PREFACE
Staphylococcus aureus is a major bacterial pathogen that causes disease worldwide. The emergence of strains that are resistant to commonly used antibiotics and the failure of vaccine development has resulted in a renewed interest in the pathophysiology of S. aureus. The staphylococcal leukocidins are a family of bi-component pore-forming toxins that are important virulence factors. During the past five years, cellular receptors have been identified for all of the bi-component leukocidins. The identification of the leukocidin receptors explains the cellular tropism and species specificity that is exhibited by these toxins, which has important biological consequences. In this review, we summarize the recent discoveries that have refueled the interest in these toxins and we provide an outlook for future research.
Staphylococcus aureus is one of the most important bacterial pathogens that has impacted human health to date1. The organism colonizes approximately 30% of the human population1, but once it invades deeper tissues, the clinical manifestations of S. aureus ranges from mild skin soft and tissue infections (SSTI) to more debilitating infections like sepsis, endocarditis, and pneumonia1. Severe S. aureus infections have a poor prognosis2, which is complicated by resistance to commonly used antibiotics3. Since there are no vaccines that are currently approved for S. aureus, there is significant interest in further understanding the pathophysiology of this bacterium. Virulence factors are crucial for the success of S. aureus in the human host4, as these factors control many aspects of its commensal and pathogenic lifestyle5–9.
An important group of staphylococcal virulence factors are the bi-component leukocidins, which are pore-forming toxins that kill immune cells (also known as leukocytes)7. Amongst leukocytes, phagocytes are required for the containment of S. aureus infection by the host9 and are considered to be the major target of the leukocidins7. Leukocidins can also target natural killer cells, dendritic cells, and T lymphocytes (Table 1)10, suggesting that these toxins can target innate and adaptive immune responses. In addition to their leukocidal activity, some leukocidins are able to lyse erythrocytes11 (Table 1). For historical reasons, these bi-component toxins are referred collectively as leukocidins or leukotoxins12. Nevertheless, S. aureus secretes other toxins that are also able to target phagocytes, lymphocytes and erythrocytes, which include alpha-toxin, beta-toxin, and the small cytotoxic peptides known as phenol soluble modulins (PSMs)8,13.
Table 1.
Leukocidins produced by human S. aureus isolates and their respective myeloid and erythroid receptors.
| Leukocidin | Receptors | Specificity1 | Species2 | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Name | Cognate pairs | Non-cognate pairing3 | Myeloid receptors | Erythroid receptors | Target Cells | Human, Rabbit, Mouse | |
| “S” type | “F” type | ||||||
| PVL | LukS-PV | LukF-PV | LukD HlgB |
C5aR1, C5aR2 | Neutrophils Monocytes Macrophages |

|
|
| LukED | LukE | LukD | LukF-PV HlgB |
CCR5, CXCR1, CXCR2 | DARC4 | Neutrophils Monocytes Macrophages Dendritic cells T-cells Erythrocytes NK cells |

|
| HlgAB5 | HlgA | HlgB | LukF-PV LukD |
CCR2, CXCR1, CXCR2 | DARC4 | Neutrophils Monocytes Macrophages Erythrocytes |

|
| HlgCB | HlgC | HlgB | LukF-PV LukD |
C5aR1, C5aR2 | Neutrophils Monocytes Macrophages |

|
|
| LukAB (LukGH) | LukA (LukH) | LukB (LukG) | None | CD11b | Neutrophils Monocytes Macrophages Dendritic cells |

|
|
Shown are the leukocytes that there are experimental data in the literature.
Based on published susceptibility of tested primary cells.
Potential non-cognate pairing of the S-component with an F-component of another leukocidin, resulting in functional mixed pores or inactive hybrid complexes depending on the pair.
DARC renders erythrocytes susceptible to the hemolytic activity of LukED and HlgAB.
HlgAB targets both human and murine CCR2 and DARC, but only human CXCR1 and CXCR2.
The bi-component leukocidins are a collection of pore-forming toxins (PFTs) that are produced by many Staphylococci. PFTs are generally secreted as inactive monomeric subunits that, upon binding to the membrane of a target host cell, multimerize, which results in the formation of a pore that spans the phospholipid bilayer and induces cell death (Figure 1). Based on the secondary structure of the membrane spanning domains, PFTs are classified into alpha-helical PFTs or beta-barrel PFTs14. Beta-barrel PFTs are further classified into cholesterol-dependent cytolysins or hemolysins; the staphylococcal bi-component leukocidins belong to hemolysin toxin class. S. aureus isolates that are associated with human infections can produce up to five different leukocidins: Panton-Valentine Leukocidin (PVL or LukSF-PV), gamma-hemolysin AB and CB (HlgAB and HlgCB), Leukocidin ED (LukED), and Leukocidin AB (LukAB, also known as LukGH)7 (Table 1).
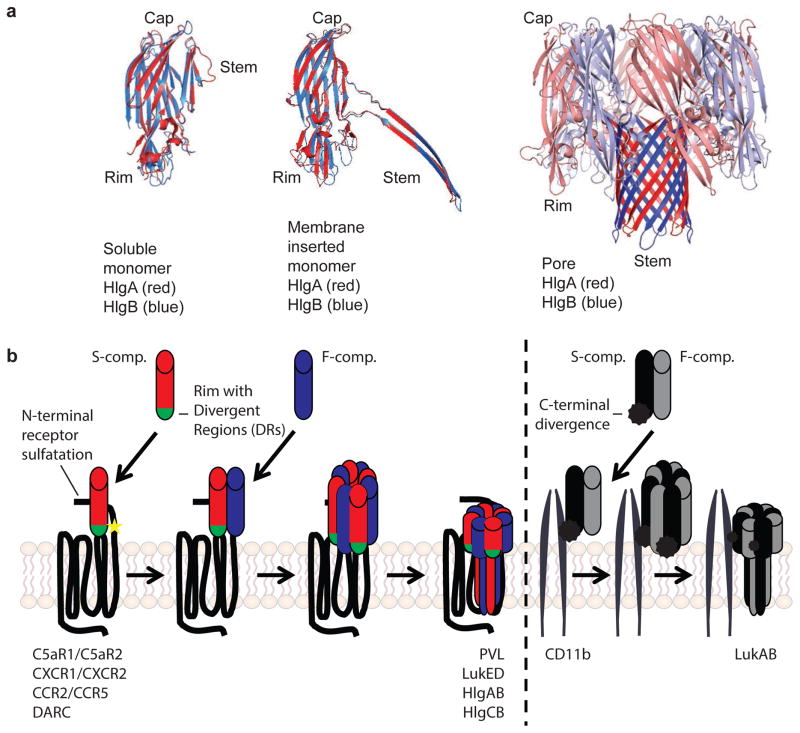
Figure 1. Pore formation by staphylococcal leukocidins.
a| Individual crystal structures of single leukocidin protein components and multimer beta-barrel leukocidin pores show high structural similarity. In soluble form, hydrophobic residues in the beta-barrel stem of both S- and F-component are covered by the cap. The rim domain of the S-component, responsible for initial binding to the host target cell, is involved in receptor recognition. Hetero-oligo-merization of the S- with the F-components induces a conformational change resulting in insertion of the hydrophobic stem into the membrane of the target cell. The resulting octameric beta-barrel pore consists of alternating four S- and four F-components. Red: HlgA; Blue: HlgB. Structural information was acquired from the Protein Data Bank, with accession numbers 2QK7 (unbound HlgA), 1LKF (unbound HlgB), and 3B07 (single HlgA and HlgB from HlgAB octamer). The major structural domains were colored using PyMOL software. Courtesy of Dr. B.W. Bardoel, University Medical Center Utrecht, The Netherlands. b| Sequences of binding and pore formation of different leukocidins to their respective receptor targets. Differences in the sequences between leukocidins targeting chemokine receptors (PVL, LukED, HlgAB, HlgCB, on the left) versus the leukocidin targeting CD11b (LukAB, on the right) are highlighted. For PVL, LukED, HlgAB, and HlgCB the initial binding of the respective S-component to its specific receptor allows secondary binding of the polymerizing F-component, hetero-oligomerization, and pore formation. In the rim domain of the S-component (labeled green), the divergent region (DR) 1 of LukE determines receptor recoginition of CCR5, while DR4 of LukE determines recognition of CXCR1 and CXCR2. The bottom loops in the rim domain of LukS-PV are essential for targeting C5aR1. The interaction of C5aR1 and C5aR2 with LukS-PV and HlgC is multi-factorial and involves the N-termini and extracellular loops of the receptors. Sulfated tyrosines in the N-termini of the receptors C5aR1 and DARC (labeled with a yellow star) are essential for interaction of the receptor with PVL and HlgAB and LukED, respectively. Uniquely, LukAB is secreted as a pre-assembled dimer. Dimerization results in high affinity for the I-domain of its receptor CD11b. Receptor recognition of LukAB is mediated by a divergent C-terminal extension of LukA (labeled with a black spike). The actual number of receptors per pore is unkown.
The other known leukocidins that are produced by S. aureus are Leukocidin MF’ (LukMF’)15 and Leukocidin PQ (LukPQ)16, however, these toxins are not found in human S. aureus isolates and are associated with zoonotic infections (Box 1). Although the first description of leukocidin activity in S. aureus culture supernatants was published around 1895, (the discoveries that lead to the identification of the leukocidins were recently reviewed elsewhere7), the molecular mechanisms that cause pore formation has remained incompletely understood.
BOX 1. Leukocidins and non-human Staphylococci.
Recent studies on bi-component leukocidins have focused on those that are produced by human S. aureus isolates. However, S. aureus can also infect different animals, including pigs, rabbits, cattle, horses, and dogs118. These zoonotic strains are likely to encode bi-component leukocidins in their genomes that enable them to target and kill neutrophils from different species. For example, LukMF’ is a toxin that was found in S. aureus isolates from bovine infections39,119. LukMF’ has an exquisite cellular tropism for bovine phagocytes, a property mediated by the targeting of CCR139, a chemokine receptor that is expressed in bovine neutrophils but not in human neutrophils. More recently, a novel bi-component leukocidin, LukPQ, was identified in S. aureus isolated from horses16 and is highly cytotoxic in equine neutrophils. LukPQ is similar to LukED (91% amino-acid identity between LukE and LukP)16, and targets the equine CXCRA and CXCR2 receptors on neutrophils.
The role of leukocidins in the pathogenesis of other Staphylococcal species is exemplified by the leukocidin LukSF-I120, which is encoded by Staphylococcus pseudintermedius, a pathogen that is primarily associated with infections of canines. LukSF-I has substantial amino acid sequence similarity with LukED, and can target canine and human phagocytes120. However, in contrast to LukED, LukSF-I has low hemolytic activity.
Bi-component leukocidin-like toxins have also been found in Staphylococcus argenteus, Staphylococcus schweitzeri and Staphylococcus delphini. S. argenteus and S. schweitzeri encode a LukAB toxin that is similar to the human LukAB leukocidin, whereas S. delphini, another zoonotic pathogen, encodes LukSF-I, which is a toxin similar to S. pseudintermedius LukSF-I.
Species diversification in leukocidins provides strong support for the notion that the targeting of phagocytes by Staphylococci is crucial for host adaptation.
Bi-component leukocidins are thought to protect S. aureus from killing by host phagocytes, however, the necessity of the apparently redundant range of phagocyte-targeting toxins is incompletely understood. The ability of the bi-component leukocidins to target and kill human leukocytes in vitro has been established and supported by over 100 years of research12, however, whether they target and kill human leukocytes in vivo to promote S. aureus infection has been controversial3. This controversy is the result of a poor understanding of the molecular mechanisms that underlies the differential cellular tropism and species specificity that is exhibited by these toxins (Table 1). Moreover, an incomplete awareness that animal leukocytes are not as susceptible to leukocidins as human leukocytes has hindered progress in understanding the contribution of the bi-component leukocidins to staphylococcal pathophysiology12,17.
During the past five years, the receptors that are targeted by the different staphylococcal leukocidins have been identified, which has helped to explain the cellular tropism and species specificity of these toxins. The identification of these receptors has also enabled the role of each individual leukocidin to be assessed. It has also heightened our understanding of the complex interplay between the different leukocidins during infection. In addition to their cytotoxic potential, the discovery of the leukocidin receptors has revealed new insights into how leukocidins modulate immune cell functions. Similarly, the identification of an erythroid receptor for the leukocidins has also provided new insights into the link between virulence and nutritional immunity. This review provides an overview of the similarities and differences in the interaction between leukocidins and their respective receptors, and discusses the implication of receptor identification for mechanisms of action of the leukocidins, their diverse roles during pathogenesis in vivo, and their potential as targets for therapeutic interventions.
LEUKOCIDINS AND RECEPTORS
Leukocidin structures
The bi-component leukocidins are PFTs that share a highly conserved structure (Figure 1a)18. The subunits of the different leukocidins have a molecular weight of approximately 33 kDa. Leukocidins have two subunits that are classified as the host cell targeting S-component (for Slow migration in chromatography columns: LukS-PV, LukE, HlgA, HlgC, LukA), and the polymerization F-component (for Fast migration: LukF-PV, LukD, HlgB and LukB) (Table 1)7. In contrast to the S-components and F-components of the other leukocidins, which are secreted as two soluble and independent monomers, LukAB is pre-assembled as a soluble heterodimer19.
Both the S-component and the F-component have three important domains: a cap, a rim, and a stem (Figure 1a). In the soluble form, the hydrophobic stem region is packed within the cap domain. The oligomerization of the leukocidins is thought to induce a conformational change in each component that causes the extension and unfolding of the stem domain, which penetrates the plasma membrane of target cells18. During oligomerization a ring-shaped pre-pore is formed, allowing the multimeric beta-strand domains of the stem regions to be inserted into the cell membrane, resulting in the formation of a pore that is 1–2 nm in diameter (Figure 1a)18,20. Structural studies of HlgAB and HlgCB have revealed that these leukocidins form an octameric pore with alternating HlgA or HlgC and HlgB subunits (Figure 1a)18,20. Similarly, LukAB was recently found to form a hetero-octomer pore21. Based on the crystal structure of the LukAB-heterodimer, the interaction sites between LukA and LukB help to explain why this toxin is pre-assembled in solution21,22. Three salt bridges between the interfaces of the cap and rim domains of LukA and LukB, that are not found in the other leukocidins, are required for the formation of LukAB-dimers in solution. Hetero-octamers have been proposed to be the preferred stable conformation for the other leukocidins such as PVL and LukED. Hexameric and heptameric pores have also been observed, but they have been hypothesized to represent intermediate structures during the formation of the stable pore23,24, however, this hypothesis remains to be tested experimentally.
For all of the bi-component leukocidins except LukAB, which binds as a pre-assembled dimer, initial binding of the S-component to the host cell is followed by the recruitment of the F-component (Figure 1b)25–27. During multimerization of the S-component and the F-component, a pre-pore is formed that eventually spans the entire membrane. F-components have also been shown to interact with the surface of neutrophils independently of the S-component28–30, nevertheless the cellular target(s) and the importance of this binding to pore-formation by the bi-component leukocidins remains to be fully elucidated. Most leukocidins can form functional pores using both cognate (for example, LukED and HlgAB) and non-cognate combinations of subunits (for example, LukE/HlgB and HlgA/LukD) (Table 1)31–33. This is true for PVL, LukED, HlgAB and HlgCB, but not for the pre-assembled LukAB dimer, which does not interact with any of the other leukocidin subunits due to its structural divergence (Table 1)21. The functional consequences of cognate and non-cognate interactions between the bi-component leukocidins will be discussed below.
Leukocidin receptors
The distinct cellular and species specificities of the bi-component leukocidins (Table 1) has provided historical clues for the involvement of specific proteinaceous host receptors. In 2011, three receptors were described for LukED and PVL in different laboratories. For LukED, C-C chemokine receptor type 5 (CCR5) was identified as a 10. Concurrently, C5a anaphylatoxin chemotactic receptor 1 (C5aR1) and C5a anaphylatoxin chemotactic receptor 2 (C5aR2) were identified as the receptors for PVL 34 (Table 1). Subsequently, receptors were identified for all of the bi-component leukocidins: in addition to CCR5, LukED also targets C-X-C chemokine receptor type 1 (CXCR1) and C-X-C chemokine receptor type 2 (CXCR2)35; HlgCB, like PVL, targets C5aR1 and C5aR236; HlgAB targets CXCR1, CXCR2, and C-C chemokine receptor type 2 (CCR2)36; LukAB targets the integrin component alpha-M (also known CD11b)37; and LukED and HlgAB both target the atypical chemokine receptor 1 (the Duffy antigen receptor for chemokines, DARC)38 (Table 1). The presence of the cognate leukocidin receptors on the surface of host target cells is required for leukocidin toxicity10,34–38. Moreover, the interspecies variations in sequence and structure of leukocidin receptors is responsible for the divergent susceptibility of immune cells from different mammalian species to the leukocidins (Table 1 and Box 2)34,36,37,39.
BOX 2. Leukocidin and receptor co-evolution.
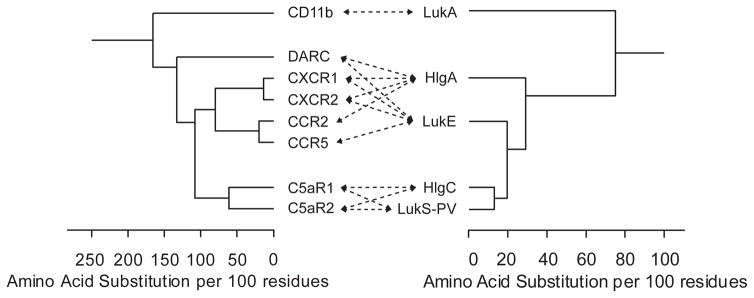
The amino acid sequence identity between the leukocidin S-components and F-components is ~30%110. Within their respective groups, the S-components and F-components share ~70% amino acid sequence identity44. Interestingly, LukAB is more divergent from the other leukocidins, with an amino acid sequence identity of approximately 30% compared to other leukocidins44. The phylogenetic association between the different leukocidin S-components from human S. aureus isolates is depicted below. It is likely that the leukocidins have evolved from a common ancestor and by gene duplication7.
Host-pathogen co-evolution is thought to be a powerful determinant in the biology of infections121. Genes involved in immune evasion play a major role in host adaptation of S. aureus48. Comparing the phylogenetic associations of the leukocidins and their cognate receptors results in an evolutionary ‘mirror’, as the divergence of the leukocidin S-components is reflected in the relatedness of the receptors that are targeted by the leukocidins. The phylogenetic associations of the human receptors that are targeted by leukocidins from human S. aureus isolates and phylogenetic associations of the leukocidin S-components from a human S. aureus isolate (USA300-FPR3575) are presented below. Arrows indicate specific interactions between leukocidin S-components and human receptors. Amino acid sequence comparisons were generated by ClustalW alignment using Lasergene MegAlign software (DNASTAR).
During evolution, structures of proteins are more conserved than the sequences of proteins. The sequence variation that evolved within the stable structure of the beta-barrel leukocidins resulted in high-affinity protein-protein interactions with diverse receptor targets and may have also enabled the emergence of species-specific mutations that allowed S. aureus to adapt to humans, it’s major mammalian host48.
Figure.
Staphylococcal leukocidins and receptors.
The majority of the receptors that are targeted by the bi-component leukocidins belong to the family of complement and chemokine receptors (Box 1), of which most are class A rhodopsin-like G-protein coupled receptors (GPCRs): C5aR1, C5aR2, CXCR1, CXCR2, CCR2, CCR5, and DARC40. These structurally and functionally related seven-transmembrane spanning receptors are involved in transducing extracellular signals to the interior of the cell via cytosolic G-proteins. With the exception of DARC, these receptors are expressed in specific leukocytes at very high levels. Key cellular functions that are regulated by G-proteins are cellular activation and migration40. In contrast, DARC is an atypical chemokine receptor that is not coupled to a G-protein41,42. CD11b is more distantly related to the family of GPCRs and a component of the Mac-1 integrin. The Mac-1 integrin is highly expressed on phagocytes, and is involved in many critical cellular functions such as phagocytosis, cell mediated killing, and chemotaxis43.
Leukocidins and receptor interactions
The identification of the leukocidin receptors has led to detailed molecular studies of the leukocidin-receptor interaction. Targeting of the receptors by leukocidins is mediated by S-components, which bind to their receptors with high affinity (all within nanomolar range)10,34–38. The S-components share 70–90% amino acid sequence identity (Box 2)44 and the alignment of leukocidin amino acid sequences revealed divergent regions (DRs) in the rim of the S-components (Figure 1a)35. For LukED, the DR1 is involved in LukE targeting of CCR5 (Figure 1b)45, whereas DR4 determines LukE targeting of CXCR1 and CXCR2, but not for CCR535 (Figure 1b). The identification of different regions in one leukocidin that are involved in the recognition of different receptors permitted to make mutant LukED toxins that only target CCR5 or CXCR1 and CXCR2, which subsequently allowed the relative importance of the different leukocidin-receptor interactions to be assessed in vivo35. For PVL, a cluster of amino acids in the bottom loops of the rim domain are essential for LukS-PV recognition of C5aR1 (Figure 1b)46. It is likely that the amino acids that are conserved in LukS-PV and HlgC determine the specificity of these toxins for C5aR1 and C5aR2, their shared receptors. The molecular mechanisms by which HlgAB targets different receptors is not understood. For LukAB, the LukA subunit has unique amino- and carboxy-terminal extensions. Remarkably, one conserved amino acid within the C-terminal extension, glutamic acid at position 323 in LukA, was found to be involved in the interaction of LukAB with CD11b19 (Figure 1b). The salt bridge that mediates the dimerization of LukA-LukB in solution also appears to be essential for this leukocidin to efficiently bind its receptor21.
The molecular determinants in the receptor for the leukocidin-receptor have also been investigated. For PVL and its receptor C5aR1, and for HlgAB and LukED and their shared erythroid receptor DARC, sulfation of tyrosines in the N-terminal receptor appears to be essential for leukocidin-receptor interactions (Figure 1b)34,38. Possibly, sulfated N-terminal tyrosines define a conserved interaction site for the leukocidins. By taking advantage of the differential species-specific engagement of PVL and HlgCB with their shared receptors C5aR1 and C5aR2, it was shown that different regions of the receptors are involved in binding and pore-formation by PVL and HlgCB47. The human specificity that is exhibited by PVL is determined by the second extracellular loop of C5aR1. In contrast to PVL, the specificity of HlgCB for C5aR1 is determined by the first and third extracellular loops of the receptor47. Differences in the interaction between PVL and HlgCB with C5aR1 have also been hypothesized, based the use of specific C5aR1-antagonists in vitro. Although the toxicity of PVL can be inhibited by C5aR inhibitors, many of the inhibitors cannot neutralize the toxicity of HlgCB47. The specificity of LukAB for human CD11b is determined by its interaction with the receptor I-domain37, the binding site of many of the CD11b ligands (Figure 1b). Except for the conserved interaction of HlgAB and LukED with the sulfated N-terminal tyrosines of DARC, both toxins differentially interact with this receptor38. An N-terminal receptor residue that is involved in LukED-mediated, but not HlgAB-mediated toxicity, is also involved in the binding the natural ligand interleukin-8 (CXCL8), supporting the notion that CXCL8 competes for receptor binding with LukE but not HlgA38. Competition of S-component binding by natural receptor ligands, and vice versa, has been shown for PVL, HlgAB, HlgCB, and LukED10,34–36,38.
Although the interactions between leukocidin subunits and their respective receptors have been investigated, the number of receptors that contribute to the formation of the leukocidin pore is unknown. Similarly, how the leukocidins transition from a receptor bound state to form octameric pores remains to be elucidated. Nevertheless, recent studies have revealed commonalities and differences in the interactions between bi-component leukocidins and their respective receptors. These differences challenge the presumed functional redundancy of this family of toxins and provide an explanation for their cellular tropism and species specificity (Box 3).
Leukocidin genomic organization, regulation and expression
S. aureus has considerable variation in its gene content between strains, both in the core genome and in the accessory genome48. The hlgACB and lukAB loci are located in the core genome and are present in over 99.5% of human S. aureus isolates. In contrast, genes that encode other leukocidins such as PVL and LukED are not as widely distributed49,50. PVL is located in the temperate phage ΦSa2 (accessory genome)51 and is found in less than 2% of all clinical isolates; however, the majority of community-acquired methicillin-resistant S. aureus isolates in the USA carry the genes that encode PVL52. LukED is encoded in the stable S. aureus pathogenicity island vSaβ53, which is present in about 70% of all clinical isolates. For PVL, HlgCB, LukED, and LukAB, the S-component and the F-component are found in an operon and is co-transcribed from a single promoter. In contrast, hlgA is transcribed as a single gene that is adjacent to the hlgCB locus54.
The expression of the bi-component leukocidins is complex and is only partially understood. However, it is clear that S. aureus combines ‘self-sensing’ and ‘host sensing’ to regulate the expression of these toxins. Globally, the quorum-sensing accessory gene regulatory (Agr) system55, the self-sensing system, regulates the expression of the leukocidin genes by controlling the production of the repressor of toxins, Rot, a HTH-type transcriptional regulator56,57. In addition, a number of transcriptional regulators that control the Agr-Rot system, for example SarA, have also been found to indirectly regulate the expression of certain leukocidins56. Interestingly, when S. aureus is in contact with neutrophils or with human blood, leukocidin expression is induced58–62. In this context, the host-sensing SaeRS two-component system, consisting of histidine protein kinase SaeS and the response regulator SaeR, is responsible for the observed enhanced leukocidin expression58–62. Interestingly, the upregulation of LukAB upon contact with neutrophils, a response that is mediated by the SaeRS system58,60,61, contributes to the ability of S. aureus to target and kill neutrophils63. Recent studies also suggest that S. aureus differentially regulates the expression of leukocidins by sensing metabolic shifts via a metabolite-sensing HTH-type transcriptional regulator known as RpiRc, providing a link between metabolism and virulence64. Although the current understanding of leukocidin gene expression is incomplete, it is clear that the expression of leukocidins is regulated in response to environmental and metabolic cues.
A question that has concerned toxin researchers is whether the toxin concentrations that are used in in vitro studies are biologically relevant during infections in vivo. In mammals, S. aureus is exposed to a range of environments and stresses, which can influence gene expression that is challenging to replicate in vitro. It is also unknown as to whether all leukocidins are expressed at the same time in vivo. Studies that have investigated the response of S. aureus to the exposure to human blood or blood components have revealed the selective expression of leukocidin genes, where HlgAB, HlgCB, and LukAB are induced62. Another study found that LukAB appears to be the most upregulated leukocidin gene when S. aureus is exposed to human neutrophils63. These observations correlate with the contribution of these leukocidins to S. aureus survival (HlgAB and HlgCB)62 or growth (LukAB)63,65,66 in ex vivo models. Most studies that have tried to quantify leukocidin levels in vivo have focused on PVL. Pus samples from human infections contains up to 399 μg/mL PVL, which is a concentration about 1,000 fold higher than needed for PVL to kill a human neutrophil67, supporting the notion that S. aureus produces sufficient amounts of these toxins to target immune cells. Recent studies using in vivo imaging systems65, mass spectometry68 and RNA-seq69 have confirmed that the leukocidins are produced during infection. These data are consistent with the observation that humans that are infected with S. aureus develop anti-leukocidin antibodies70,71. Thus these toxins are likely to contribute to the pathogenesis in a leukocyte-targeting dependent manner. The various effects of the leukocidins on cells at different concentrations will be discussed in more detail in the following section.
MOLECULAR MECHANISMS OF ACTION
Host cell death
Leukocidins can kill target cells at low concentrations (~1 nM) in vitro, and cell susceptibility is associated with receptor levels10,34,35,37,38,47,72. Pore formation ultimately leads to the demise of target cells via cell lysis, as it results in leakage of divalent cations, which are critical for cell homeostasis (Figure 2a). Over the past decade it has become clear that a large number of PFTs use cellular pathways to enhance their ability to lyse cells. One such pathway is the inflammasome pathway73. Leukocidins such as HlgAB, HlgCB, PVL and LukAB are able to activate the NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome in macrophages and monocytes74–77, promoting their lytic and pro-inflammatory activities. Leukocidin-mediated inflammasome activation appears to be dependent on the presence of the cognate cellular receptors, which are required for leukocidins to form pores, as recently shown for LukAB in human monocytes76. The mechanism by which each leukocidin activates NLRP3 is not fully understood, but it is thought that toxin-pores in the plasma membrane result in the leakage of potassium ions form the cytoplasm of target cells, which activates NLRP3 (Figure 2a)78. The ability of the leukocidins to form plasma-membrane pores33 provides an explanation as to how these different toxins that target different receptors are all able to activate NLRP3. It is also possible that differences in leukocidin potency could be explained by differences in the signaling pathways that are used by the different cellular receptors (for example, GPCR vs. integrin).
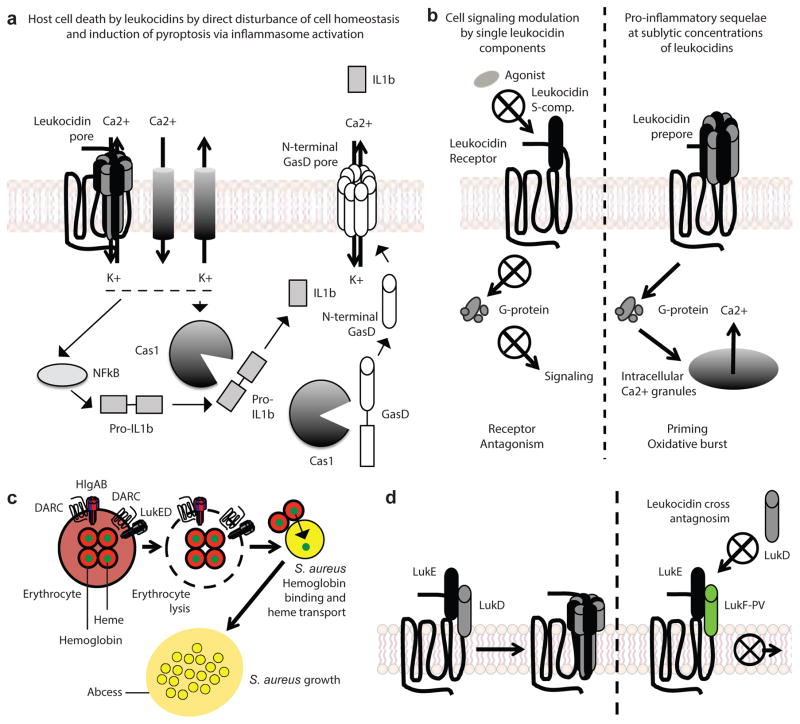
Figure 2. Leukocidins in pathophysiology.
a| Cell death by leukocidins. Disturbance of cell homeostasis by the leakage of cations through pores and by the mobilizaiton of ions through ion channels results in osmotic disbalance and inflammatory cell death, as NFkB stimulation and inflammasome activation lead to the release of pro-inflammatory cytokines and the assembly of endogenous N-terminal Gasdermin-D pores. Cas1: Caspase-1; IL1b: Interleukin-1b; GasD: Gasdermin D. b| Host cell signaling modulation by leukocidins. Depending on the receptor targeted, single leukocidin S-components in the absence of an F-component can functionally antagonize G-protein coupled receptors by preventing singnaling induced by the endogenous receptor ligand. The significance of functional antagonism by single S-components during infection is unkown. At sublytic concentrations, leukocidins prime neutrophils in a receptor-specific manner resulting in increased production of reactive oxygen species, enhanced degranulation and phagocytosis, and enhanced bactericidal activity of phagocytes. c| Leukocidins promote S. aureus growth. Hemoglobin is the most abundant source of iron in mammals, and heme iron is the preferred source of iron for S. aureus to grow. DARC is the erythroid receptor for HlgAB and LukED. Targeting of DARC by HlgAB and LukED results in hemolysis, which promotes S. aureus growth in a hemoglobin acquisition-dependent manner. d| Antagonism of leukocidins by the formation of inactive hybrid complexes as a result of sequestration of the S-component from its cognate F-component. Antagonism of cytotoxicity by non-cognate paring has been described for LukED and PVL.
After activation, NLRP3 forms a cytoplasmic protein complex together with apoptosis-associated speck-like protein containing a CARD (ASC) and Caspase-1 known as the NLRP3 inflammasome, resulting in pyroptosis, a necrosis-like cell death pathway, and a heightened production of pro-inflammatory cytokines by macrophages74–76,79. How leukocidin-mediated pores and the NLRP3 inflammasome work together to promote cell lysis is not fully understood. Recently, it has been shown that intracellular lipopolysaccharide (LPS)-induced pyroptotic cell death via caspase-4 (in mice) and Caspase-5 (in humans) mediate the cleavage of gasdermin D80,81. Cleavage of gasdermin D results in the release of the N-terminal membrane-targeting domain, which promotes the assembly of the protein into pores that disrupt the membranes of mammalian cells82,83. Moreover, the observation that Caspase-1 can also cleave gasdermin D80,84, suggests that gasdermin D-mediated pore formation could also be involved in leukocidin-mediated pyroptotic cell death. If proven, this would suggest that host cells respond to damage from PFTs by producing their own intracellular pore-forming protein to cause cell death (Figure 2a). Detailed mechanistic studies of the pathways that are involved in cell death of neutrophils – traditionally considered to be the major target of the leukocidins – are lacking, and will be required to fully understand the role of leukocidins in promoting cell death.
Modulation of host cell signaling
In addition to killing cells, leukocidins can alter cell signaling pathways at sublytic concentrations, below 1nM, in neutrophils and macrophages 75,76,79, alter the activation status of neutrophils by priming the cells34,85, and trigger the formation of neutrophil extracellular traps (Figure 2b)86. Among the toxins, PVL and LukAB have been studied the most in the context of host cell signaling. Interestingly, PVL and LukAB can alter the cell in different ways. For example, PVL primes human neutrophils, resulting in enhanced superoxide production in response to a secondary stimulus and enhanced phagocytic capacities85, whereas LukAB does not prime human neutrophils86. As single subunits, LukE, LukS-PV, HlgC, and HlgA functionally antagonize their respective receptors in vitro (Figure 2b)10,34,36. Due to the clustering of S-component and F-component genes in a single operon, the significance of functional antagonism by a single S-components during infection has not been studied. Nevertheless, studies of the non-lytic roles of the leukocidins has highlighted the complexity of how each leukocidin can alter the function of their target cell. These effects are probably due to differential signaling of the targeted receptors. Future studies are needed to further understand the effects that leukocidins have on leukocytes, and the contribution of these non-lytic effects to S. aureus pathophysiology.
More than just leukocytes
In addition to inducing cell death of phagocytes, HlgAB and LukED can also lyse erthyrocytes32,38,87 (Table 1). HlgAB and LukED promotes S. aureus growth upon erythrocyte lysis (Figure 2c)38,87, suggesting that these toxins are involved in nutrient acquisition. Indeed, lysis of erythrocytes results in the release of hemoglobin, the preferred iron source for S. aureus growth6. Iron is an essential metal for metabolism in many bacteria, and is required for S. aureus pathogenesis. A major regulator of iron acquisition, ferric uptake regulation protein (Fur), regulates the expression of LukED, HlgAB, and HlgCB88, further indicating that leukocidins may play a role in nutrient acquisition during S. aureus infection38,87.
The erythroid receptor for the leukocidins is DARC38, and S. aureus-mediated lysis of human erythrocytes is DARC-dependent (Table 1 and Figure 2c). The role of the hemolytic activity of LukED and HlgAB in vivo has been challenging to study because mutants that diminish the hemolytic activity of the leukocidins without altering the leukocidal activity of the toxins remain to be identified. However, mice that were infected with isogenic S. aureus mutants that lack LukED and HlgAB had the same phenotype as mutants lacking components of the iron-regulated surface determinant (Isd) heme-iron acquisition system89, linking hemolysis to the pathophysiology of S. aureus infection38.
Additive versus antagonistic activities of S. aureus leukocidins
Many S. aureus clinical isolates encode all five bi-component leukocidins. The assembly of cognate and non-cognate leukocidins (for example LukED or LukE/HlgB) could result in the assembly of 13 different toxin complexes that have a range of cytotoxic activities (Table 1)32,33. The differences in activities between cognate and non-cognate leukocidin subunit pairs could be due to differences in cellular receptor binding and in the formation of pores in cell membranes. Studies that investigated the killing of human monocytes by S. aureus in vitro revealed that isogenic strains that lack all of the leukocidins are less efficient at killing human monocytes compared to any single mutant strain76. Moreover, LukAB and PVL have an additive effect in the cytotoxic potential of S. aureus. As such, the leukocidin milieu at the site of infection could influence the contribution of any particular leukocidin in vivo63,66.
Leukocidins have also been shown to synergize with other virulence factors to contribute to immune subversion and pathogenesis. For example, the killing of neutrophils by PVL is enhanced by the action of PSMs8,90. Similarly, LukAB has been found to synergize with PVL and PSMs to promote S. aureus escape from macrophages after phagocytosis91. Very few studies have investigated the synergy between leukocidins and other virulence factors in vivo. Early studies demonstrated that alpha-toxin, an important PFT for S. aureus pathogensis13, and HlgAB/HlgCB together caused septic arthritis more readily compared to strains that just produced either alpha-toxin or HlgAB and HlgCB alone92. Similar results were observed in a rabbit model of endophthalmitis93. More recently, a study that aimed to identify virulence factors that are involved in subverting macrophage functions demonstrated that S. aureus that grows as a biofilm can evade phagocytosis owing to the combined activities of LukAB and alpha-toxin94. Importantly, these in vitro observations were confirmed in vivo as production of LukAB and alpha-toxin resulted in enhanced pathogenesis in a mouse model of orthopedic implant infection94. Thus, it is clear that leukocidins are able to synergize with other S. aureus virulence factors to enhance their cytotoxic potential and their contribution to S. aureus virulence.
Bi-component leukocidins have also been found to antagonize each other87. This antagonism is most evident for PVL and LukED. PVL has been shown to block LukED-mediated lysis of erythrocytes by forming complexes with LukED at the plasma membrane of erythrocytes that are impaired in pore formation87 (Figure 2d). In a series of in vivo bloodstream infections in mice, it was found that S. aureus strains that produced PVL and LukED are less virulent than strains that produced LukED alone87. Interestingly, these findings corroborate previous studies in which deletion of the pvl locus resulted in increased virulence in several murine87,95–97 and rabbit models of S. aureus infection98,99.
The virulence of S. aureus infection is remarkable, given that the organism colonizes many individuals as a commensal1. In a murine model of intranasal infection, it was shown that uncoupling of the LukED-antagonism by PVL promoted colonization of S. aureus in the lungs(Figure 2d)87. Thus, in addition to contributing to acute infection, interactions between leukocidins could also contribute to colonization. However, the role of bi-component toxins in colonization remains to be fully understood.
Together, these findings highlight the possibility that bi-component leukocidins exert different activities that result in different phenotypes when they are expressed at the same time, and that in order to fully understand the contribution of these toxins to the pathophysiology of S. aureus, we need to study them as a group.
THERAPEUTICS
Owing to the lack of new classes of antibiotics that are available to treat bacterial infections and the increase in antibiotic resistance, there is a clinical need to develop alternative antimicrobial strategies and an effective S. aureus vaccine. An important consideration in the development of new antibiotics and vaccines that target S. aureus is that the bi-component leukocidins kill phagocytes that are required for clearing the pathogen9.
The cellular receptors that are used by leukocidins are candidate drug targets for severe S. aureus infections (Figure 3). Blocking interactions between receptors and leukocidins by means of receptor antagonists has protected neutrophils against the action of some leukocidins in vitro10,36,38, however, the development of a combination therapy in which multiple receptor-leukocidin interactions are targeted could lead to adverse reactions resulting from suppressed host immune responses.
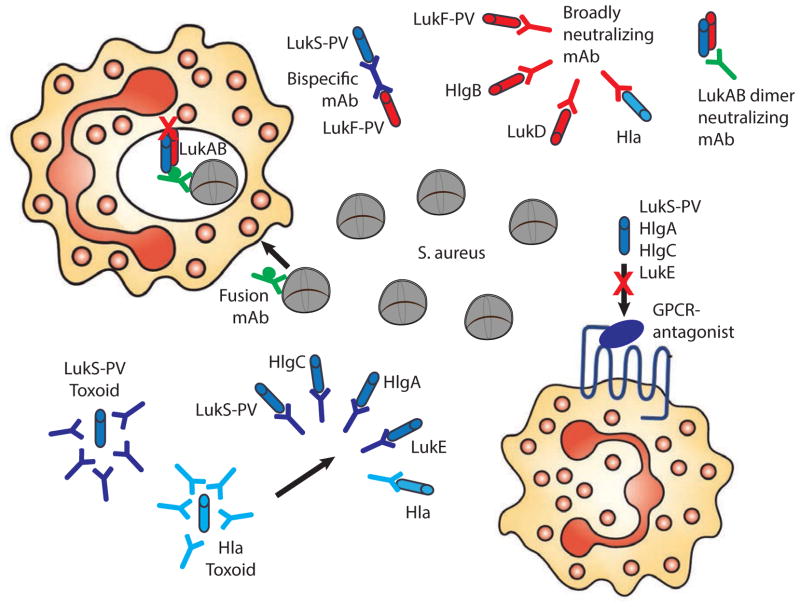
Figure 3. Strategies for the employment of leukocidins in anti-staphylococcal therapies and vaccines.
Antagonists of the receptors employed by the leukocidins can protect target cells against cytotoxicity. Bi-specific mAbs can neutralize both S- and F-components. Broadly neutralizing mAbs target multiple PFTs. A LukAB neutralizing mAb targets the LukAB dimer in solution. Fusion mAbs combine opsonophagocytic activity with neutralization of leukocidins, and potentially act intracellularly. Toxoids derived from mutant proteins induce a broadly neutralizing antibody response.
Multiple monoclonal antibodies (mAbs) that neutralize one or more toxins are currently being evaluated in clinical and preclinical trials22,100,101, and could be used to treat severe infections during the active phase or for the prevention of infections in high-risk groups (Figure 3). An engineered bi-specific tetravalent mAb was shown to neutralize PVL and HlgCB in vitro and in an inflammation model in rabbits102. More recently, a mAB that neutralizes alpha-toxin, PVL, HlgAB, HlgCB and LukED was reported to inhibit leukocidin-induced cytotoxicity in vitro, and to confer protection to S. aureus infection in mice103 and rabbits104. The broad-neutralizing activity of this mAb recognizes a shared conformational epitope in alpha-toxin and the leukocidin F-components. Owing to leukocidin structural dissimilarities, this mAb does not neutralize LukAB103. However, the identification of a series of mAbs that specifically neutralize LukAB was recently described22, this included naturally occurring mAbs isolated from infected chlidrens105. The role of LukAB in the intracellular compartment, where it promotes bacterial escape after phagocytosis37,63, is likely to pose challenges for neutralization of this toxin. Nevertheless, this obstacle could be overcome by combining opsonization and leukocidin-neutralization using a fusion antibody platform (Figure 3)101.
Many individuals have high levels of antibodies that target bi-component leukocidins70,106. Studies that have investigated protection by pre-existing leukocidin-specific antibodies have reported conflicting results97,106–109, making it difficult to evaluate the potential of a vaccine that solely targets leukocidins. The complex pathophysiology of S. aureus, together with the absence of a single dominant virulence factor, necessitates a multivalent approach and targeting bi-component leukocidins could improve the capacity of phagocytes to clear infection (Figure 3). It remains to be determined whether a vaccine that induces broad immunity against all staphylococcal beta-barrel PFTs is technically feasible. Recently, a vaccine candidate based on a mutant LukS-PV toxoid resulted in a cross-neutralizing immune response against all human leukocidins except LukAB in mice71,110. A successful multivalent vaccine will probably need to be complemented with additional targets, such as other immune modulators or cell surface proteins4,100,111. We hypothesize that previous active and passive S. aureus immunization strategies have been unsuccessful in clinical trials owing to the exclusive focus on opsonophagocytosis and the reliance on suboptimal infection animal models101,111,112. Currently, there are no reliable animal models to study S. aureus pathophysiology (Box 3)48,111.
BOX 3. Animal models and leukocidin research.
The low cytotoxic activity of PVL, LukAB, HlgCB, and HlgAB in murine PMNs compared to human PMNs suggest that the reported activities for these toxins in murine models are underestimated since murine phagocytes remain largely unaffected by these leukocidins. Currently, one of the best animal models to study PVL function is the rabbit99,122–126. PVL has been shown to enhance the early stages of bacteremic spread to the kidney in a bloodstream infection model126, and to contribute to osteomyelitis124. In a model of necrotizing pneumonia125, PVL has also been shown to be crucial for S. aureus-mediated lung injury, pulmonary edema, inflammation, increased levels of inflammatory cytokines and death. The increased lethality and pathologies of the PVL-producing strain in rabbits resulted in increased bacterial outgrowth, suggesting that this toxin also contributes to S. aureus growth in vivo. Importantly, purified PVL that was instilled into the lungs recapitulated many of the observations with live S. aureus, demonstrating that PVL alone can cause lung injury. PVL has been epidemiologically linked to SSTI, however, the contribution of PVL to SSTI is controversial98,122. Nevertheless, it is known that the administration of purified PVL into the skin of rabbits results in inflammation99 and necrosis123. Notably, data that generated using rabbit models should be interpreted with caution, as many rabbits have been previously exposed to S. aureus, resulting in the presence of antibodies127, which could mask the contribution of virulence factors to infection and pathogenesis.
More recently, the contribution of PVL to S. aureus pathogenesis has also been evaluated in humanized mice113,114. In these models, non-obese diabetic (NOD)/severe combined immune deficiency (SCID)/IL2Rγnull (NSG) mice were engrafted with primary human hematopoietic cells. Humanized mice were found to be more susceptible to S. aureus-mediated skin lesions113 and pneumonia114 compared to control mice, in a PVL-dependent manner. Together with the rabbit studies, these humanized mouse models strengthen the notion that PVL targets phagocytes during infection.
HlgAB and HlgCB have also been shown to contribute to infection. Initial studies using an endophthalmitis rabbit model revealed that the HlgAB and HlgCB toxins are involved in inflammation of the eyelid and bacterial outgrowth93. Subsequent studies revealed that the presence of HlgAB and HlgCB modestly affected weight92 and the survival of mice during bloodstream infections with S. aureus62. These data corroborate the observation that the receptor orthologues for HlgAB and HlgCB in murine neutrophils do not bind these leukocidins36. In contrast, HlgAB is able to target both human and murine CCR2 in monocytes36. In a murine peritonitis model, HlgAB promoted bacteremia in vivo in a CCR2-dependent manner36.
Initially bi-component leukocidins were only thought to target neutrophils, it is now clear that these toxins can also target natural killer cells, macrophages, dendritic cells, and T lymphocytes. The innate and adaptive immunomodulatory activity of S. aureus could prevent the development of a functional and protective immune response. However, data to support this notion are limited, as a robust model to support the study all these toxins in vivo is currently lacking.
OUTLOOK
The identification of host receptors that determine the cellular tropism and species specificity of the bi-component leukocidins has advanced our understanding of how these toxins target and kill host cells. Additional advances include uncovering the consequences of receptor binding on host cellular function. The notion that the leukocidins have multiple cellular effects in a concentration dependent manner (lytic vs. sub-lytic effects), suggest that the level of toxins that is produced during S. aureus infection could have broad effects on the host immune response. Moreover, the discovery that leukocidins can target signaling receptors suggests that these toxins can act as ligands, or even as antagonists, during infection10,34–36. It is possible that an uncharacterized role of the leukocidins is to block the function of their targeted receptors, paralyzing the migration of leukocytes.
Another important consideration is that the activities and effects of the leukocidins could vary in different target cells. A careful examination of how bi-component leukocidins target and kill different human phagocytes is required, particularly the pathways that are involved in the death of different phagocytes. The expression profiles and the tissue distribution of the known leukocidin receptors support the view that the targets of these toxins are leukocytes. However, the receptors are also expressed in non-myeloid cells, suggesting that leukocidins have the potential to contribute to S. aureus pathogenesis in a manner that does not involve targeting leukocytes.
S. aureus is a major pathogen of clinical significance, and leukocidins are a prime example that S. aureus has evolved as a highly human specific pathogen. New in vivo models are needed that better mimic the human host (Box 3). The use of humanized mice has been informative113,114, however, more work is needed to fully recapitulate the function of human neutrophils in the mouse. The identification of human specific receptors and recent advances in genome engineering with CRISPR-Cas115 suggest that developing a mouse model that is compatible with all leukocidins could be possible. Such a model would overcome the current lengthy and variable process of murine humanization, and could allow the study of the role of the leukocidins as a group to be assessed in S. aureus infection. These advances are likely to have major implications for the development of effective vaccines and novel therapeutics to prevent and treat S. aureus infections.
A question that has been challenging to address is why S. aureus produces so many different toxins that target leukocytes. These toxins are differentially regulated in vitro and in vivo63,116, supporting the hypothesis that S. aureus senses host cues to coordinate the production of leukocidins in a tissue-dependent manner. Moreover, the understanding that leukocidins are able to synergize and antagonize each other’s activities, stresses the need for further studies that consider that the contribution of leukocidins to infection could change depending on infection site, S. aureus clinical isolate, and the host.
From the host perspective, the genes that encode two leukocidin receptors, CCR5 and DARC, are under selective pressure. Cells of individuals that lack these receptors are resistant to the leukocidins that target CCR5 and DARC10,38. Moreover, large numbers of polymorphisms have been found in genes that encode other leukocidin receptors117. Future work that investigates the impact of these polymorphisms on host susceptibility to leukocidins could provide insight into observed differences in the susceptibility of humans to S. aureus infection.
With recent advances in understanding how S. aureus bi-component leukocidins target host cells, we are now able to address how these toxins contribute to S. aureus pathophysiology, which could lead to the development of novel anti-staphylococcal agents.
Acknowledgments
We apologize to authors whose relevant work was not included in this review owing to space constraints. We want to thank all the trainees that have contributed to the work on leukocidins in our laboratories and Ashira Lubkin for editing the manuscript. The work on leukocidins in the Torres laboratory is supported in part by the US National Institutes of Allergy and Infectious Diseases award numbers AI099394, AI105129, and HHSN272201400019C. V.J.T. is a Burroughs Wellcome Fund Investigator in the Pathogenesis of Infectious Diseases.
GLOSSARY
- Endocarditis
Inflammation of the inner layer of the heart, most often of infectious origin. S. aureus is a major cause of endocarditis.
- Bi-component pore-forming toxins
Toxins consisting of two protein subunits, that heter-oligomerize in order to form multimeric pores. The pores penetrate the lipid bilayer of the target cell.
- Virulence factors
Molecules produced by pathogens that contribute to the pathogenicity of the organism and that enable colonization, immune evasion and suppression, entry into and release from host cells, and the acquisition of nutrients.
- Leukocytes
Generic term to describe immune cells of hematopoietic origin.
- Phagocytes
Immune cells that specifically engulf and degrade bacteria, fungi, parasites, dead host cells and cellular and foreign debris in a process termed phagocytosis.
- Neutrophils
Abundant, short lived and motile phagocytic cells of the innate immune system that are one of the first cell types to migrate to a site of infection.
- Chemokine receptors
Cytokine receptors on the surface of mainly immune cells, that interact with specific cytokines called chemokines, and that belong to the family of G-protein coupled receptors.
- Rhodopsin-like G-protein coupled receptors
Family of receptors with a common structure comprising 7 transmembrane helices, that transduce extracellular signals through interaction with guanine nucleotide-binding (G) proteins.
- Mac-1 integrin
Macrophage-1 antigen (also known as complement receptor 3), consisting of CD11b (integrin αM) and CD18 (integrin β2).
- Core genome
Consisting of genes present in all S. aureus strains.
- Accessory genome
Consisting of genes present in subsets of S. aureus strains only.
- Self-sensing
Regulatory mechanisms of the pathogen sensing coordinative information according to stimuli provided by the population, for instance via the quorum sensing Agr system.
- Host-sensing
Regulatory mechanisms of the pathogen sensing stimuli provided by the host, for instance via the SaeRS system.
- Two-component regulatory system
Signal transduction modules consisting of a membrane receptor (sensor histidine kinase) and a transcription factor (response regulator) that enable bacteria to sense cues and coordinate a transcriptional response.
- Outgrowth
Multiplication of bacteria during an infection.
- Pus
Exudate formed at the site of infection, mainly consisting of debris from dead leukocytes.
- Necrosis
A nonapoptotic form of cell death that results in cell lysis and inflammation.
- Pyroptosis
A type of necrosis that requires pore formation in the plasma membrane and results in cell swelling, ultimately causing massive leakage of cytosolic contents, which results in inflammation.
- Inflammasomes
A multi-protein complex composed of inflammatory caspases, intracellular sensors (e.g. NOD-like Receptor proteins), and the adaptor protein ASC involved in pyroptosis and the processing of inflammatory cytokines.
- Cytokines
Small proteins involved in cell signaling that have immunomodulatory properties and act through receptors.
- Gasdermin D
a host pore forming protein responsible for cell lysis during pyroptosis.
- Activation status
State of professional phagocytes that defines the cell’s responsiveness to invading pathogens.
- Neutrophil extracellular traps
Networks of extracellular fibers, primarily composed of DNA and antimicrobial peptides, that are release from neutrophils, which bind pathogens.
- Phenol soluble modulins (PSMs)
small amphipatic peptides produce by Staphylococci that exhibit cytotoxic activity against host cells.
- Biofilms
Bacterial community typically enclosed in an extracellular polymeric substance matrix.
- Endophthalmitis
Inflammation of the internal eye, most commonly of infectious origin.
- Opsonization
Process by which a pathogen is coated with immunoglobulins or complement for ingestion and elimination by phagocytes.
- Toxoid
Toxin whose toxicity has been inactivated chemically or by mutation, while its immunogenic properties are maintained.
- Opsonophagocytosis
Process of opsonization of a micro-organism resulting in enhanced phagocytosis of the opsonized micro-organism by phagocytes.
- Peritonitis
Inflammation of the peritoneum (lining the inner abdominal wall), most often of infectious origin.
Biographies
András N. Spaan, MD/PhD
András N. Spaan is a researcher and resident in clinical microbiology at the University Medical Center in Utrecht, The Netherlands. He obtained his medical degree in 2007 at the Academic Medical Center in Amsterdam, and enrolled into the Ph.D. program on Infection and Immunity at Utrecht University in 2008 along with his training as a specialist in Clinical Microbiology. Under the supervision of Prof. van Strijp, he obtained his Ph.D. in 2015 on the identification of the staphylococcal leukocidin receptors. His board certification as a clinical specialist is foreseen in 2017, after which he will pursue his scientific career at Rockefeller University as a postdoctoral fellow under supervision of Jean-Laurent Casanova.
Jos A.G. van Strijp, PhD
Prof. van Strijp studied Biology in Utrecht, and performed his thesis on viruses, complement and neutrophils. After that he moved to Rockefeller University NY (Steinman lab) followed by a year in Boston (Golenbock lab). The last 15 years his research-focus is on immune evasion by bacteria, especially Staphylococci. His lab discovered over 30 novel immune-modulating molecules that all inhibit the human innate immune system, especially neutrophils and complement. It is now clear that this is just the tip of the iceberg. Further he explores fundamental applications in both vaccination in infectious diseases as well as the use of these evasion molecules as anti-inflammatory therapeutics. van Strijp is an Editor of many journals in Microbiology, Infectious diseases and Immunology and a regular referee and panel member on major funding schemes throughout Europe. He has supervised 35 PhD students and 15 Postdocs. van Strijp is Director of the PhD and Master Programs in Infection and Immunity (175 PhD students). Also, he is the Chair of the Graduate School of Life Sciences Utrecht (1100 Master students, 1900 PhD students).
Victor J. Torres, PhD
Prof. Torres received his B.S. degree in Industrial Microbiology in 2000 from the University of Puerto Rico Mayagüez campus. In 2000, he enrolled in the PhD program at Vanderbilt University School of Medicine, where he obtained a doctoral degree in Microbiology and Immunology by studying toxin biology under the supervision of Prof. Timothy Cover. From 2005 to 2008, he completed postdoctoral training on S. aureus pathogenesis under the tutelage of Prof. Eric Skaar, also at Vanderbilt University School of Medicine. In late 2008, he joined the faculty of the Department of Microbiology at New York University School of Medicine, where he is currently a tenured Associate Professor of Microbiology. Over the past eight years Prof. Torres has contributed extensively to the fields of S. aureus pathogenesis and toxin biology. He is also an Editor of several journals in Microbiology, Infectious diseases and Immunology, and a regular panel member on major funding agencies in the US and throughout Europe.
Footnotes
Competing interests statement
A.S.: None
J.S.: None
V.J.T.: Is and an inventor on patents and patent applications filed by New York University School of Medicine, which are currently under commercial license to Janssen Biotech Inc.
References
- 1.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Thwaites GE, et al. Clinical management of Staphylococcus aureus bacteraemia. Lancet Infect Dis. 2011;11:208–222. doi: 10.1016/S1473-3099(10)70285-1. [DOI] [PubMed] [Google Scholar]
- 3.Deleo FR, Otto M, Kreiswirth BN, Chambers HF. Community-associated meticillin-resistant Staphylococcus aureus. Lancet. 2010;375:1557–1568. doi: 10.1016/S0140-6736(09)61999-1. S0140-6736(09)61999-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thammavongsa V, Kim HK, Missiakas D, Schneewind O. Staphylococcal manipulation of host immune responses. Nat Rev Microbiol. 2015;13:529–543. doi: 10.1038/nrmicro3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammer ND, Skaar EP. Molecular mechanisms of Staphylococcus aureus iron acquisition. Annu Rev Microbiol. 2011;65:129–147. doi: 10.1146/annurev-micro-090110-102851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skaar EP, Humayun M, Bae T, DeBord KL, Schneewind O. Iron-source preference of Staphylococcus aureus infections. Science. 2004;305:1626–1628. doi: 10.1126/science.1099930. [DOI] [PubMed] [Google Scholar]
- 7.Alonzo F, 3rd, Torres VJ. The Bicomponent Pore-Forming Leucocidins of Staphylococcus aureus. Microbiol Mol Biol Rev. 2014;78:199–230. doi: 10.1128/MMBR.00055-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peschel A, Otto M. Phenol-soluble modulins and staphylococcal infection. Nat Rev Microbiol. 2013;11:667–673. doi: 10.1038/nrmicro3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spaan AN, Surewaard BG, Nijland R, van Strijp JA. Neutrophils Versus Staphylococcus aureus: A Biological Tug of War. Annu Rev Microbiol. 2013;67:629–650. doi: 10.1146/annurev-micro-092412-155746. [DOI] [PubMed] [Google Scholar]
- 10.Alonzo F, 3rd, et al. CCR5 is a receptor for Staphylococcus aureus leukotoxin ED. Nature. 2013;493:51–55. doi: 10.1038/nature11724. This manuscript described the identification of CCR5 as a receptor for LukED, linking leukocidins to evasion of both adaptive and innate immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vandenesch F, Lina G, Henry T. Staphylococcus aureus hemolysins, bi-component leukocidins, and cytolytic peptides: a redundant arsenal of membrane-damaging virulence factors? Front Cell Infect Microbiol. 2012;2:12. doi: 10.3389/fcimb.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panton PN, Valentine FCO. Staphylococcal Toxin. Lancet. 1932;219:506–508. [Google Scholar]
- 13.Berube BJ, Bubeck Wardenburg J. Staphylococcus aureus alpha-toxin: nearly a century of intrigue. Toxins (Basel) 2013;5:1140–1166. doi: 10.3390/toxins5061140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peraro MD, van der Goot FG. Pore-forming toxins: ancient, but never really out of fashion. Nat Rev Microbiol. 2016;14:77–92. doi: 10.1038/nrmicro.2015.3. Excellent review on pore-forming toxins. [DOI] [PubMed] [Google Scholar]
- 15.Prevost G, Bouakham T, Piemont Y, Monteil H. Characterisation of a synergohymenotropic toxin produced by Staphylococcus intermedius. FEBS Lett. 1995;376:135–140. doi: 10.1016/0014-5793(95)01260-9. [DOI] [PubMed] [Google Scholar]
- 16.Koop G, et al. Identification of LukPQ, a novel, equid-adapted leukocidin of Staphylococcus aureus. Sci Rep. 2017;7:40660. doi: 10.1038/srep40660. This manuscript described the identification of a novel bi-component leukocidin produced by zoonotic S. aureus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loffler B, et al. Staphylococcus aureus panton-valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog. 2010;6:e1000715. doi: 10.1371/journal.ppat.1000715. This manuscript examined the species specificity exhibited by PVL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamashita K, et al. Crystal structure of the octameric pore of staphylococcal gamma-hemolysin reveals the beta-barrel pore formation mechanism by two components. Proc Natl Acad Sci U S A. 2011;108:17314–17319. doi: 10.1073/pnas.1110402108. This manuscript described the first high resolution structure of a bi-component leukocidin octoameric pore. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DuMont AL, et al. Identification of a crucial residue required for Staphylococcus aureus LukAB cytotoxicity and receptor recognition. Infect Immun. 2014;82:1268–1276. doi: 10.1128/IAI.01444-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamashita D, et al. Molecular basis of transmembrane beta-barrel formation of staphylococcal pore-forming toxins. Nat Commun. 2014;5:4897. doi: 10.1038/ncomms5897. [DOI] [PubMed] [Google Scholar]
- 21.Badarau A, et al. Structure-function analysis of heterodimer formation, oligomerization, and receptor binding of the Staphylococcus aureus bi-component toxin LukGH. J Biol Chem. 2015;290:142–156. doi: 10.1074/jbc.M114.598110. This manuscript described the first high resolution structure of the LukAB (also known as LukGH) octoameric pore. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Badarau A, et al. Context matters: The importance of dimerization-induced conformation of the LukGH leukocidin of Staphylococcus aureus for the generation of neutralizing antibodies. MAbs. 2016;0 doi: 10.1080/19420862.2016.1215791. This manuscript described the high resolution structure of the LukAB (also known as LukGH) dimer and the identification of LukAB-sepecific antibodies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugawara-Tomita N, Tomita T, Kamio Y. Stochastic assembly of two-component staphylococcal gamma-hemolysin into heteroheptameric transmembrane pores with alternate subunit arrangements in ratios of 3:4 and 4:3. J Bacteriol. 2002;184:4747–4756. doi: 10.1128/JB.184.17.4747-4756.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das SK, Darshi M, Cheley S, Wallace MI, Bayley H. Membrane protein stoichiometry determined from the step-wise photobleaching of dye-labelled subunits. Chembiochem. 2007;8:994–999. doi: 10.1002/cbic.200600474. [DOI] [PubMed] [Google Scholar]
- 25.Colin DA, Mazurier I, Sire S, Finck-Barbancon V. Interaction of the two components of leukocidin from Staphylococcus aureus with human polymorphonuclear leukocyte membranes: sequential binding and subsequent activation. Infect Immun. 1994;62:3184–3188. doi: 10.1128/iai.62.8.3184-3188.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meunier O, et al. A predicted beta-sheet from class S components of staphylococcal gamma-hemolysin is essential for the secondary interaction of the class F component. Biochim Biophys Acta. 1997;1326:275–286. doi: 10.1016/s0005-2736(97)00031-x. [DOI] [PubMed] [Google Scholar]
- 27.Gauduchon V, Werner S, Prevost G, Monteil H, Colin DA. Flow cytometric determination of Panton-Valentine leucocidin S component binding. Infect Immun. 2001;69:2390–2395. doi: 10.1128/IAI.69.4.2390-2395.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer F, Girardot R, Piemont Y, Prevost G, Colin DA. Analysis of the specificity of Panton-Valentine leucocidin and gamma-hemolysin F component binding. Infect Immun. 2009;77:266–273. doi: 10.1128/IAI.00402-08. IAI.00402-08 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozawa T, Kaneko J, Kamio Y. Essential binding of LukF of staphylococcal gamma-hemolysin followed by the binding of H gamma II for the hemolysis of human erythrocytes. Biosci Biotechnol Biochem. 1995;59:1181–1183. doi: 10.1271/bbb.59.1181. [DOI] [PubMed] [Google Scholar]
- 30.Kaneko J, Ozawa T, Tomita T, Kamio Y. Sequential binding of Staphylococcal gamma-hemolysin to human erythrocytes and complex formation of the hemolysin on the cell surface. Biosci Biotechnol Biochem. 1997;61:846–851. doi: 10.1271/bbb.61.846. [DOI] [PubMed] [Google Scholar]
- 31.Ferreras M, et al. The interaction of Staphylococcus aureus bi-component gamma-hemolysins and leucocidins with cells and lipid membranes. Biochim Biophys Acta. 1998;1414:108–126. doi: 10.1016/s0005-2736(98)00160-6. [DOI] [PubMed] [Google Scholar]
- 32.Morinaga N, Kaihou Y, Noda M. Purification, cloning and characterization of variant LukE-LukD with strong leukocidal activity of staphylococcal bi-component leukotoxin family. Microbiol Immunol. 2003;47:81–90. doi: 10.1111/j.1348-0421.2003.tb02789.x. [DOI] [PubMed] [Google Scholar]
- 33.Reyes-Robles T, Lubkin A, Alonzo F, 3rd, Lacy DB, Torres VJ. Exploiting dominant-negative toxins to combat Staphylococcus aureus pathogenesis. EMBO Rep. 2016;17:428–440. doi: 10.15252/embr.201540994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spaan AN, et al. The staphylococcal toxin Panton-Valentine Leukocidin targets human C5a receptors. Cell Host Microbe. 2013;13:584–594. doi: 10.1016/j.chom.2013.04.006. This manuscript described the identification of C5aR1 and C5aR2 as receptors for PVL, resolving a longlasting controversy on the contribution to staphylococcal pathophysiology of PVL. [DOI] [PubMed] [Google Scholar]
- 35.Reyes-Robles T, et al. Staphylococcus aureus Leukotoxin ED Targets the Chemokine Receptors CXCR1 and CXCR2 to Kill Leukocytes and Promote Infection. Cell Host Microbe. 2013;14:453–459. doi: 10.1016/j.chom.2013.09.005. This manuscript described the identification of CXCR1 and CXCR2 as receptors for LukED, assessing the toxin-receptor interaction and its impact on neutophils during infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spaan AN, et al. The staphylococcal toxins gamma-haemolysin AB and CB differentially target phagocytes by employing specific chemokine receptors. Nat Commun. 2014;5:5438. doi: 10.1038/ncomms6438. This manuscript described the identification of the receptors for HlgAB and HlgCB, showing that HlgAB contributes to S. aureus bacteremia in a CCR2-dependent manner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DuMont AL, et al. Staphylococcus aureus LukAB cytotoxin kills human neutrophils by targeting the CD11b subunit of the integrin Mac-1. Proc Natl Acad Sci U S A. 2013;110:10794–10799. doi: 10.1073/pnas.1305121110. This manuscript described the identification of CD11b as the receptor for LukAB, linking the structural divergence of the toxin compared to other leukocidins to a unique receptor target. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spaan AN, et al. Staphylococcus aureus Targets the Duffy Antigen Receptor for Chemokines (DARC) to Lyse Erythrocytes. Cell Host Microbe. 2015;18:363–370. doi: 10.1016/j.chom.2015.08.001. This manuscript described the identificatin of the erythroid receptor targeted by HlgAB and LukED, linking staphylococcal virulence to bacterial metabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vrieling M, et al. Bovine Staphylococcus aureus Secretes the Leukocidin LukMF’ To Kill Migrating Neutrophils through CCR1. MBio. 2015;6:e00335. doi: 10.1128/mBio.00335-15. This manuscript describes the identification of the LukMF’ celluar receptors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Venkatakrishnan AJ, et al. Molecular signatures of G-protein-coupled receptors. Nature. 2013;494:185–194. doi: 10.1038/nature11896. [DOI] [PubMed] [Google Scholar]
- 41.Tournamille C, Colin Y, Cartron JP, Le Van Kim C. Disruption of a GATA motif in the Duffy gene promoter abolishes erythroid gene expression in Duffy-negative individuals. Nat Genet. 1995;10:224–228. doi: 10.1038/ng0695-224. [DOI] [PubMed] [Google Scholar]
- 42.Pruenster M, Rot A. Throwing light on DARC. Biochem Soc Trans. 2006;34:1005–1008. doi: 10.1042/BST0341005. [DOI] [PubMed] [Google Scholar]
- 43.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 44.Yoong P, Torres VJ. The effects of Staphylococcus aureus leukotoxins on the host: cell lysis and beyond. Curr Opin Microbiol. 2013;16:63–69. doi: 10.1016/j.mib.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tam K, et al. Staphylococcus aureus leukocidin LukED and HIV-1 gp120 target different sequence determinants on CCR5. mBio. doi: 10.1128/mBio.02024-16. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laventie BJ, et al. Residues essential for panton-valentine leukocidin s component binding to its cell receptor suggest both plasticity and adaptability in its interaction surface. PLoS One. 2014;9:e92094. doi: 10.1371/journal.pone.0092094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spaan AN, et al. Differential Interaction of the Staphylococcal Toxins Panton-Valentine Leukocidin and gamma-Hemolysin CB with Human C5a Receptors. J Immunol. 2015;195:1034–1043. doi: 10.4049/jimmunol.1500604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koymans KJ, Vrieling M, Gorham RD, Jr, van Strijp JA. Staphylococcal Immune Evasion Proteins: Structure, Function, and Host Adaptation. Curr Top Microbiol Immunol. 2016 doi: 10.1007/82_2015_5017. [DOI] [PubMed] [Google Scholar]
- 49.Prevost G, et al. Epidemiological data on Staphylococcus aureus strains producing synergohymenotropic toxins. J Med Microbiol. 1995;42:237–245. doi: 10.1099/00222615-42-4-237. [DOI] [PubMed] [Google Scholar]
- 50.von Eiff C, Friedrich AW, Peters G, Becker K. Prevalence of genes encoding for members of the staphylococcal leukotoxin family among clinical isolates of Staphylococcus aureus. Diagn Microbiol Infect Dis. 2004;49:157–162. doi: 10.1016/j.diagmicrobio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 51.Kaneko J, Kimura T, Narita S, Tomita T, Kamio Y. Complete nucleotide sequence and molecular characterization of the temperate staphylococcal bacteriophage phiPVL carrying Panton-Valentine leukocidin genes. Gene. 1998;215:57–67. doi: 10.1016/s0378-1119(98)00278-9. [DOI] [PubMed] [Google Scholar]
- 52.Naimi TS, et al. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA. 2003;290:2976–2984. doi: 10.1001/jama.290.22.2976. [DOI] [PubMed] [Google Scholar]
- 53.Baba T, et al. Complete genome sequence of Macrococcus caseolyticus strain JCSCS5402, [corrected] reflecting the ancestral genome of the human-pathogenic staphylococci. J Bacteriol. 2009;191:1180–1190. doi: 10.1128/JB.01058-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cooney J, Kienle Z, Foster TJ, O’Toole PW. The gamma-hemolysin locus of Staphylococcus aureus comprises three linked genes, two of which are identical to the genes for the F and S components of leukocidin. Infect Immun. 1993;61:768–771. doi: 10.1128/iai.61.2.768-771.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Novick RP, Geisinger E. Quorum sensing in staphylococci. Annu Rev Genet. 2008;42:541–564. doi: 10.1146/annurev.genet.42.110807.091640. [DOI] [PubMed] [Google Scholar]
- 56.Dunman PM, et al. Transcription profiling-based identification of Staphylococcus aureus genes regulated by the agr and/or sarA loci. J Bacteriol. 2001;183:7341–7353. doi: 10.1128/JB.183.24.7341-7353.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benson MA, et al. Evolution of hypervirulence by a MRSA clone through acquisition of a transposable element. Mol Microbiol. 2014;93:664–681. doi: 10.1111/mmi.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Flack CE, et al. Differential regulation of staphylococcal virulence by the sensor kinase SaeS in response to neutrophil-derived stimuli. Proc Natl Acad Sci U S A. 2014;111:E2037–2045. doi: 10.1073/pnas.1322125111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zurek OW, et al. The role of innate immunity in promoting SaeR/S-mediated virulence in Staphylococcus aureus. J Innate Immun. 2014;6:21–30. doi: 10.1159/000351200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Voyich JM, et al. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J Immunol. 2005;175:3907–3919. doi: 10.4049/jimmunol.175.6.3907. [DOI] [PubMed] [Google Scholar]
- 61.Voyich JM, et al. The SaeR/S gene regulatory system is essential for innate immune evasion by Staphylococcus aureus. J Infect Dis. 2009;199:1698–1706. doi: 10.1086/598967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Malachowa N, et al. Global changes in Staphylococcus aureus gene expression in human blood. PLoS One. 2011;6:e18617. doi: 10.1371/journal.pone.0018617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.DuMont AL, et al. Staphylococcus aureus elaborates leukocidin AB to mediate escape from within human neutrophils. Infect Immun. 2013;81:1830–1841. doi: 10.1128/IAI.00095-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Balasubramanian D, et al. Staphylococcus aureus Coordinates Leukocidin Expression and Pathogenesis by Sensing Metabolic Fluxes via RpiRc. MBio. 2016;7 doi: 10.1128/mBio.00818-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dumont AL, et al. Characterization of a new cytotoxin that contributes to Staphylococcus aureus pathogenesis. Mol Microbiol. 2011;79:814–825. doi: 10.1111/j.1365-2958.2010.07490.x. This manuscript described the identification and characterization of LukB as a novel and potent leukocidin produced by many S. aureus isolates, resposible for killing of human neutrophils. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ventura CL, et al. Identification of a novel Staphylococcus aureus two-component leukotoxin using cell surface proteomics. PLoS One. 2010;5:e11634. doi: 10.1371/journal.pone.0011634. This manuscript described the identification and charcterization of LukGH (also known as LukAB) in CA-MRSA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Badiou C, et al. Rapid detection of Staphylococcus aureus Panton-Valentine leukocidin in clinical specimens by enzyme-linked immunosorbent assay and immunochromatographic tests. J Clin Microbiol. 2010;48:1384–1390. doi: 10.1128/JCM.02274-09. JCM.02274-09 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Attia AS, et al. Analysis of the Staphylococcus aureus abscess proteome identifies antimicrobial host proteins and bacterial stress responses at the host-pathogen interface. Pathog Dis. 2013 doi: 10.1111/2049-632X.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Date SV, et al. Global gene expression of methicillin-resistant Staphylococcus aureus USA300 during human and mouse infection. J Infect Dis. 2014;209:1542–1550. doi: 10.1093/infdis/jit668. [DOI] [PubMed] [Google Scholar]
- 70.Thomsen IP, et al. Children with invasive Staphylococcus aureus disease exhibit a potently neutralizing antibody response to the cytotoxin LukAB. Infect Immun. 2014;82:1234–1242. doi: 10.1128/IAI.01558-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adhikari RP, et al. Antibodies to S. aureus LukS-PV Attenuated Subunit Vaccine Neutralize a Broad Spectrum of Canonical and Non-Canonical Bicomponent Leukotoxin Pairs. PLoS One. 2015;10:e0137874. doi: 10.1371/journal.pone.0137874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilke GA, Bubeck Wardenburg J. Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus alpha-hemolysin-mediated cellular injury. Proc Natl Acad Sci U S A. 2010;107:13473–13478. doi: 10.1073/pnas.1001815107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Davis B, Wen H, Ting J. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annual review of immunology. 2011;29:707–735. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mariathasan S, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. nature04515 [pii] [DOI] [PubMed] [Google Scholar]
- 75.Holzinger D, et al. Staphylococcus aureus Panton-Valentine leukocidin induces an inflammatory response in human phagocytes via the NLRP3 inflammasome. J Leukoc Biol. 2012;92:1069–1081. doi: 10.1189/jlb.0112014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Melehani JH, James DB, DuMont AL, Torres VJ, Duncan JA. Staphylococcus aureus Leukocidin A/B (LukAB) Kills Human Monocytes via Host NLRP3 and ASC when Extracellular, but Not Intracellular. PLoS Pathog. 2015;11:e1004970. doi: 10.1371/journal.ppat.1004970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Munoz-Planillo R, Franchi L, Miller LS, Nunez G. A critical role for hemolysins and bacterial lipoproteins in Staphylococcus aureus-induced activation of the Nlrp3 inflammasome. J Immunol. 2009;183:3942–3948. doi: 10.4049/jimmunol.0900729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Munoz-Planillo R, et al. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perret M, et al. Cross-talk between Staphylococcus aureus leukocidins-intoxicated macrophages and lung epithelial cells triggers chemokine secretion in an inflammasome-dependent manner. Cell Microbiol. 2012;14:1019–1036. doi: 10.1111/j.1462-5822.2012.01772.x. [DOI] [PubMed] [Google Scholar]
- 80.Shi J, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 81.Kayagaki N, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 82.Ding J, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111–116. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 83.Liu X, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.He WT, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. 2015;25:1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Graves SF, et al. Sublytic concentrations of Staphylococcus aureus Panton-Valentine leukocidin alter human PMN gene expression and enhance bactericidal capacity. J Leukoc Biol. 2012;92:361–374. doi: 10.1189/jlb.1111575. jlb.1111575 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Malachowa N, Kobayashi SD, Freedman B, Dorward DW, DeLeo FR. Staphylococcus aureus leukotoxin GH promotes formation of neutrophil extracellular traps. J Immunol. 2013;191:6022–6029. doi: 10.4049/jimmunol.1301821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yoong P, Torres VJ. Counter inhibition between leukotoxins attenuates Staphylococcus aureus virulence. Nat Commun. 2015;6:8125. doi: 10.1038/ncomms9125. This manuscript highlighted the complex interplay between the different leukocidins, impacting our understanding of the leukocidins as a group of toxins during both infection and colonization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Torres VJ, et al. Staphylococcus aureus Fur regulates the expression of virulence factors that contribute to the pathogenesis of pneumonia. Infect Immun. 2010;78:1618–1628. doi: 10.1128/IAI.01423-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Torres VJ, Pishchany G, Humayun M, Schneewind O, Skaar EP. Staphylococcus aureus IsdB is a hemoglobin receptor required for heme iron utilization. J Bacteriol. 2006;188:8421–8429. doi: 10.1128/JB.01335-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hongo I, et al. Phenol-soluble modulin alpha 3 enhances the human neutrophil lysis mediated by Panton-Valentine leukocidin. J Infect Dis. 2009;200:715–723. doi: 10.1086/605332. [DOI] [PubMed] [Google Scholar]
- 91.Munzenmayer L, et al. Influence of Sae-regulated and Agr-regulated factors on the escape of Staphylococcus aureus from human macrophages. Cell Microbiol. 2016;18:1172–1183. doi: 10.1111/cmi.12577. [DOI] [PubMed] [Google Scholar]
- 92.Nilsson IM, Hartford O, Foster T, Tarkowski A. Alpha-toxin and gamma-toxin jointly promote Staphylococcus aureus virulence in murine septic arthritis. Infect Immun. 1999;67:1045–1049. doi: 10.1128/iai.67.3.1045-1049.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Supersac G, Piemont Y, Kubina M, Prevost G, Foster TJ. Assessment of the role of gamma-toxin in experimental endophthalmitis using a hlg-deficient mutant of Staphylococcus aureus. Microb Pathog. 1998;24:241–251. doi: 10.1006/mpat.1997.0192. [DOI] [PubMed] [Google Scholar]
- 94.Scherr TD, et al. Staphylococcus aureus Biofilms Induce Macrophage Dysfunction Through Leukocidin AB and Alpha-Toxin. MBio. 2015;6 doi: 10.1128/mBio.01021-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Voyich JM, et al. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J Infect Dis. 2006;194:1761–1770. doi: 10.1086/509506. JID36574 [pii] [DOI] [PubMed] [Google Scholar]
- 96.Bubeck Wardenburg J, Palazzolo-Ballance AM, Otto M, Schneewind O, DeLeo FR. Panton-Valentine leukocidin is not a virulence determinant in murine models of community-associated methicillin-resistant Staphylococcus aureus disease. J Infect Dis. 2008;198:1166–1170. doi: 10.1086/592053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yoong P, Pier GB. Antibody-mediated enhancement of community-acquired methicillin-resistant Staphylococcus aureus infection. Proc Natl Acad Sci U S A. 2010;107:2241–2246. doi: 10.1073/pnas.0910344107. 0910344107 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kobayashi SD, et al. Comparative analysis of USA300 virulence determinants in a rabbit model of skin and soft tissue infection. J Infect Dis. 2011;204:937–941. doi: 10.1093/infdis/jir441. jir441 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Malachowa N, et al. Staphylococcus aureus leukotoxin GH promotes inflammation. J Infect Dis. 2012;206:1185–1193. doi: 10.1093/infdis/jis495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aman MJ, Adhikari RP. Staphylococcal bicomponent pore-forming toxins: targets for prophylaxis and immunotherapy. Toxins (Basel) 2014;6:950–972. doi: 10.3390/toxins6030950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sause WE, Buckley PT, Strohl WR, Lynch AS, Torres VJ. Antibody-Based Biologics and Their Promise to Combat Staphylococcus aureus Infections. Trends Pharmacol Sci. 2016;37:231–241. doi: 10.1016/j.tips.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Laventie BJ, et al. Heavy chain-only antibodies and tetravalent bispecific antibody neutralizing Staphylococcus aureus leukotoxins. Proc Natl Acad Sci U S A. 2011;108:16404–16409. doi: 10.1073/pnas.1102265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rouha H, et al. Five birds, one stone: Neutralization of alpha-hemolysin and 4 bi-component leukocidins of Staphylococcus aureus with a single human monoclonal antibody. MAbs. 2015;7:243–254. doi: 10.4161/19420862.2014.985132. This manuscript described the generation of a broadly neutralizing monoclonal antibody that blocked the majority of the leukocidins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Diep BA, et al. Improved Protection in a Rabbit Model of Community-Associated Methicillin-Resistant Staphylococcus aureus Necrotizing Pneumonia upon Neutralization of Leukocidins in Addition to Alpha-Hemolysin. Antimicrob Agents Chemother. 2016;60:6333–6340. doi: 10.1128/AAC.01213-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thomsen IP, et al. Monoclonal antibodies against the Staphylococcus aureus bicomponent leukotoxin AB (LukAB) isolated following invasive human infection reveal diverse binding and modes of action. J Infect Dis. 2017 doi: 10.1093/infdis/jix071. This manuscript described the identification and charcterization of naturraly ocuring human monoclonal antibodies isolated from a human subject infected with S. aureus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Verkaik NJ, et al. Immunogenicity of toxins during Staphylococcus aureus infection. Clin Infect Dis. 2010;50:61–68. doi: 10.1086/648673. [DOI] [PubMed] [Google Scholar]
- 107.Adhikari RP, et al. Lower antibody levels to Staphylococcus aureus exotoxins are associated with sepsis in hospitalized adults with invasive S. aureus infections. J Infect Dis. 2012;206:915–923. doi: 10.1093/infdis/jis462. [DOI] [PubMed] [Google Scholar]
- 108.Hermos CR, Yoong P, Pier GB. High levels of antibody to panton-valentine leukocidin are not associated with resistance to Staphylococcus aureus-associated skin and soft-tissue infection. Clin Infect Dis. 2010;51:1138–1146. doi: 10.1086/656742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brown EL, et al. The Panton-Valentine leukocidin vaccine protects mice against lung and skin infections caused by Staphylococcus aureus USA300. Clin Microbiol Infect. 2009;15:156–164. doi: 10.1111/j.1469-0691.2008.02648.x. CLM2648 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Karauzum H, et al. Structurally designed attenuated subunit vaccines for S. aureus LukS-PV and LukF-PV confer protection in a mouse bacteremia model. PLoS One. 2013;8:e65384. doi: 10.1371/journal.pone.0065384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Salgado-Pabon W, Schlievert PM. Models matter: the search for an effective Staphylococcus aureus vaccine. Nat Rev Microbiol. 2014;12:585–591. doi: 10.1038/nrmicro3308. [DOI] [PubMed] [Google Scholar]
- 112.Proctor RA. Is there a future for a Staphylococcus aureus vaccine? Vaccine. 2012;30:2921–2927. doi: 10.1016/j.vaccine.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 113.Tseng CW, et al. Increased Susceptibility of Humanized NSG Mice to Panton-Valentine Leukocidin and Staphylococcus aureus Skin Infection. PLoS Pathog. 2015;11:e1005292. doi: 10.1371/journal.ppat.1005292. This manuscript described the use of humanized mice to study PVL in vivo in a skin and soft tissue infection model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Prince A WH, Kitur K, Parker D. Humanized mice exhibit increased susceptibility to Staphylococcus aureus pneumonia. J Infect Dis. 2016 doi: 10.1093/infdis/jiw425. In press. This manuscript described the use of humanized mice to study PVL in vivo in a pneumonia model of infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang H, et al. One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell. 2013;154:1370–1379. doi: 10.1016/j.cell.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Alonzo F, III, et al. Staphylococcus aureus leucocidin ED contributes to systemic infection by targeting neutrophils and promoting bacterial growth in vivo. Mol Microbiol. 2012;83:423–435. doi: 10.1111/j.1365-2958.2011.07942.x. This mansucript identified LukED as a critical toxin for the lethality observed during bloodstream infection in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lek M, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fitzgerald JR. Livestock-associated Staphylococcus aureus: origin, evolution and public health threat. Trends Microbiol. 2012;20:192–198. doi: 10.1016/j.tim.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 119.Vrieling M, et al. LukMF’ is the major secreted leukocidin of bovine Staphylococcus aureus and is produced in vivo during bovine mastitis. Sci Rep. 2016;6:37759. doi: 10.1038/srep37759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Prevost G, Bouakham T, Piemont Y, Monteil H. Characterisation of a synergohymenotropic toxin produced by Staphylococcus intermedius. FEBS Letters. 1995;376:135–140. doi: 10.1016/0014-5793(95)01260-9. [DOI] [PubMed] [Google Scholar]
- 121.Woolhouse ME, Webster JP, Domingo E, Charlesworth B, Levin BR. Biological and biomedical implications of the co-evolution of pathogens and their hosts. Nat Genet. 2002;32:569–577. doi: 10.1038/ng1202-569. [DOI] [PubMed] [Google Scholar]
- 122.Lipinska U, et al. Panton-Valentine leukocidin does play a role in the early stage of Staphylococcus aureus skin infections: a rabbit model. PLoS One. 2011;6:e22864. doi: 10.1371/journal.pone.0022864. PONE-D-11-03641 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cribier B, Piemont Y, Grosshans E. Staphylococcal scalded skin syndrome in adults. A clinical review illustrated with a new case. J Am Acad Dermatol. 1994;30:319–324. doi: 10.1016/s0190-9622(94)70032-x. [DOI] [PubMed] [Google Scholar]
- 124.Cremieux AC, et al. Panton-valentine leukocidin enhances the severity of community-associated methicillin-resistant Staphylococcus aureus rabbit osteomyelitis. PLoS One. 2009;4:e7204. doi: 10.1371/journal.pone.0007204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Diep BA, et al. Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc Natl Acad Sci U S A. 2010;107:5587–5592. doi: 10.1073/pnas.0912403107. 0912403107 [pii] This manuscript demonstarted the contribution of PVL to S. aureus mediated pneumonia in rabbits. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Diep BA, et al. Contribution of Panton-Valentine leukocidin in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. PLoS One. 2008;3:e3198. doi: 10.1371/journal.pone.0003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vancraeynest D, et al. International dissemination of a high virulence rabbit Staphylococcus aureus clone. J Vet Med B Infect Dis Vet Public Health. 2006;53:418–422. doi: 10.1111/j.1439-0450.2006.00977.x. [DOI] [PubMed] [Google Scholar]