Abstract
The aryl hydrocarbon receptor repressor (AhRR) was first described as a specific competitive repressor of aryl hydrocarbon receptor (AhR) activity based on its ability to dimerize with the AhR nuclear translocator (ARNT) and through direct competition of AhR/ARNT and AhRR/ARNT complexes for binding to dioxin-responsive elements (DREs). Like AhR, AhRR belongs to the basic Helix-Loop-Helix/Per-ARNT-Sim (bHLH/PAS) protein family but lacks functional ligand-binding and transactivation domains. Transient transfection experiments with ARNT and AhRR mutants examining the inhibitory mechanism of AhRR suggested a more complex mechanism than the simple mechanism of negative feedback through sequestration of ARNT to regulate AhR signaling. Recently, AhRR has been shown to act as a tumor suppressor gene in several types of cancer cells. Furthermore, epidemiological studies have found epigenetic changes and silencing of AhRR associated with exposure to cigarette smoke and cancer development. Additional studies from our laboratories have demonstrated that AhRR represses other signaling pathways including NF-κB and is capable of regulating inflammatory responses. A better understanding of the regulatory mechanisms of AhRR in AhR signaling and adverse outcome pathways leading to deregulated inflammatory responses contributing to tumor promotion and other adverse health effects is expected from future studies. This review article summarizes the characteristics of AhRR as an inhibitor of AhR activity and highlights more recent findings pointing out the role of AhRR in inflammation and tumorigenesis.
Keywords: AhR, AhRR, inflammation, cancer, NF-κB, cytokines
Introduction
The aryl hydrocarbon receptor (AhR) belongs to the superfamily of basic Helix-Loop-Helix/Per-ARNT-Sim (bHLH/PAS) proteins and is activated by low molecular weight compounds (1–3). Upon ligand-binding in the cytoplasm, AhR shuttles in the nucleus, dimerizes with AhR nuclear translocator (ARNT) and binds to dioxin-responsive elements (DRE) in the enhancer/promoter of target genes to induce transcription. AhR target genes encode for drug-metabolizing enzymes, such as cytochrome P450 (CYP) 1A1, as well as for proteins controlling cell proliferation, differentiation, and apoptosis (1, 2). In addition, AhR activation is frequently associated with the stimulation of other signal transduction pathways, including epidermal growth factor receptor (EGFR) (4, 5), protein kinase A (PKA) (6, 7), and NF-κB signaling (7, 8).
In 1999, the team of Yoshiaki Fujii-Kuriyama screened a mouse genomic library using an AhR cDNA as hybridization probe, and discovered a novel component of AhR signaling: The AhR repressor (AhRR) (9). Meanwhile, the Ahrr gene has been identified in the human (10, 11), rat (12, 13), chicken (14), frog (15), and fish (16–18) genome.
AhRR as a feedback regulator of AhR signaling
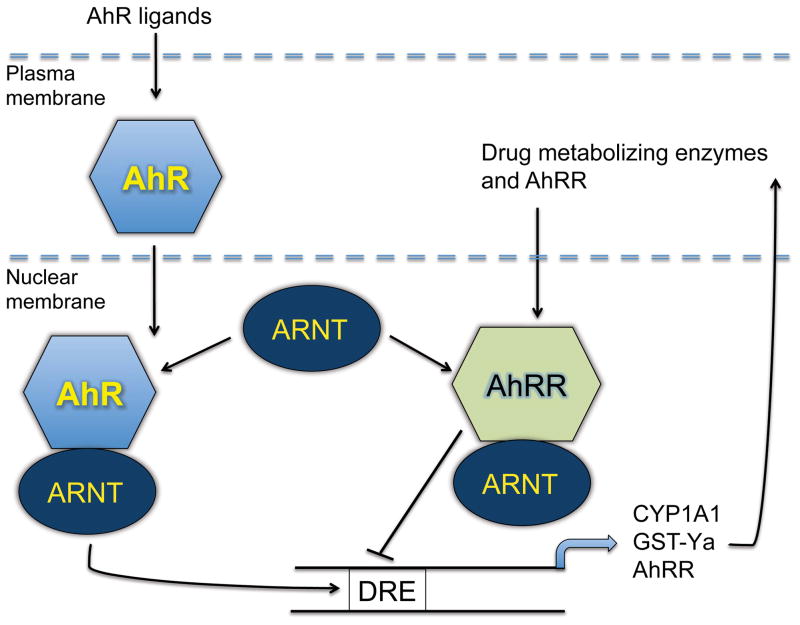
The N-terminal half of the AhRR protein has high structural similarities with AhR, i.e. it contains the DNA-binding bHLH domain and the PAS-A domain. In contrast, its C-terminal part does neither contain the PAS-B domain nor the Q-rich transactivation domain, indicating that AhRR lacks the established AhR ligand-binding domain and is transcriptionally inactive (9). AhRR expression is regulated by one or more DREs located in the enhancer/promoter sequence of the murine and human Ahrr gene (19–21), indicating that the AhR controls the expression of its own repressor protein. Indeed, overexpression experiments revealed that AhRR is capable of inhibiting AhR/ARNT-triggered transactivation of DRE-containing gene promoters by competing with AhR for both dimerization with ARNT and DRE-binding (9, 19) (figure 1). Specifically, after post-translational sumoylation (22) AhRR may recruit co-repressor molecules and histone deacetylases to DRE-containing gene promoters (21, 23, 24). The subsequent condensation of the local chromatin structure hinders a further binding of transcription factors and abrogates transcription of AhR target genes (25).
Figure 1.
Schematic illustration of the repression of the canonical AhR signaling pathway by AhRR. The ligand-activated AhR translocates into the nucleus, dimerizes with ARNT and binds on DRE sequences in the promoter region of AhR target genes, including CYP1A1 and AhRR. The increased expression of AhRR inhibits AhR activity as a result from the competition with AhR for dimerization with ARNT. Alternatively, the formation of an AhRR/ARNT complex may inhibit AhR function through binding on DRE sequences, which does not involve the competition for dimerization with ARNT.
In addition, a so-called “transrepression” model was proposed to explain the inhibitory effect of AhRR on AhR transactivation (26). The authors observed that repression of AhR-dependent gene expression by AhRR involves the N-terminal part of AhRR and does not involve a competition for ARNT. Also, DRE-binding was not necessary for AhRR’s repressive function in this study, but further contributed to it. One hypothesis of the authors is that AhRR represses AhR by competing for limiting co-activator molecules (26). Comparable transrepression models have been proposed for the interaction of AhR with NF-κB RelA (27) and EGFR (28).
Besides the DRE, the human and rodent Ahrr genes contain binding sites, which are recognized by NF-κB and zinc-finger transcription factors of the Sp1 family (13, 19–21). Whereas under inflammatory conditions the NF-κB subunit RelA may cooperate with AhR to induce AhRR expression (19, 29), Sp1-related factors may contribute to basal AhRR expression (21). In addition, nuclear factor erythroid-2-related factor-2 (Nrf2) has been recently identified to control AhRR expression in murine kidney tissue by inducing the expression of microRNA-125b (30).
Mammalian AhRR is expressed in nearly all tissues tested so far, but may be restricted to some and not all of the cell populations in a given tissue (9, 31–35). Interestingly, its expression level does not always correlate with CYP1A1 inducibility, indicating that AhRR may affect other signaling pathways and cellular functions.
The AhRR as regulator of inflammatory responses
Inflammatory processes contribute to a multitude of pathologies and have emerged as a major factor promoting cancer development (36). A link of environmental exposure with changes of inflammatory mediators and the possible consequences for carcinogenesis has been recently reviewed (37). 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is a potent tumor promoter that exerts its action via prolonged activation of AhR signaling. Studies on the mechanism of TCDD-induced liver tumor promotion revealed that inflammatory signaling and increased expression of pro-inflammatory cytokines are critical components (38). This hypothesis is also supported by findings showing that the classical AhR signaling pathway does not completely explain the toxic or carcinogenic action of dioxins. In contrast, there is evidence indicating that CYP1A1 and CYP1A2 provide protection from dioxin-induced acute hepatotoxicity and inflammation (39). On the other hand, the deficiency of CYP1A1 protected male mice from TCDD-induced wasting and lethality (40).
Increased levels of AhR as well as constitutively active AhR have been found in tumors and various cancer cell-lines (41–44). The enhanced expression of AhR in cancer may be triggered by NF-κB and STAT3 (45, 46), which could explain the positive correlation of AhR expression with an inflammatory status and inflammatory-dependent tumor development. These findings may provide a possible mechanism connecting anti-inflammatory responses of AhRR with its tumor-suppressive properties. In fact, we have created AhRR-overexpressing transgenic mice (AhRR Tg), which have significantly reduced inflammatory and acute toxic responses to TCDD compared to wild-type (wt) mice (47).
Recent studies, including our own report, revealed that the AhR is involved in immunity and bacterial lipopolysaccharide (LPS)-mediated responses in vivo (48–51). Studies on the basic mechanism of LPS tolerance found that it is mediated by the sustained silencing of a set of acute pro-inflammatory genes (52, 53). This paradigm is well-established as an effective means to help animals as well as humans survive serious infections accompanied with severe systemic inflammation. The importance of IL-1β in mediating LPS shock has been demonstrated using knockout (ko) mice that lack IL-1β-converting enzyme and are unable to produce active IL-1β and are resistant to LPS shock (54). This is particularly important since a recent report shows a reduced susceptibility towards LPS shock in AhRR-reporter and AhRR−/− mice (55). The authors found that AhRR is highly expressed in immune cells of barrier organs, and has a major impact on the regulation of inflammatory responses. AhRR prevents excessive IL-1β production in bone-marrow derived macrophages. This is consistent with a reduced expression of IL-1β found in tissues from TCDD-treated AhRR Tg mice (47). In contrast to the antagonizing effect of AhRR in response to LPS, AhR and AhRR seem to cooperate dampening intestinal inflammation (55). Similar to AhR-deficiency (56–58), AhRR−/− mice exhibited an enhanced susceptibility to dextran sodium sulfate-induced colitis. In contrast to AhR, whose expression in intestinal epithelial cells is important to maintain proper barrier functions (59), AhRR contributes to the maintenance of colonic intraepithelial lymphocytes and prevents excessive production of IL-1β and differentiation of Th17/Tc17 cells. Moreover, AhRR enhances γ-interferon production by effector T cells in the inflamed gut (55). These findings underscore that AhR and AhRR are expressed in a cell- and tissue-specific manner and may affect different target cells. Future studies with AhRR Tg and AhRR-reporter mice for instance may provide further insight into the role that AhRR plays in various inflammatory responses.
The AhRR in cancer biology
AhR is overexpressed and/or over-activated in various solid cancers (41–43), in which it may drive proliferation, apoptosis resistance, extracellular matrix degradation, and immunosuppression (60–66). Thus, AhR’s opponent may exhibit tumor-suppressive properties as depicted in figure 2. Indeed, the human Ahrr gene is located on the short arm of chromosome 5 (5p15.3), a region which is frequently deleted in various tumors (67–72), indicating the presence of a tumor suppressor gene. Frank Cuttitta and his team observed a very low AhRR expression rate in several human cancer biopsies, which was due to hypermethylation of the AhRR promoter (73). These cancers include colon, lung, esophagus and stomach tumors (73), and, interestingly, AhR is abundantly expressed in these cancer types (74–78). A low AhRR expression level that correlated with poor prognosis was also found in gastric adenocarcinomas (79). In human colorectal cancer tissue, AhRR expression correlated with CD40/CD40L signaling and histological grade. Subsequent experiments in colon cancer cell-lines revealed that CD40L treatment increases AhRR expression, resulting in a more pronounced inhibition of tumor cell growth and induction of apoptosis, respectively (80). Like other members of the tumor necrosis factor (TNF) receptor family, CD40 activation induces nuclear translocation and DNA-binding of RelB/p50 (81), which may contribute to proper anti-tumor immune responses (82), for instance in colorectal cancer (83). However, whether the CD40-dependent induction of AhRR is triggered by non-canonical NF-κB signaling remains to be elucidated. Alternatively, AhRR expression may be induced by constitutive AhR activation via CD40L. Allan and Sherr demonstrated that CD40L upregulates AhR mRNA and protein levels in B cells leading to nuclear translocation of AhR and induction of CYP1A1 in the absence of exogenous ligands (84).
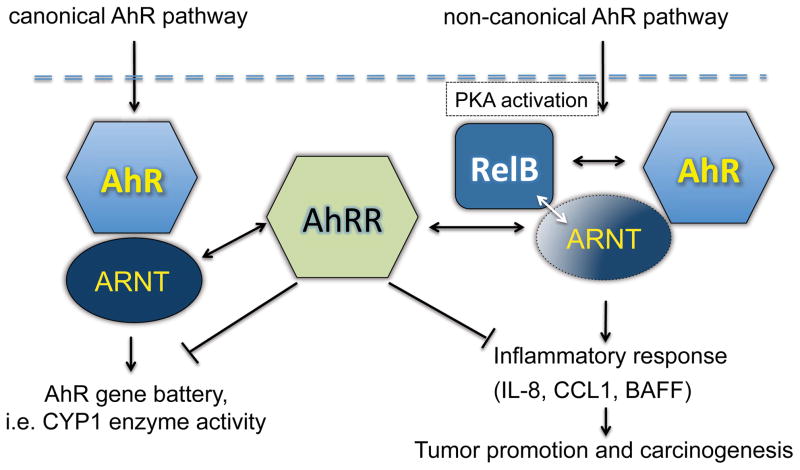
Figure 2.
Schematic illustration of the interaction of AhRR with the canonical and non-canonical AhR pathways. AhRR represses the canonical AhR pathway including the induction of CYP1A1 via competitive binding with ARNT. AhRR may protect from non-canonical AhR-mediated inflammatory responses and inflammation-dependent carcinogenesis via interaction of AhRR with RelB in complex with ARNT and/or AhR. Black double arrows indicate the interaction of AhRR with ARNT and RelB with AhR. The white double arrow indicates the interaction of ARNT with RelB. Abbreviations: Protein kinase A (PKA)
In vitro analyses of several human tumor cell-lines further underscored a tumor-suppressive function of AhRR (table 1). For example, RNAi-mediated AhRR-silencing in human lung carcinoma cells enhanced proliferation, apoptosis resistance, motility, and invasive growth (73). Transplantation of AhRR-silenced tumor cells into immune compromised mice resulted in an enhanced growth and a pronounced angiogenic potential of the tumors (73). AhRR overexpression inhibited anchorage-dependent and –independent growth as well as the angiogenic potential of lung cancer cells (73), and abrogated proliferation and AhR-mediated anti-apoptosis in breast cancer cells (85–87) (table 1). Overexpressed AhRR was clearly capable of overcoming the anti-apoptotic effect of TCDD-activated AhR in those cells. Moreover, a recent study with AhRR−/− mice showed a lower number of apoptotic cells in liver of LPS-treated mice (55), confirming the role of AhRR to resolve an anti-apoptotic response in vivo.
Table 1.
Effects of AhRR overexpression and RNAi on cell biological endpoints and gene expression in vitro.
| CELL-LINE | MANIPULATION | OUTCOME | REF # |
|---|---|---|---|
| A549 bronchoalveolar carcinoma cells | overexpression of human AhRR | diminished anchorage-dependent and -independent cell growth, reduced angiogenic potential (tube formation) | 73 |
| A549 cells | transient RNAi of AhRR | enhanced anchorage-dependent and -independent cell growth | 73 |
| BP1 mammary epithelial cells | overexpression of fish AhRR (Fundulus heteroclitus) | reduced constitutive AhR activity; reduced expression of CYP1B1 | 60 |
| MCF7 breast cancer cells | overexpression of human AhRR | reduced cell proliferation; increased expression of cyclin D1; reduced expression of E2F and cathepsin D | 86 |
| MCF7 cells | overexpression of human AhRR | reduced expression of the estrogen-responsive genes pS2, cathepsin D, and complement C3 | 87 |
| MCF7 cells | transient RNAi of AhRR | no effect on TCDD-induced expression of CYP1A1, CYP1B1, and TCDD- inducible poly (ADP-ribose) polymerase | 125 |
| MCF-10AT1 mammary epithelial cells | overexpression of murine AhRR | enhanced susceptibility towards UV-induced apoptosis | 87 |
| MCF-10A mammary epithelial cells | stable RNAi targeting AhRR | induced colony formation in soft agar | 73 |
| MCF-10F mammary epithelial cells | overexpression of fish AhRR (F. heteroclitus) | reduced cell proliferation | 85 |
| MCF-10F cells | lentiviral overexpression of fish AhRR (F. heteroclitus) | reduced constitutive AhR activity; reduced expression of CYP1B1 | 60 |
| HC11 mammary epithelial cells | overexpression of human AhRR | reduced expression of 3-casein | 126 |
| HepG2 hepatoma cells | overexpression of human AhRR | reduced expression of the estrogen-responsive genes pS2, cathepsin D, and complement C3 | 87 |
| Hs578T breast cancer cells | overexpression of fish AhRR (F. heteroclitus) | reduced constitutive AhR activity; no effect on cell proliferation | 127 |
| Hs578T cells | overexpression of fish AhRR (F. heteroclitus) | increased expression of the proto-oncogene c-myc | 128 |
In MCF7 breast cancer cells, an inhibitory effect of AhRR on the transcriptional activity of estrogen receptor-α (ERα), which was probably due to a direct protein-protein interaction, was observed (88). Interestingly, some of the ERα target genes whose expression was repressed by AhRR, such as pS2 and cathepsin D, have been previously reported to be down-regulated in TCDD-exposed MCF7 cells (89–91). Further analyses revealed that the repression of ERα target gene expression occurred in an AhR-dependent but ARNT-independent manner (91), suggesting that AhR and AhRR may directly cooperate to inhibit ERα-dependent transcription. Notably, a physical interaction between AhR and AhRR has been observed in ectopic overexpression experiments (92).
The above mentioned changes in angiogenesis and invasion (73) may be also explained by a potential crosstalk with the other binding partner of ARNT, hypoxia-inducible factor-1α (HIF-1α). The HIF-1α/ARNT complex is activated by low oxygen concentrations or oncogenic signal transduction (e.g. overactive RAS) to enable angiogenesis and ensure oxygen and nutrient supply in fast growing tumors (93). HIF-1 is also important for tissue invasion and metastasis of tumor cells and thus is a key player in cancer progression (93). Interestingly, Mark Hahn and co-workers have identified a splice variant of AhRR that lacks exon 8 and is pre-dominantly expressed in human cells and tissues (92). Overexpression experiments revealed that this AhRR variant is capable of inhibiting HIF-1-dependent transcription (92). Although further research is needed, these observations already mark AhRR as a potentially very attractive target molecule for cancer therapy.
In soft tissue angiofibroma, a histologically distinctive benign mesenchymal neoplasm of unknown cellular origin, a chromosomal rearrangement between chromosomes 5 (5p15) and 8 (8q13) resulted in the creation of a fusion protein between AhRR and nuclear receptor co-activator-2 (NCOA2) (94). The N-terminal part of the chimeric protein consists of the AhRR protein and thus harbors all domains necessary for DRE-binding. The C-terminal AhRR domain, lacking a Q-rich transactivation domain, is substituted by the NCOA2 protein, producing a fusion protein with two activation domains (94). Global gene expression analyses showing an upregulation of AhR target genes, revealed that the AhRR/NCOA2 fusion protein is able to mimic canonical AhR signaling (94). Although this chimeric AhRR/NCOA2 protein has so far only been detected in a small number of soft tissue angiofibromas, it is conceivable that an inactivation of AhRR’s repressive function through chromosomal rearrangements may also occur in other tumor types.
In context of chemical skin carcinogenesis, however, AhRR may not act as a tumor suppressor. Benzo[a]pyrene (BaP) and structurally related PAHs need to undergo CYP1A-mediated oxidations in order to unleash their carcinogenic potential (95). Accordingly, AhR−/− mice as well as transgenic mice carrying an epidermis-specific ARNT-deficiency are largely protected against the skin carcinogenicity of BaP (96–98). Thus, one would expect that AhRR−/− mice are more prone to BaP-induced skin cancer. However, a chronic carcinogenesis study on AhRR+/+ and AhRR−/− mice has shown a significantly delayed occurrence of BaP-induced skin tumors in mice that lack AhRR (99). The authors conclude that a shift of CYP1A1-driven metabolism from metabolic activation to detoxification is responsible for this unexpected outcome (99). A comparable shift in toxification/detoxification was previously discussed with regards to various CYP1-ko strains, exhibiting significantly more DNA adducts after oral BaP exposure than wt littermates (100).
Another observation that may contradict AhRR’s tumor-suppressive function is the hypomethylation of the AhRR promoter, which is frequently observed in blood and lung tissue samples from smokers (101–110). These epigenetic modifications are associated with an elevated risk to develop malignancies of the respiratory tract (104, 108, 109) and thus imply a putative role of AhRR in lung carcinogenesis. As previously discussed (106), tobacco smoke is rich in PAHs and may cause an AhR-mediated induction of AhRR gene expression, which requires chromatin relaxation associated with DNA demethylation (111). However, at least three studies found that the alterations in AhRR promoter methylation induced by prenatal maternal smoking may persist in the exposed offspring until adolescence (101, 102, 110).
With the exception of PAH-induced cancer (figure 3), the majority of the publications discussed above points to the idea that AhRR is a potent tumor suppressor protein. Its expression level may serve as a prognostic factor with low levels correlating with tumor malignancy. AhRR may inhibit proliferation and increase apoptosis susceptibility of malignant cells, and thus prevent the establishment of a tumor-promoting, pro-inflammatory microenvironment by modulating cytokine responses, and attenuating angiogenic and invasive processes. The underlying molecular mechanisms, however, are enigmatic and probably involve a crosstalk of AhRR with other signal transduction pathways including C/EBPβ and NF-κB as recently shown (47). Future studies are needed to address the regulatory mechanisms of AhRR in AhR signaling and adverse outcome pathways based on deregulated inflammatory and/or anti-apoptotic responses contributing to tumor promotion and other adverse health effects.
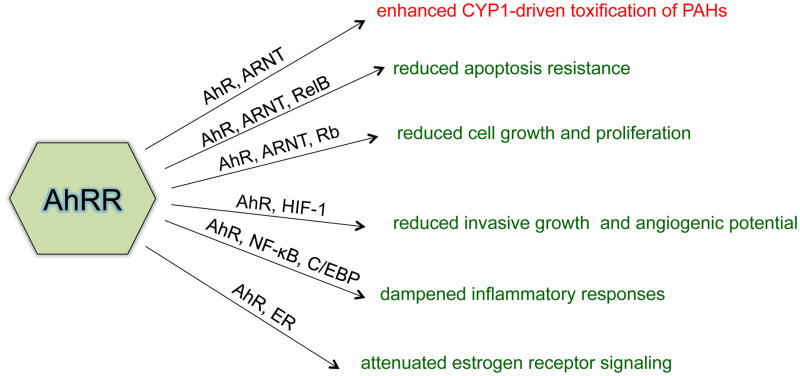
Figure 3.
Interactions of AhRR with AhR signaling pathways and possible consequences in carcinogenesis. Abbreviation: CCAAT-enhancer-binding protein (C/EBP), Estrogen receptor (ER), Hypoxia-inducible factor-1 (HIF-1), Retinoblastoma protein (Rb). In red: oncogenic properties; in green: tumor suppressive properties
Conclusion
While AhRR can effectively block AhR-dependent responses, there are many unanswered questions, including why CYP1A1 is not always suppressed when AhRR is overexpressed (112). The model of “transrepression” as described above may explain, at least partially, this observation. As AhR-driven CYP1A1 gene expression requires transcriptional co-activators, including CBP/p300 and SRC-1 (113–115), cell- or tissue-specific expression patterns of such co-factors could explain the observed discrepancy between AhRR expression and CYP1A1 inducibility.
Previous reports show that AhRR is predominantly located in the nucleus (116), however the effect of AhRR on non-canonical AhR signals (47) suggests the presence and functional activity of AhRR in the cytosol. In this context, it is noteworthy that both the interaction of AhR with HSP90 and XAP2 in the cytosol as well as the repression of AhR by AhRR involve the N-terminal domain of AhR (26, 117). Although AhRR does not contain the established AhR ligand-binding domain, it would be important to determine if the nuclear localization of AhRR or dimerization with ARNT can be stimulated by AhR agonists.
Interestingly, the NF-κB member RelB, which may interact with AhR in the non-canonical AhR pathway (118), has been found to interact with ARNT via CD30-mediated NF-κB-dependent transcription (119). RelB has been demonstrated to promote cell growth through regulation of p53 stability and retinoblastoma protein activation (120). This mechanism supports recent findings showing that promotion of certain blood cancers (e.g. human multiple myeloma and anaplastic large cell lymphoma) rely on ARNT by antagonizing RelB and p53-dependent cell-cycle arrest and apoptosis (121). Expression of RelB was also shown to be critical for survival of Hodgkin lymphoma (122). Interestingly, a recent meta-analysis found that exposure and increased blood levels of TCDD are significantly associated with the mortality caused by non-Hodgkin’s lymphoma (123). Furthermore, our previous reports strongly support the function of AhR to mediate an anti-apoptotic response in human lymphoma cells (124) and the vital role of RelB in AhR-mediated apoptotic resistance in human breast cancer cells (61, 66). Because both, AhRR and RelB, may form heterodimers with ARNT (figure 2), it is essential to understand the possible interaction of AhRR/ARNT with RelB and its consequences in regulation of cellular processes, like cell-cycling and apoptosis. Moreover, it is likely that the dominant role of AhRR in complex with ARNT is via its interaction with RelB and the non-canonical AhR pathway resulting in down-regulation of cellular inflammation, suppression of an anti-apoptotic response, and supporting its role as a tumor suppressor gene. Figure 3 summarizes the possible interactions of AhRR with AhR signaling pathways and its consequences in cellular processes and carcinogenesis.
Highlights.
The AhRR as a specific competitive repressor of AhR
The AhRR represses alternative AhR signaling pathways
The AhRR regulates inflammatory responses
The AhRR may act as a tumor suppressor gene
Acknowledgments
We thank Heike Weighardt for critical reading of the manuscript and helpful comments. This work was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health by grant number R01 ES019898 (to C.V.) and the Wilhelm Sander Foundation by grant number 2014.073.1 (to T.H.S.).
Abbreviations
- AhR
aryl hydrocarbon receptor
- AhRR
aryl hydrocarbon receptor repressor
- ARNT
AhR nuclear translocator
- BaP
Benzo[a]pyrene
- CYP
cytochrome P450
- DREs
dioxin-responsive elements
- EGFR
epidermal growth factor receptor
- bHLH/PAS
Helix-Loop-Helix/Per-ARNT-Sim
- HIF-1α
hypoxia-inducible factor-1α
- LPS
lipopolysaccharide
- NCOA2
nuclear receptor co-activator-2
- Nrf2
nuclear factor erythroid-2-related factor-2
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abel J, Haarmann-Stemmann T. An introduction to the molecular basics of aryl hydrocarbon receptor biology. Biol Chem. 2010;391:1235–1248. doi: 10.1515/BC.2010.128. [DOI] [PubMed] [Google Scholar]
- 2.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 3.Gu YZ, Hogenesch JB, Bradfield CA. The PAS superfamily: sensors of environmental and developmental signals. Annu Rev Pharmacol Toxicol. 2000;40:519–561. doi: 10.1146/annurev.pharmtox.40.1.519. [DOI] [PubMed] [Google Scholar]
- 4.Fritsche E, Schafer C, Calles C, Bernsmann T, Bernshausen T, Wurm M, Hubenthal U, Cline JE, Hajimiragha H, Schroeder P, et al. Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc Natl Acad Sci U S A. 2007;104:8851–8856. doi: 10.1073/pnas.0701764104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madhukar BV, Brewster DW, Matsumura F. Effects of in vivo-administered 2,3,7,8-tetrachlorodibenzo-p-dioxin on receptor binding of epidermal growth factor in the hepatic plasma membrane of rat, guinea pig, mouse, and hamster. Proc Natl Acad Sci U S A. 1984;81:7407–7411. doi: 10.1073/pnas.81.23.7407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oesch-Bartlomowicz B, Huelster A, Wiss O, Antoniou-Lipfert P, Dietrich C, Arand M, Weiss C, Bockamp E, Oesch F. Aryl hydrocarbon receptor activation by cAMP vs. dioxin: divergent signaling pathways. Proc Natl Acad Sci U S A. 2005;102:9218–9223. doi: 10.1073/pnas.0503488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogel CF, Sciullo E, Li W, Wong P, Lazennec G, Matsumura F. RelB, a new partner of aryl hydrocarbon receptor-mediated transcription. Mol Endocrinol. 2007;21:2941–2955. doi: 10.1210/me.2007-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian Y, Rabson AB, Gallo MA. Ah receptor and NF-kappaB interactions: mechanisms and physiological implications. Chem Biol Interact. 2002;141:97–115. doi: 10.1016/s0009-2797(02)00068-6. [DOI] [PubMed] [Google Scholar]
- 9••.Mimura J, Ema M, Sogawa K, Fujii-Kuriyama Y. Identification of a novel mechanism of regulation of Ah (dioxin) receptor function. Genes Dev. 1999;13:20–25. doi: 10.1101/gad.13.1.20. This landmark paper describes the discovery of AhRR. The authors identify AhRR by screening of a mouse genomic library with an AhR cDNA probe and subsequently amplify and clone the respective coding sequence and its corresponding promoter region. Reporter gene assays reveal that AhRR expression is regulated in an AhR/ARNT-dependent manner and ectopic overexpression experiments identify AhRR to compete with AhR for both ARNT- and DRE-binding and, thus, as a negative feedback regulator of ligand-driven AhR signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watanabe T, Imoto I, Kosugi Y, Fukuda Y, Mimura J, Fujii Y, Isaka K, Takayama M, Sato A, Inazawa J. Human arylhydrocarbon receptor repressor (AhRR) gene: genomic structure and analysis of polymorphism in endometriosis. J Hum Genet. 2001;46:342–346. doi: 10.1007/s100380170070. [DOI] [PubMed] [Google Scholar]
- 11.Fujita H, Kosaki R, Yoshihashi H, Ogata T, Tomita M, Hasegawa T, Takahashi T, Matsuo N, Kosaki K. Characterization of the aryl hydrocarbon receptor repressor gene and association of its Pro185Ala polymorphism with micropenis. Teratology. 2002;65:10–18. doi: 10.1002/tera.1093. [DOI] [PubMed] [Google Scholar]
- 12.Korkalainen M, Tuomisto J, Pohjanvirta R. Primary structure and inducibility by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) of aryl hydrocarbon receptor repressor in a TCDD-sensitive and a TCDD-resistant rat strain. Biochem Biophys Res Commun. 2004;315:123–131. doi: 10.1016/j.bbrc.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 13.Nishihashi H, Kanno Y, Tomuro K, Nakahama T, Inouye Y. Primary structure and organ-specific expression of the rat aryl hydrocarbon receptor repressor gene. Biol Pharm Bull. 2006;29:640–647. doi: 10.1248/bpb.29.640. [DOI] [PubMed] [Google Scholar]
- 14.Lee JS, Kim EY, Nomaru K, Iwata H. Molecular and functional characterization of Aryl hydrocarbon receptor repressor from the chicken (Gallus gallus): interspecies similarities and differences. Toxicol Sci. 2011;119:319–334. doi: 10.1093/toxsci/kfq336. [DOI] [PubMed] [Google Scholar]
- 15.Zimmermann AL, King EA, Dengler E, Scogin SR, Powell WH. An aryl hydrocarbon receptor repressor from Xenopus laevis: function, expression, and role in dioxin responsiveness during frog development. Toxicol Sci. 2008;104:124–134. doi: 10.1093/toxsci/kfn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans BR, Karchner SI, Franks DG, Hahn ME. Duplicate aryl hydrocarbon receptor repressor genes (ahrr1 and ahrr2) in the zebrafish Danio rerio: structure, function, evolution, and AhR-dependent regulation in vivo. Arch Biochem Biophys. 2005;441:151–167. doi: 10.1016/j.abb.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Karchner SI, Franks DG, Powell WH, Hahn ME. Regulatory interactions among three members of the vertebrate aryl hydrocarbon receptor family: AhR repressor, AhR1, and AhR2. J Biol Chem. 2002;277:6949–6959. doi: 10.1074/jbc.M110779200. [DOI] [PubMed] [Google Scholar]
- 18.Roy NK, Courtenay SC, Chambers RC, Wirgin II. Characterization of the aryl hydrocarbon receptor repressor and a comparison of its expression in Atlantic tomcod from resistant and sensitive populations. Environ Toxicol Chem. 2006;25:560–571. doi: 10.1897/05-347r.1. [DOI] [PubMed] [Google Scholar]
- 19•.Baba T, Mimura J, Gradin K, Kuroiwa A, Watanabe T, Matsuda Y, Inazawa J, Sogawa K, Fujii-Kuriyama Y. Structure and expression of the Ah receptor repressor gene. J Biol Chem. 2001;276:33101–33110. doi: 10.1074/jbc.M011497200. Reports about the chromosomal localization of human, murine and rat Ahrr and provides a detailed molecular analysis of the murine Ahrr gene promoter. By using reporter gene assays and site-directed mutagenesis, the authors provide first evidence that AhRR expression is not only regulated by the AhR/ARNT complex but also by pro-inflammatory signaling pathways, in particular by NF-κB. [DOI] [PubMed] [Google Scholar]
- 20.Cauchi S, Stucker I, Cenee S, Kremers P, Beaune P, Massaad-Massade L. Structure and polymorphisms of human aryl hydrocarbon receptor repressor (AhRR) gene in a French population: relationship with CYP1A1 inducibility and lung cancer. Pharmacogenetics. 2003;13:339–347. doi: 10.1097/01.fpc.0000054093.48725.79. [DOI] [PubMed] [Google Scholar]
- 21.Haarmann-Stemmann T, Bothe H, Kohli A, Sydlik U, Abel J, Fritsche E. Analysis of the transcriptional regulation and molecular function of the aryl hydrocarbon receptor repressor in human cell lines. Drug Metab Dispos. 2007;35:2262–2269. doi: 10.1124/dmd.107.016253. [DOI] [PubMed] [Google Scholar]
- 22.Oshima M, Mimura J, Sekine H, Okawa H, Fujii-Kuriyama Y. SUMO modification regulates the transcriptional repressor function of aryl hydrocarbon receptor repressor. J Biol Chem. 2009;284:11017–11026. doi: 10.1074/jbc.M808694200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gradin K, Toftgard R, Poellinger L, Berghard A. Repression of dioxin signal transduction in fibroblasts. Identification Of a putative repressor associated with Arnt. J Biol Chem. 1999;274:13511–13518. doi: 10.1074/jbc.274.19.13511. [DOI] [PubMed] [Google Scholar]
- 24••.Oshima M, Mimura J, Yamamoto M, Fujii-Kuriyama Y. Molecular mechanism of transcriptional repression of AhR repressor involving ANKRA2, HDAC4, and HDAC5. Biochem Biophys Res Commun. 2007;364:276–282. doi: 10.1016/j.bbrc.2007.09.131. In this paper the authors unravel the molecular mechanism by which AhRR is capable of repressing DRE-dependent gene expression. The authors show that the DRE-bound AhRR sequentially recruits the transcriptional co-repressor molecule ANKRA2 and histone deacetylases 4 and 5 to the Cyp1a1 promoter and thereby hinders the expression of the AhR target gene by closing the local chromatin. [DOI] [PubMed] [Google Scholar]
- 25.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26••.Evans BR, Karchner SI, Allan LL, Pollenz RS, Tanguay RL, Jenny MJ, Sherr DH, Hahn ME. Repression of aryl hydrocarbon receptor (AhR) signaling by AhR repressor: role of DNA binding and competition for AhR nuclear translocator. Mol Pharmacol. 2008;73:387–398. doi: 10.1124/mol.107.040204. This study reveals that repression of AhR activity by AhRR involves the N-terminal part of the AhRR protein and does not involve a competition for ARNT. Moreover, it does not per se depend on binding to DRE, but DRE-binding may contribute to the repression. A mechanism of AhRR’s action is proposed that involves “transrepression” of AhR signaling through protein-protein interactions rather than by inhibition of the formation or DNA-binding of the AhR/ARNT heterodimer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ke S, Rabson AB, Germino JF, Gallo MA, Tian Y. Mechanism of suppression of cytochrome P-450 1A1 expression by tumor necrosis factor-alpha and lipopolysaccharide. J Biol Chem. 2001;276:39638–39644. doi: 10.1074/jbc.M106286200. [DOI] [PubMed] [Google Scholar]
- 28.Sutter CH, Yin H, Li Y, Mammen JS, Bodreddigari S, Stevens G, Cole JA, Sutter TR. EGF receptor signaling blocks aryl hydrocarbon receptor-mediated transcription and cell differentiation in human epidermal keratinocytes. Proc Natl Acad Sci U S A. 2009;106:4266–4271. doi: 10.1073/pnas.0900874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tauchi M, Hida A, Negishi T, Katsuoka F, Noda S, Mimura J, Hosoya T, Yanaka A, Aburatani H, Fujii-Kuriyama Y, et al. Constitutive expression of aryl hydrocarbon receptor in keratinocytes causes inflammatory skin lesions. Mol Cell Biol. 2005;25:9360–9368. doi: 10.1128/MCB.25.21.9360-9368.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joo M, Lee C, Koo J, Kim S. miR-125b transcriptionally increased by Nrf2 inhibits AhR repressor, which protects kidney from cisplatin-induced injury. Cell Death Dis. 2013;4:e899. doi: 10.1038/cddis.2013.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernshausen T, Jux B, Esser C, Abel J, Fritsche E. Tissue distribution and function of the Aryl hydrocarbon receptor repressor (AhRR) in C57BL/6 and Aryl hydrocarbon receptor deficient mice. Arch Toxicol. 2006;80:206–211. doi: 10.1007/s00204-005-0025-5. [DOI] [PubMed] [Google Scholar]
- 32.Tsuchiya Y, Nakajima M, Itoh S, Iwanari M, Yokoi T. Expression of aryl hydrocarbon receptor repressor in normal human tissues and inducibility by polycyclic aromatic hydrocarbons in human tumor-derived cell lines. Toxicol Sci. 2003;72:253–259. doi: 10.1093/toxsci/kfg022. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto J, Ihara K, Nakayama H, Hikino S, Satoh K, Kubo N, Iida T, Fujii Y, Hara T. Characteristic expression of aryl hydrocarbon receptor repressor gene in human tissues: organ-specific distribution and variable induction patterns in mononuclear cells. Life Sci. 2004;74:1039–1049. doi: 10.1016/j.lfs.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 34.Frericks M, Meissner M, Esser C. Microarray analysis of the AhR system: tissue-specific flexibility in signal and target genes. Toxicol Appl Pharmacol. 2007;220:320–332. doi: 10.1016/j.taap.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 35.Tigges J, Haarmann-Stemmann T, Vogel CF, Grindel A, Hubenthal U, Brenden H, Grether-Beck S, Vielhaber G, Johncock W, Krutmann J, et al. The new aryl hydrocarbon receptor antagonist E/Z-2-benzylindene-5,6-dimethoxy-3,3-dimethylindan-1-one protects against UVB-induced signal transduction. J Invest Dermatol. 2014;134:556–559. doi: 10.1038/jid.2013.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson PA, Khatami M, Baglole CJ, Sun J, Harris SA, Moon EY, Al-Mulla F, Al-Temaimi R, Brown DG, Colacci A, et al. Environmental immune disruptors, inflammation and cancer risk. Carcinogenesis. 2015;36(Suppl 1):S232–S253. doi: 10.1093/carcin/bgv038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kennedy GD, Nukaya M, Moran SM, Glover E, Weinberg S, Balbo S, Hecht SS, Pitot HC, Drinkwater NR, Bradfield CA. Liver tumor promotion by 2,3,7,8-tetrachlorodibenzo-p-dioxin is dependent on the aryl hydrocarbon receptor and TNF/IL-1 receptors. Toxicol Sci. 2014;140:135–143. doi: 10.1093/toxsci/kfu065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nukaya M, Moran S, Bradfield CA. The role of the dioxin-responsive element cluster between the Cyp1a1 and Cyp1a2 loci in aryl hydrocarbon receptor biology. Proc Natl Acad Sci U S A. 2009;106:4923–4928. doi: 10.1073/pnas.0809613106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uno S, Dalton TP, Sinclair PR, Gorman N, Wang B, Smith AG, Miller ML, Shertzer HG, Nebert DW. Cyp1a1(−/−) male mice: protection against high-dose TCDD-induced lethality and wasting syndrome, and resistance to intrahepatocyte lipid accumulation and uroporphyria. Toxicol Appl Pharmacol. 2004;196:410–421. doi: 10.1016/j.taap.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 41.Bersten DC, Sullivan AE, Peet DJ, Whitelaw ML. bHLH-PAS proteins in cancer. Nat Rev Cancer. 2013;13:827–841. doi: 10.1038/nrc3621. [DOI] [PubMed] [Google Scholar]
- 42.Murray IA, Patterson AD, Perdew GH. Aryl hydrocarbon receptor ligands in cancer: friend and foe. Nat Rev Cancer. 2014;14:801–814. doi: 10.1038/nrc3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Safe S, Lee SO, Jin UH. Role of the Aryl Hydrocarbon Receptor in Carcinogenesis and Potential as a Drug Target. Toxicol Sci. 2013;135:1–16. doi: 10.1093/toxsci/kft128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehar J, Kryukov GV, Sonkin D, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stobbe-Maicherski N, Wolff S, Wolff C, Abel J, Sydlik U, Frauenstein K, Haarmann-Stemmann T. The interleukin-6-type cytokine oncostatin M induces aryl hydrocarbon receptor expression in a STAT3-dependent manner in human HepG2 hepatoma cells. FEBS J. 2013;280:6681–6690. doi: 10.1111/febs.12571. [DOI] [PubMed] [Google Scholar]
- 46.Vogel CF, Khan EM, Leung PS, Gershwin ME, Chang WL, Wu D, Haarmann-Stemmann T, Hoffmann A, Denison MS. Cross-talk between aryl hydrocarbon receptor and the inflammatory response: a role for nuclear factor-kappaB. J Biol Chem. 2014;289:1866–1875. doi: 10.1074/jbc.M113.505578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47••.Vogel CF, Chang WL, Kado S, McCulloh K, Vogel H, Wu D, Haarmann-Stemmann T, Yang G, Leung PS, Matsumura F, et al. Transgenic Overexpression of Aryl Hydrocarbon Receptor Repressor (AhRR) and AhR-Mediated Induction of CYP1A1, Cytokines, and Acute Toxicity. Environ Health Perspect. 2016;124:1071–1083. doi: 10.1289/ehp.1510194. Here we describe the generation and characterization of transgenic C57BL/6J mice that overexpress AhRR (AhRR Tg). In response to TCDD exposure, we observed a less pronounced upregulation of inflammatory cytokines, such as IL-1β, CXCL2 and CXCL3, in white adipose tissue from AhRR Tg mice. Male AhRR Tg mice are protected from high-dose TCDD-induced lethality due to reduced inflammatory responses and liver damage as indicated by lower levels of TCDD-induced alanine aminotransferase and hepatic triglycerides. Our study identifies AhRR as a previously uncharacterized regulator of specific inflammatory cytokines, which may protect from acute toxicity induced by TCDD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kimura A, Naka T, Nakahama T, Chinen I, Masuda K, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor in combination with Stat1 regulates LPS-induced inflammatory responses. J Exp Med. 2009;206:2027–2035. doi: 10.1084/jem.20090560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu D, Li W, Lok P, Matsumura F, Vogel CF. AhR deficiency impairs expression of LPS-induced inflammatory genes in mice. Biochem Biophys Res Commun. 2011;410:358–363. doi: 10.1016/j.bbrc.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sekine H, Mimura J, Oshima M, Okawa H, Kanno J, Igarashi K, Gonzalez FJ, Ikuta T, Kawajiri K, Fujii-Kuriyama Y. Hypersensitivity of aryl hydrocarbon receptor-deficient mice to lipopolysaccharide-induced septic shock. Mol Cell Biol. 2009;29:6391–6400. doi: 10.1128/MCB.00337-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bessede A, Gargaro M, Pallotta MT, Matino D, Servillo G, Brunacci C, Bicciato S, Mazza EM, Macchiarulo A, Vacca C, et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature. 2014;511:184–190. doi: 10.1038/nature13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCall KD, Holliday D, Dickerson E, Wallace B, Schwartz AL, Schwartz C, Lewis CJ, Kohn LD, Schwartz FL. Phenylmethimazole blocks palmitate-mediated induction of inflammatory cytokine pathways in 3T3L1 adipocytes and RAW 264.7 macrophages. J Endocrinol. 2010;207:343–353. doi: 10.1677/JOE-09-0370. [DOI] [PubMed] [Google Scholar]
- 53.Chen X, Yoza BK, El GM, Hu JY, Cousart SL, McCall CE. RelB sustains IkappaBalpha expression during endotoxin tolerance. Clin Vaccine Immunol. 2009;16:104–110. doi: 10.1128/CVI.00320-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, McDowell J, Paskind M, Rodman L, Salfeld J, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 55••.Brandstätter O, Schanz O, Vorac J, Konig J, Mori T, Maruyama T, Korkowski M, Haarmann-Stemmann T, von SD, Schultze JL, et al. Balancing intestinal and systemic inflammation through cell type-specific expression of the aryl hydrocarbon receptor repressor. Sci Rep. 2016;6:26091. doi: 10.1038/srep26091. This paper describes the generation of AhRR-ko and AhRR reporter mice, and the characterization of AhRR’s function under two different inflammatory conditions: LPS shock and DSS-induced colitis. The authors show that AhRR is predominantly expressed in immune cells of the skin and intestine. Whereas AhRR antagonizes the anti-inflammatory function of AhR during systemic endotoxin shock, AhR and AhRR act in concert to dampen DSS-induced intestinal inflammation. This paper highlights the importance of cell-type specific balancing of AhR/AhRR expression for responses toward microbial and environmental stressors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takamura T, Harama D, Matsuoka S, Shimokawa N, Nakamura Y, Okumura K, Ogawa H, Kitamura M, Nakao A. Activation of the aryl hydrocarbon receptor pathway may ameliorate dextran sodium sulfate-induced colitis in mice. Immunol Cell Biol. 2010;88:685–689. doi: 10.1038/icb.2010.35. [DOI] [PubMed] [Google Scholar]
- 57.Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva EF, Wilhelm C, Veldhoen M. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147:629–640. doi: 10.1016/j.cell.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 58.Singh NP, Singh UP, Singh B, Price RL, Nagarkatti M, Nagarkatti PS. Activation of aryl hydrocarbon receptor (AhR) leads to reciprocal epigenetic regulation of FoxP3 and IL-17 expression and amelioration of experimental colitis. PLoS One. 2011;6:e23522. doi: 10.1371/journal.pone.0023522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chinen I, Nakahama T, Kimura A, Nguyen NT, Takemori H, Kumagai A, Kayama H, Takeda K, Lee S, Hanieh H, et al. The aryl hydrocarbon receptor/microRNA-212/132 axis in T cells regulates IL-10 production to maintain intestinal homeostasis. Int Immunol. 2015;27:405–415. doi: 10.1093/intimm/dxv015. [DOI] [PubMed] [Google Scholar]
- 60.Yang X, Solomon S, Fraser LR, Trombino AF, Liu D, Sonenshein GE, Hestermann EV, Sherr DH. Constitutive regulation of CYP1B1 by the aryl hydrocarbon receptor (AhR) in pre-malignant and malignant mammary tissue. J Cell Biochem. 2008;104:402–417. doi: 10.1002/jcb.21630. [DOI] [PubMed] [Google Scholar]
- 61.Vogel CF, Li W, Wu D, Miller JK, Sweeney C, Lazennec G, Fujisawa Y, Matsumura F. Interaction of aryl hydrocarbon receptor and NF-kappaB subunit RelB in breast cancer is associated with interleukin-8 overexpression. Arch Biochem Biophys. 2011;512:78–86. doi: 10.1016/j.abb.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stanford EA, Wang Z, Novikov O, Mulas F, Landesman-Bollag E, Monti S, Smith BW, Seldin DC, Murphy GJ, Sherr DH. The role of the aryl hydrocarbon receptor in the development of cells with the molecular and functional characteristics of cancer stem-like cells. BMC Biol. 2016;14:20. doi: 10.1186/s12915-016-0240-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peng TL, Chen J, Mao W, Song X, Chen MH. Aryl hydrocarbon receptor pathway activation enhances gastric cancer cell invasiveness likely through a c-Jun-dependent induction of matrix metalloproteinase-9. BMC Cell Biol. 2009;10:27. doi: 10.1186/1471-2121-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 65.Goode GD, Ballard BR, Manning HC, Freeman ML, Kang Y, Eltom SE. Knockdown of aberrantly upregulated aryl hydrocarbon receptor reduces tumor growth and metastasis of MDA-MB-231 human breast cancer cell line. Int J Cancer. 2013;133:2769–2780. doi: 10.1002/ijc.28297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bekki K, Vogel H, Li W, Ito T, Sweeney C, Haarmann-Stemmann T, Matsumura F, Vogel CF. The aryl hydrocarbon receptor (AhR) mediates resistance to apoptosis induced in breast cancer cells. Pestic Biochem Physiol. 2015;120:5–13. doi: 10.1016/j.pestbp.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mitra AB, Murty VV, Singh V, Li RG, Pratap M, Sodhani P, Luthra UK, Chaganti RS. Genetic alterations at 5p15: a potential marker for progression of precancerous lesions of the uterine cervix. J Natl Cancer Inst. 1995;87:742–745. doi: 10.1093/jnci/87.10.742. [DOI] [PubMed] [Google Scholar]
- 68.Wieland I, Bohm M, Arden KC, Ammermuller T, Bogatz S, Viars CS, Rajewsky MF. Allelic deletion mapping on chromosome 5 in human carcinomas. Oncogene. 1996;12:97–102. [PubMed] [Google Scholar]
- 69.Bohm M, Kleine-Besten R, Wieland I. Loss of heterozygosity analysis on chromosome 5p defines 5p13-12 as the critical region involved in tumor progression of bladder carcinomas. Int J Cancer. 2000;89:194–197. [PubMed] [Google Scholar]
- 70.Wang VW, Bell DA, Berkowitz RS, Mok SC. Whole genome amplification and high-throughput allelotyping identified five distinct deletion regions on chromosomes 5 and 6 in microdissected early-stage ovarian tumors. Cancer Res. 2001;61:4169–4174. [PubMed] [Google Scholar]
- 71.Peralta RC, Casson AG, Wang RN, Keshavjee S, Redston M, Bapat B. Distinct regions of frequent loss of heterozygosity of chromosome 5p and 5q in human esophageal cancer. Int J Cancer. 1998;78:600–605. doi: 10.1002/(sici)1097-0215(19981123)78:5<600::aid-ijc12>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 72.Xu SF, Peng ZH, Li DP, Qiu GQ, Zhang F. Refinement of heterozygosity loss on chromosome 5p15 in sporadic colorectal cancer. World J Gastroenterol. 2003;9:1713–1718. doi: 10.3748/wjg.v9.i8.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73••.Zudaire E, Cuesta N, Murty V, Woodson K, Adams L, Gonzalez N, Martinez A, Narayan G, Kirsch I, Franklin W, et al. The aryl hydrocarbon receptor repressor is a putative tumor suppressor gene in multiple human cancers. J Clin Invest. 2008;118:640–650. doi: 10.1172/JCI30024. Reports about decreased AhRR expression rates in multiple human cancers, including breast, colon, lung, stomach, cervix, and ovary malignancies, indicating that AhRR harbors tumor suppressive properties. Further molecular analyses reveal that AhRR expression is silenced by hypermethylation of its promoter sequence, which is associated with the closure of the local chroamtin and hindering of transcription factors from DNA-binding. Additional in vitro analyses on cancer cell-lines, including ectopic overexpression and RNAi, indicate that AhRR inhibits tumor cell proliferation, invasion, motility and apoptosis resistance, thus further strengthening a tumor supressor function of AhRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang J, Zong H, Li S, Zhang D, Zhang L, Xia Q. Activation of aryl hydrocarbon receptor suppresses invasion of esophageal squamous cell carcinoma cell lines. Tumori. 2012;98:152–157. doi: 10.1177/030089161209800121. [DOI] [PubMed] [Google Scholar]
- 75.Yin J, Sheng B, Pu A, Han B, Yang K, Wang Q, Sun L, Yang H. Keratinocyte Growth Factor Regulation of Aryl Hydrocarbon Receptor Activation in Colorectal Cancer Cells. Dig Dis Sci. 2016;61:444–452. doi: 10.1007/s10620-015-3908-1. [DOI] [PubMed] [Google Scholar]
- 76.Portal-Nunez S, Shankavaram UT, Rao M, Datrice N, Atay S, Aparicio M, Camphausen KA, Fernandez-Salguero PM, Chang H, Lin P, et al. Aryl hydrocarbon receptor-induced adrenomedullin mediates cigarette smoke carcinogenicity in humans and mice. Cancer Res. 2012;72:5790–5800. doi: 10.1158/0008-5472.CAN-12-0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin P, Chang H, Tsai WT, Wu MH, Liao YS, Chen JT, Su JM. Overexpression of aryl hydrocarbon receptor in human lung carcinomas. Toxicol Pathol. 2003;31:22–30. doi: 10.1080/01926230390173824. [DOI] [PubMed] [Google Scholar]
- 78.Lai DW, Liu SH, Karlsson AI, Lee WJ, Wang KB, Chen YC, Shen CC, Wu SM, Liu CY, Tien HR, et al. The novel Aryl hydrocarbon receptor inhibitor biseugenol inhibits gastric tumor growth and peritoneal dissemination. Oncotarget. 2014;5:7788–7804. doi: 10.18632/oncotarget.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li YF, Wang DD, Zhao BW, Wang W, Yuan SQ, Huang CY, Chen YM, Zheng Y, Keshari RP, Xia JC, et al. Poor prognosis of gastric adenocarcinoma with decreased expression of AhRR. PLoS One. 2012;7:e43555. doi: 10.1371/journal.pone.0043555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou Y, Zhou SX, Gao L, Li XA. Regulation of CD40 signaling in colon cancer cells and its implications in clinical tissues. Cancer Immunol Immunother. 2016;65:919–929. doi: 10.1007/s00262-016-1847-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Coope HJ, Atkinson PG, Huhse B, Belich M, Janzen J, Holman MJ, Klaus GG, Johnston LH, Ley SC. CD40 regulates the processing of NF-kappaB2 p100 to p52. EMBO J. 2002;21:5375–5385. doi: 10.1093/emboj/cdf542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Loskog AS, Eliopoulos AG. The Janus faces of CD40 in cancer. Semin Immunol. 2009;21:301–307. doi: 10.1016/j.smim.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 83.Sun Y, Peng D, Lecanda J, Schmitz V, Barajas M, Qian C, Prieto J. In vivo gene transfer of CD40 ligand into colon cancer cells induces local production of cytokines and chemokines, tumor eradication and protective antitumor immunity. Gene Ther. 2000;7:1467–1476. doi: 10.1038/sj.gt.3301264. [DOI] [PubMed] [Google Scholar]
- 84.Allan LL, Sherr DH. Constitutive activation and environmental chemical induction of the aryl hydrocarbon receptor/transcription factor in activated human B lymphocytes. Mol Pharmacol. 2005;67:1740–1750. doi: 10.1124/mol.104.009100. [DOI] [PubMed] [Google Scholar]
- 85.Schlezinger JJ, Liu D, Farago M, Seldin DC, Belguise K, Sonenshein GE, Sherr DH. A role for the aryl hydrocarbon receptor in mammary gland tumorigenesis. Biol Chem. 2006;387:1175–1187. doi: 10.1515/BC.2006.145. [DOI] [PubMed] [Google Scholar]
- 86.Kanno Y, Takane Y, Izawa T, Nakahama T, Inouye Y. The inhibitory effect of aryl hydrocarbon receptor repressor (AhRR) on the growth of human breast cancer MCF-7 cells. Biol Pharm Bull. 2006;29:1254–1257. doi: 10.1248/bpb.29.1254. [DOI] [PubMed] [Google Scholar]
- 87.Wong PS, Li W, Vogel CF, Matsumura F. Characterization of MCF mammary epithelial cells overexpressing the Arylhydrocarbon receptor (AhR) BMC Cancer. 2009;9:234. doi: 10.1186/1471-2407-9-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kanno Y, Takane Y, Takizawa Y, Inouye Y. Suppressive effect of aryl hydrocarbon receptor repressor on transcriptional activity of estrogen receptor alpha by protein-protein interaction in stably and transiently expressing cell lines. Mol Cell Endocrinol. 2008;291:87–94. doi: 10.1016/j.mce.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 89.Kohle C, Hassepass I, Bock-Hennig BS, Walter BK, Poellinger L, McGuire J. Conditional expression of a constitutively active aryl hydrocarbon receptor in MCF-7 human breast cancer cells. Arch Biochem Biophys. 2002;402:172–179. doi: 10.1016/S0003-9861(02)00076-0. [DOI] [PubMed] [Google Scholar]
- 90.Wang F, Samudio I, Safe S. Transcriptional activation of cathepsin D gene expression by 17beta-estradiol: mechanism of aryl hydrocarbon receptor-mediated inhibition. Mol Cell Endocrinol. 2001;172:91–103. doi: 10.1016/s0303-7207(00)00379-8. [DOI] [PubMed] [Google Scholar]
- 91.Labrecque MP, Takhar MK, Hollingshead BD, Prefontaine GG, Perdew GH, Beischlag TV. Distinct roles for aryl hydrocarbon receptor nuclear translocator and ah receptor in estrogen-mediated signaling in human cancer cell lines. PLoS One. 2012;7:e29545. doi: 10.1371/journal.pone.0029545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92••.Karchner SI, Jenny MJ, Tarrant AM, Evans BR, Kang HJ, Bae I, Sherr DH, Hahn ME. The active form of human aryl hydrocarbon receptor (AhR) repressor lacks exon 8, and its Pro 185 and Ala 185 variants repress both AhR and hypoxia-inducible factor. Mol Cell Biol. 2009;29:3465–3477. doi: 10.1128/MCB.00206-09. The authors identify a novel human AhRR cDNA that lacks exon 8 of the published AhRR sequence. This transcript variant is the predominantly expressed form of AhRR in human tissues and cell-lines. It represses AhR-dependent as well as hypoxia-inducible factor-1α (HIF-1α)-1-dependent transcriptional activity, indicating that AhRR may not exclusively repress canonical AhR signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012;33:207–214. doi: 10.1016/j.tips.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94•.Jin Y, Moller E, Nord KH, Mandahl N, Von Steyern FV, Domanski HA, Marino-Enriquez A, Magnusson L, Nilsson J, Sciot R, et al. Fusion of the AhRR and NCOA2 genes through a recurrent translocation t(5;8)(p15;q13) in soft tissue angiofibroma results in upregulation of aryl hydrocarbon receptor target genes. Genes Chromosomes Cancer. 2012;51:510–520. doi: 10.1002/gcc.21939. Identifies a fusion protein of AhRR and NCOA2 that consists of the N-terminal half of AhRR, harboring the DNA-binding domain, and the C-terminal part of NCOA2, carrying two transactivation domains. Global gene expression analyses reveal that this fusion protein is capable of mimicking canonical AhR signaling. Even though the AhRR/NCOA2 fusion protein is detected only in a small number of (benign) soft tissue angiofibromas, this paper provides evidence that AhRR’s inhibitory function can be abrogated through chromosomal rearrangements. [DOI] [PubMed] [Google Scholar]
- 95.Gelboin HV. Benzo[alpha]pyrene metabolism, activation and carcinogenesis: role and regulation of mixed-function oxidases and related enzymes. Physiol Rev. 1980;60:1107–1166. doi: 10.1152/physrev.1980.60.4.1107. [DOI] [PubMed] [Google Scholar]
- 96.Shi S, Yoon DY, Hodge-Bell KC, Bebenek IG, Whitekus MJ, Zhang R, Cochran AJ, Huerta-Yepez S, Yim SH, Gonzalez FJ, et al. The aryl hydrocarbon receptor nuclear translocator (Arnt) is required for tumor initiation by benzo[a]pyrene. Carcinogenesis. 2009;30:1957–1961. doi: 10.1093/carcin/bgp201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shimizu Y, Nakatsuru Y, Ichinose M, Takahashi Y, Kume H, Mimura J, Fujii-Kuriyama Y, Ishikawa T. Benzo[a]pyrene carcinogenicity is lost in mice lacking the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A. 2000;97:779–782. doi: 10.1073/pnas.97.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nakatsuru Y, Wakabayashi K, Fujii-Kuriyama Y, Ishikawa T, Kusama K, Ide F. Dibenzo[A,L]pyrene-induced genotoxic and carcinogenic responses are dramatically suppressed in aryl hydrocarbon receptor-deficient mice. Int J Cancer. 2004;112:179–183. doi: 10.1002/ijc.20365. [DOI] [PubMed] [Google Scholar]
- 99••.Hosoya T, Harada N, Mimura J, Motohashi H, Takahashi S, Nakajima O, Morita M, Kawauchi S, Yamamoto M, Fujii-Kuriyama Y. Inducibility of cytochrome P450 1A1 and chemical carcinogenesis by benzo[a]pyrene in AhR repressor-deficient mice. Biochem Biophys Res Commun. 2008;365:562–567. doi: 10.1016/j.bbrc.2007.11.016. First generation of AhRR-ko mice. Expression analyses reveal that the expected super-induction of the Cyp1a1 gene is restricted to a few tissues, such as stomach, skin and spleen. A carcinogenesis study with BaP in wt and AhRR-ko mice shows a delayed occurrence of BaP-induced skin tumors in AhRR-ko animals. The authors propose that a shift from CYP1A1-driven toxification towards detoxification of BaP is responsible for the postponed cancer development observed in mice that lack a functional AhRR. [DOI] [PubMed] [Google Scholar]
- 100.Nebert DW, Shi Z, Galvez-Peralta M, Uno S, Dragin N. Oral benzo[a]pyrene: understanding pharmacokinetics, detoxication, and consequences--Cyp1 knockout mouse lines as a paradigm. Mol Pharmacol. 2013;84:304–313. doi: 10.1124/mol.113.086637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Novakovic B, Ryan J, Pereira N, Boughton B, Craig JM, Saffery R. Postnatal stability, tissue, and time specific effects of AhRR methylation change in response to maternal smoking in pregnancy. Epigenetics. 2014;9:377–386. doi: 10.4161/epi.27248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee KW, Richmond R, Hu P, French L, Shin J, Bourdon C, Reischl E, Waldenberger M, Zeilinger S, Gaunt T, et al. Prenatal exposure to maternal cigarette smoking and DNA methylation: epigenome-wide association in a discovery sample of adolescents and replication in an independent cohort at birth through 17 years of age. Environ Health Perspect. 2015;123:193–199. doi: 10.1289/ehp.1408614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gao X, Jia M, Zhang Y, Breitling LP, Brenner H. DNA methylation changes of whole blood cells in response to active smoking exposure in adults: a systematic review of DNA methylation studies. Clin Epigenetics. 2015;7:113. doi: 10.1186/s13148-015-0148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fasanelli F, Baglietto L, Ponzi E, Guida F, Campanella G, Johansson M, Grankvist K, Johansson M, Assumma MB, Naccarati A, et al. Hypomethylation of smoking-related genes is associated with future lung cancer in four prospective cohorts. Nat Commun. 2015;6:10192. doi: 10.1038/ncomms10192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105•.Monick MM, Beach SR, Plume J, Sears R, Gerrard M, Brody GH, Philibert RA. Coordinated changes in AhRR methylation in lymphoblasts and pulmonary macrophages from smokers. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:141–151. doi: 10.1002/ajmg.b.32021. To the best of our knowledge this is the first study correlating promoter hypomethylation of AhRR with tobacco smoking. In biomaterial from current smokers, i.e. lymphoblast DNA and RNA as well as lung alveolar macrophage DNA, the authors observe a significant hypomethylation of the Ahrr gene promoter, which was associated with reduced AhRR expression. These findings may be of relevance for smoking-related inflammatory responses and carcinogenesis of the lung. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang Y, Schottker B, Florath I, Stock C, Butterbach K, Holleczek B, Mons U, Brenner H. Smoking-Associated DNA Methylation Biomarkers and Their Predictive Value for All-Cause and Cardiovascular. Mortality. Environ Health Perspect. 2016;124:67–74. doi: 10.1289/ehp.1409020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dogan MV, Shields B, Cutrona C, Gao L, Gibbons FX, Simons R, Monick M, Brody GH, Tan K, Beach SR, et al. The effect of smoking on DNA methylation of peripheral blood mononuclear cells from African American women. BMC Genomics. 2014;15:151. doi: 10.1186/1471-2164-15-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang Y, Elgizouli M, Schottker B, Holleczek B, Nieters A, Brenner H. Smoking-associated DNA methylation markers predict lung cancer incidence. Clin Epigenetics. 2016;8:127. doi: 10.1186/s13148-016-0292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang Y, Xue Q, Pan G, Meng QH, Tuo X, Cai X, Chen Z, Li Y, Huang T, Duan X, et al. Integrated Analysis of Genome-Wide Copy Number Alterations and Gene Expression Profiling of Lung Cancer in Xuanwei, China. PLoS One. 2017;12:e0169098. doi: 10.1371/journal.pone.0169098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rotroff DM, Joubert BR, Marvel SW, Haberg SE, Wu MC, Nilsen RM, Ueland PM, Nystad W, London SJ, Motsinger-Reif A. Maternal smoking impacts key biological pathways in newborns through epigenetic modification in Utero. BMC Genomics. 2016;17:976. doi: 10.1186/s12864-016-3310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Razin A. CpG methylation, chromatin structure and gene silencing-a three-way connection. EMBO J. 1998;17:4905–4908. doi: 10.1093/emboj/17.17.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hahn ME, Allan LL, Sherr DH. Regulation of constitutive and inducible AhR signaling: complex interactions involving the AhR repressor. Biochem Pharmacol. 2009;77:485–497. doi: 10.1016/j.bcp.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Taylor RT, Wang F, Hsu EL, Hankinson O. Roles of coactivator proteins in dioxin induction of CYP1A1 and CYP1B1 in human breast cancer cells. Toxicol Sci. 2009;107:1–8. doi: 10.1093/toxsci/kfn217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hestermann EV, Brown M. Agonist and chemopreventative ligands induce differential transcriptional cofactor recruitment by aryl hydrocarbon receptor. Mol Cell Biol. 2003;23:7920–7925. doi: 10.1128/MCB.23.21.7920-7925.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Beischlag TV, Wang S, Rose DW, Torchia J, Reisz-Porszasz S, Muhammad K, Nelson WE, Probst MR, Rosenfeld MG, Hankinson O. Recruitment of the NCoA/SRC-1/p160 family of transcriptional coactivators by the aryl hydrocarbon receptor/aryl hydrocarbon receptor nuclear translocator complex. Mol Cell Biol. 2002;22:4319–4333. doi: 10.1128/MCB.22.12.4319-4333.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kanno Y, Miyama Y, Takane Y, Nakahama T, Inouye Y. Identification of intracellular localization signals and of mechanisms underlining the nucleocytoplasmic shuttling of human aryl hydrocarbon receptor repressor. Biochem Biophys Res Commun. 2007;364:1026–1031. doi: 10.1016/j.bbrc.2007.10.140. [DOI] [PubMed] [Google Scholar]
- 117.Carver LA, Bradfield CA. Ligand-dependent interaction of the aryl hydrocarbon receptor with a novel immunophilin homolog in vivo. J Biol Chem. 1997;272:11452–11456. doi: 10.1074/jbc.272.17.11452. [DOI] [PubMed] [Google Scholar]
- 118.Vogel CF, Matsumura F. A new cross-talk between the aryl hydrocarbon receptor and RelB, a member of the NF-kappaB family. Biochem Pharmacol. 2009;77:734–745. doi: 10.1016/j.bcp.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wright CW, Duckett CS. The aryl hydrocarbon nuclear translocator alters CD30-mediated NF-kappaB-dependent transcription. Science. 2009;323:251–255. doi: 10.1126/science.1162818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Iannetti A, Ledoux AC, Tudhope SJ, Sellier H, Zhao B, Mowla S, Moore A, Hummerich H, Gewurz BE, Cockell SJ, et al. Regulation of p53 and Rb links the alternative NF-kappaB pathway to EZH2 expression and cell senescence. PLoS Genet. 2014;10:e1004642. doi: 10.1371/journal.pgen.1004642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gardella KA, Muro I, Fang G, Sarkar K, Mendez O, Wright CW. Aryl hydrocarbon receptor nuclear translocator (ARNT) isoforms control lymphoid cancer cell proliferation through differentially regulating tumor suppressor p53 activity. Oncotarget. 2016;7:10710–10722. doi: 10.18632/oncotarget.7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ranuncolo SM, Pittaluga S, Evbuomwan MO, Jaffe ES, Lewis BA. Hodgkin lymphoma requires stabilized NIK and constitutive RelB expression for survival. Blood. 2012;120:3756–3763. doi: 10.1182/blood-2012-01-405951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xu J, Ye Y, Huang F, Chen H, Wu H, Huang J, Hu J, Xia D, Wu Y. Association between dioxin and cancer incidence and mortality: a meta-analysis. Sci Rep. 2016;6:38012. doi: 10.1038/srep38012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vogel CF, Li W, Sciullo E, Newman J, Hammock B, Reader JR, Tuscano J, Matsumura F. Pathogenesis of aryl hydrocarbon receptor-mediated development of lymphoma is associated with increased cyclooxygenase-2 expression. Am J Pathol. 2007;171:1538–1548. doi: 10.2353/ajpath.2007.070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125•.MacPherson L, Ahmed S, Tamblyn L, Krutmann J, Forster I, Weighardt H, Matthews J. Aryl hydrocarbon receptor repressor and TiPARP (ARTD14) use similar, but also distinct mechanisms to repress aryl hydrocarbon receptor signaling. Int J Mol Sci. 2014;15:7939–7957. doi: 10.3390/ijms15057939. This paper is representative for a handful of publications from the Matthews lab identifying TCDD-inducible poly (ADP-ribose) polymerase (TiPARP) as another important repressor of AhR signaling. In the selected study, the authors compare the molecular mechanisms through which AhRR and TiPARP inhibit AhR transactivation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Basham KJ, Leonard CJ, Kieffer C, Shelton DN, McDowell ME, Bhonde VR, Looper RE, Welm BE. Dioxin exposure blocks lactation through a direct effect on mammary epithelial cells mediated by the aryl hydrocarbon receptor repressor. Toxicol Sci. 2015;143:36–45. doi: 10.1093/toxsci/kfu203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Murray TJ, Yang X, Sherr DH. Growth of a human mammary tumor cell line is blocked by galangin, a naturally occurring bioflavonoid, and is accompanied by down-regulation of cyclins D3, E, and A. Breast Cancer Res. 2006;8:R17. doi: 10.1186/bcr1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang X, Liu D, Murray TJ, Mitchell GC, Hesterman EV, Karchner SI, Merson RR, Hahn ME, Sherr DH. The aryl hydrocarbon receptor constitutively represses c-myc transcription in human mammary tumor cells. Oncogene. 2005;24:7869–7881. doi: 10.1038/sj.onc.1208938. [DOI] [PubMed] [Google Scholar]