Abstract
Background:
Cancer survival is known to be associated with socioeconomic status. The income gap between the richer and poorer segments of the population has widened over the last 20 years in Canada. The purpose of this study was to investigate temporal trends in disparities in cancer-specific survival related to socioeconomic status in Ontario.
Methods:
There were 920 334 cancer cases between 1993 and 2009 in the Ontario Cancer Registry. We linked median household income from the Canadian census to the registry. We calculated 5-year cancer-specific survival rates for all cancers combined and for specific cancer sites by socioeconomic status quintile and year of diagnosis, and modelled time to death using Cox regression.
Results:
Between 1993 and 2009, for all cancers combined, the hazard of death decreased by 3.1% (hazard ratio [HR] 0.969 [95% confidence interval (CI) 0.967-0.971]) per year in the richest quintile and by 1.2% (HR 0.988 [95% CI 0.987-0.990]) per year in the poorest quintile. The corresponding values for breast cancer were 4.3% (HR 0.957 [95% CI 0.951-0.964]) and 2.0% (HR 0.980 [95% CI 0.975-0.986]); for lung cancer, 1.4% (HR 0.986 [95% CI 0.982-0.990]) and 0.3% (HR 0.997 [95% CI 0.995-1.000]); for colorectal cancer, 3.7% (HR 0.963 [95% CI 0.958-0.968]) and 1.8% (HR 0.982 [95% CI 0.978-0.985]); and for head and neck cancer, 3.1% (HR 0.969 [95% CI 0.958-0.979]) and 1.0% (HR 0.990 [95% CI 0.983-0.996]).
Interpretation:
Between 1993 and 2009, cancer-specific survival in Ontario improved more among patients from affluent communities than among those from poorer communities. This phenomenon cannot be explained by increased disparity in income.
Since the first report of an association between socioeconomic status and cancer survival, by Cohart1 in 1955, it has become well established that socioeconomic status is a predictor of cancer survival.2,3 This association has been observed consistently in studies from various health care systems in North America, Australia and Europe.2,3 In the late 1990s and early 2000s, investigators compared the effect of socioeconomic status on cancer survival in Ontario with that observed in the Surveillance, Epidemiology and End Results (SEER) population of the United States.4-6 It was shown that, despite Canada's system of universal health insurance, there was a significant association between socioeconomic status and survival for several major types of cancer.4 The magnitude of the association was, however, smaller in Ontario than in the US.5
Over the last 2 decades, public health agencies in Canada and elsewhere have emphasized the importance of reducing social disparities in health.7,8 At the same time, income inequality has been increasing in many countries, including Canada.9 It is not known whether these changes in income distribution have translated into an increase in income-related disparities in cancer survival.
Few studies have assessed the temporal trend in the association between socioeconomic status and cancer survival. McDonald and colleagues10 investigated this trend for head and neck cancer in Canada from 1992 to 2005 and found a significant increase in the difference in survival between the richest and poorest segments of the population for oropharynx cancer, a disease whose cause has shifted from predominantly smoking to human papillomavirus infection.11 In a prospective cohort study in the United Kingdom, Ramsay and colleagues12 investigated the same time trend by social class, defined by occupation, among 7489 men and observed no change in the association between cancer survival and occupation during their 35-year follow-up period. In the US, Niu and colleagues13 examined whether the disparity in cancer survival by insurance status changed between 1999 and 2004 and found that survival improved for privately insured patients but not for those insured by Medicaid. The objective of the current study was to determine whether the magnitude of the association between socioeconomic status and cancer survival changed between 1993 and 2009 in Ontario.
Methods
Source of data
This was a population-based retrospective study. We identified cancer cases diagnosed between 1993 and 2009 through the Ontario Cancer Registry, which provides age, sex, postal code at diagnosis, date of diagnosis, disease site, date of death and cause of death. Disease sites are coded with the use of the International Classification of Diseases for Oncology, 3rd edition. Cause of death, provided as International Classification of Diseases, 9th revision and 10th revision codes, is available up to Dec. 31, 2011, and date of death is complete up to Dec. 31, 2013.
We obtained data on gross median household income from the 1996, 2001 and 2006 Canadian censuses (Statistics Canada), at the level of enumeration area for the 1996 and 2001 censuses and dissemination area for the 2006 census. We grouped census dissemination/enumeration areas into quintiles based on median household income, with the fifth quintile (Q5) representing the communities where the wealthiest 20% of the population resided and the first quintile (Q1) representing the communities where the poorest 20% resided.
We linked median household income quintile to each cancer case through the patient's postal code by using Statistics Canada's Postal Code Conversion File Plus,14 which provides the correspondence between postal code and dissemination/enumeration area. Although the postal code and dissemination/enumeration area are similar in size, their boundaries do not correspond. In cases in which the postal code straddled the boundary of more than 1 dissemination/enumeration area, we selected the dissemination/enumeration area with the most dwellings in the postal code. We assigned the median household income from the 1996 census (1995 income), 2001 census (2000 income) and 2006 census (2005 income) to cases diagnosed in 1993-1997, 1998-2002 and 2003-2009, respectively.
Statistical analysis
We estimated 5-year cancer-specific survival for 1993-2006 (cases with complete follow-up at 5 years after diagnosis) by socioeconomic status and year of diagnosis. Five-year cancer-specific survival was calculated as 1 minus the cumulative incidence function for death from any cancer at 5 years from diagnosis. We calculated survival time (in months) from the date of diagnosis to the date of death from cancer, to the date of death from other causes (competing events) or to Dec. 31, 2011 if still alive (censored).
We used Fine-Gray subdistribution proportional hazards regression to investigate the interaction effect between socioeconomic status and year of diagnosis on time to cancer death, controlling for age and sex, and to calculate hazard ratios (HRs).15,16 All cases diagnosed between 1993 and 2009 were included in this analysis. The results were considered significant at the 0.05 level, and all tests of statistical significance were two-sided. We performed the statistical analysis using SAS version 9.4 (SAS Institute Inc.).
Ethics approval
The study obtained ethics approval from Queen's University.
Results
Study population
There were 920 334 cancer cases during the study period. Table 1 presents summary statistics for the study population. The median age was 67 years; males represented a slightly larger proportion (51.9%) than females. Fifteen percent of the patients resided in Q5 (richest), and 22.9% resided in Q1 (poorest). The annual number of incident cases increased over time, from 44 165 in 1993 to 65 522 in 2009. Breast cancer accounted for the highest proportion of all cancer cases, followed by lung, colorectal, and head and neck cancers. There was no difference in the case-mix between cancer sites across the years (Appendix 1, Supplementary Table 1, available at www.cmajopen.ca/content/5/3/E682/suppl/DC1).
Table 1: Distribution of cancer cases by study variables in Ontario, 1993-2009.
| Variable | No. (%) of cases n = 920 334 |
|---|---|
| Age, yr | |
| < 50 | 136 515 (14.8) |
| 50-59 | 154 531 (16.8) |
| 60-69 | 234 047 (25.4) |
| 70-79 | 249 900 (27.2) |
| ≥ 80 | 145 341 (15.8) |
| Sex | |
| Male | 477 336 (51.9) |
| Female | 442 998 (48.1) |
| Year | |
| 1993 | 44 165 (4.8) |
| 1994 | 44 847 (4.9) |
| 1995 | 44 738 (4.9) |
| 1996 | 46 082 (5.0) |
| 1997 | 47 804 (5.2) |
| 1998 | 49 403 (5.4) |
| 1999 | 51 164 (5.6) |
| 2000 | 52 811 (5.7) |
| 2001 | 54 559 (5.9) |
| 2002 | 55 452 (6.0) |
| 2003 | 56 098 (6.1) |
| 2004 | 58 477 (6.4) |
| 2005 | 59 971 (6.5) |
| 2006 | 61 439 (6.7) |
| 2007 | 63 909 (6.9) |
| 2008 | 63 893 (6.9) |
| 2009 | 65 522 (7.1) |
| Socioeconomic status quintile | |
| 1 (poorest) | 210 539 (22.9) |
| 2 | 197 432 (21.4) |
| 3 | 180 032 (19.6) |
| 4 | 157 701 (17.1) |
| 5 (richest) | 137 659 (15.0) |
| Missing | 36 971 (4.0) |
| Site* | |
| Breast | 124 221 (13.5) |
| Lung | 122 889 (13.4) |
| Head and neck | 30 695 (3.3) |
| Colorectal | 122 183 (13.3) |
| Other | 520 346 (56.5) |
| *The International Classification of Diseases for Oncology, 3rd edition codes for the specific cancer sites are: breast C50, lung C34, head and neck C00-C14 and C30-C32, and colorectal C18-C21 and C26.0. All sites include all malignant cancer cases except nonmelanoma skin cancer. | |
Temporal trend in survival by socioeconomic status quintiles
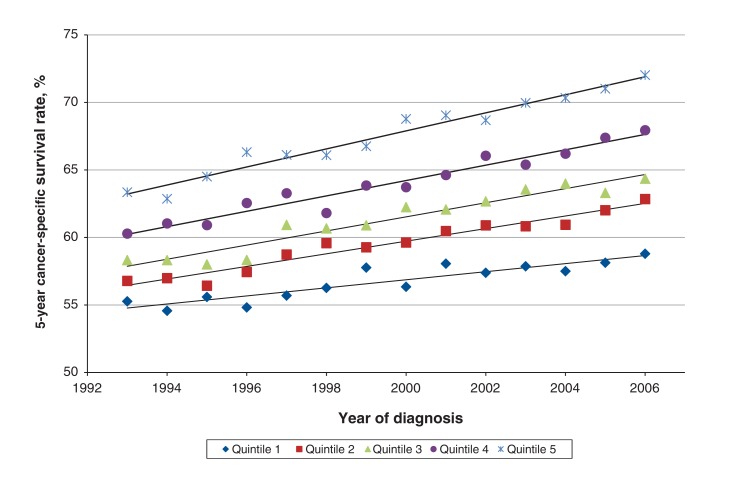
Improvement in cancer survival in Ontario between 1993 and 2006 differed by socioeconomic status quintile (Figure 1). For all cancers combined, the 5-year cancer-specific survival rate for patients in Q1 improved by 3.5 percentage points, from 55.3% (95% confidence interval [CI] 54.4%-56.2%) in 1993 to 58.8% (95% CI 58.0%-59.6%) in 2006. For patients in Q5, the rate improved by 8.6 percentage points, from 63.4% (95% CI 62.1%-64.6%) to 72.0% (95% CI 71.1%-72.9%). The difference in the survival rate between Q1 and Q5 widened from 8.1 percentage points in 1993 to 13.2 percentage points in 2006.
Figure 1.
Temporal trends in 5-year cancer-specific survival rate for all cancers in Ontario, 1993-2006, by socioeconomic status quintile (1 = poorest, 5 = richest).
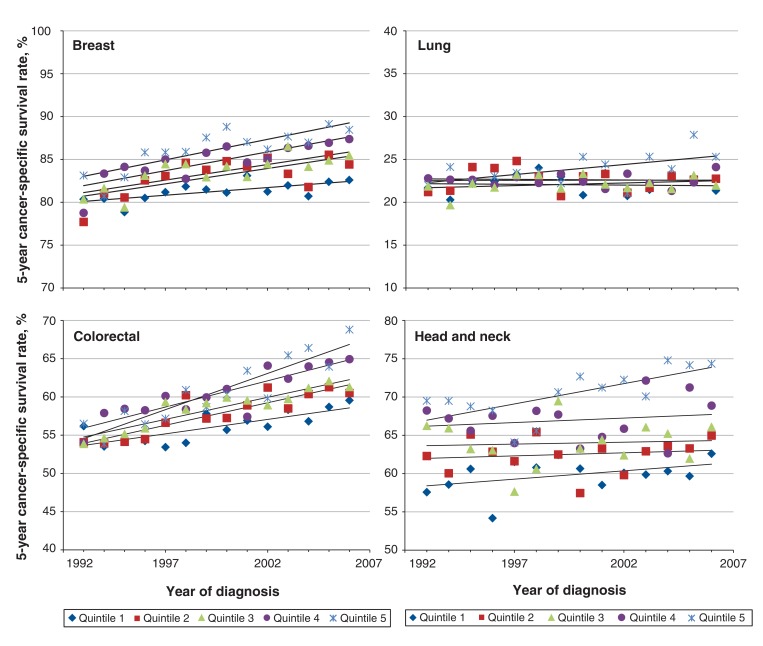
Similar temporal trends were found for specific cancer sites, but at different magnitudes (Figure 2). For breast cancer, the 5-year cancer-specific survival rate for patients in Q1 improved by 2.2 percentage points, from 80.4% (95% CI 78.3%-82.4%) to 82.6% (95% CI 80.8%-84.3%), whereas the rate for those in Q5 improved by 5.4 percentage points, from 83.1% (95% CI 80.7%-85.4%) to 88.5% (95% CI 86.8%-90.0%). The difference between Q1 and Q5 widened from 2.7 percentage points in 1993 to 5.9 percentage points in 2006. For lung cancer, the 5-year cancer-specific survival rate for patients in Q1 decreased by 0.3 percentage points, from 21.7% (95% CI 20.0%-23.6%) to 21.4% (95% CI 19.7%-23.1%). In contrast, the survival rate for those in Q5 increased by 3.3 percentage points, from 22.0% (95% CI 19.0%-25.4%) to 25.3% (95% CI 22.6%-28.3%). The difference in the survival rate between Q1 and Q5 widened from 0.3 percentage points in 1993 to 3.9 percentage points in 2006. There was no substantial change in survival over the study period for patients in Q2-Q4. For colorectal cancer, the 5-year cancer-specific survival rate among patients in Q1 increased by 3.4 percentage points, from 56.2% (95% CI 53.8%-58.6%) to 59.6% (95% CI 57.3%-61.8%), whereas the rate among those in Q5 increased by 12.3 percentage points, from 56.5% (95% CI 52.9%-60.2%) to 68.8% (95% CI 66.2%-71.4%). The difference between Q1 and Q5 widened from 0.3 percentage points in 1993 to 9.2 percentage points in 2006. Finally, for cancers of the head and neck, the 5-year cancer-specific survival rate for patients in Q1 increased by 5.0 percentage points, from 57.6% (95% CI 53.1%-62.1%) to 62.6% (95% CI 58.2%-67.0%). For those in Q5, the survival rate increased by 4.9 percentage points, from 69.5% (95% CI 62.4%-76.4%) to 74.4% (95% CI 69.1%-79.4%).
Figure 2.
Temporal trends in 5-year cancer-specific survival rates for breast cancer, lung cancer, colorectal cancer, and head and neck cancer in Ontario, 1993-2006, by socioeconomic status quintile (1 = poorest, 5 = richest).
Fitting a Fine-Gray model confirmed a significant interaction between socioeconomic status quintile and year of diagnosis after age and sex were controlled for (see Appendix 1, Supplementary Table 2, for p values). During the study period, for all cancers combined, the hazard of death decreased by 3.1% per year for patients in Q5 and by 1.2% per year for those in Q1. The corresponding values for breast cancer were 4.3% and 2.0%; for lung cancer, 1.4% and 0.3%; for colorectal cancer, 3.7% and 1.8%; and for head and neck cancer, 3.1% and 1.0%. HRs and 95% CIs are shown in column 3 of Table 2.
Table 2: Time trend in cancer survival for socioeconomic status quintiles and for selected gross median household income categories, expressed as the hazard of death per year of diagnosis from the Fine-Gray subdistribution proportional hazards regression model, for cancer cases diagnosed in Ontario, 1993-2009.
| Site | Socioeconomic status quintile* | Hazard ratio (95% CI) | Gross median household income, 2010 constant dollars | Hazard ratio (95% CI) |
|---|---|---|---|---|
| All cancers | 1 (poorest) | 0.988 (0.987-0.990) | 20 000 | 0.991 (0.989-0.993) |
| 2 | 0.983 (0.982-0.985) | 40 000 | 0.985 (0.983-0.988) | |
| 3 | 0.980 (0.978-0.981) | 60 000 | 0.980 (0.977-0.983) | |
| 4 | 0.977 (0.975-0.979) | 80 000 | 0.975 (0.971-0.978) | |
| 5 (richest) | 0.969 (0.967-0.971) | 100 000 | 0.969 (0.966-0.973) | |
| Breast | 1 | 0.980 (0.975-0.986) | 20 000 | 0.985 (0.981-0.988) |
| 2 | 0.974 (0.968-0.976) | 40 000 | 0.979 (0.975-0.983) | |
| 3 | 0.968 (0.962-0.973) | 60 000 | 0.974 (0.970-0.978) | |
| 4 | 0.966 (0.960-0.972) | 80 000 | 0.968 (0.964-0.973) | |
| 5 | 0.957 (0.951-0.964) | 100 000 | 0.963 (0.958-0.968) | |
| Lung | 1 | 0.997 (0.995-1.000) | 20 000 | 0.997 (0.995-0.999) |
| 2 | 0.997 (0.995-1.000) | 40 000 | 0.992 (0.989-0.994) | |
| 3 | 0.995 (0.992-0.997) | 60 000 | 0.986 (0.983-0.989) | |
| 4 | 0.996 (0.992-0.997) | 80 000 | 0.981 (0.977-0.984) | |
| 5 | 0.986 (0.982-0.990) | 100 000 | 0.975 (0.971-0.979) | |
| Colon-rectum | 1 | 0.982 (0.978-0.985) | 20 000 | 0.987 (0.984-0.989) |
| 2 | 0.978 (0.975-0.982) | 40 000 | 0.981 (0.978-0.984) | |
| 3 | 0.975 (0.971-0.979) | 60 000 | 0.976 (0.972-0.979) | |
| 4 | 0.971 (0.966-0.975) | 80 000 | 0.970 (0.967-0.974) | |
| 5 | 0.963 (0.958-0.968) | 100 000 | 0.965 (0.961-0.969) | |
| Head and neck | 1 | 0.990 (0.983-0.996) | 20 000 | 0.996 (0.991-1.000) |
| 2 | 0.988 (0.981-0.995) | 40 000 | 0.990 (0.985-0.995) | |
| 3 | 0.985 (0.978-0.993) | 60 000 | 0.985 (0.979-0.990) | |
| 4 | 0.979 (0.970-0.988) | 80 000 | 0.979 (0.974-0.984) | |
| 5 | 0.969 (0.958-0.979) | 100 000 | 0.974 (0.968-0.979) |
Note: CI = confidence interval.
*Distinct from gross median household income.
Temporal trend in survival by median household income
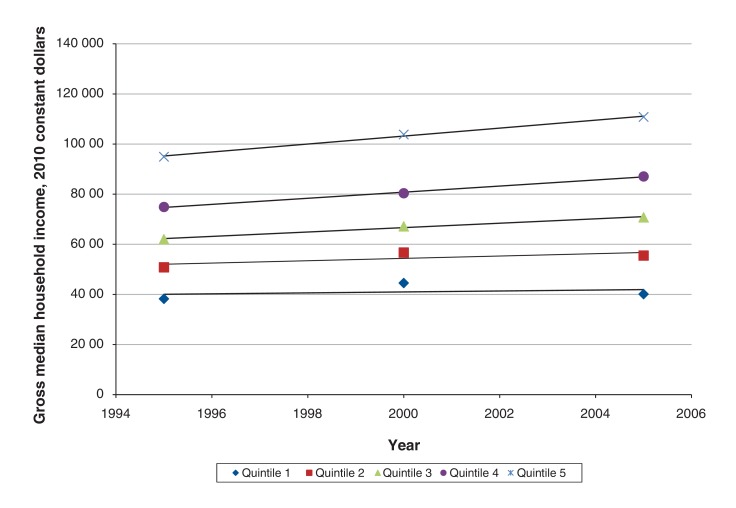
During the study period, median income in Q1 remained relatively constant, whereas median income in the higher-income quintiles increased substantially (Figure 3). As a result, the gap in median income between Q5 and Q1 widened, from $56 706 in the 1996 census to $70 693 (in 2010 constant dollars) in the 2006 census, an increase of 24.7%. Because the analysis treated the quintiles as categorical variables, the effect observed could be in part mediated by the increase in the income gap alone. To obviate this potential mediation effect, we converted median household income at the level of the dissemination/enumeration area from each census into 2010 constant dollars and used this income value to fit a second Fine-Gray model (Appendix 1, Supplementary Table 3). The model revealed significant interactions between year of diagnosis and median household income for the 5 major disease sites and for all cancers combined. The hazard of death per year of diagnosis was lower in the communities with higher median household income and higher in the communities with lower median household income (column 5 of Table 2). For example, when all cancers were combined, the hazard of death decreased by 0.9% (HR 0.991 [95% CI 0.989-0.993]) per year in the communities with a median household income of $20 000 and decreased by 3.1% (HR 0.969 [95% CI 0.966-0.973]) per year in the communities with a median household income of $100 000. This informs us that, regardless of the actual income range in 1993 and 2006, patients in the communities with the same lower income (e.g., $20 000) saw a slower improvement in survival than those in the communities with the same higher income (e.g., $100 000). Therefore, the observed disparity in the survival trend was unrelated to the increased income range over the years.
Figure 3.
Gross median household income in 2010 constant dollars for income quintiles 1-5 from the 1996, 2001 and 2006 censuses.
Interpretation
We found that, between 1993 and 2006, cancer-specific survival in Ontario improved, but the improvement was greater among patients from affluent communities than among those from poorer communities, and income-associated disparities in survival therefore widened. This phenomenon was observed for cancers of the lung, breast, colon-rectum, and head and neck as well as for all cancers combined. Furthermore, our analysis showed that the income-associated disparities in the survival trend could not be explained simply by the widened disparity in income.
The overall improvements in cancer-specific survival that we observed probably reflect earlier diagnosis owing to improved screening or improvements in treatment or both. The fact that survival improved more among patients residing in richer communities suggests that, in general, improvements in screening and treatment may have had more impact in this group than in those residing in poorer communities. The specific explanations for the widening gap in survival between richer and poorer populations probably differ among the different types of cancer. For example, for breast cancer, for which screening is known to improve outcomes at the population level,17,18 differences in the use of screening related to socioeconomic status may contribute to the differences in cancer-specific survival related to socioeconomic status. Consistent with this hypothesis, an Ontario study of breast cancer screening between 1999 and 2010 showed that mammographic screening was used less frequently in lower-income neighbourhoods.19 However, those authors did not find an increase in income-associated disparities in screening rates over time. Further study is required to determine whether disparities in the use of screening related to socioeconomic status have increased in recent years for other diseases for which screening is effective. However, differential use of screening does not offer any explanation for the widening gap in survival between richer and poorer populations for lung or head and neck cancer because no screening was routinely offered in Ontario for either of these diseases.
Multiple incremental improvements in the effectiveness of cancer treatment are probably responsible for much of the overall improvement in cancer-specific survival observed in this study. Over the last 2 decades, new and increasingly effective types of adjuvant treatment have become available for many types of cancer. Furthermore, there is evidence from Ontario that new forms of adjuvant treatment are used more frequently in richer communities.20,21 Thus, it seems probable that the more rapid adoption of effective new treatments in richer communities may be responsible for the observed increase in disparity in cancer-specific survival associated with socioeconomic status.
Comorbidity in patients with cancer has been shown to vary by socioeconomic status22,23 and has been found to be associated with poorer cancer-specific survival for some diseases, although to a lesser degree than with all-cause survival.24 Comorbidity of patients with cancer by socioeconomic status in Ontario has not been reported, and therefore it is difficult to ascertain whether the observed time trend in disparity in survival associated with socioeconomic status was related to comorbidity. Lifestyle factors as a mediator between socioeconomic status and survival have also been proposed: Woods and colleagues3 suggested that such lifestyle factors as smoking or poor diet could lead to overall poorer health of patients with cancer and therefore reduce their chance of survival. An Ontario study on disparities in tobacco use related to socioeconomic status in the general population showed no interaction between time and education between 1999 and 2006, although both smoking rates and education level increased over time.25 Further research is needed on the relation between socioeconomic status, smoking status and cancer survival to better understand the temporal trend observed in the current study.
The temporal trend in disparity in cancer survival associated with socioeconomic status that we observed has important implications for the management of cancer care. The overall improved cancer survival is consistent with improved access to diagnosis and treatments.26 However, the lesser improvement in outcomes observed in poorer populations suggests that new approaches to cancer diagnosis and treatment may be adopted more slowly among groups of lower socioeconomic status. Further study focusing on particular disease groups is required to identify the specific factors that mediate the association between socioeconomic status and survival. A better understanding of the causal pathway between socioeconomic status and cancer survival is needed to inform strategies aimed at narrowing the gap in survival between richer and poorer populations. Similar studies in various countries with different social and health care systems may also help further our understanding of this important determinant of health.
Limitations
The accuracy of classification of cause of death is a known concern for calculating cancer-specific survival. Hall and colleagues27 compared classification of cause of death in the Ontario Cancer Registry with that recorded in a clinical database for a group of patients with squamous carcinoma of the head and neck. Among the 276 patients who died from head and neck cancer, death was misclassified as noncancer death for 23 (8.3%). Misclassification from death from other causes to death from head and neck cancer also occurred. Furthermore, the authors found no difference in cancer-specific survival calculated using the Ontario Cancer Registry and that calculated using their clinical database, which could be explained if the pattern of misclassification were random. Although misclassification of cause of death does occur, there is no evidence to suggest that misclassification was responsible for the increased disparity in cancer-specific survival related to socioeconomic status observed in the current study. In addition, the measure of socioeconomic status that we used was community-level socioeconomic status and cannot be interpreted as the socioeconomic status of individual patients. Nonetheless, a previous study incorporating both community-level and individual-level socioeconomic status in a multilevel analysis showed independent effects of the 2 measures on breast cancer incidence and therefore suggested that community-level socioeconomic status may capture an unmeasured aspect of individual socioeconomic status.28
Conclusion
Despite increased awareness of the relation between socioeconomic status and cancer survival, between 1993 and 2009, cancer-specific survival in Ontario improved less among patients from poor communities than among those from affluent communities, and this phenomenon cannot be explained by increased disparity in income. The causes of the disparity need to be identified so that targeted interventions can be designed to address this issue.
Supplemental information
For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/5/3/E682/suppl/DC1.
Supplementary Material
Acknowledgements
The authors thank Cancer Care Ontario for providing the data. Thanks also go to Patti Groome, Michael Brundage, Keyue Ding and Michael McIssac for their helpful comments on the study.
Footnotes
Funding: This research was funded by Cancer Care Ontario.
References
- 1.Cohart EM. Socioeconomic distribution of cancer of the female sex organs in New Haven. Cancer. 1955;8:34–41. doi: 10.1002/1097-0142(1955)8:1<34::aid-cncr2820080104>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 2.Kogevinas M, Porta M. Socioeconomic differences in cancer survival: a review of the evidence. IARC Sci Publ. 1997;138:177–206. [PubMed] [Google Scholar]
- 3.Woods LM, Rachet B, Coleman MP. Origins of socio-economic inequalities in cancer survival: a review. Ann Oncol. 2006;17:5–19. doi: 10.1093/annonc/mdj007. [DOI] [PubMed] [Google Scholar]
- 4.Mackillop WJ, Zhang-Salomons J, Groome PA, et al. Socioeconomic status and cancer survival in Ontario. J Clin Oncol. 1997;15:1680–9. doi: 10.1200/JCO.1997.15.4.1680. [DOI] [PubMed] [Google Scholar]
- 5.Boyd C, Zhang-Salomons J, Groome PA, et al. Associations between community income and cancer survival in Ontario, Canada, and the United States. J Clin Oncol. 1999;17:2244–55. doi: 10.1200/JCO.1999.17.7.2244. [DOI] [PubMed] [Google Scholar]
- 6.Zhang-Salomons J, Qian H, Holowaty E, et al. Associations between socioeconomic status and cancer survival: choice of SES indicator may affect results. Ann Epidemiol. 2006;16:521–8. doi: 10.1016/j.annepidem.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Ontario Cancer Plan IV 2015-2019. Toronto: Cancer Care Ontario. 2014. [accessed 2015 Nov. 13]. Available https://cancercare.on.ca/common/pages/UserFile.aspx?fileId=333871.
- 8.Closing the gap in a generation: health equity through action on the social determinants of health. Final report of the Commission on Social Determinants of Health. Geneva: World Health Organization. 2008. [accessed 2015 Nov. 13]. Available www.who.int/social_determinants/thecommission/finalreport/en/
- 9.Income inequity. Ottawa: Conference Board of Canada. 2013. [accessed 2015 Nov. 13]. Available www.conferenceboard.ca/hcp/details/society/income-inequality.aspx.
- 10.McDonald JT, Johnson-Obaseki S, Hwang E, et al. The relationship between survival and socio-economic status for head and neck cancer in Canada. J Otolaryngol Head Neck Surg. 2014;43:2–8. doi: 10.1186/1916-0216-43-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta S, Kong W, Peng Y, et al. Temporal trends in the incidence and survival of cancers of the upper aerodigestive tract in Ontario and the United States. Int J Cancer. 2009;125:2159–65. doi: 10.1002/ijc.24533. [DOI] [PubMed] [Google Scholar]
- 12.Ramsay SE, Morris RW, Whincup PH, et al. Time trends in socioeconomic inequalities in cancer mortality: results from a 35 year prospective study in British men. BMC Cancer. 2014;14:474. doi: 10.1186/1471-2407-14-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niu X, Roche LM, Pawlish KS, et al. Cancer survival disparities by health insurance status. Cancer Med. 2013;2:403–11. doi: 10.1002/cam4.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Postal Code Conversion File (PCCF). Cat no 92-153-XCB. Ottawa: Geography Division, Statistics Canada. 2011. [Google Scholar]
- 15.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 16.Geskus RB. Cause-specific cumulative incidence estimation and the Fine and Gray model under both left truncation and right censoring. Biometrics. 2011;67:39–49. doi: 10.1111/j.1541-0420.2010.01420.x. [DOI] [PubMed] [Google Scholar]
- 17.Schopper D, de Wolf C. How effective are breast cancer screening programmes by mammography? Review of the current evidence. Eur J Cancer. 2009;45:1916–23. doi: 10.1016/j.ejca.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 18.Coldman A, Phillips N, Warren L, et al. Breast cancer mortality after screening mammography in British Columbia women. Int J Cancer. 2007;120:1076–80. doi: 10.1002/ijc.22249. [DOI] [PubMed] [Google Scholar]
- 19.Kiran T, Wilton AS, Moineddin R, et al. Effect of payment incentives on cancer screening in Ontario primary care. Ann Fam Med. 2014;12:317–23. doi: 10.1370/afm.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Booth CM, Nanhji S, Wei X, et al. Use and effectiveness of adjuvant chemotherapy for stage III colon cancer: a population-based study. J Natl Compr Canc Netw. 2016;14:47–56. doi: 10.6004/jnccn.2016.0006. [DOI] [PubMed] [Google Scholar]
- 21.Booth CM, Siemens DR, Li G, et al. Perioperative chemotherapy for muscle-invasive bladder cancer: a population-based outcomes study. Cancer. 2014;120:1630–8. doi: 10.1002/cncr.28510. [DOI] [PubMed] [Google Scholar]
- 22.Schrijvers CT, Coebergh JW, Mackenbach JP. Socioeconomic status and comorbidity among newly diagnosed cancer patients. Cancer. 1997;80:1482–8. [PubMed] [Google Scholar]
- 23.Grose D, Morrison DS, Devereux G, et al. Scottish Lung Cancer Forum. Comorbidities in lung cancer: prevalence, severity and links with socioeconomic status and treatment. Postgrad Med J. 2014;90:305–10. doi: 10.1136/postgradmedj-2013-132186. [DOI] [PubMed] [Google Scholar]
- 24.Søgaard M, Thomsen RW, Bossen KS, et al. The impact of comorbidity on cancer survival: a review. Clin Epidemiol. 2013;5(Suppl 1):3–29. doi: 10.2147/CLEP.S47150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reid JL, Hammond D, Driezen P. Socio-economic status and smoking in Canada, 1999-2006: Has there been any progress on disparities in tobacco use? Can J Public Health. 2010;101:73–8. doi: 10.1007/BF03405567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cancer System Quality Index (CSQI) 2016. Toronto: Cancer Quality Council of Ontario. 2016. [accessed 2017 May 10]. Available www.csqi.on.ca.
- 27.Hall S, Schulze K, Groome P, et al. Using cancer registry data for survival studies: the example of the Ontario Cancer Registry. J Clin Epidemiol. 2006;59:67–76. doi: 10.1016/j.jclinepi.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Webster TF, Hoffman K, Weinberg J, et al. Community- and individual-level socioeconomic status and breast cancer risk: multilevel modeling on Cape Cod, Massachusetts. Environ Health Perspect. 2008;116:1125–9. doi: 10.1289/ehp.10818. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.