This article provides a comprehensive review of the thoracic complications of precision cancer therapies, with an emphasis on emerging challenges resulting from novel therapies such as immune checkpoint inhibitor therapies, and serves as a practical reference guide for radiologists.
Abstract
Recent advances in understanding the molecular mechanisms of cancer have opened a new era of precision medicine for cancer treatment. Precision cancer therapies target specific molecules that are responsible for cancer development and progression, and they achieve marked treatment benefits in specific cohorts of patients. However, these therapies are also associated with a variety of complications that are often unique to specific groups of anticancer agents. The rapidly increasing use of immune checkpoint inhibitors in the treatment of various advanced malignancies has brought new challenges in diagnosing and monitoring a unique set of toxic effects termed immune-related adverse events. Familiarity with cutting-edge cancer treatment approaches and awareness of the emerging complications from novel therapies are essential for radiologists, who play a key role in the care of patients with cancer. This article provides a comprehensive review of the thoracic complications of precision cancer therapies, describes their imaging features and clinical characteristics, and discusses the role of radiologists in the diagnosis and monitoring of these entities. The authors also address the molecular mechanisms of anticancer agents that relate to thoracic complications and emphasize emerging challenges in novel cancer therapies. This article is designed to serve as a practical reference guide for day-to-day practice for radiologists in the era of precision cancer medicine.
©RSNA, 2017
SA-CME LEARNING OBJECTIVES
After completing this journal-based SA-CME activity, participants will be able to:
■ Recognize the characteristic radiologic manifestations of thoracic complications of precision cancer therapies.
■ Describe the molecular mechanisms of representative anticancer agents used in precision therapies that relate to thoracic complications.
■ Discuss the role of radiologists in detecting and monitoring thoracic complications in the era of precision medicine for cancer.
Introduction
Precision medicine approaches in oncology have brought a paradigm shift to the treatment of advanced cancers, leading to significant treatment benefits in specific cohorts of patients. However, novel agents used in precision cancer therapies are associated with a variety of thoracic complications and adverse events, which provide new challenges in clinical practice because of the rapidly increasing use of precision approaches to cancer. Imaging is a key component of assessing tumor response and evaluating adverse events during precision cancer therapies, and radiologists play a major role in detecting and monitoring adverse events at the front line of care of patients with cancer. Therefore, it is essential for radiologists to be aware of novel therapeutic agents that are increasingly being used in clinical oncology practice and to recognize radiologic manifestations of complications related to these agents (Table).
Thoracic Complications and Adverse Events Related to Precision Cancer Therapies
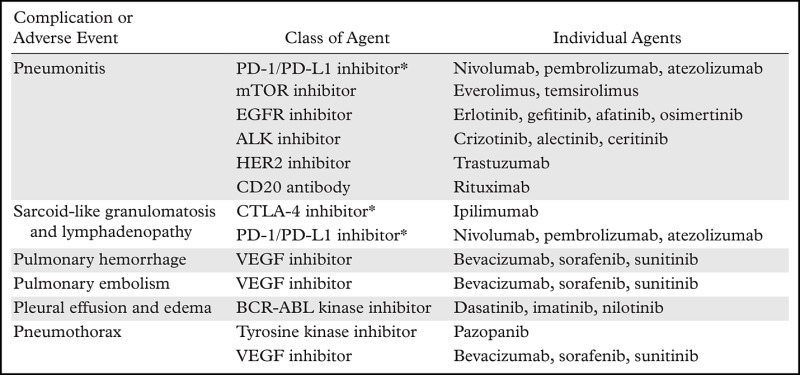
*Programmed cell death protein 1 (PD-1), programmed cell death ligand 1 (PD-L1), and cytotoxic T-lymphocyte antigen–4 (CTLA-4) inhibitors are immune checkpoint inhibitors that are associated with immune-related adverse events (irAEs).
Note.—ALK = anaplastic lymphoma kinase, EGFR = epidermal growth factor receptor, HER2 = human epidermal growth factor receptor 2, mTOR = mammalian target of rapamycin, VEGF = vascular endothelial growth factor.
This article provides a comprehensive review of thoracic complications and adverse events of precision cancer therapies, including immune checkpoint inhibitor therapy and molecular targeting therapy, and emphasizes emerging challenges in patients treated with these novel therapies. It also describes the molecular mechanisms of action for representative agents to enhance understanding and presents the radiologic features of each category of adverse events. The article is designed to serve as a practical reference guide for the day-to-day practice of thoracic radiology in the era of precision cancer therapy.
Drug-related Pneumonitis during Precision Cancer Therapies
Drug-related pneumonitis in patients treated with precision cancer therapies presents clinical challenges to cancer care providers, including radiologists, especially with the increasing use of novel molecular targeting agents and immunotherapeutic agents. A number of anticancer agents that are based on molecular targeting mechanisms or immune checkpoint inhibition have been approved for clinical use in the United States (Table), and even newer agents are in the pipeline for development and clinical testing. Drug-related pneumonitis during cancer therapy can be due to cytotoxic injuries, oxidative stress, or immune-mediated effects and represents a manifestation of the lung’s responses to these injuries. The lung’s response patterns to these injuries are often limited and show several types of histologic manifestations, with corresponding imaging patterns noted on computed tomographic (CT) images (1–3). Prior studies have described characterization of the radiologic patterns of drug-related pneumonitis according to the classification of interstitial pneumonias and related lung diseases, which is also applicable to pneumonitis related to novel precision therapies and provides important clues to accurate diagnosis in patients treated with these therapies (2,4–7).
We first discuss pneumonitis related to immune checkpoint inhibitors because it is an emerging adverse event with special clinical significance. Recent regulatory approval of a number of these agents for treatment of various tumors is rapidly accelerating their usage in the clinical setting. Next, we discuss pneumonitis associated with representative molecular targeting agents used in the clinical setting, including mTOR inhibitors, EGFR inhibitors, ALK inhibitors, HER2 inhibitors, and anti-CD20 antibodies.
Pneumonitis during Immune Checkpoint Inhibitor Therapy
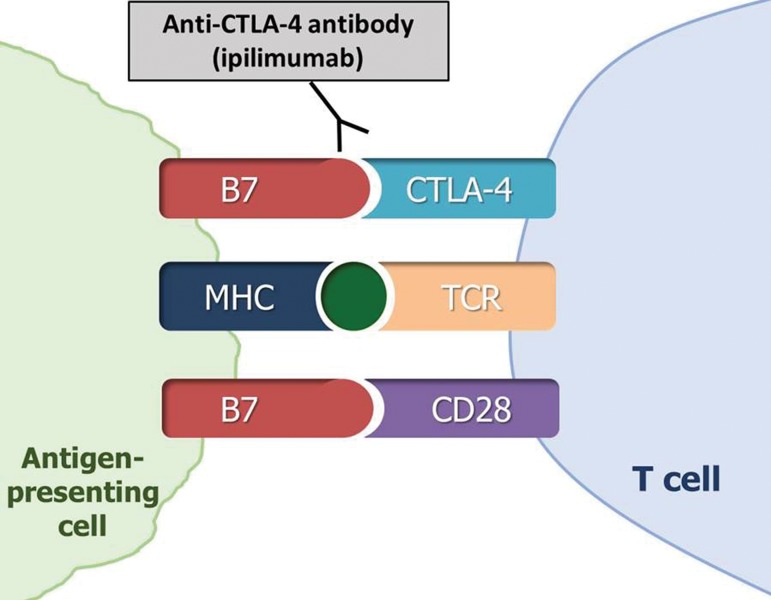
The anticancer activity of immune checkpoint inhibitor therapy occurs through the blockade of immune inhibition by tumors, which activates T-cell–mediated immune responses against tumors. In the tumor microenvironment, T-cell activation specific to cancer cells is regulated by the ligand-receptor pairs (known as immune checkpoints) among T cells, tumor cells, and other immune cells (8–14). Inhibitors of these immune checkpoint molecules, which include CTLA-4, PD-1, and PD-L1, have thus emerged as a promising treatment option for advanced malignancy (Fig 1). Some of these novel immune checkpoint inhibitors have shown marked efficacy in trials and have been approved for clinical use by the U.S. Food and Drug Administration (FDA). Currently approved agents include ipilimumab (a CTLA-4 inhibitor) for melanoma; nivolumab (a PD-1 inhibitor) for melanoma, non–small cell lung cancer (NSCLC), renal cell carcinoma, urothelial carcinoma, and Hodgkin lymphoma; pembrolizumab (a PD-1 inhibitor) for melanoma, NSCLC, and head and neck squamous cell carcinomas; and atezolizumab (a PD-L1 inhibitor) for urothelial carcinoma and NSCLC. The use of these agents is rapidly expanding in clinical oncology practice, and other new agents are currently being tested in clinical trials (8).
Figure 1a.
Diagram of immune checkpoint inhibitor therapy shows immune inhibition by tumors and its blockade as the mechanism of action. MHC = major histocompatibility complex, TCR = T-cell receptor. (a) CTLA-4 is an immune checkpoint molecule on T cells, and its interaction with the ligand B7 on antigen-presenting cells causes inhibition of the T-cell immune response against the tumor, which allows the tumor cells to evade immune attack. CTLA-4 inhibitors such as ipilimumab prevent this interaction by binding to CTLA-4 on T cells and blocking T-cell immune inhibition, thereby activating an immune response against tumor cells. (b) The binding of PD-1 on effector T cells and of its ligand PD-L1 on tumor cells delivers an inhibitory signal that decreases cytokine production and T-cell proliferation, which allows tumor cells to escape from immune response. PD-1 or PD-L1 inhibitors block the binding and prevent tumoral immune inhibition, thus inducing an antitumor immune response. PD-1/PD-L1 blockade by agents such as nivolumab, pembrolizumab, atezolizumab, and durvalumab has become a major treatment option for many types of advanced cancers. (Adapted and reprinted under a CC BY 4.0 license from reference 8.)
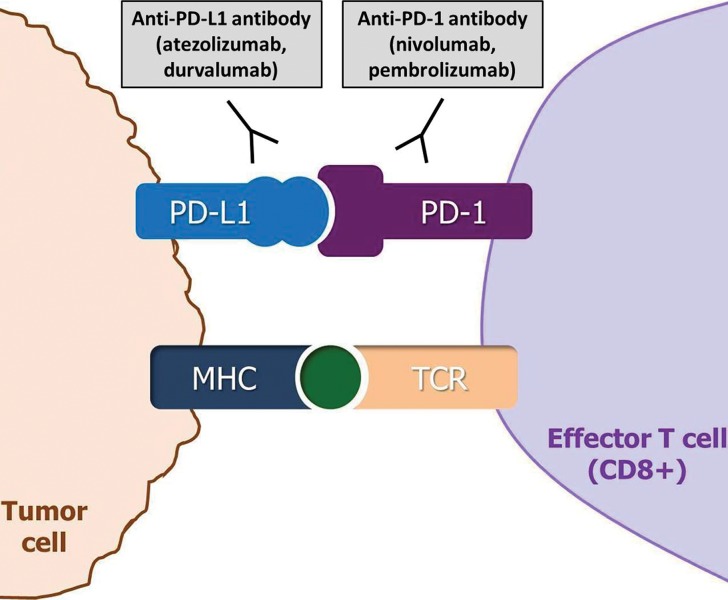
Figure 1b.
Diagram of immune checkpoint inhibitor therapy shows immune inhibition by tumors and its blockade as the mechanism of action. MHC = major histocompatibility complex, TCR = T-cell receptor. (a) CTLA-4 is an immune checkpoint molecule on T cells, and its interaction with the ligand B7 on antigen-presenting cells causes inhibition of the T-cell immune response against the tumor, which allows the tumor cells to evade immune attack. CTLA-4 inhibitors such as ipilimumab prevent this interaction by binding to CTLA-4 on T cells and blocking T-cell immune inhibition, thereby activating an immune response against tumor cells. (b) The binding of PD-1 on effector T cells and of its ligand PD-L1 on tumor cells delivers an inhibitory signal that decreases cytokine production and T-cell proliferation, which allows tumor cells to escape from immune response. PD-1 or PD-L1 inhibitors block the binding and prevent tumoral immune inhibition, thus inducing an antitumor immune response. PD-1/PD-L1 blockade by agents such as nivolumab, pembrolizumab, atezolizumab, and durvalumab has become a major treatment option for many types of advanced cancers. (Adapted and reprinted under a CC BY 4.0 license from reference 8.)
A unique set of complications related to immune checkpoint inhibitors, termed irAEs, has been recognized that can involve organs from head to toe with a variety of clinical and radiologic manifestations (8,15,16). Examples of irAEs include hypophysitis, thyroiditis, pneumonitis, hepatitis, pancreatitis, and colitis, to name a few. Among these, pneumonitis is a relatively rare but clinically serious and potentially lethal event, with several pneumonitis-related deaths reported in early trials of these agents. It therefore has been recognized as an “event of special interest” among oncology providers (17–19). In a recent meta-analysis of 20 published trials of the PD-1 inhibitors nivolumab and pembrolizumab, the incidence rate of pneumonitis was 2.7% for single-agent therapy and 6.6% for combination therapy (20). Of the tumor types NSCLC, renal cell carcinoma, and melanoma, NSCLC had higher incidence rates for both all-grade and high-grade (grade 3 or above) pneumonitis, and renal cell carcinoma had a higher incidence rate for all-grade pneumonitis, when compared with melanoma (20).
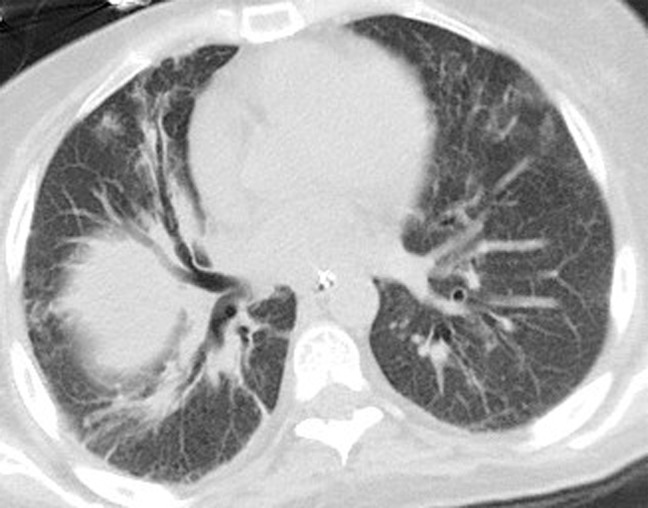
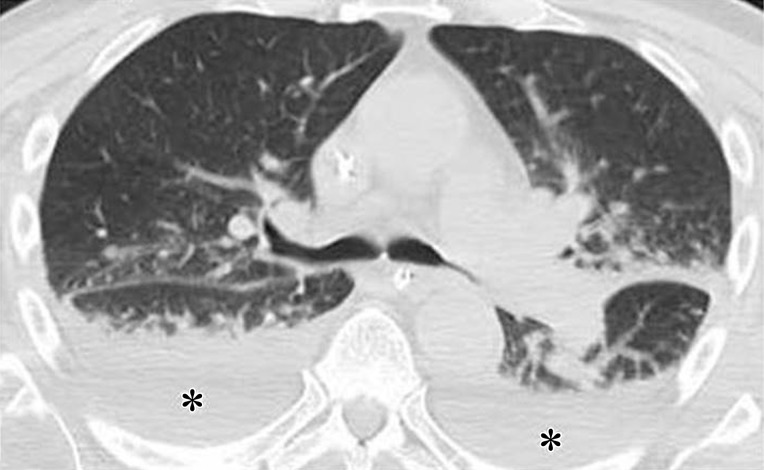
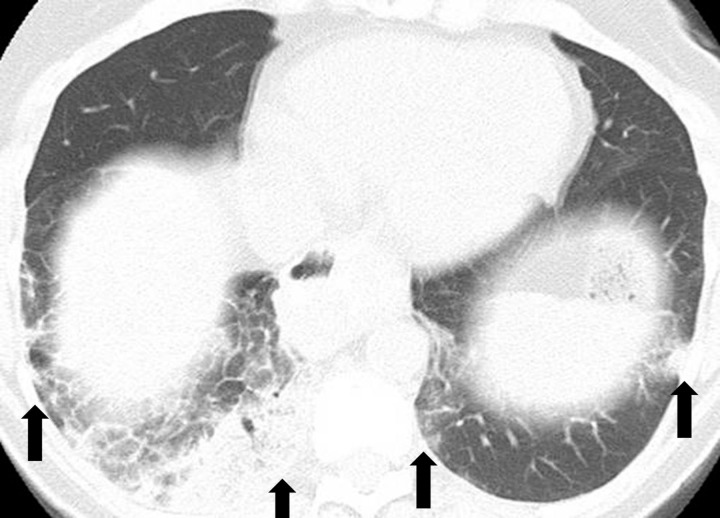
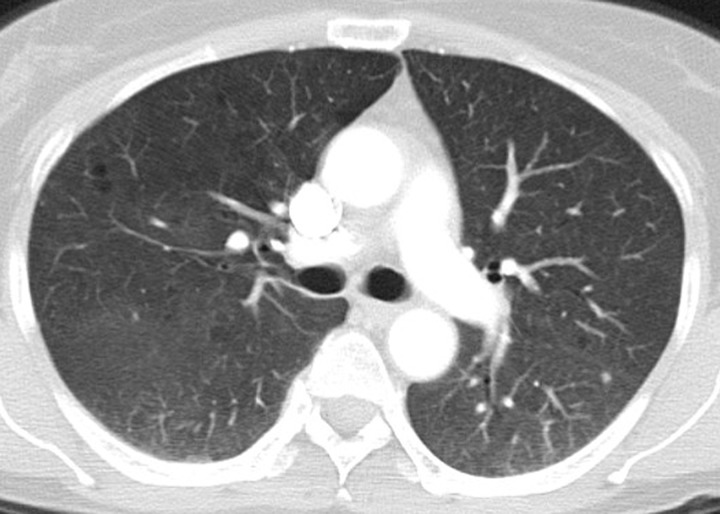
Initial reports of immune checkpoint inhibitor–related pneumonitis indicated a wide spectrum of clinical and imaging manifestations (21,22). Some patients had a fulminant clinical course, with rapidly worsening respiratory symptoms that required intensive care unit admission and intubation. These patients presented with chest CT findings of diffuse ground-glass opacities (GGOs), consolidation, and lung volume loss that were indicative of an acute interstitial pneumonia (AIP)/acute respiratory distress syndrome (ARDS) pattern (Figs 2, 3) (22). Others had a milder clinical course and imaging findings indicative of a nonspecific interstitial pneumonia (NSIP) pattern, with subtle GGOs in a peripheral and basilar distribution (Fig 4); these patients were often successfully treated with corticosteroid therapy on an outpatient basis, and some were able to restart PD-1 inhibitor therapy without recurrent pneumonitis (7,22). Additional radiologic patterns were also noted in subsequent reports, including cryptogenic organizing pneumonia (COP) and hypersensitivity pneumonitis patterns. The COP pattern typically manifests with multifocal consolidation and GGOs in a predominantly peripheral distribution (Fig 5). The hypersensitivity pneumonitis pattern manifests with diffuse GGOs and centrilobular nodularities and may also include air trapping (Fig 6) (7,21,23–28).
Figure 2a.
PD-1 inhibitor pneumonitis: AIP/ARDS pattern in a 38-year-old woman with advanced melanoma who was treated with nivolumab. Axial (a) and coronal (b) chest CT images obtained at 15 weeks of therapy show diffuse GGOs and traction bronchiectasis, with markedly decreased lung volumes seen on the coronal image and an elevated right hemidiaphragm, findings indicative of a radiologic AIP/ARDS pattern. The patient was admitted to the intensive care unit, was treated with intravenous corticosteroids, and also required infliximab (an anti–tumor necrosis factor-α immunosuppressive agent) therapy.
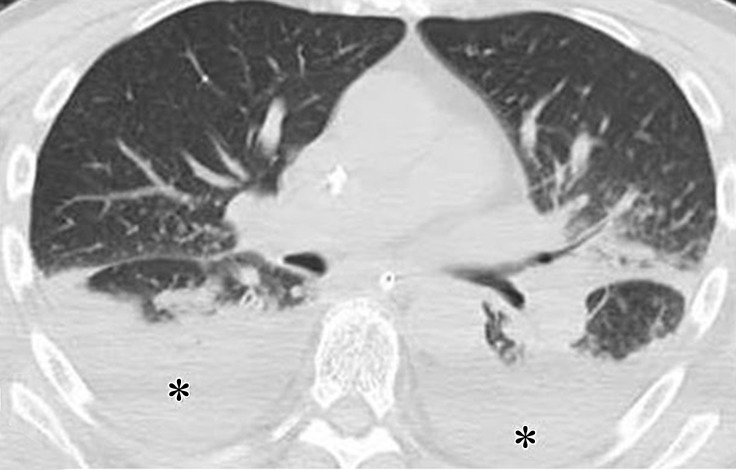
Figure 3a.
Pneumonitis with AIP/ARDS pattern in a 70-year-old man with melanoma who was treated with sequentially administered nivolumab and ipilimumab combination therapy. Axial chest CT images obtained at 5.6 months of therapy (a at the level of the carina; b at a lower level than a) show GGOs, reticular opacities, consolidation, and traction bronchiectasis, as well as pleural effusions (*) involving both lungs. (Figure reprinted from reference 7.)
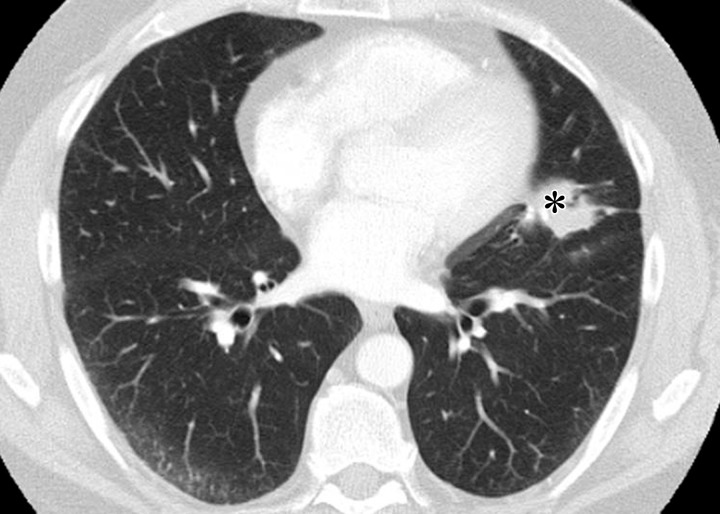
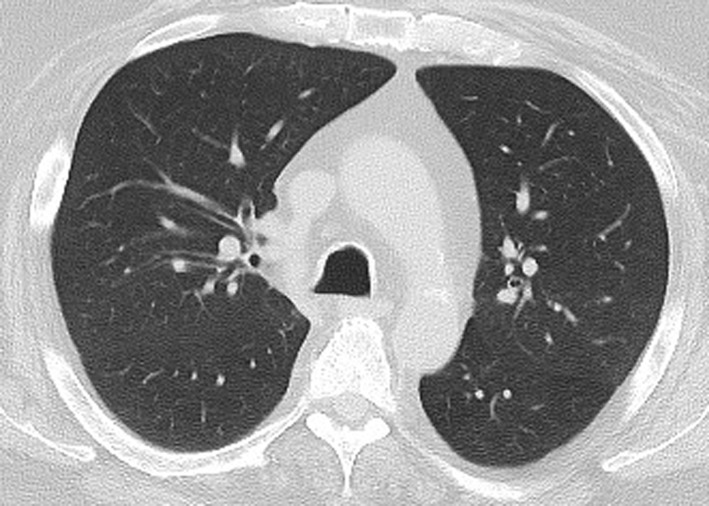
Figure 4.
PD-1 inhibitor pneumonitis: NSIP pattern in a 58-year-old man with advanced melanoma who was treated with nivolumab. Axial chest CT image obtained at 7 weeks of therapy shows GGOs and reticular opacities in a subpleural distribution, representing an NSIP pattern of PD-1 inhibitor pneumonitis. * = metastatic lesion in the lung.
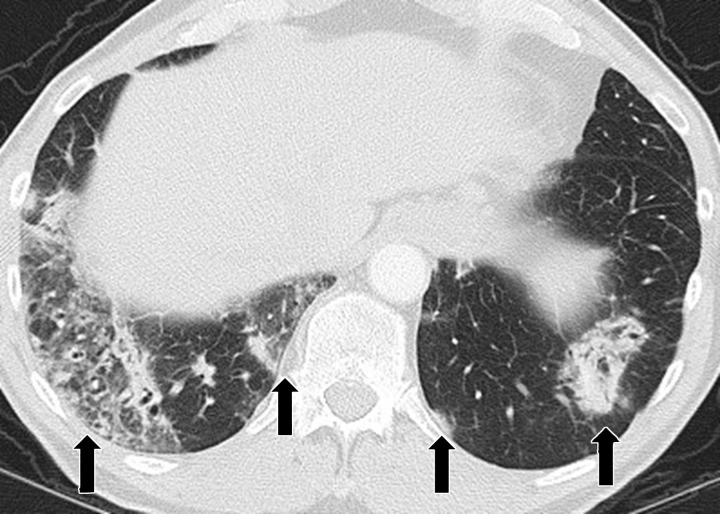
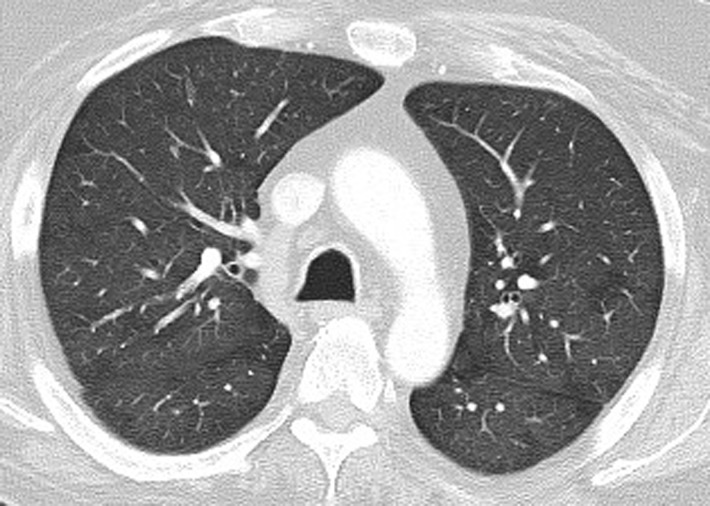
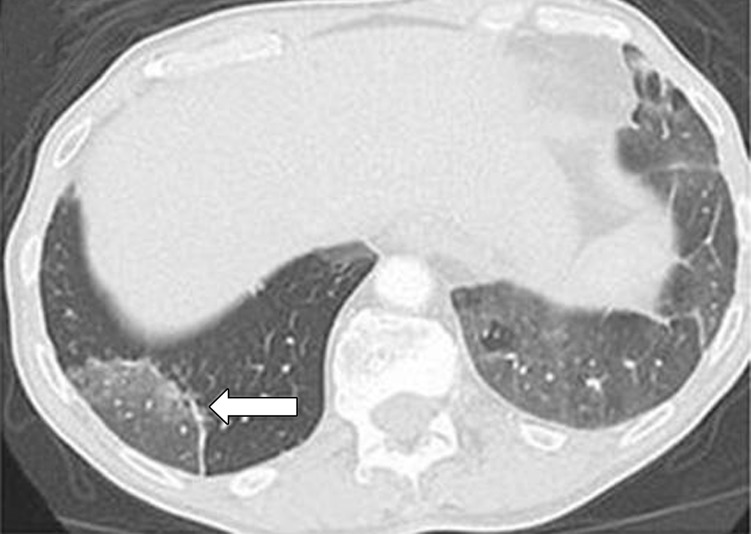
Figure 5.
PD-1 inhibitor pneumonitis: COP pattern in a 69-year-old man with advanced NSCLC who was treated with nivolumab. At 6 months of therapy, the patient presented with increased shortness of breath and cough, without fever. Axial chest CT image shows multifocal areas of consolidation and GGOs in a predominantly peripheral and basilar distribution (arrows), representing a COP pattern of PD-1 inhibitor–related pneumonitis. Bronchial dilatation was noted within the areas of consolidation. Nivolumab was withheld, and the patient was treated with corticosteroids, with subsequent improvement.
Figure 6a.
PD-1 inhibitor pneumonitis: Hypersensitivity pneumonitis pattern in a 68-year-old man with metastatic renal cell carcinoma who was treated with nivolumab and presented with a new cough at 6 months of therapy. (a) Axial chest CT image shows new multifocal GGOs in a centrilobular distribution throughout both lungs and mosaic attenuation, findings that represent pneumonitis with a hypersensitivity pneumonitis pattern. Nivolumab therapy was withheld, and the patient underwent corticosteroid therapy. (b) Axial follow-up CT image obtained after 1 month of corticosteroid therapy shows marked improvement of pneumonitis and resolution of GGOs. Nivolumab continued to be withheld, and corticosteroid therapy was tapered. At 1.5 months after completing the corticosteroid taper, without restarting nivolumab or any other systemic therapy, the patient experienced a worsening cough. (c) Axial chest CT image shows development of diffuse GGOs with areas of air trapping, findings indicative of pneumonitis with a hypersensitivy pneumonitis pattern. The radiographic pattern of this second episode is similar to that noted in the initial episode and represents pneumonitis flare.
Figure 2b.
PD-1 inhibitor pneumonitis: AIP/ARDS pattern in a 38-year-old woman with advanced melanoma who was treated with nivolumab. Axial (a) and coronal (b) chest CT images obtained at 15 weeks of therapy show diffuse GGOs and traction bronchiectasis, with markedly decreased lung volumes seen on the coronal image and an elevated right hemidiaphragm, findings indicative of a radiologic AIP/ARDS pattern. The patient was admitted to the intensive care unit, was treated with intravenous corticosteroids, and also required infliximab (an anti–tumor necrosis factor-α immunosuppressive agent) therapy.
Figure 3b.
Pneumonitis with AIP/ARDS pattern in a 70-year-old man with melanoma who was treated with sequentially administered nivolumab and ipilimumab combination therapy. Axial chest CT images obtained at 5.6 months of therapy (a at the level of the carina; b at a lower level than a) show GGOs, reticular opacities, consolidation, and traction bronchiectasis, as well as pleural effusions (*) involving both lungs. (Figure reprinted from reference 7.)
Figure 6b.
PD-1 inhibitor pneumonitis: Hypersensitivity pneumonitis pattern in a 68-year-old man with metastatic renal cell carcinoma who was treated with nivolumab and presented with a new cough at 6 months of therapy. (a) Axial chest CT image shows new multifocal GGOs in a centrilobular distribution throughout both lungs and mosaic attenuation, findings that represent pneumonitis with a hypersensitivity pneumonitis pattern. Nivolumab therapy was withheld, and the patient underwent corticosteroid therapy. (b) Axial follow-up CT image obtained after 1 month of corticosteroid therapy shows marked improvement of pneumonitis and resolution of GGOs. Nivolumab continued to be withheld, and corticosteroid therapy was tapered. At 1.5 months after completing the corticosteroid taper, without restarting nivolumab or any other systemic therapy, the patient experienced a worsening cough. (c) Axial chest CT image shows development of diffuse GGOs with areas of air trapping, findings indicative of pneumonitis with a hypersensitivy pneumonitis pattern. The radiographic pattern of this second episode is similar to that noted in the initial episode and represents pneumonitis flare.
Figure 6c.
PD-1 inhibitor pneumonitis: Hypersensitivity pneumonitis pattern in a 68-year-old man with metastatic renal cell carcinoma who was treated with nivolumab and presented with a new cough at 6 months of therapy. (a) Axial chest CT image shows new multifocal GGOs in a centrilobular distribution throughout both lungs and mosaic attenuation, findings that represent pneumonitis with a hypersensitivity pneumonitis pattern. Nivolumab therapy was withheld, and the patient underwent corticosteroid therapy. (b) Axial follow-up CT image obtained after 1 month of corticosteroid therapy shows marked improvement of pneumonitis and resolution of GGOs. Nivolumab continued to be withheld, and corticosteroid therapy was tapered. At 1.5 months after completing the corticosteroid taper, without restarting nivolumab or any other systemic therapy, the patient experienced a worsening cough. (c) Axial chest CT image shows development of diffuse GGOs with areas of air trapping, findings indicative of pneumonitis with a hypersensitivy pneumonitis pattern. The radiographic pattern of this second episode is similar to that noted in the initial episode and represents pneumonitis flare.
A recent study investigated a cohort of 20 patients treated in 10 nivolumab trials who developed PD-1 inhibitor pneumonitis. The study characterized the spectrum of clinical and imaging manifestations of the entity (7). Of the 20 patients, 10 patients had melanoma, six patients had lymphoma, and four patients had lung cancer. The toxicity grades for pneumonitis were grade 1 in five patients, grade 2 in 10 patients, and grade 3 in five patients. The median time from initiation of therapy to pneumonitis was 2.6 months (range, 0.5–11.5 months). A spectrum of radiologic patterns was noted, with the COP pattern being the most common (noted in 13 patients), followed by the NSIP pattern (three patients), the hypersensitivity pattern (two patients), and the AIP/ARDS pattern (two patients) (7). Moreover, these radiologic patterns had a significant (P = .006) association with the clinical severity of pneumonitis as assessed by toxicity grades. The AIP/ARDS pattern had the highest grade, followed by the COP pattern, while the NSIP pattern and the hypersensitivity pneumonitis pattern had lower grades (median grades of 3, 2, 1, and 1, respectively). These observations are indicative of the important role of imaging in guiding patient management in this challenging entity. The radiologic patterns may help in the triage of patients according to disease severity and in the selection of treatment options for pneumonitis (7).
Treatment of immune checkpoint inhibitor–related pneumonitis includes withholding the immune checkpoint inhibitors and administering corticosteroid therapy either orally or intravenously, depending on disease severity (7,28,29). Some cases may not readily respond to corticosteroid therapy and will require additional immunosuppressant therapy with mycophenolate mofetil, cyclophosphamide, or infliximab (an anti–tumor necrosis factor-α immunosuppressant) (7,28,29). Because the radiologic patterns reflect disease severity, recognition of these patterns may assist referring clinicians in treatment decision making regarding whether corticosteroid therapy should be administered in addition to withholding the agents, or whether immunosuppressive agents are needed in addition to corticosteroid therapy
The available data to date suggest that approximately one-third of patients are able to restart immune checkpoint inhibitor therapy after pneumonitis is successfully treated; however, 25%–28% of these patients experience recurrent pneumonitis during retreatment (7,28). Further investigations are needed to identify patients who can safely restart immune checkpoint inhibitor therapy after resolution of the initial episode of pneumonitis.
Another unique observation has been reported in a small number of patients who experienced PD-1 inhibitor–related pneumonitis. Pneumonitis may recur after completing a taper of corticosteroid therapy, in the absence of restarting immune checkpoint inhibitor or any other systemic therapy, and manifests with a radiologic pattern and clinical symptoms similar to those of the initial episode (Figs 6, 7) (21). This phenomenon is termed pneumonitis flare, seems to be unique to irAEs, and may involve underlying autoimmune mechanisms. Reported cases of pneumonitis flare were also treated successfully with corticosteroid therapy. However, more than one episode of pneumonitis flare in a patient has been noted, which indicates the clinical management challenges of this rare condition (7).
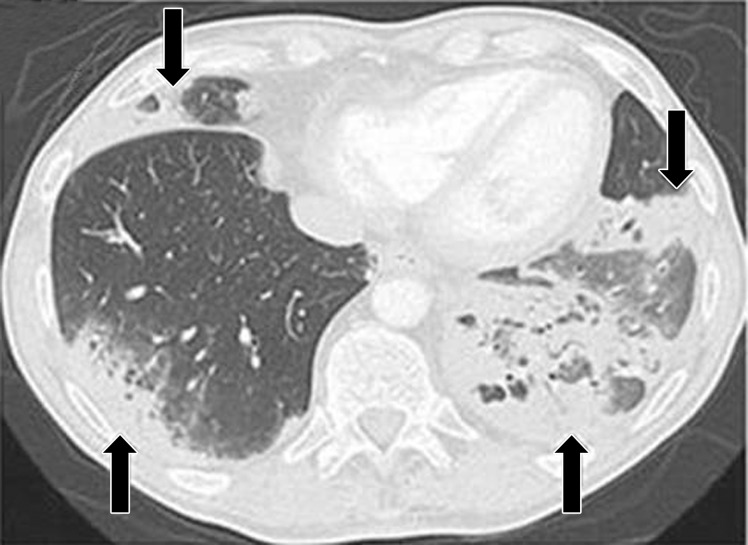
Figure 7a.
PD-1 inhibitor pneumonitis flare in a 72-year-old man with stage IV squamous NSCLC who was treated with nivolumab and presented with progressive dyspnea with cough and wheezing but no fever. (a) Axial chest CT image at 15 weeks of therapy demonstrates multifocal areas of GGOs, reticular opacities, and consolidation (arrows) involving all lobes, as well as centrilobular nodularity and traction bronchiectasis in a predominantly peripheral distribution. The overall features demonstrate a COP pattern. The patient was treated with prednisone for pneumonitis. (b) Axial follow-up CT image after 4 weeks of prednisone therapy shows a significant decrease in the findings, with residual GGOs. Note the “reversed halo” sign manifesting as a central GGO surrounded by a crecent-shaped dense airspace consolidation (arrow), a finding that has been reported as a radiologic manifestation of COP. (c) Axial CT image obtained 4 weeks after the completion of prednisone therapy shows the development of a bilateral dense consolidation with GGOs and reticular opacities (arrows) in peripheral and multifocal distributions, again demonstrating a COP pattern as noted during the first episode of PD-1 inhibitor pneumonitis. Given the similarity of the radiologic and clinical manifestations to those of the first episode, the patient restarted prednisone for treatment of pneumonitis flare. Follow-up chest CT images obtained 2 weeks after starting the second course of prednisone therapy (not shown) demonstrated a decrease in the findings, indicative of improving pneumonitis in response to corticosteroid therapy. (Figure 7 reprinted from reference 21.)
Figure 7b.
PD-1 inhibitor pneumonitis flare in a 72-year-old man with stage IV squamous NSCLC who was treated with nivolumab and presented with progressive dyspnea with cough and wheezing but no fever. (a) Axial chest CT image at 15 weeks of therapy demonstrates multifocal areas of GGOs, reticular opacities, and consolidation (arrows) involving all lobes, as well as centrilobular nodularity and traction bronchiectasis in a predominantly peripheral distribution. The overall features demonstrate a COP pattern. The patient was treated with prednisone for pneumonitis. (b) Axial follow-up CT image after 4 weeks of prednisone therapy shows a significant decrease in the findings, with residual GGOs. Note the “reversed halo” sign manifesting as a central GGO surrounded by a crecent-shaped dense airspace consolidation (arrow), a finding that has been reported as a radiologic manifestation of COP. (c) Axial CT image obtained 4 weeks after the completion of prednisone therapy shows the development of a bilateral dense consolidation with GGOs and reticular opacities (arrows) in peripheral and multifocal distributions, again demonstrating a COP pattern as noted during the first episode of PD-1 inhibitor pneumonitis. Given the similarity of the radiologic and clinical manifestations to those of the first episode, the patient restarted prednisone for treatment of pneumonitis flare. Follow-up chest CT images obtained 2 weeks after starting the second course of prednisone therapy (not shown) demonstrated a decrease in the findings, indicative of improving pneumonitis in response to corticosteroid therapy. (Figure 7 reprinted from reference 21.)
Figure 7c.
PD-1 inhibitor pneumonitis flare in a 72-year-old man with stage IV squamous NSCLC who was treated with nivolumab and presented with progressive dyspnea with cough and wheezing but no fever. (a) Axial chest CT image at 15 weeks of therapy demonstrates multifocal areas of GGOs, reticular opacities, and consolidation (arrows) involving all lobes, as well as centrilobular nodularity and traction bronchiectasis in a predominantly peripheral distribution. The overall features demonstrate a COP pattern. The patient was treated with prednisone for pneumonitis. (b) Axial follow-up CT image after 4 weeks of prednisone therapy shows a significant decrease in the findings, with residual GGOs. Note the “reversed halo” sign manifesting as a central GGO surrounded by a crecent-shaped dense airspace consolidation (arrow), a finding that has been reported as a radiologic manifestation of COP. (c) Axial CT image obtained 4 weeks after the completion of prednisone therapy shows the development of a bilateral dense consolidation with GGOs and reticular opacities (arrows) in peripheral and multifocal distributions, again demonstrating a COP pattern as noted during the first episode of PD-1 inhibitor pneumonitis. Given the similarity of the radiologic and clinical manifestations to those of the first episode, the patient restarted prednisone for treatment of pneumonitis flare. Follow-up chest CT images obtained 2 weeks after starting the second course of prednisone therapy (not shown) demonstrated a decrease in the findings, indicative of improving pneumonitis in response to corticosteroid therapy. (Figure 7 reprinted from reference 21.)
Pneumonitis Related to Molecular Targeting Therapy
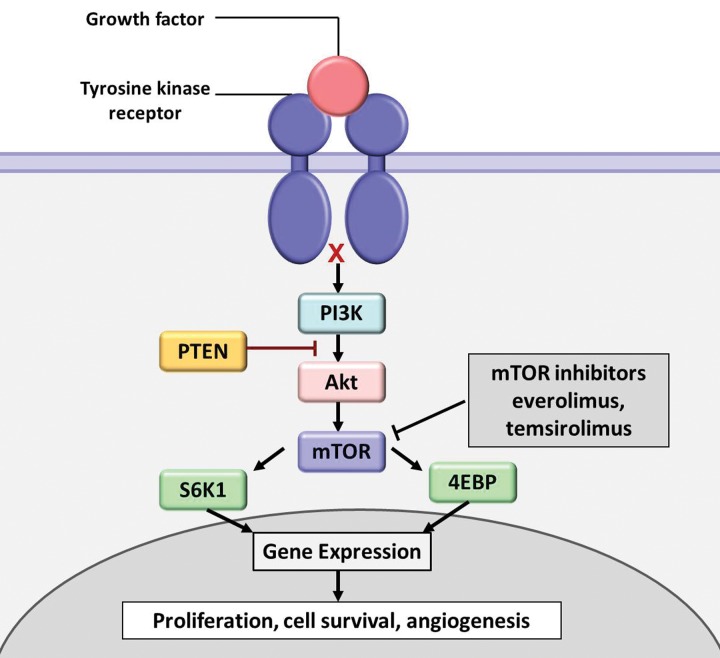
mTOR Inhibitors.—mTOR is a serine/threonine protein kinase and is a critical component of the phosphatidylinositol 3-kinase (PI3K)/Akt/mTOR pathway, which has been actively studied as an oncogenic driver in human cancers (Fig 8) (2,30). Specific inhibitors of mTOR are used as anticancer therapeutic agents, including everolimus and temsirolimus. Everolimus has been approved for treatment of advanced renal cell carcinoma, subependymal giant cell astrocytoma, advanced hormone receptor–positive HER2-negative breast cancer, and advanced pancreatic neuroendocrine tumors. Temsirolimus has been approved for renal cell carcinoma.
Figure 8.
Diagram shows a simplified scheme of the phosphatidylinositol 3-kinase (PI3K)/Akt/mTOR signaling pathway. The PI3K/Akt/mTOR pathway is an intracellular signaling pathway that regulates cell proliferation, survival, and angiogenesis. mTOR inhibitors such as everolimus and temsirolimus exert antitumor activity by inhibiting the downstream signal transduction of the pathway (30). 4EBP = eukaryotic initiation factor 4E–binding protein 1, PTEN = phosphatase and tensin homolog deleted from chromosome 10, S6K1 = S6 kinase 1.
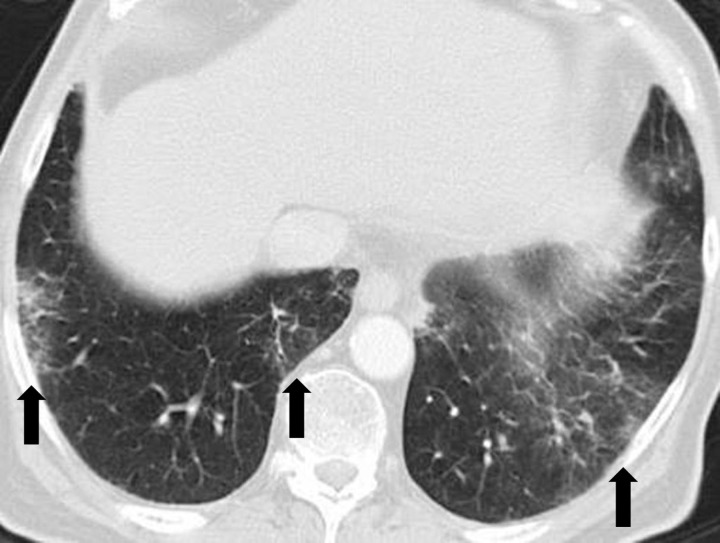
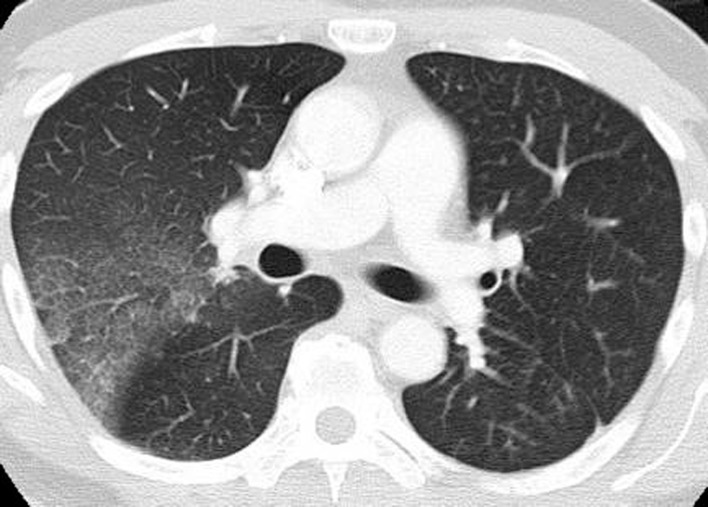
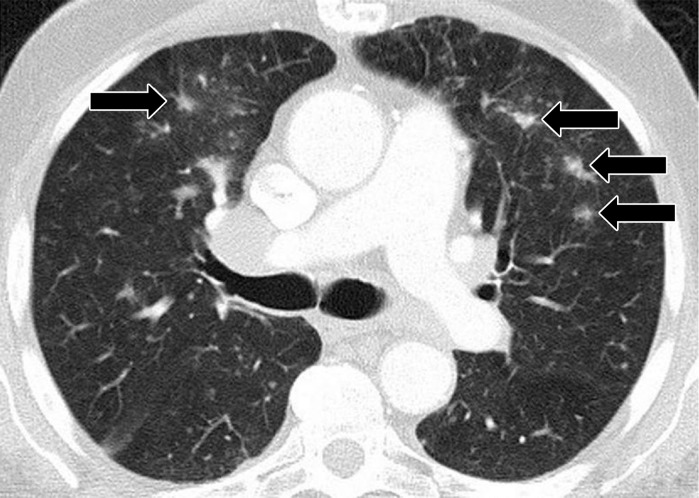
Drug-related pneumonitis is one of the major adverse events of mTOR inhibitor therapy and is well recognized as a class-effect toxicity (31). The incidence rates in prior reports were approximately 30% among patients with renal cell carcinoma, 25% in patients with NSCLC treated in trials, and 21% in patients with advanced neuroendocrine tumors (6,32–34). Prior studies described CT findings of mTOR inhibitor–related pneumonitis that included GGOs, consolidation, and reticular opacities (Fig 9) (32,33). Two recent studies described radiologic patterns of pneumonitis related to mTOR inhibitor therapy in patients with advanced neuroendocrine tumors and in trial-treated patients with Waldenström macroglobulinemia (2,6). In both cohorts, the most frequent CT findings of pneumonitis were bilateral GGOs and reticular opacities, with or without consolidation, in peripheral and lower lung distributions, indicative of a COP pattern or an NSIP pattern (Fig 10) (2,6). A minority of patients demonstrated diffuse bilateral GGOs and reticular opacities involving all lobes, indicative of a hypersensitivity pneumonitis pattern (Fig 11) (6). Similar radiologic patterns of mTOR inhibitor–related pneumonitis across different tumor types further support the concept of class-effect toxicity, a term often used to indicate the same or a very similar type of toxicity due to a group of agents that share the same mechanism of action.
Figure 9.
Pneumonitis in a 71-year-old man with metastatic renal cell carcinoma treated with temsirolimus. Axial CT image at 4 weeks of therapy shows multifocal GGOs and reticular opacities in a predominantly peripheral and basilar distribution (arrows), findings that represent mTOR inhibitor–related pneumonitis. The patient was symptomatic and was switched to an alternate therapy.
Figure 10.
Pneumonitis in a 66-year-old woman with Waldenström macroglobulinemia treated with mTOR inhibitor therapy. Axial CT image at 6 months of therapy shows consolidation, GGOs, and reticular opacities (arrows) that represent a COP pattern.
Figure 11.
Pneumonitis in a 62-year-old woman with advanced pancreatic neuroendocrine tumor treated with everolimus and temozolomide. Axial CT image obtained at 10.3 months of therapy shows diffuse bilateral GGOs and reticular opacities that are indicative of a hypersensitivity pneumonitis pattern. The patient had mild shortness of breath and was treated with prednisone.
Patient management and treatment of mTOR inhibitor–related pneumonitis depend on the clinical symptoms; asymptomatic cases with imaging abnormalities alone may be monitored closely while continuing mTOR inhibitor therapy. In symptomatic cases, mTOR inhibitors are withheld and corticosteroids may be needed, after infectious causes have been ruled out (33).
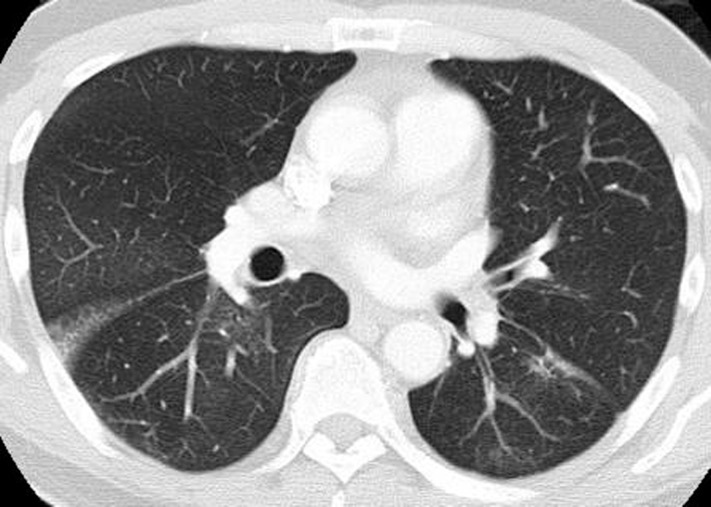
EGFR Inhibitors.—Somatic activating mutations of EGFR in NSCLC are associated with a dramatic response to EGFR inhibitors, including gefitinib, erlotinib, and afatinib, which have become a representative component of precision therapy for patients with advanced lung cancer (35–38). More recently, another novel agent, osimertinib, has also been approved for clinical use. Osimertinib is a third-generation EGFR tyrosine kinase inhibitor and targets T790M second-site EGFR that is responsible for acquired resistance to conventional EGFR inhibitors (39). Pneumonitis, although not frequent among the U.S. population, is a recognized class-effect toxicity of EGFR inhibitors and has been studied mostly in the context of erlotinib and gefitinib (Fig 12) (40–42).
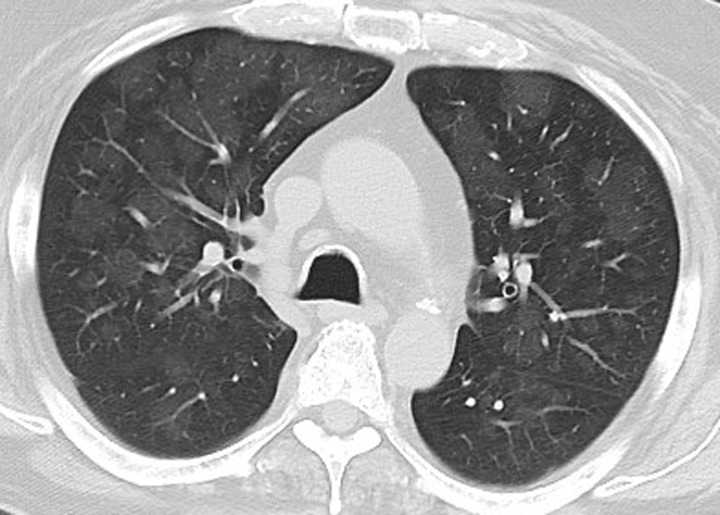
Figure 12a.
Pneumonitis in a 42-year-old man with an EGFR exon 19 deletion mutation who was treated with erlotinib in the United States. Axial chest CT images (b obtained at a lower level than a) obtained at 8 weeks of therapy show multifocal areas of GGOs in both lungs, findings that represent pneumonitis. Note the absence of traction bronchietasis or volume loss.
Figure 12b.
Pneumonitis in a 42-year-old man with an EGFR exon 19 deletion mutation who was treated with erlotinib in the United States. Axial chest CT images (b obtained at a lower level than a) obtained at 8 weeks of therapy show multifocal areas of GGOs in both lungs, findings that represent pneumonitis. Note the absence of traction bronchietasis or volume loss.
The incidence rates seem to be higher in the Japanese population (about 4%–5%), with high mortality rates of 30%–35% in both the gefitinib- and erlotinib-treated cohorts (40–42). The risk factors for pneumonitis derived from Japanese cohorts include older age, history of smoking, preexisting interstitial lung disease, poor World Health Organization performance status, short interval since NSCLC diagnosis, and 50% or lower remaining normal lung areas (41,42). Different radiologic patterns of EGFR-inhibitor–related pneumonitis have been described, including the COP pattern, hypersensitivity pneumonitis pattern, NSIP pattern, and AIP/diffuse alveolar damage (DAD) pattern (5). The DAD pattern demonstrates nonsegmental GGO or consolidation with traction bronchiectasis and volume loss at CT and has been associated with a higher mortality rate of 65% (41).
In addition, recent findings of a high incidence of severe and potentially fatal lung toxicity in patients treated with immune checkpoint inhibitors and EGFR inhibitors are raising new concerns. In an ongoing phase 1 trial, a treatment arm testing durvalumab (a PD-L1 inhibitor) plus osimertinib in patients with EGFR-mutant NSCLC reported a high rate of lung toxicity, with an incidence rate of 38% for all grades and 15% for grade 3–4 events, leading to a suspension of enrollment in this arm (43). Moreover, a report in Japan described eight cases of patients with advanced NSCLC previously treated with nivolumab who received EGFR inhibitors and then experienced severe interstitial lung disease, which was lethal in three cases (44,45). These emerging data indicate the importance of increased awareness among radiologists of the lung toxicity of EGFR inhibitors, given the rapidly expanding use of immune checkpoint inhibitors that are now approved as first-line treatments in U.S. patients with lung cancer (46,47).
ALK Inhibitors.—The ALK-rearranged oncogene is one of the molecular targets in NSCLC and is present in 2%–7% of patients with NSCLC (38,48–50). The ALK inhibitor crizotinib has shown marked efficacy in a phase 1 trial in patients with ALK-rearranged NSCLC and was approved by the FDA in 2011. Recently, two additional ALK inhibitors, alectinib and ceritinib, have also been approved for treatment of patients with disease progression or who could not tolerate crizotinib. Several reports describe drug-related pneumonitis in patients treated with ALK inhibitors (Fig 13) (51,52). Among them, a case of severe acute interstitial lung disease with rapidly deteriorating dyspnea has been described, leading to death at 3 weeks of crizotinib therapy (52). The patient demonstrated extensive bilateral GGOs at CT and diffuse alveolar damage at postmortem histologic analysis (52). Another report described a patient who presented with imaging and histologic findings of organizing pneumonia and who was able to be retreated with crizotinib. The incidence of and risk factors for ALK-inhibitor–related pneumonitis and predictors of higher- grade events require further study.
Figure 13a.
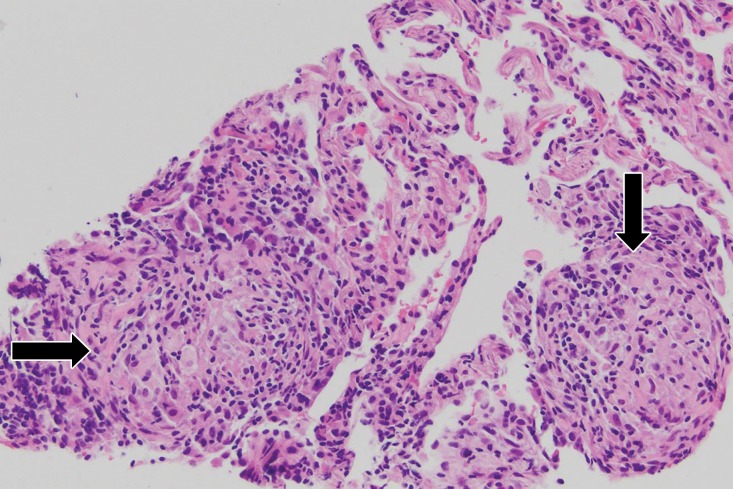
ALK inhibitor–related pneumonitis in a 55-year-old woman with ALK-positive stage IV adenocarcinoma of the lung. The patient experienced disease progression while taking a first-generation ALK inhibitor (crizotinib) and was then treated with a second-generation ALK inhibitor (ceritinib). She presented with an increasing dry cough and dyspnea at 7 months of ceritinib therapy. (a, b) Axial (a) and coronal (b) CT images show biapical consolidation and GGOs in both upper lobes (arrows) in a striking peripheral distribution, indicative of a COP pattern. (c, d) Photomicrographs from transbronchial lung biopsy specimen show organizing interstitial pneumonia characterized by alveolar interstitial widening by lymphocytic infiltrates, increased extracellular matrix material, reactive pneumocyte hyperplasia, scattered eosinophils (arrow in d), and numerous airspace foamy macrophages. There was no evidence of tumor in the biopsy specimen. (Hematoxylin-eosin stain; original magnification, ×200 in c, ×400 in d.)
Figure 13b.
ALK inhibitor–related pneumonitis in a 55-year-old woman with ALK-positive stage IV adenocarcinoma of the lung. The patient experienced disease progression while taking a first-generation ALK inhibitor (crizotinib) and was then treated with a second-generation ALK inhibitor (ceritinib). She presented with an increasing dry cough and dyspnea at 7 months of ceritinib therapy. (a, b) Axial (a) and coronal (b) CT images show biapical consolidation and GGOs in both upper lobes (arrows) in a striking peripheral distribution, indicative of a COP pattern. (c, d) Photomicrographs from transbronchial lung biopsy specimen show organizing interstitial pneumonia characterized by alveolar interstitial widening by lymphocytic infiltrates, increased extracellular matrix material, reactive pneumocyte hyperplasia, scattered eosinophils (arrow in d), and numerous airspace foamy macrophages. There was no evidence of tumor in the biopsy specimen. (Hematoxylin-eosin stain; original magnification, ×200 in c, ×400 in d.)
Figure 13c.
ALK inhibitor–related pneumonitis in a 55-year-old woman with ALK-positive stage IV adenocarcinoma of the lung. The patient experienced disease progression while taking a first-generation ALK inhibitor (crizotinib) and was then treated with a second-generation ALK inhibitor (ceritinib). She presented with an increasing dry cough and dyspnea at 7 months of ceritinib therapy. (a, b) Axial (a) and coronal (b) CT images show biapical consolidation and GGOs in both upper lobes (arrows) in a striking peripheral distribution, indicative of a COP pattern. (c, d) Photomicrographs from transbronchial lung biopsy specimen show organizing interstitial pneumonia characterized by alveolar interstitial widening by lymphocytic infiltrates, increased extracellular matrix material, reactive pneumocyte hyperplasia, scattered eosinophils (arrow in d), and numerous airspace foamy macrophages. There was no evidence of tumor in the biopsy specimen. (Hematoxylin-eosin stain; original magnification, ×200 in c, ×400 in d.)
Figure 13d.
ALK inhibitor–related pneumonitis in a 55-year-old woman with ALK-positive stage IV adenocarcinoma of the lung. The patient experienced disease progression while taking a first-generation ALK inhibitor (crizotinib) and was then treated with a second-generation ALK inhibitor (ceritinib). She presented with an increasing dry cough and dyspnea at 7 months of ceritinib therapy. (a, b) Axial (a) and coronal (b) CT images show biapical consolidation and GGOs in both upper lobes (arrows) in a striking peripheral distribution, indicative of a COP pattern. (c, d) Photomicrographs from transbronchial lung biopsy specimen show organizing interstitial pneumonia characterized by alveolar interstitial widening by lymphocytic infiltrates, increased extracellular matrix material, reactive pneumocyte hyperplasia, scattered eosinophils (arrow in d), and numerous airspace foamy macrophages. There was no evidence of tumor in the biopsy specimen. (Hematoxylin-eosin stain; original magnification, ×200 in c, ×400 in d.)
HER2 Inhibitors.—HER2 is a transmembrane tyrosine kinase receptor belonging to the EGFR family that regulates cell growth, survival, adhesion, migration, and differentiation (53). Approximately 20% of patients with breast cancer have HER2 overexpression, which benefits from HER2-directed molecular targeting therapy with HER2 inhibitors (53). Currently, HER2 inhibitors including trastuzumab, lapatinib, pertuzumab, and trastuzumab emtansine (T-DM1, an immunoconjugate that combines trastuzumab with the microtubule-targeting agent emtansine) have been approved by the FDA for treatment of breast cancer (53). Pneumonitis, although infrequent, has been reported as a lung toxic effect related to HER2 inhibitor therapy, mostly in patients treated with trastuzumab (54–56). A radiologic pattern of COP has been described that manifested with bilateral consolidation and GGOs in the lower lobes at CT, which resolved after discontinuation of trastuzumab (56). A histologic pattern of organizing pneumonia was noted in an open lung biopsy specimen obtained 1 week after discontinuation of trastuzumab (56). Some cases also require corticosteroid therapy as part of the treatment of pneumonitis (54). The exact incidence of trastuzumab-related pneumonitis remains to be further studied.
CD20 Antibody.—CD20 is an activated-glycosylated phosphoprotein expressed as a surface antigen of B cells during their differentiation (57). Molecular targeting therapy using the anti-CD20 antibody rituximab represents one of the most important advances in the treatment of lymphomas in recent decades (57,58). Rituximab is mostly used in combination with chemotherapy or radiation therapy and has substantially improved the survival of patients with lymphoma. It is also used or being investigated for the treatment of other hematologic disorders, including chronic lymphocytic leukemia and autoimmune disorders (57,58).
Pneumonitis is a well-known lung toxic effect related to rituximab therapy. In a systematic review of 121 cases of rituximab-related pneumonitis, pneumonitis occurred more frequently in male patients and during the 5th and 6th decades of life (59). Most patients were treated with rituximab in combination with other chemotherapies, while one-fourth of patients were treated with monotherapy (59). In the 21 clinical studies or trials evaluated in the review, the rate of rituximab-related pneumonitis ranged from 3.7% (one of 27 patients) to 10.0% (nine of 90 patients) (59).
CT manifestations were similar across cases, with diffuse bilateral lung opacities consisting of GGOs and consolidation (Fig 14). Treatment consisted of discontinuation of rituximab therapy and administration of corticosteroids, and most patients received concurrent empirical antibacterial and antifungal therapies. Notably, 18 of the 121 cases were fatal, which indicates the severity of this complication (59).
Figure 14.
Pneumonitis in a 65-year-old man with diffuse large B-cell lymphoma after three cycles of rituximab with cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone (R-CHOP) therapy who presented with new shortness of breath. Axial CT image shows bilateral diffuse GGOs and areas of consolidation in both lungs, with traction bronchiectasis and loss of lung volumes. The findings reflect an AIP/ARDS pattern of pneumonitis related to rituximab. Bilateral pleural effusions were also present. The patient’s condition significantly deteriorated, and he died 1 month after presentation. Autopsy results showed diffuse alveolar damage in the lungs.
Sarcoid-like Granulomatosis and Lymphadenopathy as an Immune-related Adverse Event
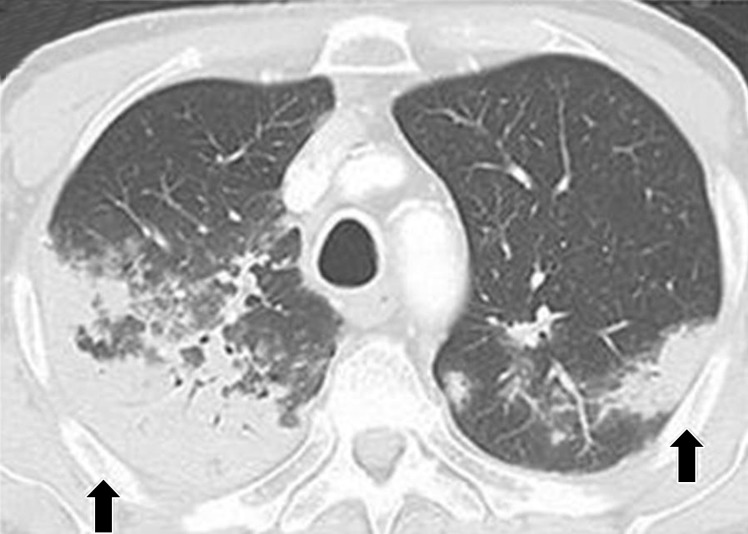
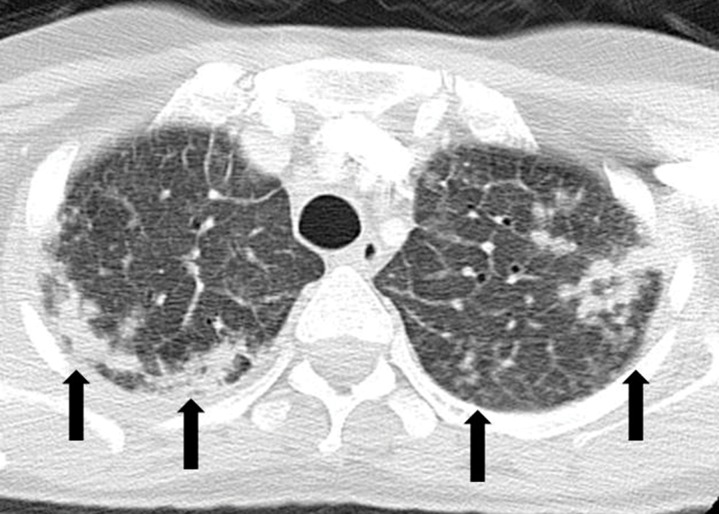
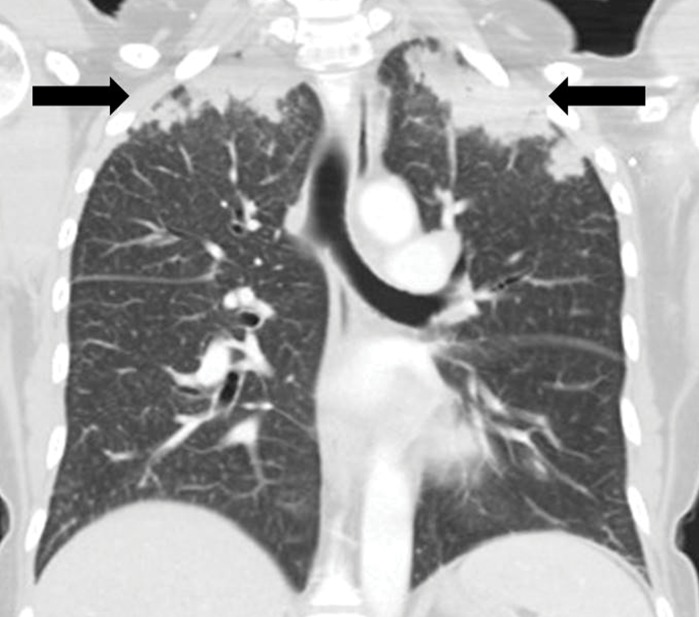
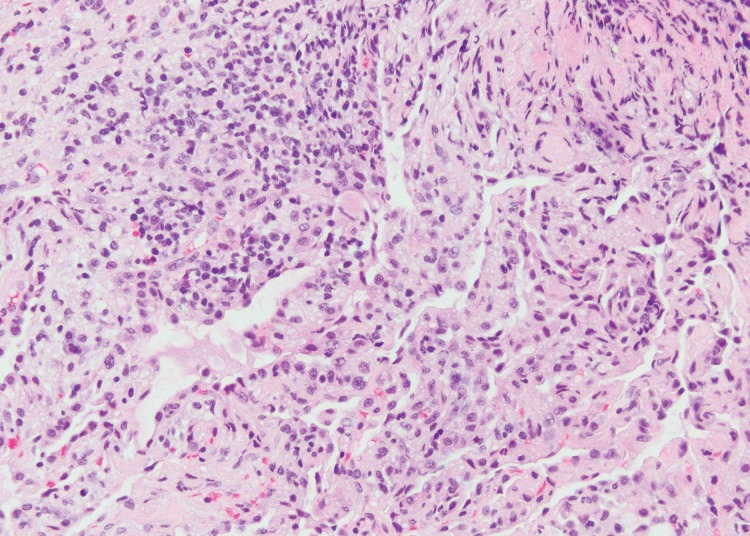
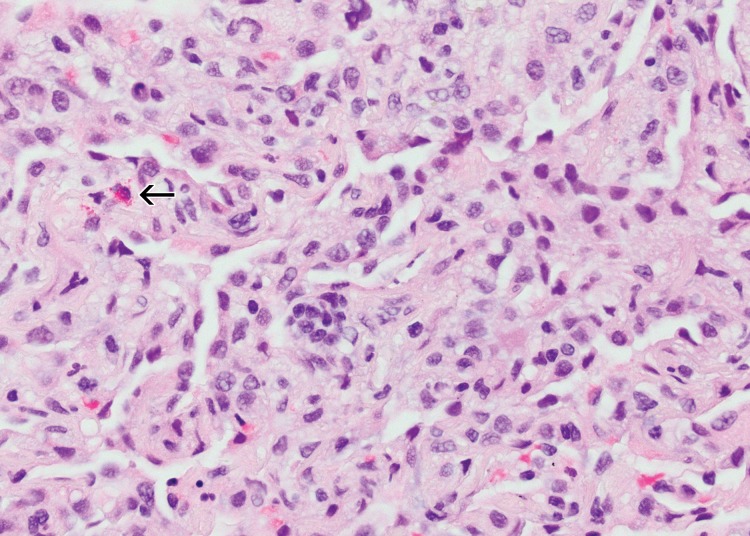
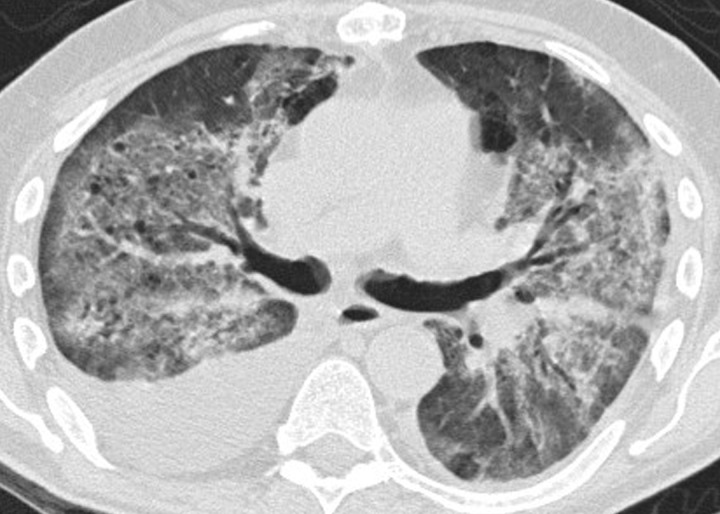
Sarcoid-like granulomatosis and lymphadenopathy is another important irAE that involves the thorax, and it is noted in 5%–7% of patients with melanoma treated with the CTLA-4 inhibitor ipilimumab (15,16). Patients often present with enlarged mediastinal and hilar lymphadenopathy, simulating findings of sarcoidosis, during treatment with immune checkpoint inhibitors. Lung parenchymal changes at CT can also be noted, with peribronchial opacities most characteristically seen in a bilateral upper lobe distribution, also simulating findings of sarcoidosis (Fig 15). The entity can be clinically silent and can resolve spontaneously without specific treatment. Because most patients receiving immune checkpoint inhibitors have advanced cancer, it is often challenging to differentiate sarcoid-like granulomatosis and lymphadenopathy from metastasis and tumor progression. An elevated angiotensin-converting enzyme level has been noted in a prior report of the entity (60); however, the level can be normal in some patients (61). Histologic sampling in these cases demonstrated granulomatous inflammation resembling sarcoidosis (Fig 16) (60,61).
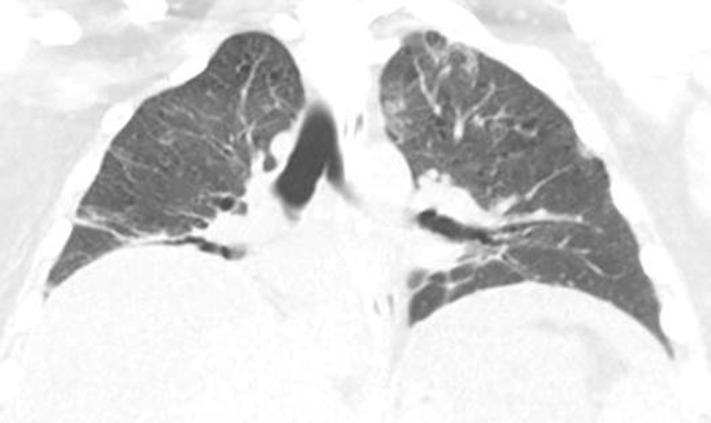
Figure 15a.
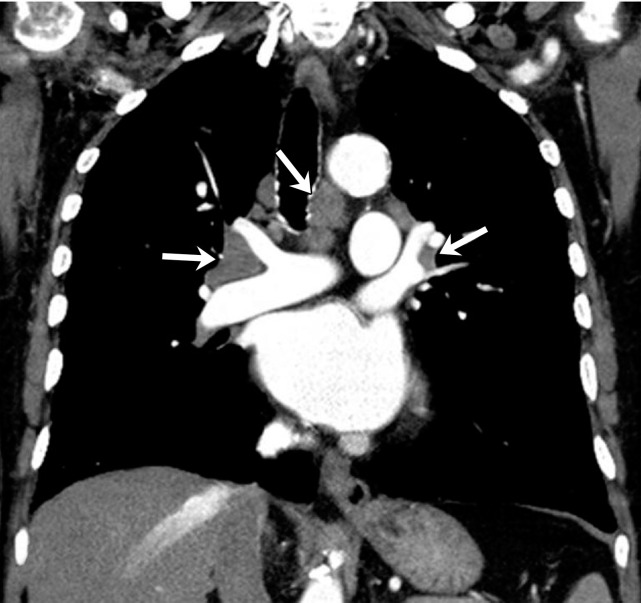
Sarcoid-like lymphadenopathy in an asymptomatic 81-year-old man with metastatic melanoma treated with ipilimumab. (a) Coronal contrast-enhanced reformatted chest CT image obtained 4.9 months after the initiation of ipilimumab therapy shows new bilateral symmetric mediastinal and hilar lymphadenopathy (arrows) resembling findings of sarcoidosis. (b) Axial CT image shows bilateral irregular and nodular parenchymal opacities (arrows) with upper- and middle-lung predominance and peribronchovascular involvement. The findings fall in the spectrum of lung parenchymal manifestations of pulmonary sarcoidosis. (Figure reprinted from reference 16.)
Figure 16a.
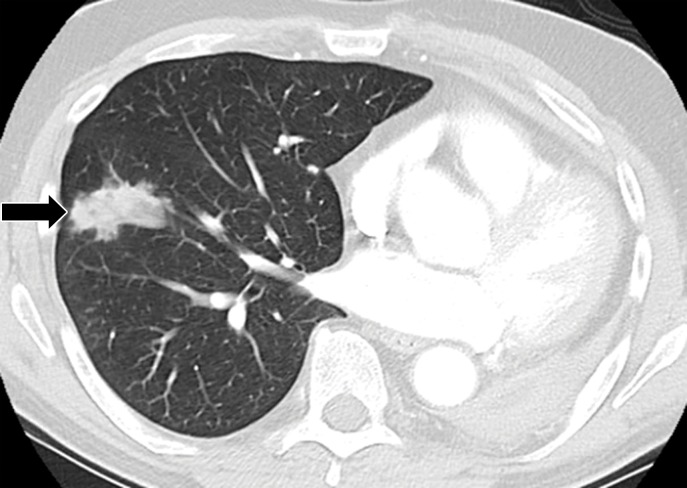
Sarcoid-like granulomatosis of the lung in an asymptomatic 75-year-old man with metastatic melanoma treated with pembrolizumab. (a) Axial CT image shows a parenchymal conglomerate opacity in the right lower lobe of the lung (arrow), which had been gradually increasing over time on serial scans obtained during therapy. Note that the patient had a previous left pneumonectomy. (b) Photomicrograph of specimen from a lung core biopsy of the right lower lobe lesion shows a sarcoid-like reaction characterized by interstitial nonnecrotizing granulomas (arrows) with associated lymphocytic infiltrates. (Hematoxylin-eosin stain; original magnification, ×200.). Gram, silver, and acid-fast bacilli stains (not shown) were negative for microorganisms.
Figure 15b.
Sarcoid-like lymphadenopathy in an asymptomatic 81-year-old man with metastatic melanoma treated with ipilimumab. (a) Coronal contrast-enhanced reformatted chest CT image obtained 4.9 months after the initiation of ipilimumab therapy shows new bilateral symmetric mediastinal and hilar lymphadenopathy (arrows) resembling findings of sarcoidosis. (b) Axial CT image shows bilateral irregular and nodular parenchymal opacities (arrows) with upper- and middle-lung predominance and peribronchovascular involvement. The findings fall in the spectrum of lung parenchymal manifestations of pulmonary sarcoidosis. (Figure reprinted from reference 16.)
Figure 16b.
Sarcoid-like granulomatosis of the lung in an asymptomatic 75-year-old man with metastatic melanoma treated with pembrolizumab. (a) Axial CT image shows a parenchymal conglomerate opacity in the right lower lobe of the lung (arrow), which had been gradually increasing over time on serial scans obtained during therapy. Note that the patient had a previous left pneumonectomy. (b) Photomicrograph of specimen from a lung core biopsy of the right lower lobe lesion shows a sarcoid-like reaction characterized by interstitial nonnecrotizing granulomas (arrows) with associated lymphocytic infiltrates. (Hematoxylin-eosin stain; original magnification, ×200.). Gram, silver, and acid-fast bacilli stains (not shown) were negative for microorganisms.
Bleeding and Thrombosis during VEGF Inhibitor Therapy
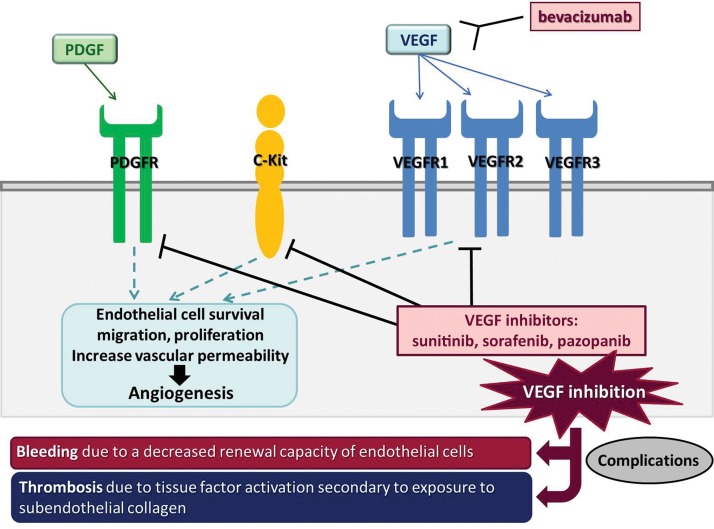
VEGF is a key factor in angiogenesis, as it regulates vascular proliferation and permeability and functions as a major endothelial mitogen (Fig 17) (62,63). VEGF inhibition may decrease the renewal capacity of the endothelial cells, leading to endothelial defects in the vascular lining and exposure of subendothelial collagen, and a decrease of matrix deposition in the supporting layers of vessels (64). In patients with cancer treated with VEGF inhibitors, major complications include (a) bleeding or hemorrhage due to the decreased renewal capacity of endothelial cells, and (b) thrombosis due to tissue factor activation secondary to exposure to subendothelial collagen (Fig 17) (64).
Figure 17.
Schematic view of the VEGF pathway and the sites of action of its inhibitors. VEGF is a key factor in angiogenesis and regulates vascular proliferation and permeability. Bevacizumab is a monoclonal antibody against VEGF and inhibits the VEGF pathway by binding to circulating VEGF. Other VEGF inhibitors, such as sunitinib, sorafenib, and pazopanib, inhibit the intracellular tyrosine kinase domain of the VEGF receptor (VEGFR); they also act on other transmembranous receptors, including platelet-derived growth factor (PDGF) receptor and C-Kit. VEGF inhibition by these agents leads to bleeding due to the decreased renewal capacity of endothelial cells, or to thrombosis due to tissue factor activation secondary to exposure to subendothelial collagen (62,63.)
Pulmonary Hemorrhage Related to VEGF Inhibitor Therapy
Pulmonary hemorrhage is increasingly noted, especially with use of antiangiogenic agents. In a phase 2 trial of bevacizumab in patients with NSCLC, bleeding was the most prominent adverse event and included minor mucocutaneous hemorrhage and major hemoptysis (65). Major hemoptysis was associated with squamous cell histology, tumor necrosis and cavitation, and disease location close to major blood vessels (65). Life-threatening or fatal hemoptysis was noted in four of 13 patients with squamous NSCLC treated with bevacizumab in the trial (65). Among patients with nonsquamous NSCLC, pulmonary hemorrhage has been reported in 2.3% (66). On CT images, pulmonary hemorrhage is associated with diffuse patchy areas of GGO, which may be accompanied by interlobular septal thickening (67).
Pulmonary Embolism Related to VEGF Inhibitor Therapy.—Pulmonary embolism is one of the major complications in patients with cancer and is associated with treatment involving VEGF inhibitors. Radiologists play an important role in the detection and monitoring of pulmonary embolism in these patients. In a phase II trial of bevacizumab in patients with colorectal cancer, thrombosis was the most significant adverse event, occurring in 19% (13 of 67) of patients (68). Of these, two patients had pulmonary embolism, which was fatal in one patient (68). Other VEGF inhibitors, such as sunitinib and sorafenib, are also associated with thrombosis and pulmonary embolism. In addition, with the technical advances in multidetector CT, clinically unsuspected pulmonary embolism may be noted incidentally in patients with cancer on routine follow-up CT scans (69). The pulmonary arteries should always be carefully screened for pulmonary embolism at CT of patients with cancer, even in the absence of clinical suspicion (Fig 18).
Figure 18.
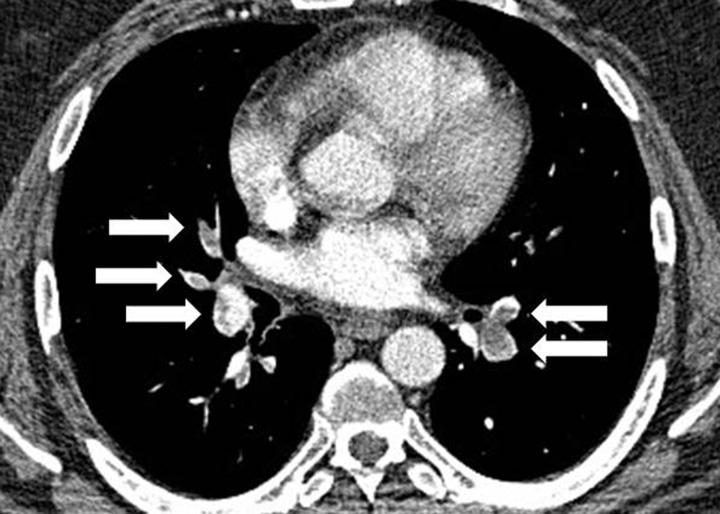
Pulmonary embolism in a 40-year-old man with glioblastoma undergoing bevacizumab therapy who presented with shortness of breath. Axial CT angiogram of the chest shows extensive filling defects involving bilateral lobar arteries and their branches (arrows), findings indicative of pulmonary embolism.
Pneumothorax Related to Antiangiogenic Therapy.—In addition to hemorrhage and embolism, pneumothorax is a rare but known complication in patients treated with antiangiogenic therapy, especially in the presence of pulmonary metastasis. Various agents have been described to lead to pneumothorax as a treatment complication, including pazopanib, bevacizumab, sorafenib, and sunitinib (Fig 19) (70–74). In a single-institute analysis of 58 patients with soft-tissue sarcoma treated with pazopanib, six patients (10%) experienced pneumothorax, with more than one episode reported in three patients (73). Although the mechanism is not entirely clear, the presence of a lung metastasis with a maximum diameter larger than 30 mm and a history of pneumothorax before pazopanib therapy were significant predictors of pneumothorax (73). Awareness of this unique complication is important for radiologists when interpreting chest imaging studies.
Figure 19a.
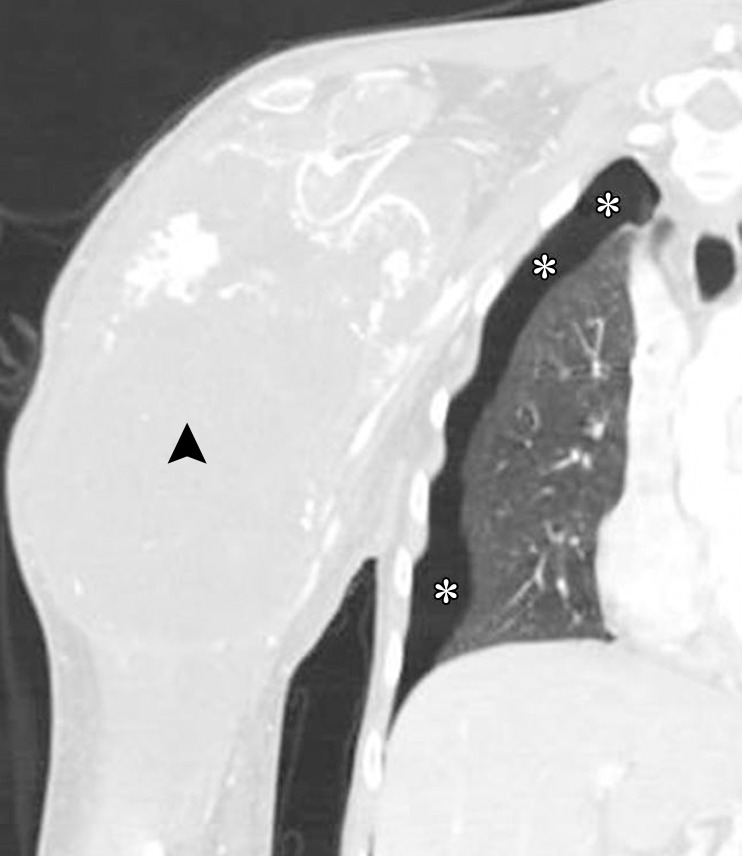
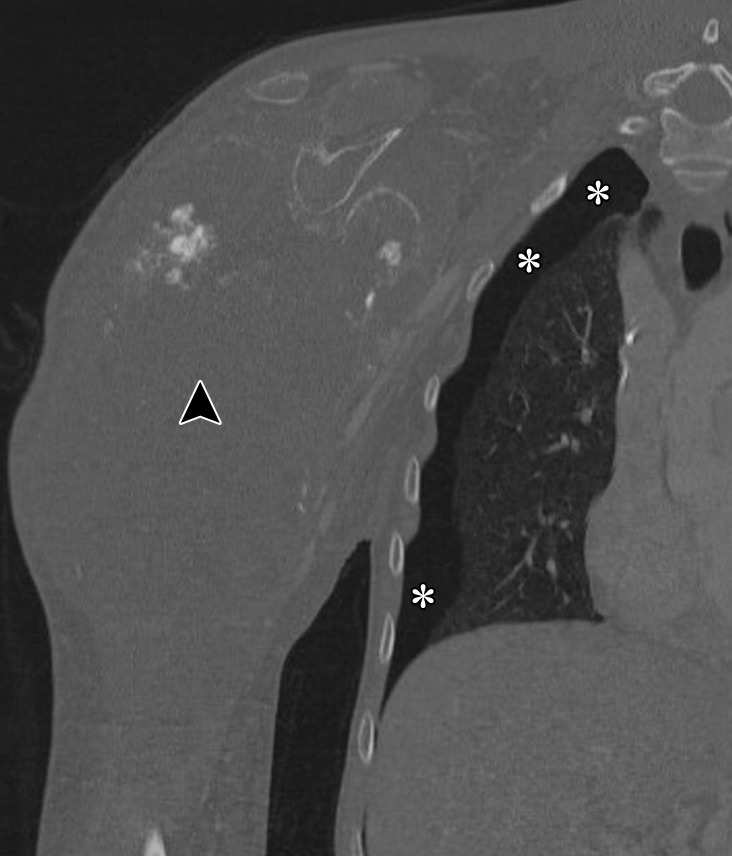
Pneumothorax in a 19-year-old man with metastatic osteosarcoma treated with pazopanib, a multitargeted tyrosine kinase inhibitor. Coronal CT images of the right shoulder with lung window (a) and bone window (b) settings show a large soft-tissue mass (arrowhead) arising from the right humerus. The finding represents primary osteosarcoma metastatic to the lung (not shown). Right pneumothorax (*) is seen and is a known complication of pazopanib therapy.
Figure 19b.
Pneumothorax in a 19-year-old man with metastatic osteosarcoma treated with pazopanib, a multitargeted tyrosine kinase inhibitor. Coronal CT images of the right shoulder with lung window (a) and bone window (b) settings show a large soft-tissue mass (arrowhead) arising from the right humerus. The finding represents primary osteosarcoma metastatic to the lung (not shown). Right pneumothorax (*) is seen and is a known complication of pazopanib therapy.
Pulmonary Edema and Pleural Effusion due to Molecular Targeting Therapy
Anticancer agents can cause injury to pneumocytes and the alveolar capillary endothelium, leading to leaky capillaries and increased permeability, which result in pulmonary edema or pleural effusions. Common responsible agents include BCR-ABL kinase inhibitors such as imatinib (used for leukemia and gastrointestinal stromal tumor), dasatinib (used for leukemia), and other newer agents in the class (Fig 20). In patients with chronic myelogenous leukemia (CML) treated in a phase 1 trial of dasatinib, 18% (15 of 84) developed pleural effusions that were treatment related. Management included diuretics, thoracentesis, or pleurodesis (75). In patients with CML treated in a randomized controlled trial of dasatinib versus imatinib, superficial edema and fluid retention were more common with imatinib, while pleural effusion was more common with dasatinib (76). In addition to effusions and pulmonary edema, subcutaneous edema can be noted as a stranding of subcutaneous fat at CT, a finding indicative of fluid retention.
Figure 20a.
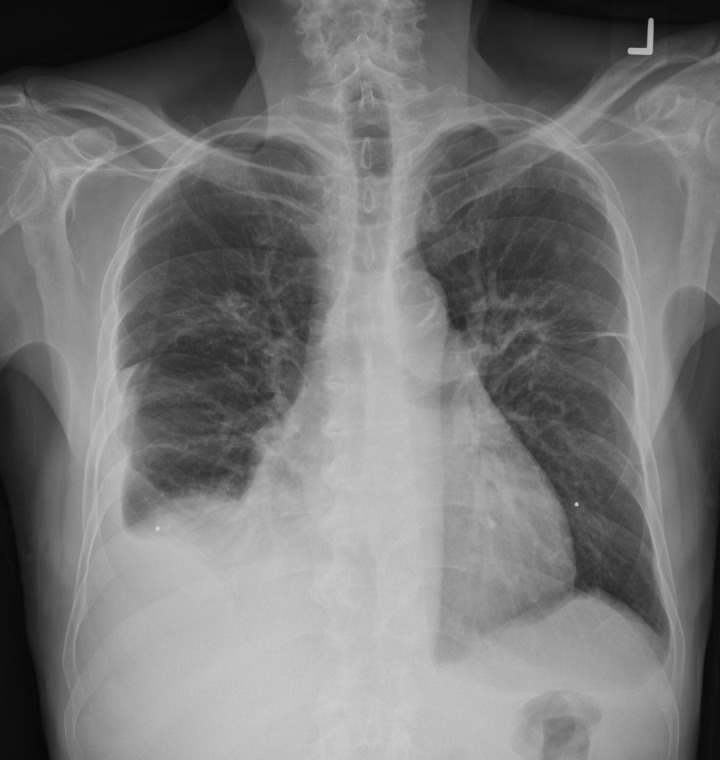
Pleural effusion in a 69-year-old man with a metastatic gastrointestinal stromal tumor treated with nilotinib, a tyrosine kinase inhibitor used for imatinib-resistant chronic myelogenous leukemia. The patient presented with increased shortness of breath and bilateral lower extremity edema. Posteroanterior (a) and lateral (b) chest radiographs show bilateral pleural effusion, with bilateral perihilar densities reflective of edema.
Figure 20b.
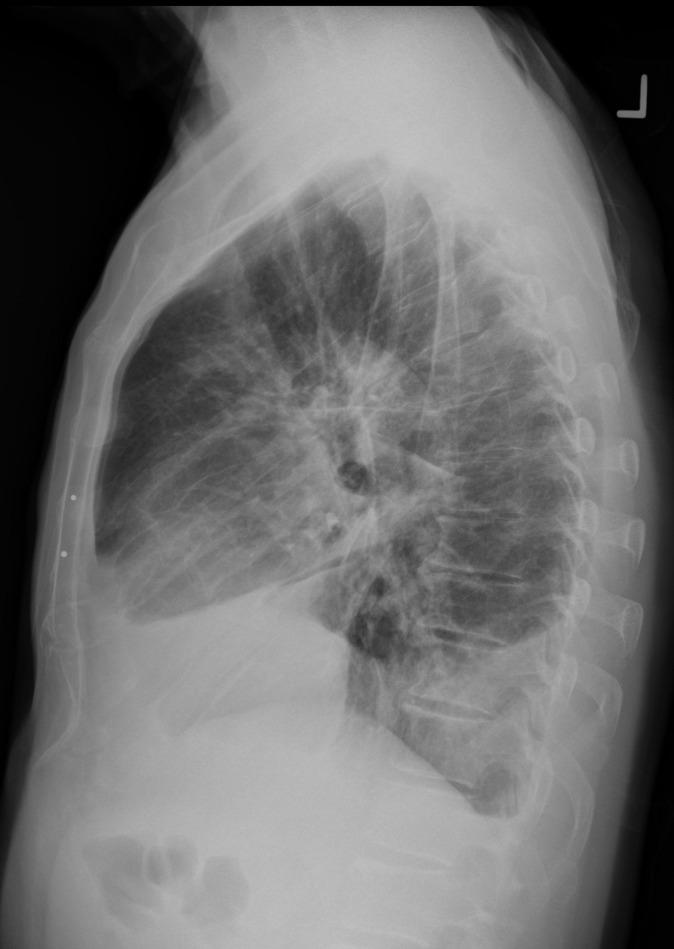
Pleural effusion in a 69-year-old man with a metastatic gastrointestinal stromal tumor treated with nilotinib, a tyrosine kinase inhibitor used for imatinib-resistant chronic myelogenous leukemia. The patient presented with increased shortness of breath and bilateral lower extremity edema. Posteroanterior (a) and lateral (b) chest radiographs show bilateral pleural effusion, with bilateral perihilar densities reflective of edema.
Radiologist’s Approach as a Member of the Multidisciplinary Team
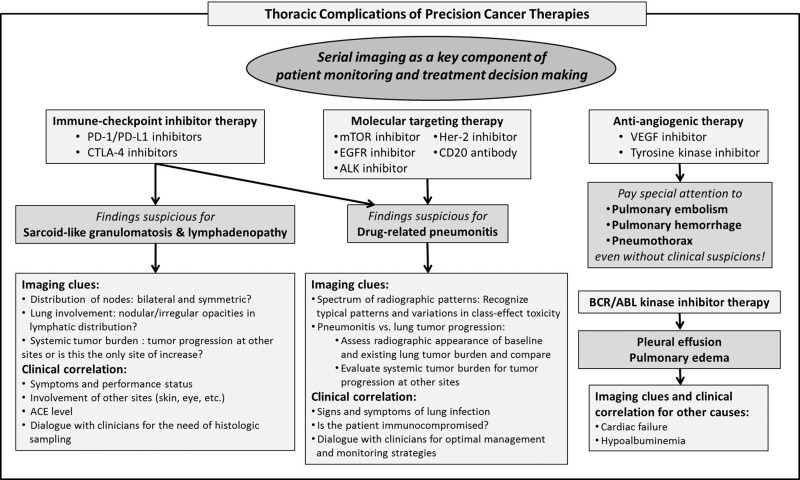
To meet increasing demands for early detection, accurate diagnosis, and optimal monitoring of complications of precision cancer therapies in state-of-the-art cancer care, radiologists should have practical strategies to approach each category of major complications (77,78). Although further efforts are needed to establish such strategies, a combination of diagnostic imaging clues and clinical dialogue in the context of precision oncology can contribute to fulfilling our mission on the multidisciplinary cancer care team (Fig 21).
Figure 21.
Diagram shows the radiologist’s approach to thoracic complications of precision cancer therapy. A combination of diagnostic clues at imaging and clinical dialogue in the context of precision oncology can contribute to accurate diagnosis and optimal patient care. ACE = angiotensin-converting enzyme.
Drug-related pneumonitis can be a major diagnostic challenge for radiologists in the era of precision cancer therapy. Recognition of the radiologic patterns of pneumonitis as class-effect toxicities is an important first step in an effective image-based approach to the entity. In patients with advanced cancer with lung involvement, distinguishing pneumonitis from tumor progression in the lungs is often a major concern for clinical providers. Careful evaluation of the imaging appearance of baseline lung tumor burden and assessment of tumor burden changes in extrathoracic sites are often helpful for distinction of these two conditions, which have different clinical management paths. In addition, exclusion of infectious causes is another important dilemma in almost all cases of pneumonitis and requires dialogue with referring clinicians regarding the detailed symptoms and immunologic status of the patient. Sarcoid-like granulomatosis and lymphadenopathy is also a source of clinical dilemma, especially regarding its differentiation from metastatic lymphadenopathy. In challenging cases, it is important to evaluate tumor burden changes other than lymphadenopathy and to obtain overall trends of the disease behavior. Pleural effusions and edema also require clinical correlation for non–drug-related causes such as cardiac failure and hypoalbuminemia. In patients treated with antiangiogenic therapy, radiologists should approach the imaging studies with heightened alert for pulmonary embolism, hemorrhage, and pneumothorax because these complications require immediate communication with referring clinicians for attention and management.
Conclusion
With the increasing use of novel precision cancer therapies in clinical oncology practice, knowledge of the imaging manifestations of thoracic complications related to specific cancer therapies has become essential for radiologists. Accurate diagnosis of thoracic complications due to novel anticancer agents is critical to maximize the benefits of precision cancer therapy. Radiologists play a major role in diagnosing and monitoring these complications at the front line of precision cancer therapy as key members of the multidisciplinary team.
Recipient of a Cum Laude award for an education exhibit at the 2016 RSNA Annual Meeting.
For this journal-based SA-CME activity, the authors M.N., H.H., and L.M.S. have provided disclosures; the other author, the editor, and the reviewers have disclosed no relevant relationships.
M.N. supported by the National Cancer Institute (1R01CA203636, 5K23CA157631).
Disclosures of Conflicts of Interest.—: M.N. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: consultant for Bristol-Myers Squibb, Toshiba Medical Systems, and WorldCare Clinical; institutional research grants from the Merck Investigator Studies Program and Toshiba Medical Systems; honorarium from Bayer. Other activities: disclosed no relevant relationships. H.H. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: grants from Toshiba Medical Systems, Canon USA, and Konica Minolta; consultant for Toshiba Medical Systems. Other activities: disclosed no relevant relationships. L.M.S. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: personal consultancy fees from Genentech. Other activities: disclosed no relevant relationships.
Abbreviations:
- AIP
- acute interstitial pneumonia
- ALK
- anaplastic lymphoma kinase
- ARDS
- acute respiratory distress syndrome
- COP
- cryptogenic organizing pneumonia
- CTLA-4
- cytotoxic T-lymphocyte antigen–4
- EGFR
- epidermal growth factor receptor
- FDA
- U.S. Food and Drug Administration
- GGO
- ground-glass opacity
- HER2
- human epidermal growth factor receptor 2
- irAE
- immune-related adverse event
- mTOR
- mammalian target of rapamycin
- NSCLC
- non–small cell lung cancer
- NSIP
- nonspecific interstitial pneumonia
- PD-1
- programmed cell death protein 1
- PD-L1
- programmed cell death ligand 1
- VEGF
- vascular endothelial growth factor
References
- 1.Erasmus JJ, McAdams HP, Rossi SE. High-resolution CT of drug-induced lung disease. Radiol Clin North Am 2002;40(1):61–72. [DOI] [PubMed] [Google Scholar]
- 2.Nishino M, Boswell EN, Hatabu H, Ghobrial IM, Ramaiya NH. Drug-related pneumonitis during mammalian target of rapamycin inhibitor therapy: radiographic pattern-based approach in Waldenström macroglobulinemia as a paradigm. Oncologist 2015;20(9):1077–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188(6):733–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Müller NL, White DA, Jiang H, Gemma A. Diagnosis and management of drug-associated interstitial lung disease. Br J Cancer 2004;91(suppl 2):S24–S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Min JH, Lee HY, Lim H, et al. Drug-induced interstitial lung disease in tyrosine kinase inhibitor therapy for non–small cell lung cancer: a review on current insight. Cancer Chemother Pharmacol 2011;68(5):1099–1109. [DOI] [PubMed] [Google Scholar]
- 6.Nishino M, Brais LK, Brooks NV, Hatabu H, Kulke MH, Ramaiya NH. Drug-related pneumonitis during mammalian target of rapamycin inhibitor therapy in patients with neuroendocrine tumors: a radiographic pattern-based approach. Eur J Cancer 2016;53:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishino M, Ramaiya NH, Awad MM, et al. PD-1 inhibitor–related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res 2016;22(24):6051–6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishino M, Tirumani SH, Ramaiya NH, Hodi FS. Cancer immunotherapy and immune-related response assessment: the role of radiologists in the new arena of cancer treatment. Eur J Radiol 2015;84(7):1259–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res 2013;19(19):5300–5309. [DOI] [PubMed] [Google Scholar]
- 10.Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy—inhibiting programmed death–ligand 1 and programmed death-1. Clin Cancer Res 2012;18(24):6580–6587. [DOI] [PubMed] [Google Scholar]
- 11.Boussiotis VA. Somatic mutations and immunotherapy outcome with CTLA-4 blockade in melanoma. N Engl J Med 2014;371(23):2230–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014;371(23):2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12(4):252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol 2013;14(12):1212–1218. [DOI] [PubMed] [Google Scholar]
- 15.Bronstein Y, Ng CS, Hwu P, Hwu WJ. Radiologic manifestations of immune-related adverse events in patients with metastatic melanoma undergoing anti-CTLA-4 antibody therapy. AJR Am J Roentgenol 2011;197(6): W992–W1000. [DOI] [PubMed] [Google Scholar]
- 16.Tirumani SH, Ramaiya NH, Keraliya A, et al. Radiographic profiling of immune-related adverse events in advanced melanoma patients treated with ipilimumab. Cancer Immunol Res 2015;3(10):1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 2015;33(18):2004–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366(26):2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372(21):2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of programmed cell death 1 inhibitor–related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol 2016;2(12):1607–1616. [DOI] [PubMed] [Google Scholar]
- 21.Nishino M, Chambers ES, Chong CR, et al. Anti-PD-1 inhibitor–related pneumonitis in non-small cell lung cancer. Cancer Immunol Res 2016;4(4):289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishino M, Sholl LM, Hodi FS, Hatabu H, Ramaiya NH. Anti-PD-1–related pneumonitis during cancer immunotherapy. N Engl J Med 2015;373(3):288–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiset PO, Shapera S, Butler MO, Tsao MS. Anti-PD-1–associated organizing pneumonia in a responding melanoma patient. Ann Oncol 2016;27(8):1649–1650. [DOI] [PubMed] [Google Scholar]
- 24.Fragkou P, Souli M, Theochari M, Kontopoulou C, Loukides S, Koumarianou A. A case of organizing pneumonia (OP) associated with pembrolizumab. Drug Target Insights 2016;10:9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gounant V, Brosseau S, Naltet C, et al. Nivolumab-induced organizing pneumonitis in a patient with lung sarcomatoid carcinoma. Lung Cancer 2016;99:162–165. [DOI] [PubMed] [Google Scholar]
- 26.Sano T, Uhara H, Mikoshiba Y, et al. Nivolumab-induced organizing pneumonia in a melanoma patient. Jpn J Clin Oncol 2016;46(3):270–272. [DOI] [PubMed] [Google Scholar]
- 27.Nishino M, Ramaiya NH, Hatabu H, Hodi FS, Armand PF. PD-1 inhibitor–related pneumonitis in lymphoma patients treated with single-agent pembrolizumab therapy. Br J Haematol doi:10.1111/bjh.14441. Published online November 11, 2016. [DOI] [PubMed]
- 28.Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol 2017;35(7):709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol 2016;2(10): 1346–1353. [DOI] [PubMed] [Google Scholar]
- 30.Holmes D. PI3K pathway inhibitors approach junction. Nat Rev Drug Discov 2011;10(8):563–564. [DOI] [PubMed] [Google Scholar]
- 31.Duran I, Siu LL, Oza AM, et al. Characterisation of the lung toxicity of the cell cycle inhibitor temsirolimus. Eur J Cancer 2006;42(12):1875–1880. [DOI] [PubMed] [Google Scholar]
- 32.Dabydeen DA, Jagannathan JP, Ramaiya N, et al. Pneumonitis associated with mTOR inhibitors therapy in patients with metastatic renal cell carcinoma: incidence, radiographic findings and correlation with clinical outcome. Eur J Cancer 2012;48(10):1519–1524. [DOI] [PubMed] [Google Scholar]
- 33.Maroto JP, Hudes G, Dutcher JP, et al. Drug-related pneumonitis in patients with advanced renal cell carcinoma treated with temsirolimus. J Clin Oncol 2011;29(13): 1750–1756. [DOI] [PubMed] [Google Scholar]
- 34.Soria JC, Shepherd FA, Douillard JY, et al. Efficacy of everolimus (RAD001) in patients with advanced NSCLC previously treated with chemotherapy alone or with chemotherapy and EGFR inhibitors. Ann Oncol 2009;20(10):1674–1681. [DOI] [PubMed] [Google Scholar]
- 35.Jänne PA, Gurubhagavatula S, Yeap BY, et al. Outcomes of patients with advanced non-small cell lung cancer treated with gefitinib (ZD1839, “Iressa”) on an expanded access study. Lung Cancer 2004;44(2):221–230. [DOI] [PubMed] [Google Scholar]
- 36.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350(21):2129–2139. [DOI] [PubMed] [Google Scholar]
- 37.Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304(5676):1497–1500. [DOI] [PubMed] [Google Scholar]
- 38.Nishino M, Hatabu H, Johnson BE, McLoud TC. State of the art: response assessment in lung cancer in the era of genomic medicine. Radiology 2014;271(1):6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372(18):1689–1699. [DOI] [PubMed] [Google Scholar]
- 40.Burotto M, Manasanch EE, Wilkerson J, Fojo T. Gefitinib and erlotinib in metastatic non-small cell lung cancer: a meta-analysis of toxicity and efficacy of randomized clinical trials. Oncologist 2015;20(4):400–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gemma A, Kudoh S, Ando M, et al. Final safety and efficacy of erlotinib in the phase 4 POLARSTAR surveillance study of 10 708 Japanese patients with non-small-cell lung cancer. Cancer Sci 2014;105(12):1584–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kudoh S, Kato H, Nishiwaki Y, et al. Interstitial lung disease in Japanese patients with lung cancer: a cohort and nested case-control study. Am J Respir Crit Care Med 2008;177(12):1348–1357. [DOI] [PubMed] [Google Scholar]
- 43.Ahn MJ, Yang J, Yu H, et al. 136O: Osimertinib combined with durvalumab in EGFR-mutant non-small cell lung cancer: results from the TATTON phase Ib trial [abstr]. J Thorac Oncol 2016;11(4 suppl):S115. [Google Scholar]
- 44.Precautions relating to interstitial lung disease during administration of epidermal growth factor receptor tyrosine kinase inhibitors . Pharmaceuticals and Medical Devices Safety Information No. 336. https://www.pmda.go.jp/files/000214024.pdf#page=4. Published September 2016.
- 45.Kotake M, Murakami H, Kenmotsu H, Naito T, Takahashi T. High incidence of interstitial lung disease following practical use of osimertinib in patients who had undergone immediate prior nivolumab therapy. Ann Oncol 2017;28(3):669–670. [DOI] [PubMed] [Google Scholar]
- 46.Johnson BE. Divide and conquer to treat lung cancer. N Engl J Med 2016;375(19):1892–1893. [DOI] [PubMed] [Google Scholar]
- 47.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non-small-cell lung cancer. N Engl J Med 2016;375(19):1823–1833. [DOI] [PubMed] [Google Scholar]
- 48.Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol 2012;13(10):1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363(18):1693–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368(25):2385–2394. [DOI] [PubMed] [Google Scholar]
- 51.Asai N, Yokoi T, Yamaguchi E, Kubo A. Successful crizotinib rechallenge after crizotinib-induced organizing pneumonia in anaplastic lymphoma kinase-rearranged non-small cell lung cancer. Case Rep Oncol 2014;7(3):681–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tamiya A, Okamoto I, Miyazaki M, Shimizu S, Kitaichi M, Nakagawa K. Severe acute interstitial lung disease after crizotinib therapy in a patient with EML4-ALK-positive non-small-cell lung cancer. J Clin Oncol 2013;31(1):e15–e17. doi:10.1200/JCO.2012.43.3730. Published online November 19, 2012. [DOI] [PubMed] [Google Scholar]
- 53.Zelnak AB, Wisinski KB. Management of patients with HER2-positive metastatic breast cancer: is there an optimal sequence of HER2-directed approaches? Cancer 2015;121(1):17–24. [DOI] [PubMed] [Google Scholar]
- 54.Pepels MJ, Boomars KA, van Kimmenade R, Hupperets PS. Life-threatening interstitial lung disease associated with trastuzumab: case report. Breast Cancer Res Treat 2009;113(3):609–612. [DOI] [PubMed] [Google Scholar]
- 55.Bettini AC, Tondini C, Poletti P, Caremoli ER, Guerra U, Labianca R. A case of interstitial pneumonitis associated with Guillain-Barré syndrome during administration of adjuvant trastuzumab. Tumori 2008;94(5):737–741. [DOI] [PubMed] [Google Scholar]
- 56.Radzikowska E, Szczepulska E, Chabowski M, Bestry I. Organising pneumonia caused by transtuzumab (Herceptin) therapy for breast cancer. Eur Respir J 2003;21(3):552–555. [DOI] [PubMed] [Google Scholar]
- 57.Shanehbandi D, Majidi J, Kazemi T, Baradaran B, Aghebati-Maleki L. CD20-based immunotherapy of B-cell derived hematologic malignancies. Curr Cancer Drug Targets 2017;17(5):423–444. [DOI] [PubMed] [Google Scholar]
- 58.Lim SH, Beers SA, French RR, Johnson PW, Glennie MJ, Cragg MS. Anti-CD20 monoclonal antibodies: historical and future perspectives. Haematologica 2010;95(1):135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hadjinicolaou AV, Nisar MK, Parfrey H, Chilvers ER, Ostör AJ. Non-infectious pulmonary toxicity of rituximab: a systematic review. Rheumatology (Oxford) 2012;51(4):653–662. [DOI] [PubMed] [Google Scholar]
- 60.Eckert A, Schoeffler A, Dalle S, Phan A, Kiakouama L, Thomas L. Anti-CTLA4 monoclonal antibody induced sarcoidosis in a metastatic melanoma patient. Dermatology 2009;218(1):69–70. [DOI] [PubMed] [Google Scholar]
- 61.Berthod G, Lazor R, Letovanec I, et al. Pulmonary sarcoid-like granulomatosis induced by ipilimumab. J Clin Oncol 2012;30(17):e156–e159. [DOI] [PubMed] [Google Scholar]
- 62.Jitawatanarat P, Wee W. Update on antiangiogenic therapy in colorectal cancer: aflibercept and regorafenib. J Gastrointest Oncol 2013;4(2):231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nazer B, Humphreys BD, Moslehi J. Effects of novel angiogenesis inhibitors for the treatment of cancer on the cardiovascular system: focus on hypertension. Circulation 2011;124(15):1687–1691. [DOI] [PubMed] [Google Scholar]
- 64.Kilickap S, Abali H, Celik I. Bevacizumab, bleeding, thrombosis, and warfarin. J Clin Oncol 2003;21(18):3542; author reply 3543. [DOI] [PubMed] [Google Scholar]
- 65.Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 2004;22(11):2184–2191. [DOI] [PubMed] [Google Scholar]
- 66.Vahid B, Marik PE. Pulmonary complications of novel antineoplastic agents for solid tumors. Chest 2008;133(2): 528–538. [DOI] [PubMed] [Google Scholar]
- 67.Souza FF, Smith A, Araujo C, et al. New targeted molecular therapies for cancer: radiological response in intrathoracic malignancies and cardiopulmonary toxicity: what the radiologist needs to know. Cancer Imaging 2014;14:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol 2003;21(1):60–65. [DOI] [PubMed] [Google Scholar]
- 69.Shinagare AB, Guo M, Hatabu H, et al. Incidence of pulmonary embolism in oncologic outpatients at a tertiary cancer center. Cancer 2011;117(16):3860–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Katta A, Fesler MJ, Tan A, Vuong G, Richart JM. Spontaneous bilateral pneumothorax in metastatic renal cell carcinoma on sunitinib therapy. Cancer Chemother Pharmacol 2010;66(2):409–412. [DOI] [PubMed] [Google Scholar]
- 71.Interiano RB, McCarville MB, Wu J, Davidoff AM, Sandoval J, Navid F. Pneumothorax as a complication of combination antiangiogenic therapy in children and young adults with refractory/recurrent solid tumors. J Pediatr Surg 2015;50(9):1484–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Verschoor AJ, Gelderblom H. Pneumothorax as adverse event in patients with lung metastases of soft tissue sarcoma treated with pazopanib: a single reference centre case series. Clin Sarcoma Res 2014;4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakano K, Motoi N, Tomomatsu J, et al. Risk factors for pneumothorax in advanced and/or metastatic soft tissue sarcoma patients during pazopanib treatment: a single-institute analysis. BMC Cancer 2016;16(1):750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nakamura T, Matsumine A, Kawai A, et al. The clinical outcome of pazopanib treatment in Japanese patients with relapsed soft tissue sarcoma: a Japanese Musculoskeletal Oncology Group (JMOG) study. Cancer 2016;122(9):1408–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med 2006;354(24):2531–2541. [DOI] [PubMed] [Google Scholar]
- 76.Kantarjian H, Pasquini R, Hamerschlak N, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia after failure of first-line imatinib: a randomized phase 2 trial. Blood 2007;109(12):5143–5150. [DOI] [PubMed] [Google Scholar]
- 77.Powell CA. Pulmonary infiltrates in a patient with advanced melanoma. J Clin Oncol 2017;35(7):705–708. [DOI] [PubMed] [Google Scholar]
- 78.Castanon E. Anti-PD1–induced pneumonitis: capturing the hidden enemy. Clin Cancer Res 2016;22(24):5956–5958. [DOI] [PubMed] [Google Scholar]