Abstract
OBJECTIVES
The purpose of this systematic literature review is to describe what is known about fragile X syndrome (FXS) and to identify research gaps. The results can be used to help inform future public health research and provide pediatricians with up-to-date information about the implications of the condition for individuals and their families.
METHODS
An electronic literature search was conducted, guided by a variety of key words. The search focused on 4 areas of both clinical and public health importance: (1) the full mutation phenotype, (2) developmental trajectories across the life span, (3) available interventions and treatments, and (4) impact on the family. A total of 661 articles were examined and 203 were included in the review.
RESULTS
The information is presented in the following categories: developmental profile (cognition, language, functional skills, and transition to adulthood), social-emotional profile (cooccurring psychiatric conditions and behavior problems), medical profile (physical features, seizures, sleep, health problems, and physiologic features), treatment and interventions (educational/behavioral, allied health services, and pharmacologic), and impact on the family (family environment and financial impact). Research gaps also are presented.
CONCLUSIONS
The identification and treatment of FXS remains an important public health and clinical concern. The information presented in this article provides a more robust understanding of FXS and the impact of this complex condition for pediatricians. Despite a wealth of information about the condition, much work remains to fully support affected individuals and their families.
Fragile X syndrome (FXS) is the most common single-gene cause of inherited intellectual disability. FXS is caused by an expanded trinucleotide repeat (CGG) on the 5′ untranslated region of the fragile x mental retardation 1 (FMR1) gene. A normal range is between 6 and 44 repeats. Individuals with 45 to 54 repeats are considered to have a gray zone or intermediate expansion. Those with 55 to 200 repeats have the premutation, which is likely to become unstable in future generations. Affected individuals with the full mutation FXS have >200 repeats. In the full mutation, methylation occurs during gestation, silencing FMR1 transcription. 1 This silencing leads to a reduction or absence of fragile X mental retardation protein (FMRP), which is needed for normal brain development. In a small number of males with the full mutation, there are no methylation patterns observed, resulting in residual levels of FMRP, which results in less impaired functioning. In females, FMRP levels are related to the X activation ratio and the amount of FMRP produced. 2 FXS results in cognitive and adaptive limitations that impact everyday function. Given that FXS is inherited, there are numerous implications for families, ranging from carrier issues and testing to family adaptation to the condition.
Policy documents by the American Academy of Pediatrics (AAP) suggest ways to identify and treat individuals with FXS. The Committee on Genetics published a clinical report on the comprehensive genetic evaluation of children with general development delays or intellectual disabilities. 3 The report recommends FXS testing as a first-line test for all boys and girls with general developmental delay or intellectual disability of unknown origin. The AAP also provides clinical guidelines on health supervision of children with FXS, 4 including information on genetic testing, recommended examinations for well-care visits, and anticipatory guidance. However, these guidelines do not provide for treatment of FXS, including the use of medications.
The purpose of this review is to describe the state of the science on FXS and research gaps to help pediatricians support patients with FXS and their families.
METHODS
Search Terms
Four overarching themes of both public health and clinical importance in FXS guided the systematic literature review: (1) epidemiology, (2) impact, (3) health care delivery, and (4) genetics. Of the topics included under these overarching areas, we focused the search on 4 topic areas for this manuscript: (1) the full mutation phenotype, (2) developmental trajectories across the life span, (3) available interventions and treatments, and (4) the impact on the family. Given that there are other reviews of the FXS phenotype, we focused on literature published in the past 6 years, but also included seminal articles outside the search dates. The other areas had more inclusive search dates. Table 1 details the search terms and inclusion/exclusion criteria for all 4 areas.
TABLE 1.
Search Terms and Inclusion/Exclusion Criteria by Topic Area
| Topic Area | Search Terms | Search Dates | Other Search Criteria |
|---|---|---|---|
| Full mutation phenotype | Fragile X syndrome, fragile X–associated disorders, fragile X premutation, fragile X carrier, fragile X–associated tremor/ataxia syndrome, or fragile X–associated primary ovarian insufficiency and phenotype, clinical presentation, clinical description, neurocognitive, cognitive, behavior, social-emotional, or language, communication | 2008–2014 | English language Human United States only |
| Developmental trajectories across the life span | Fragile X syndrome or fragile X and lifespan, developmental, longitudinal, adolescent, adult, services, or transition to adulthood | 1991–2014 | English language Human |
| Available interventions and treatments | Fragile X syndrome or fragile X and treatment, intervention, pharmacological, educational, behavioral, medication, or clinical trial | 1991–2014 | English language Human |
| Impact on family | Fragile X syndrome or fragile X and family adaptation, family impact, family outcomes, burden, or cost of care | 1991–2014 | English language Human United States only |
Abstract Review and Refinement
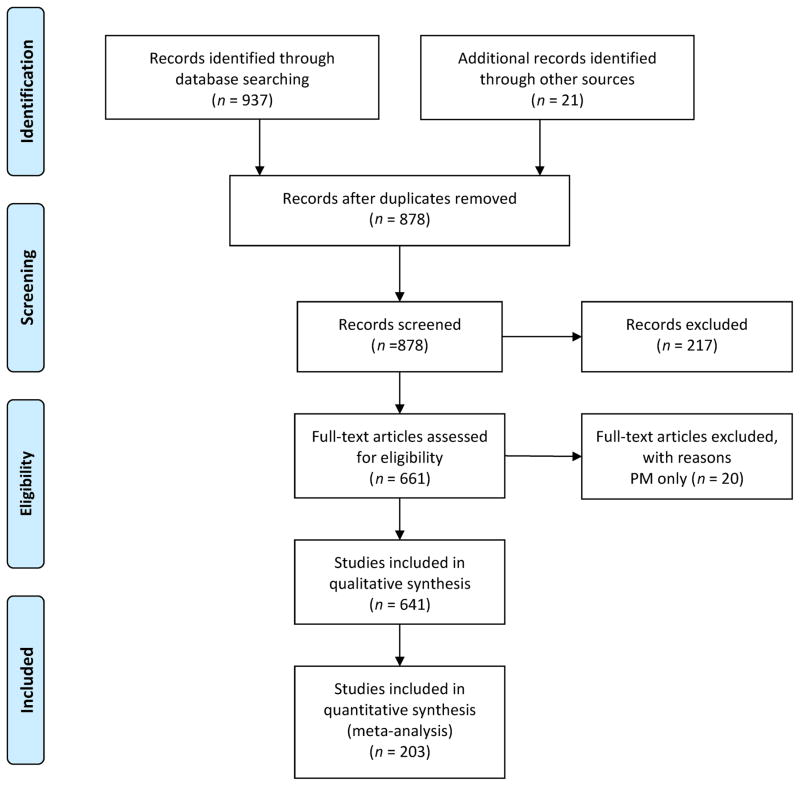
An electronic literature search was conducted by using search terms in PubMed, CINAHL, and Embase. All iterations of the search terms were combined within each topic area. For example, the interventions and treatment topic search combined either “fragile X syndrome,” or “fragile X,” with any 1 of the following: “treatment,” “intervention,” “pharmacological,” “educational,” behavioral,” “medication,” or “clinical trial.” A total of 878 unique citations matched the search criteria (Fig 1). We reviewed titles and abstracts to determine studies appropriate for inclusion. We excluded articles that either did not meet the inclusion criteria (Table 1), had a focus on basic rather than social science, or were not research studies (eg, letters to the editor). These exclusions resulted in a total of 661 articles that were reviewed. Of these, 20 were excluded because the content focused exclusively on the fragile X premutation. In sum, 203 are summarized in this review. The information from the first 2 areas of focus are presented below by profile type: (1) developmental profile, (2) social-emotional profile, and (3) medical profile. The last 2 are summarized in the Treatments and Interventions section and the Impact on the Family section.
FIGURE 1.
Articles included in the public health literature review. (Adapted from Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 6(7):e1000097.)
RESULTS
The results are organized into 5 sections. The Developmental Profile section provides an overview of research on cognitive and academic skills, language ability, functional skills, and the transition to adulthood. The Social-Emotional Profile section details the behavioral phenotype of FXS, including cooccurring conditions and other behavioral problems. The Medical Profile section describes the literature on physical features associated with FXS, seizures, sleep issues, other health issues, and physiologic characteristics. The Treatments and Interventions section summarizes research on behavioral and educational interventions, allied health services, and pharmacologic treatments. Finally, the Impact on the Family section includes research on family adaptation, such as impacts on the family environment and the financial impact of FXS.
Developmental Profile
Cognitive Development and Academic Skills
The neurocognitive profile of individuals with FXS is well described, including several recent reviews. 5–11 Boys typically have moderate to severe intellectual disability, with average IQ scores <55. Other areas of weakness include short-term and working memory, spatial memory, auditory and sequential processing, abstract thinking, executive function, and mathematical thinking. 5–11 Areas of strength for boys include receptive vocabulary, visual memory, simultaneous processing, experiential learning, and imitation.5–11 This profile of cognitive challenges persists across groups of high-, mid-, and low-functioning individuals. 12
Boys exhibit a unique cognitive profile compared with other types of intellectual or developmental disabilities or with typically developing peers. When compared with individuals with Down syndrome, boys with FXS have greater impairments in object discrimination learning, but better performance on object recognition memory and egocentric spatial learning tasks. 13 They also have significant deficits in all areas of executive function (ie, inhibition, working memory, cognitive flexibility, and planning) compared with typically developing peers. 14, 15 Boys with FXS show relatively stable deficits across most developmental domains, whereas boys with autism show more varying ability levels. 16
Girls with FXS may demonstrate similar patterns of strengths and weaknesses to boys, but are often less severely affected. 17 Girls have a higher rate of learning disability in mathematics than grade-matched comparisons. 18 Mathematical tasks requiring a conceptual understanding of numbers or amounts are most challenging for girls, 19 which may be related to delays in spatial location skills. 20
Several longitudinal studies have demonstrated that IQ scores are lower in adolescents and adults with FXS when compared with younger children with FXS. 21–26 Children make steady cognitive growth through early adolescence, at which point mental age plateaus and IQ declines. 27 Older children with FXS have a developmental growth rate 2.2 times slower than their non-FXS siblings. 27 A pattern of cognitive decline holds even when assessed by using nonverbal measures. 28,29 The increasing gap in IQ scores from typical development may be due to the nature of standardized tests (ie, increased importance of symbolic language in adolescence) as opposed to the loss of skills. 30 However, studies examining these variable rates in cognitive decline 30, 31 have suggested that methylation level, delayed frontal lobe maturation, level of FMRP production, comorbid autistic behaviors, and maternal education may be contributing factors. 28,29,32, 33
More recently, other longitudinal studies starting as young as infancy have explored the earliest age of onset of decline. Roberts et al 34 found delays beginning as early as 9 months of age. The rate of development did not change dramatically over time; however, autistic behavior was strongly related to development. Two other studies 35,36 found global delays in boys, with the rate of development being approximately half that of typically developing peers. Across these studies communication and cognition were the most affected domains of functioning.
Language Development
Many areas of language development appear to be affected in FXS, and therefore this area has been studied extensively; recent reviews provide a summary of research findings to date. 37–39
Prelinguistic
Young children with FXS often have delays in attaining early language milestones 40,41 as well as delays in nonverbal communication (ie gestures and reciprocal/symbolic behavior). 42 Children with more autistic behaviors have a negative pattern of development; initial gesture use may be negatively associated with later rates of words used. 43
Receptive
Studies of receptive language have shown mixed results. Some have found receptive vocabulary in boys with FXS to be below that of typically developing peers, even after controlling for nonverbal cognitive ability. 44,45 However, other studies report vocabulary skills on par with mental age–matched peers. 46, 47 Receptive language ability does appear to improve over time.48
Recent work has compared receptive language in individuals with FXS with other groups who have an intellectual or developmental disability. Receptive language is similar between individuals with FXS and those with autism. 49 In another set of studies, girls, but not boys, with FXS had higher receptive syntax scores than those with Down syndrome. 50, 51 Girls with FXS also have been shown to have higher scores on receptive vocabulary than on nonverbal IQ, indicating a relative strength in this area. 52
Expressive
Individuals with FXS outperform individuals with Down syndrome or autism on global measures of expressive language 53 and in general are more talkative and more intelligible. 54 When compared with mental age (MA)-matched peers, individuals with FXS often have equivalent overall expressive language skills 53 and numbers of communication units. 55 A relative strength in phonological memory may be associated with higher expressive language scores.56
Zajac et al 57,58 examined articulation in young boys with FXS compared with typically developing peers. In the first study, boys with FXS did not differ from MA-matched peers with regard to articulation rate. However, boys with comorbid FXS and autism had faster rates than the chronologically matched peers. Another study found that those with FXS, regardless of autism status, performed better than those with Down syndrome and did not differ from typically developing peers on phonological accuracy. 59 On a measure of intelligibility, those with FXS had a lower percentage of words that were understood when compared with MA-matched peers but did not differ from those with Down syndrome.
Syntax also appears to be a challenge for individuals with FXS. Boys with FXS have shorter, less complex utterances than MA-matched peers. 53,57, 60 No differences existed between boys with and without a codiagnosis of autism, but those with FXS did perform better than individuals with Down syndrome. 60,61 Sterling et al 62 found that boys with lower receptive vocabulary scores were more likely to make grammatical tense errors (eg, “She walk home.”) than those with higher receptive vocabulary scores. Nonverbal cognition and phonological working memory are other key factors that play a role in syntactic ability. 63
Pragmatics
Pragmatic development, or the ability to use language in social interactions, is an area of deficit in FXS for both boys and girls. Individuals with FXS engage in fewer conversational turns and ask fewer clarifying questions to continue the conversational topic than typically developing peers. 64, 65 Boys with both FXS and autism also appear to struggle with pragmatics. 61 When narrative language is examined, however, individuals with FXS perform similarly or better than MA-matched peers, suggesting an area of strength. 66,67 Early and sustained levels of maternal responsivity, an interaction style characterized by warmth and sensitivity, appears to play a role in many language outcomes. 68, 69
Functional Skills
Studies on functional skills and adaptive behavior in FXS have been limited. Using a large sample of parents of individuals with FXS, Bailey et al 70 reported on skill attainment in 7 areas: eating, dressing, toileting, bathing/hygiene, communication, articulation, and reading. Most adult males and females were verbal and independently used the toilet, bathed, dressed themselves, and ate. However, skills, such as using complex sentences, reading, or speaking at a typical rate, were weaknesses. Hatton et al 71 showed steady improvements in adaptive behavior over time for children up to age 12 years. Those with fewer autistic behaviors and higher levels of FMRP showed more improvements over time. In adolescents and adults, delays in communication, socialization, and daily living skills for both males and females have been documented. 72 –74 Individuals codiagnosed with autism exhibit the biggest declines in adaptive scores over time. 75–77
Transition to Adulthood
The majority of research on FXS to date has focused on children and adolescents, and therefore information about adults with FXS is limited. A large national survey showed that the majority of adult males (70%) lived with their parents, and even 50% of adult females still lived at home. 78 Many females were working full time (48%) compared with only 20% of males. More males (57%) than females (19%) needed moderate to considerable assistance with everyday life. The strongest predictor of independence was functional skill level (males) and the ability to interact appropriately (females). Adolescents and young adults with more autism symptoms had lower independent living skills, especially managing money, health and safety, and problem solving, relative to typically developing peers even after controlling for IQ. 79
Social-Emotional Profile
Cooccurring Psychiatric Diagnoses
Research on conditions that cooccur with FXS has focused primarily on attention, anxiety, autism, and other behavioral problems. These are now well-described features of the FXS phenotype that have broad implications for treatment and family adaptation.
Attention
In a large national survey of parents, 84% of males and 67% of females with the full mutation had attention problems. 80 When compared with boys with Down syndrome and typically developing peers, boys with FXS had more difficulty with attention, impulsivity and inhibition control. 81,82 A detailed exploration of these difficulties using a touch screen search task revealed several differences between boys with FXS and typically developing peers. Although there were no differences in overall search speed, boys with FXS made more errors. 83 Boys with FXS also show overall lower levels of attention, especially auditory attention, when compared with a MA-matched peers. Using a multimodal approach of visual and auditory stimuli did not improve performance.84 Boys with FXS show slight declines over time in sustained attention and larger declines in response inhibition when compared with their MA-matched peers on both visual and auditory continuous performance tests. 85 Auditory attention was associated with later IQ. 86
Recent work has studied underlying physiologic attention processes in very young children with FXS. Infants and toddlers display lower heart rate variability and shallower heart rate decelerations during attentional tasks when compared with typically developing peers. 87 This finding suggests a hampered capacity to regulate arousal levels and thus attentional behavior. 87 Visual attention was associated with the severity of autistic behaviors. 87
Anxiety
Anxiety is a pervasive concern among providers, caregivers, and individuals living with FXS. 88–90 Early work found high rates of anxiety in girls with FXS compared with typically developing peers and those with other developmental disabilities. 91–93 In a national survey, 70% of boys and 56% of girls were reported to have been treated or diagnosed with anxiety. 80 Other studies have found similarly high rates. 89,94 A recent study described an association between negative affect, a temperament construct, and later anxiety in preschool boys with FXS providing an early diagnostic method.95 A study of brain activity in the prefrontal regions found that social anxiety in FXS may be related to challenges in higher-level social cognition. 96 These findings may also explain other phenotypic traits associated with anxiety in FXS, such as poor eye contact, gaze aversion, excessive shyness, hand flapping, self-injurious behaviors, aggression, and autistic symptoms. 10,88, 97
Autism
FXS is the most common known inherited single-gene condition associated with autism. 98 Individuals with FXS can exhibit several behaviors commonly associated with autism, including difficulties with social communication, self-injurious behavior, perseverative or restricted behavior, motor stereotypies, poor eye contact, and odd or delayed speech. 99,100 Depending on the presentation of symptoms, individuals with FXS can be codiagnosed with autism spectrum disorder (ASD). Reports of comorbidity of ASD diagnoses range from 15% to 52%. 80,101 Additionally, an estimated 90% of boys with FXS exhibit at least 1 autistic behavior. 102
A series of studies suggests that children with FXS without ASD share similar profiles with children diagnosed with developmental delay, whereas children with FXS and comorbid ASD (FXS+ASD) are more similar to children with idiopathic autism. 100, 103,104 Children with FXS+ASD exhibit poorer developmental outcomes, including late-onset language milestones, weaker communication and social skills, lower adaptive behavior scores, greater behavior problems, and greater cognitive impairment than boys with FXS only or those with idiopathic autism. 16,41,105 In addition, individuals with FXS only have profiles of higher receptive than expressive language, whereas those with FXS+ASD do not exhibit this strength. 106, 107
Social impairments may be the most significant predictor differentiating individuals with FXS+ASD from individuals with FXS only. 102
Specifically, social withdrawal (eg, avoidance and indifference) and adaptive socialization behaviors (eg, recognizing emotions or appropriate social interactions) are often independent predictors of ASD in individuals with FXS. 105, 108–110 In cross-sectional 105 and longitudinal studies,101 impaired socialization was the greatest contributor to ASD diagnosis and severity in the FXS population, more so than communication or cognition. 110 Per parent report, however, repetitive and stereotyped behaviors were the strongest predictors of ASD in FXS. 102 Boys with FXS+ASD exhibited significantly less ritualistic and compulsive behavior, but increased repetitive motor behaviors compared with boys with idiopathic autism.111
Few studies have examined the severity of ASD symptoms in individuals with FXS over time, and conflicting evidence exists. Two longitudinal studies showed that behavioral profiles did not change significantly over time, with lower levels of FMRP correlated with more ASD symptoms. 99,112 However, these studies did not use gold standard ASD measures, which is the use of Diagnostic and Statistical Manual of Mental Disorders criteria by an experienced clinician, using the Autism Diagnostic Observation Schedule with or without the Autism Diagnostic Interview-Revised. More recently, the Autism Diagnostic Interview-Revised was used to examine current versus lifetime behaviors, with current scores subtracted from lifetime scores to gauge age-related improvement. By using this method, autism symptoms improved with age, with the least improvement in the Restricted Interests and Repetitive Behaviors domain. 113 In this and 1 other study 114 once IQ was controlled for, levels of FMRP were not related to ASD.
Other Behavioral Problems
Behavior problems, including tactile defensiveness, hand flapping, poor eye contact, hyperactivity, tantrums, perseveration, hyperarousal to sensory stimuli, and impulsivity are hallmark features of FXS. 115–117 Physiologic dysregulation and hyperarousal are common challenges in FXS and have been associated with avoidant behaviors. 48,118, 119 Problem behavior is one of the strongest predictors of negative outcomes for individuals with FXS and their caregivers. 120–123 Many of these behaviors can be considered behavioral markers of other comorbid diagnoses, such as anxiety or autism.
One concerning behavior not well described in the literature is aggression toward others. Survey data indicate that 38% of boys with FXS have been diagnosed or treated for aggression,80 and 24% of boys with FXS were taking medications for anger or aggression. 124 Medication use to treat anger or aggression increased significantly from early childhood through adolescence, remaining relatively constant at around 30% into the adult years. In another survey, 31% of caregivers of boys and 17% of caregivers of girls reported that they had been injured by the child (eg, knocked down or hit) at least once in the previous 12 months. 124 Parents of boys reporting injuries had a mean of 16 per year; on average, 2.7 of those injuries were serious enough to require medical care.
A recent study using parent-reported functions of behavior suggested escape as a primary function of aggression in FXS. 125 However, these reported functions are likely to be highly specific to each child, as suggested by a study of behavioral interventions in 3 children with FXS requiring considerable individualized assessment before intervention development. 126
Self-injury is also common in individuals with FXS, with prevalence rates as high as 79% of boys. 117 Compared with other genetic conditions or intellectual and developmental disability populations, self-injury is generally mild, 127 however, when it occurs, it generally occurs frequently. 117 Approximately one-third of boys exhibit severe self-injury. 117 The most commonly reported self-injury in FXS is biting of hands and fingers.128
Medical Profile
Physical Features
Boys often present with long, narrow faces; high-arched palates; prominent ears; macroorchidism (during and after puberty); hypermobility of joints; hypotonia; and flat feet. 129–131 Other conditions that are found at increased rates include cleft palates and orthopedic abnormalities, such as scoliosis or severe flat feet. 5 Adults with FXS have a shorter average height than that of the general population.132 Girls with FXS have similar physical features to boys with FXS although at lower rates. 133
Seizures
The incidence of epilepsy is between 10% and 20% for boys with FXS, with lower percentages for girls. 134 A national survey of parents found that 14% of boys and 6% of girls had experienced at least 1 seizure, 135 similar to earlier reports. 136,137 The first seizure commonly occurs between 4 and 10 years of age, and the seizures are typically focal or localized.135 Most seizures occur while awake, but approximately one-third of individuals with FXS have seizures when sleeping. 135 A key factor associated with the occurrence of seizures is whether the individual is codiagnosed with autism. 135, 138
Sleep
Over time, providers and caregivers anecdotally reported sleep problems in the FXS population, prompting several studies to explore sleep problems in FXS. 139–141 In a national survey, 32% of individuals with FXS were reported by the caregiver to experience sleep difficulties, with 85% having at least 2 problems (eg, trouble falling asleep and frequent night awakenings). Of those with problems, 47% of boys and 40% of girls were taking at least 1 medication to help with sleep. A study of sleep architecture in FXS revealed less time in bed and less time in REM sleep. 141
Other Medical and Health Problems
Several reviews highlight the medical needs of children with FXS. 4,142, 143 A recent review 143 reported higher rates of several medical conditions, including otitis media, gastrointestinal problems, and ocular disorders. However, little is known about the overall public health needs of individuals with FXS. One recent study on physical activity and obesity suggested the rate of obesity in adults with FXS is similar to the general population (30%).144 Boys with FXS, however, had higher rates of obesity (31%) compared with typically developing, same-aged peers (18%). Neither children nor adults with FXS met the recommended levels of physical activity. Other researchers have documented a Prader-Willi–like phenotype in FXS, which may explain the increased rates of obesity in a subgroup of patients. 145
Physiologic Features
Studies of brain structure using MRI have shown several enlarged regions in the brains of individuals with FXS compared with typically developing controls, including the hippocampus, amygdala, caudate nucleus, and thalamus. 119 These regions are critical in regulating cognitive and behavioral functions, such as memory and learning, information and sensory processing, and social and emotional behavior, all of which are known to be impaired in individuals with FXS. However, the cerebellar vermis and the superior temporal gyrus are smaller than those in typically developing controls. 119 MRIs of young boys with FXS show increased caudate volume when compared with typically developing peers. 146 Amygdala volume in those with FXS, although larger than controls, was not as enlarged as in individuals with ASD. 146 However, other studies have shown no differences in amygdala volume between individuals with FXS and typically developing peers.147 Functional MRIs, which predominantly have been conducted on girls, have shown different neural activity, which may help to explain the cognitive delays in the FXS population. 119, 148
In a review of neuroendocrinology studies,119 individuals with FXS were reported to have impaired hypothalamic functioning due to decreased levels or absence of FMRP. These studies may help explain the abnormal stress responses, sleep abnormalities, and physical growth patterns commonly seen in affected individuals. Other work has examined the relationship between an individual’s genetic expression, brain structure, and behavior. 149,150
Other physiologic features have also been reported. Roberts et al 151 found that the blink rate in boys with FXS was much higher than typically developing peers during passive tasks. The blink rate was correlated with problem behaviors and physiologic arousal, indicating possible underlying pathophysiological differences in dopamine functioning in individuals with FXS. 151 Studies of heart rate activity and its relationship with sensory processing have shown that boys with FXS have increased cardiac reactivity to auditory stimulus compared with typically developing peers. 152 Studies of prepulse inhibition have shown significant deficits in individuals with FXS. 153,154
Miller et al 155 demonstrated enhanced electrodermal reactivity to sensory stimuli in those with FXS, and this reactivity correlated inversely with the level of FMRP. Taken together, these results suggest that individuals with FXS have atypical physiologic characteristics when compared with typically developing peers and that these differences may be related to the behavioral profile of FXS.
Treatments and Interventions
Behavioral Interventions
Anecdotal reports suggest that reducing anxiety or sensory issues may reduce challenging behaviors in some children with FXS. 156 Several case studies suggest the use of behavioral principles, such as positive reinforcenement. 157,158 Behavioral interventions can be effective for children with FXS through the development of individualized multicomponent intervention plans implemented by parents and supported by professionals. 126 Although these studies differ in approach, they suggest that behavioral techniques can be useful for reducing challenging behaviors in children with FXS.
Cognitive/Educational Interventions
Few studies have examined cognitive or educational interventions for individuals with FXS. One interview study of professionals working with young children with FXS 159 reported that learning strategies that incorporate visually based, experiential or holistic learning were most successful. A recent case study 160 found a combination of early pharmacologic treatment combined with intensive educational interventions resulted in improved behavior and normal IQ in 2 young children with FXS. The intensive interventions included cognitive and memory games, visualized math tasks, and supplemental occupational therapy, speech-language therapy, and social skills training.
The use of a self-paced computer program (Discrete Trial Trainer [DTT]) to assess learning of basic math and geography skills by using a match-to-sample teaching procedure has been tested as a possible intervention technique. 161–164 Results showed that basic mathematical relations could be taught and were comparable with those of the control group; however, improved outcomes were not maintained, and more complex concepts (eg, equality and congruence concepts) were not learned at a rate similar to that of the control group. Additional studies using the DTT software suggest that it may be a promising approach for outcome measurement.164, 165
These studies of DTT exemplify a shift toward technological mechanisms for assessing and treating cognitive and learning impairments. Another example is the use of the Cogmed program, which can improve working memory and is being tested with individuals with FXS. 166 These computerized tools have the advantage of providing increased independent learning, are generally highly motivating, and reduce social pressure, which may affect learning in children whose anxiety is increased with social interactions. In addition, computerized protocols may be useful for research design with this population; they theoretically could be implemented anywhere at any time, thereby reducing geographical challenges.
Allied Health Services
Given the high rates of intellectual disability and comorbidities, such as autism, anxiety, and attention-deficit/hyperactivity disorder (ADHD), individuals with FXS have significant needs for specialized therapies and services, including speech-language, occupational, and physical therapies; special education services; and behavior management. In addition, individuals with FXS frequently require specialized medical care, such as consultations with developmental-behavioral pediatricians or neurologists.
Despite the clear need for services for individuals with FXS, the intensity and use of health or therapeutic services has not been well described. Three papers reporting on early intervention services 40,99, 159 all suggest that children with FXS <3 years of age commonly use speech-language, occupational, and physical therapy services. More recently, allied health service use was reported based on findings from a large survey. 160 The use of, intensity of, and parent satisfaction with speech-language, occupational, and physical therapies, as well as behavior management therapy, were examined across sex and age groups. Almost all males and half of females were receiving at least 1 of these therapies at the time of the survey. Clear declines in service use occurred across age groups, with those no longer in school (>20 years of age) receiving few services. Parents were generally satisfied with the amount and quality of services received.
One study examined parent reports of medical services and procedures that their child with FXS received during the previous year. 167 This study found that most boys and girls had at least 1 visit with their primary care physician, with an average of 2.5 visits for boys and 2.1 visits for girls in the year. Although there were few emergency department visits or inpatient care, almost all individuals with FXS required some medical specialist care (97% of boys, 95% of girls). The most common specialists seen were ophthalmologists, psychiatrists, developmental-behavioral pediatricians, and neurologists. The majority of both boys and girls were taking at least 1 prescription medication for FXS-related problem.
Pharmacologic Treatments
Pharmacotherapy is frequently used as a primary intervention to target specific symptoms for individuals with FXS. Guidelines for the suggested treatment of neuropsychiatric symptoms of FXS have been published suggesting that stimulants or selective serotonin reuptake inhibitors (SSRIs) typically work to decrease hyperactivity, cognitive deficits, and psychiatric symptoms. 115,168 A recent article reviews the treatment strategies found to be most effective for specific symptoms. 169
Anxiety Symptoms
SSRIs are the most common first-line treatment of anxiety in FXS, which is the most common reason parents seek medication treatment. 167 Case reports and survey studies suggest SSRIs are effective ~50% of the time at reducing anxiety in FXS. 170– 172 No controlled studies on the efficacy of SSRIs for reducing anxiety in FXS have been conducted. In addition, side effects, including weight loss or gain, and behavioral activation for those on SSRIs, specifically fluoxetine, have been reported.115, 172
Attention-Deficit/Hyperactivity Disorder Symptoms
Impulsivity and hyperactivity are 2 common challenges for individuals with FXS, and stimulant medications are the most common type of medication prescribed to individuals with FXS. 173 However, only 1 controlled trial conducted years ago has been carried out demonstrating efficacy. 133 In addition, stimulant use for children <5 years old can often cause irritability and, according to the AAP, is not the recommended treatment for children ≤5 years of age. 115,174 Typical doses have been found to be effective at reducing impulsivity and hyperactivity and increasing attention in a controlled trial of boys with FXS.175 Second-line treatments, recommended for those who do not respond to stimulants, include α-adrenergic receptor agonists (eg, clonidine and guanfacine). 115 Although surveys and case studies suggest clonidine can be effective 171,176 and clinical experience suggests guanfacine 115 can be helpful, no controlled trials have examined the efficacy of these medications for ADHD symptoms in FXS specifically. Two controlled studies of L-acetylcarnitine177,178 suggest some reduction of ADHD symptoms in FXS compared with placebo. More recently, valproic acid, the antiepileptic drug, was found to reduce ADHD symptoms in an open-label trial. 179
Aggression and Mood Symptoms
Aggression and irritability are behaviors of great concern to parents of individuals with FXS and are often the primary outcome of clinical trials. 169 Antipsychotic medications have been the first-line treatment of these behaviors and are effective at reducing irritability, aggression, mood instability, and perseverative behaviors in individuals with FXS. 115 In survey studies, both risperidone and aripiprazole have been reported to be effective at reducing aggression and self-injurious behaviors in FXS. 170,171 In a recent pilot, open-label study of aripiprazole, 180 10 out of 12 participants showed improvement in irritability, hyperactivity, and social behavior. Risperidone has a positive effect on irritability symptoms in individuals with autism compared with placebo. 181 However, no controlled trials of antipsychotics have been completed with individuals with FXS.
Sleep
Sleep issues are a significant concern for families of a subsample of individuals with FXS. 140 So far, melatonin is the only treatment that has been studied for individuals with FXS, with findings suggesting efficacy for increased sleep onset and duration compared with placebo. 182
Next Generation Treatments
Recent discoveries in the pathophysiology of FXS have led to excitement in the field about the development of possible therapeutic agents. Multiple reviews summarize these discoveries and the preclinical and clinical trials that are under way to examine the efficacy of these treatments. 183–187
The metabotropic glutamate receptor (mGluR) theory of FXS, first formulated by Bear et al, 188 suggests that the absence of FMRP in FXS leads to enhanced glutamatergic signaling via mGluR5, which subsequently results in increased protein synthesis and defects in synaptic plasticity. The resulting weakening of the synapse and increased number of longer immature dendritic spines is thought to explain the intellectual disability found in FXS. 130 Based on this theory, there has been a heavy focus on developing and testing agents that target mGluR5 modulation. 189–193
Based on promising findings in FMR1 knockout mice,184 several agents have been or are currently being tested in human clinical trials. These agents include the following, which are in clinical development in open-label phase II or higher clinical trials 183: (1) glutamatergics (ie, mavoglurant/AFQ056, RG7090/RO4917523, STX107, and fenobam/NPL-2009), (2) GABAergics (ie, arbaclofen, ganaxolone, and acamprosate), (3) atypical antipsychotics (ie, aripiprazole), (4) antidepressants/anxiolytics (ie, sertraline), (5) mood stabilizers (ie, lithium), and (6) antibiotics (ie, minocycline). Trials of mavoglurant (AFQ056), RG7090/RO4917523, and STX107 were proven ineffective in comparison with placebo and have been terminated. In addition, given the heterogeneity of the FXS phenotype, debate is ongoing regarding the most appropriate target patients and outcome measures that will allow for valid efficacy results.
Minocycline is a targeted treatment in FXS that lowers matrix metalloproteinase 9, an important protein for synaptic development that is elevated in FXS. A controlled trial of minocycline demonstrated efficacy in young children with improvements in behavior and moodiness. 194 Side-effects included graying of the permanent teeth, the rare occurrence of swollen joints, rash, or a lupus-like syndrome that resolves once the minocycline is discontinued.
In a retrospective study of young children on low-dose sertraline, a commonly used SSRI, those treated demonstrated improvements on both receptive and expressive language development compared with controls. 160 Sertraline stimulates neurogenesis in addition to increasing brain-derived neurotrophic factor, which can improve connectivity in the developing central nervous system. The current thinking is that low-dose sertraline may also improve anxiety and sensory hyperarousal in children <5 years of age.
Impact on the Family
Family Environment
The stress of raising a child with FXS on carrier mothers may have an impact on the family unit as well as the maternal-child relationship. Maternal depression, anxiety, and stress are related to both marital satisfaction and family cohesion in families affected by FXS. 120,195, 196 Family cohesion was slightly higher than previously reported levels of families of individuals with developmental disabilities or ASD. 195 Research on the family environment has also examined molar levels of maternal responsivity (ie, overall interaction styles), including levels of warmth, positive affect, expressed emotion, and criticism. Mothers of children with FXS display high levels of warmth and positive affect with little to no negative behaviors exhibited.197,198 These behaviors appear to be related to many child variables, as observed in unaffected populations as well, such as a child’s rate of communication 199 or developmental level. 200 Child age and frustration or help-seeking behaviors also predicted maternal encouraging/responsive behaviors. 201 Mothers of younger children and those whose children had more behavior problems had higher levels of criticism of their children.197
Financial Impact
Results of a national survey indicated that approximately half of all families experienced at least some financial burden, with almost 60% stating that someone in the family had to change work hours or stop work as a result of having a child with FXS. 202 Family (ie, not having adequate health insurance or having multiple affected sons) and child (ie, higher numbers of cooccurring conditions) factors were associated with financial burden and employment. The median out-of-pocket expenditures for families were $1900 per year, which included expenses for transportation (31%), therapy (31%), medications or other medical needs (25%), supervision (6%), recreation (4%), or other needs (3%). 202 A second survey examined the amount of paid and unpaid time spent in providing care to individuals with FXS. 124 On average, parents spent 9.2 hours per day in care or support for sons and 4.8 hours per day for daughters. The average boy with FXS also received 5.5 hours per day of paid support, and the average girl received 1.9 hours. Caregivers also had to take an average of 19.4 hours off from work each month to care for their child’s needs. In a study comparing survey responses by parents of children with FXS to those with ASD only, intellectual disability only, and ASD and intellectual disbaility, 203 a higher percentage of caregivers of children with FXS reported a negative financial and employment impact than caregivers of children with ASD only or intellectual disability only. The negative financial and employment impact of FXS was comparable to that of caring for children with ASD and intellectual disability. Greater financial and employment impact were associated with increased anxiety, seizures, irritability, and reduced thinking, reasoning, or learning ability regardless of condition.
DISCUSSION
Gaps in the Research Literature
Despite extensive literature on FXS, there are gaps that need to be addressed. Below we outline key remaining questions, which can be used to shape future research.
Are there subtypes of the FXS phenotype? There is strong evidence for the impact of FXS on cognitive and language development. Less is known about behavior problems, self-injury, and sleep issues. Research has shown differences in cognitive and behavioral profiles for individuals with FXS only and those with FXS+ASD. However, little information exists about other phenotypic subgroups within FXS. Do certain cooccurring conditions form clusters or profiles? Are there biomarkers that correlate with certain subgroups? Answering these questions is critical to additional understanding the FXS phenotype.
What are the needs of young adults, middle-aged adults, and seniors living with FXS? The vast majority of research on FXS has been conducted with children and adolescents. Longitudinal studies of age-related decline in adolescence in cognition and adaptive behavior have shown mixed results. Moreover, a large evidence base is emerging on the premutation phenotype in older adults, specifically the impact of fragile X–associated tremor-ataxia syndrome and fragile X–associated primary ovarian insufficiency. However, much less is known about FXS across the life span; in particular, studies on young adults, middle-aged adults, and seniors are lacking. Although some studies include individuals with FXS in this age range, they often focus on non–age-related topics (eg, language abilities and autism symptoms). Little is known about the transition out of school, employment opportunities, daily living and functional skills of adults, caregiving demands on families who provide assistance to adult children who are living at home, and guardianship issues. Moreover, studies examining the health and social needs of these individuals are needed.
What are the greatest public health needs of individuals with FXS? The physical features and medical problems associated with FXS have been well documented. However, little research has been conducted on public health issues related to the fragile X population, including areas such as health disparities, health literacy, access to preventative health care, health promotion activities, and health care decision-making. The communication of important health-related information to individuals with FXS is also understudied. Finally, little is known about the prevalence of noncommunicable diseases among individuals with FXS and how it compares to other intellectual and developmental disability groups as well as the general population.
What educational, behavioral, and pharmacologic treatments offer the most promise for individuals with FXS? There is a shortage of evidence regarding service use, intensity, or efficacy for school-age and preschool children with FXS, including special education eligibility and the types of educational services most commonly provided. More detailed descriptive information would lead to the development of targeted treatment options. The ability to conduct a high-quality intervention study targeting specific behaviors or learning issues in FXS is challenging given the wide range of locations of individuals with FXS. However, promising interventions targeting similar issues in populations that share symptomology (eg, ASD), as well as increasing use of technological options, suggest some directions for future intervention research. In addition, little is known regarding the efficacy of treatments. Pharmacological treatments for symptoms have been reported to be beneficial for reducing some of the core features of FXS, including impulsivity, hyperactivity, anxiety, and irritability. However, few randomized controlled trials have been conducted to prove efficacy of these medications in the FXS population. Recent work has focused on pharmacologic treatments that target the core mechanism in FXS. Much work remains not only for basic scientists but also for social scientists before FXS-specific medications are commonplace. Finally, there is a need to examine the use of medications and/or behavioral interventions in the first year of life. The efficacy of earlier treatment will need to be described if large-scale screening, like newborn screening, is to be considered. Most importantly, it has been hypothesized that the benefit of combining educational interventions with targeted psychopharmacologic interventions may prove to be more efficacious. Research on the combination of these interventions is needed.
What are the core risk and protective factors for families of individuals with FXS? What are the combinations of factors that lead to positive or negative adaptation in families of individuals with FXS? Although the field has begun to explore the economic burden of behavioral/educational and medical services, it is not well characterized for a representational sample of families affected by FXS. In addition, the cost associated with FXS has not been studied relative to other conditions. Finally, the cumulative health and financial consequences of multiple family members being affected by FMR1 mutations have not been studied. Although mental and physical health in carrier mothers is fairly well documented, it is difficult to tease apart variance caused by parenting a child with FXS versus premutation carrier status. In addition, factors such as family cohesion and marital satisfaction have not been well studied and could provide more information on possible mediating variables that may contribute to or lessen the impact on maternal outcomes. Likewise, knowledge about how FXS affects fathers and siblings, both carriers and noncarriers, is lacking. Finally, no research has been conducted on family-focused interventions that aim to improve both family and child functioning.
Implications for Pediatricians
The identification and treatment of FXS remains an important concern for pediatricians. In addition to the AAP clinical reports and recommendations already referenced, pediatricians can find clinical practice recommendations for individuals with FXS and their families developed by the Fragile X Clinical and Research Consortium (www.fragilex.org/treatment-intervention/consensus-on-clinical-practices/). The consensus documents are based on expert opinions of fragile X researchers and clinicians. The documents cover a variety of topics, such as educational guidelines, the diagnosis and treatment of associated medical conditions, and information about genetic testing. This review of the research as well as the consensus documents and AAP publications provide a wealth of information for pediatricians to help better understand and treat FXS and its associated conditions. Pediatricians can help coordinate care/therapies, provide psychopharmacologic interventions, and identify those with FXS by ordering a fragile X DNA test. In addition, pediatricians may be called on to support the larger family system, because a diagnosis of 1 individual can impact many other family members. 204 These basics are reviewed in this article, although if the pediatrician feels uncomfortable regarding these points, they can refer to a specialist, such as a developmental-behavioral pediatrician or psychiatrist, for psychopharmacologic intervention or a genetic counselor to discuss extended family involvement and reproductive options.
Acknowledgments
We thank Don Bailey, Randi Hagerman, and Julie Bolen for reviewing earlier drafts of the manuscript.
FUNDING: This work was supported in part by Centers for Disease Control and Prevention (CDC) contract 200-2007-22644-0013. The findings and conclusions in this publication are those of the authors and do not necessarily represent the views of CDC.
ABBREVIATIONS
- AAP
American Academy of Pediatrics
- ADHD
attention-deficit/hyperactivity disorder
- ASD
autism spectrum disorder
- DTT
Discrete Trial Trainer
- FMR1
fragile X mental retardation 1
- FMRP
fragile X mental retardation protein
- FXS
fragile X syndrome
- FXS+ASD
fragile X syndrome with comorbid autism spectrum disorder
- MA
mental age
- mGluR
metabotropic glutamate receptor
- SSRI
selective serotonin reuptake inhibitors
Footnotes
Drs Raspa and Wheeler conceptualized and designed the study, conducted the literature review, drafted the initial manuscript; Dr Riley conceptualized and designed the study and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: Dr Raspa received funds from the John Merck Fund for travel to a meeting co-sponsored with the Centers for Disease Control and Prevention; the other authors have indicated they have no financial relationships relevant to this article to disclose
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Malter HE, Iber JC, Willemsen R, et al. Characterization of the full fragile X syndrome mutation in fetal gametes. Nat Genet. 1997;15(2):165–169. doi: 10.1038/ng0297-165. [DOI] [PubMed] [Google Scholar]
- 2.Loesch DZ, Huggins RM, Hagerman RJ. Phenotypic variation and FMRP levels in fragile X. Ment Retard Dev Disabil Res Rev. 2004;10(1):31–41. doi: 10.1002/mrdd.20006. [DOI] [PubMed] [Google Scholar]
- 3.Moeschler JB, Shevell M Committee on Genetics. Comprehensive evaluation of the child with intellectual disability or global developmental delays. Pediatrics. 2014;134(3) doi: 10.1542/peds.2014-1839. Available at: www.pediatrics.org/cgi/content/full/134/3/e903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hersh JH, Saul RA Committee on Genetics. Health supervision for children with fragile X syndrome. Pediatrics. 2011;127(5):994–1006. doi: 10.1542/peds.2010-3500. [DOI] [PubMed] [Google Scholar]
- 5.Berry-Kravis E, Grossman AW, Crnic LS, Greenough WT. Understanding fragile X syndrome. Curr Paediatr. 2002;12(4):316–324. [Google Scholar]
- 6.Cornish K, Turk J, Hagerman R. The fragile X continuum: new advances and perspectives. J Intellect Disabil Res. 2008;52(pt 6):469–482. doi: 10.1111/j.1365-2788.2008.01056.x. [DOI] [PubMed] [Google Scholar]
- 7.Huddleston LB, Visootsak J, Sherman SL. Cognitive aspects of fragile X syndrome. Wiley Interdiscip Rev Cogn Sci. 2014;5(4):501–508. doi: 10.1002/wcs.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacquemont S, Hagerman RJ, Hagerman PJ, Leehey MA. Fragile-X syndrome and fragile X-associated tremor/ataxia syndrome: two faces of FMR1. Lancet Neurol. 2007;6(1):45–55. doi: 10.1016/S1474-4422(06)70676-7. [DOI] [PubMed] [Google Scholar]
- 9.Mazzocco MM. Advances in research on the fragile X syndrome. Ment Retard Dev Disabil Res Rev. 2000;6(2):96–106. doi: 10.1002/1098-2779(2000)6:2<96::AID-MRDD3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 10.Tranfaglia MR. The psychiatric presentation of fragile x: evolution of the diagnosis and treatment of the psychiatric comorbidities of fragile X syndrome. Dev Neurosci. 2011;33(5):337–348. doi: 10.1159/000329421. [DOI] [PubMed] [Google Scholar]
- 11.Turk J. Fragile X syndrome: lifespan developmental implications for those without as well as with intellectual disability. Curr Opin Psychiatry. 2011;24(5):387–397. doi: 10.1097/YCO.0b013e328349bb77. [DOI] [PubMed] [Google Scholar]
- 12.van der Molen MJ, Huizinga M, Huizenga HM, et al. Profiling Fragile X Syndrome in males: strengths and weaknesses in cognitive abilities. Res Dev Disabil. 2010;31(2):426–439. doi: 10.1016/j.ridd.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Kogan CS, Boutet I, Cornish K, et al. A comparative neuropsychological test battery differentiates cognitive signatures of Fragile X and Down syndrome. J Intellect Disabil Res. 2009;53(2):125–142. doi: 10.1111/j.1365-2788.2008.01135.x. [DOI] [PubMed] [Google Scholar]
- 14.Hooper SR, Hatton D, Sideris J, et al. Executive functions in young males with fragile X syndrome in comparison to mental age-matched controls: baseline findings from a longitudinal study. Neuropsychology. 2008;22(1):36–47. doi: 10.1037/0894-4105.22.1.36. [DOI] [PubMed] [Google Scholar]
- 15.Lanfranchi S, Cornoldi C, Drigo S, Vianello R. Working memory in individuals with fragile X syndrome. Child Neuropsychol. 2009;15(2):105–119. doi: 10.1080/09297040802112564. [DOI] [PubMed] [Google Scholar]
- 16.Bailey DB, Jr, Hatton DD, Mesibov G, Ament N, Skinner M. Early development, temperament, and functional impairment in autism and fragile X syndrome. J Autism Dev Disord. 2000;30(1):49–59. doi: 10.1023/a:1005412111706. [DOI] [PubMed] [Google Scholar]
- 17.Keysor CS, Mazzocco MM. A developmental approach to understanding Fragile X syndrome in females. Microsc Res Tech. 2002;57(3):179–186. doi: 10.1002/jemt.10070. [DOI] [PubMed] [Google Scholar]
- 18.Murphy MM, Mazzocco MM, Gerner G, Henry AE. Mathematics learning disability in girls with Turner syndrome or fragile X syndrome. Brain Cogn. 2006;61(2):195–210. doi: 10.1016/j.bandc.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 19.Murphy MM, Mazzocco MM. Rote numeric skills may mask underlying mathematical disabilities in girls with fragile x syndrome. Dev Neuropsychol. 2008;33(3):345–364. doi: 10.1080/87565640801982429. [DOI] [PubMed] [Google Scholar]
- 20.Mazzocco MM, Singh Bhatia N, Lesniak-Karpiak K. Visuospatial skills and their association with math performance in girls with fragile X or Turner syndrome. Child Neuropsychol. 2006;12(2):87–110. doi: 10.1080/09297040500266951. [DOI] [PubMed] [Google Scholar]
- 21.Borghgraef M, Fryns JP, Dielkens A, Pyck K, van den Berghe H. Fragile (X) syndrome: a study of the psychological profile in 23 prepubertal patients. Clin Genet. 1987;32(3):179–186. doi: 10.1111/j.1399-0004.1987.tb03351.x. [DOI] [PubMed] [Google Scholar]
- 22.Fisch GS, Arinami T, Froster-Iskenius U, et al. Relationship between age and IQ among fragile X males: a multicenter study. Am J Med Genet. 1991;38(2–3):481–487. doi: 10.1002/ajmg.1320380268. [DOI] [PubMed] [Google Scholar]
- 23.Fisch GS, Carpenter N, Howard-Peebles PN, Holden JJ, Tarleton J, Simensen R. The course of cognitive-behavioral development in children with the FMR1 mutation, Williams-Beuren syndrome, and neurofibromatosis type 1: the effect of gender. Am J Med Genet A. 2010;152A(6):1498–1509. doi: 10.1002/ajmg.a.33412. [DOI] [PubMed] [Google Scholar]
- 24.Lachiewicz AM, Gullion CM, Spiridigliozzi GA, Aylsworth AS. Declining IQs of young males with the fragile X syndrome. Am J Ment Retard. 1987;92(3):272–278. [PubMed] [Google Scholar]
- 25.Borghgraef M, Swillen A, van den Berghe H, Fryns JP. Fragile X boys: evolution of the mental age in childhood. Preliminary data on 10 prepubertal boys. Genet Couns. 1995;6(2):97–101. [PubMed] [Google Scholar]
- 26.Dykens EM, Hodapp RM, Ort S, Finucane B, Shapiro LR, Leckman JF. The trajectory of cognitive development in males with fragile X syndrome. J Am Acad Child Adolesc Psychiatry. 1989;28(3):422–426. doi: 10.1097/00004583-198905000-00020. [DOI] [PubMed] [Google Scholar]
- 27.Hall SS, Burns DD, Lightbody AA, Reiss AL. Longitudinal changes in intellectual development in children with Fragile X syndrome. J Abnorm Child Psychol. 2008;36(6):927–939. doi: 10.1007/s10802-008-9223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kover ST, Pierpont EI, Kim JS, Brown WT, Abbeduto L. A neurodevelopmental perspective on the acquisition of nonverbal cognitive skills in adolescents with fragile X syndrome. Dev Neuropsychol. 2013;38(7):445–460. doi: 10.1080/87565641.2013.820305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skinner M, Hooper S, Hatton DD, et al. Mapping nonverbal IQ in young boys with fragile X syndrome. Am J Med Genet A. 2005;132A(1):25–32. doi: 10.1002/ajmg.a.30353. [DOI] [PubMed] [Google Scholar]
- 30.Hagerman RJ, Schreiner RA, Kemper MB, Wittenberger MD, Zahn B, Habicht K. Longitudinal IQ changes in fragile X males. Am J Med Genet. 1989;33(4):513–518. doi: 10.1002/ajmg.1320330422. [DOI] [PubMed] [Google Scholar]
- 31.Curfs LM, Schreppers-Tijdink G, Wiegers A, Borghgraef M, Fryns JP. Intelligence and cognitive profile in the fra(X) syndrome: a longitudinal study in 18 fra(X) boys. J Med Genet. 1989;26(7):443–446. doi: 10.1136/jmg.26.7.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright-Talamante C, Cheema A, Riddle JE, Luckey DW, Taylor AK, Hagerman RJ. A controlled study of longitudinal IQ changes in females and males with fragile X syndrome. Am J Med Genet. 1996;64(2):350–355. doi: 10.1002/(SICI)1096-8628(19960809)64:2<350::AID-AJMG23>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 33.Bray S, Hirt M, Jo B, et al. Aberrant frontal lobe maturation in adolescents with fragile X syndrome is related to delayed cognitive maturation. Biol Psychiatry. 2011;70(9):852–858. doi: 10.1016/j.biopsych.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts JE, Mankowski JB, Sideris J, et al. Trajectories and predictors of the development of very young boys with fragile X syndrome. J Pediatr Psychol. 2009;34(8):827–836. doi: 10.1093/jpepsy/jsn129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bailey DB, Jr, Hatton DD, Skinner M. Early developmental trajectories of males with fragile X syndrome. Am J Ment Retard. 1998;103(1):29–39. doi: 10.1352/0895-8017(1998)103<0029:EDTOMW>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 36.Roberts JE, Hatton DD, Bailey DB. Development and behavior of male toddlers with fragile X syndrome. J Early Interv. 2001;24(3):207–223. [Google Scholar]
- 37.Abbeduto L, Brady N, Kover ST. Language development and fragile X syndrome: profiles, syndrome-specificity, and within-syndrome differences. Ment Retard Dev Disabil Res Rev. 2007;13(1):36–46. doi: 10.1002/mrdd.20142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fidler DJ, Philofsky A, Hepburn SL. Language phenotypes and intervention planning: bridging research and practice. Ment Retard Dev Disabil Res Rev. 2007;13(1):47–57. doi: 10.1002/mrdd.20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finestack LH, Richmond EK, Abbeduto L. Language development in individuals with fragile X syndrome. Top Lang Disord. 2009;29(2):133–148. doi: 10.1097/tld.0b013e3181a72016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brady N, Skinner D, Roberts J, Hennon E. Communication in young children with fragile X syndrome: a qualitative study of mothers’ perspectives. Am J Speech Lang Pathol. 2006;15(4):353–364. doi: 10.1044/1058-0360(2006/033). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hinton R, Budimirovic DB, Marschik PB, et al. Parental reports on early language and motor milestones in fragile X syndrome with and without autism spectrum disorders. Dev Neurorehabil. 2013;16(1):58–66. doi: 10.3109/17518423.2012.704414. [DOI] [PubMed] [Google Scholar]
- 42.Roberts JE, Mirrett P, Anderson K, Burchinal M, Neebe E. Early communication, symbolic behavior, and social profiles of young males with fragile X syndrome. Am J Speech Lang Pathol. 2002;11(3):295–304. [Google Scholar]
- 43.Flenthrope JL, Brady NC. Relationships between early gestures and later language in children with fragile X syndrome. Am J Speech Lang Pathol. 2010;19(2):135–142. doi: 10.1044/1058-0360(2009/09-0018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Price J, Roberts J, Vandergrift N, Martin G. Language comprehension in boys with fragile X syndrome and boys with Down syndrome. J Intellect Disabil Res. 2007;51(Pt 4):318–326. doi: 10.1111/j.1365-2788.2006.00881.x. [DOI] [PubMed] [Google Scholar]
- 45.Abbeduto L, Murphy MM, Kover ST, et al. Signaling noncomprehension of language: a comparison of fragile X syndrome and Down syndrome. Am J Ment Retard. 2008;113(3):214–230. doi: 10.1352/0895-8017(2008)113[214:SNOLAC]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abbeduto L, Murphy MM, Cawthon SW, et al. Receptive language skills of adolescents and young adults with down or fragile X syndrome. Am J Ment Retard. 2003;108(3):149–160. doi: 10.1352/0895-8017(2003)108<0149:RLSOAA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 47.Roberts J, Price J, Barnes E, et al. Receptive vocabulary, expressive vocabulary, and speech production of boys with fragile X syndrome in comparison to boys with down syndrome. Am J Ment Retard. 2007;112(3):177–193. doi: 10.1352/0895-8017(2007)112[177:RVEVAS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 48.Roberts JE, Mirrett P, Burchinal M. Receptive and expressive communication development of young males with fragile X syndrome. Am J Ment Retard. 2001;106(3):216–230. doi: 10.1352/0895-8017(2001)106<0216:RAECDO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 49.McDuffie A, Kover S, Abbeduto L, Lewis P, Brown T. Profiles of receptive and expressive language abilities in boys with comorbid fragile X syndrome and autism. Am J Intellect Dev Disabil. 2012;117(1):18–32. doi: 10.1352/1944-7558-117.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Finestack LH, Sterling AM, Abbeduto L. Discriminating Down syndrome and fragile X syndrome based on language ability. J Child Lang. 2013;40(1):244–265. doi: 10.1017/S0305000912000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oakes A, Kover ST, Abbeduto L. Language comprehension profiles of young adolescents with fragile X syndrome. Am J Speech Lang Pathol. 2013;22(4):615–626. doi: 10.1044/1058-0360(2013/12-0109). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sterling A, Abbeduto L. Language development in school-age girls with fragile X syndrome. J Intellect Disabil Res. 2012;56(10):974–983. doi: 10.1111/j.1365-2788.2012.01578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Finestack LH, Abbeduto L. Expressive language profiles of verbally expressive adolescents and young adults with Down syndrome or fragile X syndrome. J Speech Lang Hear Res. 2010;53(5):1334–1348. doi: 10.1044/1092-4388(2010/09-0125). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kover ST, Abbeduto L. Expressive language in male adolescents with fragile X syndrome with and without comorbid autism. J Intellect Disabil Res. 2010;54(3):246–265. doi: 10.1111/j.1365-2788.2010.01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kover ST, McDuffie A, Abbeduto L, Brown WT. Effects of sampling context on spontaneous expressive language in males with fragile X syndrome or Down syndrome. J Speech Lang Hear Res. 2012;55(4):1022–1038. doi: 10.1044/1092-4388(2011/11-0075). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pierpont EI, Richmond EK, Abbeduto L, Kover ST, Brown WT. Contributions of phonological and verbal working memory to language development in adolescents with fragile X syndrome. J Neurodev Disord. 2011;3(4):335–347. doi: 10.1007/s11689-011-9095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zajac DJ, Roberts JE, Hennon EA, Harris AA, Barnes EF, Misenheimer J. Articulation rate and vowel space characteristics of young males with fragile X syndrome: preliminary acoustic findings. J Speech Lang Hear Res. 2006;49(5):1147–1155. doi: 10.1044/1092-4388(2006/082). [DOI] [PubMed] [Google Scholar]
- 58.Zajac DJ, Harris AA, Roberts JE, Martin GE. Direct magnitude estimation of articulation rate in boys with fragile X syndrome. J Speech Lang Hear Res. 2009;52(5):1370–1379. doi: 10.1044/1092-4388(2009/07-0208). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barnes E, Roberts J, Long SH, et al. Phonological accuracy and intelligibility in connected speech of boys with fragile X syndrome or Down syndrome. J Speech Lang Hear Res. 2009;52(4):1048–1061. doi: 10.1044/1092-4388(2009/08-0001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Price JR, Roberts JE, Hennon EA, Berni MC, Anderson KL, Sideris J. Syntactic complexity during conversation of boys with fragile X syndrome and Down syndrome. J Speech Lang Hear Res. 2008;51(1):3–15. doi: 10.1044/1092-4388(2008/001). [DOI] [PubMed] [Google Scholar]
- 61.Martin GE, Losh M, Estigarribia B, Sideris J, Roberts J. Longitudinal profiles of expressive vocabulary, syntax and pragmatic language in boys with fragile X syndrome or Down syndrome. Int J Lang Commun Disord. 2013;48(4):432–443. doi: 10.1111/1460-6984.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sterling AM, Rice ML, Warren SF. Finiteness marking in boys with fragile X syndrome. J Speech Lang Hear Res. 2012;55(6):1704–1715. doi: 10.1044/1092-4388(2012/10-0106). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Estigarribia B, Martin GE, Roberts JE. Cognitive, environmental, and linguistic predictors of syntax in fragile X syndrome and Down syndrome. J Speech Lang Hear Res. 2012;55(6):1600–1612. doi: 10.1044/1092-4388(2012/10-0153). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mazzocco MM, Thompson L, Sudhalter V, Belser RC, Lesniak-Karpiak K, Ross JL. Language use in females with fragile X or Turner syndrome during brief initial social interactions. J Dev Behav Pediatr. 2006;27(4):319–328. doi: 10.1097/00004703-200608000-00007. [DOI] [PubMed] [Google Scholar]
- 65.Roberts J, Martin GE, Moskowitz L, Harris AA, Foreman J, Nelson L. Discourse skills of boys with fragile X syndrome in comparison to boys with Down syndrome. J Speech Lang Hear Res. 2007;50(2):475–492. doi: 10.1044/1092-4388(2007/033). [DOI] [PubMed] [Google Scholar]
- 66.Finestack LH, Palmer M, Abbeduto L. Macrostructural narrative language of adolescents and young adults with Down syndrome or fragile X syndrome. Am J Speech Lang Pathol. 2012;21(1):29–46. doi: 10.1044/1058-0360(2011/10-0095). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Keller-Bell YD, Abbeduto L. Narrative development in adolescents and young adults with fragile x syndrome. Am J Ment Retard. 2007;112(4):289–299. doi: 10.1352/0895-8017(2007)112[289:NDIAAY]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 68.Brady N, Warren SF, Fleming K, Keller J, Sterling A. Effect of sustained maternal responsivity on later vocabulary development in children with Fragile X Syndrome. J Speech Lang Hear Res. 2014;57(1):212–226. doi: 10.1044/1092-4388(2013/12-0341). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Warren SF, Brady N, Sterling A, Fleming K, Marquis J. Maternal responsivity predicts language development in young children with fragile X syndrome. Am J Intellect Dev Disabil. 2010;115(1):54–75. doi: 10.1352/1944-7558-115.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bailey DB, Raspa M, Holiday D, Bishop E, Olmsted M. Functional skills of individuals with fragile x syndrome: a lifespan cross-sectional analysis. Am J Intellect Dev Disabil. 2009;114(4):289–303. doi: 10.1352/1944-7558-114.4.289-303. [DOI] [PubMed] [Google Scholar]
- 71.Hatton DD, Wheeler AC, Skinner ML, et al. Adaptive behavior in children with fragile X syndrome. Am J Ment Retard. 2003;108(6):373–390. doi: 10.1352/0895-8017(2003)108<373:ABICWF>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 72.Fisch GS, Simensen R, Tarleton J, et al. Longitudinal study of cognitive abilities and adaptive behavior levels in fragile X males: a prospective multicenter analysis. Am J Med Genet. 1996;64(2):356–361. doi: 10.1002/(SICI)1096-8628(19960809)64:2<356::AID-AJMG24>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 73.Fisch GS, Carpenter N, Holden JJ, et al. Longitudinal changes in cognitive and adaptive behavior in fragile X females: a prospective multicenter analysis. Am J Med Genet. 1999;83(4):308–312. [PubMed] [Google Scholar]
- 74.Fisch GS, Carpenter NJ, Holden JJ, et al. Longitudinal assessment of adaptive and maladaptive behaviors in fragile X males: growth, development, and profiles. Am J Med Genet. 1999;83(4):257–263. doi: 10.1002/(sici)1096-8628(19990402)83:4<257::aid-ajmg5>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 75.Fisch GS, Carpenter N, Howard-Peebles PN, et al. Studies of age-correlated features of cognitive-behavioral development in children and adolescents with genetic disorders. Am J Med Genet A. 2007;143A(20):2478–2489. doi: 10.1002/ajmg.a.31915. [DOI] [PubMed] [Google Scholar]
- 76.Fisch GS, Simensen RJ, Schroer RJ. Longitudinal changes in cognitive and adaptive behavior scores in children and adolescents with the fragile X mutation or autism. J Autism Dev Disord. 2002;32(2):107–114. doi: 10.1023/a:1014888505185. [DOI] [PubMed] [Google Scholar]
- 77.Smith LE, Barker ET, Seltzer MM, Abbeduto L, Greenberg JS. Behavioral phenotype of fragile X syndrome in adolescence and adulthood. Am J Intellect Dev Disabil. 2012;117(1):1–17. doi: 10.1352/1944-7558-117.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hartley SL, Seltzer MM, Raspa M, Olmstead M, Bishop E, Bailey DB. Exploring the adult life of men and women with fragile X syndrome: results from a national survey. Am J Intellect Dev Disabil. 2011;116(1):16–35. doi: 10.1352/1944-7558-116.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hustyi KM, Hall SS, Quintin EM, Chromik LC, Lightbody AA, Reiss AL. The relationship between autistic symptomatology and independent living skills in adolescents and young adults with fragile X syndrome. J Autism Dev Disord. 2014;45(6):1836–1844. doi: 10.1007/s10803-014-2342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bailey DB, Jr, Raspa M, Olmsted M, Holiday DB. Co-occurring conditions associated with FMR1 gene variations: findings from a national parent survey. Am J Med Genet A. 2008;146A(16):2060–2069. doi: 10.1002/ajmg.a.32439. [DOI] [PubMed] [Google Scholar]
- 81.Munir F, Cornish KM, Wilding J. A neuropsychological profile of attention deficits in young males with fragile X syndrome. Neuropsychologia. 2000;38(9):1261–1270. doi: 10.1016/s0028-3932(00)00036-1. [DOI] [PubMed] [Google Scholar]
- 82.Cornish K, Sudhalter V, Turk J. Attention and language in fragile X. Ment Retard Dev Disabil Res Rev. 2004;10(1):11–16. doi: 10.1002/mrdd.20003. [DOI] [PubMed] [Google Scholar]
- 83.Scerif G, Cornish K, Wilding J, Driver J, Karmiloff-Smith A. Delineation of early attentional control difficulties in fragile X syndrome: focus on neurocomputational changes. Neuropsychologia. 2007;45(8):1889–1898. doi: 10.1016/j.neuropsychologia.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scerif G, Longhi E, Cole V, Karmiloff-Smith A, Cornish K. Attention across modalities as a longitudinal predictor of early outcomes: the case of fragile X syndrome. J Child Psychol Psychiatry. 2012;53(6):641–650. doi: 10.1111/j.1469-7610.2011.02515.x. [DOI] [PubMed] [Google Scholar]
- 85.Sullivan K, Hatton DD, Hammer J, et al. Sustained attention and response inhibition in boys with fragile X syndrome: measures of continuous performance. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(4):517–532. doi: 10.1002/ajmg.b.30504. [DOI] [PubMed] [Google Scholar]
- 86.Cornish K, Cole V, Longhi E, Karmiloff-Smith A, Scerif G. Does attention constrain developmental trajectories in fragile x syndrome? A 3-year prospective longitudinal study. Am J Intellect Dev Disabil. 2012;117(2):103–120. doi: 10.1352/1944-7558-117.2.103. [DOI] [PubMed] [Google Scholar]
- 87.Roberts JE, Hatton DD, Long AC, Anello V, Colombo J. Visual attention and autistic behavior in infants with fragile X syndrome. J Autism Dev Disord. 2012;42(6):937–946. doi: 10.1007/s10803-011-1316-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boyle L, Kaufmann WE. The behavioral phenotype of FMR1 mutations. Am J Med Genet C Semin Med Genet. 2010;154C(4):469–476. doi: 10.1002/ajmg.c.30277. [DOI] [PubMed] [Google Scholar]
- 89.Cordeiro L, Ballinger E, Hagerman R, Hessl D. Clinical assessment of DSM-IV anxiety disorders in fragile X syndrome: prevalence and characterization. J Neurodev Disord. 2011;3(1):57–67. doi: 10.1007/s11689-010-9067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gabis LV, Baruch YK, Jokel A, Raz R. Psychiatric and autistic comorbidity in fragile X syndrome across ages. J Child Neurol. 2011;26(8):940–948. doi: 10.1177/0883073810395937. [DOI] [PubMed] [Google Scholar]
- 91.Lachiewicz AM. Abnormal behaviors of young girls with fragile X syndrome. Am J Med Genet. 1992;43(1–2):72–77. doi: 10.1002/ajmg.1320430111. [DOI] [PubMed] [Google Scholar]
- 92.Lachiewicz AM, Dawson DV. Behavior problems of young girls with fragile X syndrome: factor scores on the Conners– Parent’s Questionnaire. Am J Med Genet. 1994;51(4):364–369. doi: 10.1002/ajmg.1320510413. [DOI] [PubMed] [Google Scholar]
- 93.Freund LS, Reiss AL, Abrams MT. Psychiatric disorders associated with fragile X in the young female. Pediatrics. 1993;91(2):321–329. [PubMed] [Google Scholar]
- 94.Wheeler A, Raspa M, Bann C, et al. Anxiety, attention problems, hyperactivity, and the aberrant behavior checklist in fragile X syndrome. Am J Med Genet A. 2014;164A(1):141–155. doi: 10.1002/ajmg.a.36232. [DOI] [PubMed] [Google Scholar]
- 95.Tonnsen BL, Malone PS, Hatton DD, Roberts JE. Early negative affect predicts anxiety, not autism, in preschool boys with fragile X syndrome. J Abnorm Child Psychol. 2013;41(2):267–280. doi: 10.1007/s10802-012-9671-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Holsen LM, Dalton KM, Johnstone T, Davidson RJ. Prefrontal social cognition network dysfunction underlying face encoding and social anxiety in fragile X syndrome. Neuroimage. 2008;43(3):592–604. doi: 10.1016/j.neuroimage.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sullivan K, Hooper S, Hatton D. Behavioural equivalents of anxiety in children with fragile X syndrome: parent and teacher report. J Intellect Disabil Res. 2007;51(pt 1):54–65. doi: 10.1111/j.1365-2788.2006.00899.x. [DOI] [PubMed] [Google Scholar]
- 98.Farzin F, Koldewyn K. Fragile X syndrome and autism. In: Patel VB, Preedy VR, Martin CR, editors. Comprehensive Guide to Autism. New York, NY: Springer; 2014. pp. 2743–2754. [Google Scholar]
- 99.Hatton DD, Sideris J, Skinner M, et al. Autistic behavior in children with fragile X syndrome: prevalence, stability, and the impact of FMRP. Am J Med Genet A. 2006;140A(17):1804–1813. doi: 10.1002/ajmg.a.31286. [DOI] [PubMed] [Google Scholar]
- 100.Rogers SJ, Wehner DE, Hagerman R. The behavioral phenotype in fragile X: symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. J Dev Behav Pediatr. 2001;22(6):409–417. doi: 10.1097/00004703-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 101.Hernandez RN, Feinberg RL, Vaurio R, Passanante NM, Thompson RE, Kaufmann WE. Autism spectrum disorder in fragile X syndrome: a longitudinal evaluation. Am J Med Genet A. 2009;149A(6):1125–1137. doi: 10.1002/ajmg.a.32848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brock M, Hatton D. Distinguishing features of autism in boys with fragile X syndrome. J Intellect Disabil Res. 2010;54(10):894–905. doi: 10.1111/j.1365-2788.2010.01315.x. [DOI] [PubMed] [Google Scholar]
- 103.Rogers SJ, Hepburn S, Wehner E. Parent reports of sensory symptoms in toddlers with autism and those with other developmental disorders. J Autism Dev Disord. 2003;33(6):631–642. doi: 10.1023/b:jadd.0000006000.38991.a7. [DOI] [PubMed] [Google Scholar]
- 104.Rogers SJ, Hepburn SL, Stackhouse T, Wehner E. Imitation performance in toddlers with autism and those with other developmental disorders. J Child Psychol Psychiatry. 2003;44(5):763–781. doi: 10.1111/1469-7610.00162. [DOI] [PubMed] [Google Scholar]
- 105.Kaufmann WE, Cortell R, Kau AS, et al. Autism spectrum disorder in fragile X syndrome: communication, social interaction, and specific behaviors. Am J Med Genet A. 2004;129A(3):225–234. doi: 10.1002/ajmg.a.30229. [DOI] [PubMed] [Google Scholar]
- 106.Philofsky A, Hepburn SL, Hayes A, Hagerman R, Rogers SJ. Linguistic and cognitive functioning and autism symptoms in young children with fragile X syndrome. Am J Ment Retard. 2004;109(3):208–218. doi: 10.1352/0895-8017(2004)109<208:LACFAA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 107.Lewis P, Abbeduto L, Murphy M, et al. Cognitive, language and social-cognitive skills of individuals with fragile X syndrome with and without autism. J Intellect Disabil Res. 2006;50(Pt 7):532–545. doi: 10.1111/j.1365-2788.2006.00803.x. [DOI] [PubMed] [Google Scholar]
- 108.Budimirovic DB, Bukelis I, Cox C, Gray RM, Tierney E, Kaufmann WE. Autism spectrum disorder in Fragile X syndrome: differential contribution of adaptive socialization and social withdrawal. Am J Med Genet A. 2006;140A(17):1814–1826. doi: 10.1002/ajmg.a.31405. [DOI] [PubMed] [Google Scholar]
- 109.Roberts JE, Clarke MA, Alcorn K, Carter JC, Long AC, Kaufmann WE. Autistic behavior in boys with fragile X syndrome: social approach and HPA-axis dysfunction. J Neurodev Disord. 2009;1(4):283–291. doi: 10.1007/s11689-009-9028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Budimirovic DB, Kaufmann WE. What can we learn about autism from studying fragile X syndrome? Dev Neurosci. 2011;33(5):379–394. doi: 10.1159/000330213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wolff JJ, Bodfish JW, Hazlett HC, Lightbody AA, Reiss AL, Piven J. Evidence of a distinct behavioral phenotype in young boys with fragile X syndrome and autism. J Am Acad Child Adolesc Psychiatry. 2012;51(12):1324–1332. doi: 10.1016/j.jaac.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sabaratnam M, Murthy NV, Wijeratne A, Buckingham A, Payne S. Autistic-like behaviour profile and psychiatric morbidity in Fragile X Syndrome: a prospective ten-year follow-up study. Eur Child Adolesc Psychiatry. 2003;12(4):172–177. doi: 10.1007/s00787-003-0333-3. [DOI] [PubMed] [Google Scholar]
- 113.McDuffie A, Abbeduto L, Lewis P, et al. Autism spectrum disorder in children and adolescents with fragile X syndrome: within-syndrome differences and age-related changes. Am J Intellect Dev Disabil. 2010;115(4):307–326. doi: 10.1352/1944-7558-115.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Loesch DZ, Bui QM, Dissanayake C, et al. Molecular and cognitive predictors of the continuum of autistic behaviours in fragile X. Neurosci Biobehav Rev. 2007;31(3):315–326. doi: 10.1016/j.neubiorev.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hagerman RJ, Berry-Kravis E, Kaufmann WE, et al. Advances in the treatment of fragile X syndrome. Pediatrics. 2009;123(1):378–390. doi: 10.1542/peds.2008-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hatton DD, Hooper SR, Bailey DB, Skinner ML, Sullivan KM, Wheeler A. Problem behavior in boys with fragile X syndrome. Am J Med Genet. 2002;108(2):105–116. doi: 10.1002/ajmg.10216. [DOI] [PubMed] [Google Scholar]
- 117.Symons FJ, Byiers BJ, Raspa M, Bishop E, Bailey DB. Self-injurious behavior and fragile X syndrome: findings from the national fragile X survey. Am J Intellect Dev Disabil. 2010;115(6):473–481. doi: 10.1352/1944-7558-115.6.473. [DOI] [PubMed] [Google Scholar]
- 118.Hall SS, Lightbody AA, Huffman LC, Lazzeroni LC, Reiss AL. Physiological correlates of social avoidance behavior in children and adolescents with fragile x syndrome. J Am Acad Child Adolesc Psychiatry. 2009;48(3):320–329. doi: 10.1097/CHI.0b013e318195bd15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hessl D, Rivera SM, Reiss AL. The neuroanatomy and neuroendocrinology of fragile X syndrome. Ment Retard Dev Disabil Res Rev. 2004;10(1):17–24. doi: 10.1002/mrdd.20004. [DOI] [PubMed] [Google Scholar]
- 120.Hall SS, Burns DD, Reiss AL. Modeling family dynamics in children with fragile x syndrome. J Abnorm Child Psychol. 2007;35(1):29–42. doi: 10.1007/s10802-006-9081-4. [DOI] [PubMed] [Google Scholar]
- 121.McCarthy A, Cuskelly M, van Kraayenoord CE, Cohen J. Predictors of stress in mothers and fathers of children with fragile X syndrome. Res Dev Disabil. 2006;27(6):688–704. doi: 10.1016/j.ridd.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 122.Wheeler AC, Skinner DG, Bailey DB. Perceived quality of life in mothers of children with fragile X syndrome. Am J Ment Retard. 2008;113(3):159–177. doi: 10.1352/0895-8017(2008)113[159:PQOLIM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 123.Bailey DB, Jr, Sideris J, Roberts J, Hatton D. Child and genetic variables associated with maternal adaptation to fragile X syndrome: a multidimensional analysis. Am J Med Genet A. 2008;146A(6):720–729. doi: 10.1002/ajmg.a.32240. [DOI] [PubMed] [Google Scholar]
- 124.Bailey DB, Jr, Raspa M, Bishop E, et al. Health and economic consequences of fragile X syndrome for caregivers. J Dev Behav Pediatr. 2012;33(9):705–712. doi: 10.1097/DBP.0b013e318272dcbc. [DOI] [PubMed] [Google Scholar]
- 125.Langthorne P, McGill P. An indirect examination of the function of problem behavior associated with fragile X syndrome and Smith-Magenis syndrome. J Autism Dev Disord. 2012;42(2):201–209. doi: 10.1007/s10803-011-1229-6. [DOI] [PubMed] [Google Scholar]
- 126.Moskowitz LJ, Carr EG, Durand VM. Behavioral intervention for problem behavior in children with fragile X syndrome. Am J Intellect Dev Disabil. 2011;116(6):457–478. doi: 10.1352/1944-7558-116.6.457. [DOI] [PubMed] [Google Scholar]
- 127.Richards C, Oliver C, Nelson L, Moss J. Self-injurious behaviour in individuals with autism spectrum disorder and intellectual disability. J Intellect Disabil Res. 2012;56(5):476–489. doi: 10.1111/j.1365-2788.2012.01537.x. [DOI] [PubMed] [Google Scholar]
- 128.Symons FJ, Clark RD, Hatton DD, Skinner M, Bailey DB., Jr Self-injurious behavior in young boys with fragile X syndrome. Am J Med Genet A. 2003;118A(2):115–121. doi: 10.1002/ajmg.a.10078. [DOI] [PubMed] [Google Scholar]
- 129.Garber KB, Visootsak J, Warren ST. Fragile X syndrome. Eur J Hum Genet. 2008;16(6):666–672. doi: 10.1038/ejhg.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pop AS, Gomez-Mancilla B, Neri G, Willemsen R, Gasparini F. Fragile X syndrome: a preclinical review on metabotropic glutamate receptor 5 (mGluR5) antagonists and drug development. Psychopharmacology (Berl) 2014;321(6):1217–1226. doi: 10.1007/s00213-013-3330-3. [DOI] [PubMed] [Google Scholar]
- 131.Hagerman RJ. Physical and behavioral phenotype. In: Hagerman RJ, Hagerman PJ, editors. Fragile X Syndrome: Diagnosis, Treatment and Research. Baltimore, MD: Johns Hopkins University Press; 2002. pp. 3–109. [Google Scholar]
- 132.Butler MG, Brunschwig A, Miller LK, Hagerman RJ. Standards for selected anthropometric measurements in males with the fragile X syndrome. Pediatrics. 1992;89(6 Pt 1):1059–1062. [PMC free article] [PubMed] [Google Scholar]
- 133.Hagerman RJ. Physical and behavioural phenotype. In: Hagerman RJ, Silverman AC, editors. Fragile X Syndrome: Diagnosis, Treatment and Research. Baltimore, MD: Johns Hopkins University Press; 1996. pp. 3–87. [Google Scholar]
- 134.Leung HT, Ring H. Epilepsy in four genetically determined syndromes of intellectual disability. J Intellect Disabil Res. 2013;57(1):3–20. doi: 10.1111/j.1365-2788.2011.01505.x. [DOI] [PubMed] [Google Scholar]
- 135.Berry-Kravis E, Raspa M, Loggin-Hester L, Bishop E, Holiday D, Bailey DB. Seizures in fragile X syndrome: characteristics and comorbid diagnoses. Am J Intellect Dev Disabil. 2010;115(6):461–472. doi: 10.1352/1944-7558-115.6.461. [DOI] [PubMed] [Google Scholar]
- 136.Berry-Kravis E. Epilepsy in fragile X syndrome. Dev Med Child Neurol. 2002;44(11):724–728. doi: 10.1017/s0012162201002833. [DOI] [PubMed] [Google Scholar]
- 137.Sabaratnam M, Vroegop PG, Gangadharan SK. Epilepsy and EEG findings in 18 males with fragile X syndrome. Seizure. 2001;10(1):60–63. doi: 10.1053/seiz.2000.0492. [DOI] [PubMed] [Google Scholar]
- 138.García-Nonell C, Ratera ER, Harris S, et al. Secondary medical diagnosis in fragile X syndrome with and without autism spectrum disorder. Am J Med Genet A. 2008;146A(15):1911–1916. doi: 10.1002/ajmg.a.32290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kronk R, Dahl R, Noll R. Caregiver reports of sleep problems on a convenience sample of children with fragile X syndrome. Am J Intellect Dev Disabil. 2009;114(6):383–392. doi: 10.1352/1944-7588-114.6.383. [DOI] [PubMed] [Google Scholar]
- 140.Kronk R, Bishop EE, Raspa M, Bickel JO, Mandel DA, Bailey DB., Jr Prevalence, nature, and correlates of sleep problems among children with fragile X syndrome based on a large scale parent survey. Sleep. 2010;33(5):679–687. doi: 10.1093/sleep/33.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Miano S, Bruni O, Elia M, et al. Sleep phenotypes of intellectual disability: a polysomnographic evaluation in subjects with Down syndrome and Fragile-X syndrome. Clin Neurophysiol. 2008;119(6):1242–1247. doi: 10.1016/j.clinph.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 142.Hagerman RJ, Hagerman PJ. The fragile X premutation: into the phenotypic fold. Curr Opin Genet Dev. 2002;12(3):278–283. doi: 10.1016/s0959-437x(02)00299-x. [DOI] [PubMed] [Google Scholar]
- 143.Kidd SA, Lachiewicz A, Barbouth D, et al. Fragile X syndrome: a review of associated medical problems. Pediatrics. 2014;134(5):995–1005. doi: 10.1542/peds.2013-4301. [DOI] [PubMed] [Google Scholar]
- 144.Raspa M, Bailey DB, Bishop E, Holiday D, Olmsted M. Obesity, food selectivity, and physical activity in individuals with fragile X syndrome. Am J Intellect Dev Disabil. 2010;115(6):482–495. doi: 10.1352/1944-7558-115.6.482. [DOI] [PubMed] [Google Scholar]
- 145.Nowicki ST, Tassone F, Ono MY, et al. The Prader-Willi phenotype of fragile X syndrome. J Dev Behav Pediatr. 2007;28(2):133–138. doi: 10.1097/01.DBP.0000267563.18952.c9. [DOI] [PubMed] [Google Scholar]
- 146.Hazlett HC, Poe MD, Lightbody AA, et al. Teasing apart the heterogeneity of autism: same behavior, different brains in toddlers with fragile X syndrome and autism. J Neurodev Disord. 2009;1(1):81–90. doi: 10.1007/s11689-009-9009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hoeft F, Lightbody AA, Hazlett HC, Patnaik S, Piven J, Reiss AL. Morphometric spatial patterns differentiating boys with fragile X syndrome, typically developing boys, and developmentally delayed boys aged 1 to 3 years. Arch Gen Psychiatry. 2008;65(9):1087–1097. doi: 10.1001/archpsyc.65.9.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rivera SM, Menon V, White CD, Glaser B, Reiss AL. Functional brain activation during arithmetic processing in females with fragile X syndrome is related to FMR1 protein expression. Hum Brain Mapp. 2002;16(4):206–218. doi: 10.1002/hbm.10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Reiss AL, Dant CC. The behavioral neurogenetics of fragile X syndrome: analyzing gene-brain-behavior relationships in child developmental psychopathologies. Dev Psychopathol. 2003;15(4):927–968. doi: 10.1017/s0954579403000464. [DOI] [PubMed] [Google Scholar]
- 150.Wang JY, Hessl D, Iwahashi C, et al. Influence of the fragile X mental retardation (FMR1) gene on the brain and working memory in men with normal FMR1 alleles. Neuroimage. 2013;65:288–298. doi: 10.1016/j.neuroimage.2012.09.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Roberts JE, Symons FJ, Johnson AM, Hatton DD, Boccia ML. Blink rate in boys with fragile X syndrome: preliminary evidence for altered dopamine function. J Intellect Disabil Res. 2005;49(Pt 9):647–656. doi: 10.1111/j.1365-2788.2005.00713.x. [DOI] [PubMed] [Google Scholar]
- 152.Roberts JE, Long AC, McCary LM, et al. Cardiovascular and behavioral response to auditory stimuli in boys with fragile X syndrome. J Pediatr Psychol. 2013;38(3):276–284. doi: 10.1093/jpepsy/jss114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Frankland PW, Wang Y, Rosner B, et al. Sensorimotor gating abnormalities in young males with fragile X syndrome and Fmr1-knockout mice. Mol Psychiatry. 2004;9(4):417–425. doi: 10.1038/sj.mp.4001432. [DOI] [PubMed] [Google Scholar]
- 154.Hessl D, Nguyen DV, Green C, et al. A solution to limitations of cognitive testing in children with intellectual disabilities: the case of fragile X syndrome. J Neurodev Disord. 2009;1(1):33–45. doi: 10.1007/s11689-008-9001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Miller LJ, McIntosh DN, McGrath J, et al. Electrodermal responses to sensory stimuli in individuals with fragile X syndrome: a preliminary report. Am J Med Genet. 1999;83(4):268–279. [PubMed] [Google Scholar]
- 156.Hills-Epstein J, Riley K, Sobesky W. The treatment of emotional and behavioral problems. In: Hagerman RJ, Hagerman PJ, editors. Fragile X Syndrome: Diagnosis, Treatment, and Research. 3. Baltimore, MD: Johns Hopkins University Press; 2002. [Google Scholar]
- 157.Hall SS, Maynes NP, Reiss AL. Using percentile schedules to increase eye contact in children with fragile X syndrome. J Appl Behav Anal. 2009;42(1):171–176. doi: 10.1901/jaba.2009.42-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.O’Connor JT, Sorensen-Burnworth RJ, Rush KS, Eidman SL. A mand analysis and levels treatment in an outpatient clinic. Behav Interv. 2003;18(2):139–150. [Google Scholar]
- 159.Mirrett PL, Roberts JE, Price J. Early intervention practices and communication intervention strategies for young males with fragile X syndrome. Lang Speech Hear Serv Sch. 2003;34(4):320–331. doi: 10.1044/0161-1461(2003/026). [DOI] [PubMed] [Google Scholar]
- 160.Winarni TI, Schneider A, Borodyanskara M, Hagerman RJ. Early intervention combined with targeted treatment promotes cognitive and behavioral improvements in young children with fragile x syndrome. Case Rep Genet. 2012;2012:280813. doi: 10.1155/2012/280813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hall DA, Hagerman RJ, Hagerman PJ, Jacquemont S, Leehey MA. Prevalence of FMR1 repeat expansions in movement disorders. A systematic review. Neuroepidemiology. 2006;26(3):151–155. doi: 10.1159/000091656. [DOI] [PubMed] [Google Scholar]
- 162.Hammond JL, Hirt M, Hall SS. Effects of computerized match-to-sample training on emergent fraction-decimal relations in individuals with fragile X syndrome. Res Dev Disabil. 2012;33(1):1–11. doi: 10.1016/j.ridd.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hall SS, Hammond JL, Hirt M, Reiss ALA. A ‘learning platform’ approach to outcome measurement in fragile X syndrome: a preliminary psychometric study. J Intellect Disabil Res. 2012;56(10):947–960. doi: 10.1111/j.1365-2788.2012.01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hall SS, Hustyi KM, Hammond JL, Hirt M, Reiss AL. Using discrete trial training to identify specific learning impairments in boys with fragile X syndrome. J Autism Dev Disord. 2014;44(7):1659–1670. doi: 10.1007/s10803-014-2037-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hall SS, Lightbody AA, McCarthy BE, Parker KJ, Reiss AL. Effects of intranasal oxytocin on social anxiety in males with fragile X syndrome. Psychoneuroendocrinology. 2012;37(4):509–518. doi: 10.1016/j.psyneuen.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hagerman R, Lauterborn J, Au J, Berry-Kravis E. Fragile X syndrome and targeted treatment trials. Results Probl Cell Differ. 2012;54:297–335. doi: 10.1007/978-3-642-21649-7_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bailey DB, Jr, Raspa M, Bishop E, Olmsted M, Mallya UG, Berry-Kravis E. Medication utilization for targeted symptoms in children and adults with fragile X syndrome: US survey. J Dev Behav Pediatr. 2012;33(1):62–69. doi: 10.1097/DBP.0b013e318236c0e1. [DOI] [PubMed] [Google Scholar]
- 168.Disease Management. A number of psychotropic drugs are useful in treating the various neuropsychiatric symptoms of fragile X syndrome. Drugs Ther Perspect. 2005;21(7):15–18. [Google Scholar]
- 169.Erickson CA, Wink LK, Schaefer TL, Shaffer RC. Medication management of fragile X syndrome. In: Patel VB, Preedy VR, Martin CR, editors. Comprehensive Guide to Autism. New York, NY: Springer; 2014. pp. 2773–2783. [Google Scholar]
- 170.Amaria RN, Billeisen LL, Hagerman RJ. Medication use in fragile X syndrome. Ment Health Aspects Dev Disabil. 2001;4(4):143–147. [Google Scholar]
- 171.Berry-Kravis E, Potanos K. Psychopharmacology in fragile X syndrome–present and future. Ment Retard Dev Disabil Res Rev. 2004;10(1):42–48. doi: 10.1002/mrdd.20007. [DOI] [PubMed] [Google Scholar]
- 172.Hagerman RJ, Fulton MJ, Leaman A, Riddle J, Hagerman K, Sobesky W. A survey of fluoxetine therapy in fragile X syndrome. Dev Brain Dysfunct. 1994;7(2):155–164. [Google Scholar]
- 173.Rueda JR, Ballesteros J, Tejada MI. Systematic review of pharmacological treatments in fragile X syndrome. BMC Neurol. 2009;9:53–64. doi: 10.1186/1471-2377-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wolraich M, Brown L, Brown RT, et al. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007–1022. doi: 10.1542/peds.2011-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hagerman RJ, Murphy MA, Wittenberger MD. A controlled trial of stimulant medication in children with the fragile X syndrome. Am J Med Genet. 1988;30(1–2):377–392. doi: 10.1002/ajmg.1320300138. [DOI] [PubMed] [Google Scholar]
- 176.Hagerman RJ, Riddle JE, Roberts LS, Brease K, Fulton M. Survey of the efficacy of clonidine in fragile X syndrome. Dev Brain Dysfunct. 1995;8(4–6):336–344. [Google Scholar]
- 177.Torrioli MG, Vernacotola S, Mariotti P, et al. Double-blind, placebo-controlled study of L-acetylcarnitine for the treatment of hyperactive behavior in fragile X syndrome. Am J Med Genet. 1999;87(4):366–368. [PubMed] [Google Scholar]
- 178.Torrioli MG, Vernacotola S, Peruzzi L, et al. A double-blind, parallel, multicenter comparison of L-acetylcarnitine with placebo on the attention deficit hyperactivity disorder in fragile X syndrome boys. Am J Med Genet A. 2008;146A(7):803–812. doi: 10.1002/ajmg.a.32268. [DOI] [PubMed] [Google Scholar]
- 179.Torrioli M, Vernacotola S, Setini C, et al. Treatment with valproic acid ameliorates ADHD symptoms in fragile X syndrome boys. Am J Med Genet A. 2010;152A(6):1420–1427. doi: 10.1002/ajmg.a.33484. [DOI] [PubMed] [Google Scholar]
- 180.Erickson CA, Stigler KA, Wink LK, et al. A prospective open-label study of aripiprazole in fragile X syndrome. Psychopharmacology (Berl) 2011;216(1):85–90. doi: 10.1007/s00213-011-2194-7. [DOI] [PubMed] [Google Scholar]
- 181.McCracken JT, McGough J, Shah B, et al. Research Units on Pediatric Psychopharmacology Autism Network. Risperidone in children with autism and serious behavioral problems. N Engl J Med. 2002;347(5):314–321. doi: 10.1056/NEJMoa013171. [DOI] [PubMed] [Google Scholar]
- 182.Wirojanan J, Jacquemont S, Diaz R, et al. The efficacy of melatonin for sleep problems in children with autism, fragile X syndrome, or autism and fragile X syndrome. J Clin Sleep Med. 2009;5(2):145–150. [PMC free article] [PubMed] [Google Scholar]
- 183.Jacquemont S, Berry-Kravis E, Hagerman R, et al. The challenges of clinical trials in fragile X syndrome. Psychopharmacology (Berl) 2014;231(6):1237–1250. doi: 10.1007/s00213-013-3289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pop AS, Levenga J, de Esch CE, et al. Rescue of dendritic spine phenotype in Fmr1 KO mice with the mGluR5 antagonist AFQ056/Mavoglurant. Psychopharmacology (Berl) 2014;231(6):1227–1235. doi: 10.1007/s00213-012-2947-y. [DOI] [PubMed] [Google Scholar]
- 185.Hare EB, Hagerman RJ, Lozano R. Targeted treatments in fragile X syndrome. Expert Opin Orphan Drugs. 2014;2(6):531–543. [Google Scholar]
- 186.Lozano R, Hare EB, Hagerman RJ. Modulation of the GABAergic pathway for the treatment of fragile X syndrome. Neuropsychiatr Dis Treat. 2014;10:1769–1779. doi: 10.2147/NDT.S42919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Berry-Kravis E. Mechanism-based treatments in neurodevelopmental disorders: fragile X syndrome. Pediatr Neurol. 2014;50(4):297–302. doi: 10.1016/j.pediatrneurol.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 188.Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27(7):370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 189.Burket JA, Herndon AL, Winebarger EE, Jacome LF, Deutsch SI. Complex effects of mGluR5 antagonism on sociability and stereotypic behaviors in mice: possible implications for the pharmacotherapy of autism spectrum disorders. Brain Res Bull. 2011;86(3–4):152–158. doi: 10.1016/j.brainresbull.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 190.de Vrij FM, Levenga J, van der Linde HC, et al. Rescue of behavioral phenotype and neuronal protrusion morphology in Fmr1 KO mice. Neurobiol Dis. 2008;31(1):127–132. doi: 10.1016/j.nbd.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Michalon A, Sidorov M, Ballard TM, et al. Chronic pharmacological mGlu5 inhibition corrects fragile X in adult mice. Neuron. 2012;74(1):49–56. doi: 10.1016/j.neuron.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Thomas AM, Bui N, Perkins JR, Yuva-Paylor LA, Paylor R. Group I metabotropic glutamate receptor antagonists alter select behaviors in a mouse model for fragile X syndrome. Psychopharmacology (Berl) 2012;219(1):47–58. doi: 10.1007/s00213-011-2375-4. [DOI] [PubMed] [Google Scholar]
- 193.Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major fragile X syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49(7):1053–1066. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 194.Leigh MJ, Nguyen DV, Mu Y, et al. A randomized double-blind, placebo-controlled trial of minocycline in children and adolescents with fragile x syndrome. J Dev Behav Pediatr. 2013;34(3):147–155. doi: 10.1097/DBP.0b013e318287cd17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Baker JK, Seltzer MM, Greenberg JS. Behaviour problems, maternal internalising symptoms and family relations in families of adolescents and adults with fragile X syndrome. J Intellect Disabil Res. 2012;56(10):984–995. doi: 10.1111/j.1365-2788.2012.01580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Johnston C, Hessl D, Blasey C, et al. Factors associated with parenting stress in mothers of children with fragile X syndrome. J Dev Behav Pediatr. 2003;24(4):267–275. doi: 10.1097/00004703-200308000-00008. [DOI] [PubMed] [Google Scholar]
- 197.Greenberg J, Seltzer M, Baker J, et al. Family environment and behavior problems in children, adolescents, and adults with fragile X syndrome. Am J Intellect Dev Disabil. 2012;117(4):331–346. doi: 10.1352/1944-7558-117.4.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wheeler A, Hatton D, Reichardt A, Bailey D. Correlates of maternal behaviours in mothers of children with fragile X syndrome. J Intellect Disabil Res. 2007;51(Pt 6):447–462. doi: 10.1111/j.1365-2788.2006.00896.x. [DOI] [PubMed] [Google Scholar]
- 199.Sterling AM, Warren SF, Brady N, Fleming K. Influences on maternal responsivity in mothers of children with fragile X syndrome. Am J Intellect Dev Disabil. 2013;118(4):310–326. doi: 10.1352/1944-7558-188.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sterling A, Barnum L, Skinner D, Warren SF, Fleming K. Parenting young children with and without fragile X syndrome. Am J Intellect Dev Disabil. 2012;117(3):194–206. doi: 10.1352/1944-7558-117.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wheeler AC, Hatton D, Holloway VT, et al. Maternal responses to child frustration and requests for help in dyads with fragile X syndrome. J Intellect Disabil Res. 2010;54(6):501–515. doi: 10.1111/j.1365-2788.2010.01269.x. [DOI] [PubMed] [Google Scholar]
- 202.Ouyang L, Grosse S, Raspa M, Bailey D. Employment impact and financial burden for families of children with fragile X syndrome: findings from the national fragile X survey. J Intellect Disabil Res. 2010;54(10):918–928. doi: 10.1111/j.1365-2788.2010.01320.x. [DOI] [PubMed] [Google Scholar]
- 203.Ouyang L, Grosse SD, Riley C, et al. A comparison of family financial and employment impacts of fragile X syndrome, autism spectrum disorders, and intellectual disability. Res Dev Disabil. 2014;35(7):1518–1527. doi: 10.1016/j.ridd.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Visootsak J, Hipp H, Clark H, Berry-Kravis E, Anderson T, Laney D. Climbing the branches of a family tree: diagnosis of fragile X syndrome. J Pediatr. 2014;164(6):1292–1295. [Google Scholar]