The histopathologic features of renal neoplasms that occur in older children and adolescents are reviewed, with emphasis on radiologic-pathologic correlation.
Abstract
Malignant renal tumors account for 7% of childhood cancers, and Wilms tumors are by far the most common—but not in older children and adolescents. Among individuals in the latter half of their 2nd decade of life, renal cell carcinoma (RCC) is more common than Wilms tumor. The histopathologic spectrum of RCCs in children differs from that in adults. The most common subtype of RCC in children and adolescents is Xp11.2 translocation RCC, which is distinguished by hyperattenuation at nonenhanced computed tomography, a defined capsule, and associated retroperitoneal lymphadenopathy. Papillary RCC is the second most common histologic subtype. It enhances less intensely compared with the adjacent renal parenchyma and has a propensity for calcification. Clear cell RCC is seen in patients with von Hippel–Lindau disease and is distinguished by its relatively hypervascular nature. Medullary carcinoma affects adolescents with the sickle cell trait and is characterized by an infiltrative growth pattern and extensive metastasis at presentation. Angiomyolipoma is seen in children with tuberous sclerosis complex and is often multifocal and hypervascular, with macroscopic fat. Metanephric tumors are central, circumscribed, and typically calcified. Lymphoma usually manifests as multifocal masses, but it may involve a solitary mass or infiltrative pattern. Extensive adenopathy and involvement of the gastrointestinal tract or other organs also may be seen. Primitive neuroectodermal tumor is an aggressive neoplasm that is typically quite large at diagnosis. Knowledge of the clinical, biologic, and histopathologic features of renal tumors in older children and adolescents and their effects on the imaging appearance can help the radiologist offer a useful preoperative differential diagnosis.
SA-CME LEARNING OBJECTIVES
After completing this journal-based SA-CME activity, participants will be able to:
■ Render the appropriate differential diagnosis for a renal mass in an older child or adolescent.
■ Recognize the distinguishing features of renal tumors in older children and adolescents.
■ Describe how to distinguish the various renal tumors of older children and adolescents from one another on the basis of clinical and imaging data.
Introduction
Wilms tumor is by far the most common renal neoplasm in the pediatric population; however, the majority of these tumors are diagnosed before the patient is aged 5 years. Wilms tumor and other renal neoplasms of young children are reviewed in part 1 of our two-part series on childhood renal tumors (1). A different clinical and histopathologic spectrum of renal neoplasms affects older children and adolescents (Table). In this second part of the series, we review the renal tumors seen in this older age group, with emphasis on radiologic-pathologic correlation.
Pediatric Renal Tumors That Are Common during the 2nd Decade of Life
Note.—CT = computed tomography, DSRCT = desmoplastic small round cell tumor, MR = magnetic resonance, PNET = primitive neuroectodermal tumor, RCC = renal cell carcinoma, TSC = tuberous sclerosis complex, VHL = von Hippel–Lindau.
Renal Cell Carcinoma
RCC is the second most common renal malignancy of childhood after Wilms tumor; however, it is more common than Wilms tumor in individuals who are in their 2nd decade of life. Overall, the annual incidence of pediatric RCC is approximately four cases per one million children, 30 times lower than the annual incidence of Wilms tumor (2). There is an equal gender distribution (3). Pediatric RCC is different from adult RCC. In adults, the most common RCC tumor types are clear cell carcinoma (80%–85%), papillary carcinoma (10%–15%), and chromophobe carcinoma (5%). In contrast, the most frequent types of RCC in children are translocation carcinoma (20%–47%), papillary carcinoma (17%–30%), and medullary carcinoma (11%), with chromophobe and clear cell RCCs occurring quite rarely (3,4). Furthermore, spreading of RCC to regional lymph nodes is more common with pediatric disease.
Translocation RCC
First classified as a genetically distinct subtype of RCC by the World Health Organization (5), translocation RCC accounts for one-third to nearly one-half of all pediatric RCCs (3,6). There is a slight female predominance, with a male-to-female ratio of 1.0:1.4 (3,7). Although patients may have symptoms such as flank pain, a mass, or gross hematuria, these tumors are often found incidentally (8,9).
The Xp11.2 translocation involves gene fusion and subsequent overexpression of transcription factor E3 (TFE3). Multiple variations of the Xp11.2 chromosome translocation have been reported, and all of them cause overexpression of the TFE3 gene. While the origin of these translocations is poorly understood, a known risk factor is a history of cytotoxic chemotherapy during childhood (5,9,10). In their review of 39 genetically confirmed cases, Argani et al (11) found that 15% of the cases involved treatment with cytotoxic chemotherapy.
At gross specimen inspection, translocation RCCs appear as variegated tan-yellow or brownish masses with necrotic and hemorrhagic areas (Figs 1, 2) (5,6). The tumor is usually surrounded by a thick fibrous capsule. Histologic examination reveals the tumor to be highly cellular, consisting of cells with abundant clear to mildly eosinophilic cytoplasm arranged in tubulopapillary or nested patterns (Figs 3, 4). Psammoma bodies are frequently seen (Fig 3) (5). Even with smaller (<7 cm) tumors, involvement of regional lymph nodes is commonly (in 47.5% of cases) found at evaluation of pathologic specimens from children (3). A definitive diagnosis of Xp11.2 translocation tumor is possible with the demonstration of nuclear staining for the TFE3 protein, which is highly sensitive (97.5%) and specific (99.6%) (10).
Figure 1a.
Translocation RCC in a 7-year-old girl with hematuria. (a) Bivalved gross specimen shows a well-circumscribed solid and cystic mass with hemorrhage and necrosis (curved arrow). K = adjacent kidney. (b) Axial nonenhanced CT image shows the mass (arrowhead) to be slightly hyperattenuating compared with the adjacent renal parenchyma. (c) Sagittal intravenous iodinated contrast material–enhanced reformatted CT image shows a well-circumscribed heterogeneously enhancing mass (arrowhead).
Figure 2a.
Translocation carcinoma in a 5-year-old girl. (a) Bivalved gross specimen shows a brown necrotic mass with foci of hemorrhage (arrowhead). (b) Longitudinal ultrasonographic (US) image shows a hyperechoic mass (arrowhead).
Figure 3a.
Translocation RCC in a 15-year-old girl. (a) Photomicrograph of a kidney section shows cells with voluminous clear cytoplasm (arrowhead) separated by a fibrovascular network (straight arrow). A psammomatous calcification (curved arrow) also is noted. (Hematoxylin-eosin [H-E] stain; original magnification, ×40.) (b) Longitudinal US image shows a mass (calipers) with a hyperechoic rim. (c) Axial iodinated contrast–enhanced CT image shows a fluid-attenuating portion of the mass (*) and a well-defined capsule (arrowhead). (d) Axial CT image obtained at a level slightly lower than that in c shows a more solid portion of the mass with a calcific rim (arrowhead).
Figure 4a.
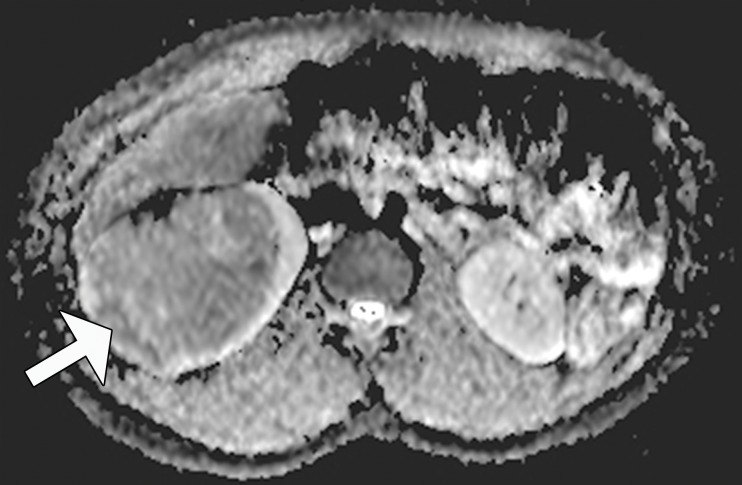
Translocation RCC in a 16-year-old girl. (a) Photomicrograph of a kidney section shows cells with abundant clear to mildly eosinophilic cytoplasm (arrowhead) in nests and papillary arrangements surrounding a fibrovascular core (arrow). (H-E stain; original magnification, ×20.) (b) Axial T2-weighted MR image shows an isointense mass with a dark rim (arrowhead), which represents the fibrous capsule. (c) Coronal gadolinium-enhanced fat-saturated T1-weighted MR image shows a heterogeneously enhancing mass with a dark rim (arrowheads). (d) Axial apparent diffusion coefficient map from an echo-planar diffusion-weighted sequence shows restricted diffusion (arrow), consistent with a densely cellular tumor.
Figure 1b.
Translocation RCC in a 7-year-old girl with hematuria. (a) Bivalved gross specimen shows a well-circumscribed solid and cystic mass with hemorrhage and necrosis (curved arrow). K = adjacent kidney. (b) Axial nonenhanced CT image shows the mass (arrowhead) to be slightly hyperattenuating compared with the adjacent renal parenchyma. (c) Sagittal intravenous iodinated contrast material–enhanced reformatted CT image shows a well-circumscribed heterogeneously enhancing mass (arrowhead).
Figure 1c.

Translocation RCC in a 7-year-old girl with hematuria. (a) Bivalved gross specimen shows a well-circumscribed solid and cystic mass with hemorrhage and necrosis (curved arrow). K = adjacent kidney. (b) Axial nonenhanced CT image shows the mass (arrowhead) to be slightly hyperattenuating compared with the adjacent renal parenchyma. (c) Sagittal intravenous iodinated contrast material–enhanced reformatted CT image shows a well-circumscribed heterogeneously enhancing mass (arrowhead).
Figure 2b.
Translocation carcinoma in a 5-year-old girl. (a) Bivalved gross specimen shows a brown necrotic mass with foci of hemorrhage (arrowhead). (b) Longitudinal ultrasonographic (US) image shows a hyperechoic mass (arrowhead).
Figure 3b.
Translocation RCC in a 15-year-old girl. (a) Photomicrograph of a kidney section shows cells with voluminous clear cytoplasm (arrowhead) separated by a fibrovascular network (straight arrow). A psammomatous calcification (curved arrow) also is noted. (Hematoxylin-eosin [H-E] stain; original magnification, ×40.) (b) Longitudinal US image shows a mass (calipers) with a hyperechoic rim. (c) Axial iodinated contrast–enhanced CT image shows a fluid-attenuating portion of the mass (*) and a well-defined capsule (arrowhead). (d) Axial CT image obtained at a level slightly lower than that in c shows a more solid portion of the mass with a calcific rim (arrowhead).
Figure 3c.
Translocation RCC in a 15-year-old girl. (a) Photomicrograph of a kidney section shows cells with voluminous clear cytoplasm (arrowhead) separated by a fibrovascular network (straight arrow). A psammomatous calcification (curved arrow) also is noted. (Hematoxylin-eosin [H-E] stain; original magnification, ×40.) (b) Longitudinal US image shows a mass (calipers) with a hyperechoic rim. (c) Axial iodinated contrast–enhanced CT image shows a fluid-attenuating portion of the mass (*) and a well-defined capsule (arrowhead). (d) Axial CT image obtained at a level slightly lower than that in c shows a more solid portion of the mass with a calcific rim (arrowhead).
Figure 3d.
Translocation RCC in a 15-year-old girl. (a) Photomicrograph of a kidney section shows cells with voluminous clear cytoplasm (arrowhead) separated by a fibrovascular network (straight arrow). A psammomatous calcification (curved arrow) also is noted. (Hematoxylin-eosin [H-E] stain; original magnification, ×40.) (b) Longitudinal US image shows a mass (calipers) with a hyperechoic rim. (c) Axial iodinated contrast–enhanced CT image shows a fluid-attenuating portion of the mass (*) and a well-defined capsule (arrowhead). (d) Axial CT image obtained at a level slightly lower than that in c shows a more solid portion of the mass with a calcific rim (arrowhead).
Figure 4b.
Translocation RCC in a 16-year-old girl. (a) Photomicrograph of a kidney section shows cells with abundant clear to mildly eosinophilic cytoplasm (arrowhead) in nests and papillary arrangements surrounding a fibrovascular core (arrow). (H-E stain; original magnification, ×20.) (b) Axial T2-weighted MR image shows an isointense mass with a dark rim (arrowhead), which represents the fibrous capsule. (c) Coronal gadolinium-enhanced fat-saturated T1-weighted MR image shows a heterogeneously enhancing mass with a dark rim (arrowheads). (d) Axial apparent diffusion coefficient map from an echo-planar diffusion-weighted sequence shows restricted diffusion (arrow), consistent with a densely cellular tumor.
Figure 4c.
Translocation RCC in a 16-year-old girl. (a) Photomicrograph of a kidney section shows cells with abundant clear to mildly eosinophilic cytoplasm (arrowhead) in nests and papillary arrangements surrounding a fibrovascular core (arrow). (H-E stain; original magnification, ×20.) (b) Axial T2-weighted MR image shows an isointense mass with a dark rim (arrowhead), which represents the fibrous capsule. (c) Coronal gadolinium-enhanced fat-saturated T1-weighted MR image shows a heterogeneously enhancing mass with a dark rim (arrowheads). (d) Axial apparent diffusion coefficient map from an echo-planar diffusion-weighted sequence shows restricted diffusion (arrow), consistent with a densely cellular tumor.
Figure 4d.
Translocation RCC in a 16-year-old girl. (a) Photomicrograph of a kidney section shows cells with abundant clear to mildly eosinophilic cytoplasm (arrowhead) in nests and papillary arrangements surrounding a fibrovascular core (arrow). (H-E stain; original magnification, ×20.) (b) Axial T2-weighted MR image shows an isointense mass with a dark rim (arrowhead), which represents the fibrous capsule. (c) Coronal gadolinium-enhanced fat-saturated T1-weighted MR image shows a heterogeneously enhancing mass with a dark rim (arrowheads). (d) Axial apparent diffusion coefficient map from an echo-planar diffusion-weighted sequence shows restricted diffusion (arrow), consistent with a densely cellular tumor.
At imaging, translocation RCCs generally have a heterogeneous appearance owing to solid and cystic components with hemorrhage, necrosis, and calcifications. The tumors arise and are centered in the medulla and contained within the kidney, although uncommon exophytic growth and renal sinus involvement have been observed (8,12,13). The retroperitoneal lymph node involvement in children, in contrast to that in adults, is pathologically common at the time of diagnosis, even with small tumors (12–15). In children, malignant adenopathy is found at pathologic evaluation in 47.5% of cases; however, it is detected at imaging—by using a threshold short-axis measurement of 1 cm—in only about 25% of cases (3,8).
There is a paucity of literature describing the appearance of translocation RCC at US. In case reports, the tumor has been described as heterogeneous, with echogenic calcifications and benign-appearing “egg-shell” calcifications (Figs 2, 3) (16,17). To minimize the risk from ionizing radiation, nonenhanced and multiphase CT examinations typically are not performed in children. However, at nonenhanced CT, Xp11.2 translocation RCC is usually hyperattenuating (45–60 HU) compared with the adjacent cortex; this is probably owing to densely cellular soft-tissue components and/or hemorrhagic or proteinaceous contents (Fig 1) (8,18,19). This feature helps to distinguish Xp11.2 translocation RCC from other RCC subtypes (15,19,20). Focal or rimlike calcifications are noted on CT images in 23.8%–60.0% of cases (Fig 3) (8,12,13,20). Koo et al (19) reported a case of Xp11.2 translocation RCC in an adult with focal fat that was visualized at CT and attributed to adipose metaplasia, mimicking the appearance of angiomyolipoma. At contrast-enhanced CT, translocation RCCs appear heterogeneous, with solid and nonenhancing cystic components, which represent old hemorrhage and/or necrosis (Fig 3) (8,13,19). The solid portions typically enhance mildly to moderately and less intensely compared with the adjacent cortex during all three phases of enhancement and enhance more intensely compared with the adjacent medulla—except during the delayed phase (8,15,20,21). Prolonged enhancement at delayed phase CT also is typical (8,13,14,20). The tumor margins are well defined, and a progressively enhancing peripheral rim, corresponding to the pathologically observed capsule, is common (76.2% of cases) and best seen on delayed phase CT images (8,13,19,20).
At T2-weighted MR imaging, the mass may appear hypointense compared with the cortex, but it is most often heterogeneous in signal intensity, regardless of its size. This is probably due to internal hemorrhage, necrosis, cystic change, and calcification (Fig 4) (8,12,13,19,22). The signal intensity of translocation RCC on T1-weighted MR images is variable and depends on the amount of hemorrhage and proteinaceous fluid in the mass, but it is often iso- to slightly hyperintense compared with the cortex (8,13). Chen et al (8) reported slight hyperintensity on diffusion-weighted MR images (b = 800 sec/mm2). Kato et al (18) reported a case in which the presence of hemosiderin was suggested at phase-shift gradient-refocused-echo MR imaging. Heterogeneous mild to moderate prolonged enhancement that is lower than that of the adjacent parenchyma is seen following the intravenous administration of gadolinium chelate (8,13). Focal areas of nonenhancement, representing cystic change or necrosis, are common (12,13). A hypointense capsule with rim enhancement is often seen (Fig 4). Renal vein thrombosis may be observed with more advanced tumors (8).
Xp11.2 translocation RCCs are thought to have an indolent course, and it is fairly common for children and adults to have advanced disease at presentation (5,9,23). Spreading to retroperitoneal lymph nodes is common at diagnosis, and metastatic spread most often involves the lung, liver, and mediastinal lymph nodes (3,9).
The mainstay of therapy is surgical resection— usually radical nephrectomy. Adequate sampling of lymph nodes is important in pediatric cases, as resection of the involved regional nodes and primary tumor leads to the best prognosis (3). Limited success has been reported with adjuvant chemotherapy involving conventional regimens and newer agents (eg, vascular endothelial growth factor tyrosine kinase inhibitors and mechanistic target of rapamycin inhibitors), so adequate surgical resection is critical (3). Early reports on nephron-sparing surgery for small tumors indicate initial success; however, the long-term outcome of this therapy has yet to be studied adequately in children (3,24).
Adult Types of RCC
RCC in children may be associated with certain predisposing conditions. Clear cell RCC is quite rare in children, but it may be seen during the 2nd decade of life in children with VHL disease. RCC in these patients, as compared with sporadic RCC, manifests at a younger age and is more often bilateral. Most affected patients have renal cysts, and 40% of them develop cystic and solid, or solid tumors that are evident at imaging (25). Additional conditions associated with VHL disease include spinal or posterior fossa hemangioblastomas, pheochromocytomas, pancreatic cysts, neuroendocrine tumors, and cystadenomas. Pediatric patients with RCC, the clear cell subtype in particular, should undergo evaluation for VHL (2). With TSC, the renal masses are more likely to represent epithelioid angiomyolipoma or perivascular epithelioid cell tumor rather than true RCC. Familial RCC is associated with a rare translocation involving chromosome 3.
RCCs of childhood have gross and microscopic findings that are similar to those in their adult counterparts (26). Papillary, chromophobe, and clear cell RCCs are circumscribed solitary masses with an expansile or ball-shaped pattern of growth. This gross morphology is similar to that of translocation RCC but in contrast to the infiltrative pattern demonstrated by medullary carcinoma (4). Without immunohistochemical analysis, many pediatric RCCs may be categorized as “RCC, unclassified,” given the considerable overlap in growth patterns (4).
Papillary RCCs consist of papillary and tubular configurations of cells, often with foamy macrophages (4,5). Type 1 and type 2 papillary RCCs can be distinguished by their single layer of cuboidal cells with scant cytoplasm (type 1) and high–nuclear-grade pseudostratified cells with eosinophilic cytoplasm (type 2) (4). Chromophobe RCC has large polygonal cells with thick-walled vessels that demonstrate eccentric hyalinization. The cytoplasm may be eosinophilic, with nuclei that may appear irregular (5). Clear cell RCC contains a network of thin-walled vessels and can demonstrate three predominant histologic patterns: acinar, solid, and alveolar (5). The pale-staining cytoplasm contains lipids and glycogen.
Cross-sectional imaging can aid in distinguishing the adult RCC types. Compared with papillary and chromophobe carcinomas, which are hypoattenuating, clear cell RCC has increased vascular density that typically results in increased enhancement following intravenous contrast material administration (Fig 5) (27). Using adjacent renal parenchyma as an internal reference for the degree of enhancement of solid portions of clear cell RCC can be helpful, as these areas of the tumor often appear to have attenuation similar to that of the adjacent renal cortex. Patterns of enhancement also may be helpful, with chromophobe carcinoma demonstrating homogeneous enhancement compared with the heterogeneous or predominantly peripheral enhancement of papillary and clear cell RCCs (27). In addition, chromophobe and papillary RCCs have an increased propensity to develop calcifications (27).
Figure 5a.
Papillary RCC in a 10-year-old boy with sepsis. (a) Axial iodinated contrast–enhanced CT image shows a mass (straight arrow) centered in the right kidney (arrowhead) that has lower enhancement than the cortex during the corticomedullary phase. Small collections of air (curved arrow) within the tumor were found at surgery to be caused by a duodenal fistula. (b) Coronal CT image shows the tumor (straight arrow), air (curved arrow), and retroperitoneal nodal metastasis (arrowhead).
Figure 5b.

Papillary RCC in a 10-year-old boy with sepsis. (a) Axial iodinated contrast–enhanced CT image shows a mass (straight arrow) centered in the right kidney (arrowhead) that has lower enhancement than the cortex during the corticomedullary phase. Small collections of air (curved arrow) within the tumor were found at surgery to be caused by a duodenal fistula. (b) Coronal CT image shows the tumor (straight arrow), air (curved arrow), and retroperitoneal nodal metastasis (arrowhead).
Differential Diagnosis for RCCs
In the setting of a solitary renal tumor, the age of the patient is the most helpful feature for narrowing the differential diagnosis. Wilms tumor is by far the most common pediatric renal malignancy. The mean patient age at diagnosis of Wilms tumor is younger than 48 months, and 95% of cases are diagnosed before the patient is aged 10 years. In the group aged 15–19 years, RCC is more common than Wilms tumor (2). Pediatric RCCs are typically smaller than Wilms tumors and more likely to calcify (in up to 25% of RCC cases vs in up to 9% of Wilms tumor cases) (28). Invasion of the renal vein may be seen with both RCC and Wilms tumor, but it may also be seen—albeit rarely—with other renal tumors that exhibit aggressive biologic behavior. Both Wilms tumor and RCC tend to spread to adjacent lymph nodes and metastasize to the lung and liver; however, RCC is more likely to involve bone (2).
In older children, in whom RCC is most common, identifying the pattern of growth as expansile (ie, ball shaped) or infiltrative (ie, ill defined and contained within the kidney) should be the next step in narrowing the differential diagnosis. Expansile RCCs in children include translocation, papillary, chromophobe, and (rarely) clear cell RCCs, whereas an infiltrative pattern may be observed in medullary carcinoma, collecting duct carcinoma, lymphoma, and inflammatory infectious processes. The degree of tumor enhancement, as compared with the renal parenchyma enhancement, also can be helpful, as clear cell RCC can be distinguished from most other renal tumors by its relatively hypervascular nature and enhancement that is equal to that of the adjacent parenchyma. Larger cystic papillary RCCs may have distinctive nodular or papillary mural excrescences extending into the cystic cavity.
Other differential considerations in older children include renal medullary carcinoma, lymphoma, and PNET. Medullary carcinoma is nearly always associated with the sickle cell trait. Lymphoma is often multifocal, but it may be unifocal, with either expansile or infiltrative patterns of growth. Lymphoma is often hypoattenuating, mimicking the appearance of cysts, and it may be distinguished by additional findings of extensive lymphadenopathy or involvement of additional organs. PNET is a highly aggressive tumor that is often much larger at the time of diagnosis than is typical of RCC.
Treatment of RCC consists mainly of surgical resection. Patients with VHL may be at risk of developing multiple tumors, and their tumors are well differentiated and of low grade. Thus, kidney-sparing treatment, including partial nephrectomy, radiofrequency ablation, and cryotherapy, is preferred (25).
Medullary Carcinoma
Renal medullary carcinoma is an aggressive tumor that affects almost exclusively older children and young adults with the sickle cell trait or heterozygous sickle cell disease (2,29). The age range of affected individuals is 5–39 years (mean age, 14.8 years) (30). Among persons younger than 25 years, males are affected three times more often than are females, but there is an equal distribution between the sexes after age 25 years (31). The most common features at presentation are gross hematuria and flank pain (30); however, hematuria is not uncommon in individuals who have the sickle trait without tumor. An abdominal mass, weight loss, and fever are less common (30).
Renal medullary carcinoma was initially described in 1995 by Davis et al (31) of the Armed Forces Institute of Pathology. This group discovered, among a group of patients with renal pelvic carcinomas, a distinct clinicopathologic subset of young patients with a particularly aggressive infiltrative tumor and sickled red blood cells (31). These tumors arise from the terminal collecting ducts or papillary epithelium, where a chronic hypoxic sickle cell trait environment causes epithelial cell proliferation (31,32). There is a predilection for the right kidney (33). At gross specimen inspection, the tumor is centered in the renal medulla, with an infiltrative growth pattern and extension into the renal collecting system (Fig 6) (31). In addition, satellite nodules are typically seen in the renal cortex and peripelvic soft tissues (Fig 7). Tumor necrosis and hemorrhage are usually identified, and calcifications are rare (2,34). The histologic appearance is variable; most often, poorly differentiated cells are arranged in a reticular pattern. However, other morphologic patterns, including microcystic, micropapillary, yolk sac–like, and adenoid cystic configurations, also are seen. The cells are found within a highly desmoplastic stroma, with a characteristic marked acute and chronic inflammatory infiltrate (Fig 7).
Figure 6a.
Renal medullary carcinoma in a 9-year-old boy with gross hematuria, who was treated for a urinary tract infection and later discovered to have the sickle cell trait. (a) Bivalved gross kidney specimen shows a central yellow-tan tumor (T) that has ill-defined margins and extends into the renal sinus. Dilated calyces (arrow) also are noted. (b) Coronal iodinated contrast–enhanced CT image shows an ill-defined mass (arrow) in the left kidney, with the reniform shape preserved; an enlarged heterogeneous retroperitoneal lymph node (arrowhead); and a dilated calyx (*).
Figure 7a.

Renal medullary carcinoma. (a) Cut gross kidney specimen from a 15-year-old boy with the sickle cell trait shows a tan-red mass. The tumor-kidney interface is lobulated, with a suggestion of satellite nodules (arrowheads). K = adjacent renal parenchyma. (b) Photomicrograph of a kidney section from a 14-year-old boy with the sickle cell trait shows an epithelial neoplasm (*) and the adjacent renal parenchyma (arrowhead), with an inflammatory reaction (arrow) between them. (H-E stain; original magnification, ×2.) (c) Longitudinal US image in the boy in a shows a homogeneous hyperechoic mass (arrow) expanding the upper pole. K = adjacent kidney. (d) Axial iodinated contrast–enhanced CT image in the boy in a shows the mass (arrow) with much lower enhancement than the adjacent kidney. Retroperitoneal nodal metastasis (arrowhead) also is seen. Images obtained through the lower region of the chest (not shown) showed multiple pulmonary nodules.
Figure 6b.
Renal medullary carcinoma in a 9-year-old boy with gross hematuria, who was treated for a urinary tract infection and later discovered to have the sickle cell trait. (a) Bivalved gross kidney specimen shows a central yellow-tan tumor (T) that has ill-defined margins and extends into the renal sinus. Dilated calyces (arrow) also are noted. (b) Coronal iodinated contrast–enhanced CT image shows an ill-defined mass (arrow) in the left kidney, with the reniform shape preserved; an enlarged heterogeneous retroperitoneal lymph node (arrowhead); and a dilated calyx (*).
Figure 7b.
Renal medullary carcinoma. (a) Cut gross kidney specimen from a 15-year-old boy with the sickle cell trait shows a tan-red mass. The tumor-kidney interface is lobulated, with a suggestion of satellite nodules (arrowheads). K = adjacent renal parenchyma. (b) Photomicrograph of a kidney section from a 14-year-old boy with the sickle cell trait shows an epithelial neoplasm (*) and the adjacent renal parenchyma (arrowhead), with an inflammatory reaction (arrow) between them. (H-E stain; original magnification, ×2.) (c) Longitudinal US image in the boy in a shows a homogeneous hyperechoic mass (arrow) expanding the upper pole. K = adjacent kidney. (d) Axial iodinated contrast–enhanced CT image in the boy in a shows the mass (arrow) with much lower enhancement than the adjacent kidney. Retroperitoneal nodal metastasis (arrowhead) also is seen. Images obtained through the lower region of the chest (not shown) showed multiple pulmonary nodules.
Figure 7c.
Renal medullary carcinoma. (a) Cut gross kidney specimen from a 15-year-old boy with the sickle cell trait shows a tan-red mass. The tumor-kidney interface is lobulated, with a suggestion of satellite nodules (arrowheads). K = adjacent renal parenchyma. (b) Photomicrograph of a kidney section from a 14-year-old boy with the sickle cell trait shows an epithelial neoplasm (*) and the adjacent renal parenchyma (arrowhead), with an inflammatory reaction (arrow) between them. (H-E stain; original magnification, ×2.) (c) Longitudinal US image in the boy in a shows a homogeneous hyperechoic mass (arrow) expanding the upper pole. K = adjacent kidney. (d) Axial iodinated contrast–enhanced CT image in the boy in a shows the mass (arrow) with much lower enhancement than the adjacent kidney. Retroperitoneal nodal metastasis (arrowhead) also is seen. Images obtained through the lower region of the chest (not shown) showed multiple pulmonary nodules.
Figure 7d.
Renal medullary carcinoma. (a) Cut gross kidney specimen from a 15-year-old boy with the sickle cell trait shows a tan-red mass. The tumor-kidney interface is lobulated, with a suggestion of satellite nodules (arrowheads). K = adjacent renal parenchyma. (b) Photomicrograph of a kidney section from a 14-year-old boy with the sickle cell trait shows an epithelial neoplasm (*) and the adjacent renal parenchyma (arrowhead), with an inflammatory reaction (arrow) between them. (H-E stain; original magnification, ×2.) (c) Longitudinal US image in the boy in a shows a homogeneous hyperechoic mass (arrow) expanding the upper pole. K = adjacent kidney. (d) Axial iodinated contrast–enhanced CT image in the boy in a shows the mass (arrow) with much lower enhancement than the adjacent kidney. Retroperitoneal nodal metastasis (arrowhead) also is seen. Images obtained through the lower region of the chest (not shown) showed multiple pulmonary nodules.
At imaging, a large central mass with an infiltrative growth pattern and extension into the cortex and renal sinus, reflecting the gross pathologic appearance, is seen (Figs 6, 7) (35–38). The tumor typically expands the kidney, but the reniform shape is preserved (35,37). Attenuation and enhancement are heterogeneous, and necrosis is extensive (35,36,38). The tumor enhances less intensely compared with the adjacent cortex and medulla during all phases of multidetector CT and at MR imaging (Figs 7, 8) (29,38). Medullary carcinoma has been reported to be hypovascular at angiography, with absence of flow at color Doppler US (35,36). Focal caliectasis without pelviectasis is a characteristic feature (Fig 6) (35–38). Medullary carcinoma is aggressive in biologic behavior, and extension into the perinephric fat and involvement of the regional lymph nodes are commonly seen (Figs 6, 7). Extensive metastatic disease is frequently found at the time of diagnosis.
Figure 8a.
Medullary carcinoma in a 21-year-old man with the sickle cell trait. (a) Axial T2-weighted MR image shows a heterogeneous, predominantly hyperintense mass (arrowhead) in the renal hilus. (b) Axial gadolinium-enhanced T1-weighted MR image shows the mass (arrowhead) with lower enhancement than the renal cortex.
Figure 8b.
Medullary carcinoma in a 21-year-old man with the sickle cell trait. (a) Axial T2-weighted MR image shows a heterogeneous, predominantly hyperintense mass (arrowhead) in the renal hilus. (b) Axial gadolinium-enhanced T1-weighted MR image shows the mass (arrowhead) with lower enhancement than the renal cortex.
The differential diagnosis for renal medullary carcinoma includes renal lymphoma, which also may grow in an infiltrative pattern. Lymphoma is usually distinguished by associated widespread lymphadenopathy and involvement of other organs. However, renal medullary carcinoma also can manifest with extensive disease, so patient age and history of the sickle cell trait are helpful clues to the diagnosis. Rhabdoid tumor and mesoblastic nephroma are additional medullary tumors with infiltrative growth patterns; however, both of these neoplasms occur in much younger patients. Infection may have an infiltrative appearance, with extension into the perinephric fat and enlargement of regional lymph nodes, mimicking the appearance of medullary carcinoma. Clinical and laboratory findings help to distinguish infection from tumor.
Advanced disease is common at the time of diagnosis, with local spread and metastatic disease involving the liver, distant lymph nodes, or lung. The tumor is resistant to chemotherapy and radiation therapy. The prognosis is dismal, with a mean survival after surgery of just 4 months (30).
Angiomyolipoma
Angiomyolipoma is a neoplasm of mixed cellular composition and is now considered part of the family of perivascular epithelioid cell tumors, which also includes pulmonary lymphangioleiomyomatosis. Sporadic angiomyolipoma is uncommon in the general population, accounting for only 1%–2% of renal tumors, and it usually occurs in middle-aged women (23). In children, angiomyolipoma is almost always associated with TSC. With TSC, mutations in the tumor suppressor genes TSC1 and TSC2 hyperactivate the mechanistic target of rapamycin–signaling pathway, decreasing the normal control of cell proliferation; this process results in tumors of multiple organs (39). Up to 80% of patients with TSC develop angiomyolipomas by a mean age of 10 years (40–42). With TSC, the tumors are more likely to be multiple, bilateral, and larger than angiomyolipomas in sporadic cases (41,42). The other renal lesions seen with TSC are less common and include isolated cysts (17%–35%), RCC (1%–2%), and polycystic kidney disease (PKD) (43). The association of TSC and PKD represents a contiguous gene syndrome owing to the proximity of the TSC2 and PKD1 genes. PKD1 is the site of the most common mutation associated with autosomal dominant PKD. A mutation involving both genes results in a combined TSC-PKD phenotype. In children, angiomyolipomas are often asymptomatic and found at surveillance imaging or the workup for TSC. The weakness of the tumor vessel walls leads to aneurysm formation, which can cause hemorrhagic complications that manifest as pain, anemia, or even hypovolemic shock. Tumors greater than 4 cm in diameter are associated with some risk of hemorrhage (44).
At gross specimen inspection, classic angiomyolipoma is seen as a sharply demarcated expansile mass centered in the cortex or medulla (Fig 9). At microscopy, tortuous blood vessels with walls composed of lipid-distended epithelioid cells that resemble adipose cells are arranged in bundles of myoid spindle cells and clear to eosinophilic cells. Vessels with normal smooth walls also are seen (Fig 9) (45). Angiomyolipomas in patients with TSC are more likely to vary from the classic sporadic (triphasic) form. They are also more likely to contain epithelial cysts, have a predominant epithelioid cell component, or be lipid poor—although the majority of these tumors contain visible lipid (46).
Figure 9a.

Angiomyolipomas in a 23-year-old woman with tuberous sclerosis. (a) Bivalved gross kidney specimen shows a yellow-tan mass (straight arrow) in the upper pole and numerous cysts (curved arrow). (b) Photomicrograph of a kidney section shows fat cells (arrow) and vessels with thick walls (arrowheads) within the tumor. (H-E stain; original magnification, ×10.) (c) Axial nonenhanced CT image shows a heterogeneous mass with fat attenuation (arrow), as well as multiple bilateral fluid-attenuating cysts (*). (d) Coronal image reformatted from CT angiography shows the enhancing components of the fat-containing tumor (arrow), as well as additional smaller enhancing tumors (arrowheads). Nonenhancing cysts (*) also are seen. Also noted are numerous small, presumed flash-filling hemangiomas in the liver.
Figure 9b.
Angiomyolipomas in a 23-year-old woman with tuberous sclerosis. (a) Bivalved gross kidney specimen shows a yellow-tan mass (straight arrow) in the upper pole and numerous cysts (curved arrow). (b) Photomicrograph of a kidney section shows fat cells (arrow) and vessels with thick walls (arrowheads) within the tumor. (H-E stain; original magnification, ×10.) (c) Axial nonenhanced CT image shows a heterogeneous mass with fat attenuation (arrow), as well as multiple bilateral fluid-attenuating cysts (*). (d) Coronal image reformatted from CT angiography shows the enhancing components of the fat-containing tumor (arrow), as well as additional smaller enhancing tumors (arrowheads). Nonenhancing cysts (*) also are seen. Also noted are numerous small, presumed flash-filling hemangiomas in the liver.
Figure 9c.
Angiomyolipomas in a 23-year-old woman with tuberous sclerosis. (a) Bivalved gross kidney specimen shows a yellow-tan mass (straight arrow) in the upper pole and numerous cysts (curved arrow). (b) Photomicrograph of a kidney section shows fat cells (arrow) and vessels with thick walls (arrowheads) within the tumor. (H-E stain; original magnification, ×10.) (c) Axial nonenhanced CT image shows a heterogeneous mass with fat attenuation (arrow), as well as multiple bilateral fluid-attenuating cysts (*). (d) Coronal image reformatted from CT angiography shows the enhancing components of the fat-containing tumor (arrow), as well as additional smaller enhancing tumors (arrowheads). Nonenhancing cysts (*) also are seen. Also noted are numerous small, presumed flash-filling hemangiomas in the liver.
Figure 9d.

Angiomyolipomas in a 23-year-old woman with tuberous sclerosis. (a) Bivalved gross kidney specimen shows a yellow-tan mass (straight arrow) in the upper pole and numerous cysts (curved arrow). (b) Photomicrograph of a kidney section shows fat cells (arrow) and vessels with thick walls (arrowheads) within the tumor. (H-E stain; original magnification, ×10.) (c) Axial nonenhanced CT image shows a heterogeneous mass with fat attenuation (arrow), as well as multiple bilateral fluid-attenuating cysts (*). (d) Coronal image reformatted from CT angiography shows the enhancing components of the fat-containing tumor (arrow), as well as additional smaller enhancing tumors (arrowheads). Nonenhancing cysts (*) also are seen. Also noted are numerous small, presumed flash-filling hemangiomas in the liver.
Visible fat in the lesion is a helpful finding at imaging, but the presence of the myoid and vascular components also contributes to the imaging appearance. At US, the fatty portions are echogenic without acoustic shadowing, and flow may be seen on color Doppler US images. The fatty components are hypoattenuating compared with fluid on CT images (Fig 9) and hyperintense on T1- and T2-weighted MR images, with suppression of the signal on fat-saturated MR images. T1-weighted gradient-recalled-echo MR sequences are useful for depicting diffuse signal loss on opposed-phase images of lipid-poor angiomyolipomas, which may not have bulk-fat signal intensity on standard T1- and T2-weighted MR images. Also, the india-ink appearance at the border between the fat-containing mass and renal parenchyma is particularly helpful for identifying small lesions (2,47,48). Vascular components may enhance intensely after the intravenous administration of iodinated contrast material or gadolinium chelate (Fig 9).
The finding of intralesional fat is highly suggestive of angiomyolipoma but not completely specific, as it may also be seen occasionally with Wilms tumor, RCC, and the very rare renal mature teratoma. Lipid-poor angiomyolipomas pose more of a diagnostic dilemma, but in the clinical setting of TSC, angiomyolipoma is by far the most likely diagnosis. Because sporadic angiomyolipomas are rare in the pediatric population, the multiplicity of lesions and other stigmata of TSC suggest the proper diagnosis. Rarely, RCC also occurs in patients with TSC, and interval follow-up to determine the tumor growth rate is required. The growth rate of angiomyolipoma is variable but generally slower than that of RCC (43). In some cases, tissue sampling may be necessary (46).
Classic angiomyolipomas are considered to be benign, although they may demonstrate locally aggressive growth. Persons with TSC have a higher risk of developing the rare variant, epithelioid angiomyoliopoma, which exhibits variable biologic behavior, including frank malignancy. Epithelioid angiomyoliopomas may invade adjacent tissues or even metastasize to the lung, liver, peritoneum, or bone during adulthood (46,49). Malignant perivascular epithelioid cell tumors frequently contain fluid-attenuating necrosis and may contain hemorrhage and calcifications but generally no fat (Fig 10) (49).
Figure 10a.
Metastatic perivascular epithelioid cell tumor in a 20-year-old woman with TSC. (a) Coronal contrast-enhanced reformatted CT image shows a large mass (arrow) with central fluid attenuation that corresponded to old hemorrhage at gross specimen inspection. No hypoattenuating fat is identified in the tumor. Linear high-attenuating calcification, bilateral fat-containing angiomyolipomas (arrowhead), and renal cysts also are seen. (b) Axial CT image obtained after resection shows hypoattenuating masses (arrowheads) in the liver and left renal bed, which were confirmed at biopsy to represent metastasis and recurrent tumor. Chest CT images (not shown) showed multiple large nodules representing metastases.
Figure 10b.
Metastatic perivascular epithelioid cell tumor in a 20-year-old woman with TSC. (a) Coronal contrast-enhanced reformatted CT image shows a large mass (arrow) with central fluid attenuation that corresponded to old hemorrhage at gross specimen inspection. No hypoattenuating fat is identified in the tumor. Linear high-attenuating calcification, bilateral fat-containing angiomyolipomas (arrowhead), and renal cysts also are seen. (b) Axial CT image obtained after resection shows hypoattenuating masses (arrowheads) in the liver and left renal bed, which were confirmed at biopsy to represent metastasis and recurrent tumor. Chest CT images (not shown) showed multiple large nodules representing metastases.
In the absence of therapy, angiomyolipomas become larger and more numerous with age, and substantial growth often occurs during puberty—particularly in girls—suggesting a hormonal influence. Lifelong surveillance with MR imaging every 1–3 years is advocated. US or CT may be used if MR imaging is not available (50). Multifocal angiomyolipomas are treated with mechanistic target of rapamycin complex 1 inhibitors with some success (39). Lesions 4 cm or greater in diameter require localized treatment if they are symptomatic. Owing to the increasing number and size of cysts and angiomyolipomas over time in persons with TSC, these individuals are at risk for premature loss of nephrons, so conservative therapy consisting of selective arterial embolization, percutaneous ablation, or partial nephrectomy is preferred (23).
Metanephric Tumors
Metanephric tumors comprise a spectrum of rare differentiated epithelial and stromal tumors that are derived from metanephric blastema and are histogenetically related to Wilms tumor (5,7). At one end of the spectrum is the purely epithelial tumor, metanephric adenoma. At the other end is the purely stromal form, metanephric stromal tumor. Between these two types is metanephric adenofibroma, which is composed of a mixture of epithelial and stromal elements. These tumors are considered to be benign; however, there have been a few reported cases of metastasis (51).
Metanephric adenoma is the most common of these tumors and occurs most often in adult women with a mean age of 41 years (age range, 14 months to 83 years). There is a female predominance, with a female-to-male ratio of 2.6:1.0 (30,52,53). Metanephric stromal tumors occur most commonly in young children with a mean age of 2 years (age range, 5 months to 15 years) (30,54). Metanephric adenofibroma most commonly affects children and young adults. The mean age of affected persons is 82.2 months (age range, 5 months to 36 years) (7). Presenting signs include pain, hematuria, hypertension, and a palpable mass, but these tumors frequently are found incidentally. Davis et al (52) found an association between metanephric adenoma and polycythemia in 12% of cases.
Histopathologic Features
Metanephric Adenoma.—At macroscopy, metanephric adenomas are usually unifocal and gray-tan to yellow, and range in greatest dimension from 0.3 to 15.0 cm (Figs 11, 12) (30,55). The tumors are well circumscribed but nonencapsulated (30). Some contain areas of hemorrhage and/or necrosis (55). At histologic analysis, metanephric adenomas are found to be composed of small uniform epithelial cells that form small acini and occasional tubular, glomeruloid, or papillary structures (Fig 13) (30,52). The cells are ovoid, with pale-staining cytoplasm and dark ovoid nuclei (30). The surrounding stroma is acellular, with fluid or a hyalinized matrix (55). In addition, these tumors are more commonly calcified than are other renal tumors (52). Glomeruloid bodies are frequently calcified, with the formation of psammoma bodies, and dystrophic calcifications within the hyalinized stroma are common (52,55).
Figure 11a.

Metanephric adenoma in a girl. (a) Sectioned gross kidney specimen shows a central yellow-tan circumscribed mass (*) with satellite nodules (arrowheads) that extend to the capsule. (b) Axial nonenhanced CT image shows a hyperattenuating mass (*) and the peripheral nodules (arrowheads). (c) Coronal T2-weighted MR image shows a central mass (arrowhead) that is hypointense compared with the renal cortex and medulla, suggesting calcification or a densely cellular tumor. (d) Coronal arterial phase MR angiogram shows the mass (arrowhead) with lower enhancement than the renal cortex.
Figure 12a.

Metanephric adenoma in a 7-year-old boy. (a) Sectioned gross specimen shows a well-circumscribed lobulated white-gray tumor (arrow) with a focal area of necrosis (arrowhead). (b) Coronal iodinated contrast–enhanced reformatted CT image shows a circumscribed hypoenhancing mass (arrow) adjacent to a cluster of coarse calcifications.
Figure 13a.
Metanephric adenoma in a 19-year-old man with an elevated hematocrit level at routine blood analysis. (a) Photomicrograph of kidney shows tightly packed tubules (arrowhead) composed of small cells with scant cytoplasm and uniform nuclei. (H-E stain; original magnification, ×10.) (b) Longitudinal color Doppler US image shows a hypoechoic mass (arrowhead) with no flow in the renal sinus echo complex. This appearance is atypical, as these lesions are characteristically hyperechoic. (c) Coronal contrast-enhanced reformatted CT image shows the mass (arrowhead) in the renal sinus has lower enhancement than the cortex.
Figure 11b.
Metanephric adenoma in a girl. (a) Sectioned gross kidney specimen shows a central yellow-tan circumscribed mass (*) with satellite nodules (arrowheads) that extend to the capsule. (b) Axial nonenhanced CT image shows a hyperattenuating mass (*) and the peripheral nodules (arrowheads). (c) Coronal T2-weighted MR image shows a central mass (arrowhead) that is hypointense compared with the renal cortex and medulla, suggesting calcification or a densely cellular tumor. (d) Coronal arterial phase MR angiogram shows the mass (arrowhead) with lower enhancement than the renal cortex.
Figure 11c.
Metanephric adenoma in a girl. (a) Sectioned gross kidney specimen shows a central yellow-tan circumscribed mass (*) with satellite nodules (arrowheads) that extend to the capsule. (b) Axial nonenhanced CT image shows a hyperattenuating mass (*) and the peripheral nodules (arrowheads). (c) Coronal T2-weighted MR image shows a central mass (arrowhead) that is hypointense compared with the renal cortex and medulla, suggesting calcification or a densely cellular tumor. (d) Coronal arterial phase MR angiogram shows the mass (arrowhead) with lower enhancement than the renal cortex.
Figure 11d.
Metanephric adenoma in a girl. (a) Sectioned gross kidney specimen shows a central yellow-tan circumscribed mass (*) with satellite nodules (arrowheads) that extend to the capsule. (b) Axial nonenhanced CT image shows a hyperattenuating mass (*) and the peripheral nodules (arrowheads). (c) Coronal T2-weighted MR image shows a central mass (arrowhead) that is hypointense compared with the renal cortex and medulla, suggesting calcification or a densely cellular tumor. (d) Coronal arterial phase MR angiogram shows the mass (arrowhead) with lower enhancement than the renal cortex.
Figure 12b.
Metanephric adenoma in a 7-year-old boy. (a) Sectioned gross specimen shows a well-circumscribed lobulated white-gray tumor (arrow) with a focal area of necrosis (arrowhead). (b) Coronal iodinated contrast–enhanced reformatted CT image shows a circumscribed hypoenhancing mass (arrow) adjacent to a cluster of coarse calcifications.
Figure 13b.
Metanephric adenoma in a 19-year-old man with an elevated hematocrit level at routine blood analysis. (a) Photomicrograph of kidney shows tightly packed tubules (arrowhead) composed of small cells with scant cytoplasm and uniform nuclei. (H-E stain; original magnification, ×10.) (b) Longitudinal color Doppler US image shows a hypoechoic mass (arrowhead) with no flow in the renal sinus echo complex. This appearance is atypical, as these lesions are characteristically hyperechoic. (c) Coronal contrast-enhanced reformatted CT image shows the mass (arrowhead) in the renal sinus has lower enhancement than the cortex.
Figure 13c.
Metanephric adenoma in a 19-year-old man with an elevated hematocrit level at routine blood analysis. (a) Photomicrograph of kidney shows tightly packed tubules (arrowhead) composed of small cells with scant cytoplasm and uniform nuclei. (H-E stain; original magnification, ×10.) (b) Longitudinal color Doppler US image shows a hypoechoic mass (arrowhead) with no flow in the renal sinus echo complex. This appearance is atypical, as these lesions are characteristically hyperechoic. (c) Coronal contrast-enhanced reformatted CT image shows the mass (arrowhead) in the renal sinus has lower enhancement than the cortex.
Metanephric Stromal Tumors.—Metanephric stromal tumors are solid but may contain large cysts; they range in size from 3 to 10 cm (Fig 14). The tumor is nonencapsulated and entraps native kidney elements, but it has a scalloped border rather than the grossly infiltrating border of mesoblastic nephroma (Fig 14). Metanephric stromal tumors are made up of spindle and stellate cells. The degree of stromal cellularity ranges from hypocellular with sclerosis to markedly hypercellular, with the latter degree of cellularity being reminiscent of the appearance of cellular mesoblastic nephroma (30). Alternating zones of myxoid hypoceullarity and collagenized hypercellularity create a characteristic nodular appearance at low microscopic field power. The finding of “onion skinning” or collarettes of condensed stromal cells surrounding entrapped renal tubules or blood vessels, similar to those seen surrounding dysplastic tubules in renal dysplasia, is a suggestive feature (Fig 14). Angiodysplasia of entrapped arteries also is a characteristic finding (30,54).
Figure 14a.
Metanephric stromal tumor. (a) Bivalved gross kidney specimen from a 3-month-old boy shows a well-defined tan-gray tumor (arrowhead) that is centered in the renal medulla and has small areas of hemorrhage. (b) Photomicrograph of a kidney section from a 2-month-old boy shows a nonencapsulated spindle cell tumor (T) on the right and the adjacent kidney on the left. (H-E stain; original magnification, ×2.) (c) Photomicrograph of a kidney section from the same boy shows the “onion-skin” or collarette appearance of concentric layers of tumor cells (arrowhead) surrounding an entrapped tubule. (H-E stain; original magnification, ×10.) (d) Longitudinal US image in the boy in a shows a hilar mass (arrowhead) with anechoic cystic components (arrow). (e) Coronal T2-weighted MR image in the boy in a shows a hilar mass (arrowhead) that is isointense compared with the renal parenchyma. There are small fluid–signal-intensity cysts (arrow) within the mass. (f) Axial gadolinium-enhanced T1-weighted fat-saturated MR image in the boy in a shows the mass (arrowhead) with lower enhancement compared with the renal parenchyma. The fluid-filled cysts (arrow) are not enhanced.
Figure 14b.
Metanephric stromal tumor. (a) Bivalved gross kidney specimen from a 3-month-old boy shows a well-defined tan-gray tumor (arrowhead) that is centered in the renal medulla and has small areas of hemorrhage. (b) Photomicrograph of a kidney section from a 2-month-old boy shows a nonencapsulated spindle cell tumor (T) on the right and the adjacent kidney on the left. (H-E stain; original magnification, ×2.) (c) Photomicrograph of a kidney section from the same boy shows the “onion-skin” or collarette appearance of concentric layers of tumor cells (arrowhead) surrounding an entrapped tubule. (H-E stain; original magnification, ×10.) (d) Longitudinal US image in the boy in a shows a hilar mass (arrowhead) with anechoic cystic components (arrow). (e) Coronal T2-weighted MR image in the boy in a shows a hilar mass (arrowhead) that is isointense compared with the renal parenchyma. There are small fluid–signal-intensity cysts (arrow) within the mass. (f) Axial gadolinium-enhanced T1-weighted fat-saturated MR image in the boy in a shows the mass (arrowhead) with lower enhancement compared with the renal parenchyma. The fluid-filled cysts (arrow) are not enhanced.
Figure 14c.
Metanephric stromal tumor. (a) Bivalved gross kidney specimen from a 3-month-old boy shows a well-defined tan-gray tumor (arrowhead) that is centered in the renal medulla and has small areas of hemorrhage. (b) Photomicrograph of a kidney section from a 2-month-old boy shows a nonencapsulated spindle cell tumor (T) on the right and the adjacent kidney on the left. (H-E stain; original magnification, ×2.) (c) Photomicrograph of a kidney section from the same boy shows the “onion-skin” or collarette appearance of concentric layers of tumor cells (arrowhead) surrounding an entrapped tubule. (H-E stain; original magnification, ×10.) (d) Longitudinal US image in the boy in a shows a hilar mass (arrowhead) with anechoic cystic components (arrow). (e) Coronal T2-weighted MR image in the boy in a shows a hilar mass (arrowhead) that is isointense compared with the renal parenchyma. There are small fluid–signal-intensity cysts (arrow) within the mass. (f) Axial gadolinium-enhanced T1-weighted fat-saturated MR image in the boy in a shows the mass (arrowhead) with lower enhancement compared with the renal parenchyma. The fluid-filled cysts (arrow) are not enhanced.
Figure 14d.
Metanephric stromal tumor. (a) Bivalved gross kidney specimen from a 3-month-old boy shows a well-defined tan-gray tumor (arrowhead) that is centered in the renal medulla and has small areas of hemorrhage. (b) Photomicrograph of a kidney section from a 2-month-old boy shows a nonencapsulated spindle cell tumor (T) on the right and the adjacent kidney on the left. (H-E stain; original magnification, ×2.) (c) Photomicrograph of a kidney section from the same boy shows the “onion-skin” or collarette appearance of concentric layers of tumor cells (arrowhead) surrounding an entrapped tubule. (H-E stain; original magnification, ×10.) (d) Longitudinal US image in the boy in a shows a hilar mass (arrowhead) with anechoic cystic components (arrow). (e) Coronal T2-weighted MR image in the boy in a shows a hilar mass (arrowhead) that is isointense compared with the renal parenchyma. There are small fluid–signal-intensity cysts (arrow) within the mass. (f) Axial gadolinium-enhanced T1-weighted fat-saturated MR image in the boy in a shows the mass (arrowhead) with lower enhancement compared with the renal parenchyma. The fluid-filled cysts (arrow) are not enhanced.
Figure 14e.
Metanephric stromal tumor. (a) Bivalved gross kidney specimen from a 3-month-old boy shows a well-defined tan-gray tumor (arrowhead) that is centered in the renal medulla and has small areas of hemorrhage. (b) Photomicrograph of a kidney section from a 2-month-old boy shows a nonencapsulated spindle cell tumor (T) on the right and the adjacent kidney on the left. (H-E stain; original magnification, ×2.) (c) Photomicrograph of a kidney section from the same boy shows the “onion-skin” or collarette appearance of concentric layers of tumor cells (arrowhead) surrounding an entrapped tubule. (H-E stain; original magnification, ×10.) (d) Longitudinal US image in the boy in a shows a hilar mass (arrowhead) with anechoic cystic components (arrow). (e) Coronal T2-weighted MR image in the boy in a shows a hilar mass (arrowhead) that is isointense compared with the renal parenchyma. There are small fluid–signal-intensity cysts (arrow) within the mass. (f) Axial gadolinium-enhanced T1-weighted fat-saturated MR image in the boy in a shows the mass (arrowhead) with lower enhancement compared with the renal parenchyma. The fluid-filled cysts (arrow) are not enhanced.
Figure 14f.
Metanephric stromal tumor. (a) Bivalved gross kidney specimen from a 3-month-old boy shows a well-defined tan-gray tumor (arrowhead) that is centered in the renal medulla and has small areas of hemorrhage. (b) Photomicrograph of a kidney section from a 2-month-old boy shows a nonencapsulated spindle cell tumor (T) on the right and the adjacent kidney on the left. (H-E stain; original magnification, ×2.) (c) Photomicrograph of a kidney section from the same boy shows the “onion-skin” or collarette appearance of concentric layers of tumor cells (arrowhead) surrounding an entrapped tubule. (H-E stain; original magnification, ×10.) (d) Longitudinal US image in the boy in a shows a hilar mass (arrowhead) with anechoic cystic components (arrow). (e) Coronal T2-weighted MR image in the boy in a shows a hilar mass (arrowhead) that is isointense compared with the renal parenchyma. There are small fluid–signal-intensity cysts (arrow) within the mass. (f) Axial gadolinium-enhanced T1-weighted fat-saturated MR image in the boy in a shows the mass (arrowhead) with lower enhancement compared with the renal parenchyma. The fluid-filled cysts (arrow) are not enhanced.
Metanephric Adenofibroma.—Metanephric adenofibroma is centered in the renal medulla and ranges in size from 1.8 to 11.0 cm (30). These tumors have indistinct borders. At microscopy, the appearance of metanephric adenofibroma ranges from predominantly stromal to nearly completely adenomatous. The epithelial component is typically circumscribed and may consist of a single nodule or multiple nodules. The stromal component typically infiltrates the native kidney in a pattern similar to that seen with intralobar nephrogenic rests. Entrapped renal elements may lead to duct dilatation and cyst formation or to increased renin production (7,30).
Imaging Features
At US, metanephric tumors are well circumscribed and have variable echogenicity, although they are typically hyperechoic (Fig 13) (55–61). This appearance may reflect psammomatous calcifications and the interfaces of numerous tubules (59). Occasionally, foci of hypoechoic necrosis or old hemorrhage may be noted (53). Metanephric stromal tumors may contain cystic components that may be large; these represent dilated entrapped tubules that are seen histopathologically (Fig 14) (2,62). Doppler US evaluation reveals a hypovascular tumor, which is reflective of the pathologic finding of scarring with few vessels (55).
At nonenhanced CT, metanephric tumors are often hyperattenuating compared with the surrounding kidney parenchyma—perhaps owing to high cellularity or extensive psammomatous calcifications (Fig 11) (55,59,61,63). The finding of a mass that is hyperechoic at US and hyperattenuating at nonenhanced CT has been described as characteristic of benign adenomas (63). Hypoattenuating areas may be seen centrally and probably represent necrosis or old hemorrhage (53,58,63). Peripheral or central calcifications are frequently seen and represent dystrophic calcification in hyalinized stroma or heterologous elements (Fig 12) (55,57,60,64). Following the intravenous administration of iodinated contrast material, the mass enhances but less intensely compared with the renal parenchyma (55,59,63). Foci of necrosis or old hemorrhage do not enhance (53). MR imaging depicts a well-circumscribed mass that is iso- to hypointense relative to the renal parenchyma on T1- and T2-weighted images—probably owing to calcification (Fig 11). After intravenous administration of gadolinium chelate, the tumor shows mild peripheral enhancement that is lower than that of the adjacent kidney parenchyma (55,56,60,63–65).
Treatment and Prognosis
Although metanephric tumors are considered to be benign, the epithelial component may be associated with the development of Wilms tumor or papillary RCC—usually in older individuals. The malignancy potential of the stromal component is unknown (30).
Most patients with metanephric tumors are treated with radical nephrectomy because a malignant tumor is suspected preoperatively. There have been several reports of a favorable outcome with partial nephrectomy for small tumors. However, given the rarity of these tumors and the risk of a malignant tumor being missed with biopsy, this approach is controversial (55,60,66,67).
Lymphoma
Primary lymphoma of the kidney is exceedingly rare because there is no renal lymphoid tissue; thus, lymphomatous involvement of the kidney occurs by way of hematogenous spread or direct extension of a retroperitoneal mass. Pediatric lymphomas involving the kidney are usually B-cell non-Hodgkin lymphomas—Burkitt lymphoma in particular. Burkitt lymphoma most commonly involves the bowel in the right lower quadrant. Other organs that may be involved include the liver, ovaries, and breasts. Affected persons are usually older than 5 years (2,47,68).
Lymphomas may affect the kidneys as solitary masses; however, they are more often multiple bilateral expansile renal masses or nodules. Less commonly, diffuse infiltration is observed, and rarely, the tumor involves only the perinephric tissues. At pathologic analysis, large masses demonstrate central hemorrhage and necrosis. At histologic examination, Burkitt lymphoma is composed of uniform medium basophilic cells interspersed with clear histiocytes that contain debris from apoptotic cells, conferring a “starry sky” appearance. Mitoses are frequent. Tumor cells express mature B-cell markers (ie, CD19, CD20, CD22, and CD79a) and a Ki-67 proliferation index of nearly 100%. The imaging appearances of lymphoma vary according to the type of tumor growth. The most common appearance is that of multiple round masses or nodules. At US, these masses or nodules may appear hyperechoic (Fig 15), but they often appear quite hypoechoic and may even show posterior acoustic enhancement, mimicking renal cysts. At CT, the masses are hypoattenuating and enhance less intensely compared with the adjacent parenchyma (Figs 15, 16). Lymphomatous lesions are more conspicuous on MR images than on CT images (68). Diffuse infiltration, usually of both kidneys, is the typical appearance of leukemic infiltration at imaging and may also be seen with lymphoma. The kidneys are enlarged but remain reniform, with decreased corticomedullary differentiation; these findings are often quite subtle (2,23,69,70).
Figure 15a.
Solitary mass of diffuse large B-cell lymphoma involving the kidney in an 11-year-old boy. (a) Longitudinal US image shows a predominantly echogenic mass (arrowhead) behind the liver (L). (b) Axial iodinated contrast–enhanced CT image shows a heterogeneously attenuating mass (arrow) involving the renal cortex and the retroperitoneum to the body wall. Punctate areas of calcification (arrowhead) are noted. (c) Coronal fused fluorine 18 fluorodeoxyglucose positron emission tomographic (PET)/CT image shows focal uptake of the radiotracer by the mass (arrowhead).
Figure 16a.
Burkitt lymphoma in a 5-year-old boy. (a) Axial contrast-enhanced CT image shows multiple hypoattenuating circumscribed masses (arrowheads) in both kidneys. (b) Axial CT image of the pelvis shows diffuse wall thickening of the ileum (arrowhead), which is typical of Burkitt lymphoma.
Figure 15b.
Solitary mass of diffuse large B-cell lymphoma involving the kidney in an 11-year-old boy. (a) Longitudinal US image shows a predominantly echogenic mass (arrowhead) behind the liver (L). (b) Axial iodinated contrast–enhanced CT image shows a heterogeneously attenuating mass (arrow) involving the renal cortex and the retroperitoneum to the body wall. Punctate areas of calcification (arrowhead) are noted. (c) Coronal fused fluorine 18 fluorodeoxyglucose positron emission tomographic (PET)/CT image shows focal uptake of the radiotracer by the mass (arrowhead).
Figure 15c.
Solitary mass of diffuse large B-cell lymphoma involving the kidney in an 11-year-old boy. (a) Longitudinal US image shows a predominantly echogenic mass (arrowhead) behind the liver (L). (b) Axial iodinated contrast–enhanced CT image shows a heterogeneously attenuating mass (arrow) involving the renal cortex and the retroperitoneum to the body wall. Punctate areas of calcification (arrowhead) are noted. (c) Coronal fused fluorine 18 fluorodeoxyglucose positron emission tomographic (PET)/CT image shows focal uptake of the radiotracer by the mass (arrowhead).
Figure 16b.
Burkitt lymphoma in a 5-year-old boy. (a) Axial contrast-enhanced CT image shows multiple hypoattenuating circumscribed masses (arrowheads) in both kidneys. (b) Axial CT image of the pelvis shows diffuse wall thickening of the ileum (arrowhead), which is typical of Burkitt lymphoma.
The finding of a solitary mass in association with lymphoma mimics that seen with primary renal tumor (Fig 15), and the appearance of multiple masses may mimic that seen with polycystic kidney disease at US. Because lymphoma rarely involves only the kidneys, additional findings of adenopathy and masses involving other organs suggest the diagnosis of lymphoma (Fig 16). The diffuse infiltration of the kidneys uncommonly seen with lymphoma is very similar at imaging to pyelonephritis. The clinical presentation and laboratory findings help to distinguish tumor from infection, and contrast-enhanced imaging of pyelonephritis reveals a striated nephrogram.
Lymphoma—Burkitt lymphoma in particular—is sensitive to chemotherapy, although surgical debulking and radiation therapy also may be required. The prognosis depends on the age of the patient and particular type of hematologic malignancy.
Other Rare Renal Tumors
Primitive Neuroectodermal Tumor
PNET is a highly aggressive small round cell tumor that most often affects bone and rarely arises in the kidney. PNETs have a characteristic chromosomal translocation, t(11;22)(q24;q12), that produces the fusion protein EWS-FLI1, which induces a Ewing sarcoma phenotype (71). Renal PNET accounts for less than 1% of pediatric renal neoplasms (72). The mean patient age at diagnosis is 24–27 years (72–74). The most common symptoms at presentation are flank pain, a mass, and hematuria. Systemic symptoms, including fever, are less frequent. Metastatic disease involving the lung, liver, and/or bone is present at diagnosis in 31%–50% of cases (73,74).
At gross specimen inspection, the tumor is typically quite large, replacing much of the kidney. Hemorrhage and necrosis are common (Figs 17, 18). The tumor may contain calcifications. Histologically, the tumor is composed of sheets and nests of small, round to ovoid blue cells that may form rosettes or pseudorosettes (Fig 17b). This appearance is very similar to that of blastemal Wilms tumor, and immunohistochemical and molecular genetic analyses are necessary to make a specific diagnosis. PNETs express neural markers, and CD99 is universally positive for PNET in the Ewing family of tumors (23,37).
Figure 17a.

PNET in a 15-year-old girl. (a) Sectioned gross kidney specimen shows a central tan-white lobular tumor (arrowhead) with small areas of hemorrhage and an adjacent intraparenchymal hematoma (arrow). (b) Photomicrograph of a kidney section demonstrates sheets of small round cells with scant cytoplasm and coarse chromatin. (H-E stain; original magnification, ×40.) (c) Coronal T2-weighted MR image shows a hypointense central mass (straight arrow) consistent with a densely cellular tumor. The hyperintense component (curved arrow) adjacent to the tumor represents the hematoma. (d) Coronal gadolinium-enhanced MR image shows the mass (arrow) enhanced but less intensely compared with the adjacent renal parenchyma.
Figure 18a.
Renal PNET in a 16-year-old girl. (a) Bisected gross kidney specimen shows a tan-brown variegated hemorrhagic mass (arrowhead) with an adjacent subcapsular hemorrhage (arrow). (b) Coronal iodinated contrast–enhanced reformatted CT image shows the upper pole mass (arrowhead) with lower enhancement compared with the adjacent renal cortex during the corticomedullary phase. The subcapsular fluid-attenuation collection (arrow) corresponds to the chronic hemorrhage seen at gross inspection.
Figure 17b.
PNET in a 15-year-old girl. (a) Sectioned gross kidney specimen shows a central tan-white lobular tumor (arrowhead) with small areas of hemorrhage and an adjacent intraparenchymal hematoma (arrow). (b) Photomicrograph of a kidney section demonstrates sheets of small round cells with scant cytoplasm and coarse chromatin. (H-E stain; original magnification, ×40.) (c) Coronal T2-weighted MR image shows a hypointense central mass (straight arrow) consistent with a densely cellular tumor. The hyperintense component (curved arrow) adjacent to the tumor represents the hematoma. (d) Coronal gadolinium-enhanced MR image shows the mass (arrow) enhanced but less intensely compared with the adjacent renal parenchyma.
Figure 17c.
PNET in a 15-year-old girl. (a) Sectioned gross kidney specimen shows a central tan-white lobular tumor (arrowhead) with small areas of hemorrhage and an adjacent intraparenchymal hematoma (arrow). (b) Photomicrograph of a kidney section demonstrates sheets of small round cells with scant cytoplasm and coarse chromatin. (H-E stain; original magnification, ×40.) (c) Coronal T2-weighted MR image shows a hypointense central mass (straight arrow) consistent with a densely cellular tumor. The hyperintense component (curved arrow) adjacent to the tumor represents the hematoma. (d) Coronal gadolinium-enhanced MR image shows the mass (arrow) enhanced but less intensely compared with the adjacent renal parenchyma.
Figure 17d.
PNET in a 15-year-old girl. (a) Sectioned gross kidney specimen shows a central tan-white lobular tumor (arrowhead) with small areas of hemorrhage and an adjacent intraparenchymal hematoma (arrow). (b) Photomicrograph of a kidney section demonstrates sheets of small round cells with scant cytoplasm and coarse chromatin. (H-E stain; original magnification, ×40.) (c) Coronal T2-weighted MR image shows a hypointense central mass (straight arrow) consistent with a densely cellular tumor. The hyperintense component (curved arrow) adjacent to the tumor represents the hematoma. (d) Coronal gadolinium-enhanced MR image shows the mass (arrow) enhanced but less intensely compared with the adjacent renal parenchyma.
Figure 18b.
Renal PNET in a 16-year-old girl. (a) Bisected gross kidney specimen shows a tan-brown variegated hemorrhagic mass (arrowhead) with an adjacent subcapsular hemorrhage (arrow). (b) Coronal iodinated contrast–enhanced reformatted CT image shows the upper pole mass (arrowhead) with lower enhancement compared with the adjacent renal cortex during the corticomedullary phase. The subcapsular fluid-attenuation collection (arrow) corresponds to the chronic hemorrhage seen at gross inspection.
At imaging, PNET appears as a poorly defined large mass, with hemorrhage and necrosis replacing the kidney (37). These tumors may appear hypo- to isoechoic, as compared with the kidney, on US images and are heterogeneous on CT images, with mild to moderate enhancement (Figs 18, 19) (23,72). At MR imaging, these tumors are iso- to hypointense compared with the renal parenchyma on T1-weighted images and variable in signal intensity on T2-weighted images owing to hemorrhage and necrosis (Fig 17) (23,70,75). A multilobulated septated appearance may be observed (76). Tumor extension into the inferior vena cava may occur (Fig 19) (72,74,75). PNET is a highly aggressive tumor that metastasizes early. Thus, the findings of complete replacement of the kidney by tumor and metastatic disease at presentation suggest the diagnosis of PNET.
Figure 19a.
PNET in a 17-year-old girl. (a) Axial delayed phase contrast-enhanced CT image shows a central mass (arrowhead) enhancing less intensely compared with the adjacent parenchyma and extension of the tumor through the renal vein (straight arrow) into the inferior vena cava. A small enhancing mass (curved arrow) also is seen in the spinal canal. (b) Axial CT image at the level of the diaphragm shows a filling defect (arrow) in the intrahepatic inferior vena cava and a left pulmonary metastatic nodule (arrowhead).
Figure 19b.
PNET in a 17-year-old girl. (a) Axial delayed phase contrast-enhanced CT image shows a central mass (arrowhead) enhancing less intensely compared with the adjacent parenchyma and extension of the tumor through the renal vein (straight arrow) into the inferior vena cava. A small enhancing mass (curved arrow) also is seen in the spinal canal. (b) Axial CT image at the level of the diaphragm shows a filling defect (arrow) in the intrahepatic inferior vena cava and a left pulmonary metastatic nodule (arrowhead).
The overall prognosis for patients with PNET is poor, as they have advanced disease at presentation. The survival rate for individuals with localized disease is about 70% but is less than 40% for those with metastatic disease (74). Initial treatment consists of nephrectomy and radiation therapy for local disease control, followed by chemotherapy.
Desmoplastic Small Round Cell Tumor
DSRCT is a rare aggressive malignancy that is part of the small round blue cell tumor family. The tumor has a strong predilection for adolescent males aged 5–30 years, with a reported male-to-female ratio of 3–5:1 (77). Diffuse primary peritoneal disease accounts for the majority of cases; however, single-organ involvement may (rarely) occur. Eleven cases of renal DSRCT have been reported (78–84). With the exception of the initial case in a 41-year-old woman, all cases occurred in children, adolescents, or young adults aged 6–20 years. Most of these patients presented with a palpable mass or gross hematuria.
Gross pathologic analysis reveals a firm lobulated gray-yellow mass with areas of central hemorrhage and/or necrosis. At histologic evaluation, nondifferentiated small round cells are arranged in well-defined nests embedded within varying amounts of desmoplastic stroma. Particularly in those cases that lack the characteristic prominent desmoplastic stroma, the histologic appearance is nonspecific and difficult to distinguish from that of other small round blue cell tumors (82,85). A highly specific cytogenetic marker is the translocation t(11;22)(p13;q12), which creates the EWS-WT1 gene fusion. At immunohistochemical analysis, cells coexpress epithelial, mesenchymal, and neural markers, and DSRCTs are hypothesized to arise from a progenitor cell with multiphenotypic potential (86).
DSRCT typically appears as disseminated peritoneal and mesenteric disease involving a large dominant mass, most often in the retrovesical region. Few reports describing the imaging features of DSRCT of the kidney exist. The mass has been described as well circumscribed and hypoechoic at US, but it may appear heterogeneous in echotexture owing to hemorrhage, necrosis, or foci of hyperechoic calcifications (80). At nonenhanced CT, the tumor may be slightly hyperattenuating compared with the normal kidney tissue, with multiple punctate or coarse calcifications (80,82). Following iodinated contrast material administration, the tumor is well circumscribed and enhances less intensely compared with the kidney parenchyma (80,87). Fluid-attenuating areas that probably represent necrosis or old hemorrhage may be seen (80,82). With DSRCT of the abdominal cavity and pelvis, the mass is generally hypo- to isointense to skeletal muscle on T1-weighted MR images and heterogeneously hyper- to isointense on T2-weighted MR images. Occasional T2-hypointense areas probably represent portions that are densely cellular or contain dense desmoplastic stroma (88). Heterogeneous enhancement is observed after intravenous administration of gadolinium chelate (88). Enlargement of adjacent lymph nodes and spread into the renal vein may be seen (79). Widespread metastases to the liver, lung, spleen, pleura, and/or soft tissue may be present at diagnosis.
DSRCT usually shows increased uptake of fluorine 18 fluorodeoxyglucose at PET, and combined PET/CT may provide useful prognostic information while aiding in the monitoring of recurrence and therapy response (89).
Treatment of DSRCTs involves surgical resection, abdominal radiation, and aggressive chemotherapy; thus, an accurate diagnosis is important. Even with intensive therapy, the prognosis remains poor, with the 5-year survival rate reported to be less than 15% (88).
Synovial Cell Sarcoma
Synovial cell sarcoma is a rare spindle cell neoplasm that most commonly occurs in the extremities of adolescents and young adults. Previously known as an embryonal sarcoma, primary renal synovial cell sarcoma was described as a distinct entity by Argani et al (90) in 2000. The age range of affected individuals is 12–59 years (mean age, 35 years), with a slight male predominance (5). Most patients present with abdominal or flank pain.
At gross specimen inspection, the tumor appears solid, with areas of hemorrhage, necrosis, and cyst formation (Fig 20) (5). Histologically, the tumor is composed of monomorphic spindle cells arranged in intersecting fascicles or solid sheets. Mitotic activity is typically seen. The tumor also contains cysts lined by eosinophilic cells with apically oriented nuclei. This lining is known as a hobnailed epithelium. The cysts probably represent entrapped tubules and may be quite dilated (5). Ninety percent of these tumors have the characteristic translocation t(X;18)(p11.2;q11) (71).
Figure 20a.
Synovial cell sarcoma of the kidney in a 15-year-old boy. (a) Sectioned gross specimen shows a well-circumscribed, tan-to-red heterogeneous necrotic mass (arrow) with focal hemorrhage (arrowhead). (b) Longitudinal US image shows a heterogeneous mass (arrow) in the lower pole of the kidney, with some nearly anechoic regions. (c) Sagittal iodinated contrast–enhanced reformatted CT image shows the mass (arrow) is predominantly cystic, with enhancing septa (arrowhead).
Figure 20b.
Synovial cell sarcoma of the kidney in a 15-year-old boy. (a) Sectioned gross specimen shows a well-circumscribed, tan-to-red heterogeneous necrotic mass (arrow) with focal hemorrhage (arrowhead). (b) Longitudinal US image shows a heterogeneous mass (arrow) in the lower pole of the kidney, with some nearly anechoic regions. (c) Sagittal iodinated contrast–enhanced reformatted CT image shows the mass (arrow) is predominantly cystic, with enhancing septa (arrowhead).
Figure 20c.
Synovial cell sarcoma of the kidney in a 15-year-old boy. (a) Sectioned gross specimen shows a well-circumscribed, tan-to-red heterogeneous necrotic mass (arrow) with focal hemorrhage (arrowhead). (b) Longitudinal US image shows a heterogeneous mass (arrow) in the lower pole of the kidney, with some nearly anechoic regions. (c) Sagittal iodinated contrast–enhanced reformatted CT image shows the mass (arrow) is predominantly cystic, with enhancing septa (arrowhead).
Imaging studies reveal a well-circumscribed solid mass with prominent fluid-attenuating areas that represent necrosis, old hemorrhage, or cysts. The cysts may predominate. The septa, cyst walls, and solid components enhance (Fig 20) (91,92). Subcapsular hemorrhage or renal vein invasion may be seen, but lymphadenopathy is absent (91,92). At MR imaging, the solid components are isointense to skeletal muscle. Hemorrhagic or proteinaceous content in the fluid component may appear bright on T1-weighted MR images (93). The mass shows the so-called triple sign of low, intermediate, and high signal intensity on T2-weighted MR images (94).
Treatment consists of radical nephrectomy and adjuvant chemotherapy. The tumor may respond to chemotherapy, but recurrence is common (5).
Conclusion
Renal tumors in older children and adolescents differ in histopathologic features and biologic behavior from those in younger children. Knowledge of the appropriate differential diagnoses and of some distinguishing clinical, histopathologic, and imaging features enables the radiologist to offer an appropriate differential diagnosis and influence proper treatment.
For this journal-based SA-CME activity, the authors, editor, and reviewers have disclosed no relevant relationships.
Supported by the American Institute for Radiologic Pathology, the Joint Pathology Center, and Uniformed Services University of the Health Sciences. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of Defense or the U.S. Government.
Abbreviations:
- DSRCT
- desmoplastic small round cell tumor
- H-E
- hematoxylin-eosin
- PNET
- primitive neuroectodermal tumor
- RCC
- renal cell carcinoma
- TSC
- tuberous sclerosis complex
- VHL
- von Hippel–Lindau
References
- 1.Chung EM, Graeber AR, Conran RM. Renal tumors of childhood: radiologic-pathologic correlation. I. The 1st decade. RadioGraphics 2016;36(2):499–522. [DOI] [PubMed] [Google Scholar]
- 2.Lowe LH, Isuani BH, Heller RM, et al. Pediatric renal masses: Wilms tumor and beyond. RadioGraphics 2000;20(6):1585–1603. [DOI] [PubMed] [Google Scholar]
- 3.Geller JI, Ehrlich PF, Cost NG, et al. Characterization of adolescent and pediatric renal cell carcinoma: a report from the Children’s Oncology Group study AREN03B2. Cancer 2015;121(14):2457–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perlman EJ. Pediatric renal cell carcinoma. Surg Pathol Clin 2010;3(3):641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eble JN, Sauter G, Epstein JI, Sesterhenn IA, eds. In: Pathology and genetics of tumours of the urinary system and male genital organs. Lyon, France: The International Agency for Research on Cancer, 2004. [Google Scholar]
- 6.Armah HB, Parwani AV. Xp11.2 translocation renal cell carcinoma. Arch Pathol Lab Med 2010;134(1):124–129. [DOI] [PubMed] [Google Scholar]
- 7.Arroyo MR, Green DM, Perlman EJ, Beckwith JB, Argani P. The spectrum of metanephric adenofibroma and related lesions: clinicopathologic study of 25 cases from the National Wilms Tumor Study Group Pathology Center. Am J Surg Pathol 2001;25(4):433–444. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Zhu Q, Li B, et al. Renal cell carcinoma associated with Xp11.2 translocation/TFE gene fusion: imaging findings in 21 patients. Eur Radiol 2017;27(2):543–552. [DOI] [PubMed] [Google Scholar]
- 9.Ross H, Argani P. Xp11 translocation renal cell carcinoma. Pathology 2010;42(4):369–373. [DOI] [PubMed] [Google Scholar]
- 10.Argani P, Lal P, Hutchinson B, Lui MY, Reuter VE, Ladanyi M. Aberrant nuclear immunoreactivity for TFE3 in neoplasms with TFE3 gene fusions: a sensitive and specific immunohistochemical assay. Am J Surg Pathol 2003;27(6):750–761. [DOI] [PubMed] [Google Scholar]
- 11.Argani P, Laé M, Ballard ET, et al. Translocation carcinomas of the kidney after chemotherapy in childhood. J Clin Oncol 2006;24(10):1529–1534. [DOI] [PubMed] [Google Scholar]
- 12.Liu K, Xie P, Peng W, Zhou Z. Renal carcinomas associated with Xp11.2 translocations/TFE3 gene fusions: findings on MRI and computed tomography imaging. J Magn Reson Imaging 2014;40(2):440–447. [DOI] [PubMed] [Google Scholar]
- 13.Wang W, Ding J, Li Y, et al. Magnetic resonance imaging and computed tomography characteristics of renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusion. PLoS One 2014;9(6):e99990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He J, Huan Y, Qiao Q, Zhang J, Zhang JS. Renal carcinomas associated with Xp11.2 translocations: are CT findings suggestive of the diagnosis? Clin Radiol 2014;69(1):45–51. [DOI] [PubMed] [Google Scholar]
- 15.Woo S, Kim SY, Lee MS, et al. MDCT findings of renal cell carcinoma associated with Xp11.2 translocation and TFE3 gene fusion and papillary renal cell carcinoma. AJR Am J Roentgenol 2015;204(3):542–549. [DOI] [PubMed] [Google Scholar]
- 16.Liang W, Xu S. Xp11.2 translocation renal cell carcinoma with egg-shell calcification mimicking a benign renal tumour: a case report. Oncol Lett 2015;10(5):3191–3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansouri D, Dimet S, Couanet D, et al. Renal cell carcinoma with an Xp11.2 translocation in a 16-year-old girl: a case report with cytological features. Diagn Cytopathol 2006;34(11):757–760. [DOI] [PubMed] [Google Scholar]
- 18.Kato H, Kanematsu M, Yokoi S, et al. Renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusion: radiological findings mimicking papillary subtype. J Magn Reson Imaging 2011;33(1):217–220. [DOI] [PubMed] [Google Scholar]
- 19.Koo HJ, Choi HJ, Kim MH, Cho KS. Radiologic-pathologic correlation of renal cell carcinoma associated with Xp11.2 translocation. Acta Radiol 2013;54(7):827–834. [DOI] [PubMed] [Google Scholar]
- 20.He J, Gan W, Liu S, et al. Dynamic computed tomographic features of adult renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusions: comparison with clear cell renal cell carcinoma. J Comput Assist Tomogr 2015;39(5):730–736. [DOI] [PubMed] [Google Scholar]
- 21.Zhu QQ, Wang ZQ, Zhu WR, Chen WX, Wu JT. The multislice CT findings of renal carcinoma associated with XP11.2 translocation/TFE gene fusion and collecting duct carcinoma. Acta Radiol 2013;54(3):355–362. [DOI] [PubMed] [Google Scholar]
- 22.Dang TT, Ziv E, Weinstein S, Meng MV, Wang Z, Coakley FV. Computed tomography and magnetic resonance imaging of adult renal cell carcinoma associated with Xp11.2 translocation. J Comput Assist Tomogr 2012;36(6):669–674. [DOI] [PubMed] [Google Scholar]
- 23.Geller E, Kochan PS. Renal neoplasms of childhood. Radiol Clin North Am 2011;49(4):689–709, vi. [DOI] [PubMed] [Google Scholar]
- 24.Gorin MA, Ball MW, Pierorazio PM, Argani P, Allaf ME. Partial nephrectomy for the treatment of translocation renal cell carcinoma. Clin Genitourin Cancer 2015;13(3):e199–e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choyke PL. Imaging of hereditary renal cancer. Radiol Clin North Am 2003;41(5):1037–1051. [DOI] [PubMed] [Google Scholar]
- 26.Lack EE, Cassady JR, Sallan SE. Renal cell carcinoma in childhood and adolescence: a clinical and pathological study of 17 cases. J Urol 1985;133(5):822–828. [DOI] [PubMed] [Google Scholar]
- 27.Kim JK, Kim TK, Ahn HJ, Kim CS, Kim KR, Cho KS. Differentiation of subtypes of renal cell carcinoma on helical CT scans. AJR Am J Roentgenol 2002;178(6):1499–1506. [DOI] [PubMed] [Google Scholar]
- 28.Lee SH, Choi YH, Kim WS, Cheon JE, Moon KC. Ossifying renal tumor of infancy: findings at ultrasound, CT and MRI. Pediatr Radiol 2014;44(5):625–628. [DOI] [PubMed] [Google Scholar]
- 29.Blitman NM, Berkenblit RG, Rozenblit AM, Levin TL. Renal medullary carcinoma: CT and MRI features. AJR Am J Roentgenol 2005;185(1):268–272. [DOI] [PubMed] [Google Scholar]
- 30.Murphy WM, Grignon DJ, Perlman EJ. Kidney tumors in children. In: Tumors of the kidney, bladder, and related urinary structures. Washington, DC: American Registry of Pathology, 2004; 1–99. [Google Scholar]
- 31.Davis CJ, Jr, Mostofi FK, Sesterhenn IA. Renal medullary carcinoma: the seventh sickle cell nephropathy. Am J Surg Pathol 1995;19(1):1–11. [DOI] [PubMed] [Google Scholar]
- 32.Prasad SR, Humphrey PA, Menias CO, et al. Neoplasms of the renal medulla: radiologic-pathologic correlation. RadioGraphics 2005;25(2):369–380. [DOI] [PubMed] [Google Scholar]
- 33.Hakimi AA, Koi PT, Milhoua PM, et al. Renal medullary carcinoma: the Bronx experience. Urology 2007;70(5):878–882. [DOI] [PubMed] [Google Scholar]
- 34.Dimashkieh H, Choe J, Mutema G. Renal medullary carcinoma: a report of 2 cases and review of the literature. Arch Pathol Lab Med 2003;127(3):e135–e138. [DOI] [PubMed] [Google Scholar]
- 35.Davidson AJ, Choyke PL, Hartman DS, Davis CJ, Jr. Renal medullary carcinoma associated with sickle cell trait: radiologic findings. Radiology 1995;195(1):83–85. [DOI] [PubMed] [Google Scholar]
- 36.Khan A, Thomas N, Costello B, et al. Renal medullary carcinoma: sonographic, computed tomography, magnetic resonance and angiographic findings. Eur J Radiol 2000;35(1):1–7. [DOI] [PubMed] [Google Scholar]
- 37.Pickhardt PJ, Lonergan GJ, Davis CJ, Jr, Kashitani N, Wagner BJ. From the archives of the AFIP: infiltrative renal lesions— radiologic-pathologic correlation. RadioGraphics 2000;20(1):215–243. [DOI] [PubMed] [Google Scholar]
- 38.Shi Z, Zhuang Q, You R, Li Y, Li J, Cao D. Clinical and computed tomography imaging features of renal medullary carcinoma: a report of six cases. Oncol Lett 2016;11(1):261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martignoni G, Pea M, Zampini C, et al. PEComas of the kidney and of the genitourinary tract. Semin Diagn Pathol 2015;32(2):140–159. [DOI] [PubMed] [Google Scholar]
- 40.Casper KA, Donnelly LF, Chen B, Bissler JJ. Tuberous sclerosis complex: renal imaging findings. Radiology 2002;225(2):451–456. [DOI] [PubMed] [Google Scholar]
- 41.Castagnetti M, Vezzù B, Laverda A, Zampieri S, Rigamonti W. Urological counseling and followup in pediatric tuberous sclerosis complex. J Urol 2007;178(5):2155–2159. [DOI] [PubMed] [Google Scholar]
- 42.Ewalt DH, Sheffield E, Sparagana SP, Delgado MR, Roach ES. Renal lesion growth in children with tuberous sclerosis complex. J Urol 1998;160(1):141–145. [PubMed] [Google Scholar]
- 43.Winterkorn EB, Daouk GH, Anupindi S, Thiele EA. Tuberous sclerosis complex and renal angiomyolipoma: case report and review of the literature. Pediatr Nephrol 2006;21(8):1189–1193. [DOI] [PubMed] [Google Scholar]
- 44.Kennelly MJ, Grossman HB, Cho KJ. Outcome analysis of 42 cases of renal angiomyolipoma. J Urol 1994;152(6 pt 1):1988–1991. [DOI] [PubMed] [Google Scholar]
- 45.Thway K, Fisher C. PEComa: morphology and genetics of a complex tumor family. Ann Diagn Pathol 2015;19(5): 359–368. [DOI] [PubMed] [Google Scholar]
- 46.Schieda N, Kielar AZ, Al Dandan O, McInnes MD, Flood TA. Ten uncommon and unusual variants of renal angiomyolipoma (AML): radiologic-pathologic correlation. Clin Radiol 2015;70(2):206–220. [DOI] [PubMed] [Google Scholar]
- 47.Gee MS, Bittman M, Epelman M, Vargas SO, Lee EY. Magnetic resonance imaging of the pediatric kidney: benign and malignant masses. Magn Reson Imaging Clin N Am 2013;21(4):697–715. [DOI] [PubMed] [Google Scholar]
- 48.Pedrosa I, Sun MR, Spencer M, et al. MR imaging of renal masses: correlation with findings at surgery and pathologic analysis. RadioGraphics 2008;28(4):985–1003. [DOI] [PubMed] [Google Scholar]
- 49.Tirumani SH, Shinagare AB, Hargreaves J, et al. Imaging features of primary and metastatic malignant perivascular epithelioid cell tumors. AJR Am J Roentgenol 2014;202(2):252–258. [DOI] [PubMed] [Google Scholar]
- 50.Krueger DA, Northrup H; International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex surveillance and management: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol 2013;49(4):255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Renshaw AA, Freyer DR, Hammers YA. Metastatic metanephric adenoma in a child. Am J Surg Pathol 2000;24(4):570–574. [DOI] [PubMed] [Google Scholar]
- 52.Davis CJ, Jr, Barton JH, Sesterhenn IA, Mostofi FK. Metanephric adenoma: clinicopathological study of fifty patients. Am J Surg Pathol 1995;19(10):1101–1114. [DOI] [PubMed] [Google Scholar]
- 53.Navarro O, Conolly B, Taylor G, Bägli DJ. Metanephric adenoma of the kidney: a case report. Pediatr Radiol 1999;29(2):100–103. [DOI] [PubMed] [Google Scholar]
- 54.Argani P, Beckwith JB. Metanephric stromal tumor: report of 31 cases of a distinctive pediatric renal neoplasm. Am J Surg Pathol 2000;24(7):917–926. [DOI] [PubMed] [Google Scholar]
- 55.Bastide C, Rambeaud JJ, Bach AM, Russo P. Metanephric adenoma of the kidney: clinical and radiological study of nine cases. BJU Int 2009;103(11):1544–1548. [DOI] [PubMed] [Google Scholar]
- 56.Amodio JB, Shapiro E, Pinkney L, et al. Metanephric adenoma in an 8-year-old child: case report and review of the literature. J Pediatr Surg 2005;40(5):e25–e28. [DOI] [PubMed] [Google Scholar]
- 57.Bastos Netto JM, Esteves TC, Mattos RD, Tibiriçá SH, Costa SM, Vieira LJ. Metanephric adenoma: a rare differential diagnosis of renal tumor in children. J Pediatr Urol 2007;3(4):340–341. [DOI] [PubMed] [Google Scholar]
- 58.Chaudhary H, Raghvendran M, Dubey D, et al. Correlation of radiological and clinical features of metanephric neoplasms in adults. Indian J Cancer 2004;41(1):37–40. [PubMed] [Google Scholar]
- 59.Fielding JR, Visweswaran A, Silverman SG, Granter SR, Renshaw AA. CT and ultrasound features of metanephric adenoma in adults with pathologic correlation. J Comput Assist Tomogr 1999;23(3):441–444. [DOI] [PubMed] [Google Scholar]
- 60.Le Nué R, Marcellin L, Ripepi M, Henry C, Kretz JM, Geiss S. Conservative treatment of metanephric adenoma: a case report and review of the literature. J Pediatr Urol 2011;7(4):399–403. [DOI] [PubMed] [Google Scholar]
- 61.Spaner SJ, Yu Y, Cook AJ, Boag G. Pediatric metanephric adenoma: case report and review of the literature. Int Urol Nephrol 2014;46(4):677–680. [DOI] [PubMed] [Google Scholar]
- 62.Bajaj SK, Misra R, Batra V, Gupta R, Bagga D. Metanephric stromal tumor: an unusual pediatric renal neoplasm. J Indian Assoc Pediatr Surg 2013;18(3):115–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Comerci SC, Levin TL, Ruzal-Shapiro C, et al. Benign adenomatous kidney neoplasms in children with polycythemia: imaging findings. Radiology 1996;198(1):265–268. [DOI] [PubMed] [Google Scholar]
- 64.Araki T, Hata H, Asakawa E, Araki T. MRI of metanephric adenoma. J Comput Assist Tomogr 1998;22(1):87–90. [DOI] [PubMed] [Google Scholar]
- 65.Hu YC, Wu L, Yan LF, Zhang W, Cui GB. The imaging features of metanephric adenoma: a case report and review of literature. Onco Targets Ther 2015;8:445–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liniger B, Wolf RW, Fleischmann A, Kluwe W. Local resection of metanephric adenoma with kidney preservation. J Pediatr Surg 2009;44(8):E21–E23. [DOI] [PubMed] [Google Scholar]
- 67.Torricelli FC, Marchini GS, Campos RS, Gil AO. Metanephric adenoma: clinical, imaging, and histological findings. Clinics (Sao Paulo) 2011;66(2):359–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McHugh K. Renal and adrenal tumours in children. Cancer Imaging 2007;7:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sheth S, Ali S, Fishman E. Imaging of renal lymphoma: patterns of disease with pathologic correlation. RadioGraphics 2006;26(4):1151–1168. [DOI] [PubMed] [Google Scholar]
- 70.Siegel MJ, Chung EM. Wilms’ tumor and other pediatric renal masses. Magn Reson Imaging Clin N Am 2008;16(3):479–497, vi. [DOI] [PubMed] [Google Scholar]
- 71.Royer-Pokora B. Genetics of pediatric renal tumors. Pediatr Nephrol 2013;28(1):13–23. [DOI] [PubMed] [Google Scholar]
- 72.Sellaturay SV, Arya M, Cuckow P, Anderson J, McHugh K, Sebire NJ. Renal primitive neuroectodermal tumor in childhood with intracardiac extension. Urology 2006;68(2):427.e13–427.e16. [DOI] [PubMed] [Google Scholar]
- 73.Thyavihally YB, Tongaonkar HB, Gupta S, et al. Primitive neuroectodermal tumor of the kidney: a single institute series of 16 patients. Urology 2008;71(2):292–296. [DOI] [PubMed] [Google Scholar]
- 74.Zöllner S, Dirksen U, Jürgens H, Ranft A. Renal Ewing tumors. Ann Oncol 2013;24(9):2455–2461. [DOI] [PubMed] [Google Scholar]
- 75.Ellinger J, Bastian PJ, Hauser S, Biermann K, Müller SC. Primitive neuroectodermal tumor: rare, highly aggressive differential diagnosis in urologic malignancies. Urology 2006;68(2):257–262. [DOI] [PubMed] [Google Scholar]
- 76.Wu CF, Wang LJ, Chen CS. Images in clinical urology: magnetic resonance imaging of primitive neuroectodermal tumor of the kidney. Urology 2000;55(2):284–285. [DOI] [PubMed] [Google Scholar]
- 77.Iyer RS, Schaunaman G, Pruthi S, Finn LS. Imaging of pediatric desmoplastic small-round-cell tumor with pathologic correlation. Curr Probl Diagn Radiol 2013;42(1):26–32. [DOI] [PubMed] [Google Scholar]
- 78.Arnold MA, Schoenfield L, Limketkai BN, Arnold CA. Diagnostic pitfalls of differentiating desmoplastic small round cell tumor (DSRCT) from Wilms tumor (WT): overlapping morphologic and immunohistochemical features. Am J Surg Pathol 2014;38(9):1220–1226. [DOI] [PubMed] [Google Scholar]
- 79.da Silva RC, Medeiros Filho P, Chioato L, Silva TR, Ribeiro SM, Bacchi CE. Desmoplastic small round cell tumor of the kidney mimicking Wilms tumor: a case report and review of the literature. Appl Immunohistochem Mol Morphol 2009;17(6):557–562. [DOI] [PubMed] [Google Scholar]
- 80.Egloff AM, Lee EY, Dillon JE, Callahan MJ. Desmoplastic small round cell tumor of the kidney in a pediatric patient: sonographic and multiphase CT findings. AJR Am J Roentgenol 2005;185(5):1347–1349. [DOI] [PubMed] [Google Scholar]
- 81.Eklund MJ, Cundiff C, Shehata BM, Alazraki AL. Desmoplastic small round cell tumor of the kidney with unusual imaging features. Clin Imaging 2015;39(5):904–907. [DOI] [PubMed] [Google Scholar]
- 82.Rao P, Tamboli P, Fillman EP, Meis JM. Primary intra-renal desmoplastic small round cell tumor: expanding the histologic spectrum, with special emphasis on the differential diagnostic considerations. Pathol Res Pract 2014;210(12):1130–1133. [DOI] [PubMed] [Google Scholar]
- 83.Collardeau-Frachon S, Ranchère-Vince D, Delattre O, et al. Primary desmoplastic small round cell tumor of the kidney: a case report in a 14-year-old girl with molecular confirmation. Pediatr Dev Pathol 2007;10(4):320–324. [DOI] [PubMed] [Google Scholar]
- 84.Wang LL, Perlman EJ, Vujanic GM, et al. Desmoplastic small round cell tumor of the kidney in childhood. Am J Surg Pathol 2007;31(4):576–584. [DOI] [PubMed] [Google Scholar]
- 85.Ordóñez NG. Desmoplastic small round cell tumor. I. A histopathologic study of 39 cases with emphasis on unusual histological patterns. Am J Surg Pathol 1998;22(11):1303–1313. [DOI] [PubMed] [Google Scholar]
- 86.Lae ME, Roche PC, Jin L, Lloyd RV, Nascimento AG. Desmoplastic small round cell tumor: a clinicopathologic, immunohistochemical, and molecular study of 32 tumors. Am J Surg Pathol 2002;26(7):823–835. [DOI] [PubMed] [Google Scholar]
- 87.Sanchez TR, Ducore J, Balagtas J, Molloy C, Wootton-Gorges SL. WARM N COLD: malignant and benign renal tumors in children. Emerg Radiol 2014;21(3):261–269. [DOI] [PubMed] [Google Scholar]
- 88.Chung EM, Biko DM, Arzamendi AM, Meldrum JT, Stocker JT. Solid tumors of the peritoneum, omentum, and mesentery in children: radiologic-pathologic correlation— from the radiologic pathology archives. RadioGraphics 2015;35(2):521–546. [DOI] [PubMed] [Google Scholar]
- 89.Ostermeier A, McCarville MB, Navid F, Snyder SE, Shulkin BL. FDG PET/CT imaging of desmoplastic small round cell tumor: findings at staging, during treatment and at follow-up. Pediatr Radiol 2015;45(9):1308–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Argani P, Faria PA, Epstein JI, et al. Primary renal synovial sarcoma: molecular and morphologic delineation of an entity previously included among embryonal sarcomas of the kidney. Am J Surg Pathol 2000;24(8):1087–1096. [DOI] [PubMed] [Google Scholar]
- 91.Gong J, Kang W, Li S, Yang Z, Xu J. CT findings of synovial sarcomas of the kidney with pathological correlation. Clin Imaging 2013;37(6):1033–1036. [DOI] [PubMed] [Google Scholar]
- 92.Lv XF, Qiu YW, Han LJ, et al. Primary renal synovial sarcoma: computed tomography imaging findings. Acta Radiol 2015;56(4):493–499. [DOI] [PubMed] [Google Scholar]
- 93.Karaosmanoğlu AD, Onur MR, Shirkhoda A, Ozmen M, Hahn PF. Unusual malignant solid neoplasms of the kidney: cross-sectional imaging findings. Korean J Radiol 2015;16(4):853–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zakhary MM, Elsayes KM, Platt JF, Francis IR. Magnetic resonance imaging features of renal synovial sarcoma: a case report. Cancer Imaging 2008;8:45–47. [DOI] [PMC free article] [PubMed] [Google Scholar]