Abstract
Macrophages are required for proper mammary gland development and maintaining tissue homeostasis. However, the mechanisms by which macrophages regulate this process remain unclear. Here, we identify STAT5 as an important regulator of macrophage function in the developing mammary gland. Analysis of mammary glands from mice with STAT5-deficient macrophages demonstrates delayed ductal elongation, enhanced ductal branching and increased epithelial proliferation. Further analysis reveals that STAT5 deletion in macrophages leads to enhanced expression of proliferative factors such as Cyp19a1/aromatase and IL-6. Mechanistic studies demonstrate that STAT5 binds directly to the Cyp19a1 promoter in macrophages to suppress gene expression and that loss of STAT5 results in enhanced stromal expression of aromatase. Finally, we demonstrate that STAT5 deletion in macrophages cooperates with oncogenic initiation in mammary epithelium to accelerate the formation of estrogen receptor (ER)-positive hyperplasias. These studies establish the importance of STAT5 in macrophages during ductal morphogenesis in the mammary gland and demonstrate that altering STAT5 function in macrophages can affect the development of tissue-specific disease.
Keywords: Mammary gland, Macrophage, STAT5, Aromatase, Estrogen, Ductal morphogenesis
1. Introduction
Recent efforts have emphasized the importance of tissue resident macrophages in regulating tissue homeostasis (Davies et al., 2013). Resident macrophages are subject to tissue programming and these macrophages exhibit distinct functions based on the tissue in which they reside and their localization within the tissue (Gabanyi et al., 2016). Macrophages have been documented in the mammary gland and have been linked to regulating the formation of epithelial structures during mammary gland development (Chua et al., 2010; Gouon-Evans et al., 2000; Gyorki et al., 2009; Ingman et al., 2006; Van Nguyen and Pollard, 2002). However, the specific mechanisms that drive macrophage function within the mammary gland have not been fully elucidated.
Elongation of the mammary ducts during puberty, which is driven by specialized structures at the tip of the ducts called terminal end buds (Bai and Rohrschneider, 2010; Humphreys et al., 1996; Kenney et al., 2001), requires a complex set of reciprocal interactions between epithelial cells and the surrounding stroma. Numerous different cell types, including innate and adaptive immune cells, contribute to signaling in the microenvironment through the production of cytokines, chemokines, growth factors, and extracellular matrix components (Cowin and Wysolmerski, 2010; Gouon-Evans et al., 2002; Ingman et al., 2006; Lilla and Werb, 2010; Plaks et al., 2015; Schwertfeger et al., 2006a). Macrophages are found in close association with the epithelium during all stages of mammary gland development, suggesting the existence of a paracrine signaling network between the two cell types (Gouon-Evans et al., 2002, 2000; Schwertfeger et al., 2006a). Previous work has shown that macrophages are essential for the ductal elongation and side-branching that normally occur during puberty (Gouon-Evans et al., 2000; Van Nguyen and Pollard, 2002). While the factors that recruit macrophages to the developing epithelial structures have been characterized, the signaling pathways that are activated in the recruited macrophages as well as the mediators produced by macrophages that regulate mammary gland development remain understudied. Mammary gland development is tightly regulated by a combination of circulating and locally derived factors, including hormones, growth factors and cytokines. The majority of studies have focused on the effects of these factors specifically on mammary epithelial cells. However, it is feasible these factors also activate signaling pathways in macrophages that contribute to their programming and function.
In many instances during tumor development, cancer cells exploit existing developmental processes to promote their own growth (Izrailit and Reedijk, 2012). These processes, which in some cases have been dormant for decades, are reactivated to provide growth factors and stimuli not typically produced in the normal environment. Thus, understanding the process of normal mammary gland development will provide valuable mechanistic insight into tumor initiation and progression. In addition to being found in the mammary gland during normal development, macrophages are often found embedded within developing tumors. These tumor-associated macrophages (TAMs) regulate many processes that are critical for tumorigenesis, such as tumor cell invasion, migration, and proliferation (Bohrer and Schwertfeger, 2012; Lin et al., 2003; Schwertfeger et al., 2006b; Wyckoff et al., 2004). Clinically, increased numbers of infiltrating TAMs are correlated with poor patient prognosis in numerous cancer types, including breast cancer (Lee et al., 1997; Leek et al., 1996; Mahmoud et al., 2012; Medrek et al., 2012). Thus, understanding the signaling pathways that control how macrophages respond to and promote tumor initiation and progression is critical for the development of novel therapeutic strategies.
The studies described here focus on identifying key signaling pathways that regulate macrophage function during ductal elongation in the mammary gland. Signal transducer and activator of transcription 5 (STAT5) is one signaling pathway that has been previously implicated in mammary epithelial cell proliferation, differentiation and survival (Cui et al., 2004; Liu et al., 1997; Miyoshi et al., 2001; Santos et al., 2010; Vafaizadeh et al., 2010; Yamaji et al., 2009). In this work, we identify a novel function of STAT5 as a regulator of macrophage function during mammary gland development and demonstrate that STAT5 is normally activated in a subset of mammary gland macrophages during development. We use a conditional knockout of STAT5 to demonstrate that the loss of STAT5 in macrophages results in altered mammary gland development that is consistent with increased estrogen production and signaling. Our studies also demonstrate that STAT5 deletion in macrophages enhances the formation of ER-positive epithelial lesions in an inducible hyperplasia model. Finally, we demonstrate that treatment of macrophages with inflammatory cytokines results in altered STAT5 binding to target sites in the Cyp19a1 locus, suggesting that exposure to an inflammatory milieu, either local or systemic, could alter the ability of resident macrophages in the mammary gland to maintain homeostasis. The results from these studies describe a novel mechanism of regulation of macrophages in the mammary gland and demonstrate that alterations in signaling pathways in these macrophages are capable of contributing to the development of tissue-specific disease. Understanding the specific mechanisms through which macrophages within the mammary gland maintain homeostasis will ultimately lead to the development of approaches that can be used to manipulate their functions for prevention and/or therapeutic purposes.
2. Materials and methods
2.1. Mice
Csf1r-iCre mice were provided by Dr. Elaine Lin (Deng et al., 2010) on the FVB background and Stat5fl/fl mice were provided by Dr. Lothar Hennighausen (Cui et al., 2004). Wild-type FVB mice were purchased from Harlan Laboratories and the Stat5fl/fl mice were backcrossed to the FVB/N background and backcrossing was verified using congenic analysis (IDEXX-RADIL, Columbia, MO). For iFGFR1 activation, mice were injected twice weekly with 1 mg/kg B/B homodimerizer (Clontech) by intraperitoneal injection as previously described (Schwertfeger et al., 2006b; Welm et al., 2002). Daily estrous staging was performed as previously described using crystal violet-stained cytology of vaginal lavage fluid (McLean et al., 2012). Two hours prior to sacrifice, mice were injected with 30 mg/kg BrdU by intraperitoneal injection. All animal care and procedures were approved by the Institutional Animal Care and Use Committee of the University of Minnesota and were in accordance with the procedures detailed in the Guide for Care and Use of Laboratory Animals.
2.2. Immunoblot analysis
Protein lysates were subjected to SDS-PAGE using 20 μg total protein. Immunoblot analysis was performed using antibodies listed in Supplemental Table 1.
2.3. Immunohistochemistry and immunofluorescence
Mammary glands were harvested, fixed in 4% paraformaldehyde for 2 h, sectioned and stained as previously described (Schwertfeger et al., 2006b) using conditions listed in Supplemental Table 1. All images were acquired using Leica LAS software.
2.4. Cell culture
HC-11 cells were maintained as previously described (Ball et al., 1988; Welm et al., 2002). RAW264.7 cells were grown in media containing DMEM (Life Technologies) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Life Technologies). Bone marrow was flushed from the femurs and tibiae of mice and plated overnight in DMEM + 10% FBS. Non-adherent cells were collected and re-plated in low-attachment plates with DMEM + 10% FBS + 20% conditioned media from L929 cells, a cell line that produces high levels of macrophage-colony stimulating factor. Differentiated macrophages were subsequently re-plated in normal tissue culture dishes for experiments.
2.5. qRT-PCR
Cells were cultured as described above, RNA harvested using TriPure Reagent (Roche) and quantitative reverse transcription PCR was done as previously described (Bohrer and Schwertfeger, 2012) using qScript cDNA SuperMix and PerfeCTa SYBR Green SuperMix (Quantabio). Gene expression was normalized to Ppib (cyclophilin B) levels. Primer sequences used are listed in Supplemental Table 2.
2.6. Mammary gland whole mounts
Mammary glands were harvested and fixed in 4% paraformaldehyde for 2 h, rinsed in 70% ethanol and stained in Carmine alum overnight. Glands were dehydrated using 70%, 95%, and 100% ethanol then cleared in xylene. Stained glands were imaged and subsequently stored in methyl salicylate. Quantification of epithelial area was performed on 8-bit images using ImageJ.
2.7. Chromatin Immunoprecipitation (ChIP)
RAW264.7 macrophages were plated at 3 × 106 cells per plate in a 10 cm plate in DMEM + 10% FBS overnight. Cells were subsequently washed and serum-starved in DMEM overnight and fixed or treated with 50 ng/mL IL-6 as indicated before being fixed. Primary BMDMs were plated at 5 × 105 cells per 6 cm plate and grown for 48 h. Cells were subsequently washed and serum-starved in DMEM for 4 h and fixed or treated with 50 ng/mL IL-6 as indicated before being fixed. ChIP was performed with a STAT5-specific antibody (sc-836X, Santa Cruz), STAT3-specific antibody (sc-482X, Santa Cruz) or non-specific rabbit IgG isotype control using Protein G magnetic beads (Active Motif). Analysis was performed using methods as previously described (Chan et al., 2015). All ChIP data presented are normalized to % input chromatin and presented as fold enrichment over IgG control. Primers sequences are listed in Supplemental Table 2.
2.8. Microarray analysis
Microarray expression analysis was performed as previously described (Reed et al., 2012). Female FVB mice were sacrificed at 6 weeks or 10 weeks of age and mammary glands were collected for analysis. Tissue was dissociated with 2 mg/mL collagenase A (Roche) for 45 min at 37 °C with gentle rocking. The solutions were vigorously shaken every 15 min and the cells were collected by centrifugation at 1500 rpm for 5 min. The cells were washed 3 times in DMEM/F-12 with 5% FBS and centrifuged at 1500 rpm for 5 min followed by 2 times at 800 rpm for 5 min. The cells were stained with CD11b-APC (Life Technologies) at a dilution of 1:200 or isotype control for 1 h at room temperature. The cells were then subsequently washed, filtered through a 40 μm filter and sorted with a triple laser MoFlo (Cytomation). RNA was isolated from CD11b+ cells sorted from 6 mice per timepoint and pooled into duplicate samples. RNA was extracted using the Arcturus PicoPure RNA Isolation Kit (Life Technologies) and hybridized to the Affymetrix MOE 2.0 microarray (Affymetrix) in the Baylor Microarray Core Facility at Baylor College of Medicine (Houston, TX). Raw data were normalized using Microarray Suite 5.0 and genes called absent in all samples were discarded, while genes that were either upregulated or downregulated at least 2-fold with a p-value of less than 0.05 were further analyzed. Data have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus data repository, accession number GSE36477.
2.9. Fluorescence-activated cell sorting (FACS)
Both #3 and both #4 mammary glands were harvested from 6-week-old female mice. Tissue was minced with scalpels and subsequently digested in 2 mg/mL collagenase A for 1 h at 37 °C. Cells were pelleted by centrifugation and stained for FACS with antibodies listed in Supplemental Table 1. Cell sorting was performed using a BD FACSAria II at the University Flow Cytometry Resource of the University of Minnesota. Sorted cells were processed for qRT-PCR as described above.
2.10. Statistical analysis
Statistical analysis was performed using Student’s unpaired, two-tailed t-test. Comparisons between multiple groups was performed using one-way ANOVA with Tukey’s multiple comparison test. Error bars represent standard error of the mean (SEM).
3. Results
3.1. STAT5 is activated in macrophages in the mammary gland
Numerous local and endocrine factors that are critical for mammary gland development potentiate downstream signaling through STAT proteins, including growth hormone, prolactin, and cytokines such as interleukin-4 (IL-4) and IL-13 (Khaled et al., 2007; Liu et al., 1997). These factors are capable of acting on both epithelial cells and stromal cells, and thus we reasoned that in addition to activation in mammary epithelial cells, STATs may also be activated in cells located within the stroma, such as macrophages, and contribute to their tissue-specific function. Additionally, it has been previously shown that the expression of transcription factors in tissue-resident macrophages is controlled by factors found in the local microenvironment (Okabe and Medzhitov, 2014; Rosas et al., 2014). Thus, we initially examined the expression of STAT family members due to their known contributions to mammary gland development. To determine whether STAT proteins are differentially expressed in macrophages derived from different tissues, gene expression of STAT family members was assessed from intraperitoneal or mammary gland macrophages from 6-week- and 10-week-old mice. Expression levels of Stat5a were significantly higher in mammary gland-derived macrophages isolated from both 6-week and 10-week-old mice compared with peritoneal macrophages (Fig. 1A). STAT5 is a transcription factor that has been well-studied as a key regulator of milk protein gene expression in mammary epithelial cells and is also a critical regulator of mammary epithelial cell proliferation, differentiation, and survival (Cui et al., 2004; Liu et al., 1997; Miyoshi et al., 2001; Santos et al., 2010; Vafaizadeh et al., 2010; Yamaji et al., 2009). STAT5 regulates various functions in a variety of immune cell subsets. In B and T cells, STAT5 is an essential regulator of development and differentiation (Yao et al., 2006), while in dendritic cells STAT5 is critical for promoting a TH2 response (Bell et al., 2013). Previous work has suggested that STAT5 may be involved in regulating IL-3-mediated polarization (Kuroda et al., 2009), however, little else is known about the role of STAT5 in macrophages.
Fig. 1.
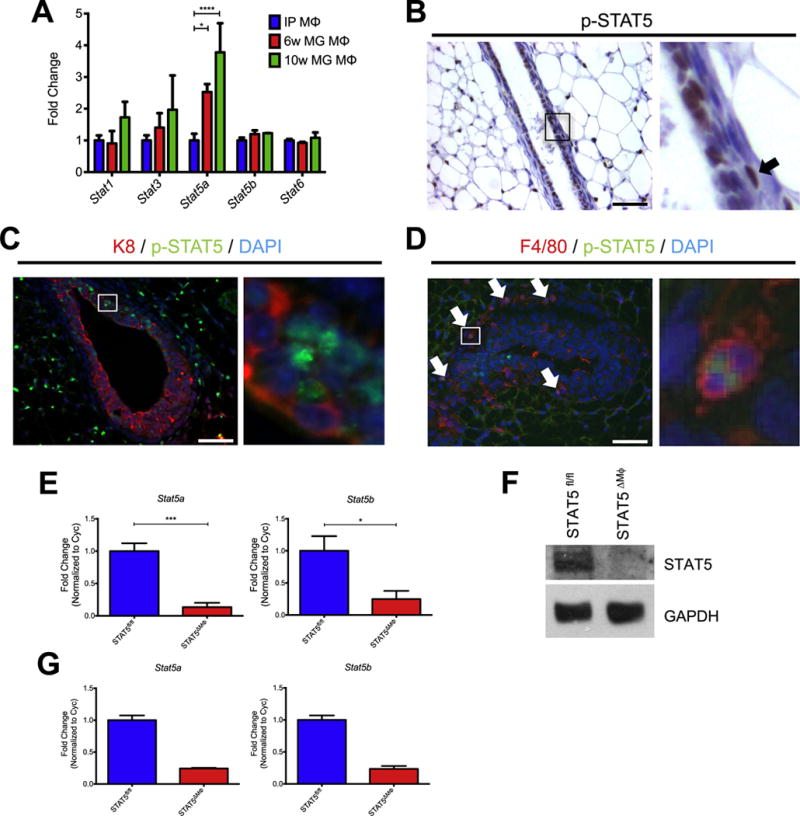
STAT5 is activated in macrophages in the mammary gland. A) Microarray gene expression from intraperitoneal (IP) or mammary gland (MG) macrophages. B) Paraffin-embedded mammary gland sections stained for p-STAT5 and counterstained with hematoxylin. Region identified in square magnified to the right and arrowhead indicates a p-STAT5+ stromal cell. C) Paraffin-embedded mammary gland sections were stained for K8 (red), p-STAT5 (green), and DAPI (blue). Region identified in square magnified to the right. D) Frozen mammary gland sections were stained for F4/80 (red), p-STAT5 (green), and DAPI (blue). Arrowheads indicate p-STAT5+ macrophages and region identified in square magnified to the right. E) Expression of Stat5a and Stat5b assessed by qRT-PCR in BMDMs from STAT5fl/fl and STAT5ΔMϕ mice. F) Immunoblot of STAT5 levels in BMDMs from STAT5fl/fl and STAT5ΔMϕ mice. GAPDH is shown as a loading control. G) Expression of Stat5a and Stat5b assessed by qRT-PCR in sorted mammary gland macrophages from STAT5fl/fl and STAT5ΔMϕ mice. Scale bars represent 50 μm. n=5, *p < 0.05, ***p < 0.001, ****p < 0.0001.
Initial studies were performed to examine the activation status of STAT5 in stromal cells in vivo. Paraffin-embedded mammary glands from pubertal wild-type mice were stained for p-STAT5, revealing numerous p-STAT5+ cells located both within and surrounding mammary ducts (Fig. 1B). To determine the identity of these cells, tissue sections were co-stained for p-STAT5 and the epithelial marker keratin 8 (K8). While some p-STAT5+ / K8+ epithelial cells were observed, as expected, we also observed activation of STAT5 in a number of stromal cells, including a large population of p-STAT5+ / K8− cells found in close proximity to the epithelium (Fig. 1C). These cells were located in a region around the terminal end buds where macrophages have been shown to reside (Gouon-Evans et al., 2002; Schwertfeger et al., 2006a). To determine whether macrophages represent a population of p-STAT5+ / K8− cells, cryosectioned mammary glands from wild-type mice were co-stained for p-STAT5 and F4/80. Numerous F4/80+ cells were observed along the neck of the terminal end bud with 30% of these cells also being p-STAT5+, demonstrating activation of STAT5 in a subset of mammary gland macrophages in vivo (Fig. 1D).
To determine the role of STAT5 signaling in macrophages during mammary gland development, a conditional knock-out approach was taken to generate mice carrying a tissue-restricted deletion of STAT5. These mice have both the Stat5a and Stat5b loci flanked by loxP recombination sites (STAT5fl/fl) and were crossed to mice harboring a transgene expressing Cre recombinase under the control of the Csf1r promoter (STAT5ΔMϕ), which deletes predominantly in myeloid cells (Deng et al., 2010). Deletion of STAT5 was verified in primary bone marrow-derived macrophages (BMDMs) by quantitative reverse transcription PCR (qRT-PCR) (Fig. 1E) and immunoblot analysis (Fig. 1F). To further confirm the deletion of STAT5, mammary glands from STAT5fl/fl and STAT5ΔMϕ mice were harvested and subjected to fluorescence-activated cell sorting. Populations of CD45+; F4/80+ cells, representing mammary gland macrophages, were collected and transcript levels of both Stat5a and Stat5b were found to be reduced in mammary gland macrophages from the STAT5ΔMϕ mice compared to littermate STAT5fl/fl control mice (Fig. 1G). The expression of Cre is limited primarily to myeloid cells and no deletion of STAT5 was detected in mammary epithelial cells (Fig. S1).
3.2. STAT5 deletion in macrophages disrupts normal mammary gland development
To assess STAT5 function in macrophages within the mammary gland, mammary gland development was analyzed in STAT5fl/fl and STAT5ΔMϕ mice. To account for morphological changes that occur during the estrous cycle, 6-week-old STAT5fl/fl and STAT5ΔMϕ mice were staged as previously described (McLean et al., 2012) and mammary glands were harvested during diestrus. Whole mount analysis revealed no differences in terminal end bud number in mammary glands from STAT5fl/fl and STAT5ΔMϕ mice (Fig. 2A and data not shown). However, a significant reduction in ductal elongation (Fig. 2B) and a significant increase in lateral branchpoints per duct (Fig. 2C) were observed in mammary glands from STAT5ΔMϕ mice compared to littermate controls. Deletion of the related transcription factor STAT3 in macrophages did not result in any gross developmental perturbations (data not shown). As an additional control, mammary glands from STAT5fl/+; Csf1r-iCre+ mice which carry a heterozygous deletion of STAT5 in cells of the myeloid lineage (STAT5fl/Δ) exhibited no defect in ductal elongation (Fig. S2A and B), suggesting that the observed changes in mammary gland development are specifically attributable to the loss of STAT5 in macrophages and not due to non-specific effects of Cre recombinase expression.
Fig. 2.
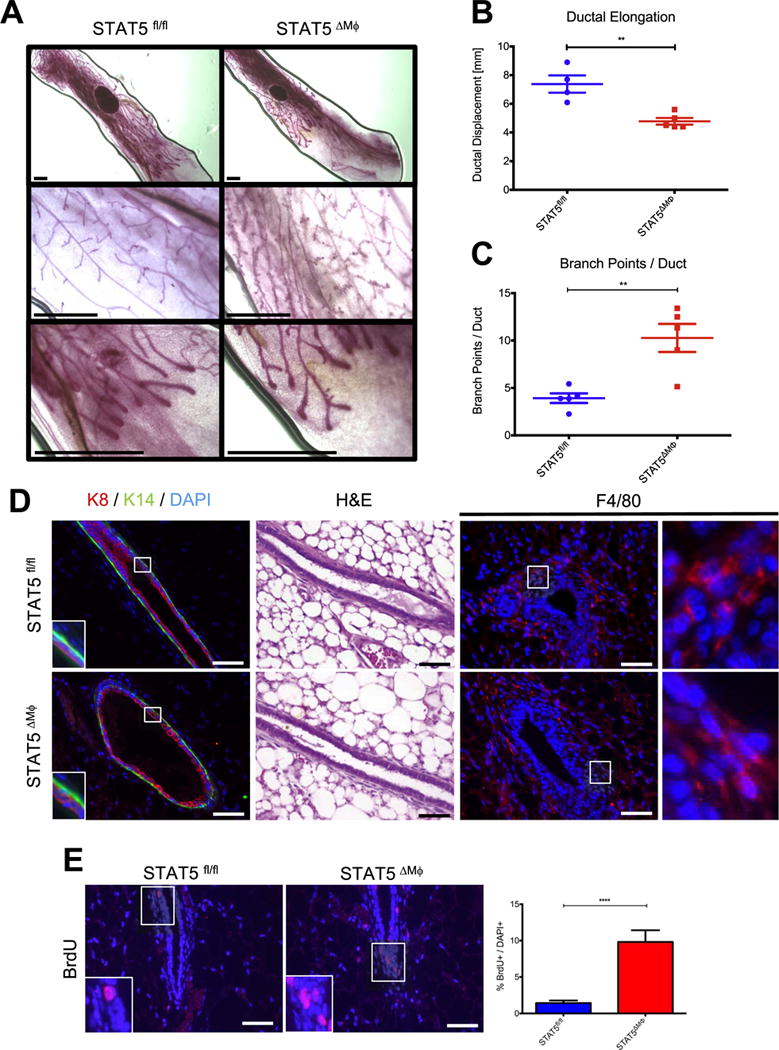
STAT5 deletion in macrophages disrupts normal mammary gland development. A) [Top] Whole mount analysis of mammary glands. [Middle] High-power magnification of mammary ducts. [Bottom] High-power magnification of terminal end buds. B) Quantification of ductal elongation in mouse mammary glands. C) Analysis of epithelial branchpoints per mammary duct. Scale bars represent 1 mm. D) [Left] Paraffin-embedded mammary gland sections were stained for K8 (red), K14 (green), and DAPI (blue). Regions identified in squares magnified in insets. [Middle] H & E staining of mammary gland sections. [Right] Paraffin-embedded mammary gland sections were stained for F4/80 (red) and DAPI (blue). Regions identified in squares magnified in insets. E) [Left] Paraffin-embedded mammary gland sections were stained for BrdU (red) to assess proliferation and counterstained with DAPI (blue). Regions identified in squares magnified in insets. [Right] Quantification of proliferating cells normalized to total number of DAPI+ cells. Scale bars represent 50 μm. n=4 (STAT5fl/fl) and n=5 (STAT5ΔMϕ), **p < 0.01, ****p < 0.0001.
To determine if the observed changes in mammary gland development were accompanied by architectural changes in the ductal structure, mammary glands from STAT5fl/fl and STAT5ΔMϕ mice were stained for K8 and keratin 14 (K14). Mammary glands from both genotypes showed normal ductal architecture, with a single layer of K8+ luminal cells surrounded by a single layer of K14+ myoepithelial cells, and no gross architectural abnormalities were noted by hematoxylin and eosin (H & E) staining (Fig. 2D). F4/80 staining demonstrated that there were no differences in macrophage recruitment to epithelial structures between mammary glands from STAT5fl/fl or STAT5ΔMϕ mice (Fig. 2D). To address whether the decreased ductal elongation and increased side branching were associated with altered rates of proliferation, BrdU incorporation was assessed by immunofluorescence. Mammary glands from the STAT5ΔMϕ mice showed a significant increase in the number of proliferating cells compared to those from STAT5fl/fl control mice (Fig. 2E). The increased proliferation is specific to established mammary ducts, as proliferation was found to be equivalent in the highly proliferative terminal end bud in mammary glands from STAT5fl/fl and STAT5ΔMϕ mice (Fig. S3). Additionally, analysis of mammary gland whole mounts from 10-week-old STAT5ΔMϕ mice indicates that ductal elongation recovers and is similar to control mice at that timepoint (Fig. S4). These data demonstrate that the loss of STAT5 signaling in macrophages impairs normal ductal development and promotes proliferation in the mammary gland.
3.3. Increased expression of estrogen receptor targets in mammary glands from STAT5ΔMϕ mice
Normal mammary gland development is tightly regulated by hormone signaling and any perturbations in hormone levels result in altered mammary gland morphology (Ciarloni et al., 2007; Mallepell et al., 2006; Shehata et al., 2014). Administration of exogenous estrogens to mice results in decreased ductal elongation and increased epithelial branching (Thomsen et al., 2006), similar to the observed phenotype in STAT5ΔMϕ mice. Therefore, experiments were performed to determine whether estrogen signaling was enhanced in mammary glands from STAT5ΔMϕ mice. To investigate the contributions of hormone signaling, six-week-old STAT5fl/fl and STAT5ΔMϕ mice were staged in their estrous cycles, inguinal mammary glands were harvested during diestrus and mammary epithelial cells were harvested following collagenase digestion. Assessment of the canonical ER targets Ctsd, Wnt4 and AREG demonstrated increased expression in mammary epithelial cells from STAT5ΔMϕ mice compared to littermate controls (Fig. 3A–C). No alterations were observed in the quantity or distribution of ER+ mammary epithelial cells between STAT5fl/fl and STAT5ΔMϕ mice (Fig. 3D and S5A), suggesting that increased ER target gene expression was not due to increased numbers of ER+ epithelial cells. Additionally, no differences in estrous cycle stages or length were observed between STAT5fl/fl and STAT5ΔMϕ mice, suggesting that the loss of STAT5 in macrophages does not affect global hormone signaling homeostasis (Fig. S5B).
Fig. 3.
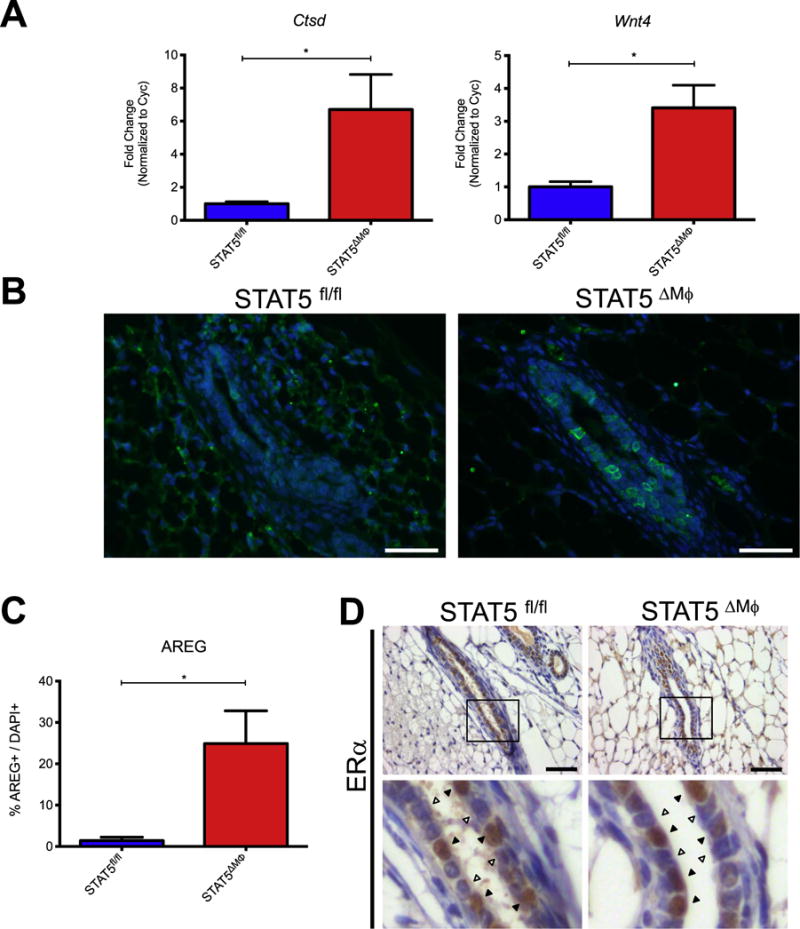
Increased expression of estrogen receptor targets in mammary glands from STAT5ΔMϕ mice. A) qRT-PCR for ER target genes in mammary epithelial cells isolated from STAT5fl/fl and STAT5ΔMϕ mice. B) Paraffin-embedded mammary gland sections were stained for AREG (green) and counterstained with DAPI (blue). C) Quantification of AREG staining normalized to total number of DAPI+ cells. D) [Top] Paraffin-embedded mammary gland sections were stained for ERα and counterstained with hematoxylin. [Bottom] Magnification of epithelial structures. Arrowheads indicate ERα+ cells. Scale bars represent 50 μm. n=4 (STAT5fl/fl) and n=5 (STAT5ΔMϕ), *p < 0.05.
3.4. Increased aromatase expression in STAT5-deficient macrophages
While we observed an increase in estrogen signaling in the mammary glands of STAT5ΔMϕ mice, we did not find significantly elevated levels of circulating estrogen (Fig. S6). Thus, a potential mechanism to explain the increased ER target gene expression observed in the STAT5ΔMϕ mice is an increased local production of estrogen. Aromatase, the protein product of the cytochrome P450, family 19, subfamily A, polypeptide 1 (Cyp19a1) gene, is a critical enzyme required for the biosynthesis of estrogen. STAT5 has been previously implicated in repression of gene expression through tetramerization and binding to tandem consensus sequences in the genome (Lin et al., 2012; Mandal et al., 2011). A putative STAT5 tetramer binding site was identified in the large first intron of the Cyp19a1 gene, approximately 700 bp downstream of the transcription start site (Fig. 4A). To model the mammary gland microenvironment in vitro, primary BMDMs were treated with conditioned media from HC-11 cells, a non-transformed mouse mammary epithelial cell line. This system has biological relevance, as BMDMs have been previously shown to be recruited to the mammary gland (O’Brien et al., 2012) and HC-11 cells retain many characteristics of normal mammary epithelium, including hormone responsiveness, milk protein production, and the ability to form mature mammary ducts in a cleared fat pad (Ball et al., 1988; Danielson et al., 1984). To determine if macrophages express aromatase in response to soluble factors produced by mammary epithelial cells, primary BMDMs were differentiated from STAT5fl/fl and STAT5ΔMϕ mice and exposed to conditioned media from HC-11 cells. Expression levels of Cyp19a1 were significantly increased in STAT5-deficient macrophages compared to STAT5fl/fl BMDMs (Fig. 4B). To assess whether STAT5 is bound to the Cyp19a1 gene under basal conditions to repress aromatase expression, chromatin immunoprecipitation (ChIP) using primers that span the predicted STAT5 binding site (Fig. 4A, arrowheads) demonstrated significantly enhanced enrichment of target DNA compared to isotype control (Fig. 4C). Complementing these studies, STAT5 ChIP in primary BMDM from STAT5fl/fl mice demonstrated enrichment for STAT5 at the Cyp19a1 locus, which is not observed in BMDM from STAT5ΔMϕ mice (Fig. 4D). These results suggest that STAT5 is a key regulator of Cyp19a1 expression in macrophages.
Fig. 4.
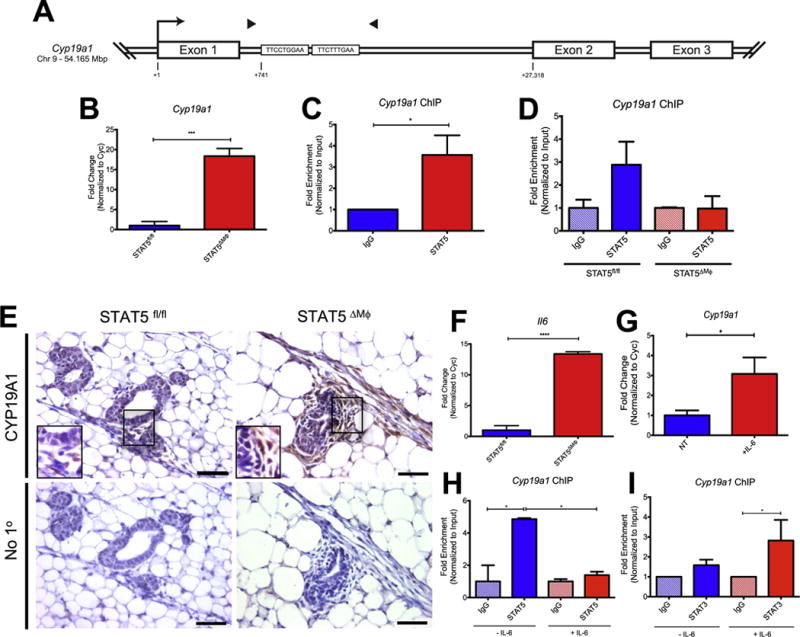
Increased aromatase expression in STAT5-deficient macrophages. A) Schematic of the Cyp19a1 locus containing STAT5 binding sites. Transcription start site (arrow) and ChIP primers (arrowheads) are shown. B) mRNA expression of Cyp19a1 in STAT5fl/fl and STAT5ΔMϕ BMDM. Representative experiment of four replicates shown. C) Fold enrichment of Cyp19a1 in RAW264.7 macrophages by ChIP using STAT5-specific antibody or isotype control. Data analyzed from five independent replicates. D) Fold enrichment of Cyp19a1 in STAT5fl/fl and STAT5ΔMϕ BMDM by ChIP using STAT5-specific antibody or isotype control. Representative experiment of three replicates shown. E) [Top] Paraffin-embedded mammary gland sections were stained for aromatase. Regions identified in squares magnified in insets. [Bottom] Immunohistochemical staining without primary antibody as negative control. F) mRNA expression of Il6 in STAT5fl/fl and STAT5ΔMϕ BMDM. G) Cyp19a1 mRNA expression in RAW264.7 macrophages stimulated with rmIL-6. n=6 per treatment. H) Fold enrichment of Cyp19a1 in RAW264.7 macrophages +/− IL-6 for 2 h by ChIP using STAT5-specific antibody or isotype control. Representative experiment of three replicates shown. I) Fold enrichment of Cyp19a1 in RAW264.7 macrophages +/− IL-6 for 30 min by ChIP using STAT3-specific antibody or isotype control. Data analyzed from three independent replicates. *p < 0.05, ***p < 0.001, ****p < 0.0001.
To confirm that aromatase expression is increased in vivo, we examined mammary glands from STAT5fl/fl and STAT5ΔMϕ mice by immunohistochemistry. Consistent with published studies (Subbaramaiah et al., 2011), immunohistochemical staining of mammary glands from control STAT5fl/fl mice revealed no detectable aromatase expression in either the stromal or epithelial cells (Fig. 4E). However, aromatase expression was readily detectable in stromal cells in mammary glands from STAT5ΔMϕ mice (Fig. 4E). The stromal cell population surrounding epithelial structures is generally comprised of multiple cell types, including macrophages and fibroblasts, suggesting that in addition to directly regulating Cyp19a1 expression, loss of STAT5 in macrophages may also lead to production of a factor that induces aromatase in other stromal cells. Thus, additional studies were performed that focus on identifying factors that could regulate Cyp19a1 expression.
3.5. Pro-inflammatory cytokines release the STAT5-dependent repression of aromatase
Local production of estrogen in the mammary gland has been implicated in the context of obesity (Subbaramaiah et al., 2011) and women with a body mass index (BMI) greater than 30 kg/m2 have a more than two-fold increased risk of developing ER+ breast cancer compared to women with BMI less than 25 kg/m2 (Ahn et al., 2007). IL-6 is a key inflammatory cytokine associated with obesity (Eder et al., 2009) and is known to induce Cyp19a1 expression in endometrial cancer stromal cells (Che et al., 2014). Because IL-6 is a key inducer of aromatase in some cell types, we set out to address whether IL-6 could regulate Cyp19a1 expression in macrophages. Analysis of cytokine expression in STAT5-deficient macrophages revealed increased expression of IL-6 (Fig. 4F). Therefore, we examined the hypothesis that IL-6 enhances expression levels of Cyp19a1 in macrophages, which has not been previously examined. IL-6 stimulation of RAW264.7 macrophages led to increased expression of Cyp19a1 (Fig. 4G). Furthermore, we found that treatment of RAW264.7 cells with IL-6 led to reduced occupancy of STAT5 at the Cyp19a1 promoter (Fig. 4H). While STAT5 appears to be a critical negative regulator of Cyp19a1 expression, we sought to identify factors downstream of IL-6 signaling which could positively regulate Cyp19a1 expression. In macrophages, the related transcription factor STAT3 is a crucial mediator of IL-6 signaling. STAT3 and STAT5 have similar consensus sequences in the genome and, in other cell types, it has been shown that the two factors can antagonize each other at the same binding site (Basu et al., 2015; Yang et al., 2011). Thus, we hypothesized that upon IL-6 stimulation, STAT3 would be recruited to the Cyp19a1 gene, displace STAT5 and activate transcription. Indeed, while STAT3 is not bound to the Cyp19a1 gene locus under basal conditions, IL-6 treatment of macrophages led to the recruitment of STAT3 to the identified binding site in the Cyp19a1 gene (Fig. 4I). These data suggest that STAT5 normally binds the Cyp19a1 gene to suppress gene expression and that exposure of macrophages to the inflammatory cytokine IL-6 leads to displacement of STAT5 from the promoter, recruitment of STAT3 and increased expression of Cyp19a1.
3.6. Accelerated formation of ER+ hyperplasias in STAT5ΔMϕ mice
Based on the findings that deletion of STAT5 in macrophages is associated with increased estrogen signaling and the enhanced production of factors involved in mammary tumorigenesis, we predicted that loss of STAT5 in macrophages would lead to the formation of an environment that is permissive for pre-neoplastic alterations. In previous studies, we have used an inducible model of fibroblast growth factor receptor 1 (FGFR1) activation to study mechanisms that drive early stages of mammary tumorigenesis (Bohrer et al., 2014; Schwertfeger et al., 2006b). These studies use transgenic mice that express an inducible FGFR1 (iFGFR1) construct, under the control of the mouse mammary tumor virus (MMTV) promoter, which can be activated by intraperitoneal injection of B/B homodimerizer (Clontech) (Welm et al., 2002). Activation of iFGFR1 in the mammary gland results in the formation of budding epithelial structures along the duct (Schwertfeger et al., 2006b; Welm et al., 2002). The inducible nature of this model allows for the temporal control of FGFR1 activation and the ability to discern how pre-existing alterations in the tissue microenvironment, such as alterations in systemic or localized inflammatory signals, contribute to early stages of tumorigenesis upon acquisition of a somatic mutation. Because the ER status of these lesions has not been previously examined, initial studies were performed to determine whether activation of inducible FGFR1 in mammary epithelial cells leads to ER+ hyperplasia. Mammary glands were isolated from MMTV-iFGFR1+ mice following 2 weeks of B/B treatment and sections were stained for ERα. The budding epithelial structures contained numerous ER+ cells (Fig. 5A), suggesting that the MMTV-iFGFR1 transgenic mice represent a relevant model for studying the formation of ER+ hyperplasias. To determine how the loss of STAT5 in macrophages affects the formation of early stage lesions in this model, STAT5fl/fl and STAT5ΔMϕ mice were crossed with the MMTV-iFGFR1 mice. Consistent with previous reports (Schwertfeger et al., 2006b; Welm et al., 2002), activation of iFGFR1 for 2 weeks in STAT5fl/fl; MMTV-iFGFR1+ mice resulted in the formation of budding epithelial structures along the duct (Fig. 5B, Left). In contrast, the same timecourse of iFGFR1 activation in STAT5ΔMϕ; MMTV-iFGFR1+ mice led to a profound increase in the size and severity of epithelial buds and instances of local hyperplasia (Fig. 5B, Right). Quantification of epithelial area demonstrated an increased amount of epithelium in the hyperplastic lesions of the STAT5ΔMϕ; MMTV-iFGFR1+ mice (Fig. 5C). Consistent with the MMTV-iFGFR1+ mice, budding epithelial structures in both STAT5fl/fl; MMTV-iFGFR1+ and STAT5ΔMϕ; MMTV-iFGFR1+ mice were also ERα+ (Fig. 5D). Analysis of BrdU incorporation revealed that while activation of FGFR1 signaling in the epithelial cells resulted in increased proliferation, the loss of STAT5 in macrophages further enhanced this proliferation (Fig. 5E). Similar to the findings in the normal mammary gland, STAT5 expression in macrophages is dispensable for their recruitment to the budding epithelial structures (Fig. S7). Taken together, these results demonstrate that STAT5 deletion in macrophages pre-disposes the mammary gland to enhanced formation of epithelial hyperplasias.
Fig. 5.
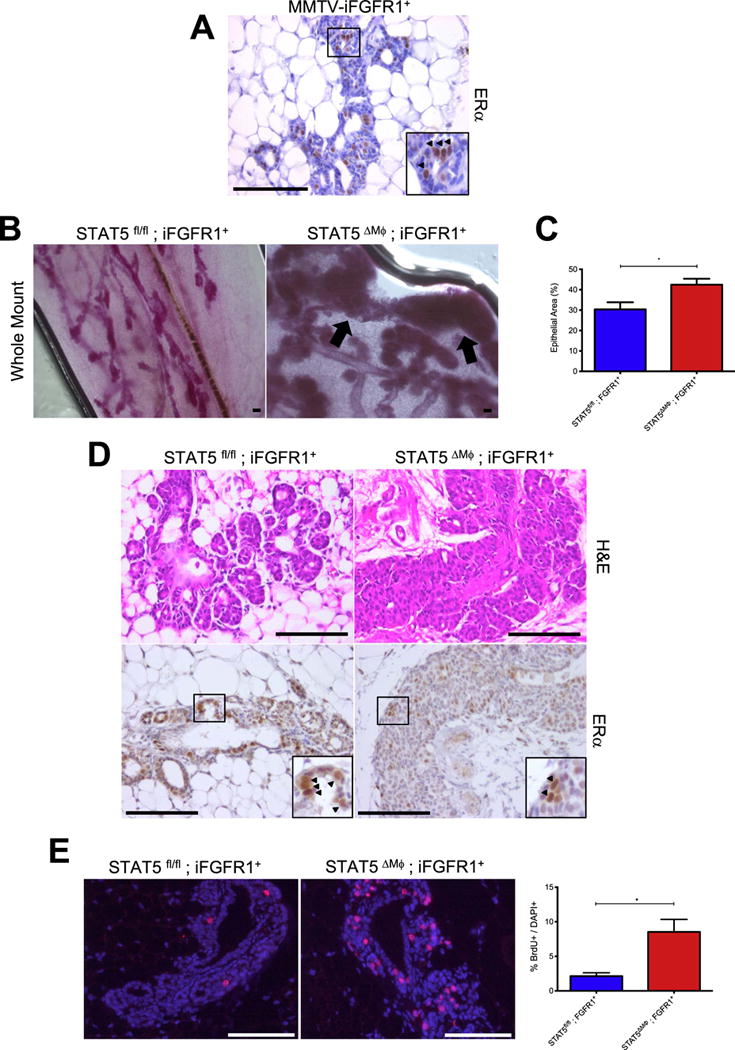
Accelerated mammary tumor initiation in STAT5ΔMϕ mice. A) Paraffin-embedded mammary gland sections from MMTV-iFGFR1+ mice after 2 weeks of iFGFR1 activation were stained for ERα. Arrowheads indicate ERα+ cells and region identified in square magnified in inset. B) Mammary gland whole mounts from mice treated with B/B dimerizer for 2 weeks. Arrows indicate regions of hyperplasia. C) Quantification of epithelial area in hyperplastic lesions from STAT5fl/fl; iFGFR1+ and STAT5ΔMϕ; iFGFR1+ mice. D) [Top] H & E staining of budding epithelial structures and hyperplasias. [Bottom] Paraffin-embedded mammary gland sections from mice after 2 weeks of iFGFR1 activation were stained for ERα. Arrowheads indicate ERα+ cells and region identified in square magnified in inset. E) [Left] Paraffin-embedded mammary gland sections from mice after 2 weeks of iFGFR1 activation were stained for BrdU (red) to assess proliferation and counterstained with DAPI (blue). [Right] Quantification of proliferating cells normalized to total number of DAPI+ cells. Scale bars represent 100 μm. *p < 0.05.
4. Discussion
Genetic ablation and biochemical depletion studies have demonstrated that macrophages play a vital role in both mammary gland development and tumorigenesis (Gouon-Evans et al., 2000; Gyorki et al., 2009; Ingman et al., 2006; O’Brien et al., 2012; Schwertfeger et al., 2006b; Van Nguyen and Pollard, 2002). The results of our studies illustrate a novel mechanism by which macrophages regulate normal mammary gland development and demonstrate that perturbations to the STAT5 signaling axis in these cells can increase susceptibility to oncogenic initiation. In the normal mammary gland, STAT5 signaling is activated in macrophages associated with epithelial structures and may be a critical factor in regulating macrophage function. Using genetic approaches, we have generated mice with a deletion of both alleles of Stat5a and Stat5b in cells of the myeloid lineage (STAT5ΔMΦ). In contrast to the global double knockout mice, STAT5ΔMΦ are viable and fertile, with no gross abnormalities apparent, demonstrating that function of STAT5 signaling in macrophages is distinct from its function in other cell types (Yao et al., 2006). Upon the loss of STAT5 in macrophages, mammary gland development is significantly altered, with decreased ductal elongation, increased lateral branching, and increased epithelial proliferation. These alterations were associated with increased levels of ER target genes. While the Csf1r-iCre transgene is expressed primarily in myeloid cells, certain subsets of splenic dendritic cells and T cells exhibit low levels of Cre-mediated gene deletion (Deng et al., 2010). Given the requirement for macrophages during mammary gland development and the well-described infiltration of macrophages (Chua et al., 2010; Gouon-Evans et al., 2000; Gyorki et al., 2009; Ingman et al., 2006; Van Nguyen and Pollard, 2002), our studies focused on this cell population. However, these studies do not rule out the possibility that deletions in small subsets of less abundant cell types may contribute to the phenotypes observed in these mice. Previous studies have investigated the role of STAT5 specifically in mammary epithelial cells. In contrast to our observations of STAT5 deletion in macrophages, mammary epithelial cell-specific deletion of STAT5 does not alter pubertal development, but instead results in decreased epithelial proliferation in response to estrogen and progesterone (Cui et al., 2004; Liu et al., 1997; Miyoshi et al., 2001; Santos et al., 2010; Vafaizadeh et al., 2010; Yamaji et al., 2009). Because secreted factors that activate STAT5 are present in the mammary gland during other stages of development, future studies will examine STAT5 function in macrophages during additional stages of mammary gland development.
Hormone signaling is a very well-regulated process in normal tissues. However, during pathologic conditions such as obesity and breast cancer, this axis can become perturbed through increased production of estrogen via the enzyme aromatase. Numerous studies have elucidated the role of hormone signaling in the developing mammary gland using tissue transplant techniques. Estrogen signaling is required in mammary epithelial cells, not stromal cells, to facilitate proper ductal elongation, as ERα-null epithelial cells remain in a rudimentary ductal tree and do not invade through the fat pad (Mallepell et al., 2006). At the same time however, an excess of estrogen signaling can also lead to reduced ductal elongation (Thomsen et al., 2006). Increased ER signaling leads to the production of growth factors, such as AREG, which can drive mammary epithelial cell proliferation and lateral branching (Brisken and O’Malley, 2010; Ciarloni et al., 2007; McBryan et al., 2007). Thus, instead of invading through the fat-pad longitudinally, increased estrogen signaling may lead to proliferation laterally across the fat pad prematurely.
While numerous roles have been ascribed to macrophages in the tumor microenvironment, less is known regarding their functions during normal mammary gland development. Consistent with their role in innate immunity, macrophages are a cell type with a high degree of plasticity and can respond to various signals in the microenvironment rapidly. Because macrophages are already associated with the developing epithelial structures, it is logical that they can also assist in orchestrating developmental processes (Gouon-Evans et al., 2000; Schwertfeger et al., 2006a; Van Nguyen and Pollard, 2002). Previous studies have shown that macrophages affect collagen organization surrounding terminal end buds in the mammary gland (Ingman et al., 2006). Our studies suggest that macrophages contribute to proper ductal elongation in a manner that requires STAT5. We have also identified a novel mechanism by which STAT5 regulates expression of aromatase, suggesting that macrophages may provide a local source of estrogen during mammary gland development. Ovarian-derived estrogen is a well-established regulator of ductal elongation in the mammary gland (Fata et al., 2001). However, given the heterogeneity of epithelial proliferation in the mammary gland, it seems feasible that macrophages may contribute small amounts of estrogen to help pattern proliferative areas or branching along the ducts. This suggests a novel potential function for tissue resident macrophages during mammary gland development and is consistent with previous studies that have suggested that macrophages are capable of synthesizing estrogen in the context of breast cancer (Mor et al., 1998). In addition, STAT5 is known to regulate expression of numerous genes in other cell types and it is possible that additional STAT5-regulated genes may contribute to macrophage function in the mammary gland (Kang et al., 2014; Metser et al., 2016; Yoo et al., 2015).
Previous work has demonstrated a role for STAT5 tetramers in repressing gene expression through chromatin modifications and STAT5 binding patterns in the genome can be affected by upstream signaling through numerous different receptors (Lin et al., 2012; Mandal et al., 2011). Additionally, many STAT5-dependent target genes are regulated by EZH2-mediated histone methylation (Yoo et al., 2015). Thus, during mammary gland development, signaling in macrophages downstream of cytokine and growth factor receptors may affect genomic STAT5 binding locations and relieve STAT5 tetramer-mediated gene repression. This may allow aromatase to be expressed and estrogen to be produced in a very local setting, acting in a short-range paracrine manner to further enhance epithelial proliferation. In addition to altering STAT5-mediated repression directly by changing tetramer binding frequency, repression can also be overcome by activating additional transcription factors with cytokines. Indeed, IL-6 treatment leads to a marked reduction in STAT5 occupancy at the Cyp19a1 promoter within 2 h of treatment and is sufficient to induce binding of STAT3 and induction of Cyp19a1 gene expression. Moreover, the increased production of IL-6 by macrophages can act on additional stromal cells, such as fibroblasts and adipocytes, to further enhance stromal aromatase expression, which could explain the enhanced expression of stromal aromatase observed in Fig. 4E. These data provide possible insight into why, in the context of obesity which is often associated with increased IL-6 levels, patients have an increased risk of developing ER+ breast cancers (Ahn et al., 2007). While our studies focus on the repressive nature of STAT5 binding at the Cyp19a1 promoter, future studies are warranted to further characterize the transcription factors involved in the positive regulation of Cyp19a1. Given that STAT3 is a major transcription factor downstream of IL-6 signaling, it is plausible that STAT3 may be one of the factors required to promote the expression of Cyp19a1. Recent studies have illustrated that the activation of STAT3 is able to antagonize STAT5 binding to consensus sites in the genome (Olson et al., 2016). Moreover, work in TH17 cells has demonstrated that retinoic acid treatment promotes STAT5 binding in the Il17a locus, while IL-1β treatment leads to the activation and the preferential recruitment of STAT3 to the binding site shared by STAT5 (Basu et al., 2015). Additionally, the loss of STAT5 led to increased binding of STAT3 to the shared consensus sequence in the Il17a locus. While our data demonstrate that STAT3 is recruited to the Cyp19a1 locus following IL-6 treatment, we cannot yet conclude whether IL-6 directly induces Cyp19a1 expression through transcriptional activation by STAT3 or indirectly through antagonizing STAT5-mediated transcriptional repression.
STAT5 signaling in epithelial cells has been shown to promote mammary tumorigenesis by regulating cell survival, in part through regulation of the phosphatidylinositol 3-kinase/Akt1 pathway (Creamer et al., 2010; Iavnilovitch et al., 2004; Ren et al., 2002; Schmidt et al., 2014). However, the function of STAT5 in stromal cells found in the tumor microenvironment is less well-characterized. Defining the role of STAT5 signaling in macrophages has implications for clinical trials in breast cancer and other malignancies. Macrophages are known to be found within breast tumors and the increased abundance of tumor-associated macrophages is correlated with poor patient outcome (Medrek et al., 2012). It has also been well-documented that genomic amplification of FGFR1 occurs in 10% of all breast cancers and drives resistance of ER+ tumors to endocrine therapy (Elbauomy Elsheikh et al., 2007; Turner et al., 2010). Using a mouse model of FGFR1 activation, we have shown that the loss of STAT5 in macrophages accelerates the formation of ER+ hyperplasias. Because activation of STAT5 and other members of the JAK/STAT signaling pathway have been linked to oncogenesis in breast cancer cells, there is currently a great interest in developing therapies to modulate the JAK/STAT signaling pathway. Ruxolitinib, an FDA-approved inhibitor of the JAK/STAT signaling pathway, is currently being explored in clinical trials with breast cancer patients (NCT02066532, NCT02041429, NCT01594216). Our data suggest that inhibition of this signaling axis in macrophages may lead to increased estrogen synthesis through the production of aromatase. While ruxolitinib has shown anti-tumor potential in pre-clinical work (Britschgi et al., 2012; Marotta et al., 2011), knowing the on-target effects in non-tumor cells will help inform clinical decision making in regards to combination therapies using ruxolitinib and aromatase inhibitors or anti-IL-6 therapeutics.
In summary, we have identified the transcription factor STAT5 as an important regulator of resident macrophage function in the developing mammary gland. Using genetic approaches, we have generated mice harboring a deletion of both alleles of Stat5a and Stat5b in macrophages. The loss of STAT5 signaling in macrophages results in altered mammary gland morphogenesis and increased expression of aromatase, correlating with increased estrogen production and ER signaling (Fig. 6). Additionally, the loss of STAT5 signaling in macrophages cooperates with FGFR1 activation in mammary epithelial cells to accelerate tumor initiation and drive the formation of ER+ mammary gland hyperplasias. Understanding how STAT5 signaling in resident macrophages shapes the microenvironment will inform future studies of modulating JAK/STAT signaling with pharmacologic inhibitors and identify new targets for combination therapies to improve treatment efficacy and patient outcome.
Fig. 6.
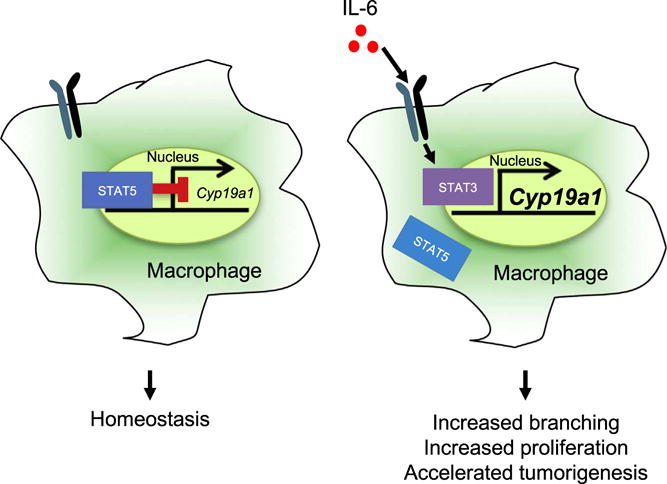
Model of STAT5-mediated repression of Cyp19a1 expression.
Supplementary Material
Acknowledgments
The authors thank Alexandra Fuher, Taylor Croissant, Alex Nguyen, Pavlina Chuntova, Sarah Kemp, Dr. Laura Bohrer, Dr. Mariya Farooqui and Dr. Scott Dehm for their technical assistance with experiments. The authors also thank Dr. Lothar Hennighausen for providing the STAT5fl/fl mice and Dr. Jeffrey Rosen for providing HC-11 cells and MMTV-iFGFR1 transgenic mice. This work was supported in part by funds from NCATS UL1TR000114 (NJB), NCI 5T32CA009138 (NJB), NCI 5R01CA132827 (KLS), DOD W81XWH-16-1-0034 (KLS) and NCI 5R21CA184541 (KLS).
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.ydbio.2017.06.007.
Footnotes
Author contributions
Conceptualization, N.J.B., M.A.F and K.L.S.; Investigation, N.J.B.; Writing – Original Draft, N.J.B. and K.L.S.; Writing – Review & Editing, N.J.B., M.A.F. and K.L.S.; Supervision, M.A.F. and K.L.S.; Funding Acquisition, K.L.S.
References
- Ahn J, Schatzkin A, Lacey JV, Albanes D, Ballard-Barbash R, Adams KF, Kipnis V, Mouw T, Hollenbeck AR, Leitzmann MF. Adiposity, adult weight change, and postmenopausal breast cancer risk. Arch Intern Med. 2007;167:2091–2102. doi: 10.1001/archinte.167.19.2091. [DOI] [PubMed] [Google Scholar]
- Bai L, Rohrschneider LR. s-SHIP promoter expression marks activated stem cells in developing mouse mammary tissue. Genes Dev. 2010;24:1882–1892. doi: 10.1101/gad.1932810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball RK, Friis RR, Schoenenberger CA, Doppler W, Groner B. Prolactin regulation of beta-casein gene expression and of a cytosolic 120-kd protein in a cloned mouse mammary epithelial cell line. EMBO J. 1988;7:2089. doi: 10.1002/j.1460-2075.1988.tb03048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, Whitley SK, Bhaumik S, Zindl CL, Schoeb TR, Benveniste EN, Pear WS, Hatton RD, Weaver CT. IL-1 signaling modulates activation of STAT transcription factors to antagonize retinoic acid signaling and control the TH17 cell-iTreg cell balance. Nat Immunol. 2015;16:286–295. doi: 10.1038/ni.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell BD, Kitajima M, Larson RP, Stoklasek TA, Dang K, Sakamoto K, Wagner KU, Reizis B, Hennighausen L, Ziegler SF. The transcription factor STAT5 is critical in dendritic cells for the development of TH2 but not TH1 responses. Nat Immunol. 2013;14:364–371. doi: 10.1038/ni.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohrer LR, Chuntova P, Bade LK, Beadnell TC, Leon RP, Brady NJ, Ryu Y, Goldberg JE, Schmechel SC, Koopmeiners JS, et al. Activation of the FGFR-STAT3 pathway in breast cancer cells induces a hyaluronan-rich microenvironment that licenses tumor formation. Cancer Res. 2014;74:374–386. doi: 10.1158/0008-5472.CAN-13-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohrer LR, Schwertfeger KL. Macrophages promote fibroblast growth factor receptor-driven tumor cell migration and invasion in a CXCR2-dependent manner. Mol Cancer Res. 2012;10:1294–1305. doi: 10.1158/1541-7786.MCR-12-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisken C, O’Malley B. Hormone action in the mammary gland. Cold Spring Harb Perspect Biol. 2010;2:a003178. doi: 10.1101/cshperspect.a003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britschgi A, Andraos R, Brinkhaus H, Klebba I, Romanet V, Müller U, Murakami M, Radimerski T, Bentires-Alj M. JAK2/STAT5 inhibition circumvents resistance to PI3K/mTOR blockade: a rationale for cotargeting these pathways in metastatic breast cancer. Cancer Cell. 2012;22:796–811. doi: 10.1016/j.ccr.2012.10.023. [DOI] [PubMed] [Google Scholar]
- Chan SC, Selth LA, Li Y, Nyquist MD, Miao L, Bradner JE, Raj GV, Tilley WD, Dehm SM. Targeting chromatin binding regulation of constitutively active AR variants to overcome prostate cancer resistance to endocrine-based therapies. Nucleic Acids Res. 2015;43:5880–5897. doi: 10.1093/nar/gkv262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che Q, Liu BY, Liao Y, Zhang HJ, Yang TT, He YY, Xia YH, Lu W, He XY, Chen Z, et al. Activation of a positive feedback loop involving IL-6 and aromatase promotes intratumoral 17β-estradiol biosynthesis in endometrial carcinoma microenvironment. Int J Cancer. 2014;135:282–294. doi: 10.1002/ijc.28679. [DOI] [PubMed] [Google Scholar]
- Chua ACL, Hodson LJ, Moldenhauer LM, Robertson SA, Ingman WV. Dual roles for macrophages in ovarian cycle-associated development and remodelling of the mammary gland epithelium. Development. 2010;137:4229–4238. doi: 10.1242/dev.059261. [DOI] [PubMed] [Google Scholar]
- Ciarloni L, Mallepell S, Brisken C. Amphiregulin is an essential mediator of estrogen receptor alpha function in mammary gland development. Proc Natl Acad Sci USA. 2007;104:5455–5460. doi: 10.1073/pnas.0611647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowin P, Wysolmerski J. Molecular mechanisms guiding embryonic mammary gland development. Cold Spring Harb Perspect Biol. 2010;2:a003251. doi: 10.1101/cshperspect.a003251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creamer BA, Sakamoto K, Schmidt JW, Triplett AA, Moriggl R, Wagner KU. Stat5 promotes survival of mammary epithelial cells through transcriptional activation of a distinct promoter in Akt1. Mol Cell Biol. 2010;30:2957–2970. doi: 10.1128/MCB.00851-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Riedlinger G, Miyoshi K, Tang W, Li C, Deng CX, Robinson GW, Hennighausen L. Inactivation of Stat5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol Cell Biol. 2004;24:8037. doi: 10.1128/MCB.24.18.8037-8047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson KG, Oborn CJ, Durban EM, Butel JS, Medina D. Epithelial mouse mammary cell line exhibiting normal morphogenesis in vivo and functional differentiation in vitro. Proc Natl Acad Sci USA. 1984;81:3756–3760. doi: 10.1073/pnas.81.12.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Zhou JF, Sellers RS, Li JF, Nguyen AV, Wang Y, Orlofsky A, Liu Q, Hume DA, Pollard JW, et al. A novel mouse model of inflammatory bowel disease links mammalian target of rapamycin-dependent hyperproliferation of colonic epithelium to inflammation-associated tumorigenesis. Am J Pathol. 2010;176:952–967. doi: 10.2353/ajpath.2010.090622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder K, Baffy N, Falus A, Fulop AK. The major inflammatory mediator interleukin-6 and obesity. Inflamm Res. 2009;58:727–736. doi: 10.1007/s00011-009-0060-4. [DOI] [PubMed] [Google Scholar]
- Elbauomy Elsheikh S, Green AR, Lambros MBK, Turner NC, Grainge MJ, Powe D, Ellis IO, Reis-Filho JS. FGFR1 amplification in breast carcinomas: a chromogenic in situ hybridisation analysis. Breast Cancer Res. 2007;9:R23. doi: 10.1186/bcr1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata JE, Chaudhary V, Khokha R. Cellular turnover in the mammary gland is correlated with systemic levels of progesterone and not 17beta-estradiol during the estrous cycle. Biol Reprod. 2001;65:680–688. doi: 10.1095/biolreprod65.3.680. [DOI] [PubMed] [Google Scholar]
- Gabanyi I, Muller PA, Feighery L, Oliveira TY, Costa-Pinto FA, Mucida D. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell. 2016;164:378–391. doi: 10.1016/j.cell.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouon-Evans V, Lin EY, Pollard JW. Requirement of macrophages and eosinophils and their cytokines/chemokines for mammary gland development. Breast Cancer Res. 2002;4:155–164. doi: 10.1186/bcr441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouon-Evans V, Rothenberg ME, Pollard JW. Postnatal mammary gland development requires macrophages and eosinophils. Development. 2000;127:2269–2282. doi: 10.1242/dev.127.11.2269. [DOI] [PubMed] [Google Scholar]
- Gyorki DE, Asselin-Labat ML, van Rooijen N, Lindeman GJ, Visvader JE. Resident macrophages influence stem cell activity in the mammary gland. Breast Cancer Res. 2009;11:R62. doi: 10.1186/bcr2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys RC, Krajewska M, Krnacik S, Jaeger R, Weiher H, Krajewski S, Reed JC, Rosen JM. Apoptosis in the terminal endbud of the murine mammary gland: a mechanism of ductal morphogenesis. Development. 1996;122:4013–4022. doi: 10.1242/dev.122.12.4013. [DOI] [PubMed] [Google Scholar]
- Iavnilovitch E, Cardiff RD, Groner B, Barash I. Deregulation of Stat5 expression and activation causes mammary tumors in transgenic mice. Int J Cancer. 2004;112:607–619. doi: 10.1002/ijc.20484. [DOI] [PubMed] [Google Scholar]
- Ingman WV, Wyckoff J, Gouon-Evans V, Condeelis J, Pollard JW. Macrophages promote collagen fibrillogenesis around terminal end buds of the developing mammary gland. Dev Dyn. 2006;235:3222–3229. doi: 10.1002/dvdy.20972. [DOI] [PubMed] [Google Scholar]
- Izrailit J, Reedijk M. Developmental pathways in breast cancer and breast tumor-initiating cells: therapeutic implications. Cancer Lett. 2012;317:115–126. doi: 10.1016/j.canlet.2011.11.028. [DOI] [PubMed] [Google Scholar]
- Kang K, Yamaji D, Yoo KH, Robinson GW, Hennighausen L. Mammary-specific gene activation is defined by progressive recruitment of STAT5 during pregnancy and the establishment of H3K4me3 marks. Mol Cell Biol. 2014;34:464–473. doi: 10.1128/MCB.00988-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney NJ, Smith GH, Lawrence E, Barrett JC, Salomon DS. Identification of stem cell units in the terminal end bud and duct of the mouse mammary gland. J Biomed Biotechnol. 2001;1:133–143. doi: 10.1155/S1110724301000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaled WT, Read EKC, Nicholson SE, Baxter FO, Brennan AJ, Came PJ, Sprigg N, McKenzie ANJ, Watson CJ. The IL-4/IL-13/Stat6 signalling pathway promotes luminal mammary epithelial cell development. Development. 2007;134:2739–2750. doi: 10.1242/dev.003194. [DOI] [PubMed] [Google Scholar]
- Kuroda E, Ho V, Ruschmann J, Antignano F, Hamilton M, Rauh MJ, Antov A, Flavell RA, Sly LM, Krystal G. SHIP represses the generation of IL-3-induced M2 macrophages by inhibiting IL-4 production from basophils. J Immunol. 2009;183:3652–3660. doi: 10.4049/jimmunol.0900864. [DOI] [PubMed] [Google Scholar]
- Lee AH, Happerfield LC, Bobrow LG, Millis RR. Angiogenesis and inflammation in invasive carcinoma of the breast. J Clin Pathol. 1997;50:669–673. doi: 10.1136/jcp.50.8.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625–4629. [PubMed] [Google Scholar]
- Lilla JN, Werb Z. Mast cells contribute to the stromal microenvironment in mammary gland branching morphogenesis. Dev Biol. 2010;337:124–133. doi: 10.1016/j.ydbio.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin EY, Jones JG, Li P, Zhu L, Whitney KD, Muller WJ, Pollard JW. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol. 2003;163:2113–2126. doi: 10.1016/S0002-9440(10)63568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JX, Li P, Liu D, Jin HT, He J, Ata Ur Rasheed M, Rochman Y, Wang L, Cui K, Liu C, et al. Critical Role of STAT5 transcription factor tetramerization for cytokine responses and normal immune function. Immunity. 2012;36:586–599. doi: 10.1016/j.immuni.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Robinson GW, Wagner KU, Garrett L, Wynshaw-Boris A, Hennighausen L. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 1997;11:179–186. doi: 10.1101/gad.11.2.179. [DOI] [PubMed] [Google Scholar]
- Mahmoud SMA, Lee AHS, Paish EC, Macmillan RD, Ellis IO, Green AR. Tumour-infiltrating macrophages and clinical outcome in breast cancer. J Clin Pathol. 2012;65:159–163. doi: 10.1136/jclinpath-2011-200355. [DOI] [PubMed] [Google Scholar]
- Mallepell S, Krust A, Chambon P, Brisken C. Paracrine signaling through the epithelial estrogen receptor alpha is required for proliferation and morphogenesis in the mammary gland. Proc Natl Acad Sci USA. 2006;103:2196–2201. doi: 10.1073/pnas.0510974103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal M, Powers SE, Maienschein-Cline M, Bartom ET, Hamel KM, Kee BL, Dinner AR, Clark MR. Epigenetic repression of the Igk locus by STAT5-mediated recruitment of the histone methyltransferase Ezh2. Nat Immunol. 2011;12:1212–1220. doi: 10.1038/ni.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marotta LLC, Almendro V, Marusyk A, Shipitsin M, Schemme J, Walker SR, Bloushtain-Qimron N, Kim JJ, Choudhury SA, Maruyama R, et al. The JAK2/STAT3 signaling pathway is required for growth of CD44⁺CD24− stem cell-like breast cancer cells in human tumors. J Clin Investig. 2011;121:2723–2735. doi: 10.1172/JCI44745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBryan J, Howlin J, Kenny PA, Shioda T, Martin F. ERalpha-CITED1 co-regulated genes expressed during pubertal mammary gland development: implications for breast cancer prognosis. Oncogene. 2007;26:6406–6419. doi: 10.1038/sj.onc.1210468. [DOI] [PubMed] [Google Scholar]
- McLean AC, Valenzuela N, Fai S, Bennett SAL. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J Vis Exp. 2012:e4389. doi: 10.3791/4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrek C, Pontén F, Jirström K, Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306. doi: 10.1186/1471-2407-12-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metser G, Shin HY, Wang C, Yoo KH, Oh S, Villarino AV, O’Shea JJ, Kang K, Hennighausen L. An autoregulatory enhancer controls mammary-specific STAT5 functions. Nucleic Acids Res. 2016;44:1052–1063. doi: 10.1093/nar/gkv999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi K, Shillingford JM, Smith GH, Grimm SL, Wagner KU, Oka T, Rosen JM, Robinson GW, Hennighausen L. Signal transducer and activator of transcription (Stat) 5 controls the proliferation and differentiation of mammary alveolar epithelium. J Cell Biol. 2001;155:531–542. doi: 10.1083/jcb.200107065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor G, Yue W, Santen RJ, Gutierrez L, Eliza M, Berstein LM, Harada N, Wang J, Lysiak J, Diano S, et al. Macrophages, estrogen and the microenvironment of breast cancer. J Steroid Biochem Mol Biol. 1998;67:403–411. doi: 10.1016/s0960-0760(98)00143-5. [DOI] [PubMed] [Google Scholar]
- O’Brien J, Martinson H, Durand-Rougely C, Schedin P. Macrophages are crucial for epithelial cell death and adipocyte repopulation during mammary gland involution. Development. 2012;139:269–275. doi: 10.1242/dev.071696. [DOI] [PubMed] [Google Scholar]
- Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157:832–844. doi: 10.1016/j.cell.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson MR, Verdan FF, Hufford MM, Dent AL, Kaplan MH. STAT3 impairs STAT5 activation in the development of IL-9-secreting T cells. J Immunol. 2016;196:3297–3304. doi: 10.4049/jimmunol.1501801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaks V, Boldajipour B, Linnemann JR, Nguyen NH, Kersten K, Wolf Y, Casbon AJ, Kong N, van den Bijgaart RJE, Sheppard D, et al. Adaptive immune regulation of mammary postnatal organogenesis. Dev Cell. 2015;34:493–504. doi: 10.1016/j.devcel.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JR, Stone MD, Beadnell TC, Ryu Y, Griffin TJ, Schwertfeger KL. Fibroblast growth factor receptor 1 activation in mammary tumor cells promotes macrophage recruitment in a CX3CL1-dependent manner. PLoS One. 2012;7:e45877. doi: 10.1371/journal.pone.0045877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren S, Cai HR, Li M, Furth PA. Loss of Stat5a delays mammary cancer progression in a mouse model. Oncogene. 2002;21:4335–4339. doi: 10.1038/sj.onc.1205484. [DOI] [PubMed] [Google Scholar]
- Rosas M, Davies LC, Giles PJ, Liao CT, Kharfan B, Stone TC, O’Donnell VB, Fraser DJ, Jones SA, Taylor PR. The transcription factor Gata6 links tissue macrophage phenotype and proliferative renewal. Science. 2014;344:645–648. doi: 10.1126/science.1251414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos SJ, Haslam SZ, Conrad SE. Signal transducer and activator of transcription 5a mediates mammary ductal branching and proliferation in the nulliparous mouse. Endocrinology. 2010;151:2876–2885. doi: 10.1210/en.2009-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt JW, Wehde BL, Sakamoto K, Triplett AA, Anderson SM, Tsichlis PN, Leone G, Wagner KU. Stat5 regulates the phosphatidylinositol 3-kinase/Akt1 pathway during mammary gland development and tumorigenesis. Mol Cell Biol. 2014;34:1363–1377. doi: 10.1128/MCB.01220-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwertfeger KL, Rosen JM, Cohen DA. Mammary gland macrophages: pleiotropic functions in mammary development. J Mammary Gland Biol. 2006a;11:229–238. doi: 10.1007/s10911-006-9028-y. [DOI] [PubMed] [Google Scholar]
- Schwertfeger KL, Xian W, Kaplan AM, Burnett SH, Cohen DA, Rosen JM. A critical role for the inflammatory response in a mouse model of preneoplastic progression. Cancer Res. 2006b;66:5676–5685. doi: 10.1158/0008-5472.CAN-05-3781. [DOI] [PubMed] [Google Scholar]
- Shehata M, van Amerongen R, Zeeman AL, Giraddi RR, Stingl J. The influence of tamoxifen on normal mouse mammary gland homeostasis. Breast Cancer Res. 2014;16:411. doi: 10.1186/s13058-014-0411-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK, Zhou XK, Blaho VA, Hla T, Yang P, et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res. 2011;4:329–346. doi: 10.1158/1940-6207.CAPR-10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Thomsen AR, Almstrup K, Nielsen JE, Sørensen IK, Petersen OW, Leffers H, Breinholt VM. Estrogenic effect of soy isoflavones on mammary gland morphogenesis and gene expression profile. Toxicol Sci. 2006;93:357–368. doi: 10.1093/toxsci/kfl029. [DOI] [PubMed] [Google Scholar]
- Turner N, Pearson A, Sharpe R, Lambros M, Geyer F, Lopez-Garcia MA, Natrajan R, Marchio C, Iorns E, Mackay A, et al. FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res. 2010;70:2085–2094. doi: 10.1158/0008-5472.CAN-09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vafaizadeh V, Klemmt P, Brendel C, Weber K, Doebele C, Britt K, Grez M, Fehse B, Desrivières S, Groner B. Mammary epithelial reconstitution with gene-modified stem cells assigns roles to Stat5 in luminal alveolar cell fate decisions, differentiation, involution, and mammary tumor formation. Stem Cells. 2010;28:928–938. doi: 10.1002/stem.407. [DOI] [PubMed] [Google Scholar]
- Van Nguyen A, Pollard JW. Colony stimulating factor-1 is required to recruit macrophages into the mammary gland to facilitate mammary ductal outgrowth. Dev Biol. 2002;247:11–25. doi: 10.1006/dbio.2002.0669. [DOI] [PubMed] [Google Scholar]
- Welm BE, Freeman KW, Chen M, Contreras A, Spencer DM, Rosen JM. Inducible dimerization of FGFR1: development of a mouse model to analyze progressive transformation of the mammary gland. J Cell Biol. 2002;157:703–714. doi: 10.1083/jcb.200107119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, Graf T, Pollard JW, Segall J, Condeelis J. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–7029. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- Yamaji D, Na R, Feuermann Y, Pechhold S, Chen W, Robinson GW, Hennighausen L. Development of mammary luminal progenitor cells is controlled by the transcription factor STAT5A. Genes Dev. 2009;23:2382–2387. doi: 10.1101/gad.1840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, Hirahara K, Sun HW, Wei L, Vahedi G, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12:247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Cui Y, Watford WT, Bream JH, Yamaoka K, Hissong BD, Li D, Durum SK, Jiang Q, Bhandoola A, et al. Stat5a/b are essential for normal lymphoid development and differentiation. Proc Natl Acad Sci USA. 2006;103:1000–1005. doi: 10.1073/pnas.0507350103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo KH, Oh S, Kang K, Hensel T, Robinson GW, Hennighausen L. Loss of EZH2 results in precocious mammary gland development and activation of STAT5-dependent genes. Nucleic Acids Res. 2015;43:8774–8789. doi: 10.1093/nar/gkv776. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


