Abstract
Chemotherapy is widely used to treat patients with systemic cancer. The efficacy of cancer therapies is frequently undermined by adverse side effects that have a negative impact on the quality of life of cancer survivors. Cancer patients who receive chemotherapy often experience chemotherapy-induced cognitive impairment across a variety of domains including memory, learning, and attention. In the current study, the impact of paclitaxel, a taxane derived chemotherapeutic agent, on episodic memory, prior learning, new learning, and reversal learning were evaluated in rats. Neurogenesis was quantified post-treatment in the dentate gyrus of the same rats using immunostaining for 5-Bromo-2′-deoxyuridine (BrdU) and Ki67. Paclitaxel treatment selectively impaired reversal learning while sparing episodic memory, prior learning, and new learning. Furthermore, paclitaxel-treated rats showed decreases in markers of hippocampal cell proliferation, as measured by markers of cell proliferation assessed using immunostaining for Ki67 and BrdU. This work highlights the importance of using multiple measures of learning and memory to identify the pattern of impaired and spared aspects of chemotherapy-induced cognitive impairment.
Keywords: Episodic memory, Learning, Reversal Learning, Chemotherapy, Neurogenesis, Rats
1. Introduction
Paclitaxel is a chemotherapeutic drug from the taxane family commonly used to treat lung, pancreatic, ovarian and breast cancers. Chemotherapeutic agents produce anti-tumor effects by blocking or suppressing proliferation of dividing cells. Paclitaxel binds to the beta subunit of tubulin which then results in the polymerization of microtubules, thereby producing apoptosis. However, the efficacy of this cancer therapy is undermined by adverse side effects including peripheral neuropathy and cognitive impairment. Taxane chemotherapeutic agents induce peripheral neuropathy characterized by sensory abnormalities including tingling, numbness, as well as shooting and burning pain (Wolf, Barton, Kottschade, Grothey, and Loprinzi, 2008); these sensory abnormalities are observed in both humans and non-human animals alike (Cavaletti, Alberti, Frigeni, Piatti, and Susani, 2011; Deng, Guindon, Vemuri, Thakur, White, Makriyannis, and Hohmann, 2012; Gutierrez-Gutierrez, Sereno, Miralles, Casado-Saenz, and Gutierrez-Rivas, 2010; Polomano, Mannes, Clark, and Bennett, 2001; Rahn, Zvonok, Thakur, Khanolkar, Makriyannis, and Hohmann, 2008; Wolf et al., 2008). Peripheral neuropathy can occur early in chemotherapy treatment and persist long after treatment cessation (Authier, Balayssac, Marchand, Ling, Zangarelli, Descoeur, Coudore, Bourinet, and Eschalier, 2009) and is reported to occur in approximately 30–40% of patients. Consequently, clinicians are forced to weigh the potential for therapeutic efficacy of dosing and administration schedule against the potential of inducing a painful state of peripheral neuropathy in patients. Reduction in cognitive function following anti-cancer treatment, referred to as chemotherapy-induced cognitive impairment (CICI) or “chemobrain” among patient groups, is another debilitating side effect of chemotherapeutic treatment. Adverse cognitive side effects include difficulty sustaining attention, poor concentration, slower processing speed, and poor learning and memory (Ahles and Saykin, 2002; Wefel and Schagen, 2012). Up to 70% of cancer survivors report cognitive deficits during and after chemotherapy treatment (Ahles and Saykin, 2007; Seigers and Fardell, 2011). CICI symptom onset occurs as early as 5 hours post-treatment and lasts up to 6 months after administration or longer (Schultz, Beck, Stava, and Vassilopoulou-Sellin, 2003). Patients treated with combination chemotherapy including paclitaxel have impairments of cognitive functioning (Ahles and Saykin, 2002; Wefel and Schagen, 2012). Specifically, patients receiving a combination of paclitaxel, 5-fluorouracik, cyclophosphamide and adriamycin showed deficits in learning and memory for up to a year following treatment (Ahles and Saykin, 2002). Furthermore, not all cancer survivors report a full CICI recovery following treatment cessation. Thus, despite paclitaxel’s low CNS penetration rate, taxane chemotherapeutic agents have a detrimental impact on cognitive functioning that have deleterious effects on patients’ quality of life. To date, no treatment for CICI has been recognized (Belzung, Wigmore, and ebrary Inc., 2013) and a greater understanding of the underlying causes and mechanisms is critical if novel therapeutics that improve quality of life in survivors are to be developed for clinical use. The mechanisms behind adverse side effects of chemotherapy on cognition remain poorly understood. Blood-brain penetration of chemotherapy drugs may affect brain structure and function (Belzung et al., 2013). Decreased cell proliferation in the hippocampus is also postulated to underlie chemotherapy-induced cognitive dysfunction (Briones and Woods, 2011; Winocur, Wojtowicz, Huang, and Tannock, 2014). A change in the level of hippocampal neurogenesis is a key biological mechanism proposed to underlie CICI. The hippocampus has been implicated in learning, memory, and spatial processing (Eichenbaum, 2000; 2007; Eichenbaum, Sauvage, Fortin, Komorowski, and Lipton, 2012; Eichenbaum, Yonelinas, and Ranganath, 2007; Ergorul and Eichenbaum, 2004; Gold, Hopkins, and Squire, 2006; Kim, Dede, Hopkins, and Squire, 2015; Shrager, Bayley, Bontempi, Hopkins, and Squire, 2007; Smith, Wixted, and Squire, 2011) and has been shown to exhibit neurogenesis in adulthood (Zhao, Deng, and Gage, 2008). In the adult mammalian brain, neurogenesis occurs in the subgranular zone (SGZ) of the dentate gyrus (DG) in the hippocampus.
Throughout this process, new dentate granule cells get integrated into existing hippocampal circuits, some of which are thought to be critical for memory (Deng, Aimone, and Gage, 2010). These newborn neurons have been shown to play a role in hippocampal function by assessing diverse hippocampal-dependent behaviors (Kee, Teixeira, Wang, and Frankland, 2007; Ramirez-Amaya, Marrone, Gage, Worley, and Barnes, 2006; Tashiro, Makino, and Gage, 2007). Adult born dentate granule cells undergo preferential recruitment into hippocampal neural circuits that mediate novelty recognition, associative learning, and spatial memory (Denny, Burghardt, Schachter, Hen, and Drew, 2012; Kee et al., 2007; Ramirez-Amaya et al., 2006). Decreased neurogenesis also accompanies deleterious effects in some forms of hippocampal-dependent learning and memory (Arruda-Carvalho, Sakaguchi, Akers, Josselyn, and Frankland, 2011; Drew, Denny, and Hen, 2010). Together, these findings suggest that hippocampal neurogenesis plays a critical role in hippocampal-dependent domains of cognition. Chemotherapy drugs suppress cell proliferation, including newly generated hippocampal neurons in both human and animal models (Pereira Dias, Hollywood, Bevilaqua, da Luz, Hindges, Nardi, and Thuret, 2014; Winocur, Wojtowicz, and Tannock, 2015). Decreased hippocampal neurogenesis has been documented following treatment with multiple chemotherapy drugs both individually (Janelsins, Roscoe, Berg, Thompson, Gallagher, Morrow, Heckler, Jean-Pierre, Opanashuk, and Gross, 2010) and when treated in combination (Briones and Woods, 2011; Christie, Acharya, Parihar, Nguyen, Martirosian, and Limoli, 2012; Winocur et al., 2015) and may accompany CICI induced by paclitaxel.
Despite the wide range of symptoms observed in human patients, only a limited range of behavioral tasks have been used to evaluate cognitive impairments attributed to chemotherapeutic treatment in rodents. To date, measures of chemotherapy-induced cognitive deficits in rodents have predominantly focused on hippocampal and frontal dependent functions including spatial navigation (Morris Water maze), novelty recognition (novel location recognition), associative learning and memory (non-match to sample and delayed non-match to sample). Chemotherapy impaired spatial memory, conditional associative learning, and discrimination learning (some of which were chronically observed (Winocur, Henkelman, Wojtowicz, Zhang, Binns, and Tannock, 2012)), and it suppressed hippocampal neurogenesis (Winocur et al., 2015).
Another domain of cognitive function relevant to memory is that of episodic memory. Episodic memory is described as the memory for unique personal past events. Elements of episodic memory include memory for what, where, and when a specific event occurred. Episodic memory encodes the origin (i.e., source) of previously encountered information (Crystal, 2016; Crystal, Alford, Zhou, and Hohmann, 2013; Johnson, 2005). Because source memory includes features that were present when the specific memory was encoded, it allows us to differentiate one episodic memory from another (Crystal and Smith, 2014; Johnson, Hashtroudi, and Lindsay, 1993; Mitchell and Johnson, 2009). While chemotherapy-induced episodic memory impairment has been reported in humans (Monje and Dietrich, 2012), this has been difficult to evaluate in animal models.
Animal models of source memory have documented that rats remember the source of encoded information (Crystal and Alford, 2014; Crystal et al., 2013; Crystal and Smith, 2014). In this approach, rats are asked to forage for distinctive flavors of food in a radial maze. Rats are able to remember what, where, and how they encountered distinct flavors using source memory (Crystal, 2016; Crystal and Alford, 2014; Crystal et al., 2013). Treatment with paclitaxel did not impair episodic memory in a source memory task, even after a long retention interval, or the development of proactive interference (Smith, Slivicki, Hohmann, and Crystal, 2017). However, paclitaxel-treated rats showed deficits in their sensitivity to changes in experimental contingencies, as assessed by reversing the source memory rule (Smith et al., 2017), a finding consistent with chemotherapy-induced reductions in cognitive flexibility.
Learning is another domain of cognition that is affected in chemotherapy patients. Notably, rule learning is a feature embedded in the source memory task used by Smith and colleagues (Smith et al., 2017). Because the source information rule reversal represented both new learning and reversal learning, it is still an open question as to whether chemotherapy impaired new learning, reversal learning, or both. An experimental design that dissociates new learning and reversal learning is therefore required to better understand cognitive deficits associated with paclitaxel-induced CICI.
Episodic memory can also be studied in rats by exploiting their well-established proficiency with odors (Panoz-Brown, Corbin, Dalecki, Gentry, Brotheridge, Sluka, Wu, and Crystal, 2016). In the item-in-context olfactory memory approach (Panoz-Brown et al., 2016), rats have been shown to remember multiple unique events and the contexts in which events occurred using episodic memory, all assessed under conditions that dissociated item in context memory from familiarity cues (Panoz-Brown et al., 2016). The olfactory preparation is also well suited for assessing multiple dimensions of cognition, including prior learning, new learning, and reversal learning (Galizio, 2016; Galizio, Miller, Ferguson, McKinney, and Pitts, 2006). However, it is not known if chemotherapy treatment differentially affects new learning and/or reversal learning. The current work presents the opportunity to validate and expand on whether paclitaxel treatments spare episodic memory and impairs learning (Smith et al., 2017).
Severity of unwanted side effects is often a dose-limiting factor for clinical use of paclitaxel (Scripture, Figg, and Sparreboom, 2006). Meanwhile, not all studies of CICI have found impairments after chemotherapy treatment (Fremouw, Fessler, Ferguson, and Burguete, 2012a; b; Long, Lee, Kelley-Bell, Spangler, Perez, Longo, de Cabo, Zou, and Rapp, 2011; Lyons, Elbeltagy, Bennett, and Wigmore, 2011). These inconsistencies across chemotherapy drugs highlight the importance of examining multiple domains of learning and memory. Multidimensional approaches serve to better characterize the pattern of spared and impaired aspects of treatment induced cognitive impairment and their underlying mechanisms.
The present study examined effects of the chemotherapeutic agent paclitaxel on prior learning, new learning, reversal learning and episodic memory. Rats trained in the item-in-context episodic memory task and multiple simple discriminations were treated with paclitaxel or vehicle (i.e., cremophor). Following treatment, episodic memory, prior learning, new learning and reversal learning were evaluated. Because of the possible confounding influence of non-mnemonic factors on cognitive measures, we examined whether chemotherapy treatment induced neuropathic pain on days in which cognitive assessments were not preformed. After behavioral testing was complete, levels of hippocampal neurogenesis in the granule cell layer of the dentate gyrus were quantified using BrdU and Ki67 immunohistochemistry in rats treated with paclitaxel or it’s cremophor-based vehicle.
2. Effects of paclitaxel on learning, memory, and hippocampal neurogenesis
2.1 Methods and materials
2.1.1 Subjects
Subjects were eleven male Sprague-Dawley rats (Harlan, Indianapolis, IN; approximately 10 months old, on average 427g at the beginning of paclitaxel treatment). Rats were individually housed in a vivarium on a 12/12-h light-dark cycle. Light onset and offset occurred at 0715 and 1950, respectively. Before the onset of paclitaxel treatment, rats completed olfactory training and memory assessments described by Panoz-Brown and colleagues (Panoz-Brown et al., 2016). Rats received 45-mg chocolate pellets (F0299; Bio-Serv, Frehchtown, NJ) during behavioral sessions followed by 15 g/day of 5012-Rat-Diet (PMI Nutrition International, St. Louis, MO). Water was available ad libitum except during experiment testing sessions. All the rats had previous olfactory training with the item-in-context episodic memory task as reported elsewhere (Panoz-Brown et al., 2016).
2.1.2 Drug preparations and administration
5-Bromo-2′-deoxyuridine (BrdU) (Sigma-Aldrich, St. Louis, MO) was dissolved in a vehicle consisting of 0.9% saline (Aquilite System; Hospira, Inc, Lake Forest, IL) containing 1N NaOH (20 mg/ml) and administered at a volume of 5 ml/kg (i.p.). Paclitaxel (Tecoland, Irvine, CA) was dissolved in a vehicle consisting of cremophor El (Sigma-Aldrich, St. Louis, MO), 95% ethanol (Sigma-Aldrich, St. Louis, MO) and saline at a ratio of 1:1:18, respectively, and was administered at a volume of 1 ml/kg.
2.1.3 Paclitaxel-induced peripheral neuropathic pain
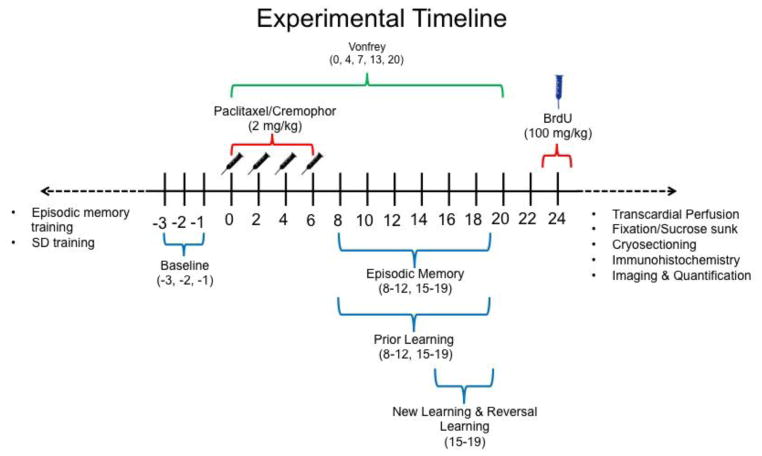
Chemotherapy-induced peripheral neuropathy was produced using paclitaxel (Polomano et al., 2001) using methods described previously by our laboratory (Deng et al., 2012; Deng, Lee, Xu, Makriyannis, and Hohmann, 2016; Rahn, Deng, Thakur, Vemuri, Zvonok, Lai, Makriyannis, and Hohmann, 2014; Rahn et al., 2008). Rats were injected with paclitaxel (2 mg/kg, i.p.; cumulative dose 8 mg/kg, i.p.) or its cremophor-based vehicle on four alternating days (day 0, 2, 4 and 6 following initiation of paclitaxel/cremophor vehicle dosing; see Figure 1).
Fig. 1.
Experimental Design in days relative to treatment onset. Paclitaxel or cremophor-based vehicle was administered on days 0, 2, 4, and 6. Item-in-context training and prior learning SD training occurred prior to day 0. Baseline was measured over three behavioral testing sessions immediately preceding treatment. Mechanical allodynia (i.e., von Frey) assessments occurred on days 0, 4, 7, 13, and 20. Post-treatment episodic memory and prior learning assessments occurred on days 8–12, and 15–19. New learning and reversal learning assessments occurred on days 15–19. Onset of BrdU occurred on day 24 post-treatment. Transcardial perfusion, fixation, cryosectioning, immunohistochemistry, imaging and quantification all occurred after day 24.
2.1.4 Mechanical allodynia assessment
Paw withdrawal thresholds to mechanical stimulation were measured using an electro von Frey anesthesiometer (IITC Life Science Inc., Woodland Hills, CA) as described previously (Deng et al., 2012; Deng et al., 2016; Rahn et al., 2014; Rahn et al., 2008). Briefly, rats were placed on an elevated mesh platform in Plexiglas observation chambers. Paw withdrawal thresholds were taken in duplicate in each paw prior to paclitaxel treatment, and on days 4, 7, 13 and 20 following the onset of paclitaxel treatment. Paw withdrawal thresholds were not measured on days when cognitive assessments occurred. Thresholds were averaged across paws.
2.1.5 Apparatus
Two arenas were used for odor presentation and served as the distinctive contexts during learning and memory assessments (each context differed in size, color and number of stimulus locations). Context A was white in color, circular in shape (94 cm in diameter) and enclosed with a 30-cm high wall, all constructed with opaque acrylic plexiglass. Eighteen circular holes (5 cm diameter, 2.5 cm deep) arranged in two concentric circles served as stimulus locations. Context B was circular in shape (46 cm in diameter), consisted of a black and white colored floor, and enclosed with transparent walls. Eight equidistant circular holes (5 cm diameter, 2.5 cm deep) served as stimulus locations that were positioned along the perimeter. White noise was used to mask outside noise during testing sessions. Following each animal’s daily session, both arenas were cleaned with 2% chlorhexidine solution.
2.1.6 Stimuli
Opaque plastic lids and condiment cups (59-ml) were used for odor presentations. During odor presentation condiment cups were firmly snapped into place so that the cup lay flush with the floor and loosely but completely covered with the odorized plastic lid. Lids were odorized by storing them in sealed plastic containers, each consisting of approximately 90 ml of oil odorant or 150 ml of spice odorant. A metal grating separated lids and odorants to prevent direct contact in the containers. Odors in the item in context episodic memory assessment included the following: almond oil, amaretto oil, asparagus, banana, blueberry oil, brandy oil, butterscotch oil, caraway seed, celery seed, chicory root, cinnamon, coffee oil, cumin, dill weed, garlic powder, hickory smoke, honey oil, horseradish, Irish cream oil, lavender, lemon zest, maple, menthol-eucalyptus, Mexican oregano, mustard seed, onion powder, orange oil, pecan oil, pineapple oil, root beer oil, rosemary leaf, sage leaf, sesame oil, spinach powder, strawberry oil, summer savory, thyme, tomato, Mexican vanilla, and watermelon oil.
Odors in the olfactory simple discrimination tasks included the following: allspice, apricot oil, blackberry oil, bubblegum oil, carob, champagne oil, cherry oil, cilantro, coconut, fenugreek, hazelnut oil, Indian curry, nutmeg, peach oil, pistachio oil, raspberry oil, sassafras oil, spearmint, white willow bark, and Worcestershire.
2.2.1 General Methods
Rats were tested once per day, approximately 5 days per week. In each session, numerous variables were randomly selected, including: context order, odor identity, and location of stimuli inside each context. In addition, although the same odors were used multiple times in each session, new lids were used in every trial to prevent the use of scent marking. During all sessions, the rat was removed from the holding cage and placed in one of the two arenas for each trial. During trials, the rat was allowed to search until a response to the designated correct choice or S+ was made, or until 2 minutes elapsed. A correct response occurred when the first response observed was made to the “new” or S+ item. If the rat selected the “old” or S−, the response was scored as incorrect the rat was allowed to navigate the arena until the correct item was selected. If a rat did not make a response before two minutes elapsed, an omission was recorded and the trial was terminated. Omission responses were scored as incorrect choices.
Experimental design and timeline are represented in Fig. 1. Rats received episodic memory training and SD pair training before paclitaxel/cremophor treatment. Cognitive tests measuring different aspects of learning and memory were administered before treatment, immediately after treatment, and one week after treatment. Baseline consisted of the average accuracy in the three behavioral testing session preceding treatment (i.e., days −3, −2, −1). Onset of treatment is represented as day 0.
2.2.2 Behavioral Procedures
The present study capitalized on the well-established proficiency of rats with olfactory information using an item-in-context memory procedure (Panoz-Brown et al., 2016). To assess item-in-context episodic memory, we asked rats to identify specific items and the context in which they were encountered for multiple unique events. In earlier training, rats were presented with multiple odors to remember across two contexts in rapid succession. In this approach, rats were trained to pick the “new” odor and avoid the “old” odor when presented with pairs of items in each trial. In each pair, one item was always “new” to the current context, while the other was always “old”; “old” items had already been presented within that context earlier in the session. To assess memory of item in context while controlling for non-episodic familiarity cues, we rapidly interleaved presentations of new odors across contexts using multiple context transitions that divided the presentation of items (i.e., odors) into multiple segments. An item-in-context procedure with three context transitions was used for the episodic memory assessment (Context A→ Context B→Context A→ Context B or ABAB). In the initial “AB” segments, rats were presented with half of the items to be remembered in each context (i.e., eight odors per context). However, in the first “AB”, it is possible for high accuracy to be achieved by using the semantic rule of “avoiding familiar items” without using episodic memory to remember the items in context. To dissociate episodic memory from non-episodic judgments of familiarity we interleaved presentations of new odors across contexts and isolated sequences of odors in the last two segments (i.e., last “AB” in ABAB) that put familiarity cues and item-in-context memory in conflict (Panoz-Brown et al., 2016). For the last “AB” segments, rats were returned to the first and second context, in sequence, and presented with the remaining half of the “new” items in each (i.e., eight new odors in each context). In order to attain high accuracy in the item-in-context memory assessments, the rats had to remember which items had been encountered in each context. For a particular pair of odors (e.g., apple and orange), we presented one item (apple), but not the other (orange), in the first context. Next, both items were presented in the second context (notably apple followed by orange). Finally, the memory assessment occurred upon return to the first context wherein the rats were confronted with a choice between apple and orange. The correct choice, based on item in context, is orange because it has not yet been presented in the first context. Notably, prior to the memory assessment, orange was presented more recently than apple. Consequently, apple would be less familiar relative to orange in the memory assessment. An animal that relied on judgments of relative familiarity would choose the apple in the memory assessment (i.e., following the semantic rule “avoid familiar items”). Our measure of accuracy is the proportion of correct choices in the item-in-context memory assessments. Thus, an animal that relied on item-in-context memory would choose orange in the memory assessment and yield above chance accuracy. On the other hand, an animal that relied on judgments of relative familiarity to pick the least familiar item would pick apple, which results in below chance accuracy. Notably, this memory assessment was, therefore, used to dissociate item-in-context memory (above chance) from judgments of relative familiarity (below chance).
Learning can also be assessed in this olfactory preparation by repeatedly presenting rats with fixed odor pairs and asking them to learn a rule. In these simple discrimination (SD) pairs, selection of one odor is always rewarded (S+), whereas selection of a foil odor is never rewarded (S−). Therefore, in order to attain high accuracy in these trials, rats are required to learn the rule of which item in the pair to choose. In the present study, rats were trained on multiple SD pairs before and after paclitaxel treatment as a means of assessing prior learning, new learning, and reversal learning (Galizio et al., 2006).
Rats were trained on an SD pair prior to drug treatment until accuracy was high; we will refer to accuracy on SD pairs that were trained before treatment as measures of prior learning. Measures of prior learning were then examined following treatment (i.e., post-treatment days 8–12, 15–19; Fig. 1). Thus, high accuracy on the SD pair before and after chemotherapy treatment suggests that the rats’ olfactory perception and motivation is intact, and that they are able to navigate the arena, and notably, that they remember the previously trained discrimination rule.
Post-treatment, we presented rats with multiple new SD pairs to learn; we refer to this as a measure of new learning. Specifically, rats were given four new SD pairs across five consecutive post-treatment sessions (i.e., post-treatment days 15–19; Fig. 1). Importantly, prior to treatment, rats had no experience with these odors. Thus, capacity for new learning following chemotherapy treatment would be represented by high accuracy in terminal performance.
Finally, in order to assess reversal learning, rats were presented with a contingency reversal for a single simple discrimination after chemotherapy treatment. Immediately following chemotherapy treatment, accuracy of a previously trained SD pair (i.e., pre-reversal SD) was documented. Next, under conditions in which pre-reversal accuracy was high, we reversed the contingency for only that simple discrimination pair, such that selection of the previous S+ was no longer rewarded and the previous S− was now rewarded; we refer to this as reversal learning. Testing for the reversed SD pair consisted of 6 trials per session across 5 consecutive days.
Typical sessions consisted of 44 trials, 32 item-in-context episodic memory trials and 12 SD trials (6 for each pair), as described above. Sessions occurring during the new learning and reversal learning assessment phase of the experiment post-treatment (i.e., days 15–19 after treatment onset) consisted of 68 total trials; 32 item-in-context episodic memory trials, 6 control SD or prior learning SD trials, 6 reversal SD trials, and 24 new learning SD trials (6 for each SD pair). Odors used in the SD task did not overlap with the pool of odors used in the item-in-context memory assessment; selection and designation of S+ and S− odors were randomly assigned to each rat. All SD assessments were conducted at the end of daily sessions after the episodic memory assessment.
2.3 Hippocampal neurogenesis
2.3.1 BrdU administration and tissue preparation for immunohistochemistry
BrdU injections were performed as described elsewhere (Rao, Hattiangady, and Shetty, 2006). Rats received four injections of BrdU (100 mg/kg, i.p), beginning 24 days following the onset of chemotherapy treatment. One injection was administered every six hours for a cumulative total of 400 mg/kg i.p. BrdU injections began 24 hours prior to transcardial perfusion with the last dose being administered 6 hours prior to perfusion. Rats were deeply anesthetized with 25% urethane, and then perfused transcardially with 0.1M phosphate-buffered saline (PBS) containing 0.1% heparin, followed by ice-cold 4% paraformaldehyde. Brains were extracted and post-fixed in the same fixative for 24 hours, then cryoprotected in 30% sucrose for at least 3 days prior to sectioning. Immunohistochemical procedures were performed in the same animals used for the behavioral experiments with one exception. One subject whose brain was inadvertently damaged during dissection was excluded from immunohistochemical studies.
2.3.2 Immunohistochemistry
Serial coronal sections (30 μm) of the entire granule cell layer (GCL) of the dentate gyrus of the hippocampus (approximately −1.80 mm:−6.30 mm from Bregma) were cut on a cryostat, mounted onto Superfrost Plus slides (VWR, West Chester, PA) and kept at −80 C° until immunostaining. In all subjects, near-adjacent sections were collected in 12 separate series. Thus, for every subject, each series contained 12 matched sections spanning the entire dentate gyrus of the hippocampus. Hippocampal sections from paclitaxel and cremophor-vehicle treated animals were processed concurrently. This method ensured that matched sections were obtained and processed at the same levels in all animals. Adjacent sections were collected in PBS and stored in antifreeze buffer (50% sucrose in ethylene glycol and 0.1 M PBS) for long term storage at −20 C°. Tissue was collected so that every 12th section was processed (i.e. within each series of sections) to ensure the same cell was not counted twice in adjacent sections. Additional sections were taken of the lateral ventricle (approximately 0.7 mm: −0.4 mm from Bregma) to confirm specificity of antibody staining. After acclimating slide-mounted tissue to room temperature, antigen unmasking was performed by incubating the slides in 10 mM sodium citrate (pH 6.0). Slides were washed thoroughly in PBS, then DNA denaturation was performed by incubating slides in pre-warmed 2N HCL at 37 C° for 30 min. Slides were then rinsed in 0.1M boric acid (pH 8.5) for 10 min, washed with PBS, then incubated in blocking buffer consisting of 10% normal goat serum and 0.1% Triton X-100 in PBS (blocking buffer was used as primary and secondary antibody diluent) for one hour. Sections were then incubated overnight at 4 C° with rat monoclonal BrdU antibody (1:200, Bio-Rad, Hercules, CA), and rabbit monoclonal Ki67 antibody (1:200, ThermoFisher Scientific, Waltham, MA). Following primary antibody incubation, slides were washed in PBS then incubated with goat-anti rat Alexa Fluor 568 and goat-anti rabbit Alexa Fluor 488 (both 1:500, ThermoFisher scientific, Waltham, MA) for 2 hours. Following secondary antibody incubation, slides were washed in PBS, incubated in DAPI (0.1 μg/ml, ThermoFisher scientific, Waltham, MA), washed in ddH20, and mounted with Aqua-Poly/Mount (Polysciences Inc., Warrington, PA). To confirm specificity of immunofluorescent labeling, separate series of tissue sections were processed using methods identical to those described above with the exception that either the primary or the secondary antibody was omitted in that series. Omission of either the primary or secondary antibody eliminated Ki67- and BrdU-like immunoreactivity.
2.3.3 Quantification and imaging of Immunohistochemistry
At least 9–12 sections per animal were quantified by an experimenter blinded to paclitaxel treatment conditions. The number of Ki67 and BrdU positive cells in the granule cell layer (GCL) and subgranular zone (SGZ) were quantified manually using a cell counter and a Leica DMLB microscope under 40× magnification. For each subject, the 8 sections displaying the greatest number of Ki67 and BrdU labeled cells were used for data analysis to ensure that sections within each series were matched between subjects and inadvertent damage to tissue sections related to cutting, immunohistochemical processing and/or mounting did not bias results. For each subject, the total number of cells positive for each marker was calculated for each subject. This single determination was then averaged across subjects for each treatment group. Photomicrographs were taken using a Nikon Eclipse NiE upright fluorescent microscope equipped with a Hamamatsu ORCA-Flash 2.8 sCMOS high resolution camera. Minimal adjustments to color histograms were performed using the levels function in Photoshop Elements 15 (Adobe Systems Inc., San Jose, CA), and all manipulations were performed uniformly to the entire image.
3. Results
3.1.1 Paclitaxel produced mechanical hypersensitivity while no weight changes were observed
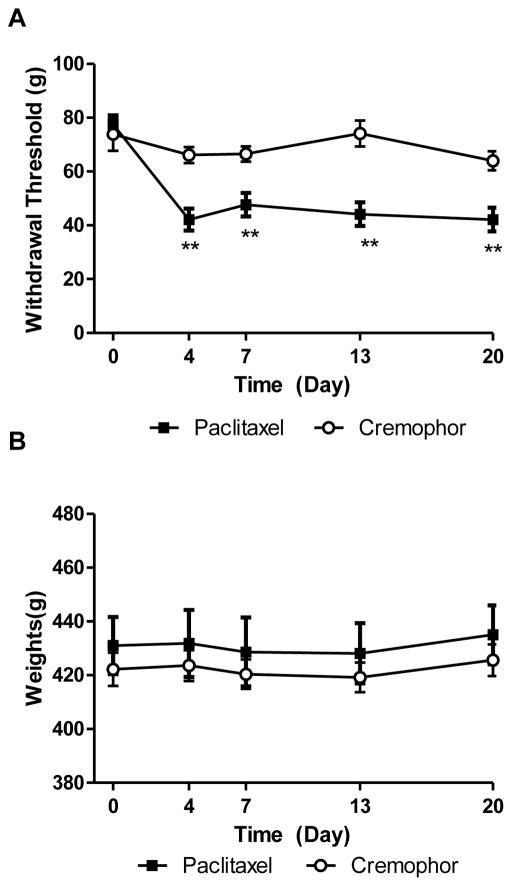
To verify effectiveness of paclitaxel-treatment, we measured the time course of paclitaxel-induced mechanical hypersensitivity in the same animals evaluated for cognitive changes. Paclitaxel produced mechanical hypersensitivity [F(1,36) = 50.47, p<0.0001; Fig. 2A], in a time dependent manner [F(4,36) = 9.259, p<0.0001; Fig. 2A] and the interaction was significant [F(4,36) =4.532, p<0.01; Fig. 2A]. Rats treated with paclitaxel displayed decreased paw withdrawal thresholds on day 4 (p<0.01), 7 (p<0.01), 13 (p<0.01), and 20 (p<0.01) after initiation of paclitaxel treatment relative to rats treated with cremophor vehicle at the same time points. Body weight did not differ between groups throughout the study [F(1,36) = 0.40, p < .05; Fig. 2B].
Fig. 2.
Paclitaxel produces mechanical hypersensitivity without altering body weight. (A) Paclitaxel treatment reduced paw withdrawal thresholds to mechanical stimulation relative to rats treated with cremophor vehicle. **p<0.01 paclitaxel- vs. cremophor-treated rats. Two-way ANOVA, two-tailed t-test. (B) Body weight did not differ between groups or relative to baseline (pre-injection) body weight. Data are expressed as mean ± SEM.
3.1. Episodic memory is spared following paclitaxel treatment
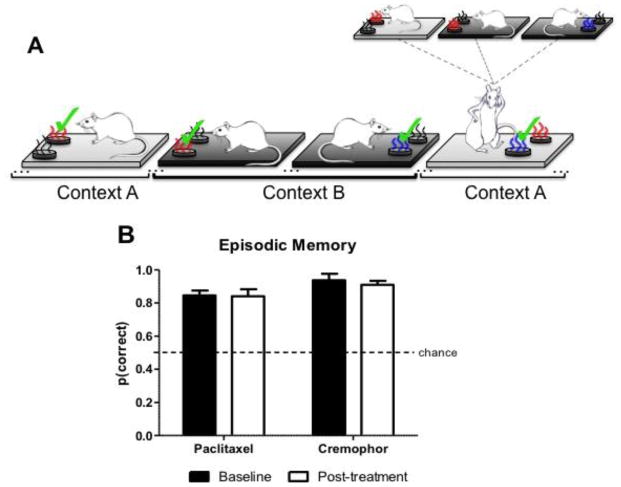
In our approach to assess episodic memory, rats were trained to pick the “new” and avoid the “old” when presented with pairs of items across multiple contexts. That is, one odor was always “new” in the current context, while the other had already been presented within that context earlier in the session (Fig. 3A). Episodic memory was examined before treatment (baseline) and after treatment (post-treatment). Baseline performance in the episodic memory test consisted of the three most recent sessions prior to paclitaxel chemotherapy treatment. Before paclitaxel treatment, the chemotherapy group (n=6) and the vehicle group (n=5) had high accuracy in the episodic memory assessment (Paclitaxel: mean=0.85, SEM=0.03; Cremophor: mean= 0.94, SEM=0.03). Post-treatment, episodic memory assessment began the week following completion of paclitaxel or cremophor vehicle treatment. After treatment, episodic memory performance was averaged across 10 sessions. Fig. 3B represents episodic memory for rats treated with paclitaxel or cremophor vehicle in baseline and post-treatment conditions. Rats treated with the chemotherapeutic agent paclitaxel were not impaired in the episodic memory assessment following treatment. This was confirmed by a 2 (baseline, post-treatment) × 2 (paclitaxel, cremophor) ANOVA on measures of episodic memory performance. No effect of the chemotherapy treatment [F(1,9)=5.08, p>.05] or decline in episodic memory performance relative to baseline was observed [F(1,9)= 0.02, p>.05], and the interaction effect was not significant [F(1,9)= 0.11, p>.05].
Fig. 3.
(A) Schematic depicting olfactory item-in-context memory procedure. Episodic item-in-context memory is dissociated from familiarity cues. Strawberry and blueberry odors are depicted here as red and blue, respectively. The “new” item in context odor is denoted by “✓“ and “old” odors previously presented in context earlier in the session are represented in grey. The presence of additional odors not shown is represented by “…” in the schematic. In the episodic memory assessment, the rats were given a choice between strawberry and blueberry. The correct item-in-context choice is blueberry because blueberry has not yet been presented in context A, even though blueberry is more familiar than strawberry. Trials depicted in (A) were randomly intermingled throughout the episodic memory part of the session. Our measure of accuracy is the proportion of choices where the “new” item-in-context odor is selected. (B) Intact item-in-context episodic memory performance is represented by a high proportion correct in the episodic memory assessment. Data are expressed as mean ± SEM (n=5–6 per group).
3.2. Prior learning is spared following paclitaxel treatment
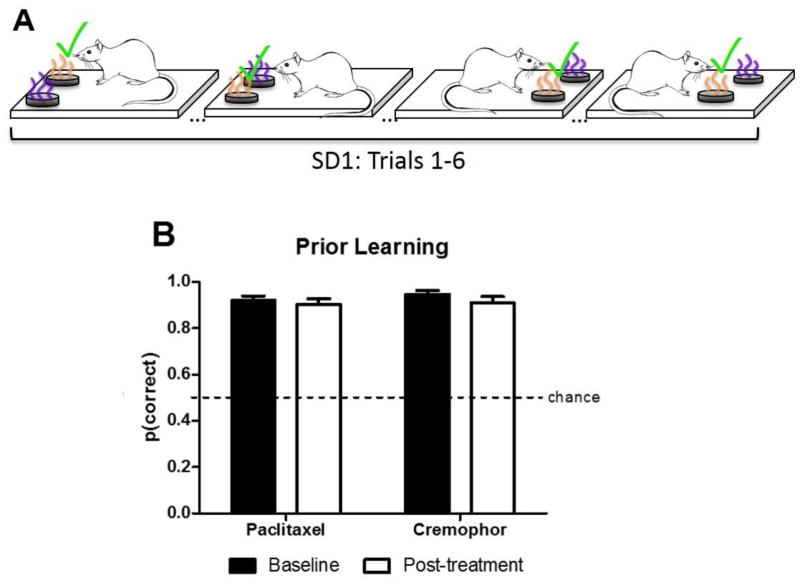
To test for prior learning memory performance and potential non-mnemonic effects chemotherapy treatment, rats were trained on fixed pairs of odors. Pairs consisted of one odor that was always rewarded and one odor that was never rewarded (Fig. 4A). Notably, because the rats were trained on the olfactory SD pairs before chemotherapy treatment, this approach measured retention of prior rule learning at a post-treatment time point. Prior learning was examined before (baseline) and after treatment (post-treatment). Baseline consisted of mean SD accuracy in the three most recent sessions prior to chemotherapy treatment. Prior to chemotherapy treatment, the chemotherapy group (n=6) and the vehicle group (n=5) had high accuracy in the SD assessment (Paclitaxel: mean=0.92, SEM=0.02; Cremophor: mean= 0.95, SEM= 0.02). Post-treatment, SD accuracy was averaged across 10 sessions following completion of chemotherapy treatment. Fig. 4B shows prior learning accuracy of rats treated with paclitaxel and cremophor in baseline and post-treatment conditions. Rats treated with paclitaxel were not impaired in the olfactory SD assessment following treatment. This was confirmed by a 2 (baseline, post-treatment) × 2 (paclitaxel, cremophor) ANOVA on measures of olfactory SD memory. No effect of the chemotherapy treatment [F(1,9)= 0.34, p >.05] or decline in performance relative to baseline [F(1,9)= 2.46, p >.05] was observed olfactory SD memory, and the interaction effect was not significant [F(1,9)= 0.36, p>.05].
Fig. 4.
Schematic depicting olfactory prior learning procedure and data. (A) Prior learning schematic. Grape and orange odors are depicted as purple and orange. The simple discrimination (SD) procedure used two fixed odors, one was designated as S+ and the other was designated as S-. Selection of the S+ was always rewarded whereas responses to the S− were never rewarded; S+ items (denoted by “✓“). Our measure of SD accuracy is the proportion of choices the S+ item is selected. (B) Prior rule learning is spared following treatment as indicated by high accuracy in the assessment in both baseline and post-treatment conditions. Data are expressed as mean ± SEM (n=5–6 per group).
3.3. Paclitaxel does not impair new learning
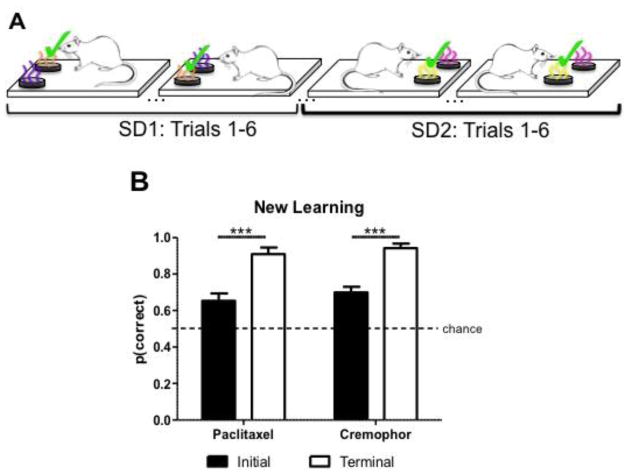
Following chemotherapy treatment, we assessed rats’ capacity for new rule learning. To assess new learning post-treatment, rats were trained on eight new olfactory SD pairs, four pairs in each context. Each pair consisted of one odor that was always rewarded, and one odor that was never rewarded.
Because rats had no training history or exposure to the newly added SD pairs before chemotherapy treatment, this approach served as a measure of new learning at a post-treatment time point. New learning was examined post-treatment after behavioral testing for retention of prior learning was complete. Capacity for new learning was examined by comparing initial accuracy and terminal accuracy for all of the new SD pairs (Fig. 5A). Initial performance in the new learning SD assessment consisted of the mean accuracy for the first 3 sessions, whereas terminal performance consisted of the mean accuracy for the last two sessions. Rats showed an increase in performance from initial to terminal with no effect of chemotherapy treatment. This finding was confirmed by a 2 (initial, terminal) × 2 (paclitaxel, cremophor) ANOVA on measures of new learning post chemotherapy treatment. When assessed on new learning during terminal performance, rats made more correct choices relative to initial performance [F(1,9)= 28.25, p < .0001; Fig. 5B]. No effect of chemotherapy group was observed [F(1,9)= 0.77, p>.05; Fig. 5B], and there was no interaction between chemotherapy group and initial versus terminal performance [F(1,9)= 0.0004, p>.05; Fig. 5B] for measures of new learning.
Fig. 5.
Schematic depicting olfactory new learning procedure and data. (A) To assess new learning, we presented the rats with several new SD pairs after treatment occurred. In each of the new SD pairs, selection of the S+ was always rewarded whereas responses to the S− were never rewarded. S+ items (denoted by “✓“). (B) Rats’ capacity for new rule learning is intact post-treatment as represented by high accuracy in terminal performance. Data are expressed as mean ± SEM (n=5–6 per group), ***p<0.001.
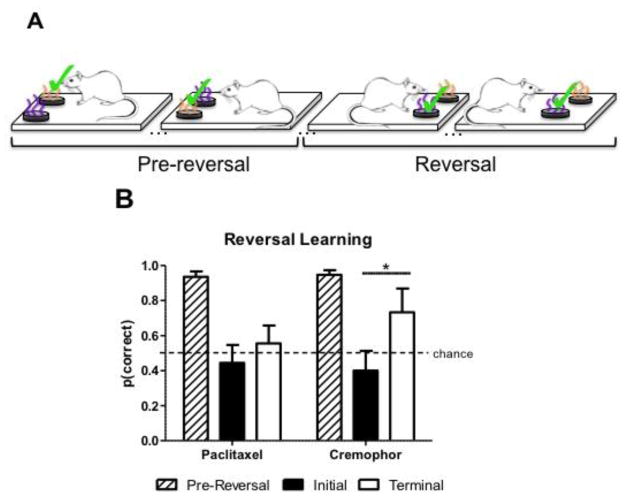
3.4. Reversal learning is impaired following paclitaxel treatment
After treatment, reversal learning was assessed using an SD contingency reversal (Fig. 6A). Fig. 6B shows initial versus terminal performance following the reversal (black and white bars, respectively, in Fig. 6B), as well as pre-reversal accuracy for the subsequently reversed SD pair (striped bars in Fig. 6B). Initial performance consisted of the mean SD accuracy for the first three reversal sessions, whereas terminal performance consisted of the mean SD accuracy for the last two reversal sessions. Prior to the reversal-learning assessment, pre-reversal accuracy of the previously trained SD pair was high post-treatment (striped bars in Fig. 6B; paclitaxel: mean=0.94, SEM=0.03; cremophor vehicle: mean=0.95, SEM=0.03). In initial performance immediately following the reversal, both groups showed substantial reduction in accuracy. In the subsequent reversal sessions, the cremophor group eventually increased accuracy over trials whereas the paclitaxel treated group did not. That is, the chemotherapy treated rats showed more perseveration in their selection of the pre-reversal contingency through initial and terminal performance relative to the cremophor treated rats. The data in Fig. 6B were subjected to a 3 (pre-reversal, initial, terminal) × 2 (paclitaxel, cremophor) ANOVA, followed by a test to compare the changes in the groups’ initial and terminal performance (Fig. 6B). The effect of the reversal contingency was significant [F(2,18)= 21.49, p < .0001]. No effect of chemotherapy treatment was observed [F(1,18)= 1.13, p >.05], and the interaction was not significant [F(2,18)= 1.59, p>.05]. Next, we evaluated the planned comparison that chemotherapy impaired reversal learning (i.e., that the paclitaxel group’s improvement in accuracy after the reversal occurs is impaired relative to the cremophor vehicle group’s improvement). To this end, we compared the magnitude of the increase in accuracy in initial and terminal conditions in paclitaxel and cremophor groups, and we used the error term from the omnibus ANOVA (MSerror= 0.0336) as recommended by Keppel and Zedeck ((Keppel and Zedeck, 1989) p. 155–156). The test revealed that the improvement in accuracy from initial to terminal time points (black and white bars, respectively, in Fig. 6B) was significantly larger in the cremophor group than in the paclitaxel group [F(1,18)= 7.35, p < 0.05].
Fig. 6.
Schematic depicting the reversal learning procedure and data. (A) Reversal learning procedure. Grape and orange odors are depicted as purple and orange. The simple discrimination (SD) procedure used two fixed odors, one was designated as S+ and the other was designated as S−. Selection of the S+ was always rewarded whereas responses to the S− were never rewarded; S+ items (denoted by “✓“). Our measure of SD accuracy is the proportion of choices the S+ item is selected. To assess reversal learning, rats were presented with a contingency reversal on a previously trained SD; depicted as pre-reversal. After treatment, pre-reversal SD accuracy was documented. Next, under conditions in which pre-reversal accuracy was high, we reversed the SD contingency such that the pre-reversal S+ (i.e., orange) was no longer rewarded and the pre-reversal S− (i.e., grape) was now rewarded. (B) Chemotherapy treatment impairs reversal learning as indicated by a significantly greater increase in initial to terminal accuracy (i.e., accuracy as a function of trials) in cremophor treated rats compared to paclitaxel treated rats. Data are expressed as mean ± SEM (n=5–6 per group), *p<0.05.
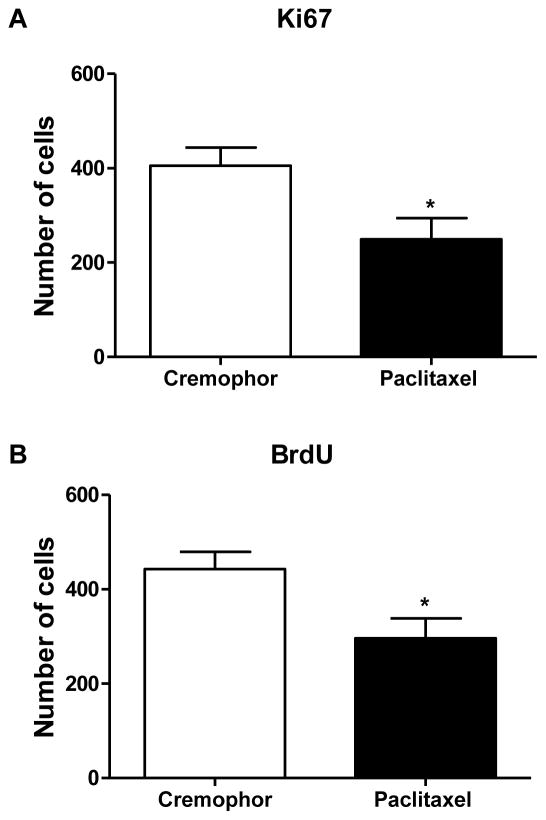
3.5 The chemotherapeutic agent paclitaxel suppressed hippocampal neurogenesis
Paclitaxel treatment reduced the number of Ki67 [t(8) = 2.64, p <0.05; Fig. 7A) and BrdU [t(8) = 2.62, p <0.05; Fig. 7B] positive cells in the granular cell layer (GCL) and SGZ relative to rats treated with cremophor vehicle in tissue dissected 25 days following the onset of chemotherapy treatment.
Fig. 7.
Chemotherapy treatment reduced markers of hippocampal neurogenesis. Paclitaxel treatment reduces Ki67 (A) and BrdU (B) immunoreactivity in the dentate gyrus of the hippocampus. Relative to cremophor vehicle-treated rats, rats treated with paclitaxel displayed decreased levels of Ki67- (p < 0.05) and BrdU-like (p < 0.05) immunoreactivity within the GCL and SGZ of the dentate gyrus of the hippocampus. Data are expressed as mean ± SEM (n=5 per group). *p<0.05 paclitaxel- vs. cremophor-treated rats. Two -tailed t-test.
4. General discussion
These experiments examined the impact of chemotherapeutic treatment with paclitaxel on learning, memory, and hippocampal neurogenesis in rodents. Our studies provide evidence that paclitaxel treatment preferentially reduces cognitive flexibility in a reversal learning task and also suppresses markers of hippocampal neurogenesis. Following paclitaxel treatment, episodic memory (i.e., item-in-context memory), prior learning, new learning, and reversal learning was assessed. In the new learning task, learning is indexed by an increase in accuracy over time (i.e., as function of trials). If chemotherapy impaired new learning in the rats, we would expect to observe no significant change in accuracy over time (i.e., initial accuracy versus terminal accuracy). Under conditions in which episodic memory and prior learning were intact, rats treated with paclitaxel showed no impairment in new rule learning. That is, following initial sessions, paclitaxel-treated rats displayed the ability to learn the new rule by attaining high terminal accuracy. Previous research has suggested that paclitaxel treatment spares source memory and spatial memory while selectively impairing learning (Smith et al., 2017). Our finding that paclitaxel treatment spares episodic memory and prior learning is consistent with results previously reported by Smith and colleagues, using a very different paradigm (Smith et al., 2017).
Next, we evaluated the effects of chemotherapy on reversal learning. In order to test reversal learning following chemotherapy treatment, we presented the rats with a contingency reversal on a previously trained simple discrimination rule (after observing high pre-reversal accuracy post-treatment). In reversing the SD rule, sensitivity to detect the change in the experimental contingency and adjust behavior accordingly is required for the rats to show improvement in accuracy over time. Thus, in the reversal task, reversal learning is indexed by improved accuracy as a function of trials over time, whereas impairment in the sensitivity to detect these changes would result in perseveration. In our analysis, rats treated with paclitaxel showed significantly less improvement in accuracy over time compared to that observed for the cremophor vehicle-treated group. In other words, rats in the vehicle group demonstrated learning of the reversal at a significantly faster rate than the group treated with chemotherapy. Paclitaxel-treated rats did not show the rate of improvement over repeated trials. Notably, these findings were observed under conditions in which episodic memory, prior learning, and new learning were not impaired. The selective impairment observed in reversal learning under conditions that show intact new learning following chemotherapy treatment is unique. Previous research examining chemotherapy induced deficits in learning utilized a measure that assessed new learning in the form of a reversal, and observed paclitaxel-induced deficits in learning. Until the current study, it was unclear whether paclitaxel had differential effects on new learning and reversal learning when tested separately. These results suggest that the learning impairment previously documented following paclitaxel treatment is selective, such that the capacity for new learning in the current study was spared while reversal learning was impaired. Thus, these findings replicate and expand our knowledge of the effects of paclitaxel on episodic memory and learning capacity. It is also worth considering the potential role of interference following the onset of the reversal. That is, the reversal task may have deleterious effects on subsequent non-reversed discriminations. Such an effect would result in decreased performance in previously trained but non-reversed simple discrimination pair. However, rats in the current study did not show evidence of negative interference on performance in the non-reversed discrimination throughout the duration of the reversal task, as indicated high performance in the non-reversed prior learning pairs (see white bars in Fig. 4B).
Based on the reversal learning deficit observed here and in prior work (Burghardt, Park, Hen, and Fenton, 2012; Smith et al., 2017; Swan, Clutton, Chary, Cook, Liu, and Drew, 2014), future research could expand on this finding by examining the effect of paclitaxel on reversal learning for multiple discriminations that reverse together or separately. Training rats on multiple simple discriminations and then reversing the reward contingency for all of the pairs following treatment could serve as a strategy to examine the effects of chemotherapy on serial reversal learning. In addition, it is currently unknown whether chemotherapy treatment has deleterious effects on learning to learn, or savings, through exposure to multiple contingency reversals. One means of examining this in future research would be to train animals on multiple simple discrimination pairs and followed by a contingency reversal for each pair separately after treatment. To this end, examining the learning curves of initial and subsequent contingency reversals would reveal intact or impaired savings following chemotherapy treatment.
Overall, no impairments in episodic memory, prior learning, or new learning were observed, suggesting that these domains of learning and memory are spared in this paclitaxel treatment regime, although reversal learning was notably impaired. Nonetheless, paclitaxel-treated animals showed marked reductions in markers of cell proliferation, assessed using immunohistochemistry for BrdU and ki67. Paclitaxel treatment significantly reduced the total number of proliferating cells in the DG and SGZ using BrdU and Ki67 immunohistochemistry. These results are consistent with previous in vitro and in vivo data showing hippocampal susceptibility to chemotherapeutic agents, even in low doses (Dietrich, Han, Yang, Mayer-Proschel, and Noble, 2006; Seigers, Schagen, Beerling, Boogerd, van Tellingen, van Dam, Koolhaas, and Buwalda, 2008; Walker, Foley, Clark-Vetri, and Raffa, 2011). Thus, even though paclitaxel is thought to minimally penetrate the CNS, it may be possible that the approach used here is sufficient to cause toxicity and damage to neural progenitor cells (Dietrich et al., 2006). This finding is consistent with deficits in reversal learning observed following suppression of adult hippocampal neurogenesis in mice (Burghardt et al., 2012). To induce suppression of adult hippocampal neurogenesis, adult mice were treated with focal X-irradiation of the hippocampus. Irradiated mice showed impaired cognitive flexibility to learning new experimental contingencies in a place avoidance task but were unimpaired in new learning (i.e. of initial zone locations) [61]. Similarly, focal X-irradiation induced suppression of adult neurogenesis in the hippocampus and discrimination performance was impaired only under conditions in which the initial discrimination was reversed (Swan et al., 2014). Together, these findings are consistent with our own work and support the hypothesis that adult hippocampal neurogenesis plays a role in reversal learning and cognitive flexibility.
Although the current study focused on paclitaxel-induced reductions of hippocampal neurogenesis as a possible mechanism underlying CICI, a related etiological concern is chemotherapy-induced reductions in gliogenesis. Additional populations of actively dividing oligodendrocyte precursor cells have been identified in subcortical white matter (Chang, Nishiyama, Peterson, Prineas, and Trapp, 2000; Dawson, Polito, Levine, and Reynolds, 2003; Geha, Pallud, Junier, Devaux, Leonard, Chassoux, Chneiweiss, Daumas-Duport, and Varlet, 2010), which are suggested to play a critical role in myelination. Decreased gliogenesis and subsequent demyelination are observed following chemotherapy and cranial irradiation. In light of this, demyelination has also been implicated as a potential mechanism of cognitive impairment (Monje and Dietrich, 2012). Our studies do not preclude the possibility that demyelination, subserved by chemotherapy-induced reductions in gliogenesis, may also contribute to the reversal learning impairments observed here.
One possible explanation for the lack of observed impairment in episodic memory, prior learning, and new learning is that enrichment from extensive cognitive training in our animals provided protection against chemobrain impairment. Prior research has linked environmental enrichment with increased hippocampal neurogenesis under conditions wherein CICI was not observed, thus resulting in a protective effect (Winocur et al., 2014; Winocur, Wojtowicz, Merkley, and Tannock, 2016). Similarly, rats used in the current study received extensive cognitive training prior to chemotherapy treatment. However, paclitaxeltreated animals here were not protected against decreased hippocampal neurogenesis nor against impairment in reversal learning. Thus, our findings may suggest that any protective role of environmental enrichment against cognitive impairment and decreased hippocampal neurogenesis may be more selective than initially thought.
Prior studies of the cognitive effects of chemotherapy after treatment have yielded mixed results. The reasons for mixed results are unclear but may relate to the cognitive assessments used or the type of cancer and treatment received. Relatively little effort has been made to associate specific cognitive changes with specific chemotherapy treatments. Future research should focus on examining the multiple aspects of cognitive function and dysfunction following treatment with additional chemotherapy drugs (e.g., methotrexate, cisplatin, doxorubicin, and fluorouracil), both individually and when treated in combination. It is also possible that CICI may heighten vulnerabilities to other cognitive impairments, including normal aging, later in life.
The present findings suggest that not all forms of cognition are detrimentally impacted by the chemotherapeutic agent paclitaxel. Paclitaxel treatment leads to selective impairments in reversal learning under conditions in which episodic memory, prior learning, and new learning are spared. This is one of few studies to investigate chemotherapy- induced cognitive impairment while assessing multiple measures of cognition, hippocampal neurogenesis, and peripheral neuropathy. Taken together, these findings shed light onto the differential effects of chemotherapies on cognition and hippocampal neurogenesis.
Fig. 8.
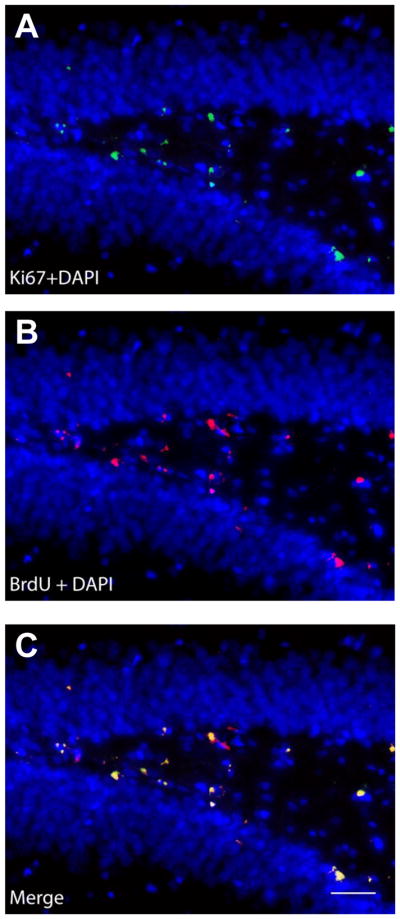
Ki67- and BrdU-like immunoreactivity in dentate gyrus of the hippocampus. Representative photomicrographs displaying (A) Ki67- (green) and (B) BrdU-like (red) immunoreactivity with DAPI counterstain (blue) in the dentate gyrus of the hippocampus. (C) The overlay image shows that Ki67 and BrdU were frequently co-expressed, based on qualitative evaluation. Photographs were taken at 40× magnification. Scale bar is equal to 20 μm.
Highlights.
Paclitaxel reduced cognitive flexibility in a reversal learning task
Paclitaxel increased susceptibility to perseveration
Paclitaxel decreased sensitivity to changes in experimental contingencies
Paclitaxel spared episodic memory, prior learning, and new learning
Paclitaxel decreased markers of cell proliferation in the hippocampus
Acknowledgments
This work was supported by R01MH098985 (to JDC), R21AG044530 (to JDC), R21AG051753 (to JDC) and CA200417 (to AGH). DPB, LMC and AES were supported by the Harlan Scholars Program. LMC was supported by a T32 training grant (DA024628). All procedures followed national guidelines and were approved by the Bloomington Institutional Animal care and Use Committee at Indiana University. We thank Sydney Brotheridge and Stefan J. Dalecki for their assistance with preliminary training.
Footnotes
Funding and disclosure
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahles TA, Saykin AJ. Breast cancer chemotherapy-related cognitive dysfunction. Clin Breast Cancer. 2002;3(Suppl 3):S84–90. doi: 10.3816/cbc.2002.s.018. [DOI] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda-Carvalho M, Sakaguchi M, Akers KG, Josselyn SA, Frankland PW. Posttraining ablation of adult-generated neurons degrades previously acquired memories. J Neurosci. 2011;31:15113–15127. doi: 10.1523/JNEUROSCI.3432-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Authier N, Balayssac D, Marchand F, Ling B, Zangarelli A, Descoeur J, Coudore F, Bourinet E, Eschalier A. Animal models of chemotherapy-evoked painful peripheral neuropathies. Neurotherapeutics. 2009;6:620–629. doi: 10.1016/j.nurt.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzung C, Wigmore P ebrary Inc. Current topics in behavioral neurosciences. Heidelberg; New York: Springer; 2013. Neurogenesis and neural plasticity; p. x.p. 401. [Google Scholar]
- Briones TL, Woods J. Chemotherapy-induced cognitive impairment is associated with decreases in cell proliferation and histone modifications. BMC Neurosci. 2011;12:124. doi: 10.1186/1471-2202-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt NS, Park EH, Hen R, Fenton AA. Adult-born hippocampal neurons promote cognitive flexibility in mice. Hippocampus. 2012;22:1795–1808. doi: 10.1002/hipo.22013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaletti G, Alberti P, Frigeni B, Piatti M, Susani E. Chemotherapy-induced neuropathy. Curr Treat Options Neurol. 2011;13:180–190. doi: 10.1007/s11940-010-0108-3. [DOI] [PubMed] [Google Scholar]
- Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci. 2000;20:6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie LA, Acharya MM, Parihar VK, Nguyen A, Martirosian V, Limoli CL. Impaired cognitive function and hippocampal neurogenesis following cancer chemotherapy. Clin Cancer Res. 2012;18:1954–1965. doi: 10.1158/1078-0432.CCR-11-2000. [DOI] [PubMed] [Google Scholar]
- Crystal JD. Animal models of source memory. J Exp Anal Behav. 2016;105:56–67. doi: 10.1002/jeab.173. [DOI] [PubMed] [Google Scholar]
- Crystal JD, Alford WT. Validation of a rodent model of source memory. Biol Lett. 2014;10:20140064. doi: 10.1098/rsbl.2014.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal JD, Alford WT, Zhou W, Hohmann AG. Source memory in the rat. Curr Biol. 2013;23:387–391. doi: 10.1016/j.cub.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal JD, Smith AE. Binding of episodic memories in the rat. Curr Biol. 2014;24:2957–2961. doi: 10.1016/j.cub.2014.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Deng L, Guindon J, Vemuri VK, Thakur GA, White FA, Makriyannis A, Hohmann AG. The maintenance of cisplatin- and paclitaxel-induced mechanical and cold allodynia is suppressed by cannabinoid CB(2) receptor activation and independent of CXCR4 signaling in models of chemotherapy-induced peripheral neuropathy. Mol Pain. 2012;8:71. doi: 10.1186/1744-8069-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Lee WH, Xu Z, Makriyannis A, Hohmann AG. Prophylactic treatment with the tricyclic antidepressant desipramine prevents development of paclitaxel-induced neuropathic pain through activation of endogenous analgesic systems. Pharmacol Res. 2016;114:75–89. doi: 10.1016/j.phrs.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny CA, Burghardt NS, Schachter DM, Hen R, Drew MR. 4- to 6-week-old adult-born hippocampal neurons influence novelty-evoked exploration and contextual fear conditioning. Hippocampus. 2012;22:1188–1201. doi: 10.1002/hipo.20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich J, Han R, Yang Y, Mayer-Proschel M, Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol. 2006;5:22. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MR, Denny CA, Hen R. Arrest of adult hippocampal neurogenesis in mice impairs single- but not multiple-trial contextual fear conditioning. Behav Neurosci. 2010;124:446–454. doi: 10.1037/a0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Comparative cognition, hippocampal function, and recollection. Comparative Cognition & Behavior Reviews. 2007:47–66. [Google Scholar]
- Eichenbaum H, Sauvage M, Fortin N, Komorowski R, Lipton P. Towards a functional organization of episodic memory in the medial temporal lobe. Neurosci Biobehav Rev. 2012;36:1597–1608. doi: 10.1016/j.neubiorev.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergorul C, Eichenbaum H. The hippocampus and memory for “what,” “where,” and “when”. Learn Mem. 2004;11:397–405. doi: 10.1101/lm.73304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremouw T, Fessler CL, Ferguson RJ, Burguete Y. Preserved learning and memory in mice following chemotherapy: 5-Fluorouracil and doxorubicin single agent treatment, doxorubicin-cyclophosphamide combination treatment. Behav Brain Res. 2012a;226:154–162. doi: 10.1016/j.bbr.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Fremouw T, Fessler CL, Ferguson RJ, Burguete Y. Recent and remote spatial memory in mice treated with cytosine arabinoside. Pharmacol Biochem Behav. 2012b;100:451–457. doi: 10.1016/j.pbb.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Galizio M. Olfactory Stimulus Control and the Behavioral Pharmacology of Remembering. Behav Anal (Wash D C) 2016;16:169–178. doi: 10.1037/bar0000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galizio M, Miller L, Ferguson A, McKinney P, Pitts RC. Olfactory repeated discrimination reversal in rats: effects of chlordiazepoxide, dizocilpine, and morphine. Behav Neurosci. 2006;120:1175–1179. doi: 10.1037/0735-7044.120.5.1175. [DOI] [PubMed] [Google Scholar]
- Geha S, Pallud J, Junier MP, Devaux B, Leonard N, Chassoux F, Chneiweiss H, Daumas-Duport C, Varlet P. NG2+/Olig2+ cells are the major cycle-related cell population of the adult human normal brain. Brain Pathol. 2010;20:399–411. doi: 10.1111/j.1750-3639.2009.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JJ, Hopkins RO, Squire LR. Single-item memory, associative memory, and the human hippocampus. Learn Mem. 2006;13:644–649. doi: 10.1101/lm.258406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Gutierrez G, Sereno M, Miralles A, Casado-Saenz E, Gutierrez-Rivas E. Chemotherapy-induced peripheral neuropathy: clinical features, diagnosis, prevention and treatment strategies. Clin Transl Oncol. 2010;12:81–91. doi: 10.1007/S12094-010-0474-z. [DOI] [PubMed] [Google Scholar]
- Janelsins MC, Roscoe JA, Berg MJ, Thompson BD, Gallagher MJ, Morrow GR, Heckler CE, Jean-Pierre P, Opanashuk LA, Gross RA. IGF-1 partially restores chemotherapy-induced reductions in neural cell proliferation in adult C57BL/6 mice. Cancer Invest. 2010;28:544–553. doi: 10.3109/07357900903405942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK. The relation between source memory and episodic memory: comment on siedlecki et Al. (2005) Psychol Aging. 2005;20:529–531. doi: 10.1037/0882-7974.20.3.529. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Hashtroudi S, Lindsay DS. Source monitoring. Psychol Bull. 1993;114:3–28. doi: 10.1037/0033-2909.114.1.3. [DOI] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- Keppel G, Zedeck S. Data analysis for research designs: analysis of variance and multiple regression/correlation approaches. New York: W.H. Freeman; 1989. [Google Scholar]
- Kim S, Dede AJ, Hopkins RO, Squire LR. Memory, scene construction, and the human hippocampus. Proc Natl Acad Sci U S A. 2015;112:4767–4772. doi: 10.1073/pnas.1503863112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JM, Lee GD, Kelley-Bell B, Spangler EL, Perez EJ, Longo DL, de Cabo R, Zou S, Rapp PR. Preserved learning and memory following 5-fluorouracil and cyclophosphamide treatment in rats. Pharmacol Biochem Behav. 2011;100:205–211. doi: 10.1016/j.pbb.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons L, Elbeltagy M, Bennett G, Wigmore P. The effects of cyclophosphamide on hippocampal cell proliferation and spatial working memory in rat. PLoS One. 2011;6:e21445. doi: 10.1371/journal.pone.0021445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK. Source monitoring 15 years later: what have we learned from fMRI about the neural mechanisms of source memory? Psychol Bull. 2009;135:638–677. doi: 10.1037/a0015849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje M, Dietrich J. Cognitive side effects of cancer therapy demonstrate a functional role for adult neurogenesis. Behav Brain Res. 2012;227:376–379. doi: 10.1016/j.bbr.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panoz-Brown D, Corbin HE, Dalecki SJ, Gentry M, Brotheridge S, Sluka CM, Wu JE, Crystal JD. Rats Remember Items in Context Using Episodic Memory. Curr Biol. 2016;26:2821–2826. doi: 10.1016/j.cub.2016.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira Dias G, Hollywood R, Bevilaqua MC, da Luz AC, Hindges R, Nardi AE, Thuret S. Consequences of cancer treatments on adult hippocampal neurogenesis: implications for cognitive function and depressive symptoms. Neuro Oncol. 2014;16:476–492. doi: 10.1093/neuonc/not321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polomano RC, Mannes AJ, Clark US, Bennett GJ. A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain. 2001;94:293–304. doi: 10.1016/S0304-3959(01)00363-3. [DOI] [PubMed] [Google Scholar]
- Rahn EJ, Deng L, Thakur GA, Vemuri K, Zvonok AM, Lai YY, Makriyannis A, Hohmann AG. Prophylactic cannabinoid administration blocks the development of paclitaxel-induced neuropathic nociception during analgesic treatment and following cessation of drug delivery. Mol Pain. 2014;10:27. doi: 10.1186/1744-8069-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn EJ, Zvonok AM, Thakur GA, Khanolkar AD, Makriyannis A, Hohmann AG. Selective activation of cannabinoid CB2 receptors suppresses neuropathic nociception induced by treatment with the chemotherapeutic agent paclitaxel in rats. J Pharmacol Exp Ther. 2008;327:584–591. doi: 10.1124/jpet.108.141994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA. Integration of new neurons into functional neural networks. J Neurosci. 2006;26:12237–12241. doi: 10.1523/JNEUROSCI.2195-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Shetty AK. The window and mechanisms of major age-related decline in the production of new neurons within the dentate gyrus of the hippocampus. Aging Cell. 2006;5:545–558. doi: 10.1111/j.1474-9726.2006.00243.x. [DOI] [PubMed] [Google Scholar]
- Schultz PN, Beck ML, Stava C, Vassilopoulou-Sellin R. Health profiles in 5836 long-term cancer survivors. Int J Cancer. 2003;104:488–495. doi: 10.1002/ijc.10981. [DOI] [PubMed] [Google Scholar]
- Scripture CD, Figg WD, Sparreboom A. Peripheral neuropathy induced by paclitaxel: recent insights and future perspectives. Curr Neuropharmacol. 2006;4:165–172. doi: 10.2174/157015906776359568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigers R, Fardell JE. Neurobiological basis of chemotherapy-induced cognitive impairment: a review of rodent research. Neurosci Biobehav Rev. 2011;35:729–741. doi: 10.1016/j.neubiorev.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Seigers R, Schagen SB, Beerling W, Boogerd W, van Tellingen O, van Dam FS, Koolhaas JM, Buwalda B. Long-lasting suppression of hippocampal cell proliferation and impaired cognitive performance by methotrexate in the rat. Behav Brain Res. 2008;186:168–175. doi: 10.1016/j.bbr.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Shrager Y, Bayley PJ, Bontempi B, Hopkins RO, Squire LR. Spatial memory and the human hippocampus. Proc Natl Acad Sci U S A. 2007;104:2961–2966. doi: 10.1073/pnas.0611233104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AE, Slivicki RA, Hohmann AG, Crystal JD. The chemotherapeutic agent paclitaxel selectively impairs learning while sparing source memory and spatial memory. Behav Brain Res. 2017;320:48–57. doi: 10.1016/j.bbr.2016.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CN, Wixted JT, Squire LR. The hippocampus supports both recollection and familiarity when memories are strong. J Neurosci. 2011;31:15693–15702. doi: 10.1523/JNEUROSCI.3438-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan AA, Clutton JE, Chary PK, Cook SG, Liu GG, Drew MR. Characterization of the role of adult neurogenesis in touch-screen discrimination learning. Hippocampus. 2014;24:1581–1591. doi: 10.1002/hipo.22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J Neurosci. 2007;27:3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EA, Foley JJ, Clark-Vetri R, Raffa RB. Effects of repeated administration of chemotherapeutic agents tamoxifen, methotrexate, and 5-fluorouracil on the acquisition and retention of a learned response in mice. Psychopharmacology (Berl) 2011;217:539–548. doi: 10.1007/s00213-011-2310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefel JS, Schagen SB. Chemotherapy-related cognitive dysfunction. Curr Neurol Neurosci Rep. 2012;12:267–275. doi: 10.1007/s11910-012-0264-9. [DOI] [PubMed] [Google Scholar]
- Winocur G, Henkelman M, Wojtowicz JM, Zhang H, Binns MA, Tannock IF. The effects of chemotherapy on cognitive function in a mouse model: a prospective study. Clin Cancer Res. 2012;18:3112–3121. doi: 10.1158/1078-0432.CCR-12-0060. [DOI] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Huang J, Tannock IF. Physical exercise prevents suppression of hippocampal neurogenesis and reduces cognitive impairment in chemotherapy-treated rats. Psychopharmacology (Berl) 2014;231:2311–2320. doi: 10.1007/s00213-013-3394-0. [DOI] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Merkley CM, Tannock IF. Environmental enrichment protects against cognitive impairment following chemotherapy in an animal model. Behav Neurosci. 2016;130:428–436. doi: 10.1037/bne0000155. [DOI] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Tannock IF. Memory loss in chemotherapy-treated rats is exacerbated in high-interference conditions and related to suppression of hippocampal neurogenesis. Behav Brain Res. 2015;281:239–244. doi: 10.1016/j.bbr.2014.12.028. [DOI] [PubMed] [Google Scholar]
- Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer. 2008;44:1507–1515. doi: 10.1016/j.ejca.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]