Summary
In Vietnam, a high prevalence of liver flukes, Clonorchis sinensis and Opisthorchis viverrini, has been reported in a number of provinces. Essential knowledge about C. sinensis infection gained from Thailand over the past decade suggests a habit of eating raw freshwater fish as a major risk factor of the infection. However, further information to confirm such relationship is needed. In the present study 1,155 villagers in 2 communes in northern Vietnam were interviewed and their stools were examined for presence of liver flukes. The prevalence of the infection was 26% and was 3.6 times higher in males than in females. The habit of eating raw fish increased a risk of C. sinensis infection by 53-fold. This article provides evidence of a strong correlation between the intensity of C. sinensis infection and the cumulative quantity of freshwater fish consumed in lifetime and suggests that simple questionnaires could be used in endemic areas to quickly identify and treat populations at risk.
Keywords: Liver flukes, Clonorchis sinensis, Questionnaire, Vietnam
Introduction
The liver flukes Clonorchis sinensis and Opistorchis viverrini cause various hepatobiliar problems and C. sinensis is a known risk factor for cholangiocarcinoma, a cancer of the bile ducts. C. sinensis and O. viverrini are known to have a freshwater snail of the genus Bithynia as a primary host and a cyprinoid fish species as a secondary host. Human infection occurs when metacercariae in flesh or skin of freshwater fish, which is the infective stage of the liver flukes, are ingested by a human host. Therefore, eating of raw freshwater fish is believed to be the leading risk factor of C. sinensis and O. viverrini infection. However, the most published descriptions of social habits regarding consumption of raw fish in relation to the infection remains anecdotal (Sithithawarn and Haswell-Elkins, 2003; Lin et al. 2005; Zhang et al. 2007) and additional information to confirm the association between raw fish consumption and the liver fluke infection is in need (Olsen et al., 2006).
World Health Organization (WHO) estimated in 2004 that approximately 1 million people have been infected with C. sinensis and O. viverrini in Vietnam. Since then, studies have been conducted in a number of communities in Vietnam. According to the extensive review by Nguyen Van De et al. (2003), C. sinensis is prevalent in northern Vietnam, while O. viverrini is predominant in central and southern Vietnam. It reported a generally higher prevalence in males than in females and an increasing prevalence with age.
The objective of the present study was to assess the prevalence and intensity of the liver fluke infection by age, gender and the habit of eating raw fish in Kim Son district, Ninh Binh province in Vietnam.
The study also evaluated the use of questionnaire as a public health tool to identify individuals at risk. This would allow targeting mass drug administration of praziquantel to a smaller group of individuals reducing logistical and drug costs.
Materials and Methods
Location and study participants
The study was conducted in 2 communes (Tan Thanh and Yen Loc) in Kim Son District, Ninh Binh province in Northern Vietnam, which was one of the target areas of national Clonorchiasis control program conducted by the Ministry of Health, Vietnam. The data was collected during the program to reduce the cost for identification of people infected with C. sinensis. Ninh Binh is a rural province, about 96 Km to the southeast of Hanoi. The two communes are located on the Red River Delta, about 18 Km from the gulf of Tonkin. The population of Tan Thanh commune and Yen Loc commune is approximately 4,700 and 8,000 people, respectively.
300 households were selected randomly from the name list of the household heads in the two communes. All members of the selected households older than 5 years of age and present on the day of the survey were enrolled in the study.
Molecular genetic techniques
C. sinensis and O. viverrini are known to have a very similar morphological feature that is difficult to distinguish from each other (WHO 1994). For differentiation of C. sinensis from O. viverrini, 315 adult worms were collected from faecal samples of 14 people in Tan Thanh commune, who were given a purgative (Mage Sulphate, Vietnam) a day before. The collected worms were investigated with molecular genetic characterization using DNA sequence.
The procedure has been described in details elsewhere (Thaenkham et al., 2007; Wongratanacheewin et al., 2002). Briefly, the DNA was extracted from 0.1 gram of adult worms and subjected to PCR amplification. Two sets of primer were used in the study. The first set was CsCoIR2 (GCC TAT AGT GAA AAG CAC CA) and CsCoIF1 (GCA TCC TGA GGT TTA TGT GT), designed using the partial sequences of mitochondrial COI gene of C. sinensis and O. viverrini (GenBank accession nos. AF096229 and AY055380, respectively). The second set was OV6F (CTG AAT CTC TCG TTT GTT CA) and OV6R (GTT CCA GGT GAG TCT CTC TA), designed using the tandem repetitive sequence of O. viverrini of Lao PDR origin (GenBank accession no. S80278). The second set was used to distinguish C. sinensis from O. viverrini. The size of the amplified PCR products were 346bp and 330bp, respectively. PCR product was electrophoresised in a 2% agalose gel and photographed under UV light.
Parasitological investigation and questionnaire
Each study participants received a plastic container and was requested to collect a single stool sample of around 10 gram and to return it to the commune health station the following day. Upon the returning of the containers, each study participant was interviewed using the simple questionnaire about gender, age and the habit of eating raw fish. Those who reported raw fish consumption were also asked about the time since when the habit started, the frequency and the amount of raw fish consumed in each event.
Stool samples were examined by Kato-Katz thick smear method (Katz et al., 1972) with template size of 41.7mg and multiplication factor of 24. The number of C. sinensis/ O. viverrini eggs present in each sample was recorded.
Informed consent was obtained from each individual participant to the study before enrolment.
Data analysis
Results of fecal examination and questionnaire survey were entered in Excel and statically analyzed using EpiInfo 3.3.2 (CDC, Atlanta, GA, USA) for prevalence and intensity of infection (epg) as measured by enumeration of eggs per gram feces. The study participants were subdivided into seven age groups: (1) <10 years; (2) 10-19 years; (3) 20-29 years; (4) 30-39 years; (5) 40-49 years; (6) 50-59 years; (7) >59 years. The univariate analysis of the relationship between the prevalence and the risk factors, namely gender, age groups, and habit of eating raw fish, was conducted. The risk factors showing a significant relationship with the prevalence were then subjected to logistic regression analysis. To examine univariate association between the intensity of infection and risk factors, geometric mean epg (logarithmic transformation of epg+1) were statistically analyzed by Mann-Whitney/Wilcoxon test (for gender and raw fish consumption) and Spearman’s rank correlation (for age groups). The factors that showed a significant association with the intensity were then subjected to multiple regression analysis to eliminate confounding factors. The "cumulative quantity of raw fish consumed" by each individual (during his lifetime) was calculated multiplying the years of raw fish consumption by the mean number of occasions in which raw fish was consumed each year and the quantity of raw fish consumed per occasion. The means of the above 4 variables stratified by the level of the intensity (<1000epg, 1000 – 5000epg, > 5000epg) was calculated and subjected to chi-square test for analysis of associations.
Results
PCR Identification of C. sinensis
Amplified PCR products of all the samples generated a PCR fragment of 346bp. On the other hand, the fragment of the tandem repetitive sequence of O. viverrini, was not amplified from any of the samples. This result indicated that the liver flukes collected from the fecal samples of villagers in Tan Thanh commune, Kim Son district, Ninh Binh province were not O. viverrini but identical to C. sinensis originating from Lao PDR.
Prevalence of infection
Stool samples were obtained from 1,155 individuals from 2 to 79 years of age. In the sample were present 97 children below 9 years of age (8.4%). The sample was constituted by 33.9% of females. The prevalence of C. sinensis infection in the sample was 26.1% with significant difference between males (33.8%) and female (11%) (p<0.01).
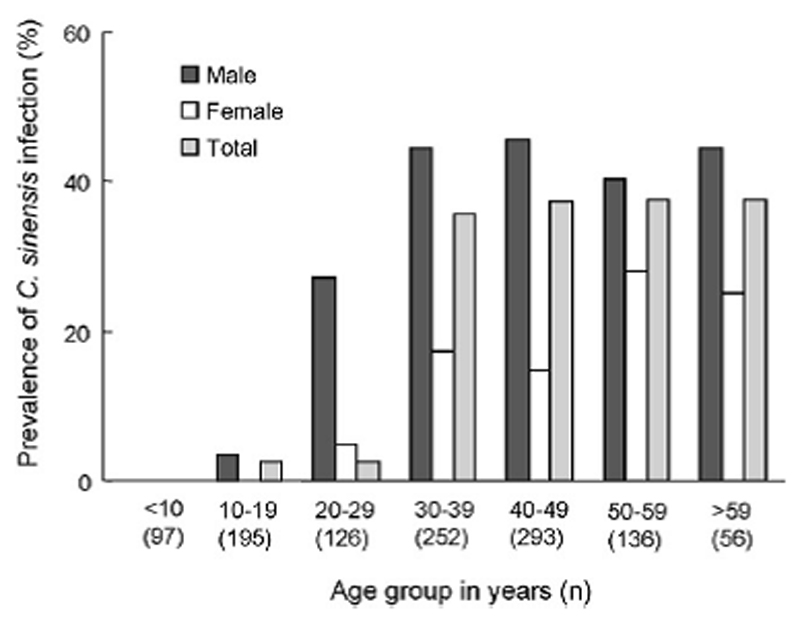
Figure 1 illustrates the prevalence of C. sinensis infection by age and gender. None of the children below 9 years of age were infected with C. sinensis. The youngest age of the infection was found to be 13, while the oldest age was 75 years old. The prevalence increased with age and reached a plateau in the 40 – 49 years age group. In the all age groups, infection rates were significantly higher in males than in females (p<0.01 for all age groups).
Figure 1.
Prevalence of C. sinensis infection by age and gender in 2 commune, Kin Som district, Ninh Binh province, Vietnam, 1999-2000 (n=1155).
Intensity of infection
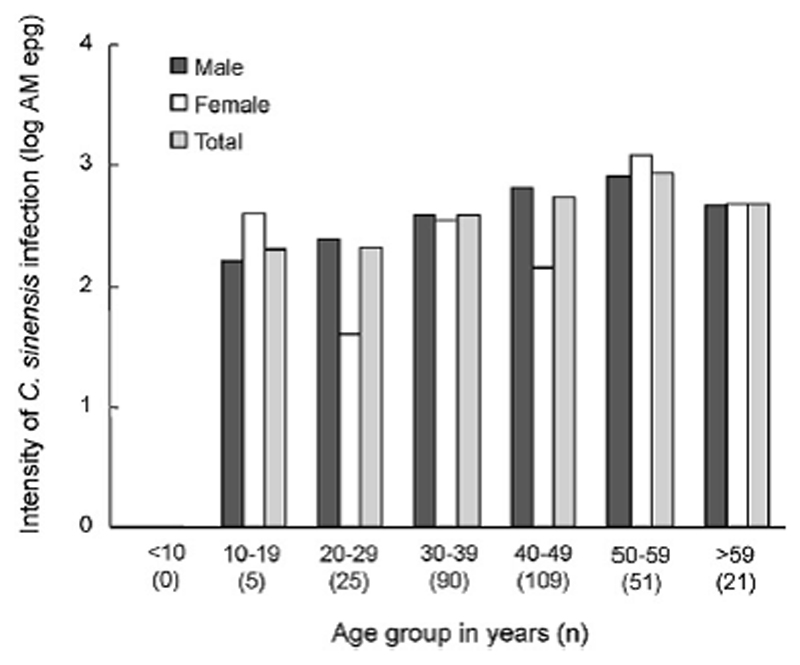
The average counts of C. sinensis eggs among 301 positive samples were 472 eggs per gram of feces (epg). The highest count was 15,801 eggs per gram feces found in a male sample at 55 years of age. Mann-Whitney/Wilcoxon test applied on the log-transformed mean egg counts showed significant difference in infection intensity between males and females (p<0.001), among different age groups (p<0.001) and between those eating raw fish and those not (p<0.001). According to multiple regression analysis, however, habit of eating raw fish was found to be the main factor associated with increased intensity (p<0.001) (Table 2). Figure 2 illustrates the arithmetic mean intensity of C. sinensis infection by age and gender. The intensity of C. sinensis infection in male population increased with age and reached its peak in the 50 – 59 years age group. On the other hand, there was no linear relationship between age and the infection intensity in female population. The peaks of the intensity in females were observed in 10 – 19 years group (391 epg) and 50 – 59 years group (1189 epg), which were even higher than those of the male population.
Table 2.
General linear model for factors associated with the intensity of C. sinensis infection in 2 commune, Kin Som district, Ninh Binh province, Vietnam, 1999-2000 (n=301).
| Variable | N | Prevalence n (%) | Crude odds ratio (95% CI) |
P-value (unadjusted)a |
Adjusted odds ratio (95% CI)b |
P-value (adjusted)c |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 764 | 258 (33.8) | 4.12 (2.91–5.86) | <0.001 | 0.67 (0.41–1.10) | 0.11 |
| Female | 391 | 43 (11.0) | ||||
| Age (years) | ||||||
| <29 | 418 | 30 (7.2) | ||||
| >29 | 737 | 271 (36.8) | 7.52 (5.04–11.23) | <0.001 | 1.07 (0.62–0.27) | 0.82 |
| Habit of eating raw fish | ||||||
| Yes | 648 | 289 (44.6) | 57.50 (26.84–123.18) | <0.001 | 53.15 (19.94–161.67) | <0.001 |
| No | 507 | 7 (3.3) | ||||
P-values from univariate analysis for each risk factor.
Odds ratios from logistic regression analysis for all the three risk factors.
P-values from logistic regression analysis for all the three risk factors.
Figure 2.
Intensity of C. sinensis infection by age and gender in 2 commune, Kin Som district, Ninh Binh province, Vietnam, 1999-2000 (n=301).
Quantity of raw fish consumption and C. sinensis infection
Consumption of raw fish was reported by 75.1% of the study population between 20 and 60 years of age (863 individuals). None of the study participants below 20 years of age reported eating raw fish. The number of males having the habit of raw fish consumption was 3 times higher than that of females.
Correlation of infection with the positive response to the questionnaire
Table 1 summarizes results of logistic regression to analyze the association between the prevalence of C. sinensis infection and report of consumption of raw fish as well as age and gender of the respondents.
Table 1.
Crude and adjusted odds ratio and 95% confidence intervals (CI) for three risk factors relative to C. sinensis infection in 2 commune, Kin Som district, Ninh Binh province, Vietnam, 1999-2000 (n=1155).
| Variable | n | Mean (range) epga | P-value (unadjusted)b | P-value (adjusted)c |
|---|---|---|---|---|
| Gender | ||||
| Male | 258 | 461.32 (0–15801) | <0.001 | 0.99 |
| Female | 43 | 138.19 (0–7797) | ||
| Age (years) | ||||
| <10 | 0 | <0.001 | 0.66 | |
| 10–19 | 5 | 196,45 (0–391) | ||
| 20–29 | 25 | 205.03 (0–2047) | ||
| 30–39 | 90 | 375.45 (0–7245) | ||
| 40–49 | 109 | 544,76 (0–9085) | ||
| 50–59 | 51 | 863.33 (0–15 801) | ||
| >59 | 21 | 468.18 (0–7797) | ||
| Habit of eating raw fish | ||||
| Yes | 289 | 497,17 (0–15 801) | <0.001 | <0.001 |
| No | 7 | 33.64 (0–2553) | ||
Arithmetic mean (range) egg counts per gram feces (epg).
P-values from univariate analysis for each risk factor.
P-values from multiple regression analysis for all the three risk factors.
The positive answer to the question about the habit of eating raw fish was found to correspond to an increased risk of infection by 53-fold (95% Cl 19.94-161.67), while gender and age were confounding factors. While approximately half of the people reporting eating raw fish have been infected (44.6%), only 3.3 % of those not reporting a habit of eating raw fish were found to be infected with C. sinensis. Habit of eating raw fish was also found to be the main factor of increased infection intensity. Sensitivity, specificity, positive and negative predictive values of screening by raw fish consumption was 0.98, 0.37, 0.45 and 0.97, respectively.
Table 3 summarizes information on cumulative quantity of raw fish consumption obtained from 159 study participants who reported raw fish consumption and were also infected with C. sinensis. The intensity of the infection illustrated a positive association with history of raw fish consumption (χ2=23.2, p<0.01), annual frequency of raw fish consumption (χ2=44.7, p<0.01), quantity of raw fish consumed per occasion (χ2=23.7, p<0.01) and the cumulative quantity of raw fish reported to be consumed by the time of the survey (χ2=73.8, p<0.01).
Table 3.
Summary of the association between the intensity of C. sinensis infection and the cumulative quantity of raw fish consumed in each interviewee's history by the time of the survey in 2 commune, Kin Som district, Ninh Binh province, Vietnam, 1999-2000 (n=159).
| Variable | n | Mean epg±SD (x103) |
|---|---|---|
| History of raw fish consumption (years)a | ||
| <10 | 71 | 0.84 ± 0.67 |
| 10–20 | 62 | 1.51 ± 1.46 |
| 20–30 | 25 | 2.62 ± 3.56 |
| >30 | 1 | 0.62 ± 0 |
| Annual frequency of raw fish consumption (times/year) | ||
| <10 | 56 | 0.56 ± 0.25 |
| 10–20 | 65 | 1.19 ± 1.09 |
| 21–40 | 38 | 2.93 ± 2.92 |
| Quantity of raw fish consumed per occasion (g) | ||
| <100 | 74 | 0.62 ± 0.37 |
| 100—200 | 63 | 1.55 ± 1.50 |
| 201–400 | 8 | 3.94 ± 3.70 |
| >400 | 14 | 1.38 ± 1.83 |
| Cumulative quantity of raw fish consumed in lifetime (g)b | ||
| <1000 | 45 | 0.41 ± 0.20 |
| 1000–2000 | 33 | 0.79 ± 0.24 |
| 2001–4000 | 28 | 0.97 ± 0.35 |
| 4001–8000 | 25 | 1.58 ± 0.84 |
| 8001–16000 | 15 | 3.50 ± 2.05 |
| >16000 | 13 | 4.51 ± 4.03 |
History of raw fish consumption by the time of the study, expressed in years.
Cumulative quantity of raw fish consumed in lifetime by the time of the study.
Discussion
The high level of C. sinensis infection documented in this study confirms the previous findings by Nguyen Van De et al. (1997), Kino et al. (1998), Le Van Chau et al. (2001), Kieu Tung Lam et al. (1992) and Nguyen Thi Hung (1999) in the same province. The study also confirmed the report of Nguyen Van De et al (2003) concerning the fact that C. sinensis is the food borne trematode specie more prevalent in northern Vietnam.
The prevalence of infection was higher in males than in females, and it increased with age in both genders. Those findings are consistent with previous reports from Vietnam as well as China, Thailand and Laos (Nontasut et al. 2003; Kieu Tung Lam et al. 1992; Chen et al.1994; Sornmani et al., 1973; Sayasone et al., 2007; Zhang et al., 2007; Lin et al., 2005) and it is probably due to the more frequent raw fish eating in males (Nontasut et al., 2003). In our study population, males had 3-fold more occasions of raw fish consumption than females.
The 3 % C. sinensis infection of the people not reporting raw fish eating is probably due to under-report or a possibility of cross-contamination during cooking process as metacercariae of C. sinensis is mucilaginous and possibly sticks to cooking utensils and could theoretically contaminate other food. The concern on using the same utensils for raw fish and cooked food has been discussed by Zhang et at (2007).
The progressive increase of prevalence and intensity of infection with age suggests a possible accumulation of C. sinensis infection over time by re-infection through repetitive consumption of raw fish in male population. The irregular pattern of the infection intensity by age in females in our study might indicate that many of the infected females are sporadically consuming raw fish or ingesting the metacercariae through cross-contamination rather than practicing a regular eating of raw fish as males commonly do.
Repetitive infection with C. sinensis is known to increase a risk of cholangiocarcinoma, a cancer of the bile duct (Parkin et al., 1993). While no reports on the prevalence of cholangiocarcinoma in Vietnam have been published so far, a high prevalence of cholangiocarcinoma and its clear association with the liver fluke infection have been increasingly revealed in Thailand (Wiwanitkit 2003; Parkin et al., 2006). This demonstrates the need for further researches on the prevalence of cholangiocarcinoma in the communities in Vietnam where a high prevalence of C. sinensis infection has been identified.
The strong association to the report in questionnaire of raw fish consumption to the prevalence and the intensity of C. sinensis infection in our opinion strongly suggest that, similarly to questionnaires used for the identification of schistosomiasis (WHO 1995), simple questionnaires investigating food habits could be used to screen population in areas where clonorchiasis is transmitted permitting to apply large scale chemotherapy with praziquantel avoiding the cost of the parasitological investigation. Finally, an importance of promoting hygienic diets and food processing was realized for prevention of C. sinensis infection.
Acknowledgements
We are indebted to the staffs of the National Institute of Veterinary Research (Hanoi) and the National Institute of Malariology, Parasitology and Entomology (Hanoi) for technical assistance. We also thank the active corporation of the authorities of the Department of Health on provincial, district and commune level as well as the people of Kim Son district.
Funding: None
Footnotes
Conflicts of interest statement: None declared
Ethical approval: The data presented in this paper was collected during the national Clonorchiasis control activity conducted by the Ministry of Health, Vietnam, in order to improve the performance of the activity. Therefore, ethical approval was not required.
Authors’ contributions
TDTC and KNV conceived and designed the study and collected the field data; TDTC administered the questionnaire and conducted molecular analysis; AY and AMcarried out the statistical analysis and interpretation of the data and prepared the manuscript; all the authors revised the manuscript and approved the final version of the manuscript. AM is the guarantor of the paper.
References
- Chen M, Lu Y, Hua X, Mott K. Progress in assessment of morbidity due to Clonorchis sinensis infection: a review of recent literature. Trop Dis Bull. 1994;91:7–65. [Google Scholar]
- Katz N, Chaves A, Pellegrino J. A simple method for quantitative stool thick-smear technique in Schistosomaiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- Lam Kieu Tung, Nguyen Thi Tan, Dang Thanh Son, Ha Viet Vien, Van Chau Le. Survey on epidemiology, treatment and prevention from Clonorchiasis in North Vietnam. Summary record of scientific research works 1986 – 1990. Hanoi: National Institute of Malariology, Parasitology and Entomology. 1992;2:30–37. (in Vietnamese) [Google Scholar]
- Kino H, Inaba H, Van De Nguyen, Van Chau Le, Son Dang Thanh, Hao Hoan Thi, Toan Nguyen Duy, Cong Le Dinh, Sano M. Epidemiology of clonorchiasis in Ninh Binh province, Vietnam. Southeast Asian J Trop Med Public Health. 1998;29:250–254. [PubMed] [Google Scholar]
- Van Chau Le, Son Dang Thanh, Hien Nguyen Thu, Van De Nguyen, Vien Ha Viet, Cong Le Dinh. Current situation of clonorchiasis infection in the Red River Delta. Malaria Parasit Dis Prevent Bull. 2001;4:96–191. [in Vietnamese] [Google Scholar]
- Lin R, Li X, Lau C, Yu S, Kawanaka M. Investigation on the epidemiological factors of Clonorchis sinensis infection in an area of south China. Southeast Asian J Trop Med Public Health. 2005;36:1114–1117. [PubMed] [Google Scholar]
- Hung Nguyen Thi, Tap Phan Huy, Vu Nguyen Cao, Xuan Pham Thi, CTV Results of survey on parasitic worm infection in delta area of Ninh Binh province, Vietnam. Malaria Parasit Dis Prevent Bull. 1990;2:77–81. [in Vietnamese] [Google Scholar]
- Van De Nguyen, Lam Keiu Tung, Van Chau Le, Son Dang Thanh, Tan Nguyen Thi, Vien Ha Viet, Chuynen Le Thi, Mai Dinh Thi, Hien Nguyen Thi. Liver fluke infection and changes of its infection rates after specific treatment. Summary record of scientific research works 1991 – 1996. Hanoi: National Institute of Malariology, Parasitology and Entomology. 1997;2:69–77. [in Vietnamese] [Google Scholar]
- Van De Nguyen, Cong Le Dinh, Cam Phung Dac, Van Chau Le, Toan Nguyen Duy, Dalsgaard A. The foodborne trematode zoonoses of Vietnam. Southeast Asian J Trop Med Public Health. 2003;34(Suppl.):12–34. [PubMed] [Google Scholar]
- Nontasut P, Thong TV, Waikagul J, Fungladda W, Imamee N, Van De Nguyen. Social and behavioral factors associated with clonorchiasis infection in one commune located in the Red River Delta of Vietnam. Southeast Asian J Trop Med Public Health. 2003;34:269–273. [PubMed] [Google Scholar]
- Olsen A, Thuan Le Khanh, Murrell D, Dalsgaard A, Johansen MV, Van De Nguyen. Cross-sectional parasitological survey for helminth infections among fish farmers in Nghe An province, Vietnam. Acta Trop. 2006;100:199–204. doi: 10.1016/j.actatropica.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Ohshima H, Srivatanakul P, Vatanasapt V. Cholangiocarcinoma: Epidemiology, mechanisms of carcinogenesis and prevention. Cancer, Epidemiology, Biomarkers and Prevention. 1993;2:537–544. [PubMed] [Google Scholar]
- Parkin DM, Srivatanakul P, Khlat M, Chenvidhya D, Chotiwan P, Insiripong S, L’abbe KA, Wild CP. Liver cancer in Thailand: I. A case – control study of cholangiocarcinoma. Intl J Cancer. 2006;48:323–328. doi: 10.1002/ijc.2910480302. [DOI] [PubMed] [Google Scholar]
- Sayasone S, Odermatt P, Phoumindr N, Vongsaravane X, Sensombath V, Phetsouvanh R, Choulamany X, Strobel M. Epidemiology of Opisthorchis viverrini in a rural district of southern Lao PDR. Trans R Soc Trop Med. 2007;101:40–47. doi: 10.1016/j.trstmh.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Sithithaworn P, Haswell-Elkins M. Epidemiology of Opisthorchis viverrini. Acta Trop. 2003;88:187–194. doi: 10.1016/j.actatropica.2003.02.001. [DOI] [PubMed] [Google Scholar]
- Sornmani S, Vivatanasesth P, Bunnag T, Intarakhao C, Harinasuta C. A study on the pattern of socioeconomic and health status in relation to parasitic diseases in the inhabitants around Ubolratana Dam in northeast Thailand. Southeast Asian J Trop Med Public Health. 1973;4:421–434. [PubMed] [Google Scholar]
- Thaenkham U, Visetsuk K, Dung Do Trung, Waikagul J. Discrimination of Opisthorchis viverrini from Haprochis Taichui using COI sequence marker. Acta Trop. 2007;103:26–32. doi: 10.1016/j.actatropica.2007.05.006. [DOI] [PubMed] [Google Scholar]
- WHO. Basic laboratory methods in medical parasitology. Geneva: World Health Organization, Geneva; 1991. (Document TDR/SER/MSR/95.2) [Google Scholar]
- WHO. Bench Aids for the diagnosis of intestinal parasites. Geneva: World Health Organization, Geneva; 1994. [Google Scholar]
- WHO. The Schistosomiasis Manual. Geneva: World health Organization; 1995. [Google Scholar]
- WHO. Report of Joint WHO/FAO workshop on Foodborne Trematode Infections in Asia. Report series Number RS/2002/GE/40 (VTN); Manila, Philippines: 2004. [Google Scholar]
- Wiwanitkit V. Clinical findings among 62 Thais with cholangiocarcinoma. Trop Med Intl Health. 2003;8:228–230. doi: 10.1046/j.1365-3156.2003.01016.x. [DOI] [PubMed] [Google Scholar]
- Wongratanacheewin S, Pumidonming W, Sermswan RW, Pipitgool V, Maleewong W. Detection of Opisthorchis viverrini in human stool specimens by PCR. J Clin Microbial. 2002;40:3879–3880. doi: 10.1128/JCM.40.10.3879-3880.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Gao S, Geng Y, Huang D, Yu L, Zhang S, Cheng J, Fu Y. Epidemiological study on Clonorchis sinensis infection in Shenzhen area of Zhujiang delta in China. Parasitol Res. 2007;101:179–183. doi: 10.1007/s00436-006-0441-3. [DOI] [PubMed] [Google Scholar]