Abstract
Glutamate signaling in the central nervous system is known to play a key role in pain regulation. AMPAkines can enhance glutamate signaling through α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. Previous studies have shown that AMPAkines are effective analgesic agents, and their site of action is likely in the brain. It is not known, however, if AMPAkines can provide complementary analgesia in combination with opioids, the most commonly used analgesics. Here, we show that the co-administration of an AMPAkine with morphine can provide additional analgesia, both in naïve rats and in rats that experience postoperative pain. Furthermore, we show that this AMPAkine can be administered directly into the prefrontal cortex to provide analgesia, and that prefrontal AMPAkine infusion, similar to systemic administration, can provide added pain relief to complement morphine analgesia.
Keywords: AMPAkines, morphine, analgesia, prefrontal cortex
Despite major advances in molecular and pharmacological pain research over the last fifty years, opioids remain the most commonly used potent analgesics. Opioid analgesia, however, has significant side effects, and the steep increase in opioid-related mortalities in chronic pain patients has been particularly alarming [1–4]. Most of these cases of mortality are related to respiratory depression [1]; thus the development of new analgesics that do not suppress the respiratory drive is urgently needed.
AMPAkines are synthetic compounds that increase the function of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors [5]. Examples of these compounds include 2,6-Difluoro-4-[2-(phenylsulfonylamino)ethylthio]-phenoxyacetamide (PEPA), CX614, CX1739, and CX546. AMPAkines bind to an allosteric site on the AMPA receptors to delay receptor desensitization so as to increase the synaptic currents carried by these receptors. These drugs have also been shown to increase levels of brain-derived neurotrophic factor (BDNF) in areas such as the cortex and hippocampus [6]. BDNF is well-known to play a role in synaptic plasticity, and its resulting increase may lead to some of the effects seen with AMPAkine treatment in the cortex [7–10]. AMPAkines are known to improve memory and cognition [6] and have been studied in a variety of neuropsychiatric diseases [11]. Two recent studies have shown that AMPAkines can relieve acute and chronic pain in several animal models, and the sites of action for these receptors are likely in the brain [12, 13]. Interestingly, AMPAkines are also known to increase the respiratory drive to antagonize hypoventilation in the context of opioids [14]. It is not known, however, if AMPAkines and opioids can provide complementary analgesia. Furthermore, while previous studies have identified the nucleus accumbens as a potential target for AMPAkines [12], other brain regions that are known to produce descending inhibitory control of nociception, such as the prefrontal cortex (PFC), may also emerge as important therapeutic targets.
In this study, we tested the analgesic efficacy of CX546 (Sigma-Aldrich), an AMPAkine which has been studied in a number of preclinical disease models [8, 9, 15–17], alone and in combination with morphine. We also examined the possibility that the PFC is a therapeutic target for such drugs. In our study, we used male Sprague-Dawley rats (Taconic Farms, Albany, NY), which were kept in the Mispro Biotech Services Facility in the Alexandria Center for Life Science, New York, NY, with controlled humidity, room temperature, and 12-hr (6:30 AM to 6:30 PM) light-dark cycle. All procedures in this study were approved by the New York University School of Medicine Institutional Animal Care and Use Committee (IACUC) as consistent with the National Institute of Health (NIH) Guide for the Care and Use of Laboratory Animals. Food and water were available ad libitum. Animals arrived to the animal facility at 250 grams and were given on average 10 days to adjust to the new environment prior to the onset of any experiments.
First, we used a traditional Hargreaves’ test to assess the rats’ threshold to acute thermal pain. During the test, rats were individually placed in clear plastic chambers. After the rats had acclimated to the environment, a mobile infrared heat generator with an aperture of 10 inch diameter (37370-Plantar Test, Ugo Basile, Italy) was aimed at the plantar surface of their hind paws (infrared intensity was 40). Latency to withdrawal to the heat source provided a measure for the threshold of thermal pain [18]. Withdrawals due to locomotion or weight shifting were not counted, and an average of five measurements at 5 minute intervals was calculated for each paw. Trials were started approximately 30 minutes after administration of either the AMPAkine or control. We administered CX546 (10mg/kg) vs Dimethyl sulfoxide (DMSO as control) systemically (via intraperitoneal injection) into the rats, and found that CX546 increased the latency to paw withdrawal, suggesting an increase in pain threshold (unpaired Student’s t test, p=0.0001) (Fig. 1A). The dosage of 10mg/kg was chosen based on previous dose-response experiments, which showed this dose to be able to provide near-maximal analgesia with no observed side effects [12, 13, 19, 20].
Figure 1.
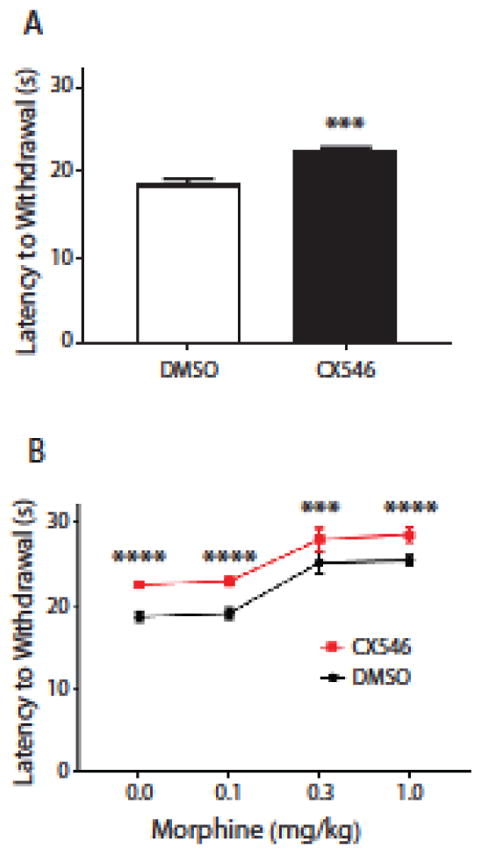
The AMPAkine CX546 produces additional pain relief after morphine administration. A. Systemic (intraperitoneal) delivery of CX546 (10mg/kg), an AMPAkine, increased the latency to paw withdrawals on the Hargreaves’ test. Unpaired Student’s t test. n=6, *** p=0.0001. B. CX546 caused an additional increase in the latency to paw withdrawals after co-administration of morphine. CX546 was administered via the intraperitoneal route, and morphine was administered subcutaneously. At each dose of morphine, compared with DMSO (control), CX546 provided a further increase in pain threshold. Two-way ANOVA with repeated measures and Bonferroni multiple comparison tests. n=6, *** p=0.0002.
Next, we injected morphine subcutaneously at various concentrations. As expected, morphine produced dose-dependent analgesic effects in prolonging the latency to paw withdrawal (Fig. 1B). Specifically, at a concentration of 0.3mg/kg, morphine is able to provide significant analgesia to elevate the threshold to acute thermal pain in rats. We then assessed if CX546 could alter the dose response curve for morphine. We administered IP CX546 or DMSO immediately after morphine injections. We were surprised to find that at each morphine concentration, the AMPAkine was able to produce additional pain relief (two way ANOVA with post-hoc Bonferroni tests, p=0.0002) (Fig. 1B). In fact, the amount of additional analgesic effect produced by CX546 was similar at each morphine concentration, suggesting that this AMPAkine and opioids can produce complementary and additive analgesic effects.
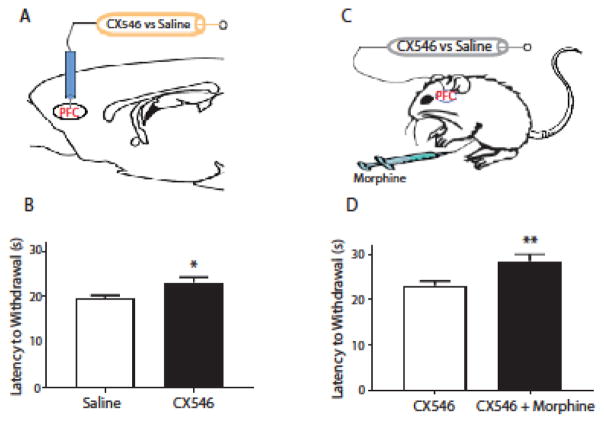
AMPAkines are known to increase excitatory glutamatergic transmissions throughout the brain. The PFC is a key region in the brain that can regulate nociception through its top-down projection to the brain stem [18, 21]. Thus, we tested whether AMPAkine administration directly into the PFC could provide analgesia (Fig. 2A, 3). In rats under anesthesia, we stereotaxically implanted bilateral 26-gauge guide cannulas into the PFC at a 12.5 degree angle with coordinates AP: +2.9 mm; ML: +/−1.6 mm; DV: −2.1 mm (PlasticsOne, Roanoke, VA) [22]. We injected 0.5 μl of either CX546 (800 μM/μl) or saline in the PFC of these rats (using PE-50 tubing attached at one end to 10-μl Hamilton syringes with 33-gauge injector cannula that extended 1.0 mm beyond the implanted guides). Injection volume was delivered bilaterally over a period of 100 sec, and injector cannulas were kept in place for an additional 60 sec prior to removal to allow slow diffusion of this solution. We performed Hargreaves’ tests 20 minutes after intracranial injections. We found that CX546, when compared with saline, prolonged paw withdrawal (unpaired Student’s t test, p=0.0124) (Fig. 2B), suggesting that this AMPAkine can indeed increase synaptic transmission through the PFC to provide pain relief.
Figure 2.
The AMPAkine CX546 can exert its analgesic effects in the prefrontal cortex (PFC). A. Schematic for the experimental design. CX546 or saline was delivered via intracranial cannulas into the prelimbic region of the PFC. Following intracranial drug delivery, rats underwent Hargreaves’ tests. B. Intra-PFC delivery of CX546 caused an increase in the latency to paw withdrawals. Unpaired Student’s t test. n=8, * p=0.0124. C. Schematic for the experimental design. CX546 (800 μM/μl) or saline was delivered via intracranial cannulas into the PFC; at approximately the same time, morphine (1mg/kg) was administered IP. Following intracranial and systemic drug deliveries, rats underwent Hargreaves’ tests. D. Intra-PFC delivery of CX546, compared with saline, caused an additional increase in the latency to paw withdrawals in rats that received morphine injections. Unpaired Student’s t test. n=8, ** p=0.0099.
Figure 3.
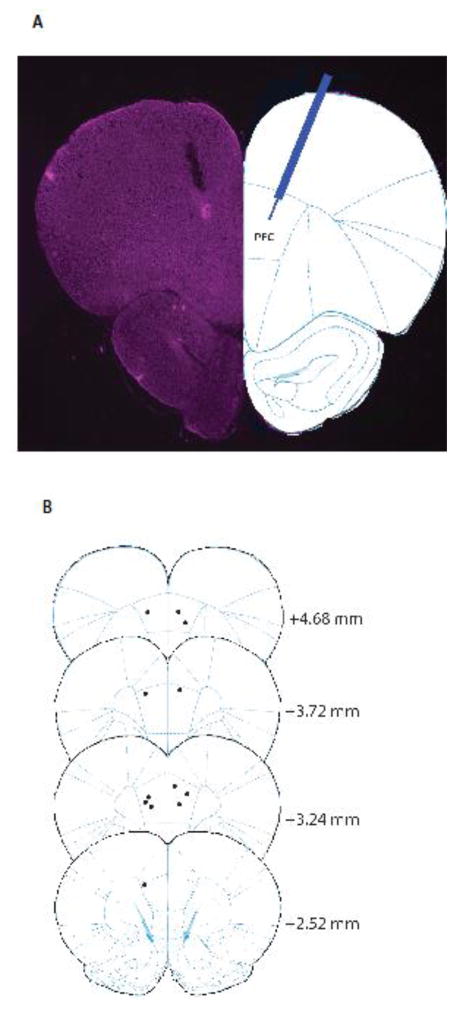
Site of intracranial drug delivery. A. Histology of a brain slice showing the injection site in the PFC. B. Schematic showing the intracranial injection sites.
We next tested if AMPAkine administration into the PFC can provide complementary analgesia when co-administered with morphine. We injected CX546 or saline (control) into the PFC, and simultaneously administered morphine subcutaneously (Fig. 2C). Saline, instead of DMSO, was used as control for intracranial injections to avoid neuronal toxicity. We found that CX546, compared with saline, provided additional pain relief in combination with morphine (unpaired Student’s t test, p=0.0099) (Fig. 2D). These results indicate that this AMPAkine can enhance PFC transmission to provide independent and additive analgesia when co-administered with opioids. Following animal sacrifice, cryogenic brain sections were collected with thickness of 20 μm using Microm HM525 Cryostat to verify cannula localization (Fig. 3).
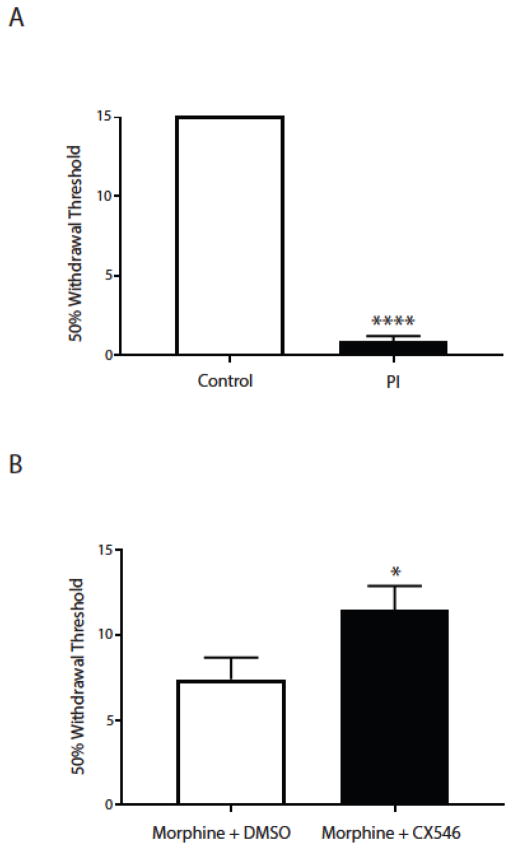
Next, we used a postoperative pain– paw incision or PI - model to verify this synergistic analgesic effect between CX546 and morphine. We made a 1.5 cm longitudinal incision through skin and fascia of the plantar aspect of the hind paw (0.5 cm from the proximal end of the heel extending to the middle of the paw) in rats under anesthesia [23]. We performed mechanical allodynia tests to verify post-incisional pain, using a Dixon up-down method with von Frey filaments (0.45, 0.75, 1.20, 2.55, 4.40, 6.10, 10.50, 15.10 g) on rats in individual plexiglass chambers over a mesh table [24]. 50% withdrawal threshold was calculated to quantify the pain response [23]. As expected, rats experienced acute incisional pain, as manifested by the presence of mechanical allodynia (unpaired Student’s t test, p<0.0001) (Fig. 4A). We administered low-dose morphine (0.2mg/kg) to all rats approximately 3 hours after paw incision, and at the same time administered CX546 (10mg/kg) or DMSO (control) systemically. We performed mechanical allodynia testing approximately 30 minutes after CX546 or DMSO administration. We found that the addition of the AMPAkine significantly reduced symptoms of mechanical allodynia (unpaired Student’s t test, p=0.0479) (Fig. 4B). Thus, co-administration of CX546 and morphine can provide complementary analgesia in this postoperative pain model.
Figure 4.
The AMPAkine CX546 produces additional pain relief after morphine administration in a paw incision (PI) model of acute postoperative pain. A. PI induces mechanical allodynia associated with acute pain. Unpaired Student’s t test. n=8, **** p<0.0001. B. The combination of CX546 and morphine provides greater relief of mechanical allodynia than morphine alone in PI-treated rats. Unpaired Student’s t test. n=8-11, * p=0.0479.
An important finding in our study is that the AMPAkine CX546 can provide additional analgesia when used in combination with morphine. By enhancing synaptic currents in the pre-Botzinger complex in the medulla [5, 25], AMPAkines can stimulate the respiratory drive to treat opioid-induced hypoventilation [14, 26]. Two recent studies have shown that AMPAkines can produce analgesia in acute and chronic pain models in rats [13, 27]. Thus, our study suggests that a combination therapy that includes both opioids and AMPAkines can achieve greater analgesia and a better safety profile.
The PFC is a critical brain region for the top-down control of sensory and affective processes [28]. A number of animal and human fMRI studies have demonstrated its importance in pain regulation [29–31]. These studies together suggest that noxious stimulation can trigger PFC activation, which in turn can provide descending pain inhibition. AMPAkines have state-dependent properties, as they have a high affinity for open but not closed AMPA receptors. Thus, these drugs are more likely to exert their pharmacological effects in neurons that respond to pain [5, 25]. Since PFC neurons are capable of responding to and subsequently decreasing acute pain, it is not surprising that AMPAkines can amplify the analgesic properties of these neurons.
The additive analgesic effects of CX546 and morphine suggest that these drugs may provide pain relief in different neural circuits. Opioids are known to exert analgesic effects primarily through μ receptors, but also κ and δ receptors, which are densely expressed in the superficial dorsal horn of the spinal cord and periaqueductal gray [32, 33]. AMPAkines, in contrast, are not known to confer analgesia in the spinal dorsal horn. In fact, increased AMPA receptor activity in the spinal dorsal horn may increase pain transmission [34]. A previous study has suggested that the nucleus accumbens is a target for AMPAkines, and our current study indicates that the PFC is another target. While opioids can also target the cortex, it is not the primary site of their analgesic action. Thus, it is not surprising that an AMPAkine delivered specifically into the PFC can have parallel, or additive, analgesic effects when administered in combination with morphine. It is likely that AMPAkines and opioids exert their analgesic effects on different sites in the nervous system.
AMPAkines are known to increase cortical and hippocampal levels of BDNF [8–10]. BDNF plays a key role in synaptic plasticity, and its increase may be responsible for some of the analgesic effects seen with AMPAkine treatment in our study. BDNF is also expressed in the spinal dorsal horn, where it can have a pro-nociceptive effect [35–37]. It is possible that there is a balance between pro- and anti-nocicpetive effects mediated by BDNF signaling. Future studies are thus needed to further elucidate the role of BDNF signaling in the molecular and cellular mechanisms of AMPAkines in the context of pain.
A potential side effect of AMPAkines is CNS hyperexcitability [5, 25]. Low-impact AMPAkines (e.g. CX1739) are less likely to result in hyper-excitability, but they may require higher doses to achieve therapeutic effects. High-impact AMPAkines such as CX546 are more potent, but they have a narrower therapeutic window. In our current study, we did not observe any behavioral side effects or evidence of hyperexcitability with a systemic dose of 10mg/kg, and previous studies using this and similar doses have also shown the safety of this dose in rats [12, 13, 19, 20]. Nevertheless, in the future, it will be important to study the analgesic properties of newer low-impact AMPAkines which are more clinically applicable.
In summary, we have shown that the combination of AMPAkine CX546 and morphine can provide complementary analgesia. The PFC is a potential therapeutic target for this AMPAkine in this complementary analgesic system. Given the role for AMPAkines in respiratory stimulation, the combination of AMPAkines and morphine should be able to provide improved pain relief with an enhanced safety profile.
HIGHLIGHTS.
We showed that an AMPAkine and morphine can provide complementary analgesia
We showed that an AMPAkine can provide analgesia by activating glutamate signaling in the prefrontal cortex
We showed that AMPAkine administration in the prefrontal cortex complements systemic morphine analgesia
Acknowledgments
This work was supported by the National Institute of General Medical Sciences (GM102691, GM115384), National Institute of Neurological Disorders and Stroke (NS100065), (Bethesda, MD, USA) and the Anesthesia Research Fund of New York University Department of Anesthesiology (New York, NY, USA).
Footnotes
Conflicts of Interest The authors declare no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dahan A, et al. Pharmacovigilance: a review of opioid-induced respiratory depression in chronic pain patients. Pain Physician. 2013;16(2):E85–94. [PubMed] [Google Scholar]
- 2.Paulozzi LJ, Budnitz DS, Xi Y. Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiol Drug Saf. 2006;15(9):618–27. doi: 10.1002/pds.1276. [DOI] [PubMed] [Google Scholar]
- 3.Ray WA, et al. Prescription of Long-Acting Opioids and Mortality in Patients With Chronic Noncancer Pain. JAMA. 2016;315(22):2415–23. doi: 10.1001/jama.2016.7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudd RA, et al. Increases in Drug and Opioid Overdose Deaths--United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2016;64(50–51):1378–82. doi: 10.15585/mmwr.mm6450a3. [DOI] [PubMed] [Google Scholar]
- 5.Arai AC, Kessler M. Pharmacology of ampakine modulators: from AMPA receptors to synapses and behavior. Curr Drug Targets. 2007;8(5):583–602. doi: 10.2174/138945007780618490. [DOI] [PubMed] [Google Scholar]
- 6.Lynch G, Gall CM. Ampakines and the threefold path to cognitive enhancement. Trends Neurosci. 2006;29(10):554–62. doi: 10.1016/j.tins.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76(2):99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Ogier M, et al. Brain-derived neurotrophic factor expression and respiratory function improve after ampakine treatment in a mouse model of Rett syndrome. J Neurosci. 2007;27(40):10912–7. doi: 10.1523/JNEUROSCI.1869-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lauterborn JC, et al. Positive modulation of AMPA receptors increases neurotrophin expression by hippocampal and cortical neurons. J Neurosci. 2000;20(1):8–21. doi: 10.1523/JNEUROSCI.20-01-00008.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rex CS, et al. Restoration of long-term potentiation in middle-aged hippocampus after induction of brain-derived neurotrophic factor. J Neurophysiol. 2006;96(2):677–85. doi: 10.1152/jn.00336.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skolnick P, Popik P, Trullas R. Glutamate-based antidepressants: 20 years on. Trends Pharmacol Sci. 2009;30(11):563–9. doi: 10.1016/j.tips.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Su C, et al. AMPAkines Target the Nucleus Accumbens to Relieve Postoperative Pain. Anesthesiology. 2016;125(5):1030–1043. doi: 10.1097/ALN.0000000000001336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le AM, LM, Su C, Zou A, Wang J. AMPAkines have novel analgesic properties in rat models of persistent neuropathic and inflammatory pain. Anesthesiology. 2014;121(5):1080–90. doi: 10.1097/ALN.0000000000000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oertel BG, et al. Selective antagonism of opioid-induced ventilatory depression by an ampakine molecule in humans without loss of opioid analgesia. Clin Pharmacol Ther. 2010;87(2):204–11. doi: 10.1038/clpt.2009.194. [DOI] [PubMed] [Google Scholar]
- 15.Nagarajan N, et al. Mechanism and impact of allosteric AMPA receptor modulation by the ampakine CX546. Neuropharmacology. 2001;41(6):650–63. doi: 10.1016/s0028-3908(01)00133-2. [DOI] [PubMed] [Google Scholar]
- 16.Procaccini C, et al. Excessive novelty-induced c-Fos expression and altered neurogenesis in the hippocampus of GluA1 knockout mice. Eur J Neurosci. 2011;33(1):161–74. doi: 10.1111/j.1460-9568.2010.07485.x. [DOI] [PubMed] [Google Scholar]
- 17.Arai AC, et al. Benzamide-type AMPA receptor modulators form two subfamilies with distinct modes of action. J Pharmacol Exp Ther. 2002;303(3):1075–85. doi: 10.1124/jpet.102.040360. [DOI] [PubMed] [Google Scholar]
- 18.Lee M, et al. Activation of corticostriatal circuitry relieves chronic neuropathic pain. J Neurosci. 2015;35(13):5247–59. doi: 10.1523/JNEUROSCI.3494-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Damgaard T, et al. Positive modulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors reverses sub-chronic PCP-induced deficits in the novel object recognition task in rats. Behav Brain Res. 2010;207(1):144–50. doi: 10.1016/j.bbr.2009.09.048. [DOI] [PubMed] [Google Scholar]
- 20.Ren J, et al. Ampakines alleviate respiratory depression in rats. Am J Respir Crit Care Med. 2006;174(12):1384–91. doi: 10.1164/rccm.200606-778OC. [DOI] [PubMed] [Google Scholar]
- 21.Cooper SJ. Anaesthetisation of prefrontal cortex and response to noxious stimulation. Nature. 1975;254(5499):439–40. doi: 10.1038/254439a0. [DOI] [PubMed] [Google Scholar]
- 22.Goffer Y, et al. Calcium-permeable AMPA receptors in the nucleus accumbens regulate depression-like behaviors in the chronic neuropathic pain state. J Neurosci. 2013;33(48):19034–44. doi: 10.1523/JNEUROSCI.2454-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64(3):493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- 24.Chaplan SR, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 25.Lynch G. Glutamate-based therapeutic approaches: ampakines. Curr Opin Pharmacol. 2006;6(1):82–8. doi: 10.1016/j.coph.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Greer JJ, Ren J. Ampakine therapy to counter fentanyl-induced respiratory depression. Respir Physiol Neurobiol. 2009;168(1–2):153–7. doi: 10.1016/j.resp.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Su C, et al. Persistent pain alters AMPA receptor subunit levels in the nucleus accumbens. Mol Brain. 2015;8(1):46. doi: 10.1186/s13041-015-0140-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10(9):1116–24. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji G, Neugebauer V. Pain-related deactivation of medial prefrontal cortical neurons involves mGluR1 and GABA(A) receptors. J Neurophysiol. 2011;106(5):2642–52. doi: 10.1152/jn.00461.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obara I, et al. Nerve injury-induced changes in Homer/glutamate receptor signaling contribute to the development and maintenance of neuropathic pain. Pain. 2013;154(10):1932–45. doi: 10.1016/j.pain.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Apkarian AV, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24(46):10410–5. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tung AS, Yaksh TL. In vivo evidence for multiple opiate receptors mediating analgesia in the rat spinal cord. Brain Res. 1982;247(1):75–83. doi: 10.1016/0006-8993(82)91029-0. [DOI] [PubMed] [Google Scholar]
- 33.Tyers MB. A classification of opiate receptors that mediate antinociception in animals. Br J Pharmacol. 1980;69(3):503–12. doi: 10.1111/j.1476-5381.1980.tb07041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tao YX. Dorsal horn alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking in inflammatory pain. Anesthesiology. 2010;112(5):1259–65. doi: 10.1097/ALN.0b013e3181d3e1ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson SW, et al. Brain-derived neurotrophic factor is an endogenous modulator of nociceptive responses in the spinal cord. Proc Natl Acad Sci U S A. 1999;96(14):7714–8. doi: 10.1073/pnas.96.14.7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kerr BJ, et al. Brain-derived neurotrophic factor modulates nociceptive sensory inputs and NMDA-evoked responses in the rat spinal cord. J Neurosci. 1999;19(12):5138–48. doi: 10.1523/JNEUROSCI.19-12-05138.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garraway SM, Huie JR. Spinal Plasticity and Behavior: BDNF-Induced Neuromodulation in Uninjured and Injured Spinal Cord. Neural Plast. 2016;2016:9857201. doi: 10.1155/2016/9857201. [DOI] [PMC free article] [PubMed] [Google Scholar]