Abstract
Background Treatment for neonatal alloimmune thrombocytopenia (NAIT) primarily involves maternal administration of intravenous immunoglobulin (IVIG) therapy and prednisone according to protocols based on risk stratification. While IVIG is generally well tolerated, hematologic side effects are a potential complication.
Case We present the successful management of a rare complication of maternal pancytopenia following standard IVIG treatment. Diagnosis was made during routine obstetric exams. Management included reducing IVIG dosage and adding daily prednisone. Additionally, infusion Lots possibly associated with the event were identified and avoided. Interventions resulted in the resolution of pancytopenia and the birth of a healthy infant without thrombocytopenia.
Conclusion Pancytopenia is a rare complication of IVIG treatment in women with pregnancies complicated by NAIT. Serial complete blood counts at the time of treatment would allow for early detection and timely management of the patient. Additionally, limiting the number of infusion Lots may decrease the chance of the described complications.
Keywords: NAIT, pancytopenia, IVIG, prednisone, blood count
Neonatal alloimmune thrombocytopenia (NAIT), affecting 1 out of every 1,000 live births in North America, is characterized by fetal thrombocytopenia due to an incompatibility between maternal and fetal platelets. 1 2 3 Paternally inherited human platelet antigens (HPAs) trigger maternal antibody formation against fetal platelets. Unlike Rh incompatibility, NAIT can present during the first pregnancy and becomes more severe in subsequent pregnancies; complications include fetal intracranial hemorrhage (ICH) and death. 1 2 Diagnosis is typically made after a previously affected pregnancy with serological confirmation of maternal antibodies against neonatal platelets. Evidence-based treatment protocols of NAIT have been stratified depending on previous pregnancy outcome and fetal complications. 4
While maternal hemolysis associated with IVIG treatment for NAIT has been reported, 5 additional cytopenias have also been associated with IVIG treatment for other disorders. 6 7 Of note, IVIG is manufactured by pooling together plasma from thousands of donors. 3 It is administered in Lots; Lot is defined as a number allotted to a pooled product to allow for tracking and quality control. 8 We present a case of maternal pancytopenia during IVIG treatment for NAIT. Subsequent testing and clinical course supported an IVIG-associated mechanism. Based on the PubMed search with keywords, such as “pregnancy” AND “intravenous immunoglobulin” AND “pancytopenia,” there has never been a published case of pancytopenia in a pregnant patient receiving IVIG treatment. Current obstetrical guidelines for the treatment of NAIT do not comment on hematologic side effects of IVIG and do not provide a standard for surveillance of maternal blood counts. We present the following case to add to the literature a rare side effect and discuss its implications in the current standard of care for NAIT.
Case Report
A 31-year-old female patient gravida 2 para 1001, with a history of a previous pregnancy affected by NAIT, presented for prenatal care in the first trimester of her second pregnancy. Her prior pregnancy had an uncomplicated prenatal course. Primary cesarean section at 40 weeks was performed following a failed vacuum extraction and a category II tracing. The female infant, 6 lbs 0 oz, had Apgar scores of 8/9 at 0.2 minute. Newborn laboratories indicated a platelet count of 6,000 × 10 3 /uL, requiring platelet transfusion and IVIG. There was no ICH, and at the age of two years, the child has been growing normally.
The detection of maternal antibodies against HPA-1a confirmed NAIT. The father's homozygosity for HPA-1a conferred a 100% chance of recurrent NAIT in a subsequent pregnancy. Maternal blood type was O positive. Medications included iron supplements and prenatal vitamins. She had no prior history of thrombocytopenia. The patient started weekly IVIG at 2 g/kg at 20 weeks gestational age (wga) per protocol for standard risk NAIT. 4
On routine obstetric exams performed at 24 + 3 wga, the CBC indicated pancytopenia of unknown etiology ( Fig. 1 , Box 1). Patient was asymptomatic, reporting no easy bruising, bleeding, or fever. At 26 + 3 wga ( Fig. 1 , Box 2), she was admitted to the hospital for fever, chills, fatigue, and continued worsening pancytopenia. The hematologist recommended a febrile neutropenia workup and antibiotics for 48 hours pending evaluation. Fever was never documented while in the hospital. The complete blood counts (CBCs) were checked daily. Chest X-ray and blood cultures were negative. Lactate dehydrogenase (LDH) and total bilirubin were normal, and Coombs' test was negative. Patient declined a bone marrow aspiration and biopsy to rule out malignant or myeloproliferative disease. Flow cytometry on peripheral blood showed no evidence of leukemia or other malignancies. A low vitamin-B 12 level was consistent with a mild macrocytic anemia and supplementation was started. Because of the possibility of IVIG adversely affecting the blood counts, at 26 + 6 wga ( Fig. 1 , Box 3), the patient was switched to an alternative standard risk regimen of IVIG 1 g/kg/week and daily 0.5 mg/kg of oral prednisone. This regimen decreased her IVIG exposure by 50%. All blood elements subsequently recovered ( Fig. 1 ).
Fig. 1.
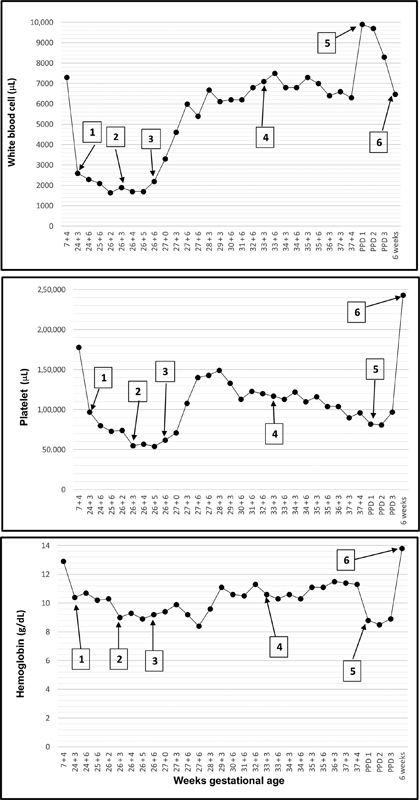
CBC trends with IVIG treatment. Patient received initial IVIG treatment at 19 + 6 wga. Box 1 : 24 + 3 wga, initial detection of pancytopenia. Box 2 : 26 + 3 wga, time of hospital admission. Box 3 : 26 + 6 wga, decreased IVIG (1 g/kg/week and daily 0.5 mg/kg of oral prednisone) and hospital discharge. Box 4 : 33 + 3 IVIG dose increased to 2 g/kg/week with daily prednisone. Box 5 : 37 + 4 wga, scheduled cesarean section. Box 6 : Six weeks postpartum exams. CBC, complete blood count; IVIG, intravenous immunoglobulin; wga, weeks gestational age.
At 33 wga, IVIG was increased to 2 g/kg/week with daily prednisone per protocol ( Fig. 1 , Box 4). A scheduled, uncomplicated repeat cesarean section at 37 weeks was completed under regional anesthesia ( Fig. 1 , Box 5). Preoperative CBC showed mild thrombocytopenia (96 × 103/uL) with normal hemoglobin and white blood cell count most consistent with gestational thrombocytopenia. Newborn platelets were 139 × 10 3 /uL and no treatment was required. Patient received postoperative venous thromboembolism prophylaxis with enoxaparin 40 mg daily and had an uncomplicated postpartum course. At 6 weeks postpartum, CBC was normal ( Fig. 1 , Box 6).
Discussion
We present a case of maternal pancytopenia associated with IVIG treatment during pregnancy complicated by NAIT. Upon initial detection with routine second trimester obstetric exams, our initial differential diagnosis included viral suppression of hematopoiesis, malignancy, and IVIG-associated complication. The negative flow cytometry and lack of elevated temperatures or other symptoms during hospital admission made the first two causes unlikely. No additional medications taken have been reported to be associated with pancytopenia. The simultaneous drop in all blood cells and the corresponding rise following the decrease in IVIG dosage with the addition of prednisone was consistent with IVIG-mediated etiology.
While only maternal hemolysis has been reported in the setting of NAIT, 5 leukopenia and thrombosis are also known complications of IVIG treatment. Leukopenia is generally self-limiting and not associated with complications. 6 Possible mechanisms involve antineutrophil antibodies derived from the pooled Lots of IVIG and immune complexes leading to neutrophil margination in vessel walls. 7 Pretreatment with prednisone has been shown to improve neutropenia by preventing this immune complex formation and complement activation. 7 Thrombosis is believed to be caused by increased plasma viscosity, increased erythrocyte aggregation, and an increase in procoagulant factors within the IVIG product. Risk reduction mechanisms involve increasing infusion time and decreasing the maximum daily dosage. 6 Hemolytic anemia is caused by the transfer of anti-A and anti-B antibodies. 9 Risk factors include higher cumulative IVIG dose, non-O recipient blood type, and recipient female gender. Chances of complication can be decreased by cross matching IVIG products and screening for high anti-A or anti-B titers. 6
IVIG is manufactured by pooling human plasma from multiple donors into Lots. 3 To decrease the quantity of donor components available to interact with the recipient, methods of reduction and filtration have been developed. 3 During our investigation, we learned that our patient was administered IVIG from as many as three different Lots during a single infusion. It is possible that the higher number of Lots used can correlate to a higher number of antibodies present, resulting in hemolysis and even in pancytopenia as reported herein. Additionally, limiting the number of Lots per infusion will allow for easier detection and avoidance of the offending Lot, should a pancytopenic event occurs.
Given the severity of the patient's pancytopenia, a change in treatment regimen was necessary. The alternate treatment regimen was selected using current recommendations for standard risk NAIT. 4 The two regimens included 2 g/kg/week of IVIG or 1 g/kg/week of IVIG with 0.5 mg/kg/day of prednisone starting at 20 wga. 4 An increase to 2 g/kg/week with the addition of 0.5 mg/kg/day of prednisone is recommended at 32 wga. 4 Selection between the two possible treatment protocols has been based on provider and patient preference and clinical correlation rather than on a specific algorithm. Based on the pancytopenia associated with the 2 g/kg IVIG infusion, our rational to change to the lower IVIG dose was to continue to treat NAIT while decreasing the dose of IVIG, thereby lowering the overall antibody exposure. Additionally, prednisone has the potential to decrease the presumed immune-mediated blood cell destruction. Research from the Netherlands has suggested that decreasing the IVIG dosage from 1.0 g/kg to 0.5 g/kg resulted in no significant difference in neonatal platelet count, 10 and a recent systematic review found little evidence that robustly supported the different IVIG dosing regimens. 11 Additionally, a recent clinical trial reported that non-O blood group mothers had hemolysis with high-dose IVIG (2 g/kg/week) but not with low-dose IVIG (1 g/kg/week). 12 Further research is required to determine the efficacy of starting with low dose of IVIG with prednisone. As seen anecdotally with our patient, a lower dose of IVIG may have the potential to decrease the chances of a reaction.
Conclusion
By presenting this case, we hope to bring to light a possible complication of IVIG treatment for NAIT and suggest that certain measures could be taken to screen for and possibly avoid pancytopenic events. Although these complications are rare and often self-limiting, our patient required a 3-day hospitalization, blood transfusion, and antibiotic regimen, none of which are without risk. While it may not be cost effective or necessary to check CBCs at each infusion, further research may determine the appropriate frequency of this laboratory test during pregnancy. In addition, to better correlate a specific IVIG Lot to a pancytopenic event, we suggest that Lot numbers should be tracked, and every effort should be made to minimize the number of Lots used for each infusion. Our report adds to the literature the need for further study of IVIG use during pregnancy and recommendations for monitoring complications related to management. 5
Financial Disclosures
There are no sources of financial support or funding to disclose for this study.
References
- 1.Berkowitz R L, Bussel J B, McFarland J G. Alloimmune thrombocytopenia: state of the art 2006. Am J Obstet Gynecol. 2006;195(04):907–913. doi: 10.1016/j.ajog.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Berkowitz R L, Lesser M L, McFarland J Get al. Antepartum treatment without early cordocentesis for standard-risk alloimmune thrombocytopenia: a randomized controlled trial Obstet Gynecol 2007110(2 Pt 1):249–255. [DOI] [PubMed] [Google Scholar]
- 3.Hooper J A.Intravenous immunoglobulins: evolution of commercial IVIG preparations Immunol Allergy Clin North Am 20082804765–778., viii [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pacheco L D, Berkowitz R L, Moise K J, Jr, Bussel J B, McFarland J G, Saade G R. Fetal and neonatal alloimmune thrombocytopenia: a management algorithm based on risk stratification. Obstet Gynecol. 2011;118(05):1157–1163. doi: 10.1097/AOG.0b013e31823403f4. [DOI] [PubMed] [Google Scholar]
- 5.Rink B D, Gonik B, Chmait R H, O'Shaughnessy R.Maternal hemolysis after intravenous immunoglobulin treatment in fetal and neonatal alloimmune thrombocytopenia Obstet Gynecol 2013121(2 Pt 2, Suppl 1)471–473. [DOI] [PubMed] [Google Scholar]
- 6.Baxley A, Akhtari M. Hematologic toxicities associated with intravenous immunoglobulin therapy. Int Immunopharmacol. 2011;11(11):1663–1667. doi: 10.1016/j.intimp.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 7.Matsuda M, Hosoda W, Sekijima Y et al. Neutropenia as a complication of high-dose intravenous immunoglobulin therapy in adult patients with neuroimmunologic disorders. Clin Neuropharmacol. 2003;26(06):306–311. doi: 10.1097/00002826-200311000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Lot Number. 2017. Available at:https://en.wikipedia.org/wiki/Lot_number. Accessed February 17, 2017
- 9.Orbach H, Katz U, Sherer Y, Shoenfeld Y. Intravenous immunoglobulin: adverse effects and safe administration. Clin Rev Allergy Immunol. 2005;29(03):173–184. doi: 10.1385/CRIAI:29:3:173. [DOI] [PubMed] [Google Scholar]
- 10.Kamphuis M, Paridaans N, Winkelhorst D et al. Lower-dose intravenous immunoglobulins for the treatment of fetal and neonatal alloimmune thrombocytopenia: a cohort study. Transfusion. 2016;56(09):2308–2313. doi: 10.1111/trf.13712. [DOI] [PubMed] [Google Scholar]
- 11.Winkelhorst D, Murphy M F, Greinacher A et al. Antenatal management in fetal and neonatal alloimmune thrombocytopenia: a systematic review. Blood. 2017;129(11):1538–1547. doi: 10.1182/blood-2016-10-739656. [DOI] [PubMed] [Google Scholar]
- 12.Lakkaraja M, Jin J C, Manotas K C et al. Blood group A mothers are more likely to develop anemia during antenatal intravenous immunoglobulin treatment of fetal and neonatal alloimmune thrombocytopenia. Transfusion. 2016;56(10):2449–2454. doi: 10.1111/trf.13779. [DOI] [PubMed] [Google Scholar]


