial cells actin cytoskeleton and the plasma membrane glypicans that subsequently mediates specific cell signaling and NO production/release (2,33–35). NO then causes localized vasodilation and acts as a free radical scavenger to help preserve the integrity of the EGL (7).
EGL Damage/Degradation
The EGL is in a constant state of synthesis and shear-induced shedding from the endothelial cells (7,28). But one of the unique qualities of an intact EGL is its ability to be compressed by as much as 20% to allow the passage of larger formed elements like white cells through the microcirculation and then restore itself to its native thickness in <1 second (2). Under conditions such as acute volume loading, hyperglycemia, hyperoxia, ischemia/reperfusion, hyperlipidemia, and inflammation, the EGL can be degraded by proteases that cause shedding of the glycocalyx proteins (2,5,36). Damage or loss of the EGL subsequently exposes underlying endothelial cells to the rheological forces of blood, which then leads to platelet, neutrophil, and endothelial activation, localized inflammation, tissue edema, and increased permeability of both solutes and solvents (4). After shedding or damage of the EGL, in vitro evidence from animal studies indicates it takes between 5 and 7 days to restore the EGL to its native thickness (37).
Shedding or damage to the EGL results in increased levels of syndecan-1 and heparan sulfate in the plasma, which can subsequently be detected by the use of a specialized laboratory test called enzyme-linked immunosorbent assay (ELISA) (23). This creates an interesting area of investigation for cardiac surgery because alterations or damage to the EGL can now be detected in humans by measuring changes in syndecan-1 and heparan sulfate levels before and after clinical interventions. Although there is evidence of endothelial glycocalyx alterations during cardiopulmonary bypass (CPB) (38), the total adverse effects of damage to this in protective layer is currently uncertain.
ACUTE VOLUME LOADING
The infusion of one or two liters of asanguineous fluid to the patient during induction of anesthesia is a common practice that contributes to hemodilution and fluid shifts during the preoperative period. The concept of fluid redistribution and hypovolemia during major (noncardiac) surgery was first reported by Shires et al. (39) over 50 years ago. At that time, it was demonstrated that in addition to surgical blood loss, there was the potential for fluid shifting because of the internal redistribution between extravascular compartments, subsequently resulting in an acute hypotensive state. Since that initial observation, vasodilation and the hypovolemia associated with fluid shifting during surgery has been described in detail (20).
During major surgery, fluid losses can exceed 3 L during the entire perioperative period (20). In anticipation of these potential fluid losses, acute volume loading of the cardiac surgical patient during the induction period has become a common anesthesia practice in both “on pump” and “off pump” procedures (20,40,41). It appears that the practice of acute preoperative volume loading during the anesthesia induction period has been practiced for many decades and seems to be based on four fundamental assumptions (16):
Dehydration occurs during the preoperative fasting period.
Increased insensible fluid loss occurs after the surgical incision is made.
Unpredictable extravascular fluid shifting and blood loss occurs during the course of surgery.
Hypervolemia is better than hypovolemia during surgery because the kidneys will always be able to compensate.
Despite these long held assumptions, evidence is accumulating against the practice of acute preoperative volume loading. The key evidence is based on the fact that hypervolemia stretches the atrial walls of the heart, which subsequently releases a natural hormone from the atrial cardiomyocytes called atrial natriuretic peptide (ANP). This natural hormone is usually released during acute or chronic hypervolemic conditions such as congestive heart failure. ANP is a short acting (approximately 20 minutes due to washout) powerful vasodilator and diuretic that functions to redistribute volume throughout the circulation. ANP also causes damage/degradation of the EGL which subsequently increases the microvascular permeability up to two fold and contributes to the formation of inflammation and edema. In other words, a state of acute hypervolemia and the sudden release of ANP before CPB will initiate the inflammatory process and establish exactly the opposite of what acute volume loading was trying to achieve, which was a stable intravascular environment (42).
There is no evidence to identify priming volumes as another contributor to acute volume loading, despite the fact that large crystalloid/colloid volumes are routinely given at the start of CPB. However, in an attempt to avoid the known poor outcomes and morbidity that are associated with excessive hemodilution and low hematocrits (43,44), every effort should still be made to reduce the crystalloid/colloid priming volumes in all patients, not just those patients who have low body weights or religious objections to red blood cell transfusions.
In addition to its effect on excessive hemodilution, it appears that it is best to avoid the practice of acute volume loading to mitigate acute damage to the EGL and the subsequent inflammatory process that follows. A fact that is often overlooked in cardiovascular perfusion is that infusion solutions should be considered as drugs with indications, contraindications, and side effects. Research suggests that the best practice is to use goal directed fluid management (matching the types and volumes of fluids to the actual clinical physiological needs) and to avoid states of acute hypervolemia during surgery, as a means for more rational use of crystalloid/colloid infusion solutions during cardiac surgery (45).
GASEOUS AND SOLID MICROEMBOLI
Historically, the focus of gaseous microemboli (GME) damage to the cerebral circulation has always been placed on the presence (or absence) of postoperative neurocognitive dysfunction associated with cardiac surgery and CPB (46,47). Unfortunately, this can be misleading because there are several other factors associated with postoperative delirium and cardiac surgery, such as patient age, patient co-morbidities, surgical procedure, length of surgery, anesthesia, and cerebral hypotension. But even though postoperative delirium is a multifactorial dilemma, we do know that GME are a significant contributing factor to this dilemma, and therefore needs to be addressed by better knowledge of the subject, improvements in techniques, and the use of effective technology. Looking for that “golden bullet” that links GME as the sole source of postoperative neurocognitive dysfunction is difficult and will likely never be found. However, this mindset tends to result in endless discussions around the limitations of microbubble size, volumes, and the use of altering the gas composition of existing gas microbubbles during extracorporeal circulation.
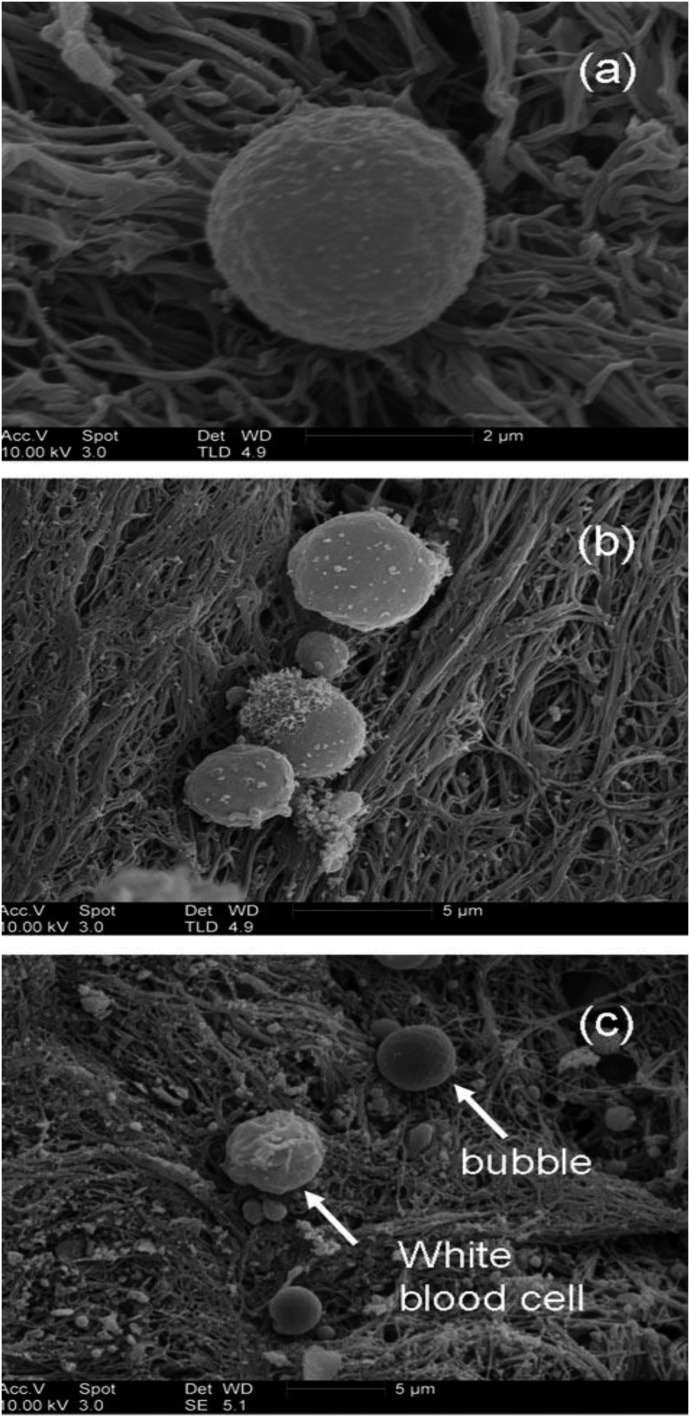
To better understand the damage caused by GME, more focus needs to be placed on the destructive nature of microbubbles that are known to damage/degrade the EGL as they pass through the microcirculation, and thereafter expose the delicate VEC to inflammation, edema, and activation of the complement/coagulation system (23,48–50). The reality is that microbubbles entering blood are immediately treated as foreign objects and are subsequently coated by protein (albumin), platelets, and leukocytes, making the surface composition and thickness of a microbubble in blood completely different than the surface composition of a microbubble in water (Figure 3). These cellular and protein deposits make the microbubble walls thicker and more difficult to break or adsorb as they pass through the microcirculation (51). Neutrophils also tend to aggregate around intravascular microbubbles in an attempt to destroy them. Platelets adhering to a microbubble’s surface also induce aggregation around that foreign object and play a role in platelet activation and thrombus generation in those areas where smaller microbubbles are able to adhere to exposed endothelial surfaces (52,53). Therefore, it appears clear that microbubbles affect thrombus formation through both activation of the coagulation cascade and the process of platelet aggregation. Clot formation at the area of microbubble tissue contact is usually followed by fibrinolysis of the thrombus material (54).
Figure 3.
SEM images obtained from thrombus exposed to Abciximab microbubbles. (A) A single bubble. (B) Three bubbles. The largest bubble is 4 μ in diameter, which is too big to be a platelet and does not resemble any other blood cell. (C) A smooth, spherical bubble, and a white blood cell. (Martin MJ, Chung EML, et al. Enhanced detection of thromboemboli with the use of targeted microbubbles. Stroke 2007;38:2726–32).
Microbubbles can also induce complement activation (C3a and C5a) at the bubble air/blood interface (55,56), which ultimately leads to the process of histamine release and the subsequent vascular permeability and the swelling that accompanies this chemical transmitter. Microbubble interaction with the microcirculation EGL can cause endothelial irritation and significant brain dysfunction, leading to swelling and intracranial hypertension (48).
In vitro investigations into the damaging effects of microbubbles on endothelial cells have demonstrated that microbubble contact with exposed endothelial cells initiates an immediate influx of calcium and causes mitochondrial dysfunction (57). Further in vitro evidence indicates that gaseous microbubble contact with exposed endothelial cells results in cell death and/or aberrant cellular function (58).
When GME enter the blood, they are transformed into a form of solid emboli with a compressible gas core as they navigate the microcirculatory pathways. As these deformable emboli pass through the cerebral microcirculation they cause damage to the EGL, leading to endothelial edema and damage to the blood brain barrier. Passage (extrusion) of further emboli through the same previously damaged vessel will lead to greater damage to that vessel (57).
The consequences of intraluminal EGL degradation from compressible microemboli are fluid extravasation from the intravascular space to the extravascular space, edema, inflammation, platelet/leukocyte activation, and damage to the blood brain barrier (59). Because of the damage of the EGL caused by microbubbles, it can be speculated that GME damage is likely a contributing factor to the postoperative neurologic dysfunction associated with CPB.
HYPEROXIA
Throughout our body there is a continual flux between oxidants and antioxidants. Oxidative stress is the loss of this ongoing flux between these two substances. Oxidative stress occurs in all cells when the cell’s natural antioxidant defense is overwhelmed by the excessive generation of reactive oxygen species (ROS) and free radicals. During periods of normal oxygen reduction (oxidation), free radicals contribute to the microbicidal activity of phagocytes, regulation of signal transduction, and gene expression. However, during periods of oxidative stress when the ROS levels increase dramatically, free radicals are instrumental in causing oxidative damage of cellular nucleic acids, lipids, and proteins (60).
Hyperoxia during CPB is best defined as a PaO2 in excess of 185 mmHg (60–65). The presence of hyperoxia during CPB induces increased levels of ROS free radicals, which subsequently cause the depolymerization of the glycosaminoglycan chains of glycocalyx proteoglycans such as heparan sulfate, chondroitin sulfate, and hyaluronic acid (66–68). Randomly increasing the FiO2–100% during CPB as a theoretical attempt to address GME will subsequently degrade/damage the glomerular EGL and do little to prevent the destructive nature of GME. As an example, the damage of glomerular endothelial cells by hyperoxia can result in the presence of proteinuria from potential kidney injury (66). It is evident that the use of hyperoxia during routine CPB contributes to patient morbidity and is not a benign process. Therefore, its use as a personal safety buffer and a theoretical treatment for GME should be avoided.
ISCHEMIA/REPERFUSION INJURY
Ischemia/reperfusion injury is recognized in a number of different organs and disease states (69). It appears that a damaged EGL plays a role in the development of ischemia/reperfusion injury. Part of the mechanism involved in this damage appears to be related to oxidative stress and its ability to degrade the EGL in all segments of the microvasculature. The subsequent exposure and dysfunction of the underlying endothelial cells results in an impaired ability of arterioles to vasodilate, capillaries that allow increased fluid extravasation, leukocytes that adhere to exposed endothelial cells, and proteins that extravasate from the venules (7,69). To date there has been no in vivo or ex vivo studies performed on coronary artery glycocalyx damage as it relates to ischemia/reperfusion and cardioplegia delivery. However, in the presence of a degraded EGL, in vivo experiments have revealed an increase in microbubble adherence to the coronary artery vasculature after the infusion of asanguineous cardioplegia, which was then subsequently reversed by the infusion of blood cardioplegia (35,70). The EGL is a dynamic structure that includes continual growth and ongoing degradation. Syndecan-1, heparan sulfate, and hyaluronic acid can be measured in the serum by using the ELISA method (71,72).
During an in vivo investigation into acute volume loading in nine patients undergoing general surgery, Chappell et al. (73) found significant increases (p < .05) in syndecan-1 and hyaluronic acid values 30 minutes after volume loading 1,326 ± 50 mL of 6% hydroxyethyl starch (Voluven). In a prospective cohort study of 75 trauma patients, Johansson and coworkers (74) found high levels of syndecan-1 to be an independent risk factor of mortality (p = .043). Rahbar et al. (75) found an association between increased levels of syndecan-1 and hyaluronic acid in trauma patients to impaired thrombin generation when colloid osmotic pressures were <16 mmHg. Some in vivo animal investigations have found better preservation of EGL in the presence of resuscitation fluids consisting of blood, plasma, and albumin (76,77), suggesting a benefit to EGL integrity when maintaining physiological levels of plasma albumin (78).
CONCLUSIONS
Understanding the physiology and pathophysiology of the EGL allows us to address technique and fluid management issues that can have an impact on damage/degradation of this layer during cardiac surgery. This includes issues such as avoiding the anesthesia practice of acute volume loading the patient before placing them on CPB, but opting instead for more volume management during this critical stage of induction and fluid management. Also, understanding the mechanisms of damage/degradation to the EGL provides us with an understanding of why all microbubbles entering the venous or arterial circulation (regardless of size or volume) have the potential to negatively impact this delicate luminal layer. We now understand how the microembolic damage to the EGL exposes the underlying endothelial cells to leukocyte/platelet interactions and the subsequent fluid shifting and edema that follows, all of which are contributing factors to neurocognitative decline. Even though there are other contributing factors to postoperative delirium, GME is also considered a contributing factor related to extracorporeal circulation. This understanding will help explain why there are no minimal safe limits when it comes to GME during CPB.
It appears that the use of serum albumin and plasma play a role in the protection and stabilization of the EGL, a fact that may influence our choices between crystalloid only primes and primes containing these natural colloids. On the other hand, there is no evidence that the use of synthetic colloids provides any protection to the EGL during CPB and in the presence of a damaged EGL, these colloids move freely between the intravascular space and the extravascular space. Evidence based literature also seems to support the use of a blood based cardioplegia over the use of an asanguineous cardioplegia solution to protect the coronary artery EGL.
Evidence also supports the avoidance of hyperoxia during CPB and the use of a more normoxic management of acid–base balance to protect the EGL. The EGL may also be the missing link that helps us understand a four-decade debate over whether colloids are better than crystalloids for fluid management. This is because we now know that in the presence of a damaged or degraded EGL, colloids will move easily through the endothelial para-cellular pathways, almost as freely as crystalloids, and therefore have been shown to be no better for fluid management than crystalloids are. This may be why in over 103 randomized control trials (14 during CPB) involving over 22,000 surgical, septic, and trauma patients (79–84), the conclusions are generally the same; that is, there is no difference in mortality outcomes, and colloids are much more expensive than crystalloids.
A clear understanding of this major intraluminal protective layer will lead cardiovascular perfusion to develop more evidence-based research on the techniques we use and the overall patient management of routine and extended extracorporeal circulation.
REFERENCES
- 1.Starling EH. On the absorption of fluids from the connective tissue spaces. J Physiol. 1896;19:312–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinbaum S, Tarbell J, Damiano E. The structure and function of the endothelial glycocalyx layer. Ann Rev Biomed Eng (NY). 2007;9:121–67. [DOI] [PubMed] [Google Scholar]
- 3.Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Arch. 2000;440:653–66. [DOI] [PubMed] [Google Scholar]
- 4.Danielli JF. Capillary permeability and edema in the perfused frog. J Cell Physiol. 1940;98:109–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambers R, Zweifach BW. Intercellular cement of capillary permeability. Physiol Rev. 1947;27:436–63. [DOI] [PubMed] [Google Scholar]
- 6.Luft JH. Fine structures of capillary and end capillary layer as revealed by ruthenium red. Fed Proc. 1966;25:1773–83. [PubMed] [Google Scholar]
- 7.Reitsma S, Slaff DW, Vink H, et al. . The endothelial glycocalyx: Composition, function and visualization. Eur J Physiol. 2007;454:345–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinbaum S. Models to solve mysteries in biomechanics at the cellular level; a new view of fiber matrix layers. Ann Biomed Eng. 1998;26:627–43. [DOI] [PubMed] [Google Scholar]
- 9.Michel CC. Fluid exchange in the microcirculation – starling: The formulation of his hypothesis of microvascular fluid exchange and its significance after 100 years. Exp Physiol. 1997;82:1–30. [DOI] [PubMed] [Google Scholar]
- 10.Rehm M, Zahler S, Lotsch M, et al. . Endothelial glycocalyx as an additional barrier determining extravasation of 6% hydroxyethyl starch or 5% albumin solutions in the coronary vascular bed. Anesthesiology. 2004;100:1211–23. [DOI] [PubMed] [Google Scholar]
- 11.Jacob M, Bruegger D, Rehm M et al. . Contrasting effects of colloid and crystalloid resuscitation fluids on cardiac vascular permeability. Anesthesiology. 2006;104:1223–31. [DOI] [PubMed] [Google Scholar]
- 12.Levick JR. Revision of the Starling principle: New views of tissue fluid balance. J Physiol. 2004;557:704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curry FR. Microvascular solute and water transport. Microcirculation. 2005;12:17–31. [DOI] [PubMed] [Google Scholar]
- 14.Woodcock TE, Woodcock TM. Revised Starling equation and the glycocalyx model of transvascular fluid exchange: An improved paradigm for prescribing intravenous fluid therapy. Br J Anaesth. 2012;108:384–94. [DOI] [PubMed] [Google Scholar]
- 15.Myburgh JA. Fluid resuscitation in acute illness: Time to reappraise the basics. N Engl J Med. 2011;364:2543–4. [DOI] [PubMed] [Google Scholar]
- 16.Chappell D, Jacob M, Paul O, et al. . Impaired glycocalyx barrier properties and increased capillary tube hematocrit. J Physiol. 2008;586:4585–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US National Research Council. Transforming Glycoscience: A roadmap for the future. 2012. Available at: http://dels.nas.edu/Report/Transforming-Glycoscience-Roadmap/13446. [PubMed]
- 18.Bartlett GR. Organization of red cell glycolytic enzymes; cell coat phosphorus transfer. Ann N Y Acad Sci. 1958;75:110–4. [DOI] [PubMed] [Google Scholar]
- 19.Varki A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature. 2007;446:1023–29. [DOI] [PubMed] [Google Scholar]
- 20.Rehm M, Haller M, Orth V, et al. . Changes in blood volume and hematocrit during acute preoperative volume loading with 5% albumin or 6% Hetastarch solutions in patients before radical hysterectomy. Anesthesiology. 2001;95:849–56. [DOI] [PubMed] [Google Scholar]
- 21.Edwards MR, Mythen MG. Fluid therapy in critical illness. Extrem Physiol Med. 2014;3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perrin RM, Harper SJ, Bates DO. A role for endothelial glycocalyx in regulating microvascular permeability in diabetes mellitus. Cell Biochem Biophys. 2007;49:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rehm M, Bruegger D, Christ F, et al. . Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation. 2007;116:1896–906. [DOI] [PubMed] [Google Scholar]
- 24.Nieuwdorp M, Marijn C, Mooij HL, et al. . Measuring endothelial glycocalyx dimensions in humans: A potential novel tool to monitor vascular vulnerability. J Appl Physiol (1985). 2008;104:845–52. [DOI] [PubMed] [Google Scholar]
- 25.Bruegger D, Jacob M, Rehm M, et al. . Atrial natriuretic peptide induces shedding of endothelial glycocalyx in coronary vascular bed of guinea pig hearts. Am J Physiol Heart Circ Physiol. 2005;289:H1993–9. [DOI] [PubMed] [Google Scholar]
- 26.Henrich M, Gruss M, Weigand MA. Sepsis induced degradation of endothelial glycocalyx. ScientificWorldJournal. 2010;10:917–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bai K, Wang W. Spatio-temporal development of the endothelial glycocalyx layer and its mechanical property in vitro. J R Soc Interface. 2012;9:2290–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bruegger D, Rehm M, Jacob M, et al. . Exogenous nitric oxide requires an endothelial glycocalyx to prevent post-ischemic coronary vascular leak in guinea pig hearts. Crit Care. 2008;12:R73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gouverneur M, Spaan JA, Pannekoek H, et al. . Fluid shear stress stimulates incorporation of hyaluronan into endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol. 2006;290:H458–52. [DOI] [PubMed] [Google Scholar]
- 30.Schott U, Solomon C, Fries D, et al. . The endothelial glycocalyx and its disruption, protection and regeneration: A narrative review. Scand J Trauma Resus Emerg Med. 2016;24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson A, Statkevicius S, Shott U, et al. . Effects of fresh frozen plasma, Ringer’s acetate and albumin on plasma volume and on circulating glycocalyx components following haemorrhagic shock in rats. Int Care Med Exp 2016;4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kozar RA, Peng Z, Zhang R, et al. . Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth Analg. 2011;112:1289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pahakis MY, Kosky JR, Dull RO, et al. . The role of endothelial glycocalyx components in mechanotransduction of fluid shear stress. Biochem Biophys Res Commun. 2007;355:228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarbell JM, Cancel LM. The glycocalyx and its significance in human medicine. J Intern Med 2016;280:97–113. [DOI] [PubMed] [Google Scholar]
- 35.Weinbaum S, Zhang X, Han Y, et al. . Mechanotransduction and flow across the endothelial glycocalyx. Proc Natl Acad Sci USA. 2003;100:7988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keller MW, Geddes L, Spotnitz W, et al. . Microcirculatory dysfunction following perfusion with hyperkalemic, hypothermic, cardioplegic solutions and blood reperfusion. Circulation. 1991;84: 2485–94. [DOI] [PubMed] [Google Scholar]
- 37.Potter DR, Jiang J, Damiano ER. The recovery time course of the endothelial cell glycocalyx in vivo and its implications in vitro. Circ Res. 2009;104:1318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boer C, Koning NJ, Van Teeffelen J, et al. . Changes in microcirculatory perfusion during cardiac surgery are paralleled by alterations in glycocalyx integrity. Crit Care. 2013;17(Suppl 2):P212. [Google Scholar]
- 39.Shires T, Williams J, et al. . Acute change in extracellular fluids associated with major surgical procedures. Ann Surg. 1961;154:803–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McIlroy DR, Kharasch ED. Acute intravascular volume expansion with rapidly administered crystalloid or colloid in the setting of moderate hypovolemia. Anesth Analg. 2003;96:1572–7. [DOI] [PubMed] [Google Scholar]
- 41.Strunden M, Heckle K, Goetz AE, et al. . Perioperative fluid and volume management: Physiological basis, tools and strategies. Ann Intensive Care. 2011;1:2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huxley VH, Scallan J. Lymphatic fluid: Exchange mechanisms and regulation. J Physiol. 2011;589:2935–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Habib RH, Zacharias A, Schwann T, et al. . Adverse effects of low hematocrit during cardiopulmonary bypass in adults: Should current practice be changed? J Thorac Cardiovasc Surg. 2003;125:1438–50. [DOI] [PubMed] [Google Scholar]
- 44.Karkouti K, Djaiani G, Borger MA, et al. . Low hematocrit during cardiopulmonary bypass is associated with increased risk of perioperative stroke in cardiac surgery. Ann Thorac Surg. 2005;80:1381–7. [DOI] [PubMed] [Google Scholar]
- 45.Chappell D, Jacob M, Hofmann-Kiefer K, et al. . A rational approach to perioperative fluid management. Anesthesiology. 2008;109:723–40. [DOI] [PubMed] [Google Scholar]
- 46.Selnes OA, McKhann GM, Borowicz LM, et al. . Cognitive and neurobehavioral dysfunction after cardiac bypass procedures. Neurol Clin. 2006;24:133–45. [DOI] [PubMed] [Google Scholar]
- 47.Newman MF, Kirchner JL, Phillips-Bute B, et al. . Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344:395–402. [DOI] [PubMed] [Google Scholar]
- 48.Stump DA. Embolic factors associated with cardiac surgery. Semin Cardiothorac Vasc Anesth. 2005;9:151–2. [DOI] [PubMed] [Google Scholar]
- 49.Barak M, Nakhoul F, Katz Y, et al. . Pathophysiology and clinical implications of microbubbles during hemodialysis. Semin Dial. 2008;21:232–8. [DOI] [PubMed] [Google Scholar]
- 50.Eckmann D, Armstead S. Influence of endothelial glycocalyx degradation and surfactants on air embolism adhesion. Anesthesiology. 2006;105:1220–7. [DOI] [PubMed] [Google Scholar]
- 51.Ohkuda K, Nakahara K, Binder A, et al. . Venous air embolism in sheep: Reversible increase in lung microvascular permeability. J Appl Phys. 1981;51:887–94. [DOI] [PubMed] [Google Scholar]
- 52.Thorsen T, Klausen H, Lie RT, et al. . Bubble induced aggregation of platelets: Effects of gas species, proteins and decompression. Undersea Hyperb Med. 1993;20:101–19. [PubMed] [Google Scholar]
- 53.Malik AB, Johnson A, Tahamont MV. Mechanisms of lung vascular injury after intravascular coagulation. Ann N Y Acad Sci. 1982;384:213–34. [DOI] [PubMed] [Google Scholar]
- 54.Lee WH, Hairston P. Structure effects on blood proteins at the gas-blood interface. Fed Proc. 1971;30:1615–20. [PubMed] [Google Scholar]
- 55.Ward CA, Koheil A, McCullough D, et al. . Activation of complement at plasma-air or serum-air interface of rabbits. J Appl Physiol. 1986;60:1651–8. [DOI] [PubMed] [Google Scholar]
- 56.Ritz-Timme S, Eckelt N, Schmidtke E, et al. . Genesis and diagnostic value of leukocyte and platelet accumulations around “air bubbles” in blood after venous air embolism. Int J Legal Med. 1998;111:22–6. [DOI] [PubMed] [Google Scholar]
- 57.Sobolewski P, Kandel J, Eckmann DM. Air bubble contact with endothelial cells causes a calcium independent loss of mitochondrial membrane potential. PLoS One. 2012;7:1–8:e4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kobayashi S, Crooks S, Eckmann DM. In vitro surfactant mitigation of gas bubble contact-induced endothelial cell death. Undersea Hyperb Med. 2011;38:27–39. [PMC free article] [PubMed] [Google Scholar]
- 59.Stump DA. Deformable emboli and inflammation: Temporary or permanent damage. J Extra Corpor Technol. 2007;39:289–90. [PMC free article] [PubMed] [Google Scholar]
- 60.Young RW. Hyperoxia: A review of the risks and benefits in adult cardiac surgery. J Extra Corpor Technol. 2012;44:241–9. [PMC free article] [PubMed] [Google Scholar]
- 61.Kagawa H, Morita K, Uno Y, et al. . Inflammatory response to hyperoxemic and normoxemic cardiopulmonary bypass in acyanotic pediatric patients. World J Pediatr Congenit Heart Surg. 2014;5: 541–5. [DOI] [PubMed] [Google Scholar]
- 62.Ihnken K, Morita K, Buckberg GD, et al. . Reduced oxygen tension during cardiopulmonary bypass limits myocardial damage in acute hypoxic immature piglet hearts. Eur J Cardiothorac Surg. 1996;10:1127–35. [DOI] [PubMed] [Google Scholar]
- 63.Fujii Y, Shirai M, Tsuchimochi H, et al. . Hyperoxic condition promotes an inflammatory response during cardiopulmonary bypass in a rat model. Artif Organs. 2013;37:1034–40. [DOI] [PubMed] [Google Scholar]
- 64.Bulutcu FS, Bayindir O, Polat B, et al. . Does normoxemic cardiopulmonary bypass prevent myocardial reoxygenation injury in cyanotic children?. J Cardiothorac Vasc Anesth. 2002;16:330–3. [DOI] [PubMed] [Google Scholar]
- 65.Joachimsson PO, Sjoberg F, Forsman M, et al. . Adverse effects of hyperoxemia during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1996;112:812–19. [DOI] [PubMed] [Google Scholar]
- 66.Singh A, Ramnath RD, Foster RR, et al. . Reactive oxygen species modulate the barrier function of the human glomerular endothelial glycocalyx. PLoS One. 2013;8:e55852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rops AL, van der Vlag J, Lensen JF, et al. . Heparan sulfate proteoglycans in glomerular inflammation. Kidney Int. 2004;65:768–85. [DOI] [PubMed] [Google Scholar]
- 68.Moseley R, Waddington R, Evans P, et al. . The chemical modification of glycosaminoglycan structure by oxygen-derived species in vitro. Biochim Biophys Acta. 1995;1244:245–52. [DOI] [PubMed] [Google Scholar]
- 69.Granger DN. Ischemia-reperfusion: Mechanisms of microvascular dysfunction and the influence of risk factors for cardiovascular disease. Microcirculation. 1999;6:167–78. [PubMed] [Google Scholar]
- 70.Lindner JR, Ismail S, Spotnitz WD, et al. . Albumin microbubble persistence during myocardial contrast echocardiography is associated with microvascular endothelial glycocalyx damage. Circulation 1998;98:2187–94. [DOI] [PubMed] [Google Scholar]
- 71.Anttonen A, Leppa S, Ruotsalainen T, et al. . Pretreatment serum syndecan-1 levels and outcome in small cell lung cancer patients treated with platinum based chemotherapy. Lung Cancer. 2003;41:171–7. [DOI] [PubMed] [Google Scholar]
- 72.Peters L, Mocroft A, Soriano V, et al. . Hyaluronic acid levels predict risk of hepatic encephalopathy and liver-related death in HIV/viral hepatitis co-infected patients. PLoS One. 2013;8:e64283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chappell D, Bruegger D, Potzel J, et al. . Hypervolemia increases release of atrial natriuretic peptide and shedding of the endothelial glycocalyx. Crit Care. 2014;18:538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johansson PI, Stensballe J, Rasmussen LS, et al. . A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann Surg. 2011;254:194–200. [DOI] [PubMed] [Google Scholar]
- 75.Rahbar E, Cardenas J, Baimukanova G, et al. . Endothelial glycocalyx shedding and vascular permeability in severely injured trauma patients. J Transl Med. 2015;13:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Filho T, Torres LN, Salgado C, et al. Plasma syndecan-1 and heparan sulfate correlate with microvascular glycocalyx degradation in hemorrhaged rats after different resuscitation fluids. Am J Physiol Heart Circ Physiol. 2016;310:H1468–78. [DOI] [PubMed] [Google Scholar]
- 77.Jacob M, Paul O, Mehringer L, et al. . Albumin augmentation improves condition of guinea pig hearts after 4 hr of cold ischemia. Transplantation. 2009;87:956–65. [DOI] [PubMed] [Google Scholar]
- 78.Becker B, Chappell D, Bruegger D, et al. . Therapeutic strategies targeting the endothelial glycocalyx: Acute deficits but great potential. Cardiovasc Res. 2010;87:300–10. [DOI] [PubMed] [Google Scholar]
- 79.Finfer S, Bellomo R, Boyce N, et al. . A comparison of albumin and saline for fluid resuscitation in the intensive care unit (SAFE Study). N Engl J Med. 2004;350:2247. [DOI] [PubMed] [Google Scholar]
- 80.Perel P, Roberts I. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. 2009;3:CD000567. [DOI] [PubMed] [Google Scholar]
- 81.Roberts I, Blackhall K, Alderson P, et al. . Human albumin solution for resuscitation and volume expansion in critically ill patients. Cochrane Database Syst Rev. 2011;11:CD001208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Myburgh JA, Finer S, Bellomo R, et al. . Hydroxyethyl starch or saline for fluid resuscitation in intensive care (CHEST Study). N Engl J Med. 2012;367:1901–11. [DOI] [PubMed] [Google Scholar]
- 83.Hartog C, Reinhart K. Hydroxyethyl starch solutions are unsafe in critically ill patients. Int Care Med. 2009;35:1337–42. [DOI] [PubMed] [Google Scholar]
- 84.Perner A, Haase N, Guttormsen A, et al. . Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis (6S Trial). N Engl J Med. 2012;367:124–34. [DOI] [PubMed] [Google Scholar]