Abstract
Aim
Polycystic kidney disease (PKD) in humans involves kidney cyst expansion beginning in utero. Recessive PKD can result in end-stage renal disease (ESRD) within the first decade, whereas autosomal dominant PKD (ADPKD), caused by mutations in the PKD1 or PKD2 gene, typically leads to ESRD by the fifth decade of life. Inhibition of mTOR signalling was recently found to halt cyst formation in adult ADPKD mice. In contrast, no studies have investigated potential treatments to prevent cyst formation in utero in recessive PKD. Given that homozygous Pkd1 mutant mice exhibit cyst formation in utero, we decided to investigate whether mTOR inhibition in utero ameliorates kidney cyst formation in foetal Pkd1 homozygous mutant mice. Methods: Pregnant Pkd1+/− female mice (mated with Pkd1+/− male mice) were treated with rapamycin from E14.5 to E17.5. Foetal kidneys were dissected, genotyped and evaluated by cyst size as well as expression of the developmental marker, Pax2.
Results
Numerous cysts were present in Pkd1−/− kidneys, which were twice the weight of wild-type kidneys. Cyst size was reduced by a third in rapamycin-treated Pkd1−/− kidney sections and kidney mass was reduced to near wild-type levels. However, total cyst number was not reduced compared with control embryos. Pax2 expression and kidney development were unaltered in rapamycin-treated mice but some lethality was observed in Pkd1−/− null embryos.
Conclusion
Rapamycin treatment reduces cyst formation in Pkd1−/− mutant mice; therefore, the prevention of kidney cyst expansion in utero by mTOR inhibition is feasible. However, selective rapamycin-associated lethality limits its usefulness as a treatment in utero.
Keywords: cystic kidney disease, developmental nephrology, mTOR, Pax2, sirolimus
Inherited polycystic kidney diseases (PKD) constitute an important subset of single-gene disorders that are transmitted as autosomal dominant (ADPKD), autosomal recessive (ARPKD) or X-linked traits, and are responsible for more than 5% of the worldwide total of end-stage renal disease.1 The development of fluid-filled cysts and progressive impairment of renal function are common features, but these disorders are distinguished from each other by different ages of onset, variable rates of renal disease progression and a diverse array of extra-renal manifestations.1,2 The two major types of PKD in humans are ADPKD and ARPKD. ARPKD may be diagnosed prenatally,3 and although less common than ADPKD, it develops in the first decade of life. ADPKD, in contrast, is a frequent genetic disorder affecting between one in 400 and one in 1000 individuals,4 characterized by progressive kidney cyst formation, beginning in utero5 and typically leading to greatly enlarged kidneys and end-stage renal disease by late middle age. Up to 10% of all patients requiring renal transplantation or dialysis have ADPKD.4 Over 85% of ADPKD cases are associated with mutations in the PKD1 gene, which codes for polycystin-1. This membrane-bound protein is postulated to be part of a cell signalling cascade involving the primary cilia at the plasma membrane; it also has a role in mediating cell adhesion at the lateral membrane.6
Multiple different treatments for ADPKD are in clinical trials, but these are limited to adults and no treatments have been evaluated for the treatment of PKD in the foetal kidney, the site of cyst development in ARPKD. A retrospective study of kidney volumes in patients with rapamycin (sirolimus) treatment as part of their renal replacement therapy showed a 24.8% decrease in the volume of their remaining cystic kidney over 24 months.7 Rapamycin administration in several mouse and rat models of PKD has been shown to slow cystic disease in adult animals.8,9 However, results of several placebo-controlled clinical trials to establish the safety and efficacy of rapamycin (also known as Rapamune), or its biochemically related analogue Everolimus, in treating ADPKD, have been mixed. One study reported no slowing of cystic kidney growth after 18 months of treatment,10 while 2 years of Everolimus treatment was able to slow the increase in total kidney volume but did not halt the progression of renal impairment.11 It was noted that treatment earlier in the course of the disease, or for a longer period, might show more of the significant benefits that have been found in the (usually) earlier onset rodent models of PKD.10
As well as inhibiting proliferation of mammalian cells, rapamycin has immunosuppressive properties and has been used in some ADPKD patients to prevent transplant rejection following renal transplantation. The mammalian target of rapamycin (mTOR) is a conserved Ser/Thr kinase, originally identified in yeast by mutations that confer resistance to the growth-inhibitory properties of rapamycin.12 Several recent studies have focused on the mTOR pathway, which is just one of a number of signalling pathways that have been implicated as being downstream of polycystin-1.7–14 The mTOR pathway integrates various upstream signals to regulate cell growth via the mTOR complex 1 (mTORC1).
The ADPKD cystic epithelium has been shown to overexpress a number of developmental genes, including PAX2,15,16 which is a transcription factor with an important role in kidney development. PAX2 expression is normally down-regulated following birth. We have previously shown that reducing Pax2 levels in a Pkd1 mutant ADPKD mouse model (by crossing Pax2 and Pkd1 heterozygous mice) resulted in a dramatic reduction in kidney size compared with the Pkd1 mouse kidneys alone, accompanied by a reduction in cyst formation.15 Others have seen a similar effect in mice with recessive PKD.16
The aim of this study was to investigate whether cyst formation in the del34 Pkd1 mouse model of ADPKD in utero is slowed by rapamycin treatment. We found that administration of rapamycin to Pkd1−/− embryonic mice was able to restore normal kidney volume and limit cyst expansion in utero, but it did not significantly change the number of cysts in comparison with untreated or vehicle-treated controls. However, rapamycin treatment resulted in significant selective lethality of Pkd1−/− null embryos, although it did not affect kidney development or change the expression pattern of Pax2. Overall, these data suggest that while the inhibition of mTOR during cyst expansion in utero is feasible, a significant level of selective rapamycin-associated lethality would likely limit its usefulness in utero.
METHODS
Mice breeding and procedures
All experiments in this study followed international principles of laboratory animal care and were approved by the University of Otago Animal Ethics Committee. Pkd1+/+ wild-type embryos and Pkd1−/− (intron 34 deleted) embryos were obtained by mating C3H Pkd1+/− parents and genotyping offspring as previously described.15 A daily rapamycin (Industrial Research Limited, Wellington, New Zealand) dose of 1 mg per kilogram of the mother’s body weight was administered every 24 h as intraperitoneal injections from E14.5 to E17.5. The dose of rapamycin was based on the reported use of 5 mg/kg in utero,17 the amount that was used daily in postnatal mice,7 and also on our own testing of several doses of rapamycin in C3H pregnant female mice in late gestation between 0.1 mg/kg and 5 mg/kg. Vehicle-treated mice were given injections of the rapamycin solvent: 21.4% DMSO, 21.4% ethanol and 57.2% saline solution (0.9% sodium chloride). Embryos were isolated at E18.5 and weighed. Embryo viability was assessed at the time of dissection by visual inspection of movement of the embryo, the opening and closing of the mouth, coloration of the foetus and the condition of the internal organs. Both kidneys were removed and their wet mass determined. One kidney, to be used for RNA and protein extraction, was frozen with liquid nitrogen and stored at −80°C. The other kidney, used for histological purposes, was fixed in 4% paraformaldehyde for 1–2 h on ice and then stored in 70% ethanol at 4°C until it was embedded in paraffin. DNA was extracted from embryo tails and genotyped by PCR for the wild-type and mutant (exon 34-deleted) alleles of Pkd1: intron 34 (wild-type and mutant reverse primer) 5′-CTTTAATCCCTGCACTCAGGA-3′, exon 34 (wild-type forward primer) 5′-CTGATCCATCAGGTACTGGCT-3′ and Neo cassette (mutant forward primer) 5′-CAGCGCATCGCCTTCTATC-3′.
Histology
Paraffin blocks were serially sectioned at 4 μm and 9–10 sections were selected per block in a randomized, systematic manner for histological analysis. Sections were mounted on glass slides and stained with haematoxylin and eosin. Images of these stained kidneys were acquired. A grid was placed over the images, and the cystic index was calculated as the percentage of grid intersection points that bisected lumen/cystic or non-lumen/cystic areas.
Analysis of cyst number and size
For the detection of the number and size of cyst/luminal spaces in renal sections, the middle section of each kidney was selected and stained with haematoxylin and eosin. Images were acquired and analysed in Photoshop using Fovea Pro (Reindeer Graphics Inc., Asheville, NC, USA). More specifically, images were normalized in tone and background, with cysts selected on the basis of colour using Photoshop’s ‘Colour Range’ selection tool, then filling the selections and thresholding the image to leave a binary silhouette of the cysts. Fovea Pro plugins were employed to calculate the data on cyst number and size in each section. Images were calibrated to present areas in square microns (μm2) with lumenal areas below 10 μm2 being discarded. Sections were scrutinized and adjusted manually to ensure all lumens were represented and that none were selected more than once. Data generated were manipulated and represented graphically in Microsoft Excel.
Real-time QRT-PCR
Total RNA was extracted from kidneys using TriReagent (Molecular Research Centre, Inc., Cincinnati, OH, USA) and an RNeasy kit (Qiagen, Valencia, CA, USA). This was then reverse-transcribed to cDNA using a mixture of oligo dT and random hexamers as primers. The real-time PCRs were carried out in duplicate using SYBR Green PCR Master Mix (Invitrogen, Carlsband, CA, USA) and an ABI 7300 machine (Applied biosystems, Carlsband, CA, USA). Gapdh and Hprt1 were used as endogenous reference genes. Primers: Gapdh forward 5′-TGCACCACCAACTGCTTAGC-3′, Gapdh reverse 5′-GGC ATGGACTGTGGTCATGA-3′; Hprt forward 5′-GAGAGGTCCTTT-3′, Hprt reverse 5′-GCTTTCCCTGGT-3′; Pax2 forward 5′-TACTCT CTCCCAGCCCTGAC-3′, Pax2 reverse 5′-CCAGGTAGAGTqGG TGCTCGT-3′.
Immunoblot analysis
Kidneys were homogenized in cell lysis buffer (50 mM Tris HCl pH 8, 150 mM NaCl, 1 mM EDTA, 1% NP40, 0.1% SDS, 0.5% sodium deoxycholate, 1 mM PMSF, 1 μM sodium orthovanadate, complete mini EDTA-free protease inhibitors (Roche Diagnostics NZ Ltd, Auckland, New Zealand), PhosStop Inhibitors (Roche)), and protein quantified using a BCA assay (Pierce, Rockford, IL, USA). Lysates were electrophoresed on 8% SDS PAGEs and transferred to polyvinylidene fluoride membranes. The following antibodies were used in detection: rabbit anti-phospho-S6-ribosomal protein (Ser235/236) 1:1000 (Cell Signaling Technology, Danvers, MA, USA), rabbit anti-S6-ribosomal protein 1:1000 (Cell Signaling Technology), rabbit anti-Pax2 1:500 (Invitrogen), HRP-conjugated anti-rabbit IgG 1:2000 (Sigma-Aldrich, St Louis, MO, USA), mouse anti-β-tubulin 1:2000 (Developmental Studies Hybridoma Bank, Iowa City, IA, USA) and HRP-conjugated anti-mouse IgG 1:2000 (Sigma-Aldrich). Immunoblotting was carried out as described (15), except that a Fujifilm LAS-3000 was used for detecting images of the S6 and phospho-S6 results.
Immunohistochemistry
Unstained paraffin-embedded sections (4 μm) were rehydrated through three changes of xylene and a graded alcohol series and then rinsed in 1× PBS. Antigens were unmasked by boiling sections in a microwave on low power in 10 mM sodium citrate buffer (pH 7.0) for 10 min. Endogenous peroxidase activity was blocked by incubating sections in 3% H2O2 in distilled water for 10 min (adult mouse sections only). Endogenous avidin/biotin binding in embryonic mouse sections was blocked using egg white and biotin (20 min each) with a PBS wash in between incubations. Sections were rinsed in PBS and processed for immunostaining using the Vector Elite ABC Universal kit (VectorLabs, Burlingame, CA, USA) and a diaminobenzidine tetrachloride (DAB) substrate kit (VectorLabs) according to the manufacturer’s protocol. Sections were counterstained with haematoxylin, dehydrated through a graded alcohol series followed by xylene and mounted with Permount (VectorLabs). The Pax2 primary antibody was rabbit anti-Pax2 (Zymed, San Francisco, CA, USA) (1:50). The secondary antibody was a biotinylated goat anti-rabbit (ABC kit; Vector Laboratories, Burlingame, CA, USA).
Pax2+/− and Pkd1−/−cond/cond : Nestincre kidney sections
Renal sections from Pkd1−/− mice that had been crossed with a Pax2+/−mutant mice, and were therefore haploinsufficient for Pax2 (details in Stayner et al.15), were subject to Pax2 immunohistochemistry as described above. Pkd1−/−cond/cond : Nestincre renal sections from 49 day-old mice that had been treated with vehicle or rapamycin for 21 days (details in Shillingford et al.9), along with age-matched controls, were also investigated using Pax2 immunohistochemical staining, as described in the previous section.
RESULTS
Pkd1−/− del34 homozygous knockout mice develop cysts in utero from embryonic day 15.5, and die just after birth.18 We treated pregnant Pkd1+/− female mice (that had been mated with Pkd1+/− male mice) with rapamycin at 1 mg per kilogram of the mother’s body weight. The drug was administered at the same time each day from embryonic day 14.5 to embryonic day 17.5 as intraperitoneal injections. Vehicle-treated mice were given injections of the rapamycin solvent alone. The pregnant mice and embryos were sacrificed at embryonic day 18.5 (just prior to birth) and the embryos isolated and weighed. Embryonic kidneys were removed, their mass determined and these were then processed for either RNA/protein extraction or histological analysis. A significant but low level of rapamycin treatment-related mortality occurred in Pkd1−/− embryos (Table 1).
Table 1.
Mortality of untreated, vehicle-treated and rapamycin-treated mouse embryos
| Treatment | Number of litters analysed† | Number of embryos analysed† | Average litter size‡ | % of embryos that were dead at E18.5§ | % of Pkd1−/− embryos that were dead at E18.5§ |
|---|---|---|---|---|---|
| Untreated | 9 | 68 | 7.6 ± 0.8 | 7.4 | 6.7 |
| Vehicle | 9 | 65 | 7.2 ± 0.6 | 7.7 | 12.5 |
| Rapamycin | 21 | 151 | 7.2 ± 0.4 | 9.9 | 43.5 |
Values are †the total number of litters or embryos analysed, ‡the mean litter size ± the margin of error and §the percentage (%) of dead embryos. A small increase in embryo death was noted following daily administration of rapamycin from E14.5 to E17.5 (1 mg/kg). However, the proportion of Pkd1−/− embryos from rapamycin-treated litters that were dead at E18.5 increased by 3.5-fold compared with vehicle treatment alone.
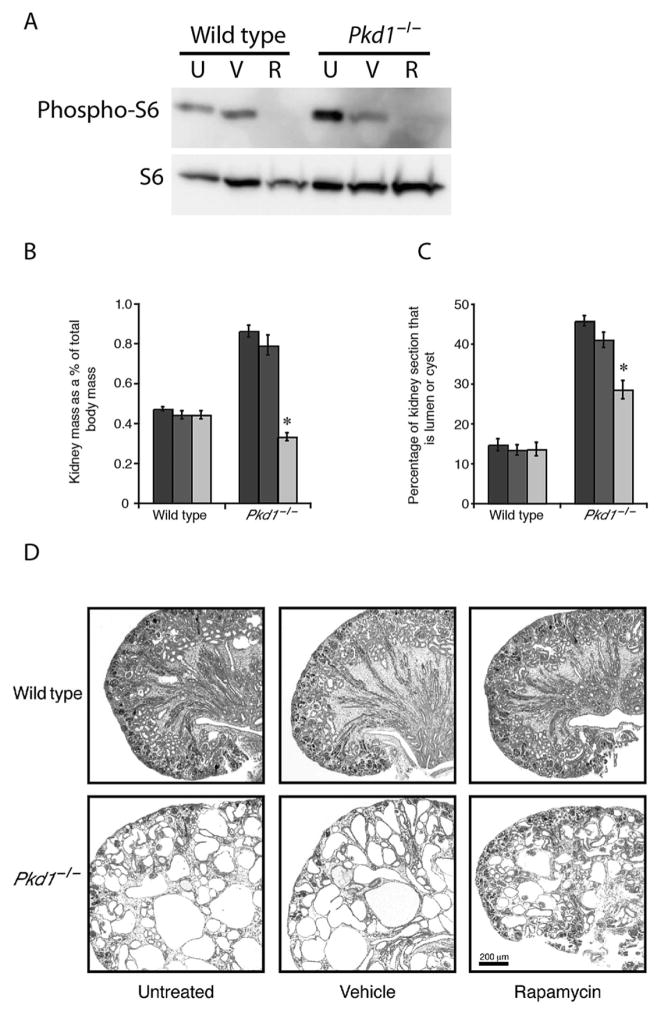
As observed in previous studies15,18 Pkd1−/− foetal kidneys which contained numerous cysts were twice the weight of wild-type kidneys (see Fig. 1, Table S1) and the relative surface area encompassed by cysts or lumen in individual sections was three times higher than in wild-type mice. The rapamycin treatment inhibited the phosphorylation of S6 protein, a downstream target of mTOR signalling, in immunoblots of wild-type as well as Pkd1−/− E18.5 kidneys (Fig. 1A), indicating that the dosage and the delivery of rapamycin in the kidneys in utero in foetal mice were adequate to inhibit the mTOR pathway. Rapamycin treatment reduced the proportion of cyst formation in Pkd1−/− kidney sections by a third, and the kidney mass in Pkd1−/− embryos exposed to rapamycin returned to near wild-type levels. When examined histologically, cysts were still apparent in the Pkd1−/−kidneys treated with rapamycin but were reduced in size (Fig. 1D).
Fig. 1.
Rapamycin treatment reduces the cystic kidney mass and lumen/cyst area, and improves the cystic phenotype in E18.5 Pkd1−/− embryos. (A) An immunoblot of kidney lysates probed with an anti-phospho-S6 antibody showed that untreated (U) and vehicle-treated (V) E18.5 wild-type and Pkd1−/− kidneys contained the phosphorylated S6 protein, whereas the rapamycin-treated (R) E18.5 wild-type and Pkd1−/− kidneys did not contain phosphorylated S6 protein. The same blot was then stripped and re-probed using anti-S6 antibody, which showed that all samples expressed S6 protein. These data confirm that the rapamycin treatment inhibited the mTOR pathway in foetal kidneys in utero. (B) The (
 ) untreated and (
) untreated and (
 ) vehicle-treated foetal kidneys from Pkd1−/− embryos were twice the weight of their wild-type counterparts. Treatment with (
) vehicle-treated foetal kidneys from Pkd1−/− embryos were twice the weight of their wild-type counterparts. Treatment with (
 ) rapamycin was successful at reducing kidney mass in Pkd1−/− embryos, such that at E18.5 they were of comparable weight to wild-type kidneys. Kidney mass is displayed as a percentage of total body mass. Error bars show standard error of the mean. n = 14–30 kidneys, both kidneys from each embryo were included. *P < 0.001 when compared with vehicle-treated Pkd1−/−. (C) The (
) rapamycin was successful at reducing kidney mass in Pkd1−/− embryos, such that at E18.5 they were of comparable weight to wild-type kidneys. Kidney mass is displayed as a percentage of total body mass. Error bars show standard error of the mean. n = 14–30 kidneys, both kidneys from each embryo were included. *P < 0.001 when compared with vehicle-treated Pkd1−/−. (C) The (
 ) untreated and (
) untreated and (
 ) vehicle-treated Pkd1−/− kidney sections were composed of approximately 40% cyst/lumen, which is three times more than that observed in wild-type kidney sections. Wild-type kidney sections were composed of approximately 15% cyst/lumen. Treatment with (
) vehicle-treated Pkd1−/− kidney sections were composed of approximately 40% cyst/lumen, which is three times more than that observed in wild-type kidney sections. Wild-type kidney sections were composed of approximately 15% cyst/lumen. Treatment with (
 ) rapamycin reduced the proportion of lumen/cyst in Pkd1−/− kidney sections by a third. Error bars show the standard error of the proportion. n = 4 kidneys in each group. *P < 0.001 when compared with vehicle-treated Pkd1−/−. (D) The cystic phenotype was visibly improved in rapamycin-treated Pkd1−/− kidneys compared with untreated and vehicle-treated kidneys. Kidneys were imaged under 50× magnification. Scale bar, 200 μm.
) rapamycin reduced the proportion of lumen/cyst in Pkd1−/− kidney sections by a third. Error bars show the standard error of the proportion. n = 4 kidneys in each group. *P < 0.001 when compared with vehicle-treated Pkd1−/−. (D) The cystic phenotype was visibly improved in rapamycin-treated Pkd1−/− kidneys compared with untreated and vehicle-treated kidneys. Kidneys were imaged under 50× magnification. Scale bar, 200 μm.
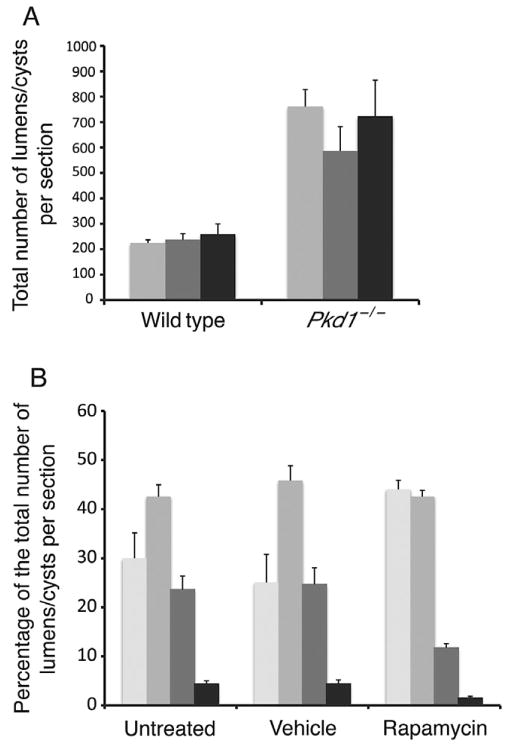
To determine whether rapamycin treatment in utero reduced total cyst number, we carried out image analysis of our untreated, vehicle-treated and rapamycin-treated kidneys. As shown in Figure 2A, treatment with rapamycin does not significantly reduce the number of cysts/lumens present in the kidney. However, rapamycin treatment does change the size distribution of the cysts (Fig. 2B). In kidneys from Pkd1−/− E18.5 embryos that have been exposed to rapamycin in utero, the percentage of cysts sized above 1000 μm2 was more than halved, and there was a corresponding increase in the number of smaller cysts (under 100 μm2, P < 0.05). Together, these data suggest that rapamycin reduced the capacity for cyst expansion, and did not significantly affect the initiation of cyst formation.
Fig. 2.
Rapamycin treatment of Pkd1−/− embryos in utero reduces cyst size but not cyst number. (A) Image analysis was used to detect the number of luminal spaces present in wild-type and Pkd1−/− E18.5 kidney sections ((
 ) untreated, (
) untreated, (
 ) vehicle-treated and (■) rapamycin-treated). There was no significant difference in the number of lumens/cysts in untreated compared with rapamycin-treated Pkd1−/− kidney sections. Error bars show standard error of the mean. n = 4–8 kidneys for each genotype and treatment group. (B) The size distributions of cystic/luminal spaces in Pkd1−/− kidney sections treated with vehicle or rapamycin were similar when compared with untreated kidney sections. However, a decrease in the largest cyst sizes (1000–9999 and 10 000+ μm2) in rapamycin-treated sections was accompanied by an increase in the number of smaller cysts/lumens (<99 μm2) (P < 0.05). Error bars show standard error of the proportion. n = 4–5 kidneys for each genotype and treatment group. Size of lumen/cyst (μm2): (
) vehicle-treated and (■) rapamycin-treated). There was no significant difference in the number of lumens/cysts in untreated compared with rapamycin-treated Pkd1−/− kidney sections. Error bars show standard error of the mean. n = 4–8 kidneys for each genotype and treatment group. (B) The size distributions of cystic/luminal spaces in Pkd1−/− kidney sections treated with vehicle or rapamycin were similar when compared with untreated kidney sections. However, a decrease in the largest cyst sizes (1000–9999 and 10 000+ μm2) in rapamycin-treated sections was accompanied by an increase in the number of smaller cysts/lumens (<99 μm2) (P < 0.05). Error bars show standard error of the proportion. n = 4–5 kidneys for each genotype and treatment group. Size of lumen/cyst (μm2): (
 ) <99; (
) <99; (
 ) 100–999; (
) 100–999; (
 ) 1000–9999; (■) 10 000+.
) 1000–9999; (■) 10 000+.
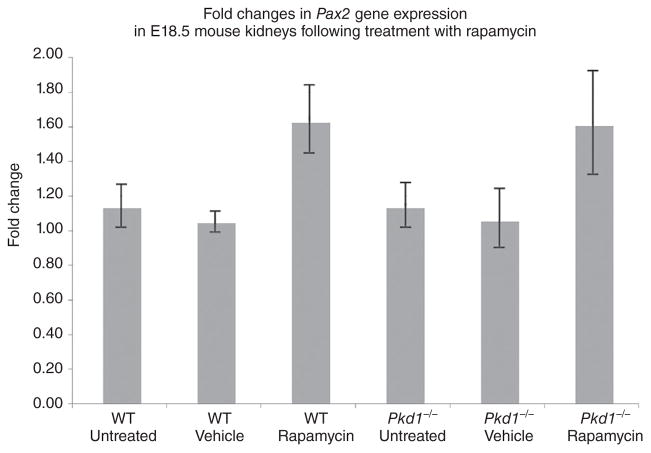
Rapamycin treatment in utero did not appear to affect normal kidney development, as the architecture of the rapamycin-treated foetal kidneys and the kidney weights appeared normal in Figure 1 and Table S1. Rapamycin is known to inhibit cell proliferation and the entry of cells into S phase; therefore, we investigated whether altered levels of the renal developmental gene, Pax2, which has previously been associated with foetal kidney epithelial cell proliferation and development,15,16,19,20 might occur following the treatment of kidneys in utero with rapamycin. RNA was prepared from the contralateral E18.5 kidney (to that used for histology) and cDNA generated for real-time QRT-PCR. As seen in Figure 3, there was an approximately 1.5-fold increase in Pax2 transcript levels following rapamycin treatment of Pkd1−/− kidneys, but this was not statistically significant.
Fig. 3.
Fold changes in Pax2 mRNA expression following treatment with rapamycin. Real-time QRT-PCR analysis of the Pax2 mRNA in E18.5 mouse kidneys following treatment with rapamycin. No significant changes in Pax2 transcript levels were observed in response to rapamycin treatment. Error bars show the standard error of the mean. n = 8 kidneys for each genotype and treatment category. WT, wild type.
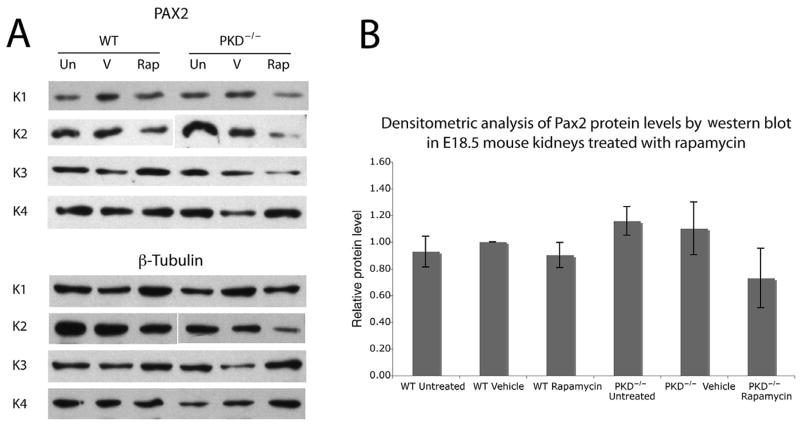
We also investigated whether changes occurred in the levels of Pax2 protein, which may indicate a change in protein turnover or degradation in response to rapamycin treatment. Figure 4 shows representative immunoblots for 24 E18.5 kidney samples probed with Pax2 (Fig. 4A, top) and the matching β-tubulin data (Fig. 4A, bottom) as well as a quantification of the bands (Fig. 4B). Although there is a small trend towards reduced Pax2 protein levels in Pkd1−/−kidneys treated with rapamycin, there was no statistically significant difference between the levels of Pax2 compared with those in the vehicle-treated Pkd1−/− kidneys.
Fig. 4.
Immunoblot analysis of Pax2 protein levels in E18.5 kidneys treated with rapamycin. (A) Immunoblot analysis of Pax2 in wild-type (WT) and Pkd1−/− kidneys (PKD−/−) after the following treatments: Un, untreated; V, vehicle only; Rap, 1 mg/mL rapamycin (daily for 4 days). The results from four separate kidneys are shown. Membranes were then stripped and re-probed with a mouse anti-β-tubulin antibody, which was used as a loading control. (B) A densitometric analysis of the Pax2 immunoblot showed no significant differences in the levels of Pax2 protein between treatment categories when compared with the loading control, β-tubulin.
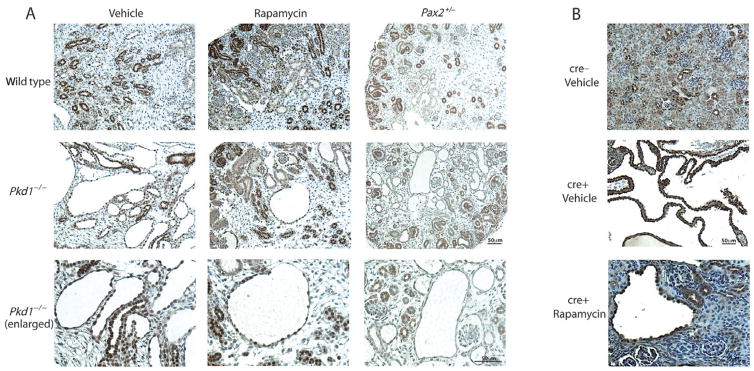
In a previously published study we observed persistence of Pax2 expression in the distal structures of cystic kidneys from E18.5 Pkd1−/− mutant foetal mice, which upon the reduction of cyst formation (by genetically reducing Pax2 dosage) was attenuated.15 We performed Pax2 immunohistochemistry on our Pkd1−/− kidneys to investigate if the distribution (rather than the amount) of Pax2 in the developing kidney may be altered after treatment with rapamycin. As shown in Figure 5A, no such difference in Pax2 expression patterns was noted following administration of rapamycin to Pkd1−/− kidneys. In addition, we investigated Pax2 expression in a postnatal model of Pkd1-induced cystogenesis, which, like our prenatal model, had been treated with rapamycin.9 Pax2 was expressed in many of the cells lining cysts in the kidney (Fig. 5B), matching what we have observed in the Pkd1−/− embryonic model of cystogenesis, and in human ADPKD.15 However, in the rapamycin-treated Pkd1cond/cond : Nestincre (cre+) mice Pax2 expression remained in the cyst-lining epithelia (Fig. 5B, lower panel), and this was indistinguishable from that in vehicle-treated mice.
Fig. 5.
Pax2 immunohistochemistry in rapamycin-treated mouse kidneys. (A) Pax2 immunohistochemistry of mouse embryonic kidneys from E18.5 (or E17.5 for the Pax2+/− mutant kidneys). The top three panels are Pkd1+/+ wild-type kidneys that have been treated with vehicle, rapamycin or carry a Pax2+/− mutation. Strong Pax2 staining is evident in the nephrogenic zone and collecting ducts. The middle three panels are Pkd1−/− mutant kidneys, with enlargements shown in the bottom three panels. Extensive cyst formation in the vehicle-treated kidneys (left column) is reduced with rapamycin treatment (middle column) or in the presence of a Pax2+/− mutation (right column). Both vehicle- and rapamycin-treated Pkd1−/− kidneys show strong Pax2 immunoreactivity in the cyst-lining epithelia. In contrast, Pax2 haploinsufficiency results in significantly reduced Pax2 expression in the cyst epithelia, when compared with the surrounding non-cystic Pax2-positive tubules (right column, and as published in Stayner et al.15). (B) Pax2 immunohistochemistry of 49 day-old Pkd1cond/cond mice (cre−) that exhibit normal kidney development and age-matched Pkd1cond/cond : Nestincre animals (cre+) with renal cysts (methods in Shillingford et al.9). Strong expression of Pax2 is noted in the cyst-lining cells of the cre+ kidneys. Daily rapamycin treatment of the cre+ animals for 21 days (from postnatal day 28) resulted in greatly reduced cyst formation (bottom panel) but Pax2 expression (positive brown stained nuclei) persisted in the cyst-lining epithelia of these postnatal kidneys, irrespective of rapamycin treatment. Scale bar, 50 μm.
DISCUSSION
Here we have shown that treatment of Pkd1−/− homozygous mutant mice with rapamycin in the period prior to and during cystogenesis (E14.5 to E17.5) was able to reduce the rate of cyst expansion in utero. However, despite the absence of any overt kidney developmental abnormalities, or differences in the amount or distribution of Pax2 expression in the rapamycin-treated kidneys, rapamycin treatment was found to result in a slight but significantly increased lethality in Pkd1−/− null mutant embryos, but at present we are unable to explain the reason for the selective lethal effect of rapamycin.
The treatment of the Pkd1−/− foetal mice with rapamycin in our experiments was started at E14.5, shortly before Pkd1 expression commences in the kidney at approximately E15.5,21 which is coincident with the appearance of cysts.15,21 The germline disruption of mTOR is known to be associated with early embryonic lethality,22,23 and rapamycin administration in embryonic mice up to E10.5 is known to be teratogenic.24 However, rapamycin treatment in our experiments did not cause any noticeable kidney developmental abnormalities. Moreover, similar to observations made previously following the treatment of E13.5 GSKβ−/− embryos for 48 h with rapamycin,17 we did not observe evidence for teratogenicity, and the wild-type embryos treated during this period did not show overt phenotypic abnormalities. These data suggest that while the mTOR pathway is required for tissue growth during embryogenesis, it may not be required for tissue patterning in the later stages of gestation.
Inhibition of mTOR with rapamycin in adult humans is associated with a broad range of side effects, and long-term administration may also block the mTORC225 arm of the mTOR complex, which is usually rapamycin-insensitive, causing off-target effects. In addition, rapamycin frequently causes a dramatic increase in proteinuria in human patients, which is often not reversible following discontinuation of the drug,26 but for which pulse treatment with rapamycin has been suggested as a possible solution.27 However, the future use of mTOR inhibitors in treating ADPKD, particularly for early and/or longer-term administration, most likely depends on a new generation of drugs that target the mTOR pathway more specifically, and/or at different locations.28,29
The data shown in Figures 3–5 suggest that although Pax2, which is associated with renal epithelial cell proliferation and differentiation15,16,19,20 and is a modifier of cyst growth,15,16 is expressed in the foetal kidneys, it is not directly or indirectly affected by inhibition of the mTOR pathway. We,15 and others,16,19,20 have demonstrated previously that persistent Pax2 expression is associated with cystic kidney disease, and that reduction of Pax2 levels in utero is associated with a delay in cyst expansion.15,16 While rapamycin treatment did not affect Pax2 mRNA levels in a renal tubular cell line,30 the experiments in that study did not investigate whether there were changes in the Pax2 protein level or the possibility of redistribution of expression patterns in foetal kidneys. Treatment of Pkd1−/− kidneys with rapamycin reduces cyst growth,9 and it is well known that rapamycin causes a G1 cell cycle arrest through inhibition of the mTOR pathway.23,24 We have shown here that while rapamycin treatment reduces cyst expansion (presumably by inhibiting proliferation), it does not reduce the total number of cysts/lumens in comparison with vehicle or untreated control Pkd1−/− mutant mice, suggesting that cysts/lumens in Pkd1−/− foetal kidneys were still able to form in the presence of rapamycin.
In conclusion, rapamycin treatment reduces the rate of cyst expansion in utero in del34 Pkd1−/− foetal mice, indicating that inhibiting the mTOR pathway without affecting kidney development is feasible. However, although rapamycin treatment did not affect gross kidney development or alter the levels or distribution of Pax2 expression, significant but low levels of late embryonic lethality were observed in PKD foetuses, and treatment with rapamycin did not appreciably reduce the total number of nascent cysts in the kidneys. As new and improved mTOR inhibitors become available it will be of interest to further determine whether these could exhibit fewer side effects than rapamycin in utero, and be more suitable for the slowing of renal cystic disease early in life.
Supplementary Material
Kidney (K) and total body weights (TBW) of rapamycin-treated and untreated wild-type and Pkd1−/−E18.5 foetal mice.
SUMMARY AT A GLANCE.
Stayner et al. investigated the effect of rapamycin on polycystic kidney disease in utero. Their data showed the potential beneficial effects of rapamycin administered during pregnancy in a rodent model of polycystic kidney disease, while cyst number remains unchanged; the size of the cysts is reduced by rapamycin treatment. Furthermore, they also showed that the inhibition of the mTOR pathway by rapamycin most likely is not mediated via Pax2 in their experimental animal model. The result provides evidence that mTOR inhibition may be therapeutically useful for slowing the progression of polycystic kidney disease with a prenatal onset.
Acknowledgments
Pkd1 del34 mice were a generous gift from Professor Jing Zhou. We thank Matt Hamilton, Cahal Mahon and Pam Cornes for genotyping the Pkd1 mice, Dr Euphemia Leung for assistance with immunoblotting and Dr Phil Sheard for assistance with image analysis. We are also grateful to Professor Paul Goodyer for his comments on the manuscript. This work was supported by a Lottery Health Grant to MRE and CS, an Otago Medical Research Foundation Laurenson Award and Healthcare Otago Charitable Trusts Grant to CS and a grant by the National Institutes of Health (DK078043) to TW. This work was also funded by a University of Otago Research Grant to MRE.
Footnotes
NOTE ADDED IN PROOF Recently, Qin et al. have also shown that rapamycin treatment did not affect Pax2 mRNA levels in immortalized murine Pkd1−/− cells. [Qin S, Taglienti M, Cai L, Zhou J, Kreidberg JA. c-Met and NF-κB-dependent overexpression of Wnt7a and -7b and Pax2 promotes cystogenesis in poly-cystic kidney disease. J. Am. Soc. Nephrol. 2012; doi: 10.1681/ASN.2011030277.]
References
- 1.Wilson PD. Polycystic kidney disease. N Engl J Med. 2004;350:151–64. doi: 10.1056/NEJMra022161. [DOI] [PubMed] [Google Scholar]
- 2.Guay-Woodford LM. Murine models of polycystic kidney disease: Molecular and therapeutic insights. Am J Physiol Renal Physiol. 2003;285:F1034–1049. doi: 10.1152/ajprenal.00195.2003. [DOI] [PubMed] [Google Scholar]
- 3.Zerres K, Mucher G, Becker J, et al. Prenatal diagnosis of autosomal recessive polycystic kidney disease (ARPKD): Molecular genetics, clinical experience, and fetal morphology. Am J Med Genet. 1998;76:137–44. [PubMed] [Google Scholar]
- 4.Torra Balcells R, Ars Criach E. Molecular diagnosis of autosomal dominant polycystic kidney disease. Nefrologia. 2011;31:35–43. doi: 10.3265/Nefrologia.pre2010.Nov.10727. [DOI] [PubMed] [Google Scholar]
- 5.Michaud J, Russo P, Grignon A, et al. Autosomal dominant polycystic kidney disease in the fetus. Am J Med Genet. 1994;51:240–46. doi: 10.1002/ajmg.1320510314. [DOI] [PubMed] [Google Scholar]
- 6.Boletta A. Emerging evidence of a link between the polycystins and the mTOR pathways. PathoGenetics. 2009;2:6. doi: 10.1186/1755-8417-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shillingford JM, Murcia NS, Larson CH, et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci US A. 2006;103:5466–71. doi: 10.1073/pnas.0509694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tao Y, Kim J, Schrier RW, Edelstein CL. Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease. J Am Soc Nephrol. 2005;16:46–51. doi: 10.1681/ASN.2004080660. [DOI] [PubMed] [Google Scholar]
- 9.Shillingford JM, Piontek KB, Germino GG, Weimbs T. Rapamycin ameliorates PKD resulting from conditional inactivation of Pkd1. J Am Soc Nephrol. 2010;21:489–97. doi: 10.1681/ASN.2009040421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serra AL, Poster D, Kistler AD, et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:820–29. doi: 10.1056/NEJMoa0907419. [DOI] [PubMed] [Google Scholar]
- 11.Walz G, Budde K, Mannaa M, et al. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:830–40. doi: 10.1056/NEJMoa1003491. [DOI] [PubMed] [Google Scholar]
- 12.Loewith R. A brief history of TOR. Biochem Soc Trans. 2011;39:437–42. doi: 10.1042/BST0390437. [DOI] [PubMed] [Google Scholar]
- 13.Wahl PR, Serra AL, Le Hir M, Molle KD, Hall MN, Wuthrich RP. Inhibition of mTOR with sirolimus slows disease progression in Han:SPRD rats with autosomal dominant polycystic kidney disease (ADPKD) Nephrol Dial Transplant. 2006;21:598–604. doi: 10.1093/ndt/gfi181. [DOI] [PubMed] [Google Scholar]
- 14.Zhou J, Brugarolas J, Parada LF. Loss of Tsc1, but not Pten, in renal tubular cells causes polycystic kidney disease by activating mTORC1. Hum Mol Genet. 2009;18:4428–41. doi: 10.1093/hmg/ddp398. [DOI] [PubMed] [Google Scholar]
- 15.Stayner C, Iglesias DM, Goodyer PR, et al. Pax2 gene dosage influences cystogenesis in autosomal dominant polycystic kidney disease. Hum Mol Genet. 2006;15:3520–28. doi: 10.1093/hmg/ddl428. [DOI] [PubMed] [Google Scholar]
- 16.Ostrom L, Tang MJ, Gruss P, Dressler GR. Reduced Pax2 gene dosage increases apoptosis and slows the progression of renal cystic disease. Dev Biol. 2000;219:250–58. doi: 10.1006/dbio.2000.9618. [DOI] [PubMed] [Google Scholar]
- 17.Liu KJ, Arron JR, Stankunas K, Crabtree GR, Longaker MT. Chemical rescue of cleft palate and midline defects in conditional GSK-3β mice. Nature. 2007;446:79–82. doi: 10.1038/nature05557. [DOI] [PubMed] [Google Scholar]
- 18.Lu W, Peissel B, Babakhanlou H, et al. Perinatal lethality with kidney and pancreas defects in mice with a targetted Pkd1 mutation. Nat Genet. 1997;17:179–81. doi: 10.1038/ng1097-179. [DOI] [PubMed] [Google Scholar]
- 19.Winyard PJD, Risdon RA, Sams VR, Dressler GR, Woolf AS. The PAX2 transcription factor is expressed in Cystic and hyperproliferative dysplastic epithelia in human kidney malformations. J Clin Invest. 1996;98:451–59. doi: 10.1172/JCI118811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dressler GR, Wilkinson JE, Rothenpieler UW, Patterson LT, Williams-Simons L, Westphal H. Deregulation of Pax-2 expression in transgenic mice generates severe kidney abnormalities. Nature. 1993;362:65–7. doi: 10.1038/362065a0. [DOI] [PubMed] [Google Scholar]
- 21.Boulter C, Mulroy S, Webb S, Fleming S, Brindle K, Sandford R. Cardiovascular, skeletal, and renal defects in mice with a targeted disruption of the Pkd1 gene. Proc Natl Acad Sci US A. 2001;98:12174–9. doi: 10.1073/pnas.211191098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gangloff YG, Mueller M, Dann SG, et al. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol Cell Biol. 2004;24:9508–16. doi: 10.1128/MCB.24.21.9508-9516.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murakami M, Ichisaka T, Maeda M, et al. mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Mol Cell Biol. 2004;24:6710–18. doi: 10.1128/MCB.24.15.6710-6718.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hentges KE, Sirry B, Gingeras AC, et al. FRAP/mTOR is required for proliferation and patterning during embryonic development in the mouse. Proc Natl Acad Sci US A. 2001;98:13796–801. doi: 10.1073/pnas.241184198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarbassov DD, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–68. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 26.Amer H, Cosio FG. Significance and management of proteinuria in kidney transplant recipients. J Am Soc Nephrol. 2009;20:2490–92. doi: 10.1681/ASN.2008091005. [DOI] [PubMed] [Google Scholar]
- 27.Wu M, Arcaro A, Varga Z, et al. Pulse mTOR inhibitor treatment effectively controls cyst growth but leads to severe parenchymal and glomerular hypertrophy in rat polycystic kidney disease. Am J Physiol Renal Physiol. 2009;297:F1597–605. doi: 10.1152/ajprenal.00430.2009. [DOI] [PubMed] [Google Scholar]
- 28.Dowling RJ, Topisirovic I, Fonseca BD, Sonenberg N. Dissecting the role of mTOR: Lessons from mTOR inhibitors. Biochim Biophys Acta. 2010;1804:433–9. doi: 10.1016/j.bbapap.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Qin S, Taglienti M, Nauli SM, et al. Failure to ubiquitinate c-Met leads to hyperactivation of mTOR signaling in a mouse model of autosomal dominant polycystic kidney disease. J Clin Invest. 2010;120:3617–28. doi: 10.1172/JCI41531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen W, Brown NS, Finn PF, Dice JF, Franch HA. Akt and mammalian target of rapamycin regulate separate systems of proteolysis in renal tubular cells. J Am Soc Nephrol. 2006;17:2414–23. doi: 10.1681/ASN.2005111157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Kidney (K) and total body weights (TBW) of rapamycin-treated and untreated wild-type and Pkd1−/−E18.5 foetal mice.