Abstract
Chondral lesions of the knee can affect the young, active population, thereby causing severe morbidity and a large economic burden. Although numerous approaches have been described in the literature, restoration of hyaline cartilage has yet to be shown. Specifically, larger, full-thickness cartilage defects remain a challenge. This Technical Note details our technique for biologic unicompartmental osteochondral allograft transplantation for the treatment of large femoral condyle articular cartilage defects.
Large chondral defects constitute a challenging disease, particularly in young, high-demand patients. Because of the avascular nature of articular cartilage, its potential for self-healing is limited and it possesses an increased propensity to progress to osteoarthritis.1, 2, 3 Based on its depth, a chondral defect can be classified as: (1) partial thickness, when confined to articular cartilage, or (2) osteochondral, or full thickness, when the defect is deep enough to affect the subchondral bone. Although no repair takes place for chondral defects, osteochondral defects, on account of the subchondral blood supply, are more amenable to attempted repair through fibrocartilage deposition.4
Among the numerous surgical options for cartilage repair, abrasion arthroplasty, subchondral drilling, and microfracture all focus on the principle of marrow stimulation to promote healing. However, marrow stimulation procedures create a hyaline-like fibrocartilage that is physiologically and mechanically inferior to hyaline cartilage.5 Moreover, they are not recommended for the treatment of large osteoarticular lesions.2, 6 Although autogenous osteochondral transfer can be used to repair cartilage defects, it is best suited for defects measuring less than 2.5 cm2 due to donor site size limitations and morbidity.7 Osteochondral allograft transplantation can be used to repair large, solitary defects through the use of fully mature articular cartilage during a single operation, while avoiding donor site morbidity.3 This Technical Note details our technique for biologic unicompartmental osteochondral allograft transplantation for the treatment of large femoral condyle articular cartilage defects.
Indications and Contraindications for Surgery
The main indication for unicompartmental osteochondral allograft transplantation is the presence of a symptomatic full-thickness articular cartilage defect of more than 3 cm2. Contraindications include an ipsilateral tibial cartilage lesion, ligamentous instability, malalignment, more than minor peripheral osteophytes, joint-space narrowing, or the absence of >50% of the meniscus in the affected compartment. Patients whose weight-bearing axis passes medial or lateral to the tibial eminences within the affected compartment should undergo a concurrent tibial osteotomy to realign the mechanical axis. Patients with >50% loss of the meniscus in the ipsilateral compartment should undergo a concurrent meniscal transplantation. Indications and contraindications are summarized in Table 1.
Table 1.
Indications and Contraindications
| Indications | Contraindications |
|---|---|
| Symptomatic full-thickness articular cartilage defect of >3 cm2 | Absolute |
| Localized grade IV unipolar lesions of the femoral condyle | “Kissing” lesion |
| Defects due to trauma, OCD, AVN, or intra-articular plateau fractures | More than minor peripheral osteophytes |
| Young, high-demand patients who are not candidates for joint replacement | Joint-space narrowing |
| Associated underlying bone defect | Rheumatoid or corticosteroid-induced osteonecrosis |
| Relative | |
| Ligamentous instability (address it first) | |
| Malalignment (address it first or concurrently) | |
| Absence of >50% of the meniscus in the affected compartment (consider meniscal transplantation) | |
| BMI >30 |
AVN, avascular necrosis; BMI, body mass index; OCD, osteochondritis dissecans.
Surgical Technique
Patient Positioning and Anesthesia
The patient is placed in the supine position on the operating table and general anesthesia is used for induction. A well-padded high-thigh tourniquet is subsequently placed on the operative leg and then a bump is placed under the knee so that it rests at approximately 30° of flexion. The contralateral leg is secured to the table in full extension with a pneumatic compression device to help prevent deep vein thrombosis. Pearls and pitfalls of this procedure are outlined in Table 2.
Table 2.
Pearls and Pitfalls
| Pearls | Pitfalls |
|---|---|
| Use the neutral, +1 mm, and −1 mm drill stops to fine-tune the graft fit | Damaging the anterior horn of the meniscus during the surgical approach |
| Use the bone graft to augment the recessed graft for a better fit | Failing to hand ream the recipient site to make minor depth adjustments |
| A guide pin or other device may be used to microfracture the recipient site to create a bleeding surface and maximize healing potential | Failing to irrigate the graft to remove antigenic material |
Objective Diagnosis
Preoperative evaluation should start with a thorough history and physical examination. Diagnostic imaging should consist of long-leg standing radiographs to assess mechanical alignment, as an osteotomy may be recommended in addition to the osteochondral allograft. Magnetic resonance imaging of the knee allows confirmation of the size and extent of the chondral lesion, as well as any concomitant ligamentous, meniscal, or other soft tissue injuries.
Operative Technique
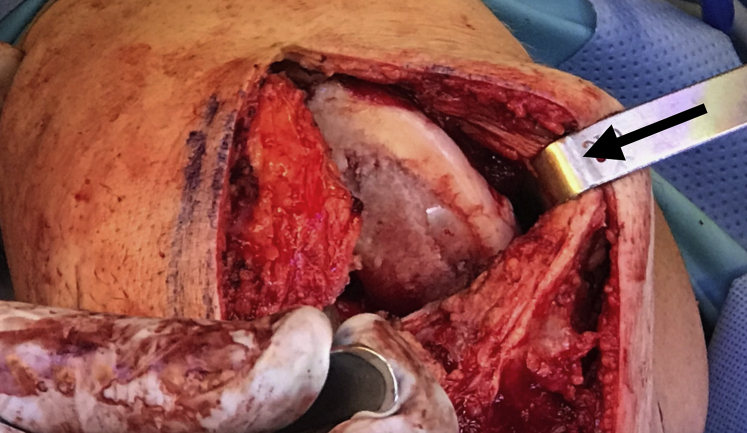
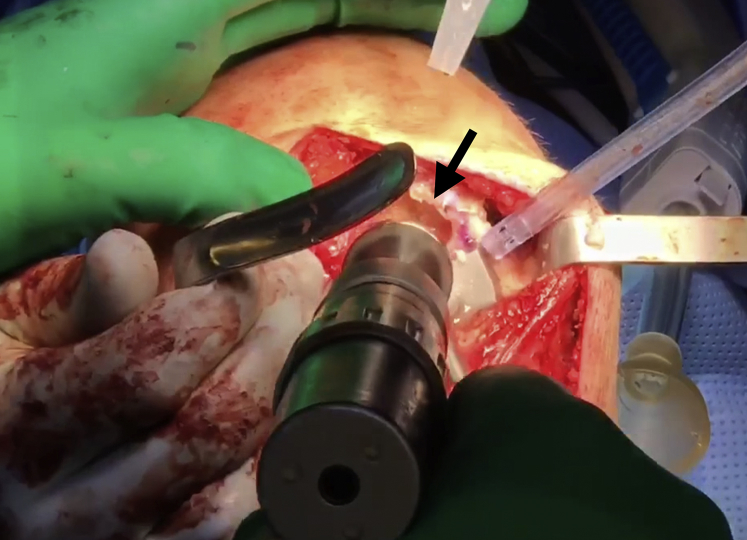
General endotracheal anesthesia may be combined with regional nerve blocks to maximize postoperative pain control. Perioperative antibiotic prophylaxis is administered intravenously before incision. A parapatellar approach (medial or lateral) is used to access the chondral lesion (Video 1). In this case, a medial parapatellar approach is employed with careful attention to avoid injury to the anterior horn of the medial meniscus. Z-retractors are placed medially and laterally to maximize exposure (Fig 1).
Fig 1.
A medial parapatellar approach is completed in the right knee with careful attention to avoid injury to the anterior horn of the medial meniscus. After careful exposure, Z-retractors (black arrow) are placed medially and laterally to fully expose the area of interest.
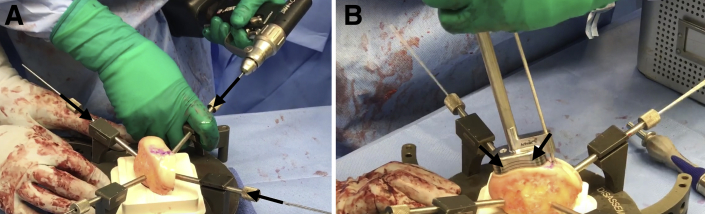
Next, the BioUni sizers are used to identify the most appropriate coverage of the lesion. While holding the selected sizer in position, an indelible marker is used to outline the sizer. In this case example, a large sizer (20 mm) is used. Care should be taken to ensure adequate native osteochondral shoulders both medially and laterally for secure graft fixation. The selected sizer used to establish the recipient defect site is then placed over the allograft condyle until the appropriate donor site has been identified. The sizer should be outlined with indelible ink while denoting the superior and inferior aspects of the donor graft. The condyle is mounted in the workstation with 2.8-mm guide pins (Fig 2A).
Fig 2.
(A) As the first step for graft preparation to treat the large femoral condyle articular cartilage defect of the right knee, the condyle allograft source is mounted on the workstation with four 2.8-mm guide pins (black arrows). (B) Once the oblong cutter has been placed flush over the allograft and a 2.8-mm guide pin has been drilled through the guide pin hole, a mallet is used to drive the oblong cutter into the graft until the third laser line is flush with the surrounding cartilage (black arrows).
Place the oblong cutter inserter into the oblong cutter and position it over the allograft until it aligns with the previously demarcated harvest site. Drill a 2.8-mm guide pin through the guide pin hole and advance it fully through the allograft. Screw the impactor handle onto the oblong cutter. Use a mallet to drive the oblong cutter into the graft until the third laser line is flush with the surrounding cartilage (Fig 2B). Insert the distractor tool into the driver handle and insert it into the oblong cutter. Remove the 2.8-mm pin and advance the distractor to remove the oblong cutter.
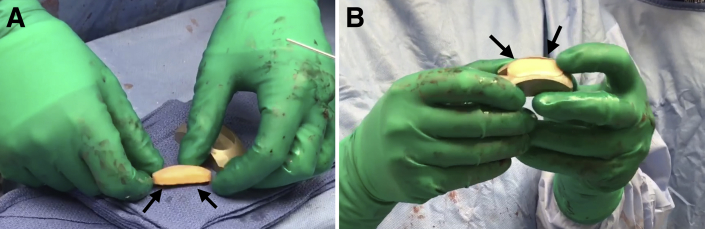
Assemble the saw depth guide over the sagittal saw guide and screw on the impactor handle. Place the assembly into the previously made cut and impact into place. Using a sagittal saw, advance the blade through the sagittal saw guide until it advances through the condyle to create the base of the graft. Remove the impactor handle and sagittal saw attachments. The donor graft will be contained within the sagittal saw depth guide. Insert the distraction tool to slowly extract the allograft implant (Fig 3A). Ensure that the marks denoting the superior and inferior aspects of the graft are visible and remark if necessary. Insert the implant into the appropriately sized donor trial to confirm sizing (Fig 3B). Thereafter, pulse lavage the graft to remove antigenic elements.
Fig 3.
(A) The distraction tool is used to slowly extract the allograft implant (black arrows) to be placed in the right knee from the condyle harvest site to be fixed in the right knee. (B) Once extracted, the allograft implant is inserted into the appropriately sized donor trial (black arrows) to confirm sizing.
Moving back to the chondral defect, place the sizer over the previously outlined recipient site while ensuring that the sizer is flush on all sides and covers the defect. Place two 4-mm drill pins into the drill holes (Fig 4A). Remove the sizer while leaving the drill pins in place (Fig 4B). Place the scoring device over the drill pins and impact it to create a cut in the cartilage approximately 2 to 3 mm deep. Place the appropriately sized drill depth guide over the bottom drill pin and advance it down to the cartilage. Place the appropriately sized reamer over the top drill pin and advance the reamer until it stops on the depth guide (Fig 5). Create a second circle by repeating this process with the opposite drill pins.
Fig 4.
Attention is then turned back to the condyle defect of the right knee. The sizer is again placed over the defect to ensure that it is flush on all sides and entirely covers the defect. (A) Then, two 4-mm drill pins are placed into the drill holes (red arrow). (B) The sizer is then removed, but the drill pins are left in place (black arrows).
Fig 5.
The corresponding drill depth guide is then placed over the bottom drill pin and advanced until it reaches the cartilage base of the right knee. After this, the appropriately sized reamer is drilled over the top drill pin and advanced until it stops at the depth guide (black arrow).
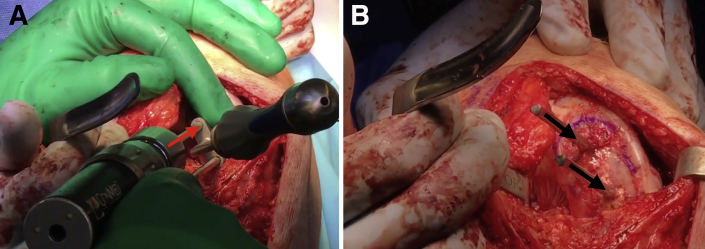
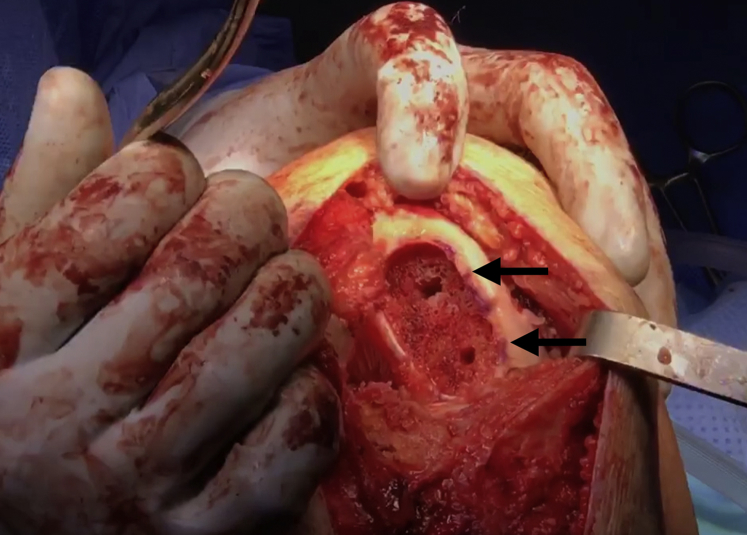
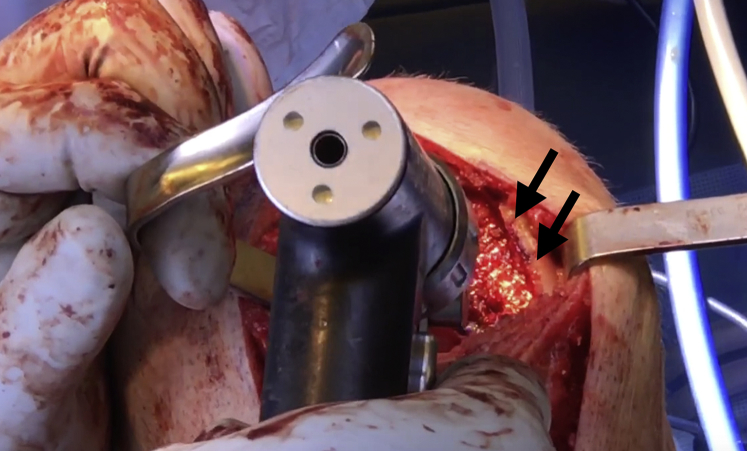
Advance the box cutter over the drill pins until the tabs on the box cutter are abutting the cancellous bone and will no longer advance. Remove the drill pins and remove any remaining cartilage and bone with a combination of curettes and rongeurs (Fig 6). Use a dilator to dilate the recipient site and confirm the fit. If the trial is proud, attach the reamer to a Jacob's chuck and ream by hand, taking care not to resect the cancellous bone too much. If the trial is recessed, autologous bone chips or demineralized bone matrix can be used to make minor adjustments. The recipient site may be microfractured with a 2.0-mm guide pin to prepare for graft implantation (Fig 7).
Fig 6.
Once the drill pins as well as any remaining cartilage and bone is removed with a combination of curettes and rongeurs, the recipient site of the right knee is nearly ready for allograft implantation (black arrows).
Fig 7.
Once the recipient site is free of drill pins as well as any excess cartilage and bone, the site of the right knee may be microfractured with a 2.0-mm guide pin (black arrows) as the final step of preparation before graft implantation.
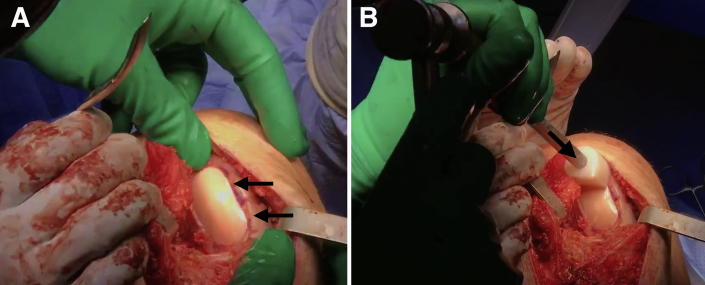
Place the graft into the recipient site by hand (Fig 8A). Gently impact the graft into place using a tamp and mallet (Fig 8B). Perform any additional procedures as indicated. Thoroughly irrigate the wound before closing the wound in a layered fashion.
Fig 8.
(A) The graft is first placed by hand (black arrows) into the recipient site of the right knee. (B) After this initial placement, a tamp and mallet (black arrow) are used to gently impact the graft securely into place.
Postoperative Rehabilitation
The patient should remain non-weight-bearing for the first 8 weeks. A supervised rehabilitation program should start immediately postoperatively. Quadriceps exercises and straight leg raises with the patient wearing a knee immobilizer should be performed 4 times daily. For the first 8 weeks, patients should use a continuous passive-motion machine at a minimum time interval of 2 hours, for a minimum of 10 hours per day. Low-impact activities are recommended for the first 12 months to allow complete healing and incorporation of the graft. All patients are advised to perform low-impact activities and to avoid high-impact activities as much as possible after this time period.
Discussion
This Technical Note details our biologic unicompartmental osteochondral allograft transplantation for the treatment of large articular cartilage defects of the femoral condyles. Early histologic analysis of osteochondral allograft transfers (OATs) has revealed high survivorship of the transferred hyaline cartilage.8 Clinical studies have shown OATs to produce a subjective improvement in pain in 74% to 85% of patients at mid-term follow-up.9, 10 Furthermore, a recent long-term follow-up study reported an osteochondral allograft survival rate of 85% at 10 years, with 74% survivorship at 15 years.9 Levy et al.10 evaluated the durability of OATs to the femoral condyles at 15-year follow-up and reported a survivorship of 82%. These encouraging graft survivorship numbers have been strengthened by outcomes studies. LaPrade et al.11 followed 23 patients for 3 years and showed improvements in Cincinnati Knee Scores and International Knee Documentation Committee scores from 52 to 68 (P < .03) after transplantation. In addition, the authors reported graft incorporation in 22 of their 23 patients (96%). McCulloch et al.12 also reported significant improvement in all subjective outcome scores in 88% (22 patients) of their patients. Given that younger patients with large chondral defects are indicated for OAT, return to activity or sport is an important consideration. Krych et al.13 reported rates of return to sport in 43 competitive athletes after allograft transfer with a mean 7.25 cm2 defect size. The authors reported that 79% of athletes could return to their preinjury level of sport and 88% of patients achieved a limited return to sport. Factors that were negatively correlated with return to sport were symptoms for more than 12 months and patients older than 25 years.
The treatment of osteochondral defects of the knee with allograft transfer offers several advantages over other surgical options. Because the allografts are acellular, they are immunologically inert, and are unlikely to be rejected by the native tissue.14 Furthermore, because allografts contain hyaline cartilage, they can be used to treat large defect without the donor site morbidity associated with autograft harvest.15
Although early laboratory and clinical studies have yielded promising results, additional long-term follow-up studies of OAT are needed. We recommend our described transplant technique, using the BioUni osteochondral allograft, for the treatment of osteochondral defects and encourage further studies to assess outcomes following our surgical technique.
Footnotes
The author reports the following potential conflicts of interest or sources of funding: M.T.P. receives consultancy fees from Arthrex and JRF Ortho; has patents issued (9226743, 20150164498, 20150150594, 20110040339); and receives royalties from Arthrex and SLACK Incorporated (publishing royalties). Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
The patient is placed in the supine position on the operating table and general anesthesia is used for induction. A well-padded high-thigh tourniquet is subsequently placed on the right operative leg and then a bump is placed under the knee so that it rests at approximately 30° of flexion. A parapatellar approach is first used for exposure of the chondral lesion. In this case, a medial parapatellar approach is employed with careful attention to avoid injury to the anterior horn of the medial meniscus. Then, Z-retractors are placed medially and laterally to fully expose the lesion. The BioUni sizers are then used to identify the necessary size of the allograft to be prepared. The outline of the sizer is then made on the recipient site of the native condyle with an indelible marker. In this case example, the size of the lesion is 20 mm total. The selected sizer is next placed over the harvest site of the condyle allograft source until the appropriate donor site has been identified. After this, the condyle allograft source is mounted in the workstation with four 2.8-mm guide pins. The oblong cutter is placed flush against the harvest site, and a mallet is used to drive the oblong cutter into the graft until the third laser line is flush with the surrounding cartilage. A sagittal saw guide along with a sagittal saw is then used to form the base of the graft. Afterward, the distraction tool allows for extraction of the allograft. The allograft is then placed in the appropriately sized donor trial to confirm sizing. After this, pulse lavage of the graft is completed to remove antigenic elements. Attention is then turned back to the recipient site, and the sizer is placed over the previously outlined recipient site while ensuring that the sizer is entirely flush. Two 4-mm drill pins are then drilled through the recipient site. The drill depth guide is then used in combination with a reamer to prepare the recipient site with the use of the 2 drill pins as guides for preparation of the site. A box cutter, dilator, curettes, and rongeurs are then used in combination for final preparation of the recipient site. After this, the site is microfractured with a 2.0-mm guide pin as the final step before graft implantation. The graft is first placed into the recipient site by hand, and then it is gently impacted into the site through use of a tamp and mallet. The wound is then thoroughly irrigated and closed in a standard layered fashion.
References
- 1.Chahla J., Dean C.S., Moatshe G., Pascual-Garrido C., Serra Cruz R., LaPrade R.F. Concentrated bone marrow aspirate for the treatment of chondral injuries and osteoarthritis of the knee: A systematic review of outcomes. Orthop J Sports Med. 2016;4 doi: 10.1177/2325967115625481. 2325967115625481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chahla J., LaPrade R.F., Mardones R. Biological therapies for cartilage lesions in the hip: A new horizon. Orthopedics. 2016;39:e715–e723. doi: 10.3928/01477447-20160623-01. [DOI] [PubMed] [Google Scholar]
- 3.Dean C.S., Chahla J., Serra Cruz R., LaPrade R.F. Fresh osteochondral allograft transplantation for treatment of articular cartilage defects of the knee. Arthrosc Tech. 2016;5:e157–e161. doi: 10.1016/j.eats.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frenkel S.R., Di Cesare P.E. Degradation and repair of articular cartilage. Front Biosci. 1999;4:D671–D685. doi: 10.2741/frenkel. [DOI] [PubMed] [Google Scholar]
- 5.Alford J.W., Cole B.J. Cartilage restoration, part 1: Basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33:295–306. doi: 10.1177/0363546504273510. [DOI] [PubMed] [Google Scholar]
- 6.Alford J.W., Cole B.J. Cartilage restoration, part 2: Techniques, outcomes, and future directions. Am J Sports Med. 2005;33:443–460. doi: 10.1177/0363546505274578. [DOI] [PubMed] [Google Scholar]
- 7.Chow J.C., Hantes M.E., Houle J.B., Zalavras C.G. Arthroscopic autogenous osteochondral transplantation for treating knee cartilage defects: A 2- to 5-year follow-up study. Arthroscopy. 2004;20:681–690. doi: 10.1016/j.arthro.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Hangody L., Kish G., Karpati Z., Szerb I., Udvarhelyi I. Arthroscopic autogenous osteochondral mosaicplasty for the treatment of femoral condylar articular defects. A preliminary report. Knee Surg Sports Traumatol Arthrosc. 1997;5:262–267. doi: 10.1007/s001670050061. [DOI] [PubMed] [Google Scholar]
- 9.Gross A.E., Kim W., Las Heras F., Backstein D., Safir O., Pritzker K.P.H. Fresh osteochondral allografts for posttraumatic knee defects: Long-term followup. Clin Orthop Relat Res. 2008;466:1863–1870. doi: 10.1007/s11999-008-0282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy Y.D., Görtz S., Pulido P.A., McCauley J.C., Bugbee W.D. Do fresh osteochondral allografts successfully treat femoral condyle lesions? Clin Orthop Relat Res. 2013;471:231–237. doi: 10.1007/s11999-012-2556-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LaPrade R.F., Botker J., Herzog M., Agel J. Refrigerated osteoarticular allografts to treat articular cartilage defects of the femoral condyles. A prospective outcomes study. J Bone Joint Surg Am. 2009;91:805–811. doi: 10.2106/JBJS.H.00703. [DOI] [PubMed] [Google Scholar]
- 12.McCulloch P.C., Kang R.W., Sobhy M.H., Hayden J.K., Cole B.J. Prospective evaluation of prolonged fresh osteochondral allograft transplantation of the femoral condyle: minimum 2-year follow-up. Am J Sports Med. 2007;35:411–420. doi: 10.1177/0363546506295178. [DOI] [PubMed] [Google Scholar]
- 13.Krych A.J., Pareek A., King A.H., Johnson N.R., Stuart M.J., Williams R.J., III Return to sport after the surgical management of articular cartilage lesions in the knee: A meta-analysis. Knee Surg Sports Traumatol Arthrosc. August 18, 2016 doi: 10.1007/s00167-016-4262-3. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 14.Arnoczky S.P. The biology of allograft incorporation. J Knee Surg. 2006;19:207–214. doi: 10.1055/s-0030-1248109. [DOI] [PubMed] [Google Scholar]
- 15.LaPrade R.F., Botker J.C. Donor-site morbidity after osteochondral autograft transfer procedures. Arthroscopy. 2004;20:e69–e73. doi: 10.1016/j.arthro.2004.06.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The patient is placed in the supine position on the operating table and general anesthesia is used for induction. A well-padded high-thigh tourniquet is subsequently placed on the right operative leg and then a bump is placed under the knee so that it rests at approximately 30° of flexion. A parapatellar approach is first used for exposure of the chondral lesion. In this case, a medial parapatellar approach is employed with careful attention to avoid injury to the anterior horn of the medial meniscus. Then, Z-retractors are placed medially and laterally to fully expose the lesion. The BioUni sizers are then used to identify the necessary size of the allograft to be prepared. The outline of the sizer is then made on the recipient site of the native condyle with an indelible marker. In this case example, the size of the lesion is 20 mm total. The selected sizer is next placed over the harvest site of the condyle allograft source until the appropriate donor site has been identified. After this, the condyle allograft source is mounted in the workstation with four 2.8-mm guide pins. The oblong cutter is placed flush against the harvest site, and a mallet is used to drive the oblong cutter into the graft until the third laser line is flush with the surrounding cartilage. A sagittal saw guide along with a sagittal saw is then used to form the base of the graft. Afterward, the distraction tool allows for extraction of the allograft. The allograft is then placed in the appropriately sized donor trial to confirm sizing. After this, pulse lavage of the graft is completed to remove antigenic elements. Attention is then turned back to the recipient site, and the sizer is placed over the previously outlined recipient site while ensuring that the sizer is entirely flush. Two 4-mm drill pins are then drilled through the recipient site. The drill depth guide is then used in combination with a reamer to prepare the recipient site with the use of the 2 drill pins as guides for preparation of the site. A box cutter, dilator, curettes, and rongeurs are then used in combination for final preparation of the recipient site. After this, the site is microfractured with a 2.0-mm guide pin as the final step before graft implantation. The graft is first placed into the recipient site by hand, and then it is gently impacted into the site through use of a tamp and mallet. The wound is then thoroughly irrigated and closed in a standard layered fashion.