To the Editor:
The increasing use of molecular diagnostics (e.g. MALDI-TOF) that facilitate speciation of coagulase negative staphylococci (CoNS), has led to enhanced detection of Staphylococcus lugdunensis as the causative agent of a variety of infections. A series of cases of infective endocarditis among patients with S. lugdunensis bacteraemia was recently reported in this journal by Non et al.1 In addition to other reports of endocarditis,2, 3, 4 this organism has also increasingly been recognized in association with invasive infections at other sites including those arising in an orthopaedic setting.5, 6 A recent retrospective study of prosthetic joint infections (PJI) in France found that S. lugdunensis may result in less favourable outcomes than other CoNS, with reported outcomes similar to S. aureus.7 The optimal management of S. lugdunensis orthopaedic infections is not established.
On this basis, we undertook a study to evaluate the contribution of S. lugdunensis to prosthetic orthopaedic infections, and to evaluate treatment and outcomes. We interrogated electronic records for patients treated under the care of Oxford trauma team and/or Bone Infection Unit, a specialist UK tertiary referral centre for bone and joint infection that handles approximately 200 revision arthroplasties each year. At this centre, patients are routinely started on broad-spectrum antimicrobials intraoperatively, after multiple samples are taken.8 Once identification and sensitivity testing are complete, antimicrobial therapy is adjusted according to susceptibility testing. Traditionally, most patients with confirmed deep bone and joint infection have been treated with six weeks of parenteral therapy before switching to oral antibiotics.
We identified cases of S. lugdunensis retrospectively, among patients age ≥18 years, by reviewing a 24 month period commencing April 2014 (when MALDI-TOF was first introduced into the diagnostic laboratory). We collated information pertaining to microbiology samples, surgical intervention, choice, route and duration of antibiotic therapy, and clinical outcomes. As a denominator group, we identified patients with S. aureus prosthetic hip and knee infections. Patient identifying information was removed before further analysis. As this was undertaken as a quality improvement project with no intervention, ethical approval was not required.
We identified 68 S. lugdunensis isolates from 20 unique patients (Suppl. data table 1). Median age was 69 years (IQR 40–86). Half of the patients were diagnosed with PJI, with knees outnumbering hips (n = 7 vs n = 3, respectively). Eight patients with S. lugdunensis were treated for infection of fracture fixation metalware and two for spinal metalware infection. A majority (17/20) of these patients had recorded surgical intervention; variable approaches were undertaken for PJI including debridement and implant retention (‘DAIR’), single stage revision and two stage revision. All of the fracture fixation devices were removed with excision of infected bone and reconstruction.
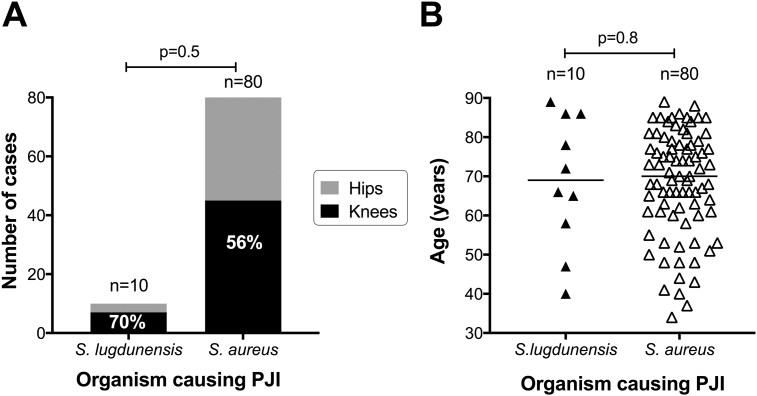
Over the same time frame, we identified 80 cases of knee and hip PJI caused by S. aureus (n = 45 vs n = 35, respectively; Suppl. data table 2). Although the distribution of S. lugdunensis between hip and knee PJI was not statistically different from that seen for S. aureus (p = 0.5, Fisher's Exact Test, Fig. 1A), an apparent enrichment of S. lugdunensis infections among prosthetic knees compared to hips is a feature that has been noted elsewhere.9 This is not as a result of a substantially greater number of knee than hip replacements (the UK National Joint Registry reports 796,636 hip and 871,472 knee replacements in England, Wales and Northern Ireland between April 2003 and December 201510). There was no difference in age between patients with S. lugdunensis vs S. aureus PJI (median 69 years vs 70 years, respectively, p = 0.8, Fig. 1B).
Figure 1.
S. lugdunensis and S. aureus as a cause of knee and hip prosthetic joint infections (PJI) in a UK cohort over two years from April 2014. A: Absolute case numbers and relative contributions of the two organisms to knee and hip PJI. Percentages shown on black bars indicate proportion of all cases that are associated with knee infection. P-value by Fisher's Exact Test. B: Age distribution of patients with S. lugdunensis and S. aureus. Horizontal lines indicate median. P-value by Mann–Whitney test.
None of the 68 S. lugdunensis isolates demonstrated resistance to glycopeptides, daptomycin, oxacillin, rifampicin, clindamycin or linezolid, but 14 isolates from 4 different patients were found to demonstrate in vitro resistance to at least one antistaphylococcal agent (co-trimoxazole, tetracycline, fucidic acid, gentamicin); Suppl. data table 3.
Details of antibiotic therapy are shown in Suppl. data table 1. Following the immediate postoperative period, nine patients continued with intravenous therapy for a median of six weeks (glycopeptide or ceftriaxone, at the discretion of the infectious diseases/microbiology consultant). A variety of oral treatment strategies were employed, with the most common being dual therapy with ciprofloxacin and rifampicin (n = 8). Therapy duration ranged from 6 to 21 weeks for 15 patients, whilst three underwent a strategy of longer term suppressive therapy on the grounds that infected metalware could not be removed or adequately debrided. We were unable to determine the antibiotic strategy retrospectively for two patients.
Follow up data were available for 16 patients (at median 118 days following operation/initiation of antimicrobials, range 43–583 days), of whom 15 (94%) had no reported evidence of ongoing or recurrent infection. One patient was found to have a minimally symptomatic persistent fracture non-union and a decision was made to continue with a suppressive strategy, without further surgery. Outcome data were unavailable for the remaining four patients, probably due to transfer out of area. There was no difference between infection outcomes for patients receiving intravenous followed by oral antimicrobial strategies, when compared to those treated with oral antimicrobials alone, although a substantially larger prospective study is required to determine whether oral therapy is non-inferior. The active approach to thorough surgical debridement in the majority of cases, together with the susceptibility data of S. lugdunensis is in keeping with the good clinical outcomes.
In conclusion, this small series confirms a niche for S. lugdunensis as a cause of orthopaedic infections involving prosthetic material. In knee and hip PJI in our centre, it accounts for cases at a ratio of 1:8 compared with S. aureus. Ascertainment of cases is likely to continue while molecular approaches to diagnostics become more widely adopted.
Optimum therapy has not been clearly defined, and we have demonstrated variation in antimicrobial prescribing practice. Given the small numbers of patients, it is not currently possible to advocate any particular management approach to S. lugdunensis prosthesis infections. However, in vitro data confirm an organism that is susceptible to conventional therapy for S. aureus, and outcomes appear to be good when patients are treated using surgical and medical management protocols equivalent to those employed for S. aureus. Further work is needed to establish with more confidence the optimum route, choice and duration of antimicrobial therapy in order to unify a treatment approach.
Authors' contributions
AL and PM conceived and designed the study. AL, NG, TP and PM collected and analysed the data. BA, AT and MM provided advice on analysis and manuscript drafting. AL and PM wrote the manuscript. All authors have seen and approved the final version.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
This study was not underpinned by any specific funding source. PM receives salary from the Wellcome Trust (grant number 110110), and was supported by a salary contribution from the NIHR during the data collection period. Some of these data were presented as a poster at the 35th annual meeting of the European Bone and Joint Infection Society (DOI: 10.13140/RG.2.2.10814.28480).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jinf.2017.05.013.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Suppl. data table 1. Characteristics of patients with Staphylococcus lugdunensis orthopaedic infections associated with prosthetic material identified in the two year period beginning April 2014.
Suppl. data table 2. Patients with Staphylococcus aureus prosthetic knee and hip joint infections identified in the two year period beginning April 2014. MSSA = methicillin susceptible S. aureus; MRSA = methicillin resistant S. aureus.
Suppl. data table 3. In vitro susceptibility testing for 67 isolates of Staphylococcus lugdunensis from 20 patients with orthopaedic infections associated with prosthetic material. MIC for each organism was ascertained by BD Phoenix; breakpoints are given in mg/L based on EUCAST data (http://www.eucast.org/clinical_breakpoints). Nd = no data.
References
- 1.Non L.R., Santos C.A. The occurrence of infective endocarditis with Staphylococcus lugdunensis bacteremia: a retrospective cohort study and systematic review. J Infect. 2017;74:179–186. doi: 10.1016/j.jinf.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Lessing M.P., Crook D.W., Bowler I.C., Gribbin B. Native-valve endocarditis caused by Staphylococcus lugdunensis. QJM. 1996;89:855–858. doi: 10.1093/qjmed/89.11.855. [DOI] [PubMed] [Google Scholar]
- 3.Matthews P.C., Missouris C.G., Jordaan J., Lessing M.P. Staphylococcus lugdunensis endocarditis following cardiac catheterisation. Int J Cardiol. 2008;130:87–88. doi: 10.1016/j.ijcard.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 4.Liu P.Y., Huang Y.F., Tang C.W., Chen Y.Y., Hsieh K.S., Ger L.P. Staphylococcus lugdunensis infective endocarditis: a literature review and analysis of risk factors. J Microbiol Immunol Infect. 2010;43:478–484. doi: 10.1016/S1684-1182(10)60074-6. [DOI] [PubMed] [Google Scholar]
- 5.Peel T.N., Cole N.C., Dylla B.L., Patel R. Matrix-assisted laser desorption ionization time of flight mass spectrometry and diagnostic testing for prosthetic joint infection in the clinical microbiology laboratory. Diagn Microbiol Infect Dis. 2015;81:163–168. doi: 10.1016/j.diagmicrobio.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 6.Douiri N., Hansmann Y., Lefebvre N., Riegel P., Martin M., Baldeyrou M. Staphylococcus lugdunensis: a virulent pathogen causing bone and joint infections. Clin Microbiol Infect. 2016;22:747–748. doi: 10.1016/j.cmi.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 7.Lourtet-Hascoet J., Bicart-See A., Felice M.P., Giordano G., Bonnet E. Staphylococcus lugdunensis, a serious pathogen in periprosthetic joint infections: comparison to Staphylococcus aureus and Staphylococcus epidermidis. Int J Infect Dis. 2016;51:56–61. doi: 10.1016/j.ijid.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Atkins B.L., Athanasou N., Deeks J.J., Crook D.W., Simpson H., Peto T.E. Prospective evaluation of criteria for microbiological diagnosis of prosthetic-joint infection at revision arthroplasty. The OSIRIS Collaborative Study Group. J Clin Microbiol. 1998;36:2932–2939. doi: 10.1128/jcm.36.10.2932-2939.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah N.B., Osmon D.R., Fadel H., Patel R., Kohner P.C., Steckelberg J.M. Laboratory and clinical characteristics of Staphylococcus lugdunensis prosthetic joint infections. J Clin Microbiol. 2010;48:1600–1603. doi: 10.1128/JCM.01769-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Joint Registry 13th Annual Report. Available at: http://www.njrcentre.org.uk/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.