Abstract
OBJECTIVE
To determine whether blacks with lower extremity peripheral artery disease (PAD) have faster functional decline than whites with PAD.
METHODS
Participants with ankle brachial index (ABI) < 0.90 were identified from Chicago medical centers and followed longitudinally. Mobility impairment and the six-minute walk were assessed at baseline and every 6 to 12 months. Mobility loss was defined as becoming unable to walk up and down a flight of stairs or walk ¼ mile without assistance.
RESULTS
Of 1,175 PAD participants, 310 (26%) were black. Median follow-up was 46.0 months. Among 727 PAD participants who walked six minutes continuously at baseline, black participants were more likely to become unable to walk six-minutes continuously during follow-up (68/178 (38.2%) vs. 157/549 (28.6%), log-rank P=0.002). Black race was associated with becoming unable to walk six minutes continuously, adjusting for age, sex, ABI, comorbidities, and other confounders (HR=1.51, 95% Confidence Interval (CI)=1.10,2.06, P=0.011). This association was attenuated after adjustment for income and education (P=0.150). Among 862 participants without baseline mobility impairment, black participants had a higher rate of mobility loss (67/212 (31.6%) vs. 169/650 (26.0%), log-rank P=0.004). Black race was associated with increased mobility loss, adjusting for potential confounders (hazard ratio (HR)=1.44, 95% CI=1.06,1.96, P=0.021). This association was attenuated after additional adjustment for income and education (P=0.348) and physical activity (P=0.086). There were no differences in average annual declines in six-minute walk, usual-paced four-meter walking velocity, or fast paced four-meter walking velocity.
CONCLUSION
Black PAD patients have higher rates of mobility loss and becoming unable to walk for six-minutes continuously. These differences appear related to racial differences in socioeconomic status and physical activity.
Journal Subject Codes: Peripheral vascular disease, race and ethnicity, complications
Keywords: Peripheral vascular disease, physical activity, exercise, prognosis
Racial disparities in treatment are well established in people with lower extremity peripheral artery disease (PAD) and critical limb ischemia (1–6). Black patients with critical limb ischemia are less likely to undergo revascularization and more likely to have amputations than white patients with critical limb ischemia (1–6).
To our knowledge, no longitudinal studies have compared mobility loss or functional decline between blacks and whites with PAD and without critical limb ischemia. Therefore, we compared mobility loss and objectively measured functional decline between blacks and whites with PAD. Based on prior cross-sectional study (7) and studies of people with critical limb ischemia (1–6), we hypothesized that black participants with PAD would have faster functional decline than white participants with PAD.
METHODS
Study Overview
Data from four prospective observational studies of patients with PAD were combined. Participants were identified from the Walking and Leg Circulation Study (WALCS), WALCS II, WALCS III, and the BRAVO Studies (8–13) (Table I). In all studies, participants were recruited from Chicago-area medical centers and followed longitudinally (8–13). Details of recruitment have been reported (8–13). Participants with PAD were identified from non-invasive vascular laboratories at multiple Chicago-area hospitals using lists of all patients who underwent lower extremity arterial testing during specific time periods and contacting those with abnormal results consistent with PAD. Participants with PAD were also identified from vascular surgery, cardiology, general medicine, endocrinology, and geriatric clinics at Chicago-area hospitals using ICD-9 codes to identify all patients with PAD who attended a clinic visit during specific time periods. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. The Institutional Review Boards of Northwestern University and all participating medical centers approved the protocol. All participants gave written informed consent. In the WALCS, WALCS II, and WALCS III cohorts, participants completed baseline testing and returned annually for up to four follow-up visits. At each annual visit, the ankle brachial index (ABI) and objective measures of lower extremity functioning were repeated. Questionnaires were re-administered to obtain data each year on mobility impairment, and current health including hospitalizations since the most recent study visit. In BRAVO, participants completed baseline testing and returned every six months for functional measures for up to 3 years. The ABI was not repeated after baseline in the BRAVO cohort, but questionnaires were administered every six-months to obtain information about the participant’s health, mobility impairment, and hospitalizations since the most recent visit.
Table I.
Characteristics of Four Cohorts Contributing Peripheral Artery Disease Participants to Analyses
| Cohort/years of study | Inclusion Criteria | Primary Aim of Study | Primary Results |
|---|---|---|---|
| WALCS 1998–2002 | Age 55 and older and ABI ≤ 0.90 | To compare baseline objective measures of lower extremity functioning and annual rates of decline in these objective measures of lower extremity functioning between participants with and without PAD. Objective measures of functioning included the six-minute walk and four-meter walking velocity. | People with PAD had greater functional impairment and faster functional decline than those without PAD, and this phenomenon was observed even in PAD participants without classical symptoms of intermittent claudication. |
| WALCS II 2002–2006 | Included participants from WALCS who consented to WALCS II and newly identified participants age 59 and older and ABI ≤0.90. | To compare baseline measures of computed tomography-measured calf skeletal muscle quantity and percent fat composition between participants with and without PAD. To determine whether smaller calf muscle quantity and higher fat composition were associated with faster functional decline among participants with PAD. | Participants with PAD had smaller calf muscle area and a higher calf muscle fat content than those without PAD. More adverse calf muscle characteristics were associated with higher rates of mobility loss. |
| WALCS III 2008–2013 | ABI < 1.00 and eligible for magnetic resonance imaging.* | To determine whether magnetic resonance imaging (MRI) measured superficial femoral artery plaque quantity and composition were associated with rates of functional decline in participants with PAD. | Greater MRI-measured plaque quantity and smaller lumen area in the superficial femoral artery were associated with greater functional impairment. |
| BRAVO 2009–2013 | Presence of PAD defined as ABI < 0.90, medical record documented lower extremity revascularization, or evidence of PAD from a medical center-based non-invasive vascular laboratory. | To determine whether inflammatory biomarkers and D-dimer levels, measured every two months, increased immediately before acute coronary events in participants with PAD. | Levels of D-dimer obtained within two months of an acute coronary event were higher than values obtained 10–32 months previously. A similar phenomenon was not observed for C-reactive protein or serum amyloid A. |
Only participants with ABI <0.90 were included in these analyses. WALCS- Walking and leg circulation study. BRAVO- Biomarker risk assessment in vulnerable outpatients. ABI- Ankle brachial index. PAD-Peripheral artery disease.
Inclusion Criterion
All participants in these analyses had a baseline ABI < 0.90.
Exclusion Criteria
In all studies, patients with dementia were excluded because of their inability to answer questions accurately. Nursing home residents were excluded because they had severely impaired functioning at baseline. Non-English-speaking patients were excluded because investigators were not fluent in non-English languages. Patients with recent major surgery were excluded because the surgery may have influenced their baseline mobility. In the WALCS, WALCS II and WALCS III cohorts, participants who were wheelchair bound or who had history of leg or foot amputations were excluded because of their severe functional impairment at baseline. Potential participants with toe or partial foot amputations were allowed to participate. In the WALCS III cohort, participants with contraindications to magnetic resonance imaging testing were excluded. We did not systematically exclude potential participants with critical limb ischemia, however participants with mobility disability or inability to perform the six-minute walk tests at baseline were not included in the mobility or six-minute walk outcome measures, respectively.
Ankle Brachial Index Measurement
A trained and certified research coordinator used a hand-held Doppler probe (Nicolet Vascular Pocket Dop II; Nicolet Biomedical Inc, Golden, Colo) to measure systolic pressures in the right and left brachial, dorsalis pedis, and posterior tibial arteries (14, 15). Research coordinators were trained by a senior staff member and certified by the principal investigator (MMM) prior to collecting data from research subjects. Each pressure was measured twice. For each leg, the ABI was calculated by dividing the mean of the dorsalis pedis and posterior tibial pressures by the mean of the 4 brachial pressures, since previous study shows that this method of ABI calculation is most closely associated with the degree of functional impairment in PAD (15). The leg with lowest ABI was used in analyses. The coefficient of variation percent for the test- re-test reliability was 5.2% in WALCS, 10.4% in WALCS II, 7.5% in WALCS III and 9.5% in the BRAVO cohort.
Race
Information on race was obtained from participants by questionnaire. Participants who were not black or white were excluded from these analyses.
Outcomes
Mobility Outcome
At baseline and at each follow-up visit, participants were asked whether they were able to walk up and down stairs to the second floor and walk ¼ mile (3 blocks), “on their own”, “with help”, or “not at all”. Incident mobility impairment, defined here as mobility loss, consisted of reporting inability to walk up and down one flight of stairs or walk ¼ mile either “at all” or “without assistance” during follow-up among participants without mobility impairment at baseline (17–19). Participant report of mobility is valid and reliable (20–22). For example, in 193 men and women age 69 and older who were interviewed twice, three weeks apart, about their mobility (measured as their ability to climb a flight of stairs and walk ¼ mile without assistance), agreement was 89% during the two independent assessments performed three weeks apart. Among participants with PAD, a randomized clinical trial reported that home-based exercise prevented mobility loss at 6-month follow-up, and findings were consistent at 12-month follow-up (24). Additional evidence from PAD participants shows that greater decline in the six-minute walk over two-year follow-up is associated with higher rates of subsequent mobility loss (25).
Six-minute walk
The six-minute walk test was performed at baseline using a standardized and well-validated protocol (8,9,12,17). Participants walked up and down a 100-foot hallway for six minutes after instructions to cover as much distance as possible. We recorded at baseline and follow-up whether participants stopped to rest for at least five seconds at least once during the six-minutes. Cardiac monitoring is not performed during the six-minute walk test. Serious adverse events during the six-minute walk occur at a rate of less than one in 7,900 tests (24).
Four-meter walking velocity
Participants were timed walking a four meter distance after instructions to walk at their “usual pace” and “fastest pace”, respectively. Usual and fast-paced four-meter walking velocity each predict mortality and mobility loss in people with and without PAD (17,18,23).
Comorbidities
Comorbidities assessed at baseline were diabetes, angina, myocardial infarction, heart failure, cancer, chronic lung disease, knee arthritis, hip arthritis, spinal stenosis, disk disease, and stroke. Disease-specific algorithms that combine data from patient report, medical record review, medications, laboratory values, and a questionnaire completed by the participant’s primary care physician were used to verify and document baseline comorbidities (27,28). The primary care physician questionnaire response rate was 78%, but the investigative team directly telephoned primary care physicians who did not return the questionnaire.
Leg Symptoms
Leg symptoms were classified using the San Diego claudication questionnaire (29). Intermittent claudication (IC) was defined as exertional calf pain that did not begin at rest, caused the patient to stop walking, and resolved within 10 minutes of rest (7–9,29). Participants without IC either reported no exertional leg symptoms (asymptomatic PAD) or had leg symptoms other than IC (7–9,29). For example, participants with leg symptoms other than IC could have exertional leg pain that does not involve the calf or that requires more than 10 minutes to resolve after rest.
Socioeconomic status
We used income and education level to classify participants’ socioeconomic status. Participants were asked their highest level of education, using a questionnaire administered by certified interviewers. Responses were categorized according to the participant’s report of their highest attained level of education as follows: a) less than high school, b) high school through college, c) at least some graduate school. Each participant’s income was categorized as either above vs. below the median income for the cohort, based on the participant’s zip code, using United States Census data linking zip code to income (30).
Physical activity level
Patient reported physical activity was measured with a questionnaire derived from the Harvard Alumni Activity Survey that has been previously validated (31–33). The physical activity question asked, “During the last week, how many city blocks or their equivalent did you walk? Let 12 city blocks equal 1 mile.” (34).
Other Measures
Height and weight were measured at baseline. Body mass index (BMI) was calculated as weight (kilograms)/(height (meters))2. Cigarette smoking history was determined with patient report.
Statistical Analyses
Two-sample t tests and Chi-square tests were used to compare continuous and binary baseline characteristics according to black vs. white race. Cox proportional hazards analyses were used to compare the first occurrence of mobility loss and becoming unable to walk for six-minutes continuously, between blacks and whites, adjusting for age, sex, race, study cohort (WALCS, WALCS II, WALCS III, or BRAVO), BMI, smoking, comorbidities, leg symptoms, and the ABI. Analyses were repeated with additional adjustment for income (above vs. below median income of cohort) and education (reference was at least some graduate school or higher education level) and for physical activity, respectively. Each cohort was followed for up to four years. Participants who did not complete their final follow-up visit by 54 months after baseline were censored. Participants who died or underwent any lower extremity revascularization during follow-up (including open revascularization, endovascular procedures, and supra-inguinal procedures) were censored at the time of death or revascularization. Repeated- measures mixed models were used to compare average annual decline in six-minute walk, four-meter walking velocity at usual pace, and four-meter walking velocity at fast pace, adjusting for age, sex, race, study cohort, BMI, smoking, comorbidities, leg symptoms, prior year performance, and the ABI. Analyses were performed using SAS statistical software (version 9.4, SAS Institute Inc, Cary, NC).
RESULTS
Of 1,433 participants with an ABI < 0.90 enrolled in the WALCS, WALCS II, WALCS III, and BRAVO cohorts, 58 did not classify themselves as either black or white, 54 died before the first follow-up visit, 43 were lost to follow-up, 54 returned for follow-up but did not repeat the outcome measures reported here, and 49 were missing one or more covariates, leaving 1,175 for analyses. Of these, 865 (74%) were white and 310 (26%) were black. Median follow-up was 46.0 months (interquartile range=24–50 months). During follow-up, 136/865 whites (15.7%) vs. 39/310 blacks (12.6%) underwent revascularization (P=0.182) and were censored at the time of revascularization.
Compared to whites, blacks with PAD were younger, and had a lower ABI value, a higher BMI, achieved lower education and had a lower median household income (Table II). Compared to whites, blacks with PAD had a lower prevalence of cancer and higher prevalences of stroke, diabetes, current smoking, and hypertension (Table II). Compared to whites with PAD, blacks with PAD had a lower prevalence of claudication symptoms, lower physical activity, and poorer functional performance at baseline (Table II).
Table II.
Baseline Characteristics of Peripheral Artery Disease Participants According to Black vs. White Race*
| White (n=865) |
Black (n=310) |
P value | |
|---|---|---|---|
| Age (years) | 71.62 (9.18) | 67.72 (9.01) | <.0001 |
| Male sex, n (%) | 532 (61.50) | 177 (57.10) | 0.174 |
| Ankle brachial index | 0.66 (0.14) | 0.63 (0.15) | 0.002 |
| Body mass index (kg/m2) | 28.26 (5.41) | 29.12 (5.82) | 0.019 |
| Current smoker, n (%) | 165 (19.08) | 100 (32.26) | <0.001 |
| Angina, n (%) | 244 (28.21) | 77 (24.84) | 0.253 |
| Myocardial infarction, n (%) | 194 (22.43) | 60 (19.35) | 0.259 |
| Heart failure, n (%) | 160 (18.50) | 62 (20.00) | 0.562 |
| Stroke, n (%) | 118 (13.64) | 69 (22.26) | <0.001 |
| Pulmonary disease, n (%) | 320 (36.99) | 113 (36.45) | 0.865 |
| Cancer, n (%) | 160 (18.50) | 38 (12.26) | 0.012 |
| Diabetes, n (%) | 264 (30.52) | 154 (49.68) | <0.001 |
| Hypertension, n (%) | 713 (82.62) | 291 (93.87) | <0.001 |
| Knee arthritis, n (%) | 104 (12.02) | 57 (18.39) | 0.005 |
| Hip arthritis, n (%) | 51 (5.90) | 19 (6.13) | 0.882 |
| Spinal stenosis, n (%) | 109 (12.60) | 25 (8.06) | 0.031 |
| Disc disease, n (%) | 285 (32.95) | 80 (25.81) | 0.020 |
| Prior lower extremity revascularization, n (%) | 338 (39.08) | 104 (33.55) | 0.085 |
| Intermittent claudication, n (%) | 244 (28.21) | 67 (21.61) | 0.018 |
| Exertional leg symptoms other than intermittent claudication, n (%) | 443 (51.21) | 187 (60.32) | |
| Asymptomatic (no exertional leg symptoms), n (%) | 178 (20.58) | 56 (18.06) | |
| Number of blocks walked in the past week | 31.12 (56.11) | 20.92 (43.45) | 0.004 |
| Baseline Functional Performance | |||
| Mobility impairment at baseline, n (%) | 172 (19.88) | 89 (28.71) | 0.001 |
| Stopped during the six-minute walk at baseline, n (%) | 243 (28.35) | 116 (37.79) | 0.002 |
| 6-min walk distance (feet) | 1157.13 (388.19) | 1056.11 (398.52) | <0.001 |
| Usual-paced four-meter walking velocity (meters/second) | 0.89 (0.21) | 0.81 (0.20) | <0.001 |
| Fastest-paced four-meter walking velocity (meters/second) | 1.20 (0.28) | 1.13 (0.30) | <0.001 |
| Baseline socioeconomic data | |||
| Median annual household income based on zip code (U.S. dollars) | 58,435 (20291) | 37,255 (14001) | <0.001 |
| Less than high school, n (%) | 62 (7.17) | 62 (20.00) | <0.001 |
| High school to college, n (%) | 604 (69.83) | 220 (70.97) | |
| Graduate school, n (%) | 199 (23.01) | 28 (9.03) | |
Data shown are mean value (standard deviation) unless otherwise indicated.
In unadjusted analyses, blacks had a higher cumulative probability of mobility loss, compared to whites (67/212 (31.6%) vs. 169/650 (26.0%), log-rank P=0.004) (Figure 1). Adjusting for age, sex, ABI, BMI, smoking, comorbidities, leg symptoms, and study cohort, black PAD participants had an increased hazard of mobility loss, compared to white PAD participants (hazard ratio=1.44, 95% Confidence Interval=1.06–1.96, P=0.021). This association was not statistically significant after additional adjustment for income and education (P=0.348) or after additional adjustment for physical activity (P=0.086), respectively (Table III). There was no significant interaction of study cohort in the association of race and mobility loss, also suggesting that the association did not change over time.
Figure 1.
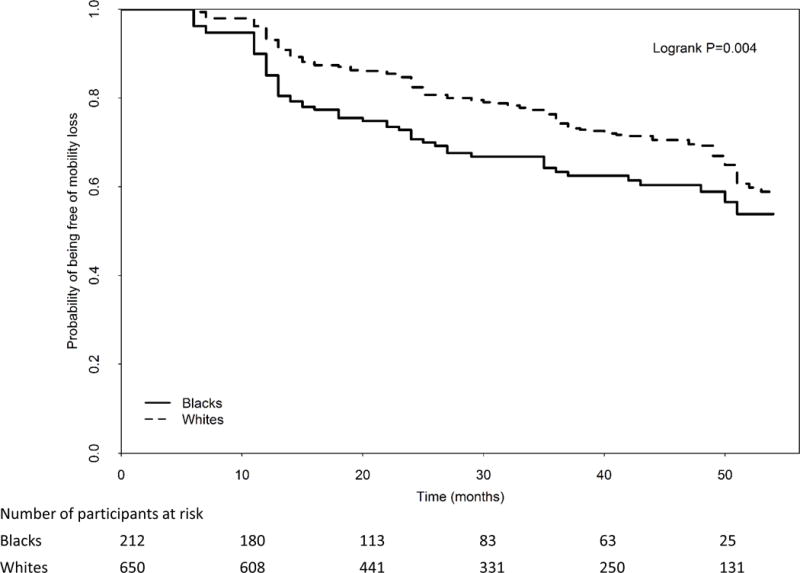
Rates of mobility loss in blacks vs. whites with peripheral artery disease (N=862)
Table III.
Adjusted associations of black vs. white race with mobility loss, inability to walk 6 minutes continuously, and lower extremity revascularization
| Hazard Ratio (95% Confidence Interval) |
P value | |
|---|---|---|
| Mobility Loss (N=862) | ||
| Model 1: Adjusting for age, sex, study cohort. | 1.54 (1.15 to 2.06) | 0.004 |
| Model 2: Adjusting for age, sex, comorbidities, smoking, BMI, leg symptoms, ABI, study cohort. | 1.44 (1.06 to 1.96) | 0.021 |
| Model 3: Adjusting for covariates in Model 2 + education and income. | 1.18 (0.84 to 1.66) | 0.348 |
| Model 4: Adjusting for covariates in Model 2 + physical activity | 1.31 (0.96 to 1.79) | 0.086 |
| Becoming unable to walk six minutes without stopping (N=727) | ||
| Model 1: Adjusting for age, sex, study cohort. | 1.67 (1.24 to 2.25) | <0.001 |
| Model 2: Adjusting for age, sex, comorbidities, smoking, BMI, leg symptoms, ABI, study cohort. | 1.51 (1.10 to 2.06) | 0.011 |
| Model 3: Adjusting for covariates in Model 2 + education and income. | 1.28 (0.91 to 1.79) | 0.150 |
| Model 4: Adjusting for covariates in Model 2 + physical activity | 1.47 (1.07 to 2.02) | 0.017 |
BMI denotes body mass index.
ABI denotes ankle brachial index.
In unadjusted analyses, blacks had a higher cumulative probability of becoming unable to walk for six minutes continuously, compared to whites (68/178 (38.2%) vs. 157/549 (28.6%) log rank P=0.002) (Figure 2). Adjusting for age, sex, ABI, BMI, smoking, comorbidities, leg symptoms, and cohort, black PAD participants had a higher rate of becoming unable to walk for six-minutes without stopping, compared to whites (hazard ratio=1.51, 95% Confidence Interval=1.10–2.06, P=0.011). This association was not statistically significant after additional adjustment for income and education (P=0.150) (Table III). The association remained statistically significant, however, after additional adjustment for baseline physical activity (P=0.017) (Table III). There was no significant interaction of study cohort in the association of race and becoming unable to walk for six minutes continuously, suggesting that the association of race with the ability to walk for six-minutes continuously did not change over time. Results shown in Table III were not substantially changed when analyses were repeated adjusting for the ABI in which the highest of the dorsalis pedis and posterior tibial pressures was used in the numerator and the highest of the left vs. right brachial artery pressure was used in the denominator.
Figure 2.
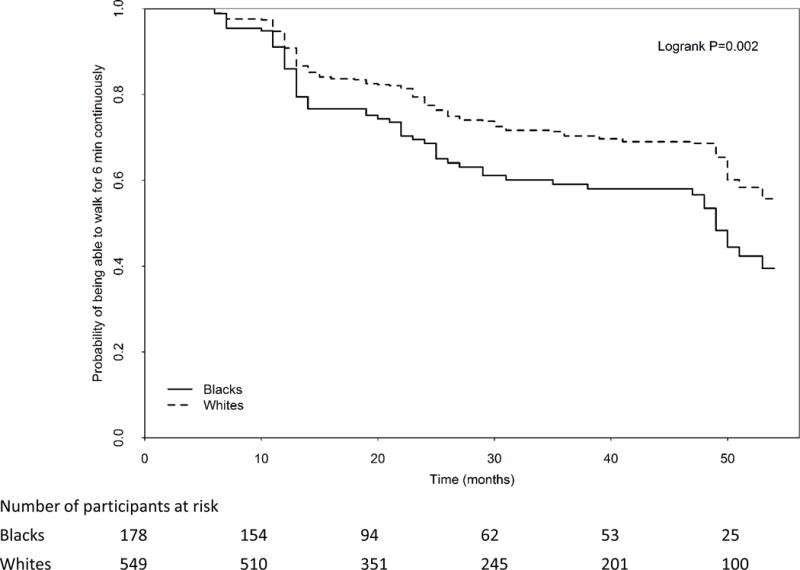
Rates of becoming unable to walk continuously for six minutes in blacks vs. whites with peripheral artery disease (N=727)
In analyses adjusting for age, sex, ABI, BMI, smoking, comorbidities, leg symptoms, study cohort, and prior year performance, there was no difference between white vs. black PAD participants in average annual decline in the six-minute walk distance (−59.2 vs. −52.8 feet per year, respectively, P=0.502), usual paced four-meter walking velocity (−0.025 vs. −0.032 meters/second, respectively, P=0.255), or fast paced four-meter walking velocity (−0.040 vs. −0.048 meters/second, respectively, P=0.249).
Because the Kaplan-Meier curve suggested that mobility loss was greatest during the first year of follow-up, we conducted post-hoc analyses in which we analyzed the association of black race with mobility loss during the first year vs. after the first year of follow-up. Rates of mobility loss during the first year of follow-up (i.e. within 13 months after enrollment) were 56/650 (8.6%) for white PAD participants and 37/212 (17.5%) for black PAD participants (P<0.001). Adjusting for age, sex, ABI, BMI, smoking, comorbidities, leg symptoms, and study cohort, black PAD participants had an increased risk of mobility loss, compared to white PAD participants during the first year of follow-up (hazard ratio=1.96, 95% Confidence Interval=1.24–3.08, P=0.004). This association was no longer statistically significant after additional adjustment for income and education status (P=0.099) but remained statistically significant after additional adjustment for physical activity level (P=0.011). When we separately analyzed rates of mobility loss after the first year of follow-up there was no difference between whites and blacks in risk of mobility loss (mobility loss: 113/507 (22.3%) vs. 30/132 (22.7%), P=0.914). Adjusting for age, sex, race, study cohort, comorbidities, ABI, BMI, leg symptoms, and smoking, the hazard ratio for mobility loss in blacks vs. whites after the first year of follow-up was: 1.035 (95% CI=0.67–1.60, P=0.877).
DISCUSSION
In 1,175 participants with PAD identified from Chicago-area medical centers and followed prospectively, black participants had higher rates of mobility loss and a higher rate of becoming unable to walk for six minutes without stopping compared to whites over 46 months follow-up. These findings were independent of adjustment for age, sex, ABI, smoking, comorbidities, and other confounders. After additional adjustment for income and education, racial differences in mobility loss and becoming unable to walk for six minutes continuously were not observed. Our findings suggest that racial differences in income and education level may explain racial differences in rates of mobility loss and becoming unable to walk for six minutes continuously among people with PAD. Blacks also had lower physical activity than whites, which may be related to lower baseline ABI values in blacks vs. whites or may be related to racial differences in environment or priorities. After adjusting for physical activity, the association of black race with mobility loss was no longer observed. However, the association of black race with becoming unable to walk for six minutes continuously remained statistically significant. This finding suggests that race differences in physical activity may explain some, but not all, of the racial differences in functional decline observed here.
Prior studies assessed associations of black race with amputation and revascularization rates in PAD patients with critical limb ischemia (1–6,35). Lefebvre et al used the Nationwide Inpatient Survey to study patients hospitalized with PAD between 2002 and 2011. Black race was associated with higher rates of trans-femoral amputation compared to non-black race and this disparity did not change over the 10 year time period of the study, even as overall amputation rates declined (35). Using data from the Nationwide Inpatient Survey, Henry et al reported that among 958,120 patients hospitalized with critical limb ischemia between 2002 and 2007, black patients were more likely than whites to undergo amputation, while white patients were more likely to undergo lower extremity revascularization (2). Racial differences in socioeconomic status and access to medical care have been cited as potential explanations for racial differences in amputation rates. However, one recent study reported racial differences in management of critical limb ischemia even within hospitals with advanced revascularization capabilities and/or located in wealthy zip codes (1). Currently, evidence is conflicting regarding whether racial differences in outcomes are explained by racial differences in factors such as socioeconomic status or education. Our findings suggest that racial differences in functional decline among people with PAD are largely explained by differences in socioeconomic status, education, and physical activity levels.
To our knowledge, no prior longitudinal studies have compared differences between blacks and whites in rates of functional decline or mobility loss in people with PAD who do not have critical limb ischemia. Lower income and education status in blacks with PAD may mediate our findings of racial differences in mobility loss and becoming unable to walk for six minutes continuously if PAD patients with lower education and income have less access to healthy lifestyles, such as healthy diet and opportunities for regular exercise, and less access to optimal medical care. For these reasons, black patients may present at a later stage of PAD, compared to white patients. Consistent with this hypothesis, black participants with PAD had lower baseline ABI values than white participants with PAD. Similarly, in a single center study, black patients with critical limb ischemia had more severe PAD than white patients with critical limb ischemia (36). We also found that black PAD participants had lower physical activity levels at baseline compared to whites and that lower physical activity levels partly explained higher rates of mobility loss in black PAD participants.
We did not identify differences in rates of average annual decline in six-minute walk distance or four-meter walking velocity between black vs. white participants with PAD. There are several potential explanations for these findings. First, it is possible that lower baseline performance in the six-minute walk and four-meter walking velocity among black PAD participants resulted in a floor effect, in which further declines in walking performance from baseline among participants were less likely. Second, it is possible that black PAD participants with the greatest declines in six-minute walk and four-meter walking velocity were less likely to return for follow-up testing than white PAD participants, resulting in the inability to detect racial differences in decline in six-minute walk and four-meter walking velocity. Third, it is possible that there is a threshold effect for decline in the six-minute walk and four meter walking velocity that black participants had already crossed at the time of study enrollment.
Our study has limitations. First, we cannot discern the specific causal pathway by which lower income, education, and physical activity levels among blacks mediate the racial differences in decline reported here. Second, there may be unmeasured confounders. Third, we did not measure severity of comorbidities at baseline. Fourth, our measure of physical activity was based on patient self-report. It is possible that an objective measure of physical activity may have altered our conclusions regarding the importance of physical activity as a mediator of racial differences in mobility loss. Fifth, the mobility loss measure is a patient-reported measure. Although the mobility loss outcome is a well validated measure of ability to walk ¼ mile and walk up and down a flight of stairs without assistance (20–22), some small cross-sectional studies suggest that patients with PAD do not accurately report their actual pain-free walking distance (37,38). Sixth, we did not assess presence of chronic kidney disease. Seventh, some of the racial differences in functional decline may have been attributable to more severe PAD among the black participants, which may not have been precisely measured by the ABI, particularly given the higher prevalence of diabetes mellitus among black PAD participants.
In summary, black patients with PAD have higher rates of mobility loss and higher rates of becoming unable to walk for six minutes continuously compared to white patients with PAD. These differences appear to be explained by lower socioeconomic status in blacks compared to whites with PAD. Racial differences in physical activity also appear to contribute to findings reported here. Further study is needed to determine whether interventions that increase physical activity levels in blacks and reduce racial disparities in income and education can eliminate racial differences in mobility loss in PAD. Recent evidence demonstrates that a home-based walking exercise intervention reduces mobility loss in patients with PAD (25). Further study is needed to determine whether increasing access of all PAD patients to home-based exercise programs can reduce the racial difference in mobility loss reported here.
Acknowledgments
The authors thank Luigi Ferrucci MD, PhD and Michael H. Criqui MD, MPH for their important contributions to the manuscript.
FUNDING SOURCES:
Funded by the National Heart Lung and Blood Institute (NHLBI); R01-HL083064, R01-HL64739, R01-HL58099, R01-HL076298, R01-HL71223, R01-HL089619, and R01-HL109244.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST DISCLOSURES
None
References
- 1.Durazzo TS, Frencher S, Gusberg R. Influence of race on the management of lower extremity ischemia: revascularization vs amputation. JAMA Surg. 2013;148:617–623. doi: 10.1001/jamasurg.2013.1436. [DOI] [PubMed] [Google Scholar]
- 2.Henry AJ, Hevelone ND, Belkin M, Nguyen LL. Socioeconomic and hospital-related predictors of amputation for critical limb ischemia. J Vasc Surg. 2011;53:330–339. doi: 10.1016/j.jvs.2010.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eslami MH, Zayaruzny M, Fitzgerald GA. The adverse effects of race, insurance status, and low income on the rate of amputation in patients presenting with lower extremity ischemia. J Vasc Surg. 2007;45:55–59. doi: 10.1016/j.jvs.2006.09.044. [DOI] [PubMed] [Google Scholar]
- 4.Feinglass J, Rucker-Whitaker C, Lindquist L, McCarthy WJ, Pearce WH. Racial differences in primary and repeat lower extremity amputation: results from a multihospital study. J Vasc Surg. 2005;41:823–829. doi: 10.1016/j.jvs.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 5.Holman KH, Henke PK, Dimick JB, Birkmeyer JD. Racial disparities in the use of revascularization before leg amputation in Medicare patients. J Vasc Surg. 2011;54:420–426. doi: 10.1016/j.jvs.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes K, Boyd C, Oyetunji T, Tran D, Chang D, Rose D, et al. Racial/ethnic disparities in revascularization in limb salvage: An analysis of the National Surgical Quality Improvement Program database. Vasc Endovascular Surg. 2014;48:402–405. doi: 10.1177/1538574414543276. [DOI] [PubMed] [Google Scholar]
- 7.Rucker-Whitaker C, Greenland P, Liu K, Chan C, Guralnik JM, Criqui MH, et al. Peripheral arterial disease in African Americans: clinical characteristics, leg symptoms, and lower extremity functioning. J Am Geriatr Soc. 2004;52:922–930. doi: 10.1111/j.1532-5415.2004.52259.x. [DOI] [PubMed] [Google Scholar]
- 8.McDermott MM, Greenland P, Liu K, Guralnik JM, Celic L, Criqui MH, et al. The ankle brachial index is associated with leg function and physical activity: the walking and leg circulation study. Ann Intern Med. 2002;136:873–883. doi: 10.7326/0003-4819-136-12-200206180-00008. [DOI] [PubMed] [Google Scholar]
- 9.McDermott MM, Greenland P, Liu K, Guralnik JM, Criqui MH, Dolan NC, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–1606. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 10.McDermott MM, Ferrucci L, Guralnik J, Tian L, Liu K, Hoff F, et al. Pathophysiological changes in calf muscle predict mobility loss at 2-year follow-up in men and women with peripheral arterial disease. Circulation. 2009;120:1048–55. doi: 10.1161/CIRCULATIONAHA.108.842328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDermott MM, Carroll TJ, Kibbe M, Kramer CM, Liu K, Guralnik JM, et al. Proximal superficial femoral artery occlusion, collateral vessels, and walking performance in peripheral artery disease. JACC Cardiovasc Imaging. 2013;6:687–94. doi: 10.1016/j.jcmg.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDermott MM, Liu K, Greenland P, Guralnik JM, Criqui MH, Chan C, et al. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA. 2004;292:453–461. doi: 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- 13.McDermott MM, Liu K, Green D, Greenland P, Tian L, Kibbe M, et al. Changes in D-dimer and inflammatory biomarkers before ischemic events in patients with peripheral artery disease: The BRAVO Study. Vascular Medicine. 2016;21(1):12–20. doi: 10.1177/1358863X15617541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, et al. Measurement and interpretation of the ankle-brachial index: A scientific statement from the American Heart Association. Circulation. 2012;126:2890–909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 15.McDermott MM, Criqui MH, Liu K, Guralnik JM, Greenland P, Martin GJ, et al. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease. J Vasc Surg. 2000;32:1164–1171. doi: 10.1067/mva.2000.108640. [DOI] [PubMed] [Google Scholar]
- 16.Shadman R, Criqui MH, Bundens WP, Fronek A, Denenberg JO, Gamst AC, et al. Subclavian artery stenosis: prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol. 2004;44:618–23. doi: 10.1016/j.jacc.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 17.McDermott MM, Guralnik JM, Ferrucci L, Liu K, Liao Y, Criqui MH. Baseline functional performance predicts the rate of mobility loss in persons with peripheral arterial disease. J Am Coll Cardiol. 2007;50:974–982. doi: 10.1016/j.jacc.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guralnik JM, Ferrucci L, Simonsick E, Salive ME, Wallace RB. Lower extremity function in persons over 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardy SE, Kang Y, Studenski SA, Degenholtz HB. Ability to walk 1/4 mile predicts subsequent disability, mortality, and health care costs. J Gen Intern Med. 2011;26:130–135. doi: 10.1007/s11606-010-1543-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kane RA, Kane RL. Assessing the elderly: A practical guide to measurement. Lexington MA: Lexington Books; 1981. [Google Scholar]
- 21.Meenan RF, Chang RW. Summary: Consensus statements. J Rheumatol. 1987;14(Suppl 15):20–21. [Google Scholar]
- 22.Smith LA, Branch LG, Scherr PA, Wetle T, Evans DA, Hebert L, et al. Short-term variability of measures of physical function in older people. J Am Geriatr Soc. 1990;38:993–998. doi: 10.1111/j.1532-5415.1990.tb04422.x. [DOI] [PubMed] [Google Scholar]
- 23.McDermott MM, Tian L, Liu K, Guralnik JM, Ferrucci L, Tan J, et al. Prognostic value of functional performance for mortality in patients with peripheral artery disease. J Am Coll Cardiol. 2008;15:1482–1489. doi: 10.1016/j.jacc.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDermott MM, Guralnik JM, Criqui MH, Liu K, Kibbe MR, Ferrucci L. Six-minute walk is a better outcome measure than treadmill walking tests in therapeutic trials of patients with peripheral artery disease. Circulation. 2014;130:61–68. doi: 10.1161/CIRCULATIONAHA.114.007002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDermott MM, Guralnik JM, Criqui MH, Ferrucci L, Liu K, Spring B, et al. Unsupervised exercise and mobility loss in peripheral artery disease: A randomized controlled trial. J Am Heart Assoc. 2015;4(5) doi: 10.1161/JAHA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDermott MM, Liu K, Ferrucci L, Tian L, Guralnik JM, Liao Y, Criqui MH. Decline in functional performance predicts later increased mobility loss and mortality in peripheral arterial disease. J Am Coll Cardiol. 2011;57(8):962–970. doi: 10.1016/j.jacc.2010.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fried LP, Kasper JD, Williamson JD, Skinner EA, Morris CD, Hochberg MC, for the Disease Ascertainment Working Group . Disease ascertainment algorithms. In: Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty ME, editors. The Women’s Health and Aging Study: Health and Social Characteristics of Older Women with Disability. Bethesda (MD): National Institute on Aging, National Institutes of Health; c1995. (Appendix E. (NIH publication; no. 95-4009)). [Google Scholar]
- 28.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Arthritis and Rheumatism. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 29.Criqui MH, Denenberg JO, Bird CE, Fronek A, Klauber MR, Langer RD. The correlation between symptoms and non-invasive test results in patients referred for peripheral arterial disease testing. Vasc Med. 1996;1:65–71. doi: 10.1177/1358863X9600100112. [DOI] [PubMed] [Google Scholar]
- 30.Jolly M, Mikolaitis RA, Shakoor N, Flogg LF, Block JA. Education, zip code based annualized household income and health outcomes in patients with lupus erythematosis. J Rheumatol. 2010;37:1150–1157. doi: 10.3899/jrheum.090862. [DOI] [PubMed] [Google Scholar]
- 31.Lee IM, Paffenbarger RS, Hsieh CC. Time trends in physical activity among college alumni, 1962–1988. Am J Epidemiol. 1992;135:912–925. doi: 10.1093/oxfordjournals.aje.a116387. [DOI] [PubMed] [Google Scholar]
- 32.Simonsick EM, Guralnik JM, Volpato S, Balfour J, Fried LP. Just get out the door! Importance of walking outside the home for maintaining mobility: Findings from the Women’s Health and Aging Study. J Am Geriatr Soc. 2005;53:198–203. doi: 10.1111/j.1532-5415.2005.53103.x. [DOI] [PubMed] [Google Scholar]
- 33.Diehr P, Williamson J, Burke GL, Psaty BM. The aging and dying processes and the health of older adults. J Clin Epidemiol. 2002;55:269–278. doi: 10.1016/s0895-4356(01)00462-0. [DOI] [PubMed] [Google Scholar]
- 34.Garg PK, Liu K, Tian L, Guralnik JM, Ferrucci L, Criqui MH, et al. Physical activity during daily life and functional decline in peripheral arterial disease. Circulation. 2009;119:251–260. doi: 10.1161/CIRCULATIONAHA.108.791491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lefebvre KM, Chevan J. The persistence of gender and racial disparities in vascular lower extremity amputation: An examination of the HCUP-NIS data (2002–2011) Vasc Med. 2015;20:51–59. doi: 10.1177/1358863X14565373. [DOI] [PubMed] [Google Scholar]
- 36.Sidawy AN, Schweitzer EJ, Neville RF, Alexander EP, Temeck BK, Curry KM. Race as a risk factor in the severity of infragenicular occlusive disease: Study of an urban hospital patient population. J Vasc Surg. 1990;11:536–543. [PubMed] [Google Scholar]
- 37.Watson CJE, Phillips D, Hands L, Collin J. Claudication distance is poorly estimated and inappropriately measured. British Journal of Surgery. 1997;84:1107–1109. [PubMed] [Google Scholar]
- 38.Frans FA, Zagers MB, Jens S, Bipat S, Reekers JA, Koelemay MJW. The relationship of walking distances estimated by the patient, on the corridor and on a treadmill, and the Walking Impairment Questionnaire in intermittent claudication. J Vasc Surg. 2013;57:720–727. doi: 10.1016/j.jvs.2012.09.044. [DOI] [PubMed] [Google Scholar]


