Abstract
In the effort of developing micro-electrochemical sensors, the miniaturization of reference electrodes has been a challenging task. In this paper, a flexible micro reference electrode with an internal electrolyte reservoir is reported. This new device is based on a unique microfabricated parylene tube structure, which is filled with Cl− rich electrolyte, into which a 50 μm diameter silver (Ag) wire covered with a 7.4 μm thick silver chloride (AgCl) layer is inserted. The distal end of the tube is filled with potassium chloride (KCl) saturated agarose gel. The Ag wire, thick AgCl layer, and internal electrolyte reservoir lead to a long operation time and a stable reference voltage. The drift over a 10-hour period has been found to be less than 2 mV. The total operation time of the device has exceeded 100 hours. Furthermore, the compatibility with microfabrication allows the integration of other components, leading to truly miniaturized electrochemical sensors or sensing systems. To prove this, we demonstrated a pH sensor by combining the reference electrode and an iridium oxide electrode monolithically integrated on the surface of the parylene tube.
Keywords: MEMS, Reference electrode, Parylene tube, pH sensor, Iridium Oxide
1. Introduction
Recently, there have been many efforts to develop miniaturized electrochemical sensors for microfluidic lab-on-chip analyses or in-vivo biological monitoring. The reference electrode is an essential component of electrochemical sensors and thus has received considerable attention [1]. The most widely used reference electrodes are Ag/AgCl (silver/silver chloride) electrodes, which are constructed by immersing a silver wire coated with AgCl in KCl (potassium chloride) or other Cl− ion-rich solution. The electrochemical reaction on the Ag/AgCl reference electrode is represented by the following equation:
Many micro reference electrodes have been developed based on this mechanism [1]. In some cases, the reference electrodes are simply thin film Ag/AgCl electrodes without a filling solution [2]. They have the advantage of a simple microfabrication process. However, a disadvantage of a thin film Ag/AgCl is a short lifetime due to the small amount of Ag/AgCl. Furthermore, their potential is very sensitive to the testing solution due to the lack of a filling solution. Therefore, their application as reference electrodes is limited. To reduce the sensitivity of these electrodes to Cl− ions and the pH value, Ag/AgCl films can be covered by gels filled with electrolytes. For example, Huang et al. used agarose gels filled with KCl, which function both as a solid internal electrolyte and an ion diffusion membrane [3]. Liao et al. mixed KCl with agar gel for the inner electrolyte and used chloroprene rubber for the liquid junction and insulator [4]. Due to the finite amount of Cl−, these types of electrodes may still suffer from stability issues during long-term applications.
To make more stable reference electrodes, there have been a number of efforts to integrate a reservoir of KCl or other Cl− ion rich solutions in these electrodes. Suzuki et al. developed a liquid-junction Ag/AgCl reference electrode with microfabrication techniques [5]. The solution reservoir was fabricated on a silicon substrate using anisotropic etching. In later work, this group formed the electrolyte layer by screen-printing a paste containing fine KCl powder and encapsulating it using silicone [6]. The electrolyte layer was then activated by injecting a solution saturated with KCl and AgCl.
The miniaturization of the reference electrode is a challenging task [1]. The lack of a reliable, long-lived reference electrode limits the performance of micro electrochemical sensors. Here we report a novel hybrid approach to developing a flexible microfabricated reference electrode with an internal electrolyte reservoir and a Ag/AgCl metal wire. This approach combines several advantages. Compared with thin films, a Ag/AgCl wire can provide larger amounts of Ag and AgCl, preventing the failure of the electrode due to the consumption of Ag or AgCl. In addition, an internal electrolyte reservoir can lead to a stable reference voltage. Unlike its glass pipette counterparts, the flexibility makes this device very robust during application. In addition, its compatibility with microfabrication allows the integration of other components, leading to truly miniaturized electrochemical sensors or sensing systems.
2. Experiments
2.1 Design and Fabrication Process
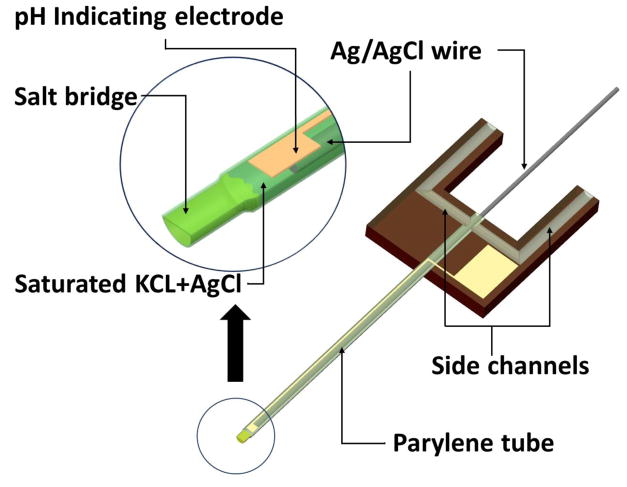
The proposed reference electrode is schematically illustrated in Figure 1. The reference electrode is formed by inserting a silver wire coated with a AgCl layer into a KCl filled parylene tube. Note that the central parylene tube is filled with electrolyte through one of the two side channels, whose inlets locate at ends of the two silicon beams. The tip of the tube is sealed with agarose gel saturated with KCl. For pH sensing, a pH indicating electrode is integrated on the top surface of the tube. The contact pad of this electrode is on the silicon pedestal, which also facilitates the handling and microfluidic coupling to the parylene tube.
Figure 1.
3D schematic of the new reference electrode.
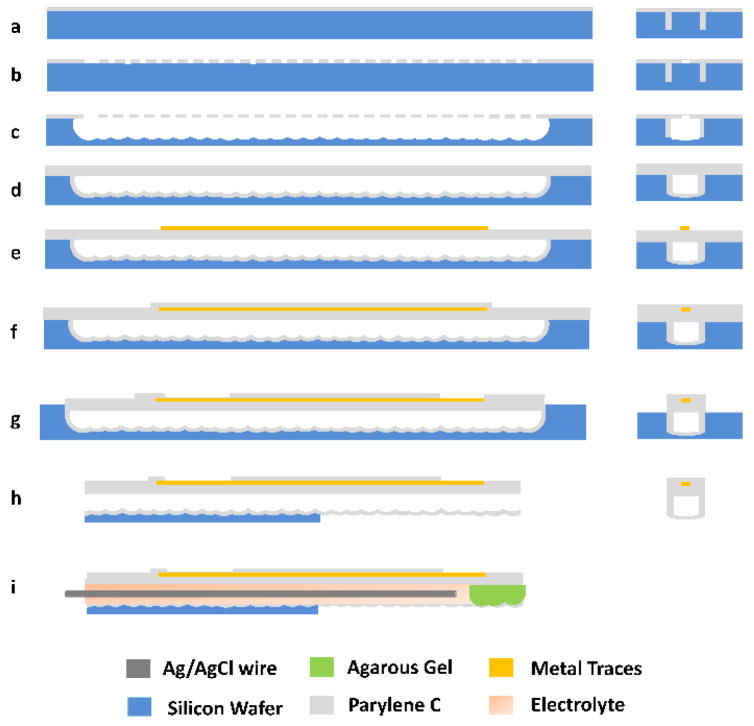
The fabrication process of parylene micro-tubes is based on XeF2 isotropic etching and parylene conformal coating [7–10]. The simplified process is summarized in Figure 2. The left column shows cross-sections along the longitudinal direction of the parylene tube, whereas the right column shows the transverse direction. The fabrication starts with the etching of 8 μm wide trenches into the silicon wafer using deep reactive ion etching (DRIE).
Figure 2.
Simplified fabrication process: (a) Parylene C coating on a silicon wafer; (b) Patterning discontinuous holes on parylene; (c) XeF2 etching to completely undercut the handling wafer and form underlying channels; (d) 2nd parylene deposition to seal the previously opened parylene windows; (e) Ti/Au deposition to form electrodes/traces/pads; (f) 3rd parylene layer to insulate the metal layer; (g) Patterning the parylene layer; (h) Backside DRIE etching to release the device from silicon wafer; and (i) Insertion of the Ag/AgCl wire and sealing of the tip with agarose gel.
Subsequently, parylene-C is deposited onto the surface of the silicon by chemical vapor deposition (CVD). Thanks to the conformal deposition of parylene, deep trenches will be refilled as shown in Figure 2a. The resulting parylene structures in trenches form sidewalls for microchannels and barb structures at the salt bridge region to mechanically anchor the cured agarose gel. In the next step shown in Figure 2b, parylene is patterned using an Al mask and oxygen reactive ion etching (RIE) to form discontinuous 8 μm × 20 μm holes that expose the silicon wafer. Then a gas phase etchant known as XeF2 is used to etch silicon beneath the holes. Since this etch is isotropic, silicon becomes undercut, and eventually, the cavities formed around each hole join to form a single continuous groove. A second deposition of parylene greater than 4 μm, shown in Figure 2d, serves to coat the walls of the XeF2 etched groove with parylene and seal the XeF2 etch holes in the surface. This creates a sealed, continuous microchannel buried beneath the silicon. Next as shown in Figure 2e, a titanium/gold thin film layer of 250 nm is deposited by electron beam evaporation and patterned to form the shapes of microelectrodes, contact pads, and traces. These metal structures are insulated by a third layer of parylene 3 μm thick, which is subsequently patterned to expose electrodes and contact pads, as shown in Figure 2f and 2g. During this etching, excess parylene around the device is also removed. Finally, as shown in Figure 2h, silicon is patterned by backside DRIE, removing silicon from parylene structures intended to be free standing, as well as shaping the silicon parts of the device. Assembly is completed with the insertion of an Ag/AgCl wire into the microchannel, loading of the electrolyte solution, and pulling of the agarose gel into the tip of the tube.
2.2 Fabricated devices
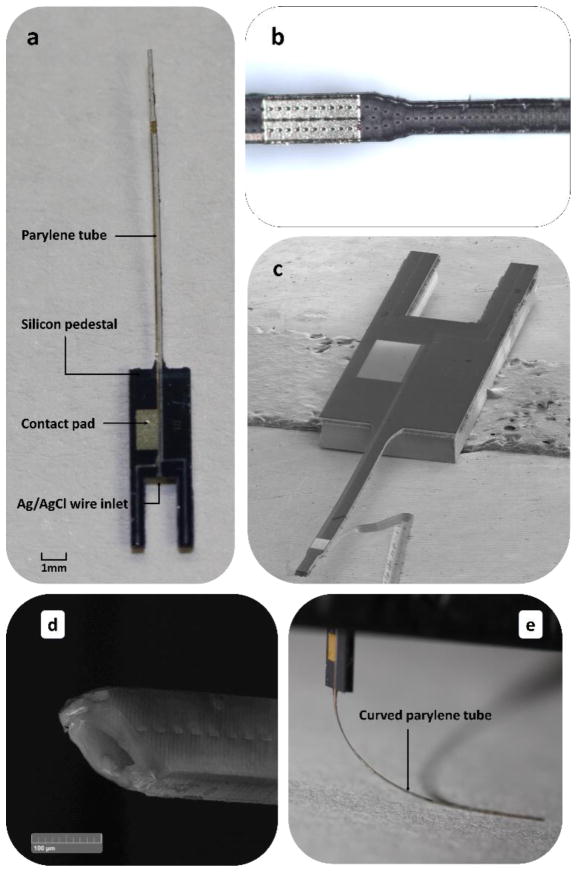
Figure 3a shows a fabricated device. The parylene tube is around 17.3 mm long. The initial width of the tube is 342 μm and it narrows down to 237 μm at the distal end. The magnified view of the distal end of the device is shown in Figure 3b, where the pH indicating electrode, arrays of sealed etching holes, and the transition from wide to narrow tubes can be clearly observed.
Figure 3.
a) Prototype of a fabricated reference electrode. b) The distal end of the parylene tube. c) SEM image of an overall view of the reference electrode. d) SEM cross-sectional view of the parylene tip. e) A curved parylene tube illustrating its flexibility.
The narrow tube is achieved by converging two rows of parylene windows into a single row for XeF2 etching. The length of the salt bridge area can be adjusted to get an appropriate impedance. Figure 3c and 3d show SEM images of the overall device and a cross-sectional view of the parylene tip, respectively. The parylene tube can be easily bent without causing any damage as illustrated in Figure 3e.
2.3 Device assembly
A silver wire with a 50 μm diameter is chosen for the reference electrode. This diameter allows the formation of a thick AgCl layer while retaining sufficient Ag, preventing the failure of the device due to the limited amount of Ag or AgCl. A simple electrochemical method is used to convert Ag to AgCl. Note that an appropriate ratio of silver and silver chloride is important for good stability and reproducibility. To achieve optimal performance, 10–25% of silver should be converted to silver chloride according to [11]. In our experiment, a constant voltage of 500 mV is applied between an Ag wire and a large Pt electrode in a 1 M KCl solution for one hour. By calculating the total amount of current, 24 % of Ag was converted, leading to a 7.4 μm thick AgCl film.
To assemble the reference electrode, a Ag/AgCl wire was first inserted into the parylene tube from the opening at the edge of the silicon interface chip. Since both the channel and wire are in micro scale, this work was performed under a microscope with a pair of fine tweezers. Once the Ag/AgCl wire reached the other end of the channel, it was stopped because of the smaller size of the salt bridge area as illustrated in Figure 3b. The Ag/AgCl wire was fixed by applying a small amount of marine epoxy at the tube inlet on the silicon pedestal. Then the inner electrolyte, saturated KCl and AgCl solution, was injected into the parylene tube using a syringe through one of the polyimide tubes glued on the two silicon beams. Note that both silicon beams host a microfluidic inlet to the parylene tube. When electrolyte was injected through one polyimide tube, the other was sealed. During this process, the device was carefully observed under a microscope to avoid air bubbles inside the tube. Next, the smaller parylene tube at the distal tip was filled with 2% agarose gel saturated with KCl, which functions as the salt bridge of the reference electrode [12]. It was achieved by applying a negative pressure to the syringe on the polyimide tube, while the probe tip was dipped into an agarose solution. The temperature of the agarose solution was kept above 80 °C during drawing to keep it in the liquid phase. The agarose solution inside the tube tip gelled quickly after the probe was removed from the hot solution. The agarose gel column is about 3 mm long. Finally, to avoid the inner aqueous electrolyte solution from evaporating, the end of the polyimide tube was sealed by epoxy. In order to maintain the integrity of the gel, the assembled device is stored in a saturated KCl solution.
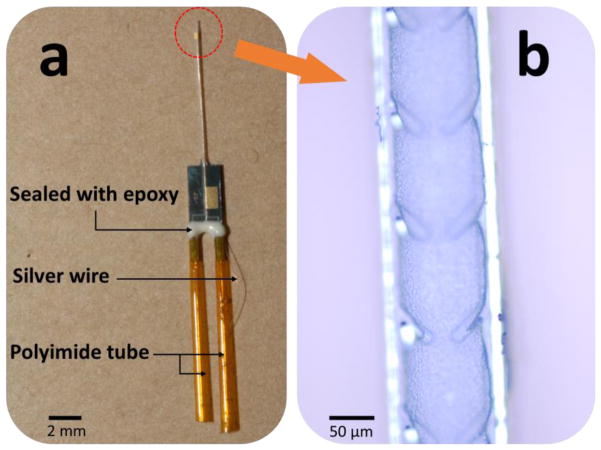
An assembled micro-reference electrode is presented in Figure 4a. Polyimide tubing is used here for the injection of the inner electrolyte and the drawing of the agarose gel to form the salt bridge as discussed previously. In order to mechanically anchor the gel, barb structures are implemented inside the tube as shown in Figure 4b.
Figure 4.
a) Prototype of an assembled reference electrode. b) Salt bridge area with barb structures.
3. Characterization of the reference electrode
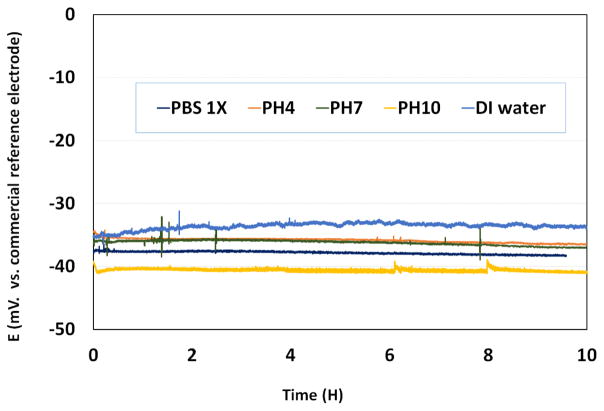
We measured the potential difference between our electrode and a commercial reference electrode in 1X phosphate buffered saline (PBS) solution, DI water and three different pH solutions as shown in Figure 5. The commercial reference electrode is a LowProfile Ag/AgCl gel electrode (3.5 mm OD) from PINE
Figure 5.
The potential difference between our reference electrode and a commercial reference electrode in different solutions.
Research Instrumentation, which is filled with 4 M KCl gel and has a typical variance of ±3–5 mV. The pH buffer solutions were purchased from EMD, and have pH values of 4, 7, and 10, respectively. The first test started with a 1X PBS solution by connecting two reference electrodes to an electrometer (Keithley 6514). The voltage difference between the two electrodes was stable at around −36 mV for the duration of a 10-hour experiment. Then, after a quick rinse, the test solution was changed to a pH 4 solution and monitored for another 10 hours. The voltage difference was still stable at around −35 mV. Then the device was tested in pH 7 and pH 10 solutions, respectively. We observed that for devices immersed in PBS 1X and pH buffers at 4, 7 and 10, the maximum deviation of the average voltage recorded over at least 10 hours was no more than 5 mV between solutions. In addition, the maximum variation of the recorded voltage over 10 hours for each solution is less than 2 mV. The device has also been tested in DI water. As can be observed, the output is less stable than other cases. However, the overall drift over the testing period is less than 5 mV.
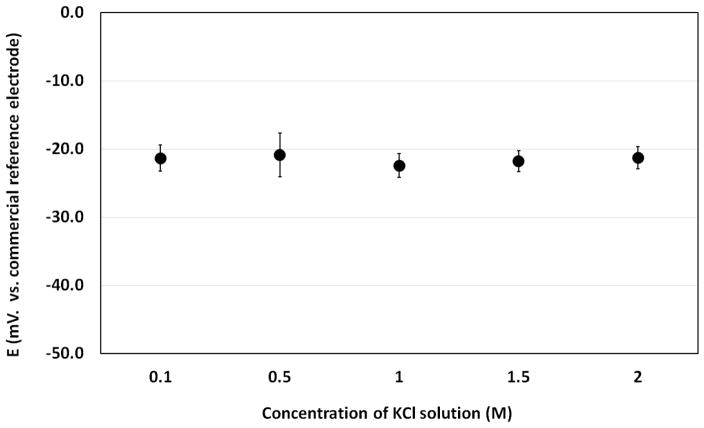
Our device has been tested in KCl solutions with different concentrations and the results are plotted in Figure 6. It can be observed that our reference electrode is insensitive to KCl concentration due to the internal electrolyte reservoir. The potential variation is less than 3 mV for KCl concentrations ranging from 0.1 M to 2 M.
Figure 6.
The potential difference between our reference electrode and a commercial reference electrode in KCl solutions with different concentration. The average value and standard deviation are calculated based on one-hour measurement.
The impedance of the new reference electrode has been characterized from 200 Hz to 20000 Hz with an LCR meter (HEWLETT PACKARD 4284A). The measured impedance is mainly resistive and approximately 60kΩ, similar to the commercial reference electrode whose resistive impedance is around 1.5 kΩ.
To further investigate the response to pH, we combined our reference electrode with a glass based commercial pH indicating electrode (Orion 350 PerpHect Meters, Thermo) and measured their voltage in different pH solutions. A sensitivity of −54.6 mV/pH is obtained, close to the ideal Nernstian slope of −59.19 mV/pH [11].
In our experiment, the overall operation time of the tested device has exceeded 100 hours. Note the operation time and stability of the reference electrode are affected by multiple factors such as the amounts of Ag/AgCl and Cl− in the electrolyte. Many micro reference electrodes have a shorter operation time compared to their macro counterparts because the limited AgCl can be rapidly consumed due to the reduction of Cl ions in the electrolyte [2]. To obtain a long operation time and stable reading for the reference electrode, we use Ag/AgCl wire instead of a thin film and integrate an internal electrolyte reservoir.
Our current device utilizes a 50 μm diameter Ag wire of which 24% is converted to AgCl. In the future, we plan to use thicker Ag wires, i.e., 75 μm. We will also integrate a larger reservoir on the silicon base in the next generation devices to significantly increase the electrolyte volume. It is also worth noting that the current reference electrode needs to be stored in a saturated KCl solution, in a way similar to commercial ones. This not only keeps a sufficient Cl− concentration but also prevents the gel from dehydration.
4. A miniaturized pH sensor based on the new reference electrode
To prove our approach can lead to miniaturized electrochemical sensors, a pH sensor has been demonstrated. The pH indicating electrode is formed by electrochemically depositing iridium oxide on the integrated gold electrode on the parylene tube surface. The electrodeposition was performed using a Princeton Applied Research Potentiostat/Galvanicstat Model 273A by sweeping a positive bias between 0 and 0.575 V (versus Ag/AgCl) at 50 mV/s for 50 cycles first, which enables the deposition of the high-quality thin film. Then a second pulse of 1Hz between 0 – 0.575 V was performed for 3200 iterations [13].
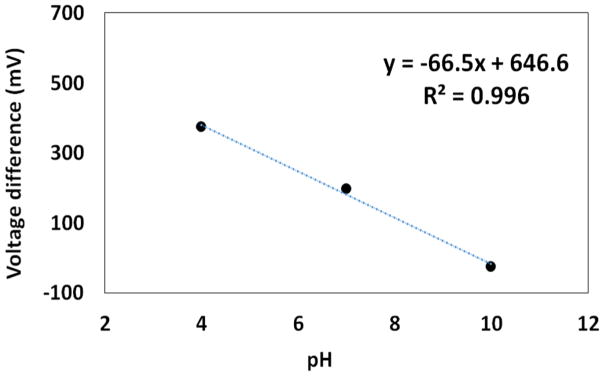
We characterized the pH sensor by measuring the voltage difference between our reference electrode and the integrated pH indicating electrode in three different pH buffer solutions for one hour. As shown in Figure 7, a sensitivity of −66.5 mV/pH was obtained with our pH indicating electrode, slightly larger than the ideal Nernstian slope of −59.16 mV/pH. The drift over one-hour period is between −5mV and −10mV, which is moderate compared to those observed by others [14, 15]. This drift is probably caused by different diffusion rates of anions and cations [15]. It has been reported that activated iridium oxide (AIROF) and electrodeposited iridium oxide (EIROF) typically exhibits a sensitivity from −60 mV/pH to −70 mV/pH [14, 16, 17]. Therefore, our miniaturized sensor has a sensitivity that matches reported values in the literature.
Figure 7.
The output voltage of our miniaturized pH sensor as a function of pH values.
5. Conclusion
A novel flexible microfabricated reference electrode has been successfully developed. By taking advantage of a unique parylene tube structure, we successfully integrated a Ag/AgCl wire and a reservoir for the inner electrolyte, leading to a long operational life and stable output. Compared to reference electrodes based on glass capillaries, the flexible parylene tube makes the new electrode more robust. Our novel micro-reference electrode exhibited good stability in PBS and pH buffer solutions with a drift of 1–2 mV over 10 hours. When the pH value is increased from 4 to 10, the voltage variation is less than 5 mV. The device has exceeded 100 operational hours without significant deviation from that observed with a commercial Ag/AgCl reference electrode. By combining a monolithically integrated IrOx pH indicating electrode, a miniaturized pH sensor has been demonstrated. This MEMS-based flexible reference electrode will be useful for 3D cell culture monitoring, in-vivo electrochemical sensing, or other applications which request miniaturized electrochemical sensors.
Highlights.
A flexible miniaturized Ag/AgCl reference electrode based on a parylene tube structure is demonstrated.
Stability and life time of the proposed reference electrode are improved by integrating an inner electrolyte reservoir and a Ag/AgCl wire.
The developed reference electrode exhibits a good stability in different pH buffer and PBS solutions.
An electrodeposited iridium oxide has been monolithically integrated to demonstrate a miniaturized pH sensor.
Acknowledgments
This work was partially supported by the Wayne State University Research Enhancement Program and NIH grant R21 CA175931.
Biographies
Zhiguo Zhao: Zhiguo Zhao received the B.S. degree from the School of Mechanical Engineering, Southwest Jiaotong University, Chengdu, China, in 2008, and MD degree from the School of Electrical and Computer Engineering, KyungPook National University, Daegu, South Korea, in 2010 under the supervision of Dr. Seongho Kong. He is currently working in Dr. Yong Xu’ lab as a Ph.D. student. His research is focused on MEMS micro pH sensors, neuroprobes for biomedical applications.
Hongen Tu: Hongen Tu received his B.S. degree in applied physics and biological engineering from the Beijing Institute of Technology, Beijing, China, in 2006 and Ph.D. degree from the Department of Electrical and Computer Engineering, Wayne State University, Detroit, MI, in 2014 under the supervision of Dr. Yong Xu. Since 2008, he has held a research assistantship with Dr. Yong Xu’s lab and focused on the research in the field of MEMS. His research interests include but not limited to flexible sensors/electronics, microfluidics for drug delivery, neurophobes for biomedical applications and cantilevers for sensing and energy harvesting.
Eric GR Kim: Eric Kim is an MD/Ph.D. student at the Wayne State University School of Medicine who is currently seeking his Ph.D. in biomedical engineering with Dr. Xu. He received his bachelor’s in biomedical engineering from Vanderbilt University in 2007. He has participated in several bioMEMS related research projects, including microfluidic toximeters, MEMS neural probes, and tactile sensors for use on the manipulator clamps of laparoscopic surgical tools. His research interests include MEMS-based devices for basic medical research, clinical analysis, and medical implantation.
Bonnie F Sloane: Bonnie Sloane is a Distinguished Professor of Pharmacology in the School of Medicine at Wayne State University. She received her B.S. and M.A. from Duke University, her Ph.D. from Rutgers University and postdoctoral training at the University of Pennsylvania. She headed Protease and Breast Cancer Biology Programs at the Karmanos Cancer Institute, co-founded the International Proteolysis Society, was a Special Assistant to the Cancer Imaging Program of the National Cancer Institute and Director of a multi-institutional DoD Breast Cancer Center of Excellence. Her primary research interest is 3D pathomimetic models for live-cell imaging of proteolytic pathways in the progression of premalignant breast disease to invasive carcinomas.
Yong Xu: Yong Xu is currently a Professor with the Department of Electrical and Computer Engineering, Wayne State University, Detroit, MI. He received the B.S. degree in electronics engineering from Tsinghua University, Beijing, China, in 1997, and the M.S. and Ph.D. degrees in electrical engineering from the California Institute of Technology, Pasadena, in 1998 and 2002, respectively. His research interests include MEMS smart skins, intelligent textiles, wearable sensors, neural implants, microfluidics, biosensors, novel packaging technology, and nanotechnology. Dr. Xu is a recipient of NSF CAREER award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shinwari MW, Zhitomirsky D, Deen IA, Selvaganapathy PR, Deen MJ, Landheer D. Microfabricated Reference Electrodes and their Biosensing Applications. Sensors. 2010;10:1679–715. doi: 10.3390/s100301679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polk BJ, Stelzenmuller A, Mijares G, MacCrehan W, Gaitan M. Ag/AgCl microelectrodes with improved stability for microfluidics. Sensors and Actuators B-Chemical. 2006;114:239–47. [Google Scholar]
- 3.Huang IY, Huang RS. Fabrication and characterization of a new planar solid-state reference electrode for ISFET sensors. Thin Solid Films. 2002;406:255–61. [Google Scholar]
- 4.Liao WY, Chou TC. Fabrication of a planar-form screen-printed solid electrolyte modified Ag/AgCl reference electrode for application in a potentiometric biosensor. Analytical Chemistry. 2006;78:4219–23. doi: 10.1021/ac051562+. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki H, Hirakawa T, Sasaki S, Karube I. Micromachined liquid-junction Ag/AgCl reference electrode. Sensors and Actuators B: Chemical. 1998;46:146–54. [Google Scholar]
- 6.Suzuki H, Shiroishi H, Sasaki S, Karube I. Microfabricated Liquid Junction Ag/AgCl Reference Electrode and Its Application to a One-Chip Potentiometric Sensor. Analytical Chemistry. 1999;71:5069–75. [Google Scholar]
- 7.Kim EGR, Tu HE, Luo H, Liu B, Bao SW, Zhang JS, et al. 3D silicon neural probe with integrated optical fibers for optogenetic modulation. Lab on a Chip. 2015;15:2939–49. doi: 10.1039/c4lc01472c. [DOI] [PubMed] [Google Scholar]
- 8.Tu HG, Chen XY, Feng XC, Xu Y. A post-CMOS compatible smart yarn technology based on SOI wafers. Sensors and Actuators a-Physical. 2015;233:397–404. [Google Scholar]
- 9.Kim EGR, John JK, Tu HG, Zheng QL, Loeb J, Zhang JS, et al. A hybrid silicon-parylene neural probe with locally flexible regions. Sensors and Actuators B-Chemical. 2014;195:416–22. [Google Scholar]
- 10.Kim E, Tu H, Lv C, Jiang HQ, Yu HY, Xu Y. A robust polymer microcable structure for flexible devices. Applied Physics Letters. 2013;102 [Google Scholar]
- 11.Gerischer H. Reference Electrodes: Theory and Practice, herausgeg. von D. J. G. Ives und G. J. Janz. Academic Press, New York—London 1961. 1. Aufl., XI, 651 S., zahlr. Abb. und Tab., geb. £ 7.3.—. Angewandte Chemie. 1961;75:879. [Google Scholar]
- 12.Kitade T, Kitamura K, Takegami S, Miyata Y, Nagatomo M, Sakaguchi T, et al. Needle-type ultra micro silver/silver chloride reference electrode for use in micro-electrochemistry. Analytical sciences : the international journal of the Japan Society for Analytical Chemistry. 2005;21:907–12. doi: 10.2116/analsci.21.907. [DOI] [PubMed] [Google Scholar]
- 13.Kim E. Dissertation. Detroit, Michigan: Wayne State University; 2016. DEVELOPMENT OF ADVANCED MULTIFUNCTIONAL NEURAL INTERFACE DEVICES. [Google Scholar]
- 14.Yao S, Wang M, Madou M. A pH electrode based on melt-oxidized iridium oxide. Journal of the Electrochemical Society. 2001;148:H29–H36. [Google Scholar]
- 15.Huang WD, Cao H, Deb S, Chiao M, Chiao JC. A flexible pH sensor based on the iridium oxide sensing film. Sensors and Actuators a-Physical. 2011;169:1–11. [Google Scholar]
- 16.Olthuis W, Robben MAM, Bergveld P, Bos M, Vanderlinden WE. PH SENSOR PROPERTIES OF ELECTROCHEMICALLY GROWN IRIDIUM OXIDE. Sensors and Actuators B-Chemical. 1990;2:247–56. [Google Scholar]
- 17.Jaworski RK, Cox JA, Strohmeier BR. Characterization of oxide films electrochemically deposited from solutions of palladium chloride and sodium hexachloroiridate. Journal of Electroanalytical Chemistry. 1992;325:111–23. [Google Scholar]