Abstract
The Hsp90/Cdc37 chaperone system interacts with and supports 60% of the human kinome. Not only are Hsp90 and Cdc37 generally required for initial folding, but many kinases rely on the Hsp90/Cdc37 throughout their lifetimes. A large fraction of these “client” kinases are key oncoproteins, and their interactions with the Hsp90/Cdc37 machinery are crucial for both their normal and malignant activity. Recently, advances in single particle cryoEM and biochemical strategies have provided the first key molecular insights into kinase:chaperone interactions. The surprising results suggest a re-evaluation of the role of chaperones in the kinase lifecycle and suggest that such interactions potentially allow for kinases to more rapidly respond to key signals while simultaneously protecting unstable kinases from degradation and suppressing unwanted basal activity.
Although initially observed decades ago, details of Hsp90-kinase interactions are just emerging
Protein kinases are widely recognized to be key players in regulating the cell cycle, cell signaling and early organism development (1, 2). Dysregulation of their normal function leads to a variety of human disease including cancer, and consequently protein kinases have become key therapeutic targets over the past decade (3). During early work on the remarkable transforming power of vSrc kinase in chick embryos, two additional proteins were discovered that closely associated with vSrc, polypeptides of 80 kDa and 50 kDa, which are now recognized to be the molecular chaperone Hsp90 and its co-chaperone Cdc37 (also referred to as p50)(4). Since then, Hsp90 has been shown to be a key molecular chaperone for ∼10% of the proteome with substrate proteins (known as clients) highly enriched for those involved in signaling and regulation, including kinases, nuclear steroid receptors, ubiquitin ligases, amyloid proteins and others (5, 6). Although initially in the shadows, over the past 10 years Cdc37 has been found to be a central player, single handedly connecting the Hsp90 chaperone system to the kinome. New advances in biophysical methods (7) and in in vitro reconstitution of Hsp90/Cdc37/kinase interactions(8) have recently yielded mechanistic and molecular insights into the role Cdc37 plays mediating interactions between Hsp90 and kinases. In this review we will discuss these recent advancements and their implications for kinase regulation.
What is a client kinase?
Although historically kinases have been broken into binary client and non-client categories, recent results affirm the view that most if not all kinases depend on and interact with the Hsp90/Cdc37 system at least during initial folding. Thus, rather than asking if a kinase is a client, the more appropriate question is where it lies in a continuum of chaperone dependence. Before discussing this more fully, it is useful to consider the two main strategies used when seeking Hsp90/Cdc37 kinase clients.
Due to the low stabilities of client kinases, studies of chaperone interactions have been predominantly limited to either cellular or cell lysate experiments. In this context, kinases have been classified as clients if they co-immunoprecipitate with Hsp90/Cdc37 and if kinase activity, typically read out indirectly via a phosphorylation cascade, decreases in response to inhibition of Hsp90's ATP cycle (See Box 1). It turns out that such loss of activity can be attributed to the fact that kinase levels plummet and/or kinases aggregate in response to Hsp90's inhibition, rather than Hsp90 providing direct activation. After Hsp90 inhibition for longer than 20 hours, this is observed for almost all the kinases, and is due to Hsp90/Cdc37 playing an important role in early folding, much as might be expected for a molecular chaperone. This is experimentally observed as a failure to either synthesize new kinases after Hsp90 inhibition or the ability to immunoprecipitate recently translated kinases with Hsp90/Cdc37 from cell lysates. Explicit examples of these are EGFR (9), ErbB3 (10), Ire1 (11), and LCK (12). Again, this behavior is observed even for kinases that are sometimes referred to as non-clients. A clear example is the pioneering in vitro reconstitution of canonically “non-client” Chk1 kinase, which when purified without proper initial chaperoning becomes seriously misfolded. By contrast, functional kinase can be obtained in the presence of the Hsp90/Cdc37 and Hsp70 systems, indicating that even a non-client kinase is biased towards an Hsp90-Cdc37 interacting state(13).
Box 1. Hsp90 undergoes large conformational changes during its ATPase cycle.
Hsp90 is an ATPase and its catalytic ability is essential for its activity. In humans there are four homologues of Hsp90, two cytosolic (α and β), one in endoplasmic reticulum and one mitochondrial, with important differences between them. Homologues differ in their ATPase rates, ranging from the human cytosolic Hsp90 which has an almost undetectable ATPase rate, to yeast cytosolic homologues, which are quite robust. A variety of co-chaperones like Aha1 or p23 can modulate Hsp90's ATPase with important functional consequences. Over the years, work via a combination of techniques from multiple labs have captured Hsp90 in drastically different conformations(Fig I). Without nucleotides or inhibitors Hsp90 exists in equilibrium of states from very open to almost completely closed. ATP binding biases this equilibrium towards a closed state, with different homologues responding differently. For example, yeast cytosolic Hsp90 almost completely shifts to the closed state and human Hsp90 populates it only transiently. The process of closure seems to be the rate-limiting step for hydrolysis. This ATP bound state used to be thought of as symmetric, but recent work on the mitochondrial homologue of Hsp90 TRAP1 shows asymmetry at the interface between the middle domain and C terminal domain, potentially formed due to strain build up. In conjunction with Cdc37, co-chaperones such as p23, or clients such as kinases, stabilize the symmetric state(Fig I). Upon hydrolysis of one ATP, the strain is released potentially forming a symmetric state. Current work from the Agard lab indicates that hydrolysis can actually lead to a switch in asymmetry, not depicted here, with the asymmetric homologue always hydrolyzing first. Upon hydrolysis of ATP by the second monomer, Hsp90 forms a transient compact ADP state before releasing the nucleotides and returning to the apo state equilibrium. Multiple Hsp90 inhibitors target the conserved nucleotide binding pocket like geldanamycin, radicicol, 17AAG, ganetespib and others. Structurally such binding seems to bias the Hsp90 towards a more compact state, which is however not as compact as ATP or ADP bound states. In vivo inhibitor treatment usually leads to release of the client, but it is important to realize that inhibition of Hsp90 is not the same as its absence, as Hsp90 stalled at some point in its cycle may still have effects on clients due to simple binding.
Figure I. Hsp90's ATPase cycle.
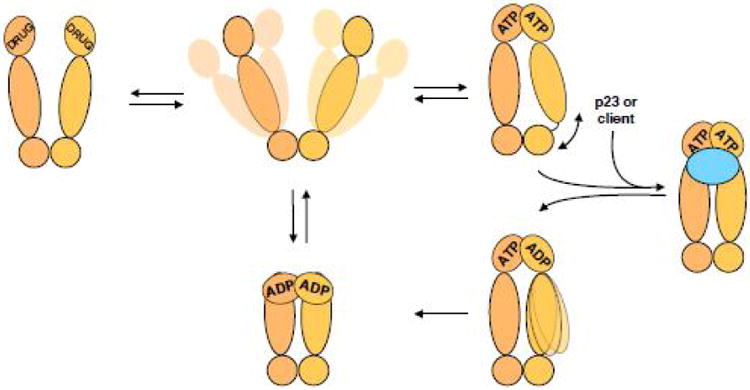
Hsp90 dimer (shades of tan) samples a variety of open states without a nucleotide. Upon binding of ATP, Hsp90 adopts a closed state with the N terminal domains rotating and dimerizing. Without additional binding partners such closed state is asymmetric at the interface between Hsp90's middle and C terminal domains. However, binding of co-chaperones like p23 or co-chaperones with clients, like Cdc37-kinase (either case is shown as blue oval) causes Hsp90 to adopt a symmetric state. ATP is then hydrolyzed in the N terminal domains, potentially sequentially with a switch in the asymmetry between the Hsp90 monomers, on the way to a more compact ADP state. Release of the nucleotide returns Hsp90 back to the equilibrium of open states. Inhibitors, like geldanamycin, 17AAG, radicicol, etc break the ATPase cycle of Hsp90, stalling it in the more compact state, yet different from the ATP bound state.
At the other end of the spectrum are kinases that historically are thought of as clients. Upon Hsp90 inhibition, levels of these kinases plummet much faster than just from disruption of newly synthesized protein, due to rapid ubiquitination and proteosomal degradation. Such kinases are constitutively addicted to Hsp90/Cdc37 even when mature, as has been shown explicitly for ErbB2 (Her2), where ErbB2 at the plasma membrane is rapidly degraded upon Hsp90 inhibition (10, 14). Notably, closely related kinases can lie at opposite ends of the Hsp90/Cdc37 dependence spectrum, with a single point mutation being able to convert one into the other. Examples include EGFR/ErbB2 (15), cSrc/vSrc (16), Cdk2/Cdk4 (17) and aRaf/cRaf (18). Many groups have used such non-client/client pairs in an effort to discover an Hsp90-interacting sequence or motif. Unfortunately, their results were only applicable to a single kinase or family, revealing no truly general feature among clients.
In 2012, the Lindquist lab heroically and quantitatively investigated the physical interactions between human kinases and Hsp90/Cdc37 (19). They observed 60% of the assayed human kinome to interact with Hsp90/Cdc37, with clients present in all of the kinase families. However, even with this vast amount of data, the authors failed to identify any global sequence determinant for Hsp90/Cdc37 interactions. Instead, the only unifying feature observed was that client kinases are generally less thermally stable than non-clients. This strongly suggested the need for detailed biochemical and biophysical investigation.
Recent rigorous in vitro work on interactions between Hsp90/Cdc37 and the constitutive client vSrc by Boczek et al. provided answers to some of the long-standing biochemical questions (20). This study is insightful for a number of reasons. First, this is the first example of being able to show using purified components that human Hsp90β (and not α) together with Cdc37, are able to safeguard vSrc from being inactivated at 30°C in an ATP dependent manner (See Box 1). Second, via mutational analysis the authors show that the interaction strength between Src and Hsp90/Cdc37 can be varied in a continuous manner from the weak/early client cSrc all the way to constitutive client vSrc. This demonstrates that fundamentally the same physical feature must be responsible for interactions across the spectrum from early folding clients to post-folding constitutive clients.
The fact that mature kinases, long post folding, depend on Hsp90/Cdc37 clearly falls outside the simplistic notion that chaperones are only needed to aid initial folding or during heat shock stress. What then is the kinase structural state that is being recognized by Hsp90/Cdc37? Also, since minimal sequence variations should be able to eliminate this post-folding need for Hsp90 why then has this dependence persisted?
Structural hallmarks of client kinases
The kinase domain has been definitively shown to be both necessary and sufficient for interaction with Hsp90/Cdc37 (9). This is curious because, structurally, eukaryotic kinase domains are all very similar with the exception of an order/disorder transition at the activation loop or movement of the αC helix (Fig 1A). The most recent data from multiple groups, along with the failure to find a general client sequence motif, argues that the relevant interaction surfaces must be present in both clients and non-clients, but it is the propensity to display these surfaces that determines interaction strength with Hsp90/Cdc37.
Figure 1. Kinase domain architecture and dynamics.
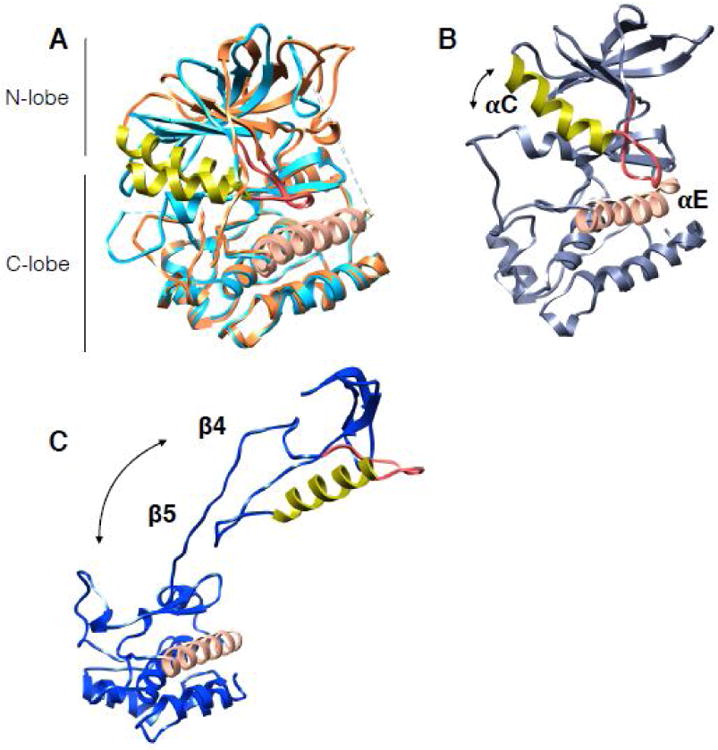
The αC helix in all structures is in lime color, adjacent αC-β4 loop is in red. The proteins have been aligned by the αE helix (salmon color helix in C-lobe). Arrows show the direction and relative magnitude of N-lobe motion in relation to C-lobe. (A) Structures of EGFR in the active state (light blue in color, PDB:2ITP) and the inactive state (piper in color, PDB:2GS7). Note the small shift in αC helix between states. (B) Snapshot from 12us all-atom MD simulation on EGFR from Shaw group displaying an opening between the kinase lobes. (C) Cdk4 kinase structure as it is in the cryoEM complex of Hsp90-Cdc37-Cdk4 (PDB:5FWL) showing dramatic unfolding between the kinase lobes and unraveling of β4- β5 strands.
The first line of evidence for this hypothesis comes from the observation that clients are less thermally stable than non-clients. Analyses of bRaf(19) and Src(20) both show that mutations which destabilize the kinase domain make for stronger Hsp90/Cdc37 clients. The converse is also true, where stabilization by the addition of drugs/nucleotides results in a decreased dependence on Hsp90/Cdc37, regardless of whether the drugs are active-site inhibitors or allosteric modulators(21). Moreover, Boczek and colleagues observed a higher rate of conformational transitions in molecular dynamics simulations of mutants that interact more strongly with Hsp90/Cdc37.
Second, recent NMR studies by the Gelis lab show that even historically non-client kinases interact with Cdc37, albeit very transiently (22). Interestingly, addition of Cdc37 alone to bRaf kinase leads to partial kinase unfolding. The residues that lose structure are the same as the ones that interact with Hsp90 alone (extremely weakly) suggesting that both Hsp90 and Cdc37 may bias the kinase towards a similar state. Importantly, drug-bound bRaf interacts transiently with Cdc37 but does not unfold, indicating that changes in kinase dynamics alone, without changes in sequence, can shift the kinase along the client strength axis.
Third, the recent cryoEM structure of an Hsp90-Cdc37-Cdk4 complex Verba et al. directly shows the kinase in an unfolded conformation, completely split between the lobes by Hsp90-Cdc37 (Fig1C) (23). Cdc37 interacts with the Cdk4 αE helix via backbone hydrogen bonds, recognizing a conserved fold and not a specific sequence. Furthermore, sequence alignment shows that there are no significant differences in the Hsp90 interacting residues between client and non-client kinases, with both being generally hydrophobic in that region.
Taken together, the most consistent interpretation is that client kinases are more dynamic (less stable) than non-clients. That is, Hsp90/Cdc37 must be recognizing a non-native, partially unfolded state with the two kinase lobes being separated. Client kinases differ from non-clients in that they significantly populate such a partially unfolded state even at physiological temperatures. This allows Cdc37 to bind and to further unfold the kinase, splitting it at the lobes and presenting regions that now can stably bind to Hsp90, as in the cryoEM structure. This mode of molecular motion, opening between the two lobes, although not as extreme as in the cryoEM structure, has been observed in molecular dynamics simulations of EGFR by the Shaw group (Fig 1B)(24), and by the Agard group when running rotamerically induced perturbations(25) on Cdk kinases (unpublished data).
How does Cdc37 interact with client kinases and promote Hsp90 function?
Cdc37 binds kinase clients without Hsp90, but Hsp90 interacts only weakly without Cdc37, therefore Cdc37 constitutes an independent kinase binding unit (8, 22). Early analysis of protein fragments identified the Cdc37 N terminal domain as the kinase interacting domain, the middle domain (residues 148-276) as a proteolytically stable Hsp90 interacting domain, and a C terminal domain of unclear function (Fig 2A)(26). In the cryoEM structure, a conserved His 20 Pro 21 Asn 22 motif in Cdc37 is seen to perfectly mimic the type 1 β-turn within the kinase αC-β4 loop, packing against the kinase αE helix. This molecular mimicry by Cdc37 prevents the N lobe from making its cognate interactions with the C lobe, stabilizing a separated, partially unfolded state, and allows Hsp90 to stabilize an even more unfolded state with Hsp90 clamping around what would be the β4- β5 strands of the N lobe (Fig 2B). Thus, Cdc37 may be playing an equivalent role to that of Hsp70 with the glucocorticoid receptor, where Hsp70 stabilizes a partially unfolded GR and then presents this state to Hsp90 (27). However, the analogy is not perfect as previous data(27) suggests that the transition to a closed Hsp90 results in active GR, whereas the observed closed Hsp90:Cdc37 complex must be fully inactive.
Figure 2. Cdc37 architecture and interactions with Cdk4 kinase in context of Hsp90.
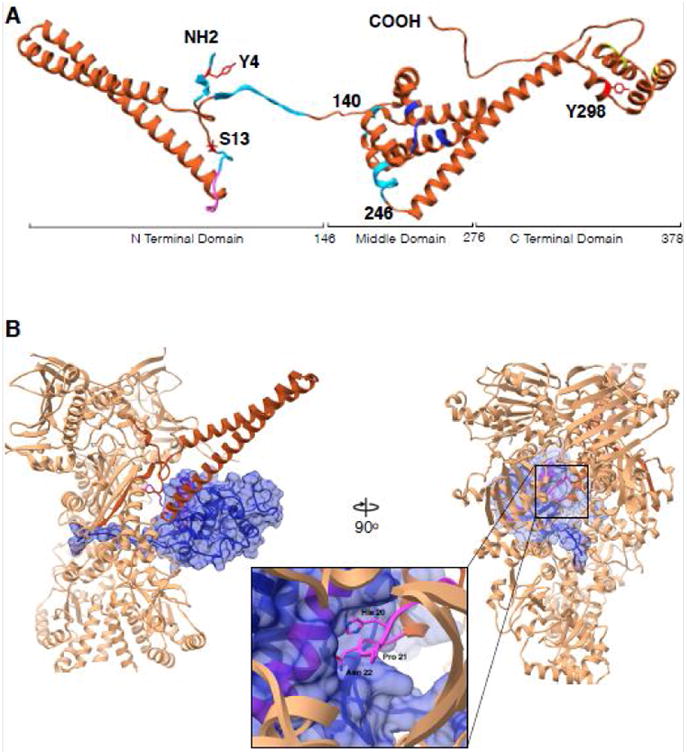
(A) Cdc37 structure synthesized from different studies. Residues 1-260 are from cryoEM structure (PDB:5FWL), 261-293 from Hsp90-Cdc37 domain crystal structure (PDB:1US7) and 294-378 from the ensemble solution NMR structure (PDB:2N5X). Cyan color indicates regions interacting with Hsp90 in Hsp90-Cdc37-Cdk4 complex, pink is the kinase interacting loop (HPN motif), blue color are Hsp90 interacting residues as in the crystal structure and in yellow are residues implicated in additional interactions with bRaf kinase by NMR. Y4, S13 and Y298 are known to be phosphorylated and are marked in red. (B) Interactions between Cdc37 and Cdk4 in the context of Hsp90. Only the Cdc37 N terminal domain is depicted for clarity (piper) interacting with the Cdk4 αE helix (pink helix within blue surface) with the loop harboring HPN motif on Cdc37 (pink, zoomed in). Kinase (omitting residues 1-85) depicted as blue transparent surface, threads β4- β5 strands through the lumen of Hsp90 with Hsp90 clamping around them at its middle domain-C terminal domain junction.
Studies of Cdc37:bRaf interactions by NMR are largely consistent with what is seen in the cryoEM structure. However, in addition to a very N terminal interaction, a number of residues in a hydrophobic patch at the very C terminal helical region of Cdc37 have altered chemical environments upon bRaf binding (Fig 2A). Speculatively, this region may provide an αC like helix for the N lobe to interact with, further stabilizing the domain separated kinase. In the cryoEM structure this region of Cdc37 is unresolved, indicating a high degree of flexibility in the quaternary complex. Mutating residues in this hydrophobic patch in human cell lines abolishes binding of Cdc37 to client kinases. This is interesting as there are previous reports of the Cdc37 C terminal domain being dispensable in yeast (28). It may be the case that a mutated C terminal region plays a dominant negative role, or there may be differences between the yeast and human systems.
Both cryoEM and NMR identify the remainder of the Cdc37 N terminal domain as an antiparallel coiled coil, consisting of about 60 residues. It is unclear why such a long coiled coil would be required to make interactions with the kinase clients. As the NMR studies were done without Hsp90, quaternary interactions are not required to stabilize the coiled coil. One possibility is that during Hsp90's cycle, Cdc37 needs to move around while still being attached to both Hsp90 and the kinase, and the long coiled coil allows this. Alternatively, this may be a docking site for a binding partner, as the coiled coils in ClpB serve as a binding site for Hsp70(29).
The Hsp90 kinase binding site observed by cryoEM is the same as the client binding site mapped on the bacterial Hsp90, showing a high degree of functional and structural conservation(30, 31). The mitochondrial Hsp90 client binding region, between the middle and C-terminal domains, has been shown to be asymmetric (32) and current work indicates a correlation between asymmetry at this interface and a differential hydrolysis rate of the two monomers. This suggests an exciting connection between client binding, establishment of asymmetry and asymmetric hydrolysis(33). It should be noted however that in the cryoEM structure of the complex, Hsp90 closely resembles a symmetric state. This may be a consequence of trapping by molybdate, or asymmetric states could be important precursors to the trapped state.
Cdc37 interactions with Hsp90
The first Hsp90-Cdc37 interaction interface was revealed in 2004 (34) via the crystal structure of a fragment of human Cdc37 containing the middle and C terminal domains (residues 148-348; Figure 2A) interacting with the N terminal domain (NTD) of yeast Hsp90. That structure is consistent with prior fragment binding assays and also explains how Cdc37 can inhibit Hsp90's ATPase. Cdc37 interactions displace the catalytic water, prevent the region called the “lid” on Hsp90 NTD from coming over the bound ATP, and would also prevent dimerization of the Hsp90 NTDs, thought to be required for ATP hydrolysis (Fig 3). Although similar interactions have been observed by NMR (35), work by Eckl and colleagues on C.elegans homologues of Hsp90 and Cdc37 demonstrated that Cdc37 binds the middle domain of Hsp90, implying that Cdc37 may bind at multiple sites (36).
Figure 3. Conformational rearrangements of Cdc37 during the Hsp90 cycle.
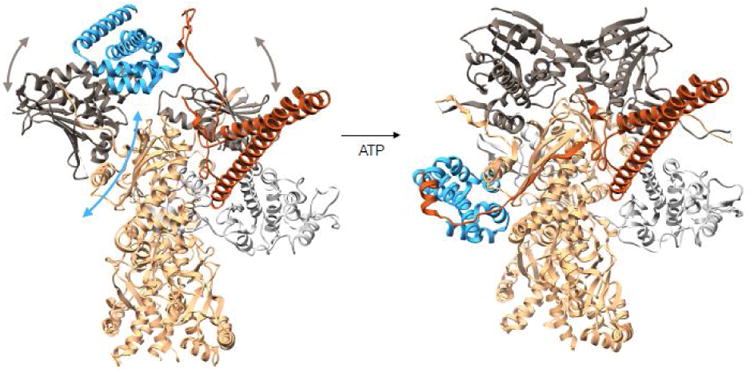
On the left is a modeled complex between Hsp90-Cdc37-Cdk4 where Cdc37-Hsp90 interactions are preserved as in the crystal structure of the fragments (PDB:1US7). The Cdc37 middle domain is colored in blue with the N terminal domain in piper, Hsp90 is in tan with N terminal domains in charcoal and Cdk4 kinase is in light gray. In this state Hsp90's N terminal domains are parted, and the domain of the monomer on the left had to undergo a rotation as to avoid clash between Hsp90's middle domain and Cdc37's middle domain. At this state, we imagine the kinase lobes to be partially open. Upon ATP binding to the Hsp90 N terminal domains, these undergo closure displacing Cdc37 down to the middle domain and stabilizing the kinase in the unfolded state, resulting in the observed cryoEM structure (domain motions are depicted with arrows). Structure on the left corresponds to state (b) in Figure 4 and structure on the right corresponds to state (c) in Figure 4.
In the cryoEM structure of Hsp90-Cdc37-Cdk4, although all the proteins are human homologues, Cdc37's middle domain is bound to the middle domain of Hsp90, a radically different interaction than the crystal structure, but consistent with the C.elegans work (Fig 3). Cdc37 contributes a strand to a β-sheet at a previously unappreciated interaction site on Hsp90's middle domain, filling a groove. The new β -strand connects Cdc37's globular middle domain with the coiled coil of the N terminal domain. The Hsp90 NTDs are dimerized, completely blocking the Cdc37 binding mode seen in the crystal structure. Five very N terminal residues of Cdc37 are making interactions with the closed Hsp90 NTDs, further stabilizing the closed state (Fig 2B). Finally, there is an extensive patch of ionic interactions between the Cdc37 N terminal region and Hsp90 middle domain, consistent with the reported salt dependence of Cdc37-Hsp90 interactions.
We propose that Cdc37 likely binds Hsp90 in two distinct modes, using two different interfaces on each molecule: one on the Hsp90 NTD, as in the crystal structure, and one on the middle domain, as seen in the cryoEM structure. We further suggest that transition between these modes is an intrinsic part of the catalytic cycle (Fig 4). Thus, the preferential interaction observed with the C. elegans Hsp90 middle domain would be a consequence of a different conformational equilibrium of C.elegans Hsp90 rather than an idiosyncratic feature of C.elegans Hsp90-Cdc37 interactions. Molecular details of this transition however are unclear, indicating a clear need to capture an Hsp90:Cdc37:kinase complex in an open Hsp90 state (perhaps analogous to that observed with Hsp90:Hsp70:and GR).
Figure 4. Hsp90-kinase cycle.
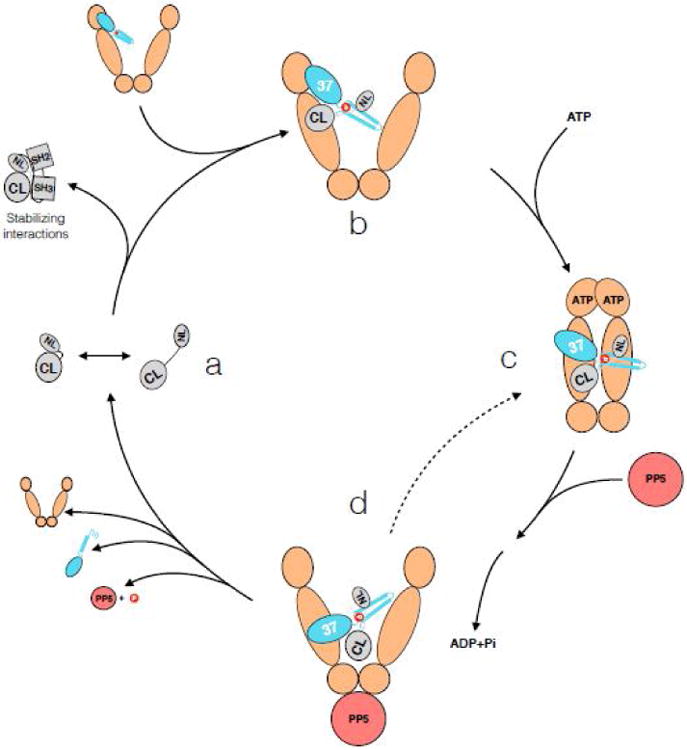
Kinases sample partially open states (CL- C-lobe, NL – N-lobe) (state a). If the kinase spends too much time open at the lobes, for example during early folded state or with a constitutive client, Cdc37 wedges in between the two kinase lobes with its N terminal domain, further unfolding the kinase (state b). Based on NMR data, the C terminus of Cdc37 also makes some kinase interactions at this point. Cdc37 interacts with the N terminal domain of an open Hsp90, utilizing the contacts as in the crystal structure (modeled in Figure 3). Upon binding ATP, Hsp90 undergoes closure, clamping around the unfolded kinase β4-β5 strands, and Cdc37 migrates to the middle domain (state c). Upon ATP hydrolysis by Hsp90 (potentially sequential, with an asymmetric state in between), Hsp90 opens, giving a chance for the kinase to fold (state d). If this was an initial folding interaction for a “non client”, or there is a stabilizing binding partner present nearby for the constitutive client, the stabilized and folded N lobe outcompetes Cdc37 for the C lobe, dissociating the kinase from the complex. However, if there are no stabilizing interactions, Hsp90 may re-bind ATP and enter another cycle (dashed line). This way, the kinase is safely held in a partially folded inactive state, always ready to interact with an appropriate binding partner, checking for its presence with the frequency of Hsp90's ATPase. Dynamic phosphorylation and dephosphorylation by Yes and CKII kinases, and PP5 phosphatase, add a layer of regulation.
Regulation of Hsp90/Cdc37/kinase interactions
Both Hsp90 and Cdc37 are intricately regulated by phosphorylation. Cdc37 can be phosphorylated on Tyr 4 and Tyr 298 by the Yes kinase (37) and on Ser 13 by CKII kinase (38). Notably, both of these kinases are also Hsp90 clients. While the effects of Tyr 4 phosphorylation are unclear, it was shown that Tyr 298 phospho-mimics lead to impaired Cdc37-kinase binding. This site is in the Cdc37 C terminal domain, corroborating recent NMR results of this domain's involvement in some step of kinase binding.
The best understood Cdc37 phosphorylation site is Ser 13, which is predominantly in the phosphorylated state in cells. Phospho-mimic or phospho-null mutations fail to produce functioning vSrc in yeast, potentially indicating that this regulatory mark has to change for the Hsp90 cycle to progress (Fig 4) (39). In the Hsp90-Cdc37-Cdk4 cryoEM structure, Cdc37 Ser 13 is clearly phosphorylated and this phosphorylation stabilizes a kinase interacting conformation of the Cdc37 N terminus via two salt bridges as well as an interaction with Hsp90. This multiplicity of ionic interactions may help explain the inability of a phospho mimetic to recapitulate the necessary interactions. This is consistent with bRaf having a slower in vitro off rate from Hsp90-Cdc37 in the phosphorylated Ser 13 state (8). Importantly however, NMR data showed no differential patterns of interaction between Cdc37 and bRaf due to Ser13 phosphorylation. This suggests that the effect is most pronounced in context with Hsp90, and/or that the in vitro system is probing the interaction in a different Hsp90 state.
Although general phosphatases can dephosphorylate Cdc37 alone or in the presence of a kinase, in the context of a quaternary complex with Hsp90 even overnight incubations with phosphatase yield no dephosphorylation. However, PP5 phosphatase has the unique ability to rapidly dephosphorylate Cdc37 even in the context of the whole complex. This is due to a TPR domain on PP5, which interacts with Hsp90 via its C terminal MEEVD motif (Fig 4). A recent crystal structure of PP5 phosphatase with a Cdc37 peptide in its active site identified a number of mutations that render PP5 catalytically dead. The authors show that such mutations cause client kinases to stay bound with the Hsp90/Cdc37/PP5 complex, indicating that Ser 13 dephosphorylation is likely important for kinase release(40). This may also have influenced the failure of Ser13 phosphomimetics. Based on the Hsp90-Cdc37-Cdk4 structure, it is clear that the N terminus of Cdc37 would have to undergo a conformational change to be accessible for dephosphorylation, however, the nature of any such change is unknown.
What are the functional consequences of kinase - Hsp90/Cdc37 interactions?
As discussed previously, the canonical interpretation of the Hsp90/Cdc37 system is that it helps fold, stabilize and activate its kinase clients. However, clear-cut evidence for activation decoupled from simple stabilization is missing. In a number of reports on ErbB2 (41, 42) and Ire1 (43), where authors carefully and promptly monitored signaling activity in response to an Hsp90 inhibitor, the kinase was actually initially activated, followed by a later degradation. Also, signaling-driven kinase activation leads to Hsp90 dissociation (as happens with GR). Taken together with the Hsp90-Cdc37-Cdk4 structure, in which the kinase is clearly inactive, this suggests a constant cycle of Hsp90/Cdc37 interactions with the most populated state in the absence of a signal being the highly repressed state observed in the cryoEM structure. Upon dissociation, clients can be activated, but if Hsp90 is inhibited, ultimately the clients become ubiquitinated and are degraded. Interestingly, studies with GR suggest that Hsp90 inhibition stabilizes a quaternary Hsp90-Hsp70-Hop-GR complex (27), which may then be on pathway to degradation.
In retrospect, it seems reasonable why a strong suppression of basal activity for highly proliferative or oncogenic kinases might be beneficial. Much less clear is why this is being done via a partially unfolded state and chaperone interactions rather than a more canonical “αC helix out” inactive state (Fig1A). One possibility is that such kinases only recently acquired their function. For example, there is ample data from the Lindquist lab showing that Hsp90 is able to buffer structurally destabilizing mutations associated with new functions arising during evolution, and perhaps the constitutively addicted kinases are examples of such clients(44, 45). However, there are constitutive kinase clients as far back as fungi, raising the question of why these kinases did not evolve away from a potentially burdensome chaperone-dependent state over the millions of years.
An exciting, albeit speculative, possibility is that for client kinases, a significantly destabilized, hence partially unfolded, native state has been evolutionarily selected for its unique functional advantages. This could come in the form of kinetic acceleration by reducing energy barriers required to undergo key conformational changes (46) (Fig 5), or through unappreciated benefits of interacting with the cellular chaperone machinery. As the active kinase depends on the precise arrangement of “spines” running through the kinase domain(47), a partially unfolded native state would necessarily be inactive. Stabilizing such a state by the chaperone machinery could strongly suppress basal activity and provide an entirely orthogonal inactivation pathway, adding an extra layer of protection from cancer as discussed above.
Figure 5. Imagined kinase folding free energy landscape.
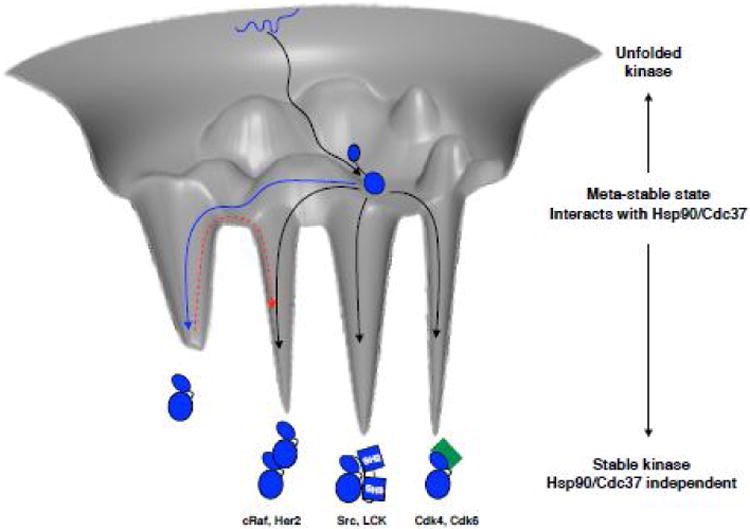
Kinase starts out in an unfolded state (depicted as blue squiggly line at the top of the folding funnel), and likely with the help of general chaperone machinery reaches a partially folded state, perhaps with a rather well folded C-lobe (black arrow to the middle of the folding funnel). From this ensemble of metastable states the kinase interacts with Hsp90-Cdc37. Non-client kinases would then readily access a distinct, well-folded native state and quickly escape the metastable ensemble (blue arrow). If the kinase is a constitutive client, it spends most of its time in the ensemble of partially folded states, without access to a stable state by itself and needs an interacting partner to be stabilized, be it cyclin (green), SH2-SH3 domains or even other kinases (black arrows into deep energy wells). Examples of client kinases representing each mode of stabilization are given below the cartoons. Non-client kinases would have to pay a considerable energy penalty to transition out of the energetically deep native state into an effector bound state (red dashed arrow). Hsp90 client kinases however, being in a structurally uncommitted state are always ready to interact with effectors, foregoing the need to pay the energy penalty. Importantly, Hsp90-Cdc37 may also catalyze the transitions between interactions with different effectors.
Some of the most important kinase family members integrate a broad array of intracellular and extracellular inputs - producing a proliferative signal if and only if the conditions are right. Inputs can be phosphorylation marks or binding interactions. The structurally uncommitted/partially unfolded state could provide a convenient platform for adding/removing such PTMs, all the while chaperone interactions provide protection from degradation. Moreover, as the chaperone-bound state would disable other kinase interactions, requiring a dependence on chaperones would allow transient resetting of interactions with binding partners to ensure that the kinase activating signals persist, as well as closely coupling growth decisions to cellular stress.
Concluding remarks
Advancements in two different areas have allowed for rapid progress in the structural understanding of Hsp90/Cdc37/kinase interactions. First, construction of a solubilized mutant of bRaf kinase that is marginally stable but still purifiable from E.coli has allowed rigorous in vitro biochemical and NMR investigations of Cdc37-kinase interactions. Second, advancements in cryoEM have allowed for direct structural investigation of Hsp90-Cdc37-kinase complexes isolated intact from eukaryotic cells. These advances have for the first time permitted the visualization of how the three proteins come together statically and in solution, providing a foundation for developing unifying mechanistic models and guiding further experimentation with the goal of understanding chaperone-mediated kinase regulation in health and disease.
These results also raise fundamental questions about nature of the “native” state, protein folding and chaperones. Traditionally, one tends to think of proteins as being confined to a distinct, well defined native state(s), and the chaperone system as only being used to aid initial folding or recovery from denaturing stresses. However, studies with Hsp90 are requiring that both of these views be significantly broadened. That is, the equilibrium between native and partially folded states may have been exploited evolutionarily by co-opting the chaperone systems to provide an additional layer of regulation or coordination with the state of the cell. More specifically, client kinases (i.e., the majority of kinases in humans) should be thought of as being intermediate between well-folded and intrinsically disordered proteins, spending much of their life in a malleable, partially unfolded state, ready to respond to the right signal. For these proteins folding is not a narrow funnel or a one-way road, but a more fully explorable and sophisticated energy landscape. Chaperones would therefore not be limited to facilitating the early, once in a lifetime folding events and dealing with non-functional aggregates, but would also carefully guide kinases throughout their function, stabilizing partially folded states and protecting them from degradation. Chaperones would also facilitate the equilibrium between these partially folded states and tightly bound complexes. The current data suggest that such behavior is not unique to kinases, but likely applies to other signaling families, like steroid receptors. Reaching a fundamental understanding of such partially unfolded states in light of evolution, although challenging, will lead to a deeper conceptual understanding of structure-function relationships, and evolutionary constraints.
Trends.
- Recent biochemistry, cryoEM and NMR experiments provide an unprecedented level of detail in understanding Hsp90-Cdc37-kinase interactions.
- Cdc37 plays a key role in bringing kinase clients to Hsp90.
- Hsp90 kinase clients continuously visit a partially unfolded state even long after folding.
- Cdc37 mimics part of the kinase, further unfolding and stabilizing the partially unfolded state.
Outstanding questions.
- What are the structures of the initial Hsp90-Cdc37-kinase complex and post-hydrolysis Hsp90-Cdc37-kinase complex?
- How does the hand off to Hsp70 occur and how is it connected to kinase degradation?
- Is an asymmetric Hsp90 state important for kinase processing?
- What are the functions of Cdc37's C terminal domain?
- What are structural effects of known phosphorylation sites?
- Is there a re-arrangement of Cdc37 on Hsp90 and how does it occur?
- How do other co-chaperones affect the Hsp90-kinase interactions?
- Are there new functionalities/benefits acquired by kinase clients due to their dependence of Hsp90/Cdc37 and meta-stable native state?
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Taylor SS, Keshwani MM, Steichen JM, Kornev AP. Evolution of the eukaryotic protein kinases as dynamic molecular switches. Philos Trans R Soc Lond B Biol Sci. 2012;367:2517–2528. doi: 10.1098/rstb.2012.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Endicott JA, Noble ME, Johnson LN. The structural basis for control of eukaryotic protein kinases. Annu Rev Biochem. 2012;81:587–613. doi: 10.1146/annurev-biochem-052410-090317. [DOI] [PubMed] [Google Scholar]
- 3.Zhang J, Yang PL, Gray NS. Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer. 2009;9:28–39. doi: 10.1038/nrc2559. [DOI] [PubMed] [Google Scholar]
- 4.Brugge J, Yonemoto W, Darrow D. Interaction between the Rous sarcoma virus transforming protein and two cellular phosphoproteins: analysis of the turnover and distribution of this complex. Mol Cell Biol. 1983;3:9–19. doi: 10.1128/mcb.3.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gierasch LM, Horwich A, Slingsby C, Wickner S, Agard D. Series in structural biology Vol 6. World Scientific; New Jersey: 2016. 1 online resource (vii, 319 pages) [Google Scholar]
- 6.Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11:515–528. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- 7.Cheng Y. Single-Particle Cryo-EM at Crystallographic Resolution. Cell. 2015;161:450–457. doi: 10.1016/j.cell.2015.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polier S, et al. ATP-competitive inhibitors block protein kinase recruitment to the Hsp90-Cdc37 system. Nat Chem Biol. 2013;9:307–312. doi: 10.1038/nchembio.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu W, et al. Sensitivity of mature Erbb2 to geldanamycin is conferred by its kinase domain and is mediated by the chaperone protein Hsp90. J Biol Chem. 2001;276:3702–3708. doi: 10.1074/jbc.M006864200. [DOI] [PubMed] [Google Scholar]
- 10.Gerbin CS, Landgraf R. Geldanamycin selectively targets the nascent form of ERBB3 for degradation. Cell Stress Chaperones. 2010;15:529–544. doi: 10.1007/s12192-009-0166-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcu MG, et al. Heat shock protein 90 modulates the unfolded protein response by stabilizing IRE1alpha. Mol Cell Biol. 2002;22:8506–8513. doi: 10.1128/MCB.22.24.8506-8513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bijlmakers MJ, Marsh M. Hsp90 is essential for the synthesis and subsequent membrane association, but not the maintenance, of the Src-kinase p56(lck) Mol Biol Cell. 2000;11:1585–1595. doi: 10.1091/mbc.11.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arlander SJ, et al. Chaperoning checkpoint kinase 1 (Chk1), an Hsp90 client, with purified chaperones. J Biol Chem. 2006;281:2989–2998. doi: 10.1074/jbc.M508687200. [DOI] [PubMed] [Google Scholar]
- 14.Chavany C, et al. p185erbB2 binds to GRP94 in vivo. Dissociation of the p185erbB2/GRP94 heterocomplex by benzoquinone ansamycins precedes depletion of p185erbB2. J Biol Chem. 1996;271:4974–4977. doi: 10.1074/jbc.271.9.4974. [DOI] [PubMed] [Google Scholar]
- 15.Xu W, et al. Surface charge and hydrophobicity determine ErbB2 binding to the Hsp90 chaperone complex. Nat Struct Mol Biol. 2005;12:120–126. doi: 10.1038/nsmb885. [DOI] [PubMed] [Google Scholar]
- 16.Xu Y, Lindquist S. Heat-shock protein hsp90 governs the activity of pp60v-src kinase. Proc Natl Acad Sci U S A. 1993;90:7074–7078. doi: 10.1073/pnas.90.15.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamphere L, et al. Interaction between Cdc37 and Cdk4 in human cells. Oncogene. 1997;14:1999–2004. doi: 10.1038/sj.onc.1201036. [DOI] [PubMed] [Google Scholar]
- 18.Grammatikakis N, Lin JH, Grammatikakis A, Tsichlis PN, Cochran BH. p50(cdc37) acting in concert with Hsp90 is required for Raf-1 function. Mol Cell Biol. 1999;19:1661–1672. doi: 10.1128/mcb.19.3.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taipale M, et al. Quantitative analysis of HSP90-client interactions reveals principles of substrate recognition. Cell. 2012;150:987–1001. doi: 10.1016/j.cell.2012.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boczek EE, et al. Conformational processing of oncogenic v-Src kinase by the molecular chaperone Hsp90. Proc Natl Acad Sci U S A. 2015;112:E3189–3198. doi: 10.1073/pnas.1424342112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taipale M, et al. Chaperones as thermodynamic sensors of drug-target interactions reveal kinase inhibitor specificities in living cells. Nat Biotechnol. 2013;31:630–637. doi: 10.1038/nbt.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keramisanou D, et al. Molecular Mechanism of Protein Kinase Recognition and Sorting by the Hsp90 Kinome-Specific Cochaperone Cdc37. Mol Cell. 2016;62:260–271. doi: 10.1016/j.molcel.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verba KA, et al. Atomic structure of Hsp90-Cdc37-Cdk4 reveals that Hsp90 traps and stabilizes an unfolded kinase. Science. 2016;352:1542–1547. doi: 10.1126/science.aaf5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shan Y, Arkhipov A, Kim ET, Pan AC, Shaw DE. Transitions to catalytically inactive conformations in EGFR kinase. Proc Natl Acad Sci U S A. 2013;110:7270–7275. doi: 10.1073/pnas.1220843110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho BK, Agard DA. Probing the flexibility of large conformational changes in protein structures through local perturbations. PLoS Comput Biol. 2009;5:e1000343. doi: 10.1371/journal.pcbi.1000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shao J, Irwin A, Hartson SD, Matts RL. Functional dissection of cdc37: characterization of domain structure and amino acid residues critical for protein kinase binding. Biochemistry. 2003;42:12577–12588. doi: 10.1021/bi035138j. [DOI] [PubMed] [Google Scholar]
- 27.Kirschke E, Goswami D, Southworth D, Griffin PR, Agard DA. Glucocorticoid receptor function regulated by coordinated action of the Hsp90 and Hsp70 chaperone cycles. Cell. 2014;157:1685–1697. doi: 10.1016/j.cell.2014.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turnbull EL, Martin IV, Fantes PA. Cdc37 maintains cellular viability in Schizosaccharomyces pombe independently of interactions with heat-shock protein 90. FEBS J. 2005;272:4129–4140. doi: 10.1111/j.1742-4658.2005.04825.x. [DOI] [PubMed] [Google Scholar]
- 29.Carroni M, et al. Head-to-tail interactions of the coiled-coil domains regulate ClpB activity and cooperation with Hsp70 in protein disaggregation. Elife. 2014;3:e02481. doi: 10.7554/eLife.02481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Street TO, et al. Cross-monomer substrate contacts reposition the Hsp90 N-terminal domain and prime the chaperone activity. J Mol Biol. 2012;415:3–15. doi: 10.1016/j.jmb.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Genest O, et al. Uncovering a region of heat shock protein 90 important for client binding in E. coli and chaperone function in yeast. Mol Cell. 2013;49:464–473. doi: 10.1016/j.molcel.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lavery LA, et al. Structural asymmetry in the closed state of mitochondrial Hsp90 (TRAP1) supports a two-step ATP hydrolysis mechanism. Mol Cell. 2014;53:330–343. doi: 10.1016/j.molcel.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elnatan Daniel, Betegon Miguel, Liu Yanxin, Ramelot Theresa, Kennedy Michael A, Agard David. Symmetry broken and rebroken during the ATP hydrolysis cycle of the mitochondrial Hsp90 TRAP1. bioRxiv. doi: 10.1101/107094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roe SM, et al. The Mechanism of Hsp90 regulation by the protein kinase-specific cochaperone p50(cdc37) Cell. 2004;116:87–98. doi: 10.1016/s0092-8674(03)01027-4. [DOI] [PubMed] [Google Scholar]
- 35.Sreeramulu S, et al. The human Cdc37.Hsp90 complex studied by heteronuclear NMR spectroscopy. J Biol Chem. 2009;284:3885–3896. doi: 10.1074/jbc.M806715200. [DOI] [PubMed] [Google Scholar]
- 36.Eckl JM, et al. Cdc37 (cell division cycle 37) restricts Hsp90 (heat shock protein 90) motility by interaction with N-terminal and middle domain binding sites. J Biol Chem. 2013;288:16032–16042. doi: 10.1074/jbc.M112.439257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu W, et al. Dynamic tyrosine phosphorylation modulates cycling of the HSP90-P50(CDC37)-AHA1 chaperone machine. Mol Cell. 2012;47:434–443. doi: 10.1016/j.molcel.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyata Y. Protein kinase CK2 in health and disease: CK2: the kinase controlling the Hsp90 chaperone machinery. Cell Mol Life Sci. 2009;66:1840–1849. doi: 10.1007/s00018-009-9152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaughan CK, et al. Hsp90-dependent activation of protein kinases is regulated by chaperone-targeted dephosphorylation of Cdc37. Mol Cell. 2008;31:886–895. doi: 10.1016/j.molcel.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oberoi J, et al. Structural and functional basis of protein phosphatase 5 substrate specificity. Proc Natl Acad Sci U S A. 2016;113:9009–9014. doi: 10.1073/pnas.1603059113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu W, Yuan X, Beebe K, Xiang Z, Neckers L. Loss of Hsp90 association up-regulates Src-dependent ErbB2 activity. Mol Cell Biol. 2007;27:220–228. doi: 10.1128/MCB.00899-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Citri A, et al. Hsp90 restrains ErbB-2/HER2 signalling by limiting heterodimer formation. EMBO Rep. 2004;5:1165–1170. doi: 10.1038/sj.embor.7400300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ota A, Wang Y. Cdc37/Hsp90 protein-mediated regulation of IRE1alpha protein activity in endoplasmic reticulum stress response and insulin synthesis in INS-1 cells. J Biol Chem. 2012;287:6266–6274. doi: 10.1074/jbc.M111.331264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lachowiec J, Lemus T, Borenstein E, Queitsch C. Hsp90 promotes kinase evolution. Mol Biol Evol. 2015;32:91–99. doi: 10.1093/molbev/msu270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Queitsch C, Sangster TA, Lindquist S. Hsp90 as a capacitor of phenotypic variation. Nature. 2002;417:618–624. doi: 10.1038/nature749. [DOI] [PubMed] [Google Scholar]
- 46.Miyashita O, Onuchic JN, Wolynes PG. Nonlinear elasticity, proteinquakes, and the energy landscapes of functional transitions in proteins. Proc Natl Acad Sci U S A. 2003;100:12570–12575. doi: 10.1073/pnas.2135471100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kornev AP, Taylor SS. Dynamics-Driven Allostery in Protein Kinases. Trends Biochem Sci. 2015;40:628–647. doi: 10.1016/j.tibs.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


