Abstract
BACKGROUND AND OBJECTIVE:
Advances in the care of patients with fragile X syndrome (FXS) have been hampered by lack of data. This deficiency has produced fragmentary knowledge regarding the natural history of this condition, healthcare needs, and the effects of the disease on caregivers. To remedy this deficiency, the Fragile X Clinic and Research Consortium was established to facilitate research. Through a collective effort, the Fragile X Clinic and Research Consortium developed the Fragile X Online Registry With Accessible Research Database (FORWARD) to facilitate multisite data collection. This report describes FORWARD and the way it can be used to improve health and quality of life of FXS patients and their relatives and caregivers.
METHODS:
FORWARD collects demographic information on individuals with FXS and their family members (affected and unaffected) through a 1-time registry form. The longitudinal database collects clinician- and parent-reported data on individuals diagnosed with FXS, focused on those who are 0 to 24 years of age, although individuals of any age can participate.
RESULTS:
The registry includes >2300 registrants (data collected September 7, 2009 to August 31, 2014). The longitudinal database includes data on 713 individuals diagnosed with FXS (data collected September 7, 2012 to August 31, 2014). Longitudinal data continue to be collected on enrolled patients along with baseline data on new patients.
CONCLUSIONS:
FORWARD represents the largest resource of clinical and demographic data for the FXS population in the United States. These data can be used to advance our understanding of FXS: the impact of cooccurring conditions, the impact on the day-to-day lives of individuals living with FXS and their families, and short-term and long-term outcomes.
In 1943, Martin and Bell1 described 11 males with severe intellectual disability (ID) in a 2-generation family. Nearly 50 years later, researchers discovered the molecular basis of what is now known as fragile X syndrome (FXS).2,3 FXS is nearly always due to an expanded CGG repeat sequence (>200 hypermethylated CGG repeats, full mutation) in the 5′ untranslated region of the FMR1 gene located at Xq27.3. FXS is the most common known inherited form of ID4 and has been reported as the most common known inherited single-gene disorder associated with autism spectrum disorder (ASD), accounting for ∼2% to 3% of all cases of ASD.5,6 Studies estimate the prevalence of FXS to be ∼1 in 4000 to 1 in 7000 in males and ∼1 in 6000 to 1 in 11 000 in females.7–10
Premutation carriers (those with 55–200 unmethylated CGG repeats) typically do not show symptoms of FXS, but are at risk for transmitting an expanded full mutation to their offspring.11,12 Approximately 1 in 500 to 1 in 800 males and 1 in 200 to 1 in 300 females are premutation carriers.9,10,13–16 In addition, premutation carriers are at high risk for fragile X–associated tremor/ataxia syndrome, a late-onset neurodegenerative disorder,17 and, among women, fragile X–associated primary ovarian insufficiency.18 Together, these 3 disorders, FXS, fragile X–associated tremor/ataxia syndrome, and fragile X–associated primary ovarian insufficiency, are referred to as fragile X–associated disorders (FXD). There are reports of other clinical manifestations among premutation carriers that may lead to expansion of this definition of FXD; however, this remains an area of active research.
Focusing on FXS, research to understand the range of phenotypic expression of the full mutation and how families adapt to FXS continues to grow. This research shows that although a classic FXS “phenotype” exists, there is considerable symptomatic variability. Males with the full mutation typically experience mild to moderate ID, but others may have more severe disabilities. Although not common, several studies have reported “high-functioning” males with intellectual ability in the normal to borderline range.19–22 One-third to one-half of females with the full mutation has intellectual function in the normal range, due to the masking effect of the normal X-chromosome FMR1 allele.23,24 Numerous associated conditions and symptoms of variable severity can occur, including ASD, attention deficits, hyperactivity/impulsivity, hyperarousal, anxiety, self-injurious behavior, sleep disturbance, seizures, and medical problems, such as frequent otitis media, strabismus, and joint hyperflexibility. However, these conditions are not universally experienced, and their actual prevalence and impact are still unknown.
Small sample sizes and referral bias of most studies make the true prevalence of associated conditions difficult to determine. For example, the prevalence of ASD among individuals with FXS varies among studies between 15% and 60%.25–29 Also, studies of cooccurring conditions have typically focused on single conditions, without examining associations with other features of FXS. Findings from a survey of >1300 FXS parents (convenience sample) indicate that parents report a higher frequency of conditions, such as seizures, ASD, and anxiety, among individuals with FXS compared with general population.30–33 Additional examination of cooccurring conditions and how they relate to other FXS features using clinical data are needed.
In addition to the limitations noted above, there is an inadequate understanding of the needs of individuals and families with FXS, and there is only a small literature on evidence-based practices for treating individuals with FXS.34,35 This limitation could have negative consequences for individuals who could benefit from intervention services as early as possible.36 To conduct well-designed clinical trials that capture the entire spectrum of the disorder, the natural history of FXS needs to be determined. To address the limitations in knowledge, 2 important resources are needed: (1) a sample of individuals with FXS and their relatives, and (2) a collection of longitudinal clinical data from individuals with FXS to facilitate answers to clinical care and public health research questions.
The Fragile X Clinical and Research Consortium (FXCRC) is a collaborative infrastructure of clinics that specializes in the care of individuals and families affected by FXS. Initially conceived by fragile X clinicians in 2006, with the support of the National Fragile X Foundation (NFXF), the FXCRC has grown to include 27 fragile X specialty clinics across the United States (see Acknowledgments for a list of FXCRC clinics). In 2008, the Centers for Disease Control and Prevention (CDC) funded the creation of a Fragile X Registry and a pilot study to test multisite clinical research in FXS. The pilot study (phase I) showed that using a consortium of clinics was an effective way to engage multiple clinics and facilitate research.29 In 2011, the CDC funded a second pilot study (phase II) to support the collection and management of longitudinal data, incorporating the existing Fragile X Registry. The result was the development and implementation of the Fragile X Online Registry With Accessible Research Database (FORWARD).
Methods
Study Design of FORWARD
FORWARD is a multisite, observational study that includes data from individuals with FXS and their family members, collected thus far by 25 of the 27 established fragile X clinics affiliated with the FXCRC (Fig 1). Each clinic must obtain institutional review board (IRB) approval from their own institutions before enrolling families in FORWARD. The goals of FORWARD are to: (1) enhance the understanding of FXS, its cooccurring conditions, and associated risk factors; (2) identify service barriers, service needs, and services used among people diagnosed with FXS and the impact of these services on health outcomes; and (3) document medical and behavioral treatments used by FXS patients and their effects on outcomes.
FIGURE 1.
Fragile X specialty clinics participating in FXCRC and contributing data to FORWARD.
There are 2 main components to FORWARD: (1) the registry, which collects primarily demographic information from families with FXD, including both affected and unaffected family members (full mutation, premutation, and noncarriers); and (2) the longitudinal database, which collects data from individuals with full mutation (FXS), currently focusing on those aged 0 to 24 years (although individuals of all ages may participate). Because this is a multisite study, standardization of clinical data collection is important. The evaluation of phase I (2008 to 2011) data collection forms led to the development of phase II (2011 to 2015) data collection forms. To facilitate data sharing across ASD and FXS data sets when possible, the forms for the longitudinal database were developed and revised by members of the FXCRC, the FORWARD project staff, and CDC collaborators, taking into account the variables used in national parent surveys, including Our Fragile X World (www.ourfragilexworld.org) and the Autism Treatment Network (www.autismspeaks.org/science/resources-programs/autism-treatment-network/what-atn). Data in the longitudinal database are collected by clinicians, primarily during a patient’s scheduled clinic visit, and by parents/caregivers. A participant is considered to have a complete set of baseline data if both a clinician report form and a parent report form have been completed and submitted. Parameters of the registry and database forms are outlined in Table 1. Additionally, there are 3 standardized parent-report instruments requested at baseline: the Social Responsiveness Scale, Second Edition (SRS-2),37 the Social Communication Questionnaire (SCQ),38 and the Aberrant Behavior Checklist–Community (ABC-C).39 The SRS-2 and SCQ only need to be entered once, whereas it is requested that the ABC-C be completed annually along with the clinician and parent report forms.
TABLE 1.
Overview of the FORWARD Study Design
| Eligibility | Registry | Longitudinal Study |
|---|---|---|
| Age | All ages | 0–24 y for compensated enrollment; however, all ages can be enrolled |
| Sex | Males and females | Males and females |
| Race/ethnicity | All race/ethnic groups | All race/ethnic groups |
| FMR1 mutation status | Noncarriers, premutation, and full mutation carriers | Full mutation carriers, including mosaics |
| Data collection source | 2-page registry form | Clinician report form |
| Self or guardian report form | Parent report form | |
| Standardized self- and parent-report behavioral assessments (ABC-C, SRS-2, SCQ) | ||
| Data domains | Demographics | Clinician report form |
| FMR1 mutation/diagnostic status | Physical exam (eg, height, weight, and head circumference) | |
| Primary spoken language | Medical history (eg, seizures, vision problems, musculoskeletal problems, chronic medical problems) | |
| Availability of participant for research projects | Language, speech, and communication | |
| Cognition | ||
| Behavior | ||
| Psychopharmacology for behaviors | ||
| Hyperarousal or sensory issues | ||
| FMR1 mutation laboratory diagnostics | ||
| Parent report form | ||
| Developmental milestones | ||
| Sleep characteristics | ||
| Education and transition history and intervention | ||
| Family support | ||
| Social participation and residential settings | ||
| Heath monitoring practices | ||
| Socioeconomic status | ||
| Data collection frequency | Once | Clinician and parent report forms: annually |
| ABC-C: annually | ||
| SRS-2 and SCQ: once |
As previously mentioned, the current data collection tools are built on lessons learned from the initial pilot study. One crucial domain to be captured in FORWARD is the level of cognitive and behavioral functioning of patients with FXS. These data are essential to evaluate the primary and secondary features of FXS. Cognitive and behavioral impairments influence almost all other domains in the life of an individual with FXS and their family. In the first pilot study (phase 1), data on this domain were often missing, because the source of the data was not from the clinic. That is, results from standardized tests were usually part of school records or assessments done at other types of clinical centers. By using the lessons learned in phase I, additional approaches were used in phase II. First, the clinician is asked in the clinician report form to provide a clinical impression of the patient’s level of cognitive function based on their interactions with the patient and available medical/education records. As in phase I, data on the measure of the full-scale IQ and related details (test score, age at testing, etc) and the composite and subscale scores from the Vineland Adaptive Behavior Scales–II40 are still requested, if available. Second, we ask the parents to complete 3 standardized behavioral assessments about their child, as described above. These combined efforts provide a more robust description of the level of impairment observed in this population.
As noted in Table 2, the longitudinal component of the FORWARD database is now coming into fruition. Data collection for FORWARD is conducted in conjunction with a clinic visit, and although most fragile X specialty clinics recommend patients be seen on at least an annual basis, this may not always occur. To help encourage continued participation, newsletters describing the status of the project are provided to clinics to distribute to participants.
TABLE 2.
Demographics of Participants Enrolled in the FORWARD Registry Between September 7, 2009 and August 31, 2014 With Known FMR1 Mutation Status (N = 1961)
| Noncarrier | Premutation Carrier | Full Mutation Carrier | |
|---|---|---|---|
| Participants, n | 65 | 541 | 1355 |
| Sex: % male | 46.2 | 8.1 | 75.6 |
| Median age, y (range) | 33 (1–80) | 40 (1–84) | 10 (0–69) |
| Ethnicity | |||
| Hispanic/Latino, % | 10.8 | 7.2 | 9.7 |
| Race | |||
| White, % | 86.2 | 94.3 | 87.9 |
| African American, % | 7.7 | 2.8 | 7.2 |
| Asian, % | 1.5 | 1.1 | 2.1 |
| Other, % | 4.6 | 1.8 | 2.9 |
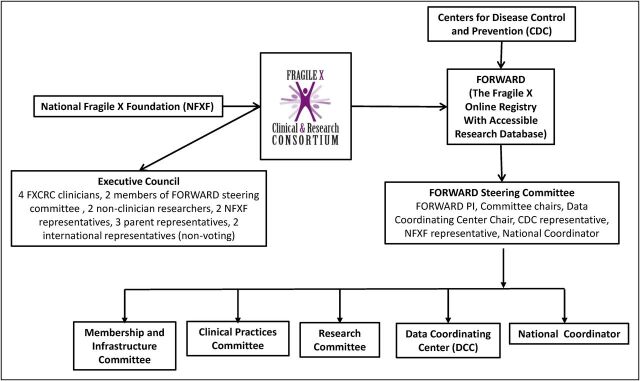
Leadership of the FXCRC is provided through the executive council, an elected body comprised of parents of children with FXS, patient advocates, clinicians, and clinical researchers. A separate steering committee was formed to oversee the implementation of FORWARD, as well as 3 other committees: (1) the membership and infrastructure committee, (2) the clinical practices committee, and (3) the research committee. A national coordinator (NC) facilitates communication between these committees, provides support to the clinics to enhance ongoing participation in the project, helps to manage data collection and monitor data quality, and facilitates analysis. Figure 2 provides an overview of the administrative structure.
FIGURE 2.
Administrative structure of the FXCRC and the FORWARD longitudinal study.
Clinic-based Recruitment
Recruitment for this study is clinic based, and, therefore, only individuals choosing to attend specialty clinics are approached to participate in FORWARD. Additionally, sometimes it is not possible to approach all clinic patients due to the circumstances of the visit, and, of those who are approached, not everyone participates. To understand some of the differences in the characteristics of individuals who participate in the database and those who do not participate, clinics have been asked to implement, with IRB approval, nonenrollment logs to gather a minimal set of data. Because the registry entails only a simple 2-page form completed by a family member compared with the more intensive database component of FORWARD, it may enroll a larger proportion of the eligible clinic participants. Demographic data from the registry, used in combination with the nonenrollment log, will help determine whether patients in the database are representative of all individuals with FXS attending fragile X clinics.
Clinic Compensation
To enhance the participation of clinics in FORWARD and to support data collection and entry, the fragile X specialty clinics are compensated for their efforts. They are compensated for obtaining initial IRB approval and subsequently for data collection. The latter is based on the number and type of forms submitted to FORWARD for each study participant. To reduce the burden on clinics, the Data Coordinating Center (DCC) can enter data for clinics from the deidentified clinical forms. These combined efforts have contributed to enhance the clinics’ capacity to participate in this longitudinal study.
Data Management
The registry and longitudinal database were developed under secure data transmission and centralized housing of all data. The DCC at Columbia University is responsible for the development and maintenance of the system and thoroughly tests each component of the online data entry system before making it available to the users. User-specific identification and passwords are managed centrally by the DCC. Programmed checks (eg, out-of-range checks for variables) are incorporated to ensure high data quality. For longitudinal data collection, data checks ensure that individual patterns of change over time represent reasonable trajectories. If anomalies are detected, queries are generated and sent to the source clinic for verification or possible correction.
The identity of FORWARD participants in the online system is known only to clinic staff. The individual clinics are the gatekeepers of their participants’ contact information and keep the link between the participant’s unique identifier in the online system and the name and contact information of the individual patient or family member. An additional level of security has been created by FXCRC policy, the role-based access control. Each site-based coordinator can enter, edit, and view data on patients and family members registered from his/her site, but cannot see data entered by other sites. In phase II of the project, only the DCC has access to deidentified individual-level data for purposes of quality control and analyses.
The DCC and the NC monitor progress, create monthly reports enumerating the number of forms of each type submitted by each clinic, and provide compensation to clinics for their data collection. To facilitate longitudinal data collection, the DCC provides regular reports to the clinics to identify participants who are due for an annual follow-up examination. The steering committee is responsible for the oversight of all requests for data analyses and access to FORWARD participants for recruitment into other studies.
Quality Assurance and Quality Control
The NC conducts site visits, hosts webinars, and communicates with participating clinics to help clinics with IRB submissions, implementation of research protocols, and identification of methods to improve data collection and entry. These efforts are complemented by a yearly FXCRC meeting that brings the clinics together to discuss successes and challenges with regard to participating in FORWARD and FXCRC, share ideas for ways to improve, collaboratively think about appropriate uses for these data, and brainstorm about ways to build on these efforts.
In addition to the ongoing quality control measures implemented by the DCC and the NC, several strategies have been conducted to improve data quality: a data review workgroup conducted an in-depth assessment of data quality, evaluating each variable for outliers and potential errors, and documenting any identified limitations; a process evaluation was conducted to assess recruitment and data collection processes. This process helped inform the consistency of data collection and, together with the data review results, is being used to improve the data collection process moving forward.
Because this is a multisite study, participants may be seen at >1 clinic over the course of the study time line. Because the clinics assign unique identifiers and the DCC does not have access to participant identifiers, it is difficult to identify data that ought to be linked but are not. The registry form inquires whether the individual has been seen at any other fragile X specialty clinic. If a clinician thinks the participant has contributed data from >1 site based on their responses, they contact the NC. The NC facilitates communication between the clinics to confirm the linkage. If confirmed, a new umbrella unique identifier is assigned to the participant, linking his or her data. Completion of these comprehensive quality control measures has resulted in the development of FORWARD’s first fixed data set (FORWARD data set version 1.0), representing baseline data collected for the longitudinal database from September 7, 2012 to August 31, 2014 and linked data from the registry form for participants in the longitudinal database.
Results
All individuals diagnosed with FXS who attend a fragile X specialty clinic and all family members (including premutation carriers and those with no FMR1 mutations) are eligible to participate in the FORWARD registry. From September 7, 2009 to August 31, 2014, 2376 individuals registered, the majority of whom reported their FMR1 mutational status (n = 1961), and the remaining 17% did not know, had unrelated testing, or chose not to respond. Table 2 provides the demographic data of those with known mutational status. As expected for this FXS clinic–based ascertainment, the majority (69%) of tested individuals carry the full mutation and most are male (76%) (Table 2). The majority of premutation carriers are female (92%), and they are primarily the mothers of individuals diagnosed with FXS. Race and ethnicity are reported by the respondent (ie, self-report or parent/caregiver report). Overall, 9% of registrants reported their ethnicity as Hispanic and ∼10% reported their racial group as nonwhite (Table 2).
The participants enrolled in the FORWARD longitudinal database are a subset of those in the FORWARD registry, because only those who have been diagnosed with the full mutation and who attend a fragile X clinic are eligible for this component. Table 3 shows the number of completed forms in the FORWARD database collected from September 7, 2009 to August 31, 2014. As expected for this X-linked disorder, 78% of the participants are male (Table 4). As observed in the FORWARD registry, the vast majority of participants in the FORWARD database are non-Hispanic (90%) and white (88%), as reported by the parent or caregiver. The current data collection focuses on individuals with FXS ages 0 to 24 years, and, as such, ∼94% of participants are ≤24 years of age. The age group frequencies of participants attending the clinic at baseline did not differ by sex (Pearson χ2; P > .10), with the majority being <15 years of age (74%) (Table 4).
TABLE 3.
Summary of the FORWARD Longitudinal Database Forms Completed for Participants Diagnosed With FXS Between September 7, 2012 and August 31, 2014
| Form Completed | Baseline Assessment | Follow-up Assessment |
|---|---|---|
| Clinician report form | 713 | 178 |
| Parent report form | 549 | 127 |
| ABC-C | 557 | 131 |
| SCQ | 534 | N/A |
| SRS-2 | 457 | N/A |
| Both clinician and parent report forms, n | 503 | 114 |
N/A, not applicable.
TABLE 4.
Demographics of Participants Diagnosed With FXS Enrolled Between September 7, 2012 and August 31, 2014 in the FORWARD Longitudinal Database
| Male | Female | |
|---|---|---|
| Participants, N | 558 (78.3%) | 155 (21.7%) |
| Ethnicity | ||
| Hispanic/Latino, % | 9.0 | 12.3 |
| Race | ||
| White, % | 88.7 | 87.1 |
| African American, % | 7.2 | 8.4 |
| Asian, % | 2.0 | 2.6 |
| Other, % | 2.2 | 1.9 |
| Age group, y | ||
| 0–4, % | 18.3 | 16.8 |
| 5–9, % | 28.5 | 31.0 |
| 10–14, % | 26.2 | 28.4 |
| 15–19, % | 13.1 | 13.5 |
| 20–24, % | 7.2 | 5.9 |
| >24, % | 6.8 | 4.5 |
There is a wide range of ID and of behavioral problems among individuals diagnosed with FXS, and this is reflected by the patients enrolled in FORWARD. Table 5 provides an overview of the cognitive function of participants using the clinician’s global impression. For patients <7 years of age, clinicians reported whether there was evidence of developmental delay. Among these younger individuals, all male participants and 70.1% of female participants with the full mutation were reported to have evident developmental delays, with frequency differences by sex being statistically significant (Pearson χ2; P < .0001). Among the patients ≥7 years of age, we present a finer scale of the impression of the level of impairment. Among male patients, ∼60% fall into the moderate ID range, whereas the majority of female patients fall into the borderline to mild ID range (64%). The differences observed among male and female patients in the level of impairment are statistically significant (Pearson χ2; P < .0001).
TABLE 5.
General Description of ID Level and ASD Diagnosis of Participants Diagnosed With FXS Enrolled Between September 7, 2012 and August 31, 2014 in the FORWARD Longitudinal Database
| Male | Female | |
|---|---|---|
| Clinician’s summary of overall ID level | ||
| Participants <7 y of age, N | 162 | 41 |
| % with developmental delay | 100 | 70.7 |
| Participants ≥7 y of age, N | 362 | 106 |
| No ID, % | 0.6 | 21.7 |
| Borderline ID, % | 3.6 | 31.1 |
| Mild ID, % | 22.9 | 33.0 |
| Moderate ID, % | 59.9 | 12.3 |
| Severe ID, % | 12.4 | 1.9 |
| Profound ID, % | 0.6 | 0 |
| Current diagnosis of ASD | ||
| Participants ≥3 y of age, N | 449 | 136 |
| % with ASD diagnosis | 49.9 | 16.9 |
To provide an indicator of the presence of behavioral problems, we present one of many parameters in the FORWARD longitudinal database: the percent of participants ≥3 years of age who have a current diagnosis of ASD, as reported by the clinician. The status of ASD was reported for 93% of participants. Half of the male participants (50%) and 17% of the female participants have a current diagnosis of ASD (Table 5) (Pearson χ2; P < .0001).
Discussion
The FXCRC was initiated by clinicians and researchers to form a research structure that eventually would lead to better care of individuals and families diagnosed with FXD. FORWARD is the only such structure of this kind in the United States, collecting longitudinal data based on both clinician and parent report. The results from FORWARD will provide a forum to better understand the natural history of FXS, identify important gaps in our knowledge about the needs of FXS patients and their families, and guide clinical care. The data collected through FORWARD will provide essential information to assist health care professionals in their goal of providing excellent care with the long-term objective of improving health outcomes. The FORWARD registry also provides centralized data that can be used to facilitate recruitment into research efforts to identify important pharmacologic, behavioral, and educational interventions.
The FORWARD structure is clinic-based, similar to that for disorders such as spina bifida41 and cystic fibrosis.42 This structure differs from other clinical databases, such as Duchenne Connect, that collect self-reported clinical information from individuals with a specific disorder and their families (eg, see ref 43). The advantage of the clinic-based system is that data are collected by a clinician at the time of the clinic visit, maximizing completeness and accuracy of these data.
A limitation of FORWARD is that enrollment occurs only through fragile X specialty clinics. This enrollment only through clinics has the potential to lead to a biased representation of family demographics and severity of the syndrome. Families who have access to more resources or live in urban areas with increased access to clinicians specializing in FXS may be more represented in FORWARD. Additionally, it may be that affected individuals who attend fragile X specialty clinics have more severe problems than those with milder symptoms, or it may be that the most severely affected patients cannot be seen in clinic because their behavior prevents them from being brought in for medical care. Methods, such as the nonenrollment log described above, will help determine whether FORWARD represents those who attend fragile X clinics, but cannot inform whether FORWARD represents those diagnosed with FXS in general.
However, the data on the clinician’s global impression of cognitive impairment and the presence of ASD in male and female participants in FORWARD is comparable to that observed in the literature and, thus, is encouraging in terms of being representative of individuals with FXS (Table 5). For example, the majority of male participants have moderate ID and female participants have borderline to mild ID (Table 5), similar to that reported previously.34 Also, results on the percent of patients with a current diagnosis of ASD are similar to those obtained from a national survey of parents with children diagnosed with FXS. In that survey, 46% of males and 16% of females with FXS had ASD by parent report,30 and in FORWARD, 50% of males and 17% of females with FXS had ASD (Table 5). Use of other data collection methods, such as Our Fragile X World’s parent-report survey, will complement these clinic-based data and collectively provide a more robust characterization of FXS and identify needs of individuals and families.
Based on current data (Tables 2 and 4), FORWARD is probably missing a proportion of racial/ethnic minorities either because they are not diagnosed or because they are not attending fragile X clinics. Many reasons for not attending a clinic can be considered, such as: the lack of a clinical referral or awareness of the existence of a clinic; cost barriers related to travel to the clinic or lack of insurance to cover care beyond the diagnosis; language barriers; cultural or religious beliefs; and many others. The FXCRC has relied on community support networks, sponsored by the NFXF, to educate stakeholders in their regions about the benefits of the fragile X clinics, and it hopes to increase the overall number and to have a wider distribution of racial/ethnic minority patients attending fragile X specialty clinics. Additionally, to improve recruitment for the Spanish-speaking population, most of the parent-report FORWARD materials have been translated into Spanish and administered at clinics in locations that serve this population.
The experience and tools used to create FORWARD can serve as a model for other FXS studies in other countries and for studies of other ID disorders. For example, the data collection tools used in FORWARD were shared with a group of fragile X clinicians and researchers in Germany working to create a similar data resource, referred to as EXPLAIN.44 The potential outcome will be the ability to compare and contrast clinical outcomes of FXS in different clinical and cultural settings. Use of the same tools and variables in longitudinal studies of other neurologic disorders will also increase understanding of the commonalities among ID and behavioral disorders and potentially lead to new discoveries.
Conclusions
The FORWARD longitudinal database includes complete baseline data on >900 individuals with FXS, representing the largest resource of clinical data for the FXS population in the United States. These data can now be used as a resource by the clinical and research communities interested in advancing our understanding of FXS, including the impact of cooccurring conditions, the impact on the day-to-day life of individuals living with FXS and their families, and short-term and long-term outcomes. The availability of longitudinal data is currently limited; however, the intent is to continue collecting longitudinal data with the goal of providing a valuable resource to the FXS community.
Acknowledgments
We thank the NFXF for their support, encouragement, and commitment to establish the FXCRC. We thank Don Bailey, Melissa Raspa, and Anne Wheeler for their input into FORWARD. We thank Rebecca Nash for her assistance with graphics. Importantly, FORWARD can only exist through the efforts of individuals who have FXS and their families and the clinicians and teams who care for them.
To acknowledge the work of the fragile X specialty clinics participating in FXCRC (25 of which have contributed data to FORWARD), we list the clinical director(s), their affiliation, and the year they joined the FXCRC. The list is alphabetical by state.
Glossary
- ABC-C
Aberrant Behavior Checklist–Community
- ASD
autism spectrum disorder
- CDC
Centers for Disease Control and Prevention
- DCC
Data Coordinating Center
- FORWARD
Fragile X Online Registry With Accessible Research Database
- FXCRC
Fragile X Clinical and Research Consortium
- FXD
fragile X–associated disorder
- FXS
fragile X syndrome
- ID
intellectual disability
- IRB
institutional review board
- NC
national coordinator
- NFXF
National Fragile X Foundation
- SCQ
Social Communication Questionnaire
- SRS-2
Social Responsiveness Scale, Second Edition
Footnotes
Dr Sherman drafted the original manuscript; Drs Riley and Kidd revised and edited the manuscript for review by all other authors; Dr Andrews conducted analyses for the data presented; Drs Andrews, Brown, Berry-Kravis, Kaufmann, Kidd, and Sherman have been involved in all aspects of design, implementation, and evaluation; Dr Riley has been involved in the implementation and evaluation of FORWARD; Dr Swanson, Mr Miller, and Ms Lincoln were involved in the initial design and implementation; and all authors read, edited, and approved the final version for submission.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
FUNDING: Supported by cooperative agreements 5U01DD000231 and 5U19DD000753-02 with the Centers for Disease Control and Prevention.
References
- 1.Martin JP, Bell J. A pedigree of mental defect showing sex linkage. J Neurol Psychiatry. 1943;6(3–4):154–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verkerk AJ, Pieretti M, Sutcliffe JS, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65(5):905–914 [DOI] [PubMed] [Google Scholar]
- 3.Yu S, Pritchard M, Kremer E, et al. Fragile X genotype characterized by an unstable region of DNA. Science. 1991;252(5009):1179–1181 [DOI] [PubMed] [Google Scholar]
- 4.Baird G, Cook EH, Happe FG, et al. Neurodevelopmental disorders. In: Diagnostic and Statistical Manual of Mental Disorders DSM-5. 5th ed. Washington, DC: American Psychiatric Publishing; 2013:31–86 [Google Scholar]
- 5.Schaefer GB, Mendelsohn NJ. Genetics evaluation for the etiologic diagnosis of autism spectrum disorders. Genet Med. 2008;10(1):4–12 [DOI] [PubMed] [Google Scholar]
- 6.Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics. 2004;113(5). Available at: www.pediatrics.org/cgi/content/full/113/5/e472 [DOI] [PubMed] [Google Scholar]
- 7.Crawford DC, Acuña JM, Sherman SL. FMR1 and the fragile X syndrome: human genome epidemiology review. Genet Med. 2001;3(5):359–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coffee B, Keith K, Albizua I, et al. Incidence of fragile X syndrome by newborn screening for methylated FMR1 DNA. Am J Hum Genet. 2009;85(4):503–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tassone F, Iong KP, Tong TH, et al. FMR1 CGG allele size and prevalence ascertained through newborn screening in the United States. Genome Med. 2012;4(12):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunter J, Rivero-Arias O, Angelov A, Kim E, Fotheringham I, Leal J. Epidemiology of fragile X syndrome: a systematic review and meta-analysis. Am J Med Genet A. 2014;164A(7):1648–1658 [DOI] [PubMed] [Google Scholar]
- 11.Nolin SL, Sah S, Glicksman A, et al. Fragile X AGG analysis provides new risk predictions for 45-69 repeat alleles. Am J Med Genet A. 2013;161A(4):771–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nolin SL, Glicksman A, Ding X, et al. Fragile X analysis of 1112 prenatal samples from 1991 to 2010. Prenat Diagn. 2011;31(10):925–931 [DOI] [PubMed] [Google Scholar]
- 13.Cronister A, Teicher J, Rohlfs EM, Donnenfeld A, Hallam S. Prevalence and instability of fragile X alleles: implications for offering fragile X prenatal diagnosis. Obstet Gynecol. 2008;111(3):596–601 [DOI] [PubMed] [Google Scholar]
- 14.Hantash FM, Goos DM, Crossley B, et al. FMR1 premutation carrier frequency in patients undergoing routine population-based carrier screening: insights into the prevalence of fragile X syndrome, fragile X-associated tremor/ataxia syndrome, and fragile X-associated primary ovarian insufficiency in the United States. Genet Med. 2011;13(1):39–45 [DOI] [PubMed] [Google Scholar]
- 15.Seltzer MM, Baker MW, Hong J, Maenner M, Greenberg J, Mandel D. Prevalence of CGG expansions of the FMR1 gene in a US population-based sample. Am J Med Genet B Neuropsychiatr Genet. 2012;159B(5):589–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rousseau F, Rouillard P, Morel ML, Khandjian EW, Morgan K. Prevalence of carriers of premutation-size alleles of the FMRI gene--and implications for the population genetics of the fragile X syndrome. Am J Hum Genet. 1995;57(5):1006–1018 [PMC free article] [PubMed] [Google Scholar]
- 17.Hagerman RJ, Leehey M, Heinrichs W, et al. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology. 2001;57(1):127–130 [DOI] [PubMed] [Google Scholar]
- 18.Allingham-Hawkins DJ, Babul-Hirji R, Chitayat D, et al. Fragile X premutation is a significant risk factor for premature ovarian failure: the International Collaborative POF in fragile X study--preliminary data. Am J Med Genet. 1999;83(4):322–325 [PMC free article] [PubMed] [Google Scholar]
- 19.Smeets HJ, Smits AP, Verheij CE, et al. Normal phenotype in two brothers with a full FMR1 mutation. Hum Mol Genet. 1995;4(11):2103–2108 [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, Taylor AK, Bridge JA. FMR1 fully expanded mutation with minimal methylation in a high functioning fragile X male. J Med Genet. 1996;33(5):376–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wöhrle D, Salat U, Gläser D, et al. Unusual mutations in high functioning fragile X males: apparent instability of expanded unmethylated CGG repeats. J Med Genet. 1998;35(2):103–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han XD, Powell BR, Phalin JL, Chehab FF. Mosaicism for a full mutation, premutation, and deletion of the CGG repeats results in 22% FMRP and elevated FMR1 mRNA levels in a high-functioning fragile X male. Am J Med Genet A. 2006;140(13):1463–1471 [DOI] [PubMed] [Google Scholar]
- 23.Abrams MT, Reiss AL, Freund LS, Baumgardner TL, Chase GA, Denckla MB. Molecular-neurobehavioral associations in females with the fragile X full mutation. Am J Med Genet. 1994;51(4):317–327 [DOI] [PubMed] [Google Scholar]
- 24.Loesch DZ, Huggins RM, Hagerman RJ. Phenotypic variation and FMRP levels in fragile X. Ment Retard Dev Disabil Res Rev. 2004;10(1):31–41 [DOI] [PubMed] [Google Scholar]
- 25.Kaufmann WE, Cortell R, Kau AS, et al. Autism spectrum disorder in fragile X syndrome: communication, social interaction, and specific behaviors. Am J Med Genet A. 2004;129A(3):225–234 [DOI] [PubMed] [Google Scholar]
- 26.Lewis P, Abbeduto L, Murphy M, et al. Cognitive, language and social-cognitive skills of individuals with fragile X syndrome with and without autism. J Intellect Disabil Res. 2006;50(Pt 7):532–545 [DOI] [PubMed] [Google Scholar]
- 27.Harris SW, Hessl D, Goodlin-Jones B, et al. Autism profiles of males with fragile X syndrome. Am J Ment Retard. 2008;113(6):427–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loesch DZ, Bui QM, Dissanayake C, et al. Molecular and cognitive predictors of the continuum of autistic behaviours in fragile X. Neurosci Biobehav Rev. 2007;31(3):315–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kidd SA, Lachiewicz A, Barbouth D, et al. Fragile X syndrome: a review of associated medical problems. Pediatrics. 2014;134(5):995–1005 [DOI] [PubMed] [Google Scholar]
- 30.Bailey DB Jr, Raspa M, Olmsted M, Holiday DB. Co-occurring conditions associated with FMR1 gene variations: findings from a national parent survey. Am J Med Genet A. 2008;146A(16):2060–2069 [DOI] [PubMed] [Google Scholar]
- 31.Symons FJ, Byiers BJ, Raspa M, Bishop E, Bailey DB. Self-injurious behavior and fragile X syndrome: findings from the national fragile X survey. Am J Intellect Dev Disabil. 2010;115(6):473–481 [DOI] [PubMed] [Google Scholar]
- 32.Kronk R, Bishop EE, Raspa M, Bickel JO, Mandel DA, Bailey DB Jr. Prevalence, nature, and correlates of sleep problems among children with fragile X syndrome based on a large scale parent survey. Sleep. 2010;33(5):679–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berry-Kravis E, Raspa M, Loggin-Hester L, Bishop E, Holiday D, Bailey DB. Seizures in fragile X syndrome: characteristics and comorbid diagnoses. Am J Intellect Dev Disabil. 2010;115(6):461–472 [DOI] [PubMed] [Google Scholar]
- 34.Hagerman RJ, Berry-Kravis E, Kaufmann WE, et al. Advances in the treatment of fragile X syndrome. Pediatrics. 2009;123(1):378–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berry-Kravis E, Knox A, Hervey C. Targeted treatments for fragile X syndrome. J Neurodev Disord. 2011;3(3):193–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCary LM, Roberts JE. Early identification of autism in fragile X syndrome: a review. J Intellect Disabil Res. 2013;57(9):803–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Constantino JN, Todd RD. Intergenerational transmission of subthreshold autistic traits in the general population. Biol Psychiatry. 2005;57(6):655–660 [DOI] [PubMed] [Google Scholar]
- 38.Rutter M, Bailey A, Lord C, eds. Manual for the Social Communication Questionnaire. Los Angeles, CA: Western Psychological Services; 2003 [Google Scholar]
- 39.Aman MG, Singh NN, Stewart AW, Field CJ. The aberrant behavior checklist: a behavior rating scale for the assessment of treatment effects. Am J Ment Defic. 1985;89(5):485–491 [PubMed] [Google Scholar]
- 40.Sparrow S, Balla D, Cicchetti D. Vineland Scales of Adaptive Behavior. Circle Pines, MN: American Guidance Service; 1984 [Google Scholar]
- 41.Thibadeau JK, Ward EA, Soe MM, et al. Testing the feasibility of a National Spina Bifida Patient Registry. Birth Defects Res A Clin Mol Teratol. 2013;97(1):36–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morgan WJ, Butler SM, Johnson CA, et al. Epidemiologic study of cystic fibrosis: design and implementation of a prospective, multicenter, observational study of patients with cystic fibrosis in the U.S. and Canada. Pediatr Pulmonol. 1999;28(4):231–241 [DOI] [PubMed] [Google Scholar]
- 43.Rangel V, Martin AS, Peay HL. DuchenneConnect registry report. PLoS Curr. 2012;4:RRN1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haessler F, Gaese F, Colla M, et al. EXPLAIN Fragile-X: an explorative, longitudinal study on the characterization, treatment pathways, and patient-related outcomes of fragile X syndrome. BMC Psychiatry. 2013;13:339. [DOI] [PMC free article] [PubMed] [Google Scholar]